Abstract
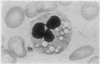
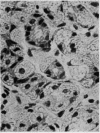
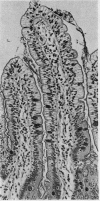
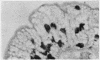
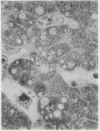
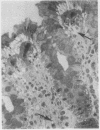
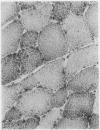
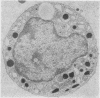
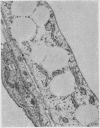
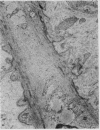
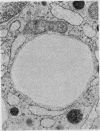
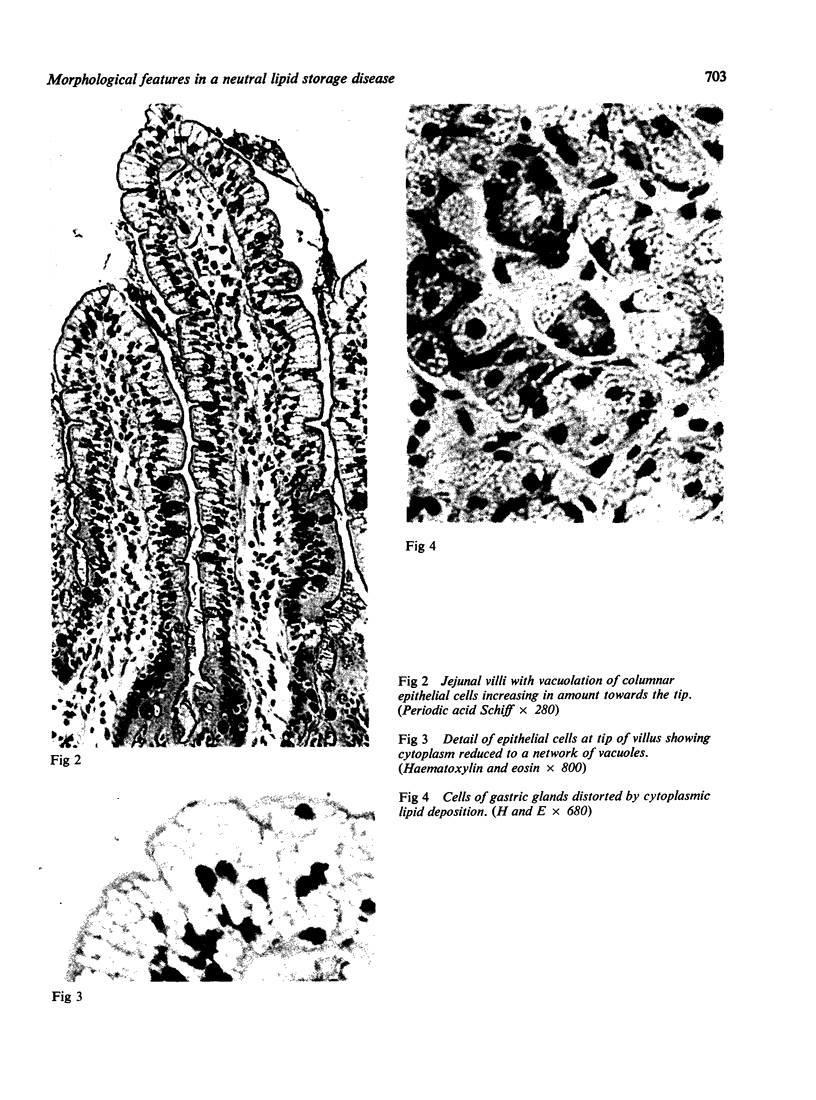
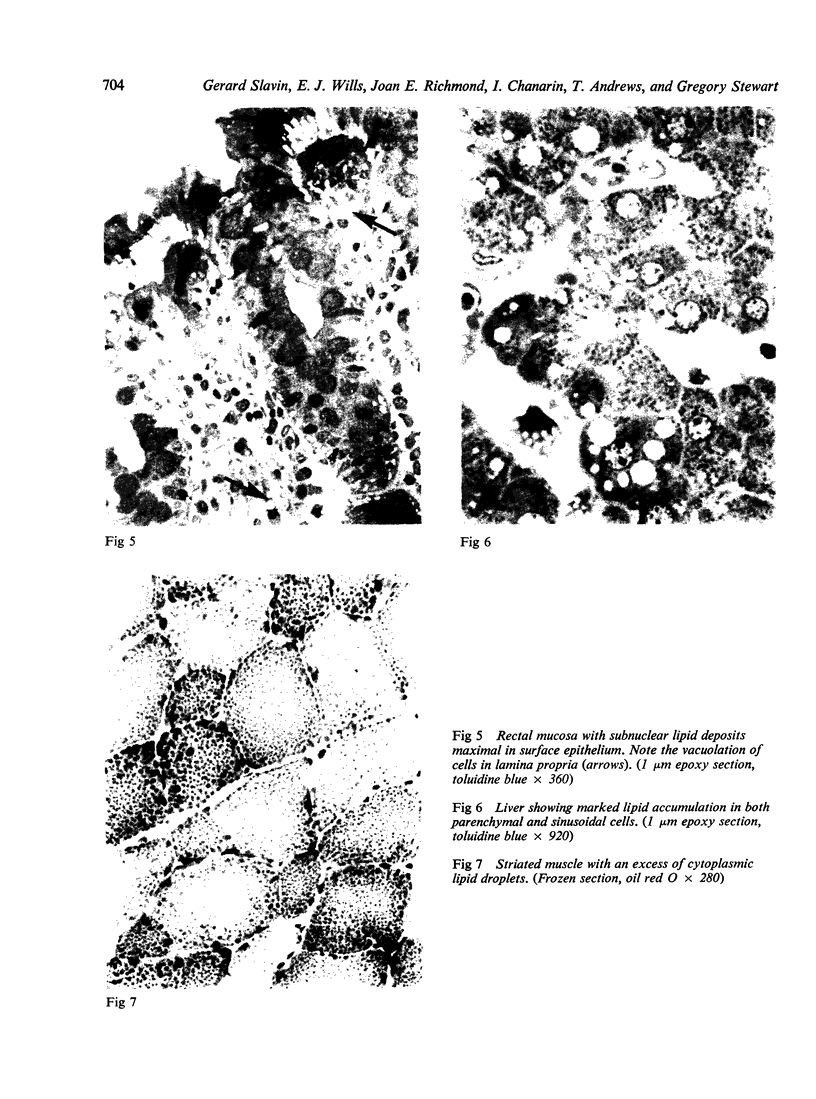
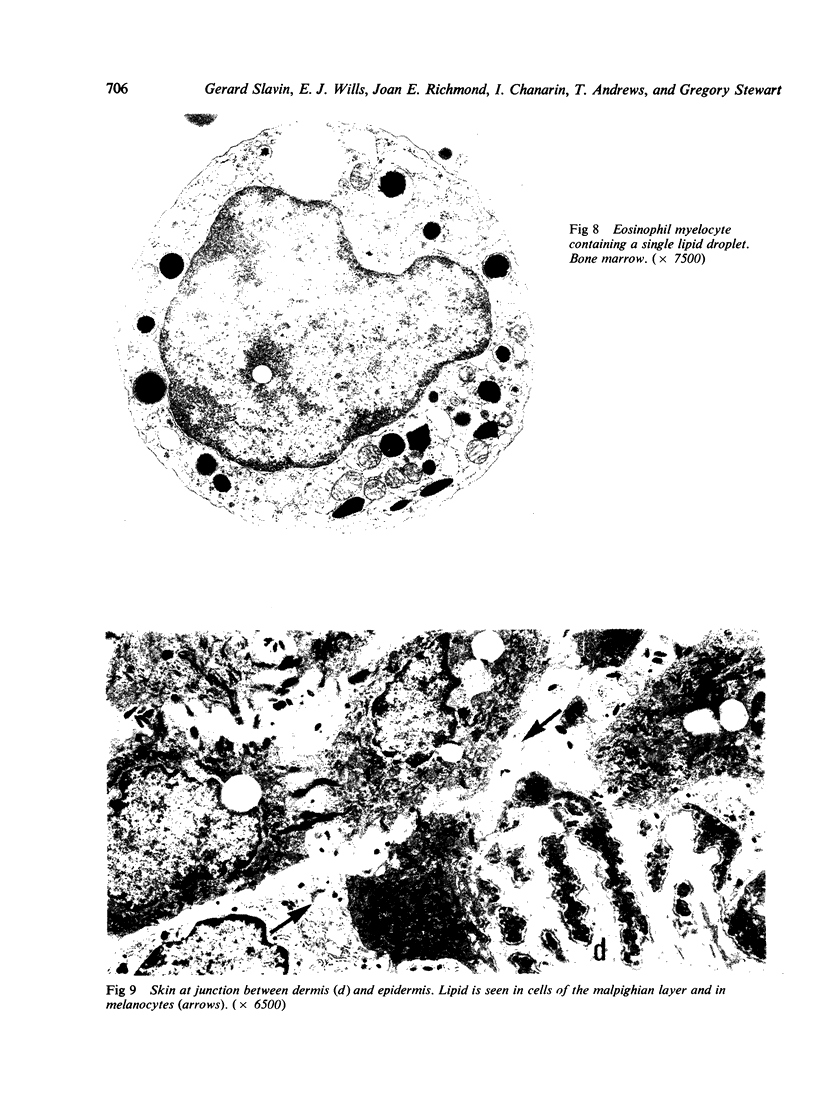
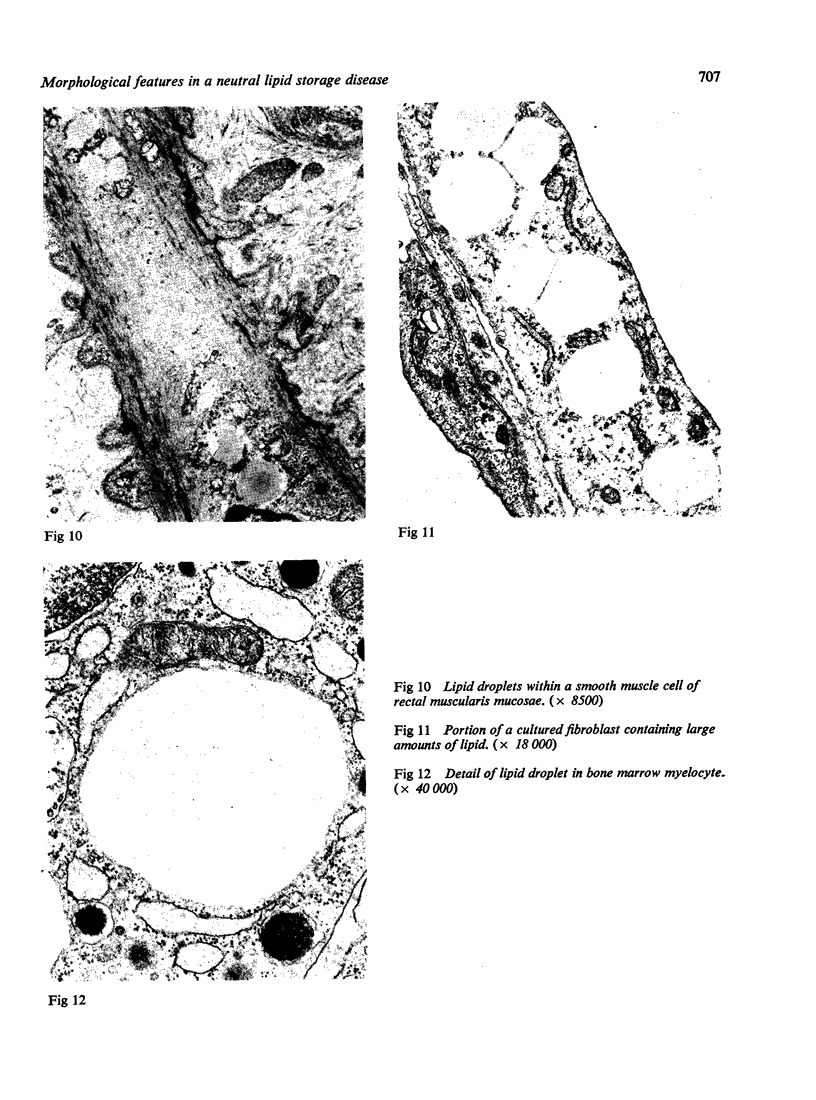
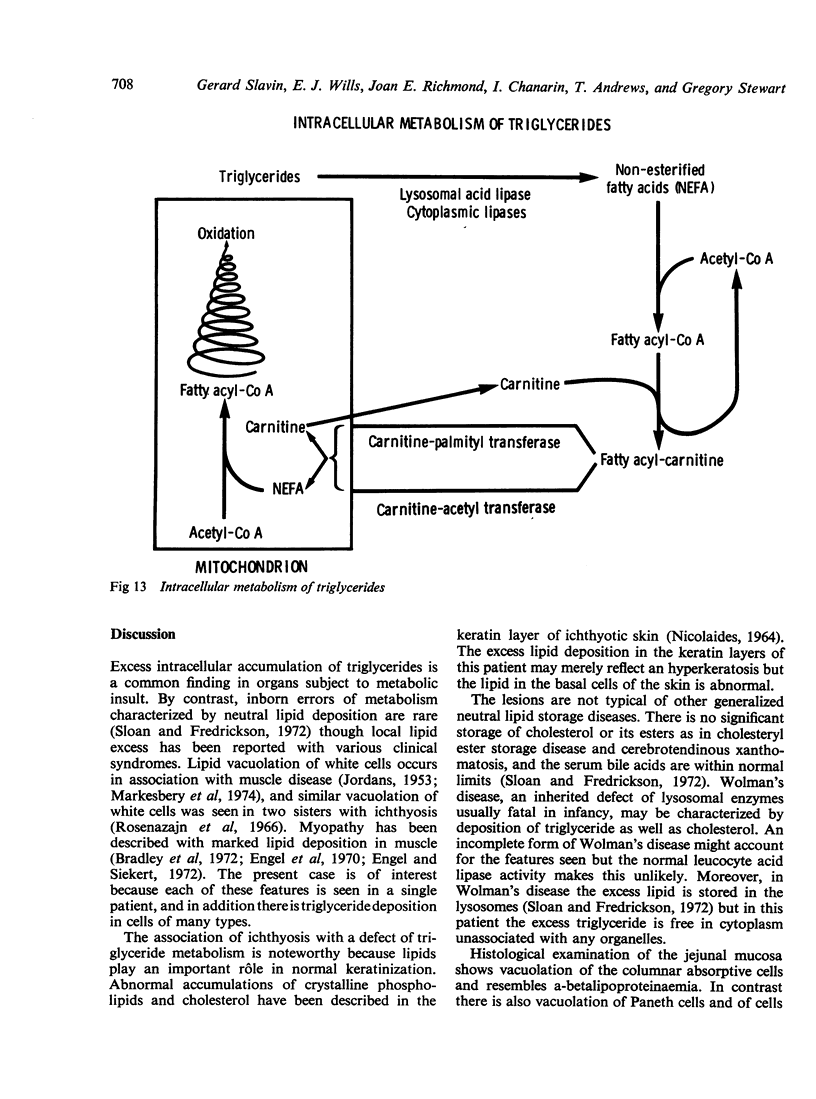
The morphological changes in a patient with a generalized storage disease characterized by the intracellular deposition of neutral lipid are described. There is widespread accumulation of lipid in the cytoplasm of many cells and in occasional nuclei. Diagnosis may be facilitated by the recognition of clear vacuoles in the cytoplasm of granulocytes in blood films. In jejunal biopsies vacuolation of the epithelial cells may simulate the appearances of a-betalipoproteinaemia. The lipid inclusions consist largely of normal triglycerides and are free in the cytoplasm, unassociated with any organelle. The biochemical basis of the lesions is uncertain. Although there are lipoprotein abnormalities the primary defect appears to be intrinsic to the cell and may involve either a defective cytoplasmic lipase or an impaired uptake and utilization of fatty acids by mitochondria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R. A method of preparing peripheral leucocytes for electron microscopy. J Ultrastruct Res. 1965 Oct;13(3):263–268. doi: 10.1016/s0022-5320(65)80075-2. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M., Park D. C., Hudgson P., Gardner-Medwin D., Pennington R. J., Walton J. N. A myopathy associated with lipid storage. J Neurol Sci. 1972 Jun;16(2):137–154. doi: 10.1016/0022-510x(72)90083-4. [DOI] [PubMed] [Google Scholar]
- Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975 Mar 8;1(5957):553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 1973 Mar 2;179(4076):899–902. doi: 10.1126/science.179.4076.899. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Siekert R. G. Lipid storage myopathy responsive to prednisone. Arch Neurol. 1972 Aug;27(2):174–181. doi: 10.1001/archneur.1972.00490140078011. [DOI] [PubMed] [Google Scholar]
- Engel W. K., Vick N. A., Glueck C. J., Levy R. I. A skeletal-muscle disorder associated with intermittent symptoms and a possible defect of lipid metabolism. N Engl J Med. 1970 Mar 26;282(13):697–704. doi: 10.1056/NEJM197003262821301. [DOI] [PubMed] [Google Scholar]
- Harriman D. G., Reed R. The incidence of lipid droplets in human skeletal muscle in neuromuscular disorders: a histochemical, electron-microscopic and freeze-etch study. J Pathol. 1972 Jan;106(1):1–24. doi: 10.1002/path.1711060102. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDANS G. H. The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB.). Acta Med Scand. 1953;145(6):419–423. doi: 10.1111/j.0954-6820.1953.tb07038.x. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery W. R., McQuillen M. P., Procopis P. G., Harrison A. R., Engel A. G. Muscle carnitine deficiency. Association with lipid myopathy, vacuolar neuropathy, and vacuolated leukocytes. Arch Neurol. 1974 Nov;31(5):320–324. doi: 10.1001/archneur.1974.00490410068007. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Prineas J., Ng R. C. Ultrastructural features of intracellular lipid in normal human muscle. Neurology. 1967 Nov;17(11):1092–1098. doi: 10.1212/wnl.17.11.1092. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik M., Hansen J. L. Mitochondria in degenerating and regenerating skeletal muscle. Arch Pathol. 1969 Jun;87(6):601–608. [PubMed] [Google Scholar]
- Rozenszajn L., Klajman A., Yaffe D., Efrati P. Jordans' anomaly in white blood cells. Report of case. Blood. 1966 Aug;28(2):258–265. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]