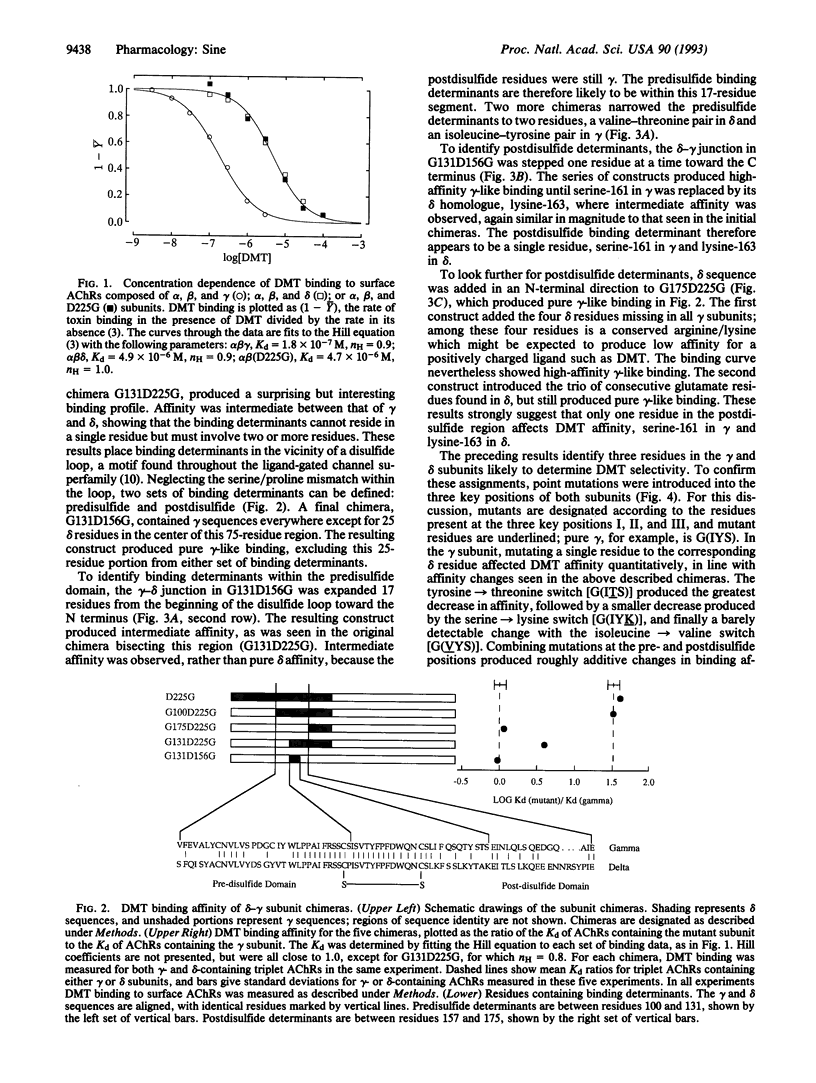
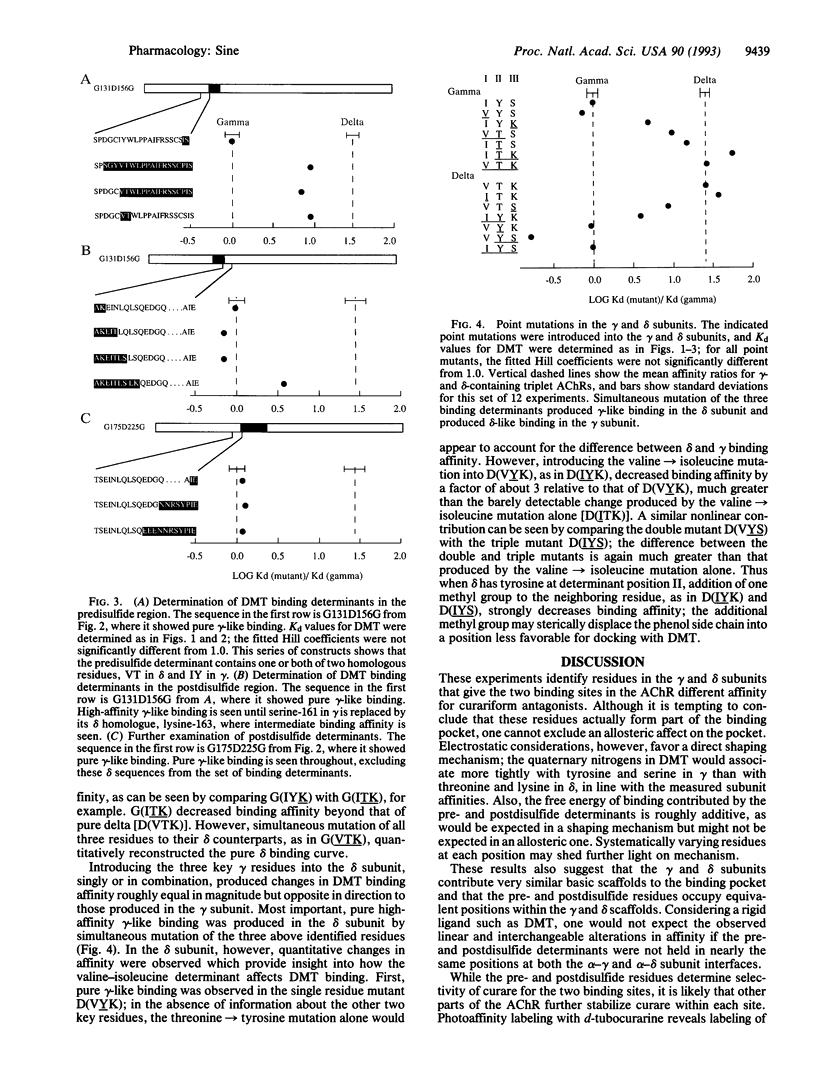
Abstract
The acetylcholine receptor from vertebrate skeletal muscle is a transmembrane channel that binds nerve-released acetylcholine to elicit rapid transport of small cations. Composed of two alpha subunits and one beta, one gamma, and one delta subunit, the receptor is a cooperative protein containing two sites that bind agonists, curariform antagonists, and snake alpha-toxins. Until recently the two binding sites were thought to reside entirely within each of the two alpha subunits, but affinity labeling and expression studies have demonstrated contributions by the gamma and delta subunits. Affinity labeling and mutagenesis studies have identified residues of the alpha subunit that contribute to the binding site, but the corresponding gamma- and delta-subunit residues remain unknown. By making gamma-delta chimeras and following the nearly 100-fold difference in curare affinity for the two binding sites, the present work identified residues of the gamma and delta subunits likely to be near the binding site. Two sets of binding determinants were identified in homologous positions of the gamma and delta subunits. The determinants lie on either side of a disulfide loop found within the major extracellular domain of the subunits. This loop is common to all acetylcholine, gamma-aminobutyrate, and glycine receptor subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Yoshihara C. M., Blackmer K., Kintner C. R., Burden S. J. Regulation of acetylcholine receptor transcript expression during development in Xenopus laevis. J Cell Biol. 1988 Feb;106(2):469–478. doi: 10.1083/jcb.106.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990 Oct;5(4):383–392. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989 Sep;3(3):349–357. doi: 10.1016/0896-6273(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski C., Kaufmann C., Karlin A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6285–6289. doi: 10.1073/pnas.90.13.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Noda M., Takai T., Tanabe T., Kayano T., Shimizu S., Tanaka K., Takahashi H., Hirose T., Inayama S. Primary structure of delta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur J Biochem. 1985 May 15;149(1):5–13. doi: 10.1111/j.1432-1033.1985.tb08885.x. [DOI] [PubMed] [Google Scholar]
- LaPolla R. J., Mayne K. M., Davidson N. Isolation and characterization of a cDNA clone for the complete protein coding region of the delta subunit of the mouse acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. S., Gunn R. B., Kopito R. R. Functional differences among nonerythroid anion exchangers expressed in a transfected human cell line. J Biol Chem. 1991 Jun 25;266(18):11448–11454. [PubMed] [Google Scholar]
- Luther M. A., Schoepfer R., Whiting P., Casey B., Blatt Y., Montal M. S., Montal M., Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989 Mar;9(3):1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef P., Mauron A., Stalder R., Alliod C., Ballivet M. Structure linkage, and sequence of the two genes encoding the delta and gamma subunits of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7975–7979. doi: 10.1073/pnas.81.24.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Equilibrium binding of [3H]tubocurarine and [3H]acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry. 1979 Nov 27;18(24):5464–5475. doi: 10.1021/bi00591a032. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Kubo T., Perski H. J., Takahashi H., Noda M., Numa S. Cloning and sequence analysis of human genomic DNA encoding gamma subunit precursor of muscle acetylcholine receptor. Eur J Biochem. 1985 Jan 2;146(1):15–22. doi: 10.1111/j.1432-1033.1985.tb08614.x. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J Biol Chem. 1991 Oct 15;266(29):19369–19377. [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M. Functional properties of human skeletal muscle acetylcholine receptors expressed by the TE671 cell line. J Biol Chem. 1988 Dec 5;263(34):18052–18062. [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Relationship between reversible antagonist occupancy and the functional capacity of the acetylcholine receptor. J Biol Chem. 1981 Jul 10;256(13):6692–6699. [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Takai T., Noda M., Furutani Y., Takahashi H., Notake M., Shimizu S., Kayano T., Tanabe T., Tanaka K., Hirose T. Primary structure of gamma subunit precursor of calf-muscle acetylcholine receptor deduced from the cDNA sequence. Eur J Biochem. 1984 Aug 15;143(1):109–115. doi: 10.1111/j.1432-1033.1984.tb08348.x. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Stein E., Barg B., Konno T., Koenen M., Kues W., Criado M., Hofmann M., Sakmann B. Primary structure and functional expression of the alpha-, beta-, gamma-, delta- and epsilon-subunits of the acetylcholine receptor from rat muscle. Eur J Biochem. 1990 Dec 12;194(2):437–448. doi: 10.1111/j.1432-1033.1990.tb15637.x. [DOI] [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]



