Summary
Background
Sodium glucose co‐transporter 2 (SGLT2) inhibitors are a new class of pharmacologic agents developed for the treatment of type 2 diabetes mellitus (T2DM). Their unique mechanism of action is independent of pancreatic beta‐cell function or the degree of insulin resistance, giving these agents the potential for use in combination with any of the existing classes of glucose‐lowering agents, including insulin. This makes SGLT2 inhibitors an option for patients with long‐standing T2DM, but they also have a promising role for early intervention in T2DM, and that role is explored in this review.
Methods
A literature search was performed to identify relevant English language articles relating to SGLT2 inhibitors, particularly dapagliflozin, canagliflozin and empagliflozin.
Results
Clinical trials of dapagliflozin, canagliflozin and empagliflozin, given as monotherapy or in combination with other glucose‐lowering agents, reported clinically significant improvements in glycaemic control, body weight and systolic blood pressure. SGLT2 inhibitors were well tolerated and had a generally favourable safety profile. Few serious adverse events have been reported to date. The frequency of hypoglycaemic events was low, similar to that of placebo, and the choice of co‐administered glucose‐lowering agent was the major determinant of hypoglycaemic risk. Increased genital and urinary tract infections were consistently reported with SGLT2 inhibitors.
Conclusions
SGLT2 inhibitors, with their unique insulin‐independent mode of action, could have a significant impact on the early management of T2DM, by addressing some of the specific risk factors associated with this disease. SGLT2 inhibitors induce beneficial changes in a number of cardiovascular risk factors, such as lowering blood pressure and body weight, in addition to improved glycaemic control, although information on clinical cardiovascular outcomes is currently limited.
Review criteria
A MEDLINE search was performed to identify relevant English language articles relating to SGLT2 inhibitors, and particularly those currently with US marketing approval; namely, dapagliflozin, canagliflozin and empagliflozin. Abstracts were obtained from the websites of major diabetes and endocrinology congresses. Additional data were obtained from the websites of the European Medicines Agency, US Food and Drug Administration and the pharmaceutical companies sponsoring the development of individual SGLT2 inhibitors.
Message for the clinic
SGLT2 inhibitors, with their unique insulin‐independent mode of action, could have a significant impact on the early management of T2DM, by addressing some of the specific risk factors associated with this disease. SGLT2 inhibitors induce beneficial changes in a number of cardiovascular risk factors, such as lowering blood pressure and body weight, in addition to improved glycaemic control.
Introduction
Diabetes mellitus is a significant health burden in the United States, occurring in 9.3% of the population (approximately 29.1 million individuals) 1. Type 2 diabetes mellitus (T2DM) is more common than type 1, and accounts for 90–95% of all cases of diabetes mellitus 2. T2DM is a complex cardio‐metabolic disorder characterised by insulin resistance, pancreatic beta‐cell failure and hyperglycaemia 3. People with T2DM are at increased risk of developing macrovascular complications (coronary artery disease, peripheral artery disease and stroke), as well as microvascular complications (diabetic retinopathy, nephropathy and neuropathy). Early and effective intervention in T2DM to obtain good glycaemic control is vital to reduce the risks of long‐term diabetic complications 4. The benefits of early and intensive glycaemic control in reducing microvascular complications in T2DM are well established 5, 6, 7, 8, and these benefits are maintained over the long‐term 9. Results from randomised controlled trials (RCTs) have not shown the same consistency regarding reductions in macrovascular complications 9, 10; however, several meta‐analyses of RCTs reported tight glycaemic control had a positive effect on cardiovascular outcomes 11, 12, 13.
Lifestyle modification, particularly regarding weight control in overweight/obese individuals, is a crucial component of T2DM therapy, but most patients eventually require glucose‐lowering pharmacotherapy to control hyperglycaemia. Although initial drug monotherapy is recommended, usually with metformin 14, given the progressive nature of T2DM, combination therapy is eventually required for most patients to achieve adequate glycaemic control. A number of classes of glucose‐lowering agents are available, but some of them are associated with side effects (e.g. weight gain, hypoglycaemia) that need to be considered when the choice of pharmacotherapy is made. Thus, there is a continual need for novel T2DM pharmacotherapies with improved efficacy and safety/tolerability.
Sodium glucose co‐transporter 2 (SGLT2) inhibitors are a new class of pharmacologic agents for T2DM treatment; they reduce hyperglycaemia by targeting the kidney to promote urinary glucose excretion. SGLT2 inhibitors have a unique mechanism of action that is independent of pancreatic beta‐cell function or the degree of insulin resistance, conferring these agents the potential to be used at any stage of the disease, and in combination with any of the existing classes of glucose‐lowering agents, including insulin. In turn, this would allow them to be used at any stage of disease course, and may have created the perception that they are particularly appropriate for patients with long‐standing T2DM. The aim of this review is to examine the evidence supporting the role of SGLT2 inhibitors as an early intervention in patients recently diagnosed with T2DM.
Methods
To identify relevant English language articles relating to SGLT2 inhibitors, a MEDLINE search was performed using ‘SGLT2’ as a search term, as well as the individual drug names for SGLT2 inhibitors with marketing approvals in the US; namely, dapagliflozin, canagliflozin and empagliflozin. Characteristics of clinical trials to be included in the review were not pre‐defined, although detailed review of efficacy and safety was restricted to phase 3 studies. Key parameters reviewed were the changes in glycated haemoglobin (HbA1c) levels, fasting plasma glucose (FPG) levels, body weight and blood pressure (BP). Abstracts were obtained from the websites of major diabetes and endocrinology congresses, and were included if the corresponding manuscript had not been published. Additional data were obtained from the websites of the European Medicines Agency, US Food and Drug Administration, and the website of the pharmaceutical companies sponsoring the development of individual SGLT2 inhibitors.
Early intervention in T2DM
Given the complex nature of T2DM, there is agreement that drug treatment should be tailored to each patient, according to their individual glycaemic target (i.e. HbA1c) and other factors, such as duration and stage of disease, life expectancy, risk of hypoglycaemia and risk of cardiovascular disease (CVD) 15. The recommended glycaemic target for many non‐pregnant adults with T2DM is < 7.0% 14. This can be individualised so that a more stringent target (e.g. < 6.5%) is applied to a newly diagnosed person with no complications (e.g. without CVD) 14. Some clinicians believe the target HbA1c should be reduced further to ≤ 6.0% in newly diagnosed T2DM patients with no CVD 16. Conversely, a less stringent target (e.g. < 8.0%) could be applied to T2DM patients with advanced CVD, reduced life expectancy and multiple comorbidities. Whatever the precise goal, it is also well established that early and effective intervention in T2DM provides a greater opportunity to reduce the risks of long‐term diabetes complications 15.
As described in a recent review by DeFronzo and colleagues, an individual has already lost approximately 80% of their beta‐cell function by the time a diagnosis of T2DM is made; thus, drug therapy must be started promptly to compensate for the progressive beta‐cell failure that is already well established in such individuals 16. They suggest treatment should be based on the reversal of known pathogenic abnormalities (beta‐cell failure and insulin resistance) and not simply on HbA1c reductions 16. To accomplish this, they proposed early combination therapy with thiazolidinediones (TZDs) and glucagon‐like peptide 1 receptor (GLP‐1R) agonists added to metformin, as these agents improve and preserve beta‐cell function, and TZDs are also potent insulin sensitizers while GLP‐1R agonists promote weight loss 17, 18, 19, 20, 21, 22. However, these drugs have limitations; for example, TZDs are associated with weight gain, fluid retention and bone fractures 21, 23, whereas GLP‐1R agonists are given via subcutaneous injection, and are associated with gastrointestinal side effects 24. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors provide an alternative incretin‐based option to GLP‐1R agonists, but are weight‐neutral rather than associated with weight loss 25. Thus, there remains a need for additional treatment options in the early stages of T2DM.
SGLT2 inhibitors: background and mechanism of action
In addition to the core pathologic defects of beta‐cell failure and insulin resistance, a number of other factors contribute to disease progression in T2DM. Together, these have been termed the ‘ominous octet’, as shown in Figure 1. The dysregulation of kidney‐mediated maintenance of glucose homoeostasis is one component of the ominous octet 3. Renal glucose resorption capacity is increased in individuals with diabetes 26, 27, 28, and the kidneys continue to reabsorb glucose even when plasma glucose concentrations are high, with levels that usually exceed the transport maximum of glucose of healthy individuals. This leads to the continuous movement of glucose from the kidneys into the circulation, even in the presence of hyperglycaemia, thus perpetuating hyperglycaemia and increasing the risk for diabetes‐associated complications. In addition, renal gluconeogenesis is elevated in patients with T2DM, resulting in increased glucose release in these individuals. Renal gluconeogenesis is negatively regulated by insulin and renal glucose production increases with increasing insulin resistance, with 40% of the increased endogenous glucose release in patients with T2DM attributable to increased renal gluconeogenesis 29.
Figure 1.
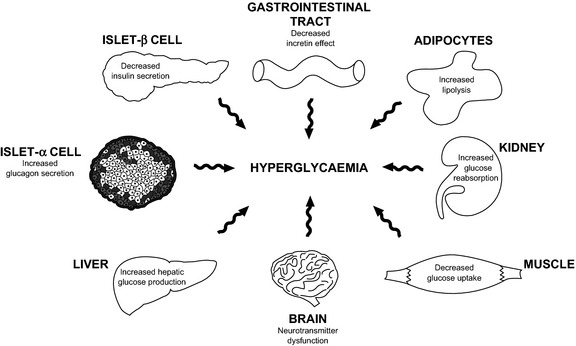
The ominous octet. In addition to the insulin resistance in the muscle and liver, and impaired insulin secretion in the β‐cell, the fat cell (accelerated lipolysis), gastrointestinal tract (incretin deficiency/resistance), α‐cell (hyperglucagonaemia), kidney (increased glucose reabsorption) and brain (insulin resistance) all play important roles in the development of glucose intolerance in type 2 diabetic individuals 3. Reproduced with permission from DeFronzo R et al. Diabetes, 2009; 58:773–795. Copyright ©2009 American Diabetes Association. All rights reserved
SGLT2 is a low‐affinity, high‐capacity glucose transporter located in the early part of the proximal tubule, involved in the reabsorption of the vast majority (~90%) of glucose in the kidney 30. As the actions of SGLT2 promote glucose conservation and the maintenance of plasma glucose concentrations, inhibition of SGLT2 may have the opposite effect; namely, to reduce hyperglycaemia by stimulating urinary glucose excretion 31. Observations in individuals with SGLT2 gene alterations suggest that functional depletion of SGLT2 may not have long‐term deleterious effects, at least in the individuals followed up to date. The resulting disorder, known as familial renal glucosuria, causes urinary glucose excretion, with the amount of glucose excreting ranging from < 10 g/day to > 200 g/day 32, 33. Affected individuals are usually otherwise asymptomatic 33, and the condition is not known to be associated with T2DM or other pathological sequelae.
As the action of SGLT2 is independent of insulin, its inhibition should not be influenced by pancreatic beta‐cell mass or function, or by the degree of insulin resistance present. Therefore, SGLT2 inhibitors have the potential to be used at any stage of T2DM. They may even have the potential to show efficacy as the disease progresses, unlike some other types of antidiabetes agents that show a decline in glucose‐lowering potential caused by their dependence on beta‐cell function (e.g. sulfonylureas or glinides). Additionally, the non–insulin‐dependent mechanism of action of SGLT2 inhibitors gives them the potential to be used in combination with any of the existing classes of glucose‐lowering agents, including insulin. Other metabolic characteristics of SGLT2 inhibitors may also be anticipated. For example, SGLT2 inhibitors should not increase the risk of hypoglycaemia, as inhibition of SGLT2 does not affect endogenous glucose production 34, does not stimulate insulin release when glucose levels decline and does not cause urinary glucose excretion when plasma glucose levels fall below threshold values 35, 36, 37. SGLT2 inhibitors should also promote some weight loss 36, resulting from the reduction in available calories caused by urinary glucose excretion. This ability to induce weight loss, along with the ability to act as a diuretic, would also suggest a potential BP‐lowering effect for SGLT2 inhibitors 38.
Clinical experience with SGLT2 inhibitors
Dosing, pharmacology and current indications
Dapagliflozin, canagliflozin and empagliflozin are approved for use in the US and European Union (Table 1) 39, 40, 41, 42, 43, 44. In addition, various fixed‐dose combination products involving an SGLT2 inhibitor plus a second oral glucose‐lowering agent (including metformin, metformin extended‐release and DPP‐4 inhibitors) are in clinical development. A fixed‐dose combination product containing dapagliflozin plus metformin (in 5/850 mg and 5/1000 mg tablets) was recently granted marketing authorisation in the EU 45, and an extended‐release version of this combination has been recently approved for use in the US (in 5/500 mg, 10/500 mg, 5/1000 mg and 10/1000 mg tablets) 46. A fixed‐dose combination of canagliflozin plus metformin has also been granted marketing authorisation, both in the EU (in 50/850 mg, 150/850 mg, 50/1000 mg and 150/1000 mg) and the US (in 50/500 mg, 150/500 mg, 50/1000 mg and 150/1000 mg) 47, 48.
Table 1.
SGLT2 inhibitors approved for use in the US
| Drug | Approved dosages | Selectivity for SGLT2 vs. SGLT1 | Indications* | Dose adjustment in renal impairment† |
|---|---|---|---|---|
| Canagliflozin 43 | 100 mg, 300 mg | > 250‐fold | Adjunct to diet and exercise to improve glycaemic control in adults with T2DM |
No dose adjustment needed in pts with eGFR ≥ 60 ml/min/1.73 m2
Limited to 100 mg in pts with eGFR 45 to < 60 ml/min/1.73 m2 Should not be initiated in pts with eGFR < 45 ml/min/1.73 m2 Contraindicated in severe renal impairment (eGFR ≤ 30 ml/min/1.73 m2), end‐stage renal disease, or dialysis |
| Dapagliflozin 41 | 5 mg, 10 mg | > 1200‐fold | Adjunct to diet and exercise to improve glycaemic control in adults with T2DM |
No dose adjustment needed in pts with eGFR ≥ 60 ml/min/1.73 m2
Should not be initiated in pts with eGFR < 60 ml/min/1.73 m2 Contraindicated in severe renal impairment, end‐stage renal disease, or dialysis |
| Empagliflozin 40 | 10 mg, 25 mg | > 2500‐fold | Adjunct to diet and exercise to improve glycaemic control in adults with T2DM |
No dose adjustment is needed in pts with eGFR ≥ 45 ml/min/1.73 m2
Should not be initiated in pts with eGFR < 45 ml/min/1.73 m2 Contraindicated in severe renal impairment, end‐stage renaldisease, or dialysis |
*Indications shown are for US prescribing information. In the EU, all three drugs shown are indicated as monotherapy when diet and exercise alone do not provide adequate glycaemic control in pts for whom the use of metformin is considered inappropriate because of intolerance or contraindications 39, 42, 44. †Use in specific populations and contraindications are based on US prescribing information at the time of writing; EU advice may differ. Metabolism of dapagliflozin, canagliflozin and empagliflozin occurs in the liver and kidneys, and elimination of the drugs occurs predominantly via faeces but also in the urine 40, 111, 112. T2DM, type 2 diabetes mellitus; pts, patients; eGFR, estimated glomerular filtration rate.
As new agents, SGLT2 inhibitors are beginning to be incorporated in treatment guidelines. The 2013 American Association of Clinical Endocrinologists algorithm included SGLT2 inhibitors as a therapeutic alternative in patients with T2DM in whom metformin is not tolerated or otherwise contraindicated 49. The algorithm also stated that SGLT2 inhibitors could be used as add‐on therapy to two or three other agents, including insulin, in patients who would benefit from weight loss 49. In 2015, the American Diabetes Association and European Association for the Study of Diabetes issued an update to their joint position statement, including SGLT2 inhibitors among the options for second‐line therapy after metformin, and as alternative first‐line options in patients with contraindications to metformin, or as add‐on to insulin to improve glycaemic control and reduce the requirement for insulin 50.
Efficacy
A summary of efficacy data from the main phase 3 clinical trials of dapagliflozin, canagliflozin and empagliflozin are presented in Table 2 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69.
Table 2.
Efficacy data summary from the main phase 3 clinical trials of dapagliflozin, canagliflozin and empagliflozin*
| Study details (N §) | Treatment, dose (mg/day) | Change from baseline† , ‡ | |||
|---|---|---|---|---|---|
| HbA1c (%) | FPG (mg/dl) | Body weight (kg) | SBP (mmHg) | ||
| Dapagliflozin | |||||
| Monotherapy, 24 weeks (N = 485) 51 | Pbo | −0.23 | −4.1 | −2.2 | −0.9 |
| Dapa 5/10 | −0.77 to −0.89 | −24.1 to −29.6 | −2.8 to −3.6 | −2.3 to −5.2 | |
| Monotherapy (A1c ≥10.1), 24 weeks (N = 73) 51 | Dapa 5/10 | −2.88/−2.66 | −77.1/−84.3 | −2.1/−1.9 | −5.7/−2.5 |
| Add‐on to MET, 24 weeks (N = 546) 52 | Pbo | −0.30 | −5.95 | −0.9 | −0.2 |
| Dapa 5/10 | −0.70/−0.84 | −21.44/−23.42 | −3.0/−2.9 | −4.3/−5.1 | |
| Initial combination with MET XR, 24 weeks (N = 1244) 53 | Pbo + MET XR | −1.35 to −1.44 | −33.51 to −34.78 | −1.29 to −1.36 | −1.2 to −1.8 |
| Dapa 5/10 + MET XR | −1.98 to −2.05 | −60.36 to −61.09 | −2.66 to −3.33 | −2.9 to −3.30 | |
| Add‐on to SU (GLIM), 24 weeks (N = 597) 54 | Pbo | −0.13 | −1.98 | −0.72 | −1.2 |
| Dapa 5/10 | −0.63 to −0.82 | −21.26 to −28.47 | −1.56 to −2.26 | −4.0 to −5.0 | |
| Add‐on to DPP4i (SITA) ± MET, 24 weeks (N = 432) 55 | Pbo + SITA | 0.1 | 4.6 | −0.1 | −4.2 |
| Dapa 10 + SITA | −0.5 | −22.0 | −1.9 | −6.6 | |
| Pbo + SITA + MET | −0.0 | 3.0 | −0.5 | −5.5 | |
| Dapa 10 + SITA + MET | −0.4 | −26.2 | −2.4 | −5.3 | |
| Add‐on to MET, 52 weeks (N = 814) 56 | Dapa 2.5–10 | −0.52 | −22.34 | −3.22 | −4.3 |
| GLIP 5–20 | −0.52 | −18.74 | 1.44 | 0.8 | |
| Add‐on to TZD, 48 weeks (N = 420) 57 | Pbo | −0.54 | −13.1 | 2.99 | 2.0 |
| Dapa 5/10 | −0.95 to −1.21 | −22.8 to −33.1 | 0.69 to 1.35 | −1.0 to −2.2 | |
| Add‐on to INS (≥ 30 units/day) ± OAD, 48 weeks (N = 800) 58 | Pbo | −0.47 | N/r | 0.82 | −1.49 |
| Dapa 5/10 | −0.96 to −1.01 | N/r | −1.00 to −1.61 | −4.09 to −4.33 | |
| Canagliflozin | |||||
| Monotherapy, 26 weeks (N = 584) 59 | Pbo | 0.14 | 9.00 | −0.5 | 0.4 |
| Cana 100/300 | −0.77 to −1.03 | −27.03 to −34.23 | −2.5 to −3.4 | −3.3 to −5.0 | |
| Monotherapy (A1c>10.0 ≤ 12.0) (N = 94) 59 | Cana 100/300 | −2.1 to −2.6 | −81.1 to −86.5 | −3.0 to −3.8 | −4.5 to −5.0 |
| Add‐on to MET, 52 weeks (N = 1450) 60 | GLIM 1–8 | −0.81 | −18.0 | 0.7 | 0.2 |
| Cana 100/300 | −0.82 to −0.93 | −25.2 to −27.0 | −3.7 to −4.0 | −3.3 to −4.6 | |
|
Add‐on to MET, 52 weeks (N = 1284) 61
(26 weeks Pbo + comparator; 26 weeks comparator) |
SITA 100 | −0.73 | −17.7 | −1.3% | −0.7 |
| Cana 100/300 | −0.73 to −0.88 | −26.2 to −35.2 | −3.8 to −4.2% | −3.5 to −4.7 | |
| Add‐on to MET + SU, 52 weeks (N = 755) 62 | SITA 100 | −0.66 | −2.2 | 0.1 | 0.9 |
| Dapa 300 | −1.03 | −28.7 | −2.3 | −5.1 | |
| Add‐on to MET + SU, 26 weeks (N = 469) 63 | Pbo | −0.13 | 3.60 | −0.8 | −2.7 |
| Cana 100/300 | −0.85 to −1.06 | −18.02 to −30.63 | −1.9 to −2.5 | −4.3 to −4.9 | |
| Add‐on to MET + TZD (PIO), 26 weeks (+26‐week extension) (N = 342) 64 | Pbo | −0.26 | 2.5 | −0.2 | −1.2 |
| Cana 100/300 | −0.89 to −1.03 | −26.8 to −33.2 | −2.6 to −3.8 | −4.7 to −5.3 | |
|
Add‐on to INS (≥ 30 units/day) ± OADs Substudy efficacy duration 18 weeks (N = 1708) 65 |
Cana 100/300¶ | −0.65 to −0.73¶ | −22.52 to −29.01¶ | −1.9 to −2.4%¶ | −2.6 to −4.4¶ |
| Empagliflozin | |||||
| Monotherapy, 24 weeks (N = 899) 66 | Pbo | 0.08 | 11.7 | −0.33 | −0.3 |
| SITA 100 | −0.66 | −6.85 | 0.18 | 0.5 | |
| Empa 10/25 | −0.66 to −0.78 | −19.5 to −24.5 | −2.26 to −2.48 | −2.9 to −3.7 | |
| Monotherapy (A1c > 10.0), 24 weeks (N = 87) 66 | Empa 25 | −3.70 | −84.3 | −2.43 | −4.0 |
| Add‐on to MET, 24 weeks (N = 637) 67 | Pbo | −0.13 | 6.38 | −0.45 | −0.4 |
| Empa 10/25 | −0.70 to −0.77 | −20.04 to −22.28 | −2.08 to −2.46 | −4.5 to −5.2 | |
| Add‐on to MET + SU, 24 weeks (N = 666) 68 | Pbo | −0.17 | 5.52 | −0.39 | −1.4 |
| Empa 10/25 | −0.77 to −0.82 | −23.27 to −23.30 | −2.16 to −2.39 | −3.5 to −4.1 | |
| Add‐on to TZD (PIO) ± MET, 24 weeks (N = 498) 69 | Pbo | −0.11 | 6.47 | 0.34 | 0.7 |
| Empa 10/25 | −0.59 to 0.72 | −17.0 to −22.0 | −1.47 to −1.62 | −3.1 to −4.0 | |
| Add‐on to MET, 104 weeks (N = 1549) 79 | GLIM 1–4 | −0.55 | −3.06 | 1.3 | 2.5 |
| Empa 25 | −0.66 | −15.32 | −3.1 | −3.1 | |
*All included studies were conducted in adults (≥ 18 years old). †Data for high glycaemic subgroups are presented for monotherapy studies only. ‡Data are presented as reported in each publication; the range of changes shown is for approved doses of the drug only. §Number of patients randomized. ¶Adjusted mean difference from placebo. HbA1c, glycated haemoglobin; FPG, fasting plasma glucose; SBP, systolic blood pressure; Pbo, placebo; Dapa, dapagliflozin; A1c, glycated haemoglobin; MET, metformin; GLIP, glipizide; XR, extended‐release formulation; SU, sulfonylurea; GLIM, glimepiride; DPP4i, dipeptidyl peptidase‐4 inhibitor; SITA, sitagliptin; TZD, thiazolidinedione; INS, insulin; OAD, oral antidiabetes drug; N/r, not reported (in original publication); Cana, canagliflozin; PIO, pioglitazone; Empa, empagliflozin.
Glycaemic control
In patients with T2DM, treatment with the SGLT2 inhibitors dapagliflozin, canagliflozin and empagliflozin, given as monotherapy and/or in combination with other antidiabetes agents, produced clinically and statistically significant improvements in HbA1c vs. placebo. In some cases, improvements were also significantly greater than with active comparators; for example, comparison of efficacy data for an SGLT2 inhibitor (canagliflozin) vs. a DPP‐4 inhibitor (sitagliptin) is presented in Figure 2 62. Data on HbA1c and FPG from individual clinical trials are presented in Table 2.
Figure 2.
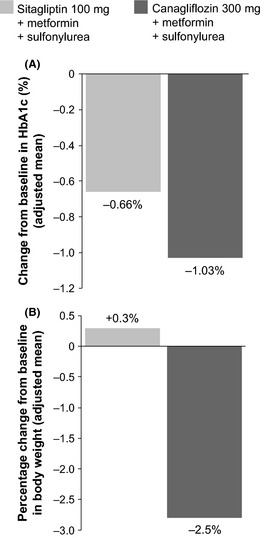
Efficacy data for an SGLT2 inhibitor vs. a DPP‐4 inhibitor. Panel A shows a greater reduction in HbA1c from baseline to week 52 in patients receiving canagliflozin (N = 377) vs. sitagliptin (N = 378), when both agents were given in combination with metformin and a sulfonylurea. Mean baseline HbA1c was 8.12% and 8.13% in the canagliflozin and sitagliptin groups, respectively. As shown in panel B, there was a decrease in body weight from baseline to week 52 in the canagliflozin group, compared with an increase in body weight for the sitagliptin group. DPP‐4, dipeptidyl peptidase‐4; HbA1c, glycated haemoglobin 62
In addition to mean changes in blood glucose levels, attainment of the widely used HbA1c target of < 7.0% was measured, as this outcome provides a guide to the likelihood of achieving goals in practice. All three SGLT2 inhibitors significantly increased the odds of achieving goal, although differences between trials in the placebo group attainment rate was notable, presumably reflecting baseline characteristics of patients in individual trials. In treatment‐naïve patients in whom hyperglycaemia was insufficiently controlled with diet and exercise alone, 24 weeks of monotherapy with dapagliflozin 10 mg led to 51% of patients achieving HbA1c < 7.0%, vs. 32% of those in the placebo group 51. Treatment with dapagliflozin added on to stable metformin led to significantly greater proportions of subjects in the dapagliflozin 5‐mg and 10‐mg groups achieving HbA1c < 7.0% after 24 weeks vs. placebo (37.5% and 40.6%, respectively, vs. 25.9% with placebo) 52. Canagliflozin monotherapy given for 26 weeks resulted in 44.5% and 62.4% of subjects receiving 100 mg and 300 mg, respectively, achieving HbA1c < 7.0%, vs. 20.6% of those on placebo 59. When canagliflozin was used with metformin, HbA1c < 7.0% occurred in 54% and 60% of subjects receiving 100 mg and 300 mg, respectively, vs. 56% of those receiving the active comparator glimepiride 60. Empagliflozin monotherapy given for 24 weeks led to HbA1c < 7.0% in 35.3% and 43.6% of subjects receiving empagliflozin 10 mg and 25 mg, respectively, vs. 37.5% for the active comparator group (sitagliptin 100 mg), and 12.0% in the placebo group 66. When empagliflozin was given in combination with metformin, HbA1c < 7.0% occurred in 37.7% and 38.7% of empagliflozin 10‐mg and 25‐mg groups, respectively, vs. 12.5% of the placebo group 67.
As the mechanism of action of SGLT2 inhibitors relies on the glomerular filtration rate, reduced efficacy is predicted in patients with impaired renal function. Dapagliflozin did not improve HbA1c in patients with T2DM and moderate renal impairment (eGFR ≥ 30 to < 60 ml/min/1.73 m2) after 52 weeks 70, whereas canagliflozin 100 mg and 300 mg significantly lowered HbA1c compared with placebo in patients with T2DM and eGFR ≥ 30 to < 50 ml/min/1.73 m2 after 26 weeks, with placebo‐corrected changes of −0.30% and −0.40%, respectively 71. In patients with T2DM and moderate renal impairment (eGFR ≥ 30 to < 60 ml/min/1.73 m2), empagliflozin 25 mg significantly lowered HbA1c vs. placebo with a placebo‐corrected mean treatment difference of −0.42% at week 24 72.
Blood pressure
A recent meta‐analysis of 27 RCTs, predominantly involving dapagliflozin (n = 12) and canagliflozin (n = 9), reported that SGLT2 inhibitor use was associated with a statistically significant reduction in systolic blood pressure (SBP) from baseline (−4.0 mmHg; 95% confidence interval [CI], −4.4, −3.5; Cochrane p = 0.986; I 2 = 0%) 73. This reduction was similar when placebo‐controlled RCTs and active‐controlled RCTs were pooled separately 73. A meta‐analysis of 10 dapagliflozin RCTs reported that decreases in SBP (seated) were greater in the dapagliflozin group compared with the placebo group [weighted mean difference (WMD): −3.57 mmHg; 95% CI, −4.38, −2.77; p < 0.00001; I 2 = 0%) 74. For canagliflozin, a pooled analysis of six phase 3 RCTs recorded placebo‐corrected reductions in SBP of −3.3 mmHg and −4.5 mmHg with 100 mg and 300 mg, respectively 75. For empagliflozin, a pooled analysis of four phase 3 RCTs investigating empagliflozin as monotherapy or add‐on therapy (with metformin, metformin plus sulfonylurea or pioglitazone ± metformin) reported reductions in SBP for empagliflozin groups vs. placebo (placebo‐corrected change from baseline −3.4 mmHg and −3.8 mmHg for empagliflozin 10 mg and 25 mg, respectively) 76. Furthermore, a study using ambulatory BP monitoring for patients with T2DM and hypertension found that empagliflozin 10 mg and 25 mg significantly reduced mean 24‐h SBP vs. placebo (−2.95 and −3.68 mmHg vs. +0.48 mmHg, respectively; p < 0.001 vs. placebo for each dose) 77.
Body weight
In the aforementioned meta‐analysis, when the data from 16 RCTs were pooled together, compared with control, SGLT2 inhibitor use was associated with statistically significant reduction in body weight from baseline (WMD: −1.9 kg; 95% CI, −2.5, −1.2), which was greater in the active‐controlled RCTs than the placebo‐controlled RCTs 73. The body weight reduction observed in a 2‐year trial of dapagliflozin added to metformin (−4.5 kg for dapagliflozin plus metformin vs. −2.1 kg for placebo plus metformin) was principally caused by a reduction in body fat mass, as shown by dual energy X‐ray absorptiometry, which demonstrated the weight loss is because of caloric loss caused by urinary glucose excretion and not simply because of fluid loss 78. This was confirmed in a 1‐year trial of canagliflozin vs. glimepiride (both administered with metformin), where approximately two‐thirds of the reduction in body weight was from body fat mass (−3.7 to −4.0 kg for canagliflozin groups vs. +0.7 kg for glimepiride) 60, as well as a 2‐year study of empagliflozin vs. glimepiride (again both administered as add‐on to metformin), in which nearly 90% of weight loss with empagliflozin was because of a reduction in fat mass 79.
Pancreatic beta‐cell function
T2DM patients receiving SGLT2 inhibitor therapy showed improvements in pancreatic beta‐cell function as measured by Homeostasis Model Assessment 2 (HOMA‐2%B). For dapagliflozin given as monotherapy or as add‐on to metformin, the placebo‐corrected mean improvement in HOMA‐2%B across dapagliflozin groups (2.5 mg, 5 mg and 10 mg doses) ranged from 13.2% to 17.3% for monotherapy and from 8.3% to 13.4% as add‐on to metformin 80. In an additional study, dapagliflozin given as add‐on to sitagliptin (± metformin), showed 24.9% increase in beta‐cell function, per HOMA‐2%B analysis, vs. a 5.2% increase for the placebo group 55. There are some further early clinical data suggesting improvements in insulin resistance with dapagliflozin 81. For canagliflozin given as monotherapy or as add‐on to metformin plus sulfonylurea, the mean improvement in HOMA‐2%B for canagliflozin 300 mg was 22.8% for monotherapy (placebo‐corrected, week 26) 59, and 12.6% as add‐on to metformin plus sulfonylurea (active control‐corrected, week 52) 62. In this latter canagliflozin study, other indices of beta‐cell function were evaluated (including proinsulin/insulin ratio, and the proinsulin/C‐peptide ratio) 62. The data reflected improvements in beta‐cell function, which was deemed to be due either to the reversal of glucotoxicity, or the ‘unloading’ of the beta‐cell as systemic glucose levels decrease 82. A recent study examining the metabolic response to empagliflozin in T2DM patients, using a model to reconstruct insulin secretion and its control by glucose 83, found that beta‐cell glucose sensitivity was enhanced and insulin sensitivity improved following empagliflozin therapy 84. Increased insulin sensitivity following SGLT2 inhibitor therapy in T2DM was also reported in a recently published study using dapagliflozin 85. Interestingly, despite reducing FPG, SGLT2 inhibition with dapagliflozin or empagliflozin increased endogenous glucose production, and this may be at least partially explained by a concentration change in the insulin‐to‐glucagon ratio that was observed with SGLT2 inhibitor therapy 84, 85.
Safety
A summary of safety data from the main phase 3 clinical trials of dapagliflozin, canagliflozin and empagliflozin are presented in Table 3 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69. Events of interest from these trials included hypoglycaemia, urinary tract infection (UTI), and genital mycotic infection. Dapagliflozin, canagliflozin and empagliflozin were generally well tolerated when given as monotherapy and in all combination therapies used to date, and trials have reported few serious adverse events.
Table 3.
Safety data summary from the main phase 3 clinical trials of dapagliflozin, canagliflozin and empagliflozin*
| Reference | Study details | Treatment and dose (mg/day) | Patients with a special interest adverse event† | ||
|---|---|---|---|---|---|
| Hypoglycaemia (%) | Urinary tract infection (%) | Genital infection (%)‡ | |||
| Dapagliflozin | |||||
| Monotherapy 51 | Phase 3, 24 weeks | Pbo | 2.7 | 4.0 | 1.3 |
| Dapa 5/10 | 0 to 2.9 | 5.7 to 12.5 | 2.6 to 12.9 | ||
| Monotherapy (A1c ≥ 10.1) 51 | Phase 3, 24 weeks | Dapa 5/10 | 2.9/0 | 8.8/15.4 | 5.9/17.9 |
| Add‐on to MET 52 | Phase 3, 24 weeks | Pbo | 3 | 8 | 5 |
| Dapa 5/10 | 4/4 | 7/8 | 13/9 | ||
| Initial combination with MET XR 53 | Phase 3, 24 weeks | Pbo + MET XR | 0–2.9 | 7.5 | 2.0–2.4 |
| Dapa 5 + MET XR | 2.6 | 7.7 | 6.7 | ||
| Dapa 10 + MET XR | 3.3 | 7.6 | 8.5 | ||
| Add‐on to SU (GLIM) 54 | Phase 3, 24 weeks | Pbo | 4.8 | 0.7 | 0.7 |
| Dapa 5 | 6.9 | 6.9 | 6.2 | ||
| Dapa 10 | 7.9 | 5.3 | 6.6 | ||
| Add‐on to DPP4i (SITA) 55 | Phase 3, 24 weeks§ | Pbo | 1.8 | 4.0 | 0.4 |
| Dapa 10 | 2.7 | 4.9 | 8.4 | ||
| Add‐on to MET 56 | Phase 3, 52 weeks | GLIP 5–20 | 39.7 | 6.4 | 2.7 |
| Dapa 2.5–10 | 3.4 | 10.8 | 12.3 | ||
| Add‐on to TZD (PIO) 57 | Phase 3, 48 weeks | Pbo | 0.7 | 7.9 | 2.9 |
| Dapa 5/10 | 2.1/0 | 8.5/5.0 | 9.2/8.6 | ||
| Add‐on to INS (≥ 30 units/day) ± OADs 58 | Phase 3, 48 weeks | Pbo | 51.8 | 5.1 | 2.5 |
| Dapa 5 | 55.7 | 10.8 | 9.9 | ||
| Dapa 10 | 53.6 | 10.2 | 10.7 | ||
| Canagliflozin | |||||
| Monotherapy 59 | Phase 3, 26 weeks | Pbo | 2.6 | 4.2 | 2.1 (M0%, F3.8%) |
| Cana 100 | 3.6 | 7.2 | 6.2 (M2.5%, F8.8%) | ||
| Cana 300 | 3.0 | 5.1 | 6.6 (M5.6%, F7.4%) | ||
| Monotherapy (A1c >10.0 ≤ 12.0) 59 | Phase 3, 26 weeks | Cana 100 | N/r | 6.4 | 12.7 (M4.3%; F20.8%) |
| Cana 300 | N/r | 4.5 | 4.5 (M5.3%; F4.0%) | ||
| Add‐on to MET 60 | Phase 3, 52 weeks | GLIM 1–8 | 34 | 5 | 1.7 (M1%, F2%) |
| Cana 100 | 6 | 6 | 8.9 (M7%, F11%) | ||
| Cana 300 | 5 | 6 | 11.1 (M8%, F14%) | ||
| Add‐on to MET 61 | Phase 3, 52 weeks¶ | SITA 100 | 4.1 | 6.3 | 1.9 (M1.2%, F2.6%) |
| Cana 100 | 6.8 | 7.9 | 8.4 (M5.2%, F11.3%) | ||
| Cana 300 | 6.8 | 4.9 | 6.5 (M2.4%, F9.9%) | ||
| Add‐on to MET + SU 62 | Phase 3, 52 weeks | SITA 100 | 40.7 | 5.6 | 2.1 (M0.5%, F4.3%) |
| Cana 300 | 43.2 | 4.0 | 11.9 (M9.2%, F15.3%) | ||
| Add‐on to MET + SU 63 | Phase 3, 52 weeks** , †† | Pbo | 17.9 | 7.7 | 3.2 (M1.3%; F5.0%) |
| Cana 100 | 33.8 | 8.3 | 13.3 (M7.9%; F18.5%) | ||
| Cana 300 | 36.5 | 8.3 | 11.5 (M5.7%; F18.8%) | ||
| Add‐on to MET + TZD (PIO) 64 | Phase 3, 52 weeks†† , ‡‡ | Pbo / SITA | 4.4 | 7.8 | 2.6 (M0%; F7.7%) |
| Cana 100 | 6.1 | 5.3 | 8.0 (M3.9%; F16.7%) | ||
| Cana 300 | 6.1 | 7.9 | 12.3 (M4.8%; F21.6%) | ||
| Add‐on to INS (≥ 30 units/day) ± OADs 65 | Phase 3, 18 weeks efficacy substudy | Pbo | 37 | 2.1 | (M0.5%, F2.2%) |
| Cana 100 | 49 | 2.3 | (M4.0%, F11.8%) | ||
| Cana 300 | 48 | 3.4 | (M8.3%, F9.9%) | ||
| Empagliflozin | |||||
| Monotherapy 66 | Phase 3, 24 weeks | Pbo | <1 | 5 (M2%, F9%) | 0 |
| SITA 100 | <1 | 5 (M3%, F9%) | 1 (M1%, F1%) | ||
| Empa 10 | <1 | 7 (M2%, F15%) | 3 (M3%, F4%) | ||
| Empa 25 | <1 | 5 (M1%, F13%) | 4 (M1%, F9%) | ||
| Monotherapy (A1c > 10.0) 66 | Empa 25 | 0 | 3 (M3%; F4%) | 1 (M2%; F0%) | |
| Add‐on to MET 67 | Phase 3, 24 weeks | Pbo | 0.5 | 4.9 (M2.6%, F7.7%) | 0 |
| Empa 10 | 1.8 | 5.1 (M0%, F12.0%) | 3.7 (M0.8%, F7.6%) | ||
| Empa 25 | 1.4 | 5.6 (M0.8%, F11.8%) | 4.7 (M0.8%, F9.7%) | ||
| Add‐on to MET + SU 68 | Phase 3, 24 weeks | Pbo | 8.4 | 8.0 (M2.7%, F13.3%) | 0.9 (M0.9%, F0.9%) |
| Empa 10 | 16.1 | 10.3 (M2.7%, F18.0%) | 2.7 (M0.9%, F4.5%) | ||
| Empa 25 | 11.5 | 8.3 (M0%, F17.5%) | 2.3 (M0.9%, F3.9%) | ||
| Add‐on to TZD (PIO) ± MET 69 | Phase 3, 24 weeks | Pbo | 1.8 | 16.4 (M8.2%, F22.8%) | 2.4 (M1.4%, F3.3%) |
| Empa 10 | 1.2 | 17.0 (M3.6%, F30.5%) | 8.5 (M7.2%, F9.8%) | ||
| Empa 25 | 2.4 | 11.9 (M2.4%, F21.7%) | 3.6 (M1.2%, F6.0%) | ||
| Add‐on MET | Phase 3, 104 weeks | GLIM 1‐4 | 25 | 13 (M5%, F23%) | 2 (M1%, F3%) |
| Empa 25 | 4 | 14 (M7%, F22%) | 12 (M9%, F15%) | ||
*All included studies were conducted in adults (≥ 18 years). †Data are presented as reported in each publication, the changes (range where applicable) is for approved doses of the drug only. ‡Genital mycotic infection specified in canagliflozin studies. §Safety data provided for entire cohort only. ¶26 weeks Pbo + SITA; 26 weeks SITA only. **26 weeks + 26 weeks extension. †† Safety data reported at week 52. ‡‡ 26 weeks + 26 weeks extension, Pbo group switched to SITA during extension. Pbo, placebo; M, male; F, female; Dapa, dapagliflozin; A1c, glycated haemoglobin; MET, metformin; XR, extended‐release formulation; SU, sulfonylurea; GLIM, glimepiride; DPP4i, dipeptidyl peptidase‐4 inhibitor; SITA, sitagliptin; GLIP, glipizide; TZD, thiazolidinedione; PIO, pioglitazone; INS, insulin; OAD, oral antidiabetes drug; Cana, canagliflozin; Empa, empagliflozin.
Hypoglycaemia
Monotherapy with dapagliflozin, canagliflozin or empagliflozin was not associated with an increased risk of hypoglycaemia 51, 59, 66. Clinical trials show the frequency of hypoglycaemia with SGLT2 inhibitor combination therapy appears to be dependent upon the choice of glucose‐lowering therapy that is co‐administered: an increased frequency of hypoglycaemic events is reported when used in combination with insulin or sulfonylureas (see Table 3). These data correlate with behaviour predicted of SGLT2 inhibitors; namely, as SGLT2 inhibitors act independently of insulin to reduce blood glucose levels, no increased risk of hypoglycaemia is anticipated when used in combination with drugs that do not affect insulin levels (e.g. metformin or TZDs). Conversely, when given with insulin or insulin secretagogues such as sulfonylureas, the reduced blood glucose levels would be expected to increase the risk of hypoglycaemic risk (unless the dosage of insulin/sulfonylurea was reduced). This is stated in the prescribing information, in which consideration of a lower dose of insulin or an insulin secretagogue is advised to reduce the risk of hypoglycaemia 39, 40, 41, 43.
Genital mycotic infection
Monotherapy with dapagliflozin, canagliflozin or empagliflozin was associated with an increased incidence in symptoms suggestive of genital mycotic infection 51, 59, 66. These events were more common in women than in men, where subgroup analyses by gender were available 51, 59, 66. A similar trend was observed with add‐on combination therapy involving dapagliflozin, canagliflozin and empagliflozin, and was consistent across studies (Table 3). This was confirmed by analyses of pooled data from phase 3 trials of these three SGLT2 inhibitors 86, 87, 88. Furthermore, the majority of these events were mild in severity and responded to standard therapies, and very few patients discontinued treatment because of these events 86, 87, 88.
Urinary tract infection
The association of SGLT2 inhibitors with UTIs is less straightforward 89. Treatment with canagliflozin (100 and 300 mg) and dapagliflozin (5 and 10 mg) has been shown to be accompanied by a slightly increased incidence of UTIs compared with placebo 90, 91. The infections were generally mild to moderate or similar in severity to infections in the control groups, clinically manageable and did not lead to study discontinuations; furthermore, there was no meaningful increase in upper UTIs 91. For empagliflozin (10 and 25 mg), systematic review and meta‐analysis of available data have not shown evidence of increased risk of UTIs compared with placebo 88, 92. The empagliflozin prescribing information notes an increased risk of UTIs in elderly patients (aged ≥ 75 years) to 15.7% and 15.1% with 10 mg and 25 mg, respectively, vs. 10.5% with placebo 40, as well as increased risk in patients with worsening renal impairment, similar to what has been observed with canagliflozin, although details of these empagliflozin analyses have yet to be published 40, 71.
Bone safety
Bone fractures were more common in patients with T2DM and moderate renal impairment who were receiving dapagliflozin than those receiving placebo (7.7% vs. 0% for dapagliflozin groups and placebo, respectively) 70; however, there was no evidence that dapagliflozin induced bone demineralisation or increased fracture rates in individuals with either normal renal function or mild renal impairment 42. Additional data revealed no meaningful changes, compared with placebo, in markers of bone turnover or bone mineral density from baseline over 102 weeks for dapagliflozin added to metformin 78. A regulatory authority assessment of bone safety with canagliflozin treatment (~10,000 patients with T2DM from phase 3 trials) reported apparent canagliflozin‐associated increases in overall fractures (2.5% and 2.3% for 100 mg and 300 mg, respectively) vs. control groups (1.7%) 93. The regulators noted that, while small, these differences approached statistical significance, and concluded that canagliflozin demonstrated a modest dose‐dependent increase in bone resorption, which may contribute to bone fragility 93. For empagliflozin, a pooled analysis of more than 11,000 patients with T2DM (from phase 1, 2 and 3 trials) reported no increase in bone fractures with empagliflozin vs. placebo (1.6% and 1.1% for empagliflozin 10 mg and 25 mg, respectively, and 1.6% for placebo) 94. However, longer term studies of SGLT2 inhibitors are required to determine fracture rates or changes in bone mineral density, and bone safety is being followed in postmarketing studies mandated by the regulatory agencies for empagliflozin, dapagliflozin and canagliflozin 42, 95, 96.
Volume depletion
Osmotic diuresis associated with use of SGLT2 inhibitors may result in intravascular volume contractions, and to adverse events associated with volume depletion such as hypotension (orthostatic, ambulatory and systolic), dehydration, postural dizziness and syncope. In a pooled analysis of five placebo‐controlled trials, adverse events related to volume depletion were reported in 0.5% and 0.3% of patients on empagliflozin 10 mg and 25 mg, respectively, compared with 0.3% with placebo 40. Across 12 dapagliflozin studies, 0.6% of patients on dapagliflozin 5 mg and 0.8% on 10 mg had volume depletion events vs. 0.4% with placebo 41. In a pooled analysis of eight trials comparing canagliflozin with placebo or an active comparator, adverse events related to volume depletion were reported in 2.3% and 3.4% of patients on canagliflozin 100 mg and 300 mg, respectively, vs. 1.5% with comparators 43. Elderly patients, patients with impaired renal function, with low SBP or on diuretics are at particular risk of symptomatic hypotension on initiating SGLT2 inhibitors and it is recommended that the volume status of these patients is assessed and corrected prior to treatment initiation and monitored afterwards 40, 41, 43.
Neoplasia
An imbalance in cases of bladder cancer was observed in clinical trials of dapagliflozin (newly diagnosed bladder cancer where study drug exposure was ≥ 1 year at the time of diagnosis: four cases in patients on dapagliflozin vs. no cases in patients on placebo/comparator) 41. Consequently, the prescribing information advises that dapagliflozin should not be used in patients with active bladder cancer, and should be used with caution in patients with a prior history of bladder cancer 41. The overall incidence of bladder cancer was low in safety analyses across the clinical programs for canagliflozin (canagliflozin, five cases; comparators, four cases) and empagliflozin (empagliflozin, two cases; comparators, no cases), and prescribing information does not currently advise against use in patients with a prior history of bladder cancer 39, 40, 43.
Lipid levels
Low‐density lipoprotein cholesterol (LDL‐C) is an independent predictor of cardiovascular risk 97. Dose‐related increases in LDL‐C have been reported with canagliflozin 98. In data pooled from four 26‐week RCTs, the mean percentage increase from baseline in LDL‐C for 100 mg and 300 mg canagliflozin relative to placebo were 4.5% and 8.0%, respectively 43. Statistically significant increases in high‐density lipoprotein cholesterol (HDL‐C) from baseline were observed with canagliflozin in four of eight placebo‐controlled phase 3 trials, but decreases in triglyceride levels with canagliflozin were small and were generally not statistically significant 93. A review of dapagliflozin RCTs reported that overall small mean changes in HDL‐C (+2.1% to +9.3%), triglycerides (−0.9 to −10.6%) and LDL‐C (−0.5 to +9.5%) levels were observed in patients receiving dapagliflozin, but the effect on lipid levels was clinically insignificant in the individual studies 99. For empagliflozin, a pooled analysis of four RCTs reported small increases in HDL‐C and LDL‐C and small decreases in triglycerides with empagliflozin compared with placebo after 24 weeks (HDL‐C: +0.07 mmol/l [2.70 mg/dl] for both empagliflozin 10 mg and 25 mg doses, vs. 0.00 mmol/l for placebo; p < 0.001 vs. placebo for both doses; LDL‐C: +0.08 mmol/l [+3.10 mg/dl] and +0.10 mmol/l [+3.87 mg/dl] for empagliflozin 10 mg and 25 mg, respectively, vs. +0.02 mmol/l [+0.77 mg/dl] for placebo; p < 0.01 vs. placebo for 25 mg dose; triglycerides: −0.11 mmol/l [−9.73 mg/dl] and −0.02 mmol/l [−1.77 mg/dl] for empagliflozin 10 mg and 25 mg, respectively, vs. +0.03 mmol/l [+2.65 mg/dl] for placebo; p < 0.05 vs. placebo for 10‐mg dose) 76. Monitoring of LDL‐C and treatment by standard care is recommended after initiating treatment with SGLT2 inhibitors 40, 41, 43.
Cardiovascular safety
Although SGLT2 inhibitors appear to have a beneficial effect on cardiovascular risk factors such as HbA1c, body weight and BP 100, there is a lack of data on clinical outcomes such as stroke, myocardial infarction (MI) and cardiovascular death. A recent report based on simulation modelling described significant reductions in the risk of MI, stroke, cardiovascular death and all‐cause death that could be expected with SGLT2 inhibitor treatment vs. standard care 101.
Raised uric acid levels are associated with ischaemic heart disease and stroke 102, 103. Reductions in mean blood uric acid levels (from normal baseline levels) to week 24 of up to −55.32 μmol/l (−0.93 mg/dl) were observed in an analysis of four dapagliflozin RCTs 99. Decreases in serum urate were observed after 26 weeks for canagliflozin in add‐on combination therapy compared with placebo (−8.8% and −9.4% for canagliflozin 100 mg and 300 mg, respectively, vs. +0.7% for placebo) 63, and after 52 weeks compared with active comparator (−9.9% and −10.3% for canagliflozin 100 mg and 300 mg, respectively, vs. +8.0% for glimepiride 60; and −6.5% for canagliflozin 300 mg vs. +6.2% for sitagliptin) 62. Empagliflozin reduced blood uric acid vs. placebo at week 24 in a pooled analysis of four RCTs [−28.95 μmol/l (−0.49 mg/dl) and −29.55 μmol/l (−0.50 mg/dl) for empagliflozin 10 mg and 25 mg, respectively, vs. +1.03 μmol/l (+0.02 mg/dl) for placebo; p < 0.001 vs. placebo for both dose groups] 76.
Large cardiovascular trials are underway for dapagliflozin, canagliflozin and empagliflozin. The Dapagliflozin Effect on Cardiovascular Events (DECLARE; ClinicalTrials.gov identifier: NCT01730534) began recruitment in 2013, with the intention of recruiting 27,000 patients with T2DM and a high risk of cardiovascular events. The first recruitment phase of the Canagliflozin Cardiovascular Assessment Study (CANVAS; ClinicalTrials.gov identifier: NCT01‐032629) 104 was completed in 2012 with the interim analysis showing no significant increase in risk of cardiovascular events 105, and the Empagliflozin Cardiovascular Outcome Event Trial (EMPA‐REG OUTCOME™, ClinicalTrials.gov identifier: NCT01131676) 96 has also completed recruitment. A cardiovascular trial for the SGLT2 inhibitor ertugliflozin has also recently started recruitment (Cardiovascular Outcomes Following Treatment with Ertugliflozin in Participants with Type 2 Diabetes Mellitus and Established Vascular Disease; ClinicalTrials.gov identifier: NCT01986881).
Discussion
Clinical trials of dapagliflozin, canagliflozin and empagliflozin have investigated their efficacy, safety and tolerability as monotherapy and as add‐on combination therapy with other anti‐diabetes agents, including metformin, sulfonylureas, TZDs, DPP‐4 inhibitors and insulin. These SGLT2 inhibitors produced clinically significant reductions in HbA1c, FPG, body weight and SBP. In terms of safety, dapagliflozin, canagliflozin and empagliflozin were well tolerated and had a generally favourable safety profile, similar to that of placebo. To date, few serious adverse events have been reported from clinical trials. The frequency of hypoglycaemic events was low, similar to that of placebo, and lower than comparators known to have increased risk of hypoglycaemia, with the choice of co‐administered glucose‐lowering agent being the major determinant of hypoglycaemic risk 79. Genital infections and UTIs have been consistently reported with SGLT2 inhibitors, and reported episodes were mostly mild and non‐recurrent.
As described by the Global Partnership for Effective Diabetes Management, overall risk factor management in the early treatment of T2DM is vital 4, 15. This encompasses good glycaemic control as the basis of such management to prevent the onset or delay the progression of diabetes complications 4, and the reduction of cardiovascular risk factors to improve patient outcomes 4. Clinical trial data demonstrate that SGLT2 inhibitors induce beneficial changes in a number of cardiovascular risk factors, such as lowering BP and body weight, in addition to decreasing HbA1c. However, currently available information on clinical outcomes such as stroke, MI and cardiovascular death is limited, and we must await the completion of the various ongoing cardiovascular trials. In addition, the potential renal effect of SGLT2 inhibitors beyond lowering blood glucose, identified from preliminary clinical data, may influence the natural course of renal function decline in individuals with diabetes mellitus.
There is preliminary evidence to suggest that SGLT2 inhibitors may have a protective effect on the kidney beyond blood glucose lowering 106. Like agents that block the renin–angiotensin system, SGLT2 inhibitors also reduce single‐nephron glomerular filtration rate in the chronically altered kidney function states, although by different mechanisms, and SGLT2 inhibitors also cause modest reductions in plasma uric acid, as well as SBP (described above) 106. Evidence for this renoprotective effect is supported by a pilot study in type 1 diabetes (T1DM) subjects with renal hyperfiltration who were given short‐term treatment with empagliflozin 107. Empagliflozin led to a significant reduction in hyperfiltration during clamped euglycaemic and hyperglycaemic conditions, probably by affecting tubular‐glomerular feedback mechanisms; in contrast, renal hemodynamic parameters were unchanged in T1DM subjects with normal renal function 107. Empagliflozin was also associated with a decline in arterial stiffness in young patients with T1DM 108, and empagliflozin improved glycaemic control when given as an adjunctive to insulin in a recent proof‐of‐concept study 109. Preliminary results describing the use of dapagliflozin in T1DM have also been presented 110.
The novel mechanism of action of SGLT2 inhibitors makes them suitable for use in combination with any glucose‐lowering agent, including insulin. The complementary effects of SGLT2 inhibition with other antihyperglycaemic agents may provide additional benefits to T2DM patients, such as glucose lowering plus weight loss, without increasing the risk of hypoglycaemia. Obviously, the ideal combination therapy using SGLT2 inhibitors would need to be tailored to an individual's risk factors. The fixed‐dose combination products emerging onto the market, or soon to do so, may provide further options in this area. Moreover, as SGLT2 inhibitors are not dependent on the production of insulin, they could be used at any stage of T2DM, from newly diagnosed patients to those with long‐standing disease. Even in T2DM patients already receiving insulin, SGLT2 inhibitors may provide an alternative to increasing the dose or frequency of insulin.
In conclusion, the available evidence suggests that SGLT2 inhibitors could have a significant impact on the early management of patients with recent‐onset T2DM, either as monotherapy or in combination with other classes of glucose‐lowering agents, by addressing many of the overall risk factors associated with this disease. The anticipated ability of SGLT2 inhibitors to alleviate known cardiovascular risk factors such as BP and body weight also supports their use early in the management of T2DM. The prevention of vascular complications is crucial in the early treatment of T2DM, and ongoing outcome studies within the SGLT2 inhibitors class will explore whether their mechanism of action has such potential beyond lowering glucose.
Author contributions
The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The author received no direct compensation related to the development of the manuscript.
Acknowledgements
Writing support was provided by Debra Brocksmith, MB ChB, PhD, Dhinakaran Sambandan, PhD, and Geraldine Thompson of Envision Scientific Solutions, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosures
Advisory Boards for Janssen, Novo Nordisk, Abbott Diabetes, AstraZeneca, Sanofi, Lilly. Speakers' Bureaus for Janssen, Novo Nordisk, AstraZeneca, Boehringer Ingelheim‐Lilly.
References
- 1. Centers for Disease Control and Prevention (CDC) . National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. http://www.cdc.gov/diabetes/pubs/statsreport14.htm (accessed 8 August 2014).
- 2. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Suppl 1): S81–90. [DOI] [PubMed] [Google Scholar]
- 3. DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey CJ, Aschner P, Del Prato S et al. Individualized glycaemic targets and pharmacotherapy in type 2 diabetes. Diab Vasc Dis Res 2013; 10: 397–409. [DOI] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53. [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–65. [PubMed] [Google Scholar]
- 7. Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkubo Y, Kishikawa H, Araki E et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–17. [DOI] [PubMed] [Google Scholar]
- 9. Holman RR, Paul SK, Bethel MA et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–89. [DOI] [PubMed] [Google Scholar]
- 10. Hemmingsen B, Lund SS, Gluud C et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 11: CD008143. [DOI] [PubMed] [Google Scholar]
- 11. Ray KK, Seshasai SR, Wijesuriya S et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet 2009; 373: 1765–72. [DOI] [PubMed] [Google Scholar]
- 12. Turnbull FM, Abraira C, Anderson RJ et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52: 2288–98. [DOI] [PubMed] [Google Scholar]
- 13. Mannucci E, Monami M, Lamanna C et al. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta‐analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2009; 19: 604–12. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical care in diabetes ‐ 2014. Diabetes Care 2014; 37(Suppl 1): S14–80. [DOI] [PubMed] [Google Scholar]
- 15. Del Prato S, LaSalle J, Matthaei S, Bailey CJ. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010; 64: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Eldor R, Abdul‐Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013; 36(Suppl 2): S127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunck MC, Corner A, Eliasson B et al. Effects of exenatide on measures of beta‐cell function after 3 years in metformin‐treated patients with type 2 diabetes. Diabetes Care 2011; 34: 2041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bunck MC, Diamant M, Corner A et al. One‐year treatment with exenatide improves beta‐cell function, compared with insulin glargine, in metformin‐treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang AM, Jakobsen G, Sturis J et al. The GLP‐1 derivative NN2211 restores beta‐cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003; 52: 1786–91. [DOI] [PubMed] [Google Scholar]
- 20. Gastaldelli A, Ferrannini E, Miyazaki Y et al. Thiazolidinediones improve beta‐cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292: E871–83. [DOI] [PubMed] [Google Scholar]
- 21. Kahn SE, Haffner SM, Heise MA et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43. [DOI] [PubMed] [Google Scholar]
- 22. Kahn SE, Lachin JM, Zinman B et al. Effects of rosiglitazone, glyburide, and metformin on beta‐cell function and insulin sensitivity in ADOPT. Diabetes 2011; 60: 1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz AV, Sellmeyer DE, Vittinghoff E et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 2006; 91: 3349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–705. [DOI] [PubMed] [Google Scholar]
- 25. Deacon CF. Dipeptidyl peptidase‐4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 2011; 13: 7–18. [DOI] [PubMed] [Google Scholar]
- 26. Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 1971; 28: 101–9. [DOI] [PubMed] [Google Scholar]
- 27. Rahmoune H, Thompson PW, Ward JM et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes 2005; 54: 3427–34. [DOI] [PubMed] [Google Scholar]
- 28. Vestri S, Okamoto MM, de Freitas HS et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol 2001; 182: 105–12. [DOI] [PubMed] [Google Scholar]
- 29. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010; 27: 136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–94. [DOI] [PubMed] [Google Scholar]
- 31. Abdul‐Ghani MA, Norton L, Defronzo RA. Role of sodium‐glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011; 32: 515–31. [DOI] [PubMed] [Google Scholar]
- 32. Santer R, Kinner M, Lassen CL et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003; 14: 2873–82. [DOI] [PubMed] [Google Scholar]
- 33. Santer R, Calado J. Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010; 5: 133–41. [DOI] [PubMed] [Google Scholar]
- 34. McCrimmon RJ, Evans ML, Jacob RJ et al. AICAR and phlorizin reverse the hypoglycemia‐specific defect in glucagon secretion in the diabetic BB rat. Am J Physiol Endocrinol Metab 2002; 283: E1076–83. [DOI] [PubMed] [Google Scholar]
- 35. Rossetti L, Smith D, Shulman GI et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987; 79: 1510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han S, Hagan DL, Taylor JR et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 2008; 57: 1723–9. [DOI] [PubMed] [Google Scholar]
- 37. Sha S, Devineni D, Ghosh A et al. Canagliflozin, a novel inhibitor of sodium glucose co‐transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011; 13: 669–72. [DOI] [PubMed] [Google Scholar]
- 38. Oliva RV, Bakris GL. Blood pressure effects of sodium‐glucose co‐transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 2014; 8: 330–9. [DOI] [PubMed] [Google Scholar]
- 39. European Medicines Agency . Jardiance [summary of product characteristics] June 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002677/WC500168592.pdf (accessed 5 March 2015).
- 40. Boehringer Ingelheim Pharmaceuticals Inc . Jardiance (empagliflozin) tablets, for oral use [prescribing information] August 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204629s000lbl.pdf (accessed 5 March 2015).
- 41. AstraZeneca Pharmaceuticals LP . Farxiga (dapagliflozin) tablets, for oral use [prescribing information] August 2014. http://www.azpicentral.com/farxiga/pi_farxiga.pdf#page=1 (accessed 5 March 2015).
- 42. European Medicines Agency . Assessment report: Forxiga (dapagliflozin). Procedure no. EMEA/H/C/002322, 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002322/WC500136024.pdf (accessed 10 April 2014).
- 43. Janssen Pharmaceuticals Inc . Invokana (canagliflozin) tablets, for oral use [prescribing information] May 2014. http://www.invokanahcp.com/prescribing-information.pdf (accessed 2 March 2015).
- 44. European Medicines Agency . Invokana [summary of product characteristics] September 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002649/WC500156456.pdf (accessed 5 March 2015).
- 45. European Medicines Agency . Xigduo [summary of product characteristics] October 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002672/WC500161033.pdf (accessed 5 March 2015).
- 46. AstraZeneca Pharmaceuticals LP . Xigduo XR (dapagliflozin and metformin HCl extended‐release) tablets, for oral use [prescribing information] October 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205649s000lbl.pdf (accessed 5 March 2015).
- 47. Janssen Pharmaceuticals Inc . Invokamet (canagliflozin and metformin hydrochloride) tablets, for oral use [prescribing information] August 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204353s000lbl.pdf (accessed 3 March 2015).
- 48. European Medicines Agency . Vokanamet [summary of product characteristics] May 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002656/WC500166670.pdf (accessed 5 March 2015).
- 49. Garber AJ, Abrahamson MJ, Barzilay JI et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract 2013; 19(Suppl 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–9. [DOI] [PubMed] [Google Scholar]
- 51. Ferrannini E, Ramos SJ, Salsali A et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care 2010; 33: 2217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bailey CJ, Gross JL, Pieters A et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–33. [DOI] [PubMed] [Google Scholar]
- 53. Henry RR, Murray AV, Marmolejo MH et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 2012; 66: 446–56. [DOI] [PubMed] [Google Scholar]
- 54. Strojek K, Yoon KH, Hruba V et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2011; 13: 928–38. [DOI] [PubMed] [Google Scholar]
- 55. Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care 2014; 37: 740–50. [DOI] [PubMed] [Google Scholar]
- 56. Nauck MA, Del Prato S, Meier JJ et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care 2011; 34: 2015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosenstock J, Vico M, Wei L et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012; 35: 1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilding JP, Woo V, Soler NG et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 2012; 156: 405–15. [DOI] [PubMed] [Google Scholar]
- 59. Stenlof K, Cefalu WT, Kim KA et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15: 372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cefalu WT, Leiter LA, Yoon KH et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013; 382: 941–50. [DOI] [PubMed] [Google Scholar]
- 61. Lavalle‐Gonzalez FJ, Januszewicz A, Davidson J et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56: 2582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schernthaner G, Gross JL, Rosenstock J et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care 2013; 36: 2508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilding JP, Charpentier G, Hollander P et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 2013; 67: 1267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forst T, Guthrie R, Goldenberg R et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014; 16: 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matthews DR, Fulcher G, Perkovic V et al. Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co‐transporter 2 (SGLT2), added‐on to insulin therapy +/‐ oral agents in type 2 diabetes. Diabetologia 2012; 55: S314 (Abstract 764). [Google Scholar]
- 66. Roden M, Weng J, Eilbracht J et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–19. [DOI] [PubMed] [Google Scholar]
- 67. Häring HU, Merker L, Seewaldt‐Becker E et al. Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2014; 37: 1650–9. [DOI] [PubMed] [Google Scholar]
- 68. Häring HU, Merker L, Seewaldt‐Becker E et al. Empagliflozin as add‐on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2013; 36: 3396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kovacs CS, Seshiah V, Swallow R et al. Empagliflozin improves glycaemic and weight control as add‐on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab 2013; 16: 147–58. [DOI] [PubMed] [Google Scholar]
- 70. Kohan DE, Fioretto P, Tang W, List JF. Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yale JF, Bakris G, Cariou B et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barnett AH, Mithal A, Manassie J et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–84. [DOI] [PubMed] [Google Scholar]
- 73. Baker WL, Smyth LR, Riche DM et al. Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens 2014; 8: 262–75. [DOI] [PubMed] [Google Scholar]
- 74. Zhang M, Zhang L, Wu B et al. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev 2014; 30: 204–21. [DOI] [PubMed] [Google Scholar]
- 75. Weir MR, Januszewicz A, Gilbert RE et al. Lower blood pressure (BP) with canagliflozin (cana) in subjects with type 2 diabetes mellitus (T2DM). Diabetes 2013; 62 (Supplement 1): (Abstract 1077‐P). [Google Scholar]
- 76. Hach T, Gerich J, Salsali A et al. Empagliflozin improves glycemic parameters and cardiovascular risk factors in patients with type 2 diabetes (T2DM): pooled data from four pivotal phase III trials. Diabetes 2013; 62 (Supplement 1): Abstract 69‐LB. [Google Scholar]
- 77. Tikkanen I, Narko K, Zeller C et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38: 420–8. [DOI] [PubMed] [Google Scholar]
- 78. Bolinder J, Ljunggren O, Johansson L et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2013; 16: 159–69. [DOI] [PubMed] [Google Scholar]
- 79. Ridderstrale M, Andersen KR, Zeller C et al. Comparison of empagliflozin and glimepiride as add‐on to metformin in patients with type 2 diabetes: a 104‐week randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Diabetes Endocrinol 2014; 2: 691–700. [DOI] [PubMed] [Google Scholar]
- 80. Salsali A, Bastien A, Mansfield T et al. Dapagliflozin improves hyperglycemia and beta‐cell function without increasing hypoglycemic episodes in patients with type 2 diabetes mellitus. American Association of Clinical Endocrinologists (AACE), San Diego, CA, April 13–17, 2011. Abstract 204.
- 81. Mudaliar S, Henry RR, Boden G et al. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther 2014; 16: 137–44. [DOI] [PubMed] [Google Scholar]
- 82. Wajchenberg BL. Clinical approaches to preserve beta‐cell function in diabetes. Adv Exp Med Biol 2010; 654: 515–35. [DOI] [PubMed] [Google Scholar]
- 83. Mari A, Schmitz O, Gastaldelli A et al. Meal and oral glucose tests for assessment of beta‐cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 2002; 283: E1159–66. [DOI] [PubMed] [Google Scholar]
- 84. Ferrannini E, Muscelli E, Frascerra S et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Merovci A, Solis‐Herrera C, Daniele G et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Johnsson KM, Ptaszynska A, Schmitz B et al. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013; 27: 479–84. [DOI] [PubMed] [Google Scholar]
- 87. Nyirjesy P, Sobel JD, Fung A et al. Genital mycotic infections with canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014; 30: 1109–19. [DOI] [PubMed] [Google Scholar]
- 88. Kim G, Gerich JE, Salsali A et al. Empagliflozin (EMPA) increases genital infections but not urinary tract infections (UTIs) in pooled data from four pivotal phase III trials. Diabetes 2013; 62 (Supplement 1): LB21 (Abstract 74‐LB). [Google Scholar]
- 89. Geerlings S, Fonseca V, Castro‐Diaz D et al. Genital and urinary tract infections in diabetes: impact of pharmacologically‐induced glucosuria. Diabetes Res Clin Pract 2014; 103: 373–81. [DOI] [PubMed] [Google Scholar]
- 90. Johnsson KM, Ptaszynska A, Schmitz B et al. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013; 27: 473–8. [DOI] [PubMed] [Google Scholar]
- 91. Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co‐transporter 2 inhibitor. Postgrad Med 2014; 126: 7–17. [DOI] [PubMed] [Google Scholar]
- 92. Liakos A, Karagiannis T, Athanasiadou E et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 984–93. [DOI] [PubMed] [Google Scholar]
- 93. US Food and Drug Administration . FDA briefing document: Invokana (canagliflozin) tablets. NDA 204042, 2013. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf (accessed 10 April 2014).
- 94. Wanner C, Toto RD, Gerich J et al. No increase in bone fractures with empagliflozin (EMPA) in a pooled analysis of more than 11,000 patients with type 2 diabetes (T2DM). J Am Soc Nephrol 2013; 24: 205A (Abstract TH‐PO452). [Google Scholar]
- 95. Nigro SC, Riche DM, Pheng M, Baker WL. Canagliflozin, a novel SGLT2 inhibitor for treatment of type 2 diabetes. Ann Pharmacother 2013; 47: 1301–11. [DOI] [PubMed] [Google Scholar]
- 96. Zinman B, Inzucchi SE, Lachin JM et al. Rationale, design, and baseline characteristics of a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG OUTCOME). Cardiovasc Diabetol 2014; 13: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Turner RC, Millns H, Neil HA et al. Risk factors for coronary artery disease in non‐insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316: 823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rodriguez‐Gutierrez R, Gonzalez‐Saldivar G. Canagliflozin. Cleve Clin J Med 2014; 81: 87–8. [DOI] [PubMed] [Google Scholar]
- 99. Ptaszynska A, Hardy E, Johnsson E et al. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 2013; 125: 181–9. [DOI] [PubMed] [Google Scholar]
- 100. Basile JN. The potential of sodium glucose cotransporter 2 (SGLT2) inhibitors to reduce cardiovascular risk in patients with type 2 diabetes (T2DM). J Diabetes Complications 2013; 27: 280–6. [DOI] [PubMed] [Google Scholar]
- 101. Dziuba J, Alperin P, Racketa J et al. Modeling effects of SGLT‐2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab 2014; 16: 628–35. [DOI] [PubMed] [Google Scholar]
- 102. Kim SY, Guevara JP, Kim KM et al. Hyperuricemia and coronary heart disease: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken) 2010; 62: 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim SY, Guevara JP, Kim KM et al. Hyperuricemia and risk of stroke: a systematic review and meta‐analysis. Arthritis Rheum 2009; 61: 885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Neal B, Perkovic V, de Zeeuw D et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) ‐ a randomized placebo‐controlled trial. Am Heart J 2013; 166: 217–23 e11. [DOI] [PubMed] [Google Scholar]
- 105. Center for Drug Evaluation and Research . Summary review. Invokana (canagliflozin), 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf (accessed 29 October 2014).
- 106. Gilbert RE. Sodium‐glucose linked transporter‐2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int 2014; 86: 693–700. [DOI] [PubMed] [Google Scholar]
- 107. Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–97. [DOI] [PubMed] [Google Scholar]
- 108. Cherney DZ, Perkins BA, Soleymanlou N et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Perkins BA, Cherney DZI, Partridge H et al. Sodium‐glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8‐week open‐label proof‐of‐concept trial. Diabetes Care 2014; 37: 1480–3. [DOI] [PubMed] [Google Scholar]
- 110. Henry RR, Rosenstock J, Chalamandaris AG et al. Exploring the potential of dapagliflozin in type 1 diabetes: phase 2a pilot study. Diabetes 2013; 62: (Abstract 70‐LB). [Google Scholar]
- 111. Kasichayanula S, Liu X, Lacreta F et al. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium‐glucose co‐transporter type 2. Clin Pharmacokinet 2014; 53: 17–27. [DOI] [PubMed] [Google Scholar]
- 112. Lamos EM, Younk LM, Davis SN. Canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, for the treatment of type 2 diabetes mellitus. Expert Opin Drug Metab Toxicol 2013; 9: 763–75. [DOI] [PubMed] [Google Scholar]


