Abstract
The ly-6 proteins are a large family of proteins that resemble the snake three finger alpha toxins such as α-bungarotoxin and are defined by their multiple cysteine residues. Multiple members of the ly-6 protein family can modulate nicotinic signaling including lynx1, lynx2, slurp-1, slurp-2 and prostate stem cell antigen (PSCA). Consistent with the expression of multiple nicotinic receptors in bronchial epithelium, multiple members of the nicotinic-modulatory ly-6 proteins are expressed in lung including lynx1 and lynx2. We studied the role of lynx1 as an exemplar of the role of ly-6 proteins in lung. Our data demonstrates that lynx1 acts as a negative modulator of nicotinic signaling in normal and neoplastic lung. In normal lung lynx1 serves to limit the ability of chronic nicotine exposure to increase levels of nicotinic receptors and also serves to limit the ability of nicotine to upregulate levels of GABAA receptors in lung. In turn this allows lynx1 to limit the ability of nicotine to upregulate levels of mucin which is mediated by GABAergic signaling. This suggests that lynx1- mimetics may have potential for treatment of asthma and COPD. In that most lung cancer cells also express nicotinic receptor and lynx1 we examined the role of lynx-1 in lung cancer. Lynx1 levels are decreased in lung cancers compared to adjacent normal lung. Knockdown of lynx1 by siRNAs increased growth of lung cancer cells while expression of lynx1 in lung cancer cell decreased cell proliferation. This suggests that lynx1 is an endogenous regulator of lung cancer growth. Given that multiple small molecule negative and positive allosteric modulators of nicotinic receptors have already been developed, this suggests that lynx1 is a highly druggable target both for development of drugs that may limit lung cancer growth as well as for drugs that may be effective for asthma or COPD treatment.
Keywords: Ly6, lynx1, nicotinic receptor, lung cancer, bronchial epithelium, lynx2
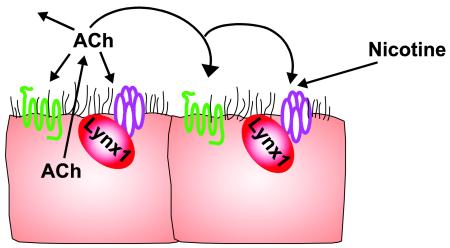
Graphical abstract
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are widely expressed in lung as part of a nonneuronal paracrine and autocrine network as well as for targets of traditional sympathetic and parasympathetic signaling. Acetylcholine is synthesized by bronchial epithelial cells and pulmonary neuroendocrine cells from which it is released to feedback on itself (autocrine), on neighboring cells (paracrine) or to be circulated in blood to effect distal cells (endocrine) [1,2]. Many aspects of nonneuronal acetylcholine are similar to neuronal acetylcholine signaling; indeed it is likely that cholinergic signaling evolved in simple epithelial structures prior to being borrowed for synaptic signaling [3-5]. Non-neuronal cholinergic signaling uses the same nAChRs as does cholinergic signaling and the nAChRs in non-neuronal networks are modulated by ly-6 family members just as neuronal nAChRs are.
The ly-6 proteins are a large family of small proteins related to snake α-neurotoxins [6,7] such as the α7 nAChR antagonist α-bungarotoxin. Ly-6 proteins are also known as 3 finger proteins because of their conserved structure of 3 fingers anchored by cysteine bonds surrounding a hydrophobic core. In humans more than 25 genes encoding ly-6 proteins have been identified, most with multiple forms produced by alternate splicing [7-9]. Most of the ly-6 proteins are membrane bound, anchored by a GPI linkage; however some of family members lack the GPI linkage and are secreted. The GPI linkage can be cleaved by phospholipases so it is likely that some of the family members can exist in both membrane-bound and secreted forms.
Consistent with the similarity of structure of the mammalian ly-6 proteins to α-bungarotoxin, many of the ly-6 proteins modulate nAChR signaling. Miwa et al [10,11] used similarity to α-bungarotoxin to identify a mammalian α-bungarotoxin-like protein in mouse brain which they named lynx1 and then demonstrated it was a negative allosteric regulator of α4β2 and α7 nAChR receptors. Subsequently lynx1 has also been shown to modulate α6-containing nAChR [12] as well as shifting nAChR stoichiometry from high sensitivity (alpha4)2 (beta2)3 to low sensitivity (alpha4)3 (beta2)2 nAChR through interaction as a chaperone in the endoplasmic reticulum [13]. In addition to lynx1, Miwa and co-workers also identified a second ly-6 protein in brain, lynx2 and showed it too was a negative regulator of α7 nAChR [14]. Of note, the cDNA for lynx2 had in fact been previously described twice before, though not in the context of nAChR regulation, and named both lypd1 and PHTS which is indicative of the nomenclature problems surrounding the ly-6 proteins, in which many of the family members have been described multiple times and given multiple names.
Lynx1 and lynx2 are not the only ly-6 proteins that regulate nAChR. The ly-6 protein, slurp-1 was initially characterized for its role in the skin disease Mal de Maleda [15,16] and is a positive regulator of α7 nAChR [17,18]. Slurp-1 differs from most of the ly-6 proteins as it lacks a GPI linkage and is secreted. Slurp-2 is encoded by an alternate transcript of the LYNX1 gene and has been reported to be a negative nAChR regulator [19]. Slurp-1, like slurp-2, lacks a GPI-anchor and is secreted. Prostate stem cell antigen (PSCA) has been shown by Nishi and co-workers to be yet another ly-6 protein that negatively regulates α7 nAChR [20]. The Pates (named for prostate and testis expression, the site of their original characterization) are another group of ly6 proteins, of which human Pate B has been shown to be a positive regulator of α7 [21]. Thus lynx1, lynx2, Slurp-1, Slurp-2, PSCA and Pate-B are ly-6 proteins that have been demonstrated to modulate nAChR activity but it is likely that other members of the ly-6 family will also turn out to modulate nicotinic signaling.
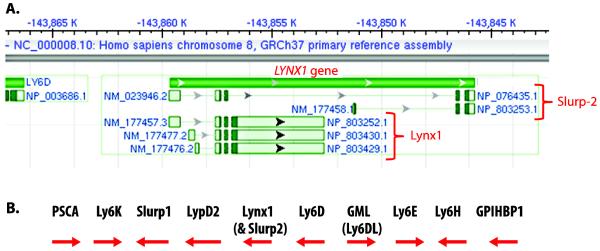
As shown in figure 1A, the LYNX1 gene gives rise to both the lynx1 and slurp-2 proteins through alternate splicing. Such alternate splicing is typical of the ly-6 family, with most of the ly-6 genes giving rise to multiple proteins. Another characteristic of the ly-6 genes is that many of the genes fall in gene clusters. The 10-gene cluster on chromosome 8 that encompasses LYNX1 is shown in figure 1B. Another ly-6 gene cluster encodes the PATE genes on chromosome 11. Lynx2 however (encoded by the LYPD1 gene) occurs by itself on chromosome 2.
Figure 1. Chromosomal structure of the LYNX1 gene and locus.
A. The gene is named LYNX1, and alternate splicing gives rise to the lynx1 protein and to two slurp-2 proteins. Slurp-2 exists in a short and long form and both forms of slurp-2 lack the exon of lynx1 which encodes the GPI linkage thereby resulting in the secretory nature of Slurp-2. The shorter form of slurp-2 has no amino acids in common with the lynx1 protein while the longer form of slurp-2 shares the second coding exon of the lynx1 gene with the lynx1 protein. B. The LYNX1 gene occurs in a cluster of 10 ly-6 proteins spanning approximately 650,000 bases.
Given the expression of multiple ly-6 modulators of nAChR activity in brain, our previous characterization of multiple nAChR subtypes in airway epithelium [22,23] raised the question of whether ly-6 proteins are similarly expressed in normal lung to modulate nicotinic signaling in lung and the potential role of such ly-6 proteins in lung cancer. In this review, we discuss data on the roles of nAChR-modulatory ly-6 proteins in normal and neoplastic lung.
2. Materials and Methods
2.1 Drugs and epithelial cell cultures
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Utilization Committee. Bronchial epithelial cell cultures were established from lungs obtained from rhesus macaques (newborn to 2 years old) undergoing necropsy from protocols not expected to alter lung function as described by Wu et al [24,25] with modifications as previously described by Fu et al [3]. All drugs used for the study were obtained from Sigma (St. Louis, MO).
2.2 Immunohistochemistry
Single and dual immunohistochemistry shown in figure 2 was performed after Proskocil et al [1]. The antibody used for lynx1 was as described and validated by Sekhon et al [26]. Localization of expression of lynx1 in pulmonary neuroendocrine cells was determined by colocalization with serotonin as described by Fu and Spindel [27]. The antibody used for α7-nAChR staining was rat monoclonal 219 generously provided by Jon Lindstrom [28] as previously described by Sekhon et al [22] and validated by comparison to fluorescently-labeled α-bungarotxon toxin binding. Other antibodies used were sc-135296 for lynx2 (goat anti-lypd1, Santa Cruz Biotechnology, Dallas TX) and sc-98140 for slurp1 (goat anti-slurp1, Santa Cruz Biotechnology). These antibodies were validated by RT-PCR detection of target molecules in same tissues.
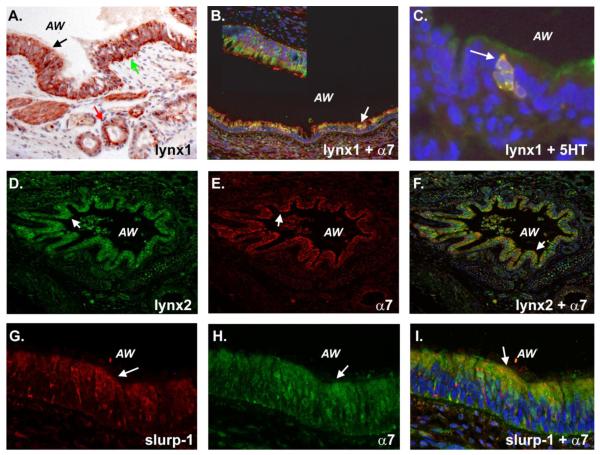
Figure 2. Expression of nAChR-modulatory ly-6 proteins in monkey lung.
A. Lynx1-staining seen in airway epithelial cells (black arrow shows apical staining and green arrow shows basal staining) and submucosal glands (red arrow) of a large airway. AW = airway. B. Colocalization of lynx1 (red) with α7 (green) in airway epithelial cells (white arrow). Inset box shows section of same field at higher magnification (blue = DAPI). C. Expression of lynx1 in pulmonary neuroendocrine cells as defined by co-expression with serotonin (white arrow). Green = lynx1, red = serotonin, blue = DAPI). D. Expression of lynx2 in monkey lung airway epithelial cells (white arrow). E. Expression of α7 in monkey lung airway epithelial cells (white arrow). F. Co-expression of lynx2 and α7 in airway epithelial cells (white arrow) (red = lynx lynx2, green = α7, blue = DAPI). G. Expression of slurp-1 in monkey lung airway epithelial cells (white arrow). H. Expression of α7 in monkey lung airway epithelial cells (white arrow). I. Co-expression of slurp-1 and α7 in airway epithelial cells (white arrow) (red = slurp-1, green = α7, blue = DAPI). Immunohistochemistry was performed as described in the materials and methods.
2.3 qPCR and siRNA knockdown
Quantitative PCR on lynx1, nAChR, GABA receptors and mucin in bronchial epithelial cells was performed using primers and probes as described by Fu et al [29]. ON-TARGET plus siRNAs for lynx1 and negative control siRNA were purchased from Dharmacon (Lafayette, CO). siRNAs were transfected at a concentration 100 - 150 nM with DharmaFECT 1 according to the manufacturer’s instruction. Forty-eight hours after transfection, cells were harvested for real-time PCR or for Western blotting. The lynx1 siRNA (TCAGCAACATCGAGAACTT) was chosen to be distinct from the other transcripts of the lynx1 gene and was 100% homologous to NM_001266622. The lynx1 siRNA caused a 75% decrease in lynx1 siRNA levels as measured by qPCR and confirmed by western blotting. Quantification of lynx1 RNA levels in human tumors was as described by Song et al [30]. All human samples were deidentified prior to receipt.
2.4 Lentiviral transduction of lynx1
The human lynx1 cDNA was subcloned into the lentiviral shuttle vector pLVI-IRES which contains a CMV promoter to drive cDNA expression and a bicistronic-expressed GFP marker. Lentivirus was prepared and titrated as previously described [31]. A549 cells were infected at a ratio of 5 transforming units per cell. One week post infection, cells were sorted by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA) and used for studies shown in figure 4.
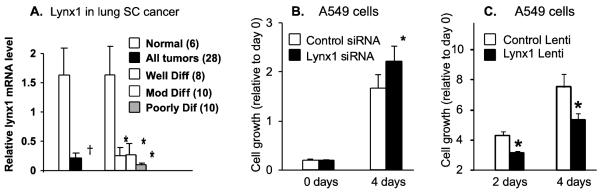
Figure 4. Lynx1 expression and function in lung cancers cells.
A. Relative RNA levels of Lynx1 in squamous cell carcinomas compared to normal and also plotted according to degree of differentiation. †p< .03 by t-test, *p<.05 compared to normal tissue by Tukey-Kramer after 1 WAY ANOVA. Number of samples of each grade is shown in figure legend in parentheses. Figure modified after Song et al [30]. B. siRNA knockdown of lynx1 increased cell growth of A549 lung adenocarcinoma cells. Levels of lynx1 were decreased by ~70% by the siRNA knockdown (data not shown). Data are mean ± SD of 20 replicates in two separate experiments. * p < 0.05 versus control siRNA. C. Effect of increased lynx1 expression in A549 cells. A lentivirus expressing lynx1 was prepared and A549 cells transduced with the lynx-1 lentiviral vector or a control lentivirus. Lynx1 protein expression was highly expressed after transduction (data not shown). Cell growth is shown relative to day 0. * p < .05 compared to controls.
2.5 Statistics
All data are given as mean (± SEM), and statistical comparisons were made using ANOVA followed by post-hoc multiple comparisons or unpaired, two-tailed Student’s t-tests.
3. nAChR-modulatory ly-6 proteins in normal lung
3.1 Expression of nAChR-modulatory ly-6 proteins in normal lung
To examine this we initially sequenced cDNAs from monkey lung and showed the presence of lynx1 [26] mRNAs in lung. Next we prepared antibodies to lynx1 and showed that lynx1 was co-expressed in airway epithelial cells along with nAChR (Fig 2, A-C). Consistent with a negative regulatory action of lynx1 on effects of nicotine on lung development, exposure of monkeys to prenatal nicotine throughout gestation increased levels of lynx1 mRNA and protein in lung [26].
3.2 Regulation of nAChR expression by lynx1 and downstream sequelae in lung
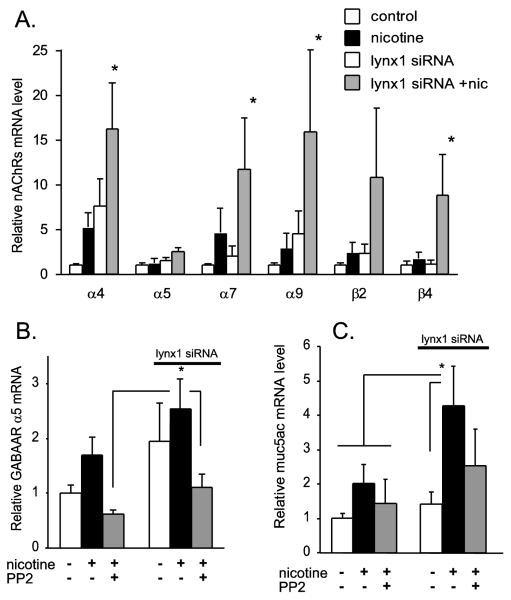
Multiple reports both from our laboratory and others have demonstrated that chronic nicotine exposure increases levels of nicotinic receptors. Mechanisms appear to involve both increased gene transcription [32,33] and stabilization of receptor proteins [34]. This is significant because upregulation of nAChRs expression plays a role in pathophysiologic responses to smoking as well as describing likely responses to endogenous release of epithelial ACh. Therefore if lynx1 is a significant modulator of nicotinic signaling, it should modulate how levels of nicotinic receptors respond to nicotine exposure. This is indeed the case as shown in figure 3A in which siRNA knockdown of lynx1 increased basal levels of nAChRs and significantly potentiated the rise in nicotine-induced increases in nAChRs levels. This shows that lynx1 acts as endogenous break to this aspect of nicotinic signaling.
Figure 3. Regulation of nicotinic signaling and mucin expression by lynx1 in airway epithelial cells.
A. Effect of lynx1 siRNA knockdown on the ability of nicotine (1 μM for 48 hours) to increase levels of nAChR subunits. Values are expressed as means ± SE. B. siRNA knockdown of lynx1 increased the nicotine-induced upregulation GABAAR α5 (n= 8 per group). Relative fold change of each condition versus control with error bars showing SEM (*p< 0.05 compared to control by Fisher’s multiple comparison tests after 1-way ANOVA). The Src inhibitor PP2 (1 μM) decreased the ability of nicotine (1 μM) and lynx1 knockdown to increase GABAAR α5 mRNA expression in cultured BEC. p<0.05 for nicotine + siRNA-treated group compared to groups shown by Fisher’s multiple comparison tests after 1-way ANOVA. C. The nicotine-induced increase in mucin mRNA was blocked by PP2. PP2 also blocked the additional increase in mucin mRNA caused by lynx1 knockdown. * p<0.05 for nicotine + siRNA-treated group compared to groups shown by Fisher’s multiple comparison tests after 1-way ANOVA. (n= 5 per group). Drugs and siRNAs were added to cultures 48 hours prior to harvesting of cells. The figure was modified from Fu et al [29].
The physiologic significance of the ability of lynx1 to act as a break on nicotinic signaling is shown in figure 3B & C. Our laboratory has previously reported that one function of nicotinic signaling in airway epithelium is to regulate levels of GABA signaling in airway epithelium [3,29]. This is important because GABA signaling in airway epithelium plays a critical role in regulation of mucin secretion and asthma development as reported by Xiang et al [35]. As shown in figure 3B, lynx1 decreased the ability of nicotine to increase GABAA receptor levels in airway epithelium which translates to decreased levels of GABAA-mediated currents in airway epithelial cells [3]. The mechanism of regulation appears to involve Src signaling as the effects of lynx1 knockdown on GABA receptor expression are blocked by the Src inhibitor PP2 (Fig. 3B, C). This is consistent with reports from Dasgupta and coworkers that some non-neuronal signaling events of α7 are mediated by Src [36,37]. The ability of lynx1 to modulate levels of GABA signaling in turn translates to lynx1 modulation of levels of mucin expression (Fig. 3C) [3,35]. This therefore shows a clear role for lynx1 in airway physiology as well as suggesting that nicotinic negative allosteric modulators might have clinical potential for modulating mucus production in asthma and COPD.
Underlying mechanisms of signaling by the ly-6 proteins still remains poorly understood. It is not clear the extent to which binding sites for the ly-6 proteins overlap with the site of conventional nAChR ligands such as acetylcholine, with the allosteric sites that modulate small molecule allosteric modulator binding sites [38] or to a different site altogether. In addition how ly-6 binding may initiate signalling is not clear. Signalling my result in modification of ion channel activity, activation of kinases secondary to receptor conformation shift, or a mix of both mechanisms. In particular, the relative importance, or lack therof, of ion-channel activity in ly-6 protein-mediated signaling remains to be answered, especially in non-excitable cells.
Additional evidence for the importance of the ly-6 proteins in epithelial functioning is provided by the work of Grando and co-workers who have shown that slurp-2 stimulated keratinocyte proliferation, migration and inhibited apoptosis, potentially acting through the α3 nAChR [19,39]. By contrast slurp-1 appears to stimulate apoptosis of keratinocytes [40].
4.0 Lynx1 and lung cancer
Multiple laboratories have demonstrated that nicotine and nicotinic receptors stimulate the growth of lung cancers [30,41-44]. Nicotine stimulates growth of lung cancer cells through nicotinic receptors and ACh released by airway epithelial cells stimulates growth of lung cancer cells through both nicotinic and muscarinic mechanisms [41]. Consistent with the secretion of ACh by airway epithelial cells [1,2], most lung cancers also secrete ACh which acts as an autocrine growth factor for lung cancer [41]. Thus lung cancer cell growth can be stimulated by endogenous ACh release and by exogenous nicotine. This nicotinic regulation of lung cancer cell growth suggests that lynx1 acting as a nicotinic modulator may regulate lung cancer cell growth. The potential role of lynx1 in modulating lung cancer cell growth is further supported by the increase in cholinergic signaling that has been reported in lung cancers in which levels of ACh and nicotinic receptors are increased in most lung cancers [30] and of course by the fact that most lung cancers occur in smokers, so there is also chronic exposures of the lung cancers to nicotine.
The potential role for lynx1 in regulating lung cancer cell growth is also supported by our previous report that levels of lynx1 are decreased in most lung cancers as compared to adjacent normal tissue [30]. This is shown in figure 4A, and as can be seen level of lynx1 are significantly decreased in lung cancer compared to normal lung and the magnitude of this decrease increases as the tumors become less differentiated. This raises the key question of whether levels of lynx1 can modulate growth of lung cancer cells? As shown in figure 4B, siRNA knockdown of lynx1 significantly increased growth of lung cancer cells. This suggests that the decreased levels of lynx1 in lung cancers shown in figure 4A may have significance in terms of rate of growth of lung cancers. What about the converse; can increased levels of lynx1 inhibit lung cancer cell growth. To investigate this we constructed a lentiviral vector expressing lynx1 and infected A549 lung cancer cells. Infection of cells with this vector significantly increased levels of lynx1 both at the mRNA and protein level (data not shown) and as shown in figure 4C, this resulted in significantly decreased growth of the A549 cells compared to cells infected with a control lentivirus. This confirms the role of lynx1 as a modulator of lung cancer cell growth and suggests that small molecule mimetics of lynx1 could have therapeutic potential in lung cancer. A key question yet to be answered is the exact target of lynx1 to modulate lung cancer cell growth since lynx1 interacts with α7, α4β2 and α6 containing receptors [10-13]. Indeed it may be that one reason why lynx1 significantly modulates lung cancer cell growth is its promiscuity in interacting with multiple nAChR subtypes, each of which has effects on lung cancer cell growth. If this is the case this serves to further emphasize the potential importance of lynx1 as a therapeutic target.
While we have focused on lynx1 here, there is also evidence supporting roles of other nAChR-modifying ly-6 proteins in cancer. PSCA was identified in part due to its ability to modify neuroblastoma growth [20] and polymorphisms in PSCA are associated with clinical course of gastric carcinomas [45]. Likewise the ability of lynx2 to modulate alpha7 signaling suggests that it may similarly modulate lung cancer cell growth.
5.0 Conclusions
The expression of multiple ly-6 proteins that regulate nAChR function in bronchial epithelium suggests an important role for these proteins. It is striking that the same cell types can express three or more ly-6 proteins that all appear to regulate α7 function. While it is likely that the different proteins link to different mediators, this does suggest key roles both for the ly-6 proteins and the nAChR they regulate. It is also likely that the different ly-6 proteins have different specificities and affinities for the multiple nAChR expressed in bronchial epithelial cells and the cancers arising from those cells. Given that a number of small molecule allosteric modulators for nAChR, both positive and negative [46-48], have already been reported for nAChR, this shows that the ly-6 proteins are a highly druggable target, that can yield new classes of drugs that modulate airway growth, mucin production, bronchial reactivity, bronchial secretion and cancer growth and development.
It is however important to point out that there is still much we do not know about the nAChR-modulating ly-6 proteins. Do multiple ly-6 proteins bind to a single receptor, do they bind only to alpha subunits like acetylcholine or can they bind to both alpha and beta subunits? Is there cooperativity when multiple ly-6 proteins bind to the same receptor and what is the mechanism by which these proteins signal? Future studies can be expected to answer these important questions.
Highlights.
There are multiple ly-6 proteins that modulate nicotinic receptor (nAChR) signaling
Ly-6 proteins that modulate nAChR include lynx1, lynx2, slurp-1, slurp-2 and PSCA
Lynx1 modulates nicotinic signaling in normal lung to regulate mucin secretion
Lynx1 modulates lung cancer cell growth
Lynx1 is a potential therapeutic target for COPD and lung cancer
The family of ly-6 proteins needs a unified nomenclature
Acknowledgements
This research was supported by NIH grants CA151601, HL087710 and OD011092
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine Is an Autocrine or Paracrine Hormone Synthesized and Secreted by Airway Bronchial Epithelial Cells. Endocrinology. 2004;145:2498–506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- [2].Wessler I, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- [3].Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol. 2011;44:222–9. doi: 10.1165/rcmb.2010-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, et al. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE. 2007;2:e506. doi: 10.1371/journal.pone.0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kosik KS. Exploring the early origins of the synapse by comparative genomics. Biol Lett. 2009;5:108–11. doi: 10.1098/rsbl.2008.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsetlin V. Snake venom alpha-neurotoxins and other 'three-finger' proteins. Eur J Biochem. 1999;264:281–6. doi: 10.1046/j.1432-1327.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- [7].Tsetlin VI. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends Pharmacol Sci. 2015;36:109–23. doi: 10.1016/j.tips.2014.11.003. [DOI] [PubMed] [Google Scholar]
- [8].Galat A, Gross G, Drevet P, Sato A, Menez A. Conserved structural determinants in three-fingered protein domains. FEBS J. 2008;275:3207–25. doi: 10.1111/j.1742-4658.2008.06473.x. [DOI] [PubMed] [Google Scholar]
- [9].Miwa JM, Lester HA, Walz A. Optimizing cholinergic tone through lynx modulators of nicotinic receptors: implications for plasticity and nicotine addiction. Physiology (Bethesda ) 2012;27:187–99. doi: 10.1152/physiol.00002.2012. [DOI] [PubMed] [Google Scholar]
- [10].Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- [11].Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–14. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- [12].O'Neill HC, Wageman CR, Baddick C, Parker RL, Drenan RM, McIntosh JM, Miwa JM, Grady SR. Effect of Lynx1 genotype on synaptosomal activity of alpha6beta2*-nAChR. Society for Neuroscience; 42nd Annual Meeting; 2012. Abstract# 640.09. [Google Scholar]
- [13].Nichols WA, Henderson BJ, Yu C, Parker RL, Richards CI, Lester HA, Miwa JM. Lynx1 Shifts alpha4beta2 Nicotinic Receptor Subunit Stoichiometry by Affecting Assembly in the Endoplasmic Reticulum. J Biol Chem. 2014 doi: 10.1074/jbc.M114.573667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tekinay AB, Nong Y, Miwa JM, Lieberam I, Ibanez-Tallon I, Greengard P, Heintz N. A role for LYNX2 in anxiety-related behavior. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fischer J, Bouadjar B, Heilig R, Huber M, Lefevre C, Jobard F, et al. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum Mol Genet. 2001;10:875–80. doi: 10.1093/hmg/10.8.875. [DOI] [PubMed] [Google Scholar]
- [16].Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–24. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- [17].Horiguchi K, Horiguchi S, Yamashita N, Irie K, Masuda J, Takano-Ohmuro H, et al. Expression of SLURP-1, an endogenous alpha7 nicotinic acetylcholine receptor allosteric ligand, in murine bronchial epithelial cells. J Neurosci Res. 2009;87:2740–7. doi: 10.1002/jnr.22102. [DOI] [PubMed] [Google Scholar]
- [18].Narumoto O, Niikura Y, Ishii S, Morihara H, Okashiro S, Nakahari T, et al. Effect of secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor-related peptide-1 (SLURP-1) on airway epithelial cells. Biochem Biophys Res Commun. 2013;438:175–9. doi: 10.1016/j.bbrc.2013.07.048. [DOI] [PubMed] [Google Scholar]
- [19].Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208:238–45. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- [20].Hruska M, Keefe J, Wert D, Tekinay AB, Hulce JJ, Ibanez-Tallon I, Nishi R. Prostate stem cell antigen is an endogenous lynx1-like prototoxin that antagonizes alpha7-containing nicotinic receptors and prevents programmed cell death of parasympathetic neurons. J Neurosci. 2009;29:14847–54. doi: 10.1523/JNEUROSCI.2271-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levitin F, Weiss M, Hahn Y, Stern O, Papke RL, Matusik R, et al. PATE gene clusters code for multiple, secreted TFP/Ly-6/uPAR proteins that are expressed in reproductive and neuron-rich tissues and possess neuromodulatory activity. J Biol Chem. 2008;283:16928–39. doi: 10.1074/jbc.M801454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–47. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- [24].Wu R, Martin WR, Robinson CB, St George JA, Plopper CG, Kurland G, et al. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol. 1990;3:467–78. doi: 10.1165/ajrcmb/3.5.467. [DOI] [PubMed] [Google Scholar]
- [25].Robinson CB, Wu R. Culture of conducting airway epithelial cells in serum-free medium. J Tiss Cult Meth. 1991;13:95–102. [Google Scholar]
- [26].Sekhon HS, Song P, Jia Y, Lindstrom J, Spindel ER. Expression of lynx1 in developing lung and its modulation by prenatal nicotine exposure. Cell Tissue Res. 2005;320:287–97. doi: 10.1007/s00441-005-1077-9. [DOI] [PubMed] [Google Scholar]
- [27].Fu XW, Spindel ER. Recruitment of GABA(A) Receptors in Chemoreceptor Pulmonary Neuroepithelial Bodies by Prenatal Nicotine Exposure in Monkey Lung. Adv Exp Med Biol. 2009;648:439–45. doi: 10.1007/978-90-481-2259-2_50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lindstrom J, Schoepfer R, Whiting P, Anand R, Conroy WG, Saedi MS, Das M. Monoclonal antibody probes for nicotinic receptors of muscles and nerves. Biochem Soc Trans. 1991;19:115–20. doi: 10.1042/bst0190115. [DOI] [PubMed] [Google Scholar]
- [29].Fu XW, Rekow SS, Spindel ER. The ly-6 protein, lynx1 is an endogenous inhibitor of nicotinic signaling in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2012;303:L661–L668. doi: 10.1152/ajplung.00075.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dissen GA, Lomniczi A, Neff TL, Hobbs TR, Kohama SG, Kroenke CD, et al. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49:70–7. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA, et al. Nicotine induces the up-regulation of the alpha7-nicotinic receptor (alpha7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J Biol Chem. 2013;288:33049–59. doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: acceleration of sequential expression of alpha5 and alpha7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 2008;22:1356–68. doi: 10.1096/fj.07-9965.com. [DOI] [PubMed] [Google Scholar]
- [34].Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–9. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- [35].Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, et al. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–7. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- [36].Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–8. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [38].Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A. 2008;105:14686–91. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chernyavsky AI, Kalantari-Dehaghi M, Phillips C, Marchenko S, Grando SA. Novel cholinergic peptides SLURP-1 and -2 regulate epithelialization of cutaneous and oral wounds. Wound Repair Regen. 2012;20:103–13. doi: 10.1111/j.1524-475X.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125:1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- [41].Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–21. [PubMed] [Google Scholar]
- [42].West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schuller HM, Nylen ES, Park P, Becker KL. Nicotine, acetylcholine and bombesin are trophic growth factors in neuroendocrine cell lines derived from experimental hamster lung tumors. Life Sci. 1990;47:571–8. doi: 10.1016/0024-3205(90)90618-2. [DOI] [PubMed] [Google Scholar]
- [44].Quik M, Chan J, Patrick J. alpha-Bungarotoxin blocks the nicotinic receptor mediated increase in cell number in a neuroendocrine cell line. Brain Res. 1994;655:161–7. doi: 10.1016/0006-8993(94)91610-1. [DOI] [PubMed] [Google Scholar]
- [45].Lu Y, Chen J, Ding Y, Jin G, Wu J, Huang H, et al. Genetic variation of PSCA gene is associated with the risk of both diffuse- and intestinal-type gastric cancer in a Chinese population. Int J Cancer. 2010;127:2183–9. doi: 10.1002/ijc.25228. [DOI] [PubMed] [Google Scholar]
- [46].Gill-Thind JK, Dhankher P, D'Oyley JM, Sheppard TD, Millar NS. Structurally similar allosteric modulators of alpha7 nicotinic acetylcholine receptors exhibit five distinct pharmacological effects. J Biol Chem. 2014 doi: 10.1074/jbc.M114.619221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nirogi R, Goura V, Abraham R, Jayarajan P. alpha4beta2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur J Pharmacol. 2013;712:22–9. doi: 10.1016/j.ejphar.2013.04.021. [DOI] [PubMed] [Google Scholar]
- [48].Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]