Abstract
Cardiometabolic diseases, which include obesity, diabetes, hypertension and cardiovascular disease, are associated with reduced quality of life and reduced life expectancy. Unfortunately, racial/ethnic and socioeconomic disparities in these diseases exist such that minority populations, such as African Americans and Hispanics, and those of lower socioeconomic status, experience a greater burden. Several reports have indicated that there are differences in sleep duration and quality that mirror the disparities in cardiometabolic disease. The goal of this paper is to review the association between sleep and cardiometabolic disease risk because of the possibility that suboptimal sleep may partially mediate the cardiometabolic disease disparities. We will review both experimental studies that have restricted sleep duration or impaired sleep quality and examined biomarkers of cardiometabolic disease risk, including glucose metabolism and insulin sensitivity, appetite regulation and food intake, and immune function. We will also review observational studies that have examined the association between habitual sleep duration and quality and the prevalence or risk of obesity, diabetes, hypertension and cardiovascular disease. Many experimental and observational studies do support an association between suboptimal sleep and increased cardiometabolic disease risk.
Keywords: diabetes, hypertension, inflammation, epidemiology
1. Introduction
Cardiometabolic disease refers to obesity, diabetes and cardiovascular disease (CVD), which are grouped together because they are related and share risk factors. For example, approximately 70% of total mortality in type 2 diabetes is due to cardiovascular disease, and individuals with the metabolic syndrome, which is a clustering of risk factors including obesity, dyslipidemia, high blood pressure and insulin resistance, are at increased risk of developing type 2 diabetes and CVD [1]. Unfortunately, the prevalence of these diseases is not distributed equally among the different racial/ethnic and socioeconomic groups in the US. Obesity rates are higher in African Americans (45%) and Mexican-Americans (36.8%) compared to whites (30%) [2] and African-Americans and Mexican-Americans are at greater risk of developing diabetes compared to non-Hispanic whites [3]. The prevalence of hypertension is also higher among African-American adults compared to non-Hispanic whites [4]. Persons with lower socioeconomic status are also at an increased risk of cardiometabolic disease [5, 6]. Cardiometabolic disease is associated with reduced quality of life, decreased life expectancy and increased economic burden [7–10] and due to the disparities in cardiometabolic disease, minorities and people from lower socioeconomic groups suffer a greater burden.
There are disparities in optimal sleep duration and sleep quality that parallel the disparities in cardiometabolic disease. Minority groups, particularly African Americans, have shorter habitual sleep duration and poorer habitual sleep quality based on objectively-measured wrist actigraphy compared to non-Hispanic whites [11, 12]. In addition, several studies that used polysomnography (PSG) consistently observed lower amounts of slow wave sleep and reduced sleep efficiency among African Americans compared to non-Hispanic whites [13–16]. A recent meta-analysis of data from 14 studies that enrolled people without suspected sleep disorders and used either actigraphy or PSG demonstrated that on average African Americans sleep almost 30 minutes less, take 6 minutes longer to fall sleep, have 5% lower sleep efficiency and have 8% less SWS than non-Hispanic whites [17]. In addition, sleep disorders such as obstructive sleep apnea (OSA) may be more common, go undiagnosed more frequently and may be treated less efficaciously in African American patients [18, 19]. OSA is associated with poor sleep quality and often reduced sleep duration. Papers in this special issue also report sleep disparities.
Thus, cardiometabolic disease disparities and sleep disparities seem to exist, which begs the question of whether suboptimal sleep may partially mediate the cardiometabolic disease disparities. In order for this to be true, suboptimal sleep needs to be associated with increased cardiometabolic disease risk. Therefore, the aim of this paper is to review the association between sleep duration or sleep quality and cardiometabolic disease risk factors in both experimental and observational studies. Note that obstructive sleep apnea, a sleep disorder associated with cardiometabolic diseases, is beyond the scope of this article (please see [20] for a review of this topic). The next step for research would be to explicitly test whether sleep partially mediates cardiometabolic disease disparities.
2. Sleep loss and its role in the development of cardiometabolic disorders: What do experimental studies tell us?
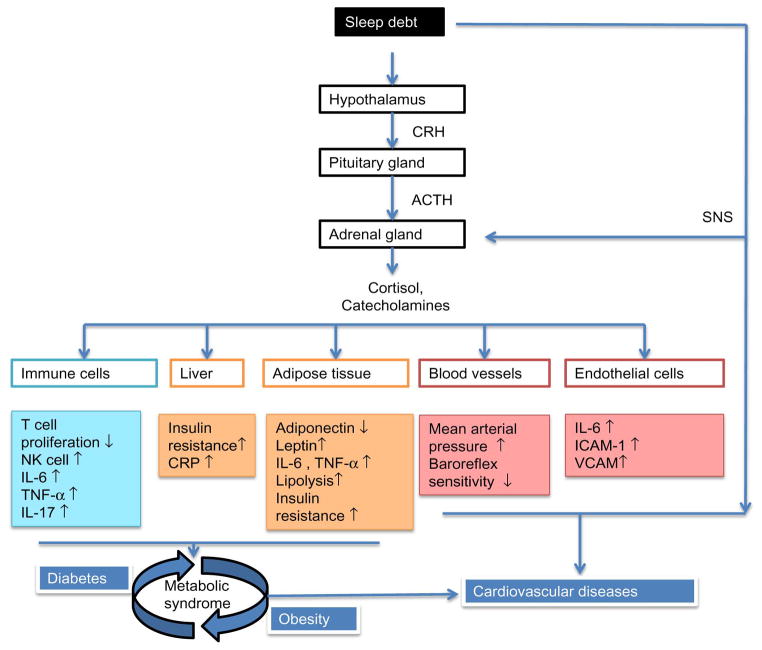
Sleep is an important and complex physiological process that is critical for maintaining metabolic homeostasis. Disturbances in metabolic homeostasis can increase the risk for developing cardiometabolic diseases, such as diabetes, obesity and heart diseases. Mounting evidence suggests that suboptimal sleep is a possible risk factor in the development of cardiometabolic disease. The effects of experimental sleep manipulation, which includes sleep deprivation or fragmentation, on cardiometabolic measures will be discussed in the following paragraphs. It is important to recognize that these effects of sleep are not isolated to any one of these single outcomes. Cardiometabolic diseases often share overlapping and intertwined pathways of sleep-regulated metabolic function, and perturbations in any one pathway may lead to increased risk of multiple metabolic disorders (see Figure 1). Controlled laboratory studies of sleep restriction and/or sleep fragmentation have shown that sleep curtailment under conditions of total sleep deprivation (TSD; continuous wake) or partial sleep deprivation (PSD; reduced sleep period) even for a night, impairs metabolic function and homeostasis.
Figure 1. Contributions of sleep debt in the pathophysiology of cardiometabolic diseases.
Suboptimal sleep leads to metabolic dysfunction via activation of stress-immune axis. Acute sleep loss can trigger metabolic distress in immune cells, hepatocytes, adipocytes, endothelial cells and blood vessels by disturbing their homeostatic regulation. Prolonged activation along with defective counter-regulatory mechanisms as a consequence of chronic sleep deficiency may lead to the development of cardiometabolic diseases.
Natural killer (NK) cell, interleukin (IL), tumor necrosis factor (TNF), c-reactive protein (CRP), intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), sympathetic nervous system (SNS).
2.1 Sleep and Diabetes
2.1.1 Glucose metabolism
In healthy individuals, glucose homeostasis is a tightly regulated balance between glucose release into the blood and glucose utilization to maintain a plasma glucose level of approximately 5 mmol/L. A decrease in plasma glucose levels suppresses the release of insulin by pancreatic β-cells, activates the sympathetic nervous system and triggers the release of pancreatic α-cell glucagon, catecholamines, cortisol and growth hormone (GH). These changes will collectively inhibit glucose uptake and increase hepatic glucose release into the blood. On the other hand, an increase in plasma glucose will lead to the stimulation of insulin secretion and suppression of glucagon to restore normal glucose levels. One of the key regulatory factors in glucose metabolism is insulin. Insulin regulates glucose metabolism through both direct actions (binding to insulin receptors in the muscle and adipose tissue to stimulate glucose uptake) and indirect actions (suppression of glucose release from the liver and kidney, inhibition of free fatty acid (FFA) release). Insulin resistance is the decreased ability of cells to respond to insulin. This generally leads to an abnormal increase in pancreatic insulin secretion to regulate plasma glucose uptake. When this compensation from the pancreatic β-cells becomes insufficient, it leads to type 2 diabetes mellitus (T2DM).
Spiegel et al demonstrated a direct effect of sleep restriction on glucose metabolism [21]. In this study, glucose tolerance measures were taken after one week of partial sleep deprivation of 4 hours in bed per night for 6 nights (sleep-debt) and after 6 nights of 12 hours per night in bed (sleep-recovery). A 40% decrease in the rate of glucose clearance (glucose tolerance) and a 30% decrease in glucose effectiveness, the ability of glucose to mediate its own disposal independent of insulin, were observed during the sleep-debt condition. This was the first evidence showing sleep loss can impair glucose metabolism. Subsequent studies further corroborated this finding. Acute partial sleep restriction of 4 hours in bed for one night when compared with a night of unrestricted sleep showed an increase in endogenous glucose production (p=0.017) and a decrease in glucose disposal rate (p=0.009) from a hyperinsulinemic clamp assessment indicating a decrease in hepatic and peripheral insulin sensitivities [22]. In another study, 2 nights of sleep curtailment to 4 hours in bed per night in healthy men reported a significant decrease in insulin sensitivity compared to 2 nights of unrestricted sleep [23]. In other studies that enforced partial sleep deprivation (5 hours in bed) for one week in men [24] and 4 days of decreasing sleep curtailment (7 hours, 6 hours, 4 hours, 4 hours on consecutive nights) in women [25] also observed decreased insulin sensitivity and glucose tolerance. The chronic effects of sleep loss on alterations in glucose metabolism were further demonstrated by a randomized crossover study comparing 5.5 hours in bed per night for 2 weeks to 8.5 hours in bed per night for 2 weeks in 11 healthy, overweight individuals. This sleep restriction period was associated with reduced oral glucose tolerance and insulin sensitivity [26]. Quality of sleep also affects insulin sensitivity as demonstrated by a study that fragmented sleep across the night [27], and another that suppressed Stage N3 (deep non-REM sleep or slow wave sleep [SWS]) [28]. In both of these studies decreased glucose tolerance and insulin sensitivity were observed. A single night of SWS or REM suppression was compared to a night of uninterrupted sleep in 16 healthy men and insulin sensitivity was decreased after SWS-suppression (−15%, p<0.001) but not REM suppression [29].
These studies collectively suggest that sleep durations of 6 hours or less across multiple consecutive nights and poor sleep quality has profound effects on glucose metabolism and homeostasis.
2.1.2 Counter-regulatory molecules
Other counter-regulatory hormones coordinate glucose homeostasis and are impacted by sleep curtailment, including glucagon [30] and catecholamines [31]. The alterations in the secretory profiles of these molecules may also contribute to the changes in glucose regulation observed after sleep restriction. Two crossover studies of a single night of total sleep [32] or partial sleep [30] deprivation showed decreased basal glucagon levels, however catecholamines levels remained unchanged. In another study of partial sleep deprivation, one night of partial sleep deprivation was associated with significantly increased catecholamine levels [31]. A similar finding was also reported in a crossover study of 14 healthy males during 24-h continuous wakefulness [33]. They reported an increase in nocturnal and daytime levels of norepinephrine (p<0.05). Another study that included both men and women reported an increase in nocturnal and 24-h epinephrine (p<0.05) and nocturnal norepinephrine (p<0.05) during 2 weeks of 4.5 hours in bed per night compared with 8.5 hours in bed per night [26]. Three days of 4 hours in bed per night compared to 9 hours in bed per night for 3 days in adolescent boys resulted in an increase in fasting insulin (+59%, p=0.001), fasting C-peptide (+24%, p<0.001) and post-prandial C-peptide (+11%, p=0.018), in addition to a 24% decrease in 24-hour epinephrine levels (p=0.013) [34]. The combination of elevated insulin and C-peptide levels and unchanged glucose and glucagon levels indicate a reduced sensitivity to insulin. These studies point to a suppressive action of sleep loss on pancreatic α-cell glucagon secretion irrespective of glycemic level. The pancreatic glucose regulation could be partly under the control of the autonomous nervous system.
2.1.3 Adipokines
In addition to skeletal muscle and liver, another important insulin-sensitive peripheral tissue is the adipose tissue, which is comprised mainly of adipocytes, fibroblasts, immune cells and other cell types. It secretes various peptides and proteins collectively called adipokines, and include leptin, adiponectin, omentin, resistin, TNF-α and IL-6. The white adipose tissue is not only an energy-storage depot; it is also an endocrine regulator of energy homeostasis. Acute sleep restriction of 4.5 hours in bed per night for 4 days was compared to 8.5 hours in bed per night for 4 days in a randomized cross-over study [35]. The phosphorylation of Akt is a crucial early step in insulin signaling in the adipocytes, and the effect of increasing doses of insulin on the phosphorylation of Akt was reduced by 30% (p=0.01) after acute sleep deprivation, which indicates decreased insulin sensitivity in the adipose tissue. There was also a parallel reduction in total body insulin sensitivity (p=0.02). The actions of insulin through the Akt pathway in adipose tissue include leptin secretion and fat metabolism and therefore alterations in the insulin sensitivity of the adipose tissue could have important metabolic consequences.
One of the most studied adipokines in relation to sleep duration is leptin, which is produced by the adipocytes in proportion to the mass of the adipose tissue. Leptin signals satiety through its interactions with the appetite-regulating centers in the brain; hence it is called the satiety hormone. Leptin is regulated by insulin through glucose metabolism (i.e. by food intake) and it also has a diurnal rhythm that peaks at the nocturnal sleep phase and declines in the morning a few hours after a rise in levels of another adipokine called adiponectin [36, 37]. Continuous total sleep deprivation for 88 hours in healthy men (body mass index (BMI) range: 20–34.5 kg/m2) decreased the nocturnal amplitude of leptin (p<0.001) [38]. Similarly, 4 hours in bed per night for 6 days significantly decreased leptin’s amplitude by 20% when compared with 12 hours in bed per night for 6 nights in 11 lean men [39]. Partial sleep deprivation of 4 hours in bed for 2 days also decreased leptin levels (−18%, p=0.04) in 12 healthy men (BMI: 23.6 ±2.0 kg/m2) [40]. The effects of sleep restriction on leptin levels in subsequent studies have been mixed, with some studies reporting an increase in leptin [25, 41–45] while others reported no significant difference [33, 46–51] after sleep restriction. Among the studies that did find an increase in leptin levels, one measured the energy balance of the subjects and found energy balance to be positive during sleep loss in comparison to after habitual sleep [25]. Leptin increased in parallel with increase in energy intake and energy expenditure [25], while other studies either reported no significant differences in energy intake [42, 45, 52] or did not evaluate energy expenditure [44]. Among studies that reported no significant difference in leptin, some did not assess energy intake or controlled calorie intake [33, 46, 51] while others reported increase in calorie intake without any changes in energy expenditure [47, 49, 50]. Finally, a recent study of sleep restriction observed decreased leptin levels, consistent with the Spiegel et al study described above. In this study, 19 healthy men were sleep restricted by 1.5 hours each night for 3 weeks [53]. In the sleep-restricted group, morning leptin levels after three weeks of sleep restriction were reduced (p=0.023) compared to baseline. The subjects were allowed to eat ad libitum but no documentation of calorie intake during those 3 weeks was reported. Interpreting the discrepant results regarding sleep restriction and leptin levels is further complicated by the dynamic relationship between leptin, food intake and body fat changes, and these three components are not always measured and/or controlled.
Adiponectin is another adipocyte-derived hormone that has been known to have anti-inflammatory properties. Decreased plasma levels of adiponectin have been associated with increased insulin resistance. Like leptin, adiponectin has an internal circadian rhythm that peaks late in the morning and decreases during nocturnal sleep [37]. In one study 74 healthy adults were allowed 4 hours in bed per night for 5 days (sleep restriction) after 2 nights of 10 hours in bed (baseline) [52]. A decrease in plasma adiponectin levels after sleep restriction compared to baseline was observed among Caucasian women (p = 0.028), but an increase in adiponectin after sleep restriction compared to baseline was observed among African American women (p = 0.006) despite similar adiponectin levels at baseline in both racial groups. No significant alterations of adiponectin were observed among men [52]. An increase in leptin under conditions of leptin resistance and a decrease in adiponectin contribute to the pathogenesis of insulin resistance and diabetes in overweight or obese individuals. Other adipokines that are altered under controlled conditions of sleep loss are TNF-α and IL-6, which will be discussed below in section 2.4.2.
In conclusion, sleep loss has a deleterious effect on insulin sensitivity and overall glucose homeostasis through alterations in several key metabolic hormones, including insulin, leptin and other adipokines.
2.2 Sleep and Obesity
Experimental studies of sleep restriction have demonstrated an effect of short sleep on markers of positive energy balance such as decreased energy expenditure (EE), increased feelings of hunger and appetite, increased food intake and changes in appetite regulating hormones. The effects of acute sleep curtailment on energy homeostasis could lead to weight gain overtime if sleep loss is extended for longer periods.
Studies have examined the effects of sleep restriction on hunger and food intake. For example, a single night of TSD or PSD (4.5 hours in bed/night) leads to a significant increase in hunger [51]. Partial sleep restriction of 4 hours in bed per night for 2 consecutive days induced an increase in hunger and appetite (+23–24%, p<0.01) especially for foods with high carbohydrate content such as sweets and salty snacks (p<0.02) [40]. In 14 healthy women (BMI: 20.0–36.6 kg/m2), 4 nights of consecutively increasing sleep curtailment (7 h, 6 h, 6 h and 4 h sleep/night) with an ad libitum diet had a profound effect on total energy intake (+20%, p<0.05) with no changes in energy expenditure [25]. A single night of 4 hours in bed, when compared to 8 hours in bed, increased total calorie intake by 22% (p<0.01) [54]. This increase in food consumption was mostly during breakfast and dinner, particularly under free-living conditions, and was preceded by greater sensation of hunger. A significantly higher preference for fatty foods was also noted on the day after sleep restriction, particularly at dinner. In another study, participants were restricted to two-thirds of their habitual sleep time for 8 days and their caloric consumption was measured [47]. The wake time for all participants was constant and ad libitum food intake was allowed. Under these conditions, caloric intake increased by 677 kcal/d (p=0.014) in the sleep deprived state. Mimicking a typical work-week, 31 participants in another study were allowed to sleep for 4 hours per night for 5 days and a control group of 6 participants were allowed 10 hours in bed [55]. Sleep-restricted subjects consumed more calories (+30% of daily caloric requirement, p=0.003) during days with restricted bedtime compared with control subjects during corresponding days. In a study that limited time in bed to 5.5 hours in bed per night for 2 weeks with ad libitum access to food and snacks did not observe changes in caloric intake during meals or energy expenditure, but calories from snacks increased (p <0.04) [26]. In another study, 15 men and 15 women were restricted to 4 hours in bed per night for 5 days after 9 hours in bed per night for 5 days [56]. The diet on the first 4 days was controlled, while ad libitum food intake was allowed on the last day. Participants consumed more energy on day 5 after short sleep (2813.6 ± 593.0 kcal) than after 9 hours in bed (2517.7 ± 593.0 kcal; p = 0.023). This effect was mostly due to increased consumption of fat (p =0.01), notably saturated fat (p = 0.038) after short sleep. This study also observed differences in energy intake between men and women. Women had a 15.3% increase in energy intake after short sleep relative to habitual sleep (p = 0.07), while men had a 9.2% increase (p = 0.14). The increase in the consumption of fatty foods also varied between women (+39%, p=0.007) and men (+10.3%, p=0.32). When comparing these studies, the different effects on food intake could be attributed to differences in study design such as availability of types of food, snacks, and participant characteristics such as age, gender and BMI. These studies bolster the notion that sleep loss could lead to increased appetite, particularly for unhealthy foods, could promote weight gain.
Specific sleep stages may be related to hunger and appetite. Specifically, inverse associations between REM sleep duration and hunger (p<0.031) and between stage 2 sleep and appetite for sweet (p<0.015) and salty (p<0.046) foods were detected in one study [57]. This study also observed that Stage 2 sleep percentage was inversely related to energy consumed (p<0.024) and the percentages of Stage 2 sleep (p<0.005), SWS (p<0.008), and REM sleep (p<0.048) were inversely related to fat intake, while SWS (p<0.040) and REM sleep durations (p<0.050) were inversely related to carbohydrate intake. Thus, more REM and more SWS were associated with less fat and carbohydrate intake. In another study that used PSG, a night of fragmented sleep in male subjects resulted in a decrease in REM (p<0.05) and an increase in the post-dinner desire-to-eat ratings (p<0.05) the following day [46]. Thus, specific sleep stages, such as REM and SWS, may be particularly important in appetite regulation.
A few studies noted differences in energy expenditure (EE) under conditions of sleep deprivation. In one study of 5 hours in bed per night for 5 days, showed an increase in 24-h EE (+5%, p<0.01) when compared with 9 hours in bed per night for 5 days [41]. A parallel increase in food intake (+6%, p<0.05) was observed. Post-dinner snacking increased with a greater preference for carbohydrates. This may be attributed to the increased energy required to stay awake during periods of wakefulness and ad libitum availability of food. The study participants had an overall positive energy balance with an average yet statistically insignificant increase in weight gain in the 5-h per night sleep condition. Other studies have shown a decrease in EE following sleep loss. In 14 normal-weight men in the morning after 24-h of continuous wakefulness both resting and postprandial EE were assessed along with hunger ratings before the standardized breakfast. The resting (−5%, p<0.05) and postprandial (−20%, p<0.001) EE were significantly reduced after one night of total sleep deprivation with a significant increase in hunger ratings while no changes in ad libitum food intake in the afternoon was observed [33]. One night of TSD in 7 healthy participants increased EE by 32% during the night of TSD and then decreased by 4% during the recovery night when compared to baseline [58]. Some studies also showed that sleep restriction decreased physical activity, which may contribute to lower EE during sleep debt. In 15 healthy men spontaneous physical activity was recorded by accelerometer during a 15-h daytime period after 2 nights of restricted bedtime (4.5 hours in bed per night) [49]. Sleep deprivation significantly decreased physical activity (p=0.008) and also shifted the physical activity towards lower intensity activity (p<0.05) without any changes in energy intake. Another study of a single night of sleep restricted to 4 hours in bed per night resulted in a 22% increase in caloric intake (p<0.01) with a significant increase in physical activity recorded by an actimeter despite an increase in sleepiness [54]. The latter study allowed ad libitum food intake and that may be reflected in the increased calorie intake with increased physical activity. The data summarized above suggests that increased food intake during insufficient sleep is a physiological adaptation to provide energy needed to sustain additional wakefulness; yet when food is easily accessible, intake surpasses need. The increase in appetite and food intake coinciding with decreases in physical activity and energy expenditure under sleep-deprived conditions could result in overeating.
In addition to the homeostatic system, the hedonic system also plays an important role in regulating food intake. After one night of TSD, 16 healthy normal-weight men choose larger food portions (+14%, p=0.02) in correspondence with increased hunger status [59]. The portions of snacks consumed after breakfast also increased (+16%, p=0.02). TSD [60] and PSD [61] lead to greater stimulation of brain regions in response to hedonic food stimuli. The overall neuronal activity measured by fMRI in response to food stimuli was greater after sleep restriction than habitual sleep. The areas of brain with increased neuronal activity after sleep deprivation were associated with reward centers. Another study showed sleep loss decreases activity in the appetite evaluation regions within human frontal cortex and insula cortex during food desirability choices combined with an increase or amplification of activity within the amygdala promoting a desire for high-calorie foods [62].
Sleep restriction is also known to alter the hormonal profile of appetite regulating hormones: anorexigenic leptin (discussed previously) and orexigenic ghrelin. While ghrelin and leptin levels remain unchanged in some studies [46, 47, 49, 50], a few others have shown significant changes after sleep deprivation. A randomized crossover study of 16 healthy normal-weight men resulted in an increase in hunger ratings parallel to an increase in morning plasma ghrelin levels (+13%, p=0.04) after one night of TSD when compared to 8 hours in bed sleep opportunity [59]. A similar study with 14 men also showed elevations in morning ghrelin levels (p<0.02) following the night after TSD [33]. In yet another study with 9 healthy normal-weight men plasma ghrelin levels were 22% higher after total SD than after 7 hours of sleep (p <0.05) [51]. Feelings of hunger were elevated, whereas morning leptin concentrations remained unaffected. In a randomized crossover study of 12 healthy normal-weight men, 2 consecutive nights of PSD (4 hours in bed) was compared with 2 consecutive nights of 10 hours in bed [40]. Ghrelin levels were elevated (+28%, p <0.04), along with an increase in subjective hunger and appetite (p<0.01). In another study of 27 normal-weight men and women, 3 nights of 4 hours in bed (PSD) was compared with 9 hours in bed under conditions of controlled calorie intake [48]. Total morning ghrelin increased (p<0.05) in men but not in women. On the contrary, in a study of 16 healthy normal-weight adults (8 men and 8 women), 24-h ghrelin levels decreased after a night of PSD (5 hours in bed) in comparison to baseline (−30%, p<0.001), while the 24-h leptin levels increased (+22%, p<0.05) under the same conditions [41]. Although the changes in hormone levels promoted satiety and reduced hunger, there was a significant increase in overall food intake promoting overeating in this study. Thus, the association between ghrelin and sleep loss is not consistent, which may be due to duration and severity of sleep restriction as well as to the complex association between food intake and regulation of this hormone.
If sleep loss increases appetite and subsequently food intake without a compensatory and equivalent increase in energy expenditure, then sleep loss could increase risk of weight gain and obesity.
2.3 Sleep and Cardiovascular diseases
2.3.1 Blood pressure
Circulation of blood by the cardiovascular system is partially mediated through the regulation of blood pressure (BP). Baroreflex modulated by behavioral and physiological changes contributes to control of arterial blood pressure through the autonomous nervous system and baroreceptors. In a normotensive person the BP circadian rhythm is characterized by a steep nocturnal/sleep decrease called BP dipping and a rise in the wake-associated elevations of BP. Non-dipping in BP is associated with CVD risk [63] and has been associated with poor sleep [64]. Increased BP during sleep deprivation could be due to a combination of factors such as increased sympathetic outflow to the heart or periphery, decreased baroreflex sensitivity and a resetting of the baroreflex set-point to a higher level.
After a night of total sleep deprivation in 8 healthy men and women, the systolic BP (SBP) (p=0.002) and diastolic BP (DBP) (p=0.05) were significantly higher than after a night of regular sleep. However, the morning plasma catecholamines and heart rate remain unchanged [65]. In another study of 6 healthy men, TSD increased diastolic BP (p=0.02) and increased arterial baroreflex set-point by 12 mm Hg (p<0.05) [66]. No changes in morning plasma catecholamines, including epinephrine and norepinephrine, dopamine, or cortisol were recorded. These two studies attribute the effects of sleep deprivation on baroreflex to changes in peripheral and central mechanisms but did not observe an increase in their measures of sympathetic nervous activity. This could be due to the subjects resting in a supine position throughout the study [67]. This scenario is unlikely to occur in the real world, as it may be difficult to stay awake recumbently when sleep pressure is high.
A single night of sleep restriction in normotensive subjects from 8 hours in bed to 5 hours in bed was associated with an increase in the morning to mid-afternoon SBP and heart rate (p<0.05) [68]. When the PSD duration was increased to 4 hours in bed for 6 consecutive nights, a significant decrease in heart rate variability was observed (p<0.02), which indicates elevated sympathovagal balance [21]. Another study found no significant differences in SBP, DBP, or mean arterial pressure (MAP) after 36 hours of continuous sleep deprivation in 18 subjects seated in an upright position while the measurements were taken, but arterial baroreflex sensitivity significantly decreased [69]. This study also observed a significant increase in sympathetic modulation of BP variability and reduction in parasympathetic modulation of heart rate variability, which suggests increased sympathetic nervous activity. In summary, the above studies demonstrate the effects of acute sleep loss on HRV, BP and arterial baroreflex sensitivity and a prolonged effect of sleep loss on these cardiac measures could increase the risk for heart diseases.
2.3.2 Endothelial dysfunction
The endothelium, a thin layer of cells that forms the inner surface of blood vessels, controls vascular tone, inflammatory response and coagulation [70]. Endothelial function is clinically assessed as the degree of impaired vasodilation measured in arterial and venous circulation by changes in vessel diameter [71, 72]. Impairment of endothelial function is a mechanism in the development of CVD [73] and also an independent predictor of CVD risk [74]. In a randomized controlled study of 15 healthy lean participants (10 men, 5 women), partial sleep deprivation (two thirds of regular sleep duration) for 8 days showed a significant impairment in arterial endothelial function (−4.4%, p<0.01) when compared to the control group [75]. In another randomized crossover study of 18 healthy male participants, partial sleep deprivation (<5 hours) for 5 days was compared to regular sleep (>7 hours) and a significant reduction in venous endothelial function was observed [76]. Similarly, acute total sleep deprivation (TSD) also altered endothelial-dependent arterial [77] and microvascular activities [78] in healthy participants. Thus, experimental sleep loss appears to impair endothelial function.
Sleep debt impacts many of the risk factors for CVD, including increased blood pressure, insulin resistance, obesity, unhealthy diet, and lower physical activity as suggested by the controlled sleep deprivation studies discussed previously. In addition to these risk factors, cardiometabolic diseases, which are known to be inflammation-associated pathological states, can be exacerbated by the activation of stress and immune systems during prolonged cumulative sleep restriction.
2.4 Potential Mechanisms that relate sleep to cardiometabolic disease risk
2.4.1 Stress response
Several studies have shown an activation of the hypothalamic-pituitary-adrenal (HPA) stress axis in response to sleep restriction. The HPA axis is the principal mediator of the neuroendocrine stress system and seems to play a central role in sleep debt induced metabolic impairment. Activation of HPA axis initiates hypothalamic secretion of corticotropin-releasing hormone (CRH) leading to the secretion of glucocorticoids (cortisol) by the adrenal cortex. Cortisol inhibits the activation of the HPA axis through a negative feedback loop to the hypothalamus and pituitary. The circadian rhythm of the HPA axis is regulated via the suprachiasmatic nuclei (central pacemaker) in the brain. Cortisol rhythmicity is a critical regulatory component of the sleep/wake cycle. In a healthy person with sufficient nocturnal sleep cortisol levels begin to increase toward the end of their sleep period resulting in a morning surge in cortisol levels that peaks shortly after awakening. After this peak, the negative feedback mechanism begins to suppress the HPA axis, leading ultimately to a long quiescent period of low cortisol levels reaching its nadir 1–2 hours around sleep onset.
Total sleep deprivation of more than 24 hours [79–81] or partial deprivation of 3–4 hours in bed each night for 1–3 days [34, 44, 82] showed no significant change in morning cortisol levels after sleep deprivation. The same trend was observed for levels of plasma adrenocorticotropic hormone (ACTH), which is produced by the pituitary in response to CRH [83]. In other studies of partial sleep deprivation (3–4 hours in bed per night) where bedtimes were either delayed or wake times were advanced, decreased morning cortisol levels were observed in men [84, 85] and women [45]. REM sleep has been shown to be positively correlated with morning cortisol levels [86] but inversely associated with afternoon cortisol levels [87] and could partly explain decreased morning cortisol levels after sleep restriction. It is possible that sleep restriction causes a shift in the time of morning cortisol rise and/or the prolonged wake time activates the HPA axis through the night decreasing the amplitude of morning cortisol peak. This in addition to the lack of repeated blood sampling before and after morning wake time suggests the possibility that the actual peak of cortisol may have been missed. Another possibility is the rate at which the morning cortisol levels decrease following its peak is slower after a night of sleep restriction resulting in elevated evening cortisol levels. This was shown in the study of acute sleep restriction (3 hours in bed for one night) [45] and prolonged sleep restriction (4 hours in bed per night for 5 days) [23, 42]. In the first study, a significant reduction in morning cortisol (p=0.02) and an elevation in evening cortisol (p=0.04) as a result of slower decline of daytime cortisol (p=0.04) was observed after sleep restriction [42, 45]. In the second study, an increase in cortisol (p=0.002) but not ACTH levels was observed after 5 days of sleep deprivation [42Schmid, 2011 #6886]. Cortisol was 15.5% higher after sleep restriction but decreased during the day due its internal rhythmicity. An increase in evening cortisol levels was a result of a slower decline of daytime cortisol. The elevation of evening cortisol was recorded in other studies of sleep restriction as well [85, 88, 89]. [42]Cortisol levels also increased after sleep fragmentation when compared with non-fragmented sleep (p<0.05) [46]. This difference is attributed to a lower morning rise in cortisol and higher evening cortisol level [46]. A controlled study that simulated habitual sleep patterns of a typical week included 5 days of sleep restriction (work week) followed by 2 days of extended sleep opportunity (weekends). No changes in 24-h cortisol was observed when comparing sleep restriction to baseline, however a decrease in 24-h cortisol levels during sleep extension (p<0.05) was observed. [90]. In summary, cortisol levels appear to increase as a result of sleep loss and these elevated levels could exacerbate sleep disturbances. Higher cortisol levels are associated with insulin resistance hours [91, 92] and therefore could be one mechanism through which sleep loss impairs glucose metabolism. HPA axis activation can also regulate immune cell traffic [93] and provides another possible link between sleep and chronic-inflammation based diseases.
An additional or alternative pathway linking sleep, stress and glucose metabolism involves gene expression. Partial sleep restriction for 4 hours in bed per night for 5–7 days activated several gene pathways, notably those that were involved in the stress response and apoptosis, as measured by gene microarray [94, 95]. A similar differential gene expression was observed after a single day of prolonged wakefulness [96]. These studies elucidate the possibility that a mechanism alternative to cortisol-HPA-stress axis may be activated. Sleep deprivation showed differential expression of stress-related genes particularly those related to endoplasmic reticulum (ER) stress. ER stress can lead to protein misfoldings that activate the unfolded protein response (UPR) pathway. The cell produces heat shock proteins (HSP) to counter the perturbations caused by ER stress, but when it fails to check the UPR stimulation, it triggers cell apoptosis. For example, activation of the ER stress pathway in macrophages could lead to apoptosis, which is detrimental in atherosclerotic lesions.
2.4.2 Sleep and Immune system
Chronic inflammation, a risk factor for CVD, is caused by disruption to immune system homeostasis. The immune cells follow the 24 hour biological rhythm that is regulated by the central clock, and sleep is partly regulated by proinflammatory cytokines. Some of the key sleep-associated inflammatory measures include cytokines such as TNF-α, IL-1, IL-2, IL-6 and IL-10 and immune cells such as neutrophils, monocytes, lymphocytes. The mechanism of activation and regulation of this neuroendocrine-immune axis is not entirely known. Sleep deprivation disturbs the circadian rhythm of the immune cells leading to disruptions of the physiological rhythms that could lead to metabolic and endocrine consequences. It has been shown that white blood cells (WBCs) such as neutrophils and natural killer (NK) cells peak during the light phase of the sleep/wake cycle and reach their nadir during the dark cycle, while the circulating monocytes, T and B lymphocytes peak during the early stages of nocturnal sleep and decrease during the day [97]. A circadian rhythm in cytokines has also been observed. TNF-α, IL-1, IL-2, IL-6 and IL-12 (proinflammatory) reach their peaks during the sleep phase while IL-10 (anti-inflammatory) reaches its maximum levels proceeding wake initiation.
Total sleep deprivation (TSD) of 40 hours or more in humans alters both the cellular and cytokine patterns of immune cells. A delay in the rhythm of lymphocytes and monocytes by 3 to 6 hours was observed in TSD compared to normal sleep. Lymphocytes, monocytes and natural killer (NK) cells were significantly higher during the night of wakefulness and remained high the following day when compared to baseline [81, 98]. Two nights of TSD increased neutrophils and leukocytes but the levels returned to baseline after sleep recovery [99]. On the contrary, the leukocyte numbers measured in the morning after >40h of TSD showed a decrease in NK cell counts (p=0.001) and an increase in monocytes (p=0.017) [81]. Partial sleep deprivation (PSD) is a better representation of habitual sleep loss in the population than TSD. Studies that followed a PSD sleep protocol showed variable results in leukocytes count as well. An increase in WBC count (p=0.03) and neutrophils (p=0.01) was observed the morning after 3 nights of PSD to 4 hours in bed per night [100]. No changes in monocytes, lymphocytes and CRP were reported. The increase in leukocyte numbers persisted even after a night of sleep recovery following acute partial sleep loss [101]. Further analysis showed a significant increase in neutrophil populations. Another study reported a decline in NK cell activity by 30% (p<0.01) after a night of PSD (4 hours per night) towards the later part of sleep [102]. This was restored after sleep recovery the following night. Partial sleep deprivation during the early part of sleep for one night showed no changes in WBC count across baseline, PSD and recovery nights [103]. However, granulocyte numbers significantly decreased while lymphocyte numbers increased the morning after PSD. Recovery night restored the immune cell numbers to baseline values. Simultaneous decrease in NK cell activity (p<0.05) was observed after accounting for fluctuations in NK cell count between baseline and PSD suggesting an impairment in its activity independent of decrements in the number of NK cells. An inhibitory effect of sleep restriction on NK cell count and activity were observed. In parallel, a significant reduction in T helper cell IL-2 (an activator of NK cells) production was observed. This reduction in NK cell activity persisted even after a night of recovery sleep. When T lymphocytes collected after sleep restriction were incubated with monocytes after baseline sleep, a decrease in IL-2 production signified the effect of partial sleep deprivation on T cell function. A night of TSD elevated neutrophil counts and altered subpopulations of circulating neutrophils the following morning [104]. An increase in immature cells and CD11b surface marker was observed. The elevation of CD11b and CD35 on neutrophils is observed in chronic inflammatory diseases like asthma. This suggests nocturnal wakefulness may promote a pro-inflammatory state of circulating immune cells. The effect of sleep deprivation on WBC numbers have been mixed across studies and this variation could be explained by differences in blood sampling frequency, time of day and sample heterogeneity. Changes in immune cell numbers after SD, for the most part, returned to baseline levels after recovery sleep. This could suggest an alteration in their redistribution between the lymphoid organs and peripheral tissue. This could potentially play a role if sleep activates inflammation in peripheral tissues like adipose tissue and thereby modulating cellular insulin sensitivity.
Effects of sleep loss on cytokine gene and protein expression have also been recorded. The two most commonly assessed cytokines are IL-6 and TNF-α. These proinflammatory cytokines are primarily monocyte derived and are elevated during slow wave sleep and reach their nadir in the morning [105]. After a night of TSD, mean daytime IL-6 values were significantly higher (p<0.05) than baseline and a nighttime undersecretion (p<0.05) was reported [106]. When compared with a night of continuous wakefulness, sleep elucidated an increase in the concentrations of soluble IL-6 receptors (sIL-6R) but not membrane-bound IL-6R which negatively regulate IL-6 trans-signaling [107]. No changes in IL-6 producing monocytes were observed. TSD was associated with an increase in proinflammatory IL-1β [108]; however data on IL-6 have been mixed. Continuous wakefulness resulted in the increase in IL-6 [88, 109, 110], decrease in IL-6 [111] or no measurable changes [97, 108]. This could in part relate to IL-6 cytokine’s dual pro- and anti- inflammatory roles. Continuous wakefulness of 40 hours significantly increased TNF-α (p<0.01) but not its receptor, TNFR1 [112]. No changes in IL-6 and CRP levels were documented.
PSD for one night (4 hours in bed) showed a significant increase in nocturnal IL-6 levels compared to baseline [113]. Blood was sampled during the nocturnal sleep period. However daytime IL-6 levels after PSD was not measured. Another study of acute partial restriction of REM sleep across 4 days showed no changes in cytokines IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ but a significant increase in T lymphocytes and IgA. However, another study that included a week of PSD from 8 hours in bed to 6 hours in bed for 7 nights showed elevated levels of 24-h IL-6 (p<0.01) and TNF-α (p<0.05), indicating a pro-inflammatory shift [114]. A similar increase in proinflammatory cytokines IL-1β (137%, p<0.05), IL-6 (163%, p<0.05) and IL-17 (138%, p<0.05) was observed in stimulated peripheral blood mononuclear cells (PBMC) collected after 5 nights of PSD to 4 hours in bed [115]. A possible difference in results could be attributed to limits in detection of low concentrations of plasma cytokine levels owing to their short half-life and sensitivity of assay used for its detection. Moreover, frequency of sampling may vary; some studies include a single morning sample and local inflammation caused by repeated blood sampling via catheter insertion could also confound the results [116]. Stimulation of PBMC in vitro, could detect low-grade inflammation that may not be captured in plasma samples.
Recently analysis of the transcriptome of blood cells after sleep deprivation has shed light on the differential expression of several genes; a common thread being alteration of immune pathways. Whole genome microarrays complemented with pathway and transcription factor analysis of blood samples comparing experimentally induced PSD of 4 hours in bed per night for 5 days to baseline and post-recovery periods revealed that 33% of the 25 most up-regulated genes were related to immune function including IL-8 biosynthesis regulation (p<0.001), lymphocyte activation (p<0.02) and NF-kB signaling (p< 0.005) besides several genes related to stress signaling [94]. Among the 25 most down-regulated genes 19 were associated with immune system including NK cell function. Cumulative sleep restriction (SR) resulted in an increase in IL-8 production along with activation of TLRs, NF-kB and AP-1. These pathways point towards the activation of pro-inflammatory cascade through the innate immune system. This study also showed a shift towards Th2 cell type while the total number of T cells remained constant. This, along with the gene expression data, suggests acute SR activates the innate and adaptive immune systems. In addition, one night of TSD significantly decreased the number of pre-mDC producing IL-12, a main inducer of Th1 response [117]. Compared to a night of sustained wakefulness, sleep suppressed IL-4 producing Th2 cells [118]. The Th1/Th2 balance measured by IFN-γ/IL-4 producing T cells was enhanced by SWS. During the REM phase of sleep, the Th balance reversed. But the ratio of Th2/Th1 was lower (p<0.01) during sleep than wakefulness.
Natural regulatory T cells (nTreg) are key regulators of adaptive immune responses that are regulated by IL-2 production. They are known to attenuate activated immune responses and control systemic immune homeostasis. Following one night of TSD, nTregs known to exhibit its highest suppressive activity during sleep, was diminished [119]. To put it in context, the increase in pro-inflammatory cytokines during sleep will consequently lead to subsequent increases in the suppressive activity of nTregs to maintain immune homeostasis. In this study, the levels of IL-2 and T cell proliferation were sleep-independent; however the same was not true for nTreg activity. A diminished nTreg suppressive activity rhythm might be a factor in the aggravation of the immune system post sleep deprivation. PSD during early part of the sleep cycle showed an increase in monocytic IL-6 and TNF-α when compared to uninterrupted sleep [120]. An increase in mRNA transcripts of IL-6 and TNF-α genes were also observed. Bioinformatic analysis suggested the inflammatory response was mediated by NF-kB signaling pathway. A significant increase in IL-8 and IL-1β gene transcripts was also observed. The data from the gene array was limited by sample size (n=5). In the morning after a night of PSD to 4 hours in bed, the activation of NF-kB was greater than baseline or sleep recovery mornings [121]. Effects of acute SR on blood transcriptome was accessed at time points distributed across the day following 1 week of <6h or 8.5 h of sleep each night [95]. Loss of circadian rhythmicity of genes associated with peripheral circadian clocks, oxidative stress, inflammation and metabolism were observed after acute SR. Another study collected blood samples the morning after normal sleep, after 48 hours of prolonged wakefulness and after 12 hours of recovery sleep to study the changes in blood transcriptome [96]. Differentially expressed genes were identified to represent NK cell signaling, endoplasmic reticulum stress, oxidative phosphorylation, mTOR signaling, representing a shift towards inflammatory stress response.
In conclusion, sleep loss alters the immune homeostasis and tilts the scales towards proinflammation. Even if recovery sleep has been shown to restore the immune balance, chronic sleep deprivation for weeks, months and even years, could potentially activate a state of chronic low-grade inflammation that could cause metabolic distress.
3. Habitual sleep and the development of cardiometabolic disorders: What do observational studies tell us?
Experimental studies conducted in the controlled environment of the laboratory provide detailed information about physiological changes associated with sleep restriction or impairments in sleep quality. These studies, however, are brief, lasting only a few weeks. Chronic conditions like obesity, diabetes and CVD develop over longer periods of time and therefore we need to understand whether habitual sleep patterns are also associated with chronic disease risk. Observational, epidemiologic studies can shed some light in this area. It is important to keep in mind while interpreting the results from these studies that many of these studies had to rely on subjective estimates of sleep duration or sleep quality. Self-reported sleep duration has been only moderately associated with objectively-estimated sleep duration [122, 123]. Nonetheless, numerous studies have observed consistent associations using subjective sleep assessments that support the findings of the laboratory studies; sleep deficiency appears to be associated with cardiometabolic disease.
3.1 Habitual Sleep and Obesity or BMI
A multitude of observational studies have examined the association between sleep and obesity or BMI. Over 60 published articles have reported significant cross-sectional associations between short sleep duration (generally <6 hours per night) and increased prevalence of obesity or higher BMI (see [124–126] for reviews). These associations have been observed in both adults and children and from a variety of countries, including the US and countries in Europe and Asia. Many of these studies also observed higher mean BMI associated with longer sleep durations (generally >8 hours per night), indicating a U-shaped association between self-reported sleep duration and BMI where both short and long sleepers have higher average BMI. In addition to sleep duration, worse subjective sleep quality has also been associated with higher BMI [127, 128]. One meta-analyses of data from many of these cross-sectional studies found that short sleep duration (<5 hours per night) significantly predicted prevalent obesity in adults (pooled odds ratio [OR] was 1.55, 95% confidence interval [CI]: 1.43–1.68) [129]. Thus, most published studies have reported a significant cross-sectional association between inadequate sleep (too short or too long) and higher BMI or the presence of obesity. One important limitation of these studies is that that sleep was self-reported. A few studies have examined objectively-estimated sleep duration. For example, a study in the US that collected at least 3 days of wrist actigraphy in over 600 middle-aged adults found that shorter average sleep duration was associated with higher average BMI in cross-sectional analysis [130]. A study in Brazil also collected actigraphy data for at least 3 days and recorded one night of PSG in 1,074 adults and found that obese and overweight subjects had shorter sleep durations based on actigraphy and PSG and less slow-wave sleep (SWS) and REM sleep, although these comparisons were unadjusted [131]. A study of over 2,700 older men (≥65 years) in the U.S. recorded one night of PSG and found that those with the lowest percentage of SWS had the highest mean BMI and largest mean waist circumference, but they did not find an association between SWS and percent body fat [132]. Thus, there is some evidence for a cross-sectional association between objectively characterized sleep and body size. Of course, another important limitation of these studies is that they are cross-sectional and therefore causal direction cannot be inferred. Indeed, the association between sleep and BMI could be bidirectional. It is well-known that obesity is a major risk factor for obstructive sleep apnea, a sleep disorder that is associated with increased risk of cardiometabolic diseases (see [20] and [133] for reviews on OSA).
Some prospective studies examined sleep and change in body size measures in adults (see Table 1 for details on these studies). Four of these studies did not find a statistically significant association between sleep duration and change in body size [130, 134–136]. Some studies reported a significant association between shorter sleep and weight gain or increased BMI among adult men and/or women [137–145] and sleep disturbances and increased weight gain in women [146]. Two studies observed associations between longer sleep durations and increased weight gain [141, 142] and in a weight loss study that compared an exercise program to a stretching program, women who slept less at follow-up lost more weight [147]. In a US study of African-American and Hispanic adults that measured height and weight as well as subcutaneous and visceral fat, participants aged 18–39 years who reported sleeping ≤5 hours per night experienced a larger increase in BMI, visceral fat and subcutaneous fat over 5 years compared to those aged 18–39 years who reported sleeping 6–7 hours per night [142]. In this same study, sleep duration was not associated with change in BMI or body fat among those aged 40 years or older [142]. A few studies reported sex differences in these associations, but which sex demonstrated a significant association was not consistent. For example, one study found an effect in men but not women [141] while another study saw an effect in women but not men [146]. Some, but not all, prospective studies found longitudinal associations between short sleep duration and increases in body weight and some found long sleep associated with greater weight gain or less weight loss. In addition, some of these studies suggest that there may be important age or gender differences, which require further examination.
Table 1.
Summary of prospective studies that examined the association between habitual sleep and changes in body size
| Authors | Follow up period | Method of Sleep Assessment | Sample | Results | Country |
|---|---|---|---|---|---|
| Adults | |||||
| Hairston et al Sleep 2010 [142] | 5 years | Self-report | Age: 18–81 years N=1,107 (IRAS Family Study); |
Significant associations were observed in subjects aged 18-<40 years but not ≥40 years. These associations found that compared to those reporting sleeping 6–7 hours/night, those sleeping ≤5 hours/night and those reporting sleeping ≥8 hours/nigh had a larger increase in BMI (LS Mean = 2.7 kg/m2 for ≤5h, 0.91 kg/m2 for 6–7h and 1.81 kg/m2 for ≥8h), a larger increase in subcutaneous fat measured by CT (LS Mean = 68.21 cm2 for ≤5h, 26.93 cm2 for 6–7h and 47.54 cm2 for ≥8h) and a larger increase in visceral fat measured by CT (LS Mean = 11.89 cm2 for ≤5h, −1.46 cm2 for 6–7h and 5.64 cm2 for ≥8h) adjusting for age, sex, race, and baseline fat. | US |
| Hasler et al, 2004 [137] | 21 years | Self-report | Age: 19 years at baseline n=496 (Zurich Cohort Study) |
Longitudinal analysis resulted in an odds ratio of 0.50 for sleep duration (in hours) predicting obesity. | Switzerland |
| Chaput et al, 2008 [139] | 6 years | Self-report | Age: 21–64 years n=276 men and women (Quebec Family Study). |
In fully-adjusted model, short sleepers gained more weight: 5–6 hours/night gained 1.84 (95% CI 1.08, 2.61) kg compared to those sleeping 7–8 hours/night. Long sleepers (9–10 hours/night) did not differ from 7–8 hour sleepers. | Canada |
| Gangwisch et al, 2005 [134] | 8–10 years | Self-report | Age: 32–86 years n=9,588 men and women (NHANES) |
No significant prospective association | US |
| Gunderson et al [143] | 6 months | Self-report | Age: mean 33.0 (SD 4.7) n=940 postpartum women |
Women who slept ≤5 hours/night at 6 months postpartum had increased odds for substantial weight retention (≥5 kg above pre-pregnancy weight) at follow up compare to women sleeping 7 hours/night (OR 3.13, 95% CI 1.42, 6.94). | US |
| Lauderdale et al 2009 [130] | 5 years | Actigraphy | Age: 35–50 years at baseline n=612 men and women (CARDIA) |
No significant prospective association | US |
| Watanabe et al, 2010 [141] | 1 year | Self-report | Age: mean 39.8 years N=34,852 men and women | Compared to men sleeping 7-<8 hours/night, men who slept <5 hours/night, 5-<6 hours/night or ≥9 hours/night had a larger increase in BMI (betas: <5: .016, 95% CI .003, .146); 5-<6: .013, 95% CI .001, .061); ≥9: .018, 95% CI .079, .34). No significant associations were observed in women. | Japan |
| Patel et al, 2006 [138] | 16 years | Self-report | Age: 39–65 years n=68,183 women (Nurse’s Health Study) |
Women who slept ≤ 5 hours/night gained 1.14 kg (95% CI: 0.49, 1.79) and those sleeping 6 hours/night gained 0.71 kg (95% CI: 0.41, 1.00) more than those sleeping 7 hours/night adjusting for age and baseline BMI. | US |
| Lyytikainen et al [146] | 5–7-years | Self-report | Age: 40–60 years N= 7,022 male and female municipal employees (Helsinki Health Study) |
After adjusting for age, women with frequent trouble falling asleep (OR 1.65; 95% CI 1.22, 2.22), women frequently waking up several times per night (OR 1.49; 95% CI 1.22, 1.81) or reporting trouble staying asleep (OR 1.41; 95% CI 1.13, 1.75) were more likely to have gained 5 kg or more than women without such sleep problems. No significant associations were observed in men | Finland |
| Stranges, AJE 2008 [135] | 5–6 years | Self-report | Age: 44–65 y n=10,308 men and women (Whitehall Study) |
No significant prospective association | UK |
| Nishiura et al, 2010 [144] | 4 years | Self-report | Age: mean 47.8 years N=3,803 male white-collar workers |
Men sleeping <5 hours/night, as compared with those sleeping 7 hours/night, increased their BMI by 0.15 kg/m2 more over four years (95% CI: 0.03, 0.27), after adjustment for age, baseline BMI, lifestyle behavior, and medication. | Japan |
| Appelhans et al [136] | Average of 4.6 years | Actigraphy | Age: 48–59 years N=310 women (Study of Women’s Health Across the Nation). |
No significant prospective association. | US |
| Xiao [145] | Average 7.5 years | Self-report | Age: 51–72 years n=83,377 men and women (National Institutes of Health-AARP Diet and Health Study) |
Compared with 7–8 hours of sleep, shorter sleep (<5 hours or 5–6 hours) was associated with greater weight gain (in kilograms; men: for <5 hours, β = 0.66, 95% CI: 0.19, 1.13, and for 5–6 hours, β = 0.12, 95% CI: −0.02, 0.26; women: for <5 hours, β = 0.43, 95% CI: 0.00, 0.86, and for 5–6 hours, β = 0.23, 95% CI: 0.08, 0.37). Adjusting for mediators (physical activity level, overall time spent sitting, total caloric intake, and the intake of fruits and vegetables, whole grains, and total fat.) diminished associations. |
US |
| Littman et al2006 [147] | 1 year | Self-report | Age: 50–75 years n= 173 overweight, sedentary postmenopausal women randomized to an exercise or stretching weight loss program |
Exercisers who slept less at follow-up than at baseline lost more weight than those whose sleep stayed the same or those who slept more. Stretchers who napped less at follow up gained more weight and both exercisers and stretchers whose trouble falling asleep increased gained more weight. | US |
| Lopez-Garcia AJCN 2008 [140] | 2 years | Self-report | Age: ≥60 years n=3,235 men and women |
In fully adjusted model, the association between sleep duration and odds of gaining ≥5 kg over the follow-up period was significant in women only: ≤5 hours/night: 3.41 (1.34–8.69); 6 hours/night: not significant; 7 hours/night referent; 8 hours/night: 3.03 (1.29–7.12); 9 hours/night 3.77 (1.55–9.17); ≥10 hours/night not significant. | Spain |
3.2 Habitual sleep, appetite regulation and dietary behavior
Quite a few observational studies have examined the relationship between habitual sleep characteristics and levels of hormones involved in appetite regulation and/or dietary behavior. For example, in cross-sectional analysis of data from 740 men and women in the Quebec Family Study, leptin levels in those sleeping 5–6 hours per night were approximately 15–17% lower than predicted based on body fat alone [148]. The Wisconsin Sleep Cohort Study, which enrolled state employees aged 30–60 years, found that average habitual sleep duration was positively associated with leptin levels independently of BMI (beta = 0.11, p=0.01) [149]. The Women’s Health Initiative prospective Observational Study (WHI-OS), which enrolled postmenopausal women, found that women reporting ≤6 hours of sleep per night had lower fasting leptin concentrations than those reporting ≥8 hours of sleep per night adjusting for age, race, total body fat mass, and smoking status [150]. A fourth study found the opposite association; shorter sleep was associated with higher leptin levels. Specifically, this study examined fasting leptin levels and self-reported sleep duration in a sample of 254 Taiwanese adults and found that women sleeping <6.5 hours per night had an increased odds of having hyperlipidemia, defined as leptin levels in the upper quartile (OR = 2.15, 95% CI 0.99, 4.78, p=.055) compared to women who slept at least 6.5 hours per night [151], although statistical significance was not quite reached. In men there was a similar but not statistically significant association (OR = 4.98, 95% CI = 0.80–42.4, p=.096). Although most of these epidemiologic studies are consistent with the laboratory studies, other studies did not observe a significant association between self-reported sleep duration and leptin levels. Women in the Nurse’s Health Study returned a blood sample through the mail for assessment of leptin, and no association was found between self-reported sleep duration and leptin levels [152]. A study among 173 overweight, sedentary postmenopausal women aged 50–74 year also did not observe a significant cross-sectional association between self-reported sleep duration and fasting leptin or total ghrelin levels [147]. Finally, in a sleep extension study, 80 obese men and premenopausal women aged 18–50 years who were habitual short sleepers, no association was observed between average sleep duration or sleep efficiency from two weeks of wrist actigraphy recordings and fasting levels of leptin [153]. Two studies looked at appetite hormones in relation to sleep measured via PSG. The Wisconsin Sleep Cohort Study found that total sleep time from one night of polysomnography was inversely associated with ghrelin levels (beta = −0.69, p=0.008) but was not associated with leptin levels [149]. The Cleveland Family Study in adults (average age was 45 years) recorded one night of PSG with bedtimes were at 23:00 and wake times at 6:45 for everyone and collected a fasting blood sample the following morning. They found that shorter TST and less REM sleep was associated with higher leptin levels, while SWS was not associated with leptin [154]. Because time in bed was the same for all individuals, it raises the question of how the measurements from one night of PSG are impacted by varying degrees of sleep debt within individuals. In other words, more REM sleep on the PSG could represent a REM rebound due to habitual sleep restriction, but habitual sleep duration was not reported or included in the analyses. The discrepant results concerning the association between sleep and leptin among these studies may be due to unmeasured confounding, differences in the association between sleep and appetite regulation in obese versus lean adults, or methodological issues such as self-reported sleep duration or sample collection.
Recently, many observational studies that have examined cross-sectional associations between habitual sleep duration, sleep quality or sleep problems and dietary intake or dietary patterns have been published (see Table 2 for details about these studies).
Table 2.
Summary of studies that examined the association between habitual sleep and dietary patterns or behavior.
| Study | n | Age range or mean (SD) and sex | Sleep Measure | Dietary Measure | Result summary |
|---|---|---|---|---|---|
| Soares et al, 2011 [155] | 870 | Undergraduate students aged 17–25 years in Portugal | 2 questions addressing difficulties initiating sleep (DIS) and difficulties maintaining sleep (DMS). A sleep disturbance index (SDI) was the sum of DIS and DMS scores | Eating Attitudes Test-40 used to measure: (1) diet concerns (DC), (2) bulimic behavior (BB) and (3) social pressure to eat (SPE) | In the total sample and in males, BB and SPE dimensions were significantly associated with sleep difficulties (DIS, DMS, SDI). In females, BB was significantly associated with sleep difficulties (SDI, DIS; P < 0.01). |
| Nakade et al, 2009 [156] | 800 | Female students aged 18–29 years in Japan | healthy sleep habits based on self-reported sleep quality, sleep hours, regularity of sleep-wake cycles, and sleep satisfaction | excerpt from an Examination of Eating Habits to assess drinking, smoking, and regularity of meals | Participants with healthier sleep habits were significantly more likely to eat breakfast regularly and less likely to eat “midnight snacks” |
| Hicks et al, 1986 [157] | 31 | College students in the US (age not specified) | Self-report | Meal record for one day | Short-sleepers (average 6h/night) were more likely to deviate from the normal three meals/day pattern, and they showed a tendency to eat more small meals or snacks than longer sleepers (≥8h/night). |
| Haghighatdoost et al, 2012 [158] | 410 | Female students aged 18–28 y in Iran | Sleep duration based on physical activity record for 24 h during a weekday and weekend day | Food frequency questionnaire | Compared to subjects in the highest tertile of sleep duration (>8 h/d), subjects in the lowest tertile of sleep duration (<6 h/d) had a higher intake of energy (2406 ± 825 versus 2092 ± 700 kcal/d; P < 0.01). Mean percentages of protein and carbohydrate intake were 14% and 58% in the lowest tertile and 19% and 51.6% in the highest, respectively (P < 0.05). Intake of fruits, whole grains, beans, niacin, vitamin C, and vitamin B12 was lower among those in the lowest tertile compared to the highest tertile. Note that these differences are adjusted only for total energy intake. |
| Galli et al, 2013 [159] | 118 | Obese men and premenopausal women aged 18–50 years who are short sleepers and enrolled in a sleep extension study in the US | 14-days of wrist actigraphy and assessment of sleep apnea was assessed using a portable screening device | 3-day food diary | After adjustment for apnea severity, age, gender, BMI, and race/ethnicity a 30-min shorter sleep duration was associated with 83-kcal greater energy intake on average. RDI was inversely related to carbohydrate intake and positively associated with fat intake. Sleep duration was also inversely associated with alcohol intake after adjusting for age, gender and BMI or ethnicity/race. |
| Chaput et al, 2012 [160] | 703 | Quebec Family Study: men and women aged 18–64 years in Canada | Self-report | 3-day food record | After adjusting for covariates, short sleep duration (≤77h/night) was associated with an increase in the odds of exceeding the recommendations weekly alcohol intake of 14 drinks for men and 7 drinks for women compared to those sleeping between 7 and 8 h (OR 1.87, 95% CI 1.03–3.54, both sexes combined). Long sleepers (≥9h/night) did not differ from 7–8 h sleepers. |
| Crispim et al, 2011 [161] | 52 | Women and men aged 19–45 years in Brazil | Nocturnal polysomnography (PSG) | 3-day food diary immediately prior to the PSG | Greater nocturnal intake (dinner plus any late night snacks) of fat was associated with lower sleep efficiency, greater REM latency, higher percentage of N2, lower percentage of REM and greater WASO after adjustment for age, gender, and socioeconomic status |
| Imaki et al 2002 [162] | 12,333 | Male employees aged 20–59 years at a chemical plant in Japan | Self-report with 3 response options: <6; 6.1 to 8.9; ≥9 hours | dietary habits assessed with 7 items: (1) number of meals per day (1, 3 times; 2, twice; 3, irregular); (2) snack between meals (yes or no); (3) eating out (frequently or rarely); (4) daily diet pattern (regular or irregular); (5) intake of vegetables in the diet (ample or none); (6) seasoning of food (strongly salty, conventional or lightly seasoned); (7) oily food items (preferred or not desired much). | Short sleepers (≤6 hours per night) were more likely to eat meals irregularly, snack between meals and eat outside the home than those reporting sleeping between 6.1 and 8.9 hours. (Long sleepers were not analyzed because the prevalence was only 1%) |
| Shi et al, 2008 [163] | 2828 | Adults ≥20 years old in China | Self-report | 3-day food records. | In adjusted models, subjects who slept for < 7 h/night had significantly higher percentage of energy intake from fat (1.5% more per day) and significantly lower percentage from carbohydrates (1.8% per day) than those who slept for 7–9 h per day. |
| Parvaneh et al, 2014 [164] | 226 | Men and women aged 20–55 years old in Iran | Sleep Habit Heart Questionnaire | Three-days of 24-hour dietary recall | Sleep duration showed significant but weak positive correlations with energy intake (r = 0.174), carbohydrate intake (r = 0.154) and fat intake (r = 0.141). |
| Zadeh et al, 2011 [165] | 87 | Men & women aged 21–45 years in India | Insomnia Screening Index. | 3-day food diary | Presence of insomnia was associated with lower caloric intake, lower protein intake and lower carbohydrate intake compared to normal sleepers. |
| Patterson et al, 2014 [166] | 223 | UCLA Energetics Study: African-American and non-Hispanic whites aged 21–69 y in the US | Self-report | Energy intake = total energy expenditure from doubly labeled water technique | Compared with ≤6 h/d, participants who reported longer sleep consumed fewer total kilocalories per day (−50 kcal/day for 7 h, −41 kcal/day for 8 h, and 187 kcal/day for ≥9 h. |
| Rontoyanni et al, 2007 [167] | 30 | Women 30–60 years of age in Greece | Sleep Habits Questionnaire and a 7-d sleep diary | Two 24-h dietary recall | Weak positive association between sleep duration and saturated fat (r = 0.392, P < 0.05). No significant association between sleep duration and energy intake or a preference for fat or carbohydrate consumption. |
| Hart et al, 2012 [168] | 318 | Women in the US in clinical weight loss trial who were ≥ 30 years old, overweight or obese and reported ≥ 10 urinary incontinent episodes per week | PSQI for time in bed (TIB) | Block Food Frequency Questionnaire | TIB was not associated with any of the dietary composition variables measured on the Block FFQ. |
| Kim et al, 2011 [169] | 27,983 | Sisters of women who had breast cancer. Ages 35–74 years. | Self-report | Assessed meal and snack frequencies (days/week) via self-report modified Block FFQ | Tendency for eating during conventional hours (from breakfast to dinner) increased with increasing sleep duration up to 7–7.9 h. Dominance of snacking over meals decreased with increasing sleep duration. Women with long daily sleep duration (≥10 h) were similar to those with less than normal sleep duration (<6 h) |
| Nishiura et al 2010 [170] | 2632 | Male employees aged 40–59 years with a BMI < 25 kg/m2 at a gas company in Japan | Self-report | Dietary patterns: preference for fatty food, skipping breakfast, snacking, and eating out (all yes or no responses) | In unadjusted analysis, the prevalence of a preference for fatty foods, skipping breakfast and eating out was highest among the shortest sleepers (<6 hours). |
| Grandner et al [171] [172] | 5587 – sleep duration analysis 4552 – sleep problems analysis | NHANES 2007–2008: men and women with a mean age of 46 years in the US | Self-reported sleep duration and sleep problems. | 24-h recall, guided by a structured interview | In adjusted models, total energy intake did not differ by sleep duration but number of foods in the diet was significantly lower in the very short (<5h), short (5–6h) and long sleep (≥9h) duration groups compared to those reporting sleeping 7–8 hours per night. Very short sleepers also reported eating fewer carbohydrates and less protein than those reporting sleeping 7–8 hours per night. Daytime sleepiness was associated with increased total calorie intake. Difficulty maintaining sleep was associated with less variety in diet. Sleep duration groups and the four sleep difficulty groups were associated with differences in micronutrient intake. |
| Grandner et al 2010 [173] | 459 | Women’s Health Initiative: postmenopausal women aged 50–81 years in the US | 7 days wrist actigraphy | Food Frequency Questionnaire (FFQ) | Nocturnal sleep duration was significantly negatively correlated with amount of fat (r= −0.185 p=0.0004) and calories (r= −0.162 p=0.002) consumed after adjusting for age, education, income, exercise, BMI and total dietary grams |
| Stern et al, 2014 [150] | 769 | Women’s Health Initiative: postmenopausal women with a median age of 63 years in the US | Self-report Insomnia Scale | Food Frequency Questionnaire | Women who reported sleeping ≤6 h/night demonstrated a 1% increase in mean dietary energy intake relative to women who reported sleeping 7 hr/night. WHI insomnia scale was not significantly associated with diet quality, dietary energy intake, or the macronutrient composition of dietary energy intake. |
Among studies that relied on self-reported measures of sleep duration, shorter sleep durations were associated with greater total energy intake [150, 158, 159, 164, 166, 174], greater fat intake [163, 164], greater carbohydrate intake [158, 164] and greater alcohol intake [159, 160]. However, one study found lower caloric intake among people with insomnia [165] and two studies did not observe significant associations between habitual sleep and dietary intake [167, 168]. Several studies also found an association with optimal sleep (not short sleepers and/or better sleep quality) and healthier eating habits, such as eating breakfast, eating more regular meals, snacking less, eating at home more often and a greater variety of foods in the diet [156, 157, 162, 169–172]. Sleep disturbances among undergraduate students in Portugal were associated with more bulimic behavior and more social pressure to eat [155]. A study of 459 postmenopausal women used one week of wrist actigraphy to estimate sleep duration and found that sleep duration was negatively associated with amount of fat and calories consumed even after adjusting for BMI and physical activity [173]. These data indicate that at a specific level of BMI and physical activity, a woman who sleeps less consumes more calories, particularly from fat, on average than a woman sleeping more. One study of adult women and men aged 19–45 years examined diet and sleep architecture using PSG and found that greater nocturnal (dinner plus any late night snacks) intake of fat was associated with lower sleep efficiency, greater REM latency, higher percentage of N2, lower percentage of REM and greater WASO after adjustment for age, gender, and socioeconomic status [161]. These results indicate that dietary intake can impact sleep and therefore the sleep-diet relationship may be bidirectional.
Differences in dietary intake could partly explain the association between short sleep duration and weight gain since dietary intake is associated with weight gain [175]. Recently, a meta-analysis compared the effect of alcohol, sleep deprivation and TV watching on food intake using 23 controlled, laboratory studies in healthy participants and found that the effect was greatest for alcohol (Cohen’s d = 1.03; 95% CI: 0.66, 1.4; P , 0.001) followed by sleep deprivation (Cohen’s d = 0.49; 95% CI: 0.11, 0.88; P , 0.05) [176]. Thus, habitual sleep loss could result in significant weight gain and one pathway though which this could happen is increasing energy intake.
3.3 Habitual sleep and diabetes risk
Several observational studies have reported cross-sectional associations between inadequate sleep and greater prevalence of diabetes or impaired glucose tolerance (see [177] for another recent review). Sleep quality is generally worse based on both subjective and objective estimates in people with type 2 diabetes (T2DM) [178]. Furthermore, among persons with T2DM, poorer glycemic control is associated with worse sleep quality and increased sleep disordered breathing [179–181]. Indeed, obstructive sleep apnea seems to be highly prevalent among patients with type 2 diabetes, with estimates ranging from 58 to 86% [20]. Although the results of these studies indicate that inadequate sleep is associated with diabetes, the direction of causality cannot be determined. Insufficient sleep may increase the risk of developing diabetes, as the laboratory studies suggest, or, conversely, having diabetes could impair sleep quality. Still if inadequate sleep can impair glucose control among patients with T2DM, then we need to understand better how to improve sleep in this population in order to identify whether such interventions can help to improve disease management and reduce the risk of complications.
A recent cross-sectional study in China simultaneously examined self-reported sleep duration (<6, 6–8 and >8 h) and subjective sleep quality (poor vs good) in relation to the presence of impaired fasting glucose (IFG) [182]. People with IFG are considered to have “pre-diabetes” and therefore at risk of developing T2DM. Compared to good sleepers who sleep 6–8 hours all other groups had increased odds of having IFG. Poor sleepers who sleep <6 hours had the highest odds (OR 6.37, 95%CI 4.66 to 8.67) and good sleepers who sleep >8 hours had the second highest odds of IFG (OR 3.17, 95%CI 2.29 to 4.41) [182]. These results highlight the importance of both sleep duration and sleep quality and underscore the need to understand better the health risk associated with long sleep.
Prospective studies provide stronger evidence for a causal link between inadequate sleep and the development of cardiometabolic diseases. Many studies reported increased odds of diabetes associated with short sleep duration (≤5h and/or ≤6h) and some observed a U-shaped association [183–187]. Furthermore, impaired sleep quality, such as difficulty initiating or maintaining sleep, has been associated with increased odds of developing diabetes in multiple studies [188–193]. Note that one study did not find a significant association between sleep and incident diabetes [194], while one study observed a significant association in men only [191]. A meta-analysis of 10 prospective studies examined the association between the incidence of diabetes and either short self-reported sleep duration, long self-reported sleep duration or subjective sleep disturbances [195]. The estimated pooled OR for short sleep was 1.28 (95% CI 1.03–1.6), however, there was a significant gender difference. The OR was 2.07 (95% CI 1.16–3.72) for men and 1.07 (95% CI 0.90–1.28) for women, indicating a stronger association among men. The pooled OR for long sleep was 1.48 (95% CI 1.13–1.96), indicating that overall there is a significant U-shaped association between self-reported sleep duration and incident diabetes. Finally, both difficulty initiating sleep (pooled OR 1.57, 95% CI 1.25–1.97) and difficulty maintaining sleep (pooled OR 1.84, 95% CI 1.39–2.43) significantly predicted incident diabetes. Another meta-analysis examined the association between sleep duration and the metabolic syndrome, which is characterized by a combination of abdominal adiposity, dyslipidemia, elevated glucose and elevated blood pressure [196]. The authors identified 12 cross-sectional studies and 3 cohort studies, and estimated for short sleep durations (<5 to 6 hours/night) an OR of 1.27 (95% CI 1.10, 1.48) in the cross-sectional studies and 1.62 (95% CI 0.74, 3.55) in the 3 cohort studies. For long sleep durations (>8 to 10 hours/night), the OR was 1.23 (95% CI 1.02, 1.49) in the cross-sectional studies and 1.62 (95% CI 0.86, 3.04) in the cohort studies. Thus the prospective associations were not significant in this meta-analysis of 3 studies. Overall, prospective epidemiologic studies suggest that subjective short or long sleep duration and poor sleep quality may predict the development of diabetes. Whether or not strategies to improve sleep can help reduce the risk of diabetes or improve glucose control in diabetes patients has yet to be determined.
3.4 Habitual sleep and cardiovascular disease risk
Self-reported short sleep and subjectively poor sleep quality have been associated with higher average blood pressure or higher prevalence of hypertension in cross-sectional studies [197–203]. Some of these studies also observed higher blood pressure among long sleepers [198, 203] and two of these studies observed a significant association only in women [200, 202]. Analysis of NHANES did find that self-reported short sleep duration was associated with both self-reported chronic disease (e.g. obesity and hypertension) as well as objectively-measured markers of chronic disease (e.g. obesity and hyperlipidemia) [204]. Two studies among elderly adults found no association between habitual sleep duration and blood pressure [205, 206], which suggests that associations between sleep and blood pressure may be modified by age. In one study that recorded wrist actigraphy among adults aged 35–50 years, shorter sleep duration and lower sleep quality were both associated with higher blood pressure [207]. A small study among 23 healthy, young adults aged 18–28 years examined sleep efficiency based on wrist actigraphy in relation to nocturnal blood pressure dipping, which is a phenomenon associated with lower cardiovascular disease risk. They found that those with an average sleep efficiency <85% had a blunted nocturnal dip in systolic blood pressure compared to those with a sleep efficiency ≥85% [208]. Thus, cross-sectional studies generally support a relationship between inadequate sleep and higher mean blood pressure, but the causal direction cannot be determined and the strength of these associations may be modified by gender or age.
Several prospective epidemiologic studies have examined various cardiovascular outcomes in relation to habitual sleep. Analysis of the National Health and Nutrition Examination Survey (NHANES) data in the US found that short sleepers (≤5 hours per night) had increased odds of incident hypertension over 8–10 years (OR 1.32, 95% CI 1.02–1.71) compared to those reporting sleeping 7–8 hours per night after adjusting for numerous potential confounders [209]. In the study of adults aged 35–50 years that estimated sleep duration using actigraphy, shorter sleep was significantly associated with incident hypertension over 5 years (OR 0.72; 95% CI: 0.55, 0.95) [207] and incident coronary artery calcification (OR = 0.67 per hour, p=.011; 95% CI 0.49–0.91), which is a predictor of the development of coronary heart disease [210]. The Nurses’ Health Study found a significantly increased risk of incident coronary heart disease over 10 years associated with short (≤5 hours per night) self-reported sleep duration (risk ratio 1.45; 95% CI: 1.1–1.92) and long (≥9 hours per night) self-reported sleep duration (risk ratio 1.38; 95% CI: 1.03–1.86) compared to those sleeping 8 hours per night [211]. A few studies have examined cardiovascular disease mortality in relation to self-reported sleep duration, but none found a significant association in fully adjusted models [212–214]. Poor subjective sleep quality was also associated with increased coronary artery disease mortality in men (RR: 3.1, 95%CI 1.5–6.3) [212]. A study in the US of over 3,400 men and women 35 years of age or older reported a significant increase of cardiovascular disease events in those who complained of insomnia every day compared to those without any insomnia complaint (RR: 1.78, 95%CI 1.03–3.08) [214]. Overall, there is some evidence that inadequate sleep is associated with cardiovascular disease and hypertension, but more rigorous studies are required to fully understand whether inadequate sleep actually leads to cardiovascular disease.
4. Summary
Experimental studies have found associations between sleep restriction or sleep fragmentation and impairments in glucose metabolism, alterations in appetite regulation, and increased inflammation, all of which would increase the risk of developing cardiometabolic disease. Observational studies have reported many cross-sectional associations between self-reported short and/or long sleep duration and prevalent obesity, diabetes and hypertension. Fewer prospective studies exist and results are often mixed. Some demonstrated an association between sleep duration and weight gain or incident cardiometabolic disease.
Future Directions
Future research should focus on novel interventions to modify sleep. These studies could explicitly test whether extending sleep duration in short sleepers, restricting time in bed in long sleepers, improving sleep quality or treating sleep disorders such as OSA can lead to improvements in cardiometabolic health. Thus these interventions could be behavioral (changing bed times) or therapeutic (CPAP for OSA; medications for insomnia), depending on the specific sleep problem. Finally, studies should investigate whether sleep deficiency partially mediates cardiometabolic disease disparities between racial/ethnic or socioeconomic groups. If so, these novel interventions and the therapeutics to improve sleep could help to reduce the associated health disparities, but they must be explicitly tested in the disadvantaged groups.
Acknowledgments
Dr. Knutson is partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK095207) and by the National Institute for Occupational Safety and Health (R01OH009482). Ms. Rangaraj is supported by the University of Chicago Diabetes Research and Training Center, which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK020595).
References Cited
- 1.Vasudevan AR, Ballantyne CM. Cardiometabolic risk assessment: an approach to the prevention of cardiovascular disease and diabetes mellitus. Clin Cornerstone. 2005;7:7–16. doi: 10.1016/s1098-3597(05)80063-8. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, Treatment, and Control of Diagnosed Diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol. 2008;18:222–9. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–5. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 6.Elovainio M, Ferrie JE, Singh-Manoux A, Shipley M, Batty GD, Head J, Hamer M, Jokela M, Virtanen M, Brunner E, Marmot MG, Kivimaki M. Socioeconomic differences in cardiometabolic factors: social causation or health-related selection? Evidence from the Whitehall II Cohort Study, 1991–2004. Am J Epidemiol. 2011;174:779–89. doi: 10.1093/aje/kwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66:1044S–50S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 8.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 9.Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22:149–64. doi: 10.2165/00019053-200422030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–51. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 11.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 12.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–7. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 13.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Amer J Hum Biol. 2002;14:321–6. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 15.Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr, Buysse DJ, Kamarck TW, Owens JF, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–6. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Owens JF, Sowers M. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12:209–14. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 19.Jean-Louis G, von Gizycki H, Zizi F, Dharawat A, Lazar JM, Brown CD. Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med. 2008;4:421–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24:703–15. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 22.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 23.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatakis KA. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. CHEST Journal. 2010;137:95. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog N, Jauch-Chara K, Hyzy F, Richter A, Friedrich A, Benedict C, Oltmanns KM. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. 2013;38:2075–82. doi: 10.1016/j.psyneuen.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Schmid SM, Jauch-Chara K, Hallschmid M, Schultes B. Mild Sleep Restriction Acutely Reduces Plasma Glucagon Levels in Healthy Men. The Journal of Clinical Endocrinology & Metabolism. 2009;94:5169–73. doi: 10.1210/jc.2009-0969. [DOI] [PubMed] [Google Scholar]
- 31.Irwin M, Thompson J, Miller C, Gillin J, Ziegler M. Effects of Sleep and Sleep Deprivation on Catecholamine and Interleukin-2 Levels in Humans: Clinical Implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 32.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep Loss Alters Basal Metabolic Hormone Secretion and Modulates the Dynamic Counterregulatory Response to Hypoglycemia. The Journal of Clinical Endocrinology & Metabolism. 2007;92:3044–51. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- 33.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, Born J, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. The American Journal of Clinical Nutrition. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 34.Klingenberg L, Chaput J-P, Holmback U, Visby T, Jennum P, Nikolic M, Astrup A, Sjodin A. Acute Sleep Restriction Reduces Insulin Sensitivity in Adolescent Boys. Sleep. 2013;36:1085–90. doi: 10.5665/sleep.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. Journal of Clinical Endocrinology & Metabolism. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 38.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 41.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds AC, Dorrian J, Liu PY, Van Dongen HPA, Wittert GA, Harmer LJ, Banks S. Impact of Five Nights of Sleep Restriction on Glucose Metabolism, Leptin and Testosterone in Young Adult Men. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson NS, Banks S, Dinges DF. Sleep Restriction Is Associated With Increased Morning Plasma Leptin Concentrations, Especially in Women. Biological research for nursing. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pejovic S, Vgontzas AN, Basta M, Tsaoussoglou M, Zoumakis E, Vgontzas A, Bixler EO, Chrousos GP. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Gonnissen HKJ, Hursel R, Rutters F, Martens EAP, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. The British Journal of Nutrition. 2013;109:748–56. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 47.Calvin AD, Carter RE, Adachi T, Macedo P, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of Experimental Sleep Restriction on Caloric Intake and Activity Energy Expenditure. Chest. 2013 doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St-Onge M-P, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrère B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 50.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 52.Simpson NS, Banks S, Arroyo S, Dinges DF. Effects of sleep restriction on adiponectin levels in healthy men and women. Physiology & behavior. 2010;101:693–8. doi: 10.1016/j.physbeh.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson MD, Russell-Jones D, Umpleby AM, Dijk D-J. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism. 2013;62:204–11. doi: 10.1016/j.metabol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 55.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. SLEEP. 2013 doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, Roychoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge M-P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;303:R883–R9. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. The Journal of Physiology. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hogenkamp PS, Nilsson E, Nilsson VC, Chapman CD, Vogel H, Lundberg LS, Zarei S, Cedernaes J, Rångtell FH, Broman J-E, Dickson SL, Brunstrom JM, Benedict C, Schiöth HB. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology. 2013;38:1668–74. doi: 10.1016/j.psyneuen.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Benedict C, Brooks SJ, O’Daly OG, Almen MS, Morell A, Aberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, Larsson EM, Schioth HB. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 61.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nature communications. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level--the “normotensive non-dipper” paradox. Chronobiol Int. 2013;30:87–98. doi: 10.3109/07420528.2012.701127. [DOI] [PubMed] [Google Scholar]
- 64.Ulu SM, Ulu S, Ulasli SS, Yaman G, Ahsen A, Ozkececi G, Yuksel S. Is impaired sleep quality responsible for a nondipping pattern even in normotensive individuals? Blood Press. Monit. 2013;18:183–7. doi: 10.1097/MBP.0b013e3283624b03. [DOI] [PubMed] [Google Scholar]
- 65.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique [see comment] Sleep. 2003;26:986–9. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 67.Holmes AL, Burgess HJ, Dawson D. Effects of sleep pressure on endogenous cardiac autonomic activity and body temperature. J Appl Physiol. 2002;92:2578–84. doi: 10.1152/japplphysiol.01106.2001. [DOI] [PubMed] [Google Scholar]
- 68.Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–5. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 69.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, DeMeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 70.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40L–8L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 71.Vogel F. Measurement of endothelial function by brachial artery flow-mediated vasodilation. Am J Cardiol. 2001;88:31E–4E. doi: 10.1016/s0002-9149(01)01764-7. [DOI] [PubMed] [Google Scholar]
- 72.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–43. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landmesser U, Drexler H. The clinical significance of endothelial dysfunction. Curr Opin Cardiol. 2005;20:547–51. doi: 10.1097/01.hco.0000179821.11071.79. [DOI] [PubMed] [Google Scholar]
- 74.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 75.Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK. Experimental sleep restriction causes endothelial dysfunction in healthy humans. Journal of the American Heart Association. 2014;3:e001143. doi: 10.1161/JAHA.114.001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H, Jr, Lorenzi-Filho G. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol (1985) 2012;113:232–6. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 77.Amir O, Alroy S, Schliamser JE, Asmir I, Shiran A, Flugelman MY, Halon DA, Lewis BS. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93:947–9. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 78.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Beers PV, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 79.González-Ortiz M, Martínez-Abundis E, Balcázar-Muñoz BR, Pascoe-González S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes, Nutrition & Metabolism. 2000;13:80–3. [PubMed] [Google Scholar]
- 80.Tobaldini E, Cogliati C, Fiorelli EM, Nunziata V, Wu MA, Prado M, Bevilacqua M, Trabattoni D, Porta A, Montano N. One night on-call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. European Journal of Internal Medicine. 2013;24:664–70. doi: 10.1016/j.ejim.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 81.Heiser P, Dickhaus B, Schreiber W, Clement HW, Hasse C, Hennig J, Remschmidt H, Krieg JC, Wesemann W, Opper C. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur Arch Psychiatry Clin Neurosci. 2000;250:16–23. doi: 10.1007/pl00007534. [DOI] [PubMed] [Google Scholar]
- 82.Kerkhofs M, Boudjeltia KZ, Stenuit P, Brohée D, Cauchie P, Vanhaeverbeek M. Sleep restriction increases blood neutrophils, total cholesterol and low density lipoprotein cholesterol in postmenopausal women: A preliminary study. Maturitas. 2007;56:212–5. doi: 10.1016/j.maturitas.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Wu H, Zhao Z, Stone WS, Huang L, Zhuang J, He B, Zhang P, Li Y. Effects of sleep restriction periods on serum cortisol levels in healthy men. Brain Res Bull. 2008;77:241–5. doi: 10.1016/j.brainresbull.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 85.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 86.Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond) 2012;36:1346–52. doi: 10.1038/ijo.2011.250. [DOI] [PubMed] [Google Scholar]
- 87.Gonnissen HKJ, Mazuy C, Rutters F, Martens EAP, Adam TC, Westerterp-Plantenga MS. Sleep Architecture When Sleeping at an Unusual Circadian Time and Associations with Insulin Sensitivity. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 89.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 90.Pejovic S, Basta M, Vgontzas AN, Kritikou I, Shaffer ML, Tsaoussoglou M, Stiffler D, Stefanakis Z, Bixler EO, Chrousos GP. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. American journal of physiology Endocrinology and metabolism. 2013;305:E890–6. doi: 10.1152/ajpendo.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plat L, Byrne MM, Sturis J, Polonsky KS, Mockel J, Féry F, Van Cauter E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270:E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- 92.Plat L, Féry F, L’Hermite-Balériaux M, Mockel J, Van Cauter E. Metabolic effects of short-term physiological elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–92. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 93.Trifonova ST, Zimmer J, Turner JD, Muller CP. Diurnal redistribution of human lymphocytes and their temporal associations with salivary cortisol. Chronobiology International: The Journal of Biological & Medical Rhythm Research. 2013;30:669–81. doi: 10.3109/07420528.2013.775654. [DOI] [PubMed] [Google Scholar]
- 94.Aho V, Ollila HM, Rantanen V, Kronholm E, Surakka I, van Leeuwen WMA, Lehto M, Matikainen S, Ripatti S, Harma M, Sallinen M, Salomaa V, Jauhiainen M, Alenius H, Paunio T, Porkka-Heiskanen T. Partial Sleep Restriction Activates Immune Response-Related Gene Expression Pathways: Experimental and Epidemiological Studies in Humans. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0077184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JCY, Santhi N, von Schantz M, Smith CP, Dijk D-J. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–E41. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellegrino R, Sunaga DY, Guindalini C, Martins RCS, Mazzotti DR, Wei Z, Daye ZJ, Andersen ML, Tufik S. Whole blood genome-wide gene expression profile in males after prolonged wakefulness and sleep recovery. Physiological Genomics. 2012;44:1003–12. doi: 10.1152/physiolgenomics.00058.2012. [DOI] [PubMed] [Google Scholar]
- 97.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 98.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruiz FS, Andersen ML, Martins RC, Zager A, Lopes JD, Tufik S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immunity. 2012;18:44–54. doi: 10.1177/1753425910385962. [DOI] [PubMed] [Google Scholar]
- 100.Boudjeltia KZ, Faraut B, Stenuit P, Esposito MJ, Dyzma M, Brohee D, Ducobu J, Vanhaeverbeek M, Kerkhofs M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: A pilot study. Vascular Health and Risk Management. 2008;4:1467–70. doi: 10.2147/vhrm.s3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, Stenuit P, Franck T, Antwerpen PV, Vanhaeverbeek M, Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–8. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 103.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 104.Christoffersson G, Vågesjö E, Pettersson US, Massena S, Nilsson EK, Broman J-E, Schiöth HB, Benedict C, Phillipson M. Acute sleep deprivation in healthy young men: Impact on population diversity and function of circulating neutrophils. Brain Behav Immun. doi: 10.1016/j.bbi.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 105.Sothern RB, Roitman-Johnson B, Kanabrocki EL, Yager JG, Roodell MM, Weatherbee JA, Young MR, Nenchausky BM, Scheving LE. Circadian characteristics of circulating interleukin-6 in men. The Journal of Allergy and Clinical Immunology. 1995;95:1029–35. doi: 10.1016/s0091-6749(95)70104-4. [DOI] [PubMed] [Google Scholar]
- 106.Vgontzas AN. Circadian Interleukin-6 Secretion and Quantity and Depth of Sleep. The Journal of Clinical Endocrinology & Metabolism. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 107.Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J. Sleep enhances IL-6 trans-signaling in humans. The FASEB Journal. 2006;20:2174–6. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- 108.Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- 109.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 110.Vgontzas AN. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. The Journal of Clinical Endocrinology & Metabolism. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 111.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 112.Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318–24. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Redwine L, Hauger R, Gillin J, Irwin M. Effects of Sleep and Sleep Deprivation on Interleukin-6, Growth Hormone, Cortisol, and Melatonin Levels in Humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 114.Vgontzas AN. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. The Journal of Clinical Endocrinology & Metabolism. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 115.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haack M, Reichenberg A, Kraus T, Schuld A, Yirmiya R, Pollmächer T. Effects of an intravenous catheter on the local production of cytokines and soluble cytokine receptors in healthy men. Cytokine. 2000;12:694–8. doi: 10.1006/cyto.1999.0665. [DOI] [PubMed] [Google Scholar]
- 117.Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. SLEEP-NEW YORK THEN WESTCHESTER. 2007;30:401. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- 118.Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–8. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 119.Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155:231–8. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 121.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep Loss Activates Cellular Inflammatory Signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kline CE, Zielinski MR, Devlin TM, Kripke DF, Bogan RK, Youngstedt SD. Self-reported long sleep in older adults is closely related to objective time in bed. Sleep Biol Rhythms. 2010;8:42–51. doi: 10.1111/j.1479-8425.2009.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 125.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gonnissen HK, Adam TC, Hursel R, Rutters F, Verhoef SP, Westerterp-Plantenga MS. Sleep duration, sleep quality and body weight: parallel developments. Physiol Behav. 2013;121:112–6. doi: 10.1016/j.physbeh.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 127.Asplund R, Aberg H. Sleep complaints in women of ages 40–64 years in relation to sleep in their parents. Sleep Med. 2001;2:233–7. doi: 10.1016/s1389-9457(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 128.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 129.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–13. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moraes W, Poyares D, Zalcman I, de Mello MT, Bittencourt LR, Santos-Silva R, Tufik S. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 2013;14:312–8. doi: 10.1016/j.sleep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 132.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–51. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 134.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate Sleep as a Risk Factor for Obesity: Analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 135.Stranges S, Cappuccio FP, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167:321–9. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Appelhans BM, Janssen I, Cursio JF, Matthews KA, Hall M, Gold EB, Burns JW, Kravitz HM. Sleep duration and weight change in midlife women: the SWAN sleep study. Obesity (Silver Spring) 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hasler G, Buysse D, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rössler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-Year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 138.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between Reduced Sleep and Weight Gain in Women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 141.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–7. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33:289–95. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM, Gillman MW. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167:178–87. doi: 10.1093/aje/kwm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishiura C, Hashimoto H. A 4-Year Study of the Association between Short Sleep Duration and Change in Body Mass Index in Japanese Male Workers. J Epidemiol. 2010;20:385–90. doi: 10.2188/jea.JE20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao Q, Arem H, Moore SC, Hollenbeck AR, Matthews CE. A large prospective investigation of sleep duration, weight change, and obesity in the NIH-AARP Diet and Health Study cohort. Am J Epidemiol. 2013;178:1600–10. doi: 10.1093/aje/kwt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lyytikainen P, Lallukka T, Lahelma E, Rahkonen O. Sleep problems and major weight gain: a follow-up study. Int J Obes (Lond) 2011;35:109–14. doi: 10.1038/ijo.2010.113. [DOI] [PubMed] [Google Scholar]
- 147.Littman AJ, Vitiello MV, Foster-Schubert K, Ulrich CM, Tworoger SS, Potter JD, Weigle DS, McTiernan A. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2006;31:466–75. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 148.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 149.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring) 2014;22:E55–61. doi: 10.1002/oby.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koo M, Lai NS, Chiang JK. Short duration of sleep is associated with hyperleptinemia in Taiwanese adults. J Clin Sleep Med. 2013;9:1049–55. doi: 10.5664/jcsm.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–40. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 153.Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G, Study NSE. No association between leptin levels and sleep duration or quality in obese adults. Obesity (Silver Spring) 2011;19:2433–5. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soares MJ, Macedo A, Bos SC, Maia B, Marques M, Pereira AT, Gomes AA, Valente J, Nogueira V, Azevedo MH. Sleep disturbances, body mass index and eating behaviour in undergraduate students. J Sleep Res. 2011;20:479–86. doi: 10.1111/j.1365-2869.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 156.Nakade M, Takeuchi H, Kurotani M, Harada T. Effects of meal habits and alcohol/cigarette consumption on morningness-eveningness preference and sleep habits by Japanese female students aged 18–29. J Physiol Anthropol. 2009;28:83–90. doi: 10.2114/jpa2.28.83. [DOI] [PubMed] [Google Scholar]
- 157.Hicks RA, McTighe S, Juarez M. Sleep duration and eating behaviors of college students. Percept Mot Skills. 1986;62:25–6. doi: 10.2466/pms.1986.62.1.25. [DOI] [PubMed] [Google Scholar]
- 158.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28:1146–50. doi: 10.1016/j.nut.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 159.Galli G, Piaggi P, Mattingly MS, de Jonge L, Courville AB, Pinchera A, Santini F, Csako G, Cizza G. Inverse relationship of food and alcohol intake to sleep measures in obesity. Nutrition & diabetes. 2013;3:e58. doi: 10.1038/nutd.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chaput JP, McNeil J, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with greater alcohol consumption in adults. Appetite. 2012;59:650–5. doi: 10.1016/j.appet.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 161.Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med. 2011;7:659–64. doi: 10.5664/jcsm.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002;21:115–20. doi: 10.2114/jpa.21.115. [DOI] [PubMed] [Google Scholar]
- 163.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 164.Parvaneh K, Poh BK, Hajifaraji M, Ismail MN. Sleep deprivation is related to obesity and low intake of energy and carbohydrates among working Iranian adults: a cross sectional study. Asia Pacific journal of clinical nutrition. 2014;23:84–90. doi: 10.6133/apjcn.2014.23.1.02. [DOI] [PubMed] [Google Scholar]
- 165.Zadeh SS, Begum K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr Res Pract. 2011;5:230–5. doi: 10.4162/nrp.2011.5.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Patterson RE, Emond JA, Natarajan L, Wesseling-Perry K, Kolonel LN, Jardack P, Ancoli-Israel S, Arab L. Short sleep duration is associated with higher energy intake and expenditure among African-American and non-Hispanic white adults. J Nutr. 2014;144:461–6. doi: 10.3945/jn.113.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition. 2007;23:773–7. doi: 10.1016/j.nut.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 168.Hart CN, Fava JL, Subak LL, Stone K, Vittinghoff E, Demos K, O’Brien E, Cairns A, Wing R. Time in Bed is Associated with Decreased Physical Activity and Higher BMI in Women Seeking Weight Loss Treatment. ISRN obesity. 2012;2012 doi: 10.5402/2012/320157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–95. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep. 2010;33:753–7. doi: 10.1093/sleep/33.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71–80. doi: 10.1016/j.appet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11:180–4. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Golley RK, Maher CA, Matricciani L, Olds TS. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond) 2013;37:546–51. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 175.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chapman C, Benedict C, Brooks S, Schiöth H. Lifestyle determinants of the drive to eat: a meta-analysis. The American journal of clinical nutrition. 2012;96:492–7. doi: 10.3945/ajcn.112.039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. The lancet. Diabetes & endocrinology. 2014 doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 178.Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, Passera P, Tibaldi P, Tomelini M, Cavallo F, Ghigo E, Porta M. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225–9. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- 179.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 180.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–6. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lou P, Chen P, Zhang L, Zhang P, Chang G, Zhang N, Li T, Qiao C. Interaction of sleep quality and sleep duration on impaired fasting glucose: a population-based cross-sectional survey in China. BMJ open. 2014;4:e004436. doi: 10.1136/bmjopen-2013-004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 184.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 185.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs R, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep Duration as a Risk Factor for Diabetes Incidence in a Large US Sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaput JP, Despres JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10:919–24. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 187.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–3. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 189.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–9. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 190.Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–41. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 191.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 192.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–7. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 194.Bjorkelund C, Bondyr-Carlsson D, Lapidus L, Lissner L, Mansson J, Skoog I, Bengtsson C. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28:2739–44. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 195.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009 doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutrition & diabetes. 2013;3:e65. doi: 10.1038/nutd.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Houyez F, Degoulet P, Cittee J, Fouriaud C, Jacquinet-Salord MC, Lang T, Aime F. Sommeil et hypertension artérielle. Arch Mal Coeur Vaiss. 1990;83:1085–8. [PubMed] [Google Scholar]
- 198.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 199.Kotani K, Saiga K, Sakane N, Mu H, Kurozawa Y. Sleep status and blood pressure in a healthy normotensive female population. Int J Cardiol. 2007;125:425–7. doi: 10.1016/j.ijcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 200.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kawabe H, Saito I. Does short sleep duration in daily life affect morning home blood pressure? Evaluation in Japanese people. Clin Exp Hypertens. 2008;30:183–90. doi: 10.1080/10641960802064575. [DOI] [PubMed] [Google Scholar]
- 202.Stang A, Moebus S, Mohlenkamp S, Erbel R, Jockel KH. Gender-specific associations of short sleep duration with prevalent hypertension. Hypertension. 2008;51:e15–6. doi: 10.1161/HYPERTENSIONAHA.107.108456. author reply e7. [DOI] [PubMed] [Google Scholar]
- 203.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–7. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 204.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Medicine. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van den Berg JF, Tulen JH, Neven AK, Hofman A, Miedema HM, Witteman JC, Tiemeier H. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50:585–9. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- 206.Lima-Costa MF, Peixoto SV, Rocha FL. Usual sleep duration is not associated with hypertension in Brazilian elderly: The Bambui Health Aging Study (BHAS) Sleep Med. 2008;9:806–7. doi: 10.1016/j.sleep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 207.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ross AJ, Yang H, Larson RA, Carter JR. Sleep Efficiency and Nocturnal Hemodynamic Dipping in Young, Normotensive Adults. Am J Physiol Regul Integr Comp Physiol. 2014 doi: 10.1152/ajpregu.00211.2014. [DOI] [PubMed] [Google Scholar]
- 209.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 210.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 212.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 213.Heslop P, Smith G, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Medicine. 2002;3:305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 214.Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]