Abstract
The mechanoelectrical transducer (MET) channels located at the stereocilia tip of cochlear hair cells are crucial to convert the mechanical energy of sound into receptor potentials, but the identity of its pore‐forming subunits remains uncertain. Piezo1, which has been identified in the transcriptome of mammalian cochlear hair cells, encodes a transmembrane protein that forms mechanosensitive channels in other tissues. We investigated the properties of the MET channel in outer hair cells (OHCs) of Piezo1 mice (postnatal day 6–9). The MET current was elicited by deflecting the hair bundle of OHCs using sinewave and step stimuli from a piezo‐driven fluid jet. Apical and basal OHCs were investigated because the properties of the MET channel vary along the cochlea. We found that the maximal MET current amplitude and the resting open probability of the MET channel in OHCs were similar between Piezo1 +/− haploinsufficient mice and wild‐type littermates. The sensitivity to block by the permeant MET channel blocker dihydrostreptomycin was also similar between the two genotypes. Finally, the anomalous mechano‐gated current, which is activated by sheer force and which is tip‐link independent, was unaffected in OHCs from Piezo1 +/− haploinsufficient mice. Our results suggest that Piezo1 is unlikely to be a component of the MET channel complex in mammalian cochlear OHCs.
Keywords: Cochlea, electrophysiology, hair cell, mechanoelectrical transduction, Piezo1
Introduction
The hair cells of the organ of Corti within the mammalian cochlea convert mechanical stimuli into receptor potentials. The hair bundle atop each cell contains multiple rows of stereocilia of increasing height, which are deflected in response to sound. The stereocilia are connected by tip links in the direction of optimal mechanosensitivity (Pickles et al. 1984; Schwander et al. 2010), such that their deflections toward the tallest stereocilia elicit excitatory responses. The mechanoelectrical transducer (MET) channel complex, which allows the influx of positive ions into the hair cells, resides in the region where the tip link inserts into the tip of the lower stereocilia (Beurg et al. 2009). In recent years, four molecules have been identified as likely components of the MET channel complex: TMHS/LHFPL5 (Xiong et al. 2012), TMIE (Zhao et al. 2014), TMC1 (Kawashima et al. 2011; Pan et al. 2013; Kurima et al. 2015), and TMC2 (Kawashima et al. 2011; Kurima et al. 2015). While TMHS/LHFPL5 and TMIE are unlikely to form the pore‐forming subunits of the MET channel (Xiong et al. 2012; Zhao et al. 2014), mutations in tmc1 and tmc2 have been shown to affect the MET channel (Kawashima et al. 2011; Pan et al. 2013; Corns et al. 2016). The possibility that other proteins could be contributing to the MET channel pore in hair cells has been suggested by the fact that an anomalous MET current, which is independent of tiplinks and is instead evoked by sheer stress (Marcotti et al. 2014), has been shown to be present in mice lacking both TMC1 and TMC2 (Beurg et al. 2014).
In this study, we investigated Piezo1, which has been shown to constitute the pore‐forming subunit of some mechanosensitive channels (Coste et al. 2010, 2012). A transcriptome analysis has demonstrated that Piezo1 (Fam38A) is expressed in mammalian cochlear hair cells (Liu et al. 2014), suggesting that it could play a role in mechanosensory transduction in these cells. Although homozygous mutant (Piezo1 −/−) mice are embryonic lethal (Li et al. 2014; Ranade et al. 2014a), Piezo1 +/− mice show haploinsufficiency, which affects the coupling between sheer stress and Ca2+ entry into endothelial cells (Li et al. 2014). In cochlear OHCs, we found no differences in maximal MET current amplitude, reversal potential, adaptation, or blocking potency of DHS between Piezo1 +/− and Piezo +/+ mice. Moreover, the anomalous MET current (Marcotti et al. 2014) was still present in Piezo1 +/− OHCs.
Methods
Animals and genotyping
All experiments were performed in accordance with Home Office regulations under the Animals (Scientific Procedures) Act 1986 and following approval by the University of Sheffield Ethical Review Committee. Piezo1 +/− mice were obtained from David J. Beech (University of Leeds, Leeds, UK) and maintained on a C57BL/6J background. Genotype was determined by PCR analysis of DNA from tail clips (Li et al. 2014). Recordings were made from mice of either sex.
Hearing test
To test whether the hearing in Piezo1 haploinsufficient mice was affected, we performed a visual observation of the Preyer reflex, which is a back‐flick of the pinna in response to sound. Control and haploinsufficient mice were placed in a large plastic box and subjected to a 20 kHz tone burst with an intensity of 95 dB sound pressure level from distances of 20 cm above the head, using a custom‐built device provided by the Medical Research Council Institute of Hearing Research (Nottingham, UK).
Tissue preparation
To study OHCs in acutely dissected organs of Corti, mice were killed by cervical dislocation, the cochlea removed and the organ of Corti dissected in extracellular solution composed of (in mmol/L): 135 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 d‐glucose, 10 HEPES‐NaOH, 2 Na‐pyruvate. Amino acids and vitamins (Eagle's MEM) were added from concentrates (pH 7.5, 308 mOsm/kg). Once dissected, the apical and basal coils of the organ of Corti were transferred to a microscope chamber containing extracellular solution and viewed on a Leica DMLFS microscope (Leica Micro Systems, Wetzlar, Germany) through a long working‐distance 63× water‐immersion objective.
Whole‐cell patch clamp
Recordings were made from postnatal day 6 (P6) to P9 OHCs at room temperature (20–25°C) using an Optopatch amplifier (Cairn Research Ltd, Faversham, UK). Patch pipettes with a typical resistance of 2–4 MΩ were pulled from soda glass capillaries. In order to reduce the fast electrode capacitative transient, the shank of each capillary was coated with surf wax (Mr Zoggs Sex Wax, CA). Pipettes were filled with an intracellular solution of composition (in mmol/L): 106 l‐glutamic acid, 20 CsCl, 10 Na2‐phosphocreatine, 3 MgCl2, 1 EGTA‐CsOH, 5 Na2ATP, 5 HEPES, and 0.3 GTP (adjusted to pH 7.28 with 1 mol/L CsOH; 294 mOsm/kg). An l‐glutamic acid‐based intracellular solution was used as it preserves cellular ultrastructure and improves the stability of recordings (Kay 1992). A similar solution has extensively been used for investigating the biophysical properties of mammalian cochlea hair cells (e.g., Moser and Beutner 2000; Corns et al. 2014). Data acquisition was performed using pClamp software (Molecular Devices, Sunnyvale, CA) using a Digidata 1440A. Data were filtered at 5 kHz (8‐pole Bessel). Offline data analysis was performed using Origin software (OriginLab, Northampton, MA). Membrane potentials were corrected for a liquid junction potential of −11 mV measured between electrode and bath solution.
Hair bundle stimulation
Mechanoelectrical transducer currents were elicited using a fluid jet from a pipette driven by a 25‐mm diameter piezoelectric disc (Kros et al. 1992; Corns et al. 2014). The fluid jet pipette tip had a diameter of 8–10 μm and was positioned at about 8 μm from the hair bundles, which elicited a maximal MET current. Mechanical stimuli were applied as steps or 50 Hz sinusoids (filtered at 1 kHz, 8‐pole Bessel). The tiplink independent anomalous current was elicited by applying unphysiologically strong mechanical stimuli causing a sheer force displacement of the cuticular plate of OHCs (Marcotti et al. 2014). The conversion value used to express the piezo‐driver voltage into bundle displacement during normal fluid jet stimulation was as previously reported when using the above standard recording conditions (10 nm/V: Corns et al. 2014).
Dihydrostreptomycin application
In order to test the effects of the MET channel blocker dihydrostreptomycin (DHS, Sigma, Gillingham, UK) on the MET current, OHCs were superfused with a normal extracellular solution containing 10 μmol/L DHS, which in these cells represents its half‐blocking concentration near −80 mV (Marcotti et al. 2005). The stock solution of DHS (100 mmol/L: molecular weight = 730.7) was prepared using extracellular solution (see above). During the recordings, the DHS test solution was present in the fluid jet and also superfused via a pipette positioned orthogonally to the axis of mechanical sensitivity of the hair bundle. To ensure complete exchange of the normal extracellular solution with that containing DHS, the test solution was sucked into the fluid jet prior to stimulation of the hair bundles.
Statistical analysis
Statistical comparisons of means were made by one‐way ANOVA, with P < 0.05 selected as the criterion for statistical significance. All values are quoted as mean ± SEM.
Results
Hearing in Piezo1 haploinsufficient mice was visually tested by probing for the presence of a Preyer's reflex in response to a 20 kHz tone burst. Although the Piezo1 +/− mice responded similarly to littermate controls (n = 3 each genotype; >3 months of age), a Preyer's reflex test cannot detect less severe forms of sensorineural deafness (Jero et al. 2001). In addition, the absence of a hearing phenotype would not preclude the involvement of Piezo1 in mechano‐electrical transduction since, for example, the loss of TMC2 does not confer hearing loss despite affecting the MET current in the immature cochlear hair cells (Kawashima et al. 2011).
To investigate the possible involvement of Piezo1 in hair cells, MET currents in apical and basal OHCs were elicited by displacing stereociliary hair bundles with sinewave stimuli from a piezoelectric fluid jet. A positive driver voltage to the fluid jet pushed solution out, deflecting the hair bundles in the excitatory direction toward the tallest stereocilia. At negative membrane potentials, large inward MET currents were recorded in all OHCs tested (Fig. 1A, B). At a membrane potential of −121 mV, the maximum amplitude of the MET current in apical OHCs was similar between Piezo1 +/+ (−1812 pA ± 61, n = 10) and Piezo1 +/− (−1692 pA ± 84; n = 16) mice. The MET current amplitude normally increases from the low‐frequency OHCs in the apex to the high‐frequency cells in the base (Ricci 2002; Johnson et al. 2011; Kim and Fettiplace 2013). We found that the maximal MET current in basal OHCs was similar between the two genotypes (Piezo1 +/+: −2351 pA ± 69, n = 8; Piezo1 +/−: −2189 pA ± 113; n = 7), and it was significantly larger (P < 0.001) than that recorded in apical OHCs in both Piezo1 +/+ and Piezo1 +/− mice. As the membrane potential was stepped to more depolarized values, the inward MET current decreased in size at first and then reversed to become an outward current (Fig. 1). The reversal potential of the MET current did not vary between apical OHCs from Piezo1 +/+ (−1.3 mV ± 0.7, n = 10) and Piezo1 +/− (−1.8 mV ± 0.4, n = 16) mice (Fig. 1C), or between basal OHCs (Piezo1 +/+: −3.2 mV ± 0.4, n = 8; Piezo1 +/−: −3.4 mV ± 0.5, n = 7, Fig. 1D).
Figure 1.

Maximal amplitude of the mechanoelectrical transducer (MET) current in outer hair cells (OHCs) is not affected in Piezo1 +/− haploinsufficient mice. Saturating MET currents in apical OHCs from Piezo1 +/+ (A, P7) and Piezo1 +/− (B, P6) mice in response to 50 Hz sinusoidal force stimuli to the hair bundles at membrane potentials of −121 and +99 mV. Driver voltage (DV) stimuli to the fluid jet are shown above the traces, with positive deflections of the DV being excitatory. The arrows and arrowheads indicate the closure of the transducer channel in response to inhibitory bundle stimuli at −121 and +99 mV, respectively. Average peak to peak MET current–voltage curves from apical (C) and basal (D) OHCs of Piezo1 +/+ (10 apical and 8 basal OHCs) and Piezo1 +/− (16 apical and 7 basal OHCs) mice. Recordings were obtained by mechanically stimulating the hair bundles of OHCs while stepping their membrane potential from −121 to +99 mV in 20 mV increments.
The resting MET current, which is defined as the current flowing into the channel in the absence of bundle stimulation, can be measured from the difference between the holding current and the current observed during inhibitory bundle stimulation, that is, when negative driver voltages pull solution into the fluid jet and completely close the MET channel. The resting MET current was represented as a proportion of the total MET current (i.e., resting open probability, P o). At the negative membrane potentials of −121 mV, the resting P o of apical (0.077 ± 0.008, n = 16) and basal (0.078 ± 0.012, n = 7) OHCs from Piezo1 +/− mice was similar to that observed in OHCs from their wild‐type littermates (Piezo1 +/+ apical: 0.076 ± 0.006, n = 10; basal: 0.067 ± 0.004, n = 8), and in agreement with previous finding in normal wild‐type mice (Géléoc et al. 1997; Stauffer and Holt 2007; Corns et al. 2014). The resting P o of the MET channel in wild‐type mice normally increases when stepping the membrane potential to positive values near the Ca2+ equilibrium potential (Piezo1 +/+ apical: 0.40 ± 0.04, n = 10; basal: 0.30 ± 0.03, n = 8; see also Fig. 1A). This is because while increasing Ca2+ influx through the MET channels promotes adaptation, thus closing some channels, reducing its influx increases the channel P o (Assad et al. 1989; Crawford et al. 1991; Ricci et al. 1998; Corns et al. 2014). Similar increases in P o were also observed in OHCs from Piezo1 +/− mice (apical: 0.38 ± 0.04, n = 16; basal: 0.33 ± 0.03, n = 7; see also Fig. 1B).
To investigate further the adaptation properties of the MET current in Piezo1 +/− mice, the hair bundles of OHCs were stimulated with step stimuli. While holding the cell at −81 mV, small nonsaturating bundle displacements in the excitatory direction caused a time‐dependent decline of the MET current (e.g., MET current adaptation) in both Piezo1 +/+ and Piezo1 +/− OHCs (Fig. 2A, B: left panels). Adaptation is a crucial property of the MET channel allowing the resetting of its operating range (Crawford et al. 1991; Fettiplace and Hackney 2006). Adaptation was similar between the two genotypes, exhibiting a fast time constant (Piezo1 +/+: 1.35 ± 0.57 ms, n = 4; Piezo1 +/−: 1.02 ± 0.12 ms, n = 4) and a slow time constant (Piezo1 +/+: 21 ± 7 ms; Piezo1 +/−: 20 ± 5 ms). MET current adaptation was lost and the channel resting P o increased on stepping the membrane potential to +99 mV (Fig. 2A, B: right panels), a condition that prevents or strongly reduces Ca2+ entry via the MET channels. As for the sinewave stimuli, the P o of the MET current at −81 and +99 mV did not differ between wild type and Piezo1 +/− in both apical and basal coil OHCs (Fig. 2E).
Figure 2.
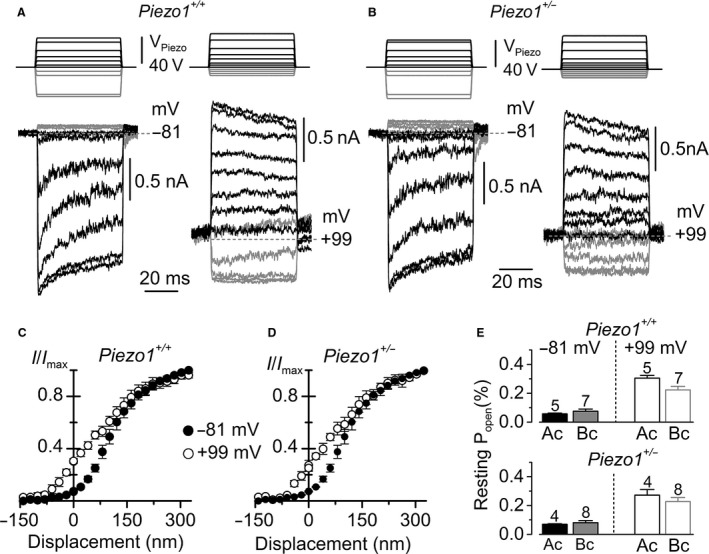
Mechanoelectrical transducer (MET) current adaptation in apical or basal outer hair cells (OHCs) is similar between Piezo1 +/+ and Piezo1 +/− mice. A and B, Force step stimuli of 50 ms duration (top) elicited MET currents (bottom panels). Recordings were obtained at −81 mV (left panels) and +99 mV (right panels) from apical OHCs of Piezo1 +/+ (A) and Piezo1 +/− (B) mice. At −81 mV, positive driver voltages (excitatory stimuli) elicited large inward currents that adapted or decline over time for intermediate stimuli for both Piezo1 +/+ and Piezo1 +/− OHCs; in the inhibitory direction (negative driver voltages) the step stimuli closed the small MET current that was available at rest (gray traces). At +99 mV (right panels), adaptation was absent and the resting MET current was increased in both genotypes. C and D, Normalized peak MET current recorded from apical OHCs of P7 Piezo1 +/+ (n = 5) and P7–P8 Piezo1 +/− (n = 4) mice, respectively. The MET currents were recorded at the holding potential of −81 and +99 mV from the same OHCs and plotted as a function of bundle displacement. The Ca2+‐dependent adaptive leftward shift of the MET current at +99 mV was normal in OHCs from both genotypes. E, Resting P open at −81 and +99 mV obtained from apical (Ac) and basal (Bc) coil OHCs in the two different genotypes.
We then investigated whether the MET current in Piezo1 +/− mice was affected by the aminoglycoside dihydrostreptomycin (DHS), a large polycation known to block (Ohmori 1985; Kroese et al. 1989; Ricci 2002; Marcotti et al. 2005) and permeate (Marcotti et al. 2005) the MET channel in vertebrate hair cells. Using 50 Hz sinusoidal stimulation, we recorded the MET current by stepping the membrane between −161 and +99 mV in 20 mV increments from OHCs of both Piezo1 +/+ and Piezo1 +/− mice before (Fig. 3A, B, left panels) and during the superfusion of 10 μmol/L extracellular DHS (Fig. 3A, B, right panels). The peak‐to‐peak MET current–voltage curves revealed that, as previously described (Marcotti et al. 2005), extracellular DHS caused a voltage‐dependent block of the MET current with positive membrane potentials relieving the block in both genotypes (Fig. 3C, D). The block of the MET current by DHS at negative membrane potentials was partially relieved for values negative to about −80 mV, indicating that the drug was pushed from its binding site and forced through the channel pore into the cytoplasm when sufficient electrical driving force was applied (Marcotti et al. 2005). When the MET current amplitude in the presence of DHS was normalized to that recorded during control conditions (I DHS/I control) in the same OHCs and at −81 mV, we found a similar level of DHS block between Piezo1 +/+ (0.48 ± 0.01, n = 8) and Piezo1 +/− (0.50 ± 0.02, n = 6) mice.
Figure 3.

The mechanoelectrical transducer (MET) current in Piezo1 +/− and Piezo1 +/+ outer hair cells (OHCs) is similarly reduced by the channel blocker dihydrostreptomycin. A and B, Saturating MET currents recorded from apical OHCs of Piezo1 +/+ (A, P7) and Piezo1 +/− (B, P8) mice elicited by fluid jet stimulation to the hair bundles (top) superimposed to voltage steps between −161 and +99 mV in 20 mV increments. For clarity, only voltage steps of −121 and +99 mV are shown. Recordings were performed before (left panels) and during (right panels) the extracellular application of 10 μmol/L dihydrostreptomycin (DHS). C and D, Average saturating MET current–voltage curves for OHCs from the apical coil of P7 Piezo1 +/+ (C, n = 8) and P7–P8 Piezo1 +/− (D, n = 6) mice. Note the relief of the block at the most negative potentials.
The Piezo1 channel responds to sheer stress caused by frictional force (Bae et al. 2013; Li et al. 2014; Ranade et al. 2014a). Therefore, we tested whether the anomalous MET current present in wild‐type cochlear hair cells (Beurg et al. 2014; Marcotti et al. 2014) was affected in Piezo1 +/− mice. This anomalous current is not tip link mediated and can be elicited under a variety of conditions (Marcotti et al. 2014), including mechanical overstimulation of the cuticular plate. We recorded MET currents from apical coil OHCs of both Piezo1 +/+ and Piezo1 +/− animals before (Fig. 4A, D) and after (Fig. 4B, E) irreversible damage to the hair bundles by overstimulation. After this procedure, we were able to record the anomalous currents in both Piezo1 +/+ and Piezo1 +/− OHCs by applying strong sinusoidal stimuli with a fluid jet positioned in close proximity to the cuticular plate and the damaged hair bundle (Fig. 4C, F). While the normal MET current was elicited during positive driver voltages (e.g., excitatory bundle deflections: arrow in Fig. 4A, D), the anomalous current was obtained during the negative phase of the sinewave stimulus, which is normally associated with inhibitory responses (arrow in Fig. 4C, F). We found that the amplitude of the anomalous current at 81 mV was similar between Piezo1 +/+ (−102 ± 44 pA, n = 3) and Piezo1 +/− (−87 ± 12 pA, n = 5) OHCs.
Figure 4.

The anomalous mechanoelectrical transducer (MET) current is present in Piezo1 +/− mice. A–F, MET currents recorded from apical P7 outer hair cells (OHCs) of Piezo1 +/+ (A–C) and Piezo1 +/− (D–F) mice with an intact hair bundle (A and D), after being damaged by repeated strong stimulation (B and E) and obtained by applying strong mechanical stimulation to the cuticular plate and the damaged hair bundle (C and F), which has previously been used to elicit the anomalous MET current (Marcotti et al. 2014). Note that the anomalous MET current is obtained using negative stimuli (C and F: arrows), which normally closes the MET current available at rest (A and D: arrows).
Discussion
In this work, we found no evidence for profound hearing loss in Piezo1 +/− mice, and more specifically, we established that the size of the MET current and its biophysical properties such as Ca2+‐dependent adaptation and resting open probability were similar between Piezo1 +/+ and Piezo1 +/− OHCs, which show haploinsufficiency for the protein (Li et al. 2014). Moreover, the recently described anomalous current activated by sheer force exerted onto the cuticular plate (Beurg et al. 2014; Marcotti et al. 2014) was unaffected by Piezo1 haploinsufficiency.
The full molecular identity of the MET channel in mammalian cochlear hair cells is still unknown. TMC1 and TMC2 are candidate subunits based on several lines of evidence (Kawashima et al. 2011; Pan et al. 2013; Corns et al. 2016) including the fact that they are required for mechanotransduction in hair cells (Kawashima et al. 2011). TMC1 and TMC2 have also been shown to localize at the tip of the shorter stereocilia (Kurima et al. 2015) where the MET channels are located (Beurg et al. 2009), can restore sensory transduction when exogenously expressed in mice that are deficient of such proteins (Askew et al. 2015), and in humans mutations in TMC1 cause dominant progressive hearing loss (DFNA36) and recessive profound congenital deafness (DFNB7/B11) (Kurima et al. 2002). However, recent studies have shown that an additional mechano‐activated current is present in TMC1/TMC2 double knockouts (Beurg et al. 2014). This anomalous current is elicited by applying unphysiologically strong mechanical stimuli eliciting a sheer force displacement onto the cuticular plate of hair cells (Beurg et al. 2014; Marcotti et al. 2014). The presence of this current has led to the suggestion that the channels underlying it could be the MET channel precursor in cochlear hair cells, although the biophysical and pharmacological properties of the anomalous channel are somewhat different from those of the normal MET current (Marcotti et al. 2014).
Piezo1 and Piezo2 are part of a conserved family of nonselective cation channels that are activated by mechanical stimuli (Coste et al. 2010, 2012) and have no sequence similarity with any other channel (Coste et al. 2010; Nilius and Honoré 2012). Expression profiles of mRNA for Piezo1 and Piezo2 are found in numerous mechanosensitive tissues (Coste et al. 2010). While Piezo2 is required for touch sensation in the mouse (Ranade et al. 2014b; Woo et al. 2014) and is associated with rapidly adapting mechanically stimulated currents in neurons of the dorsal root ganglion (Coste et al. 2010; Ranade et al. 2014b), Piezo1 is expressed in endothelial cells and red blood cells where it detects frictional force (sheer stress) (Bae et al. 2013; Li et al. 2014; Ranade et al. 2014a). Moreover, Piezo1 (Fam38A) has been identified in the transcriptomes of cochlear hair cells (Liu et al. 2014). Piezo proteins form a homotetramer of about 1.2 MDa and reconstitution experiments of Piezo1 in the lipid bilayer resulted in the recording of a spontaneous cation current, indicating that it is able to form a channel‐forming protein (Coste et al. 2012). The ability of an E2133K point mutation in Piezo1 to modify the channel unitary conductance, Ca2+ permeability, and the block potency of the polycationic blocker ruthenium red (Coste et al. 2015), suggests that Piezo1 constitutes a pore‐forming subunit. This was confirmed by the recently described structure of Piezo1, which has highlighted a central pore module (Ge et al. 2015). Our data demonstrated that despite Piezo1 being an excellent candidate for mediating mechanosensory transduction in cochlear hair cells, Piezo1 haploinsufficiency did not alter any of the biophysical properties of the MET current recorded from apical or basal cochlear OHCs nor in the anomalous current activated by sheer stimuli.
Conclusion
Our findings show that Piezo1 haploinsufficiency (Piezo1 +/−) has no functional consequences on the MET current (Figs. 1, 2, 3) and the tip‐link independent anomalous current activated by sheer force (Fig. 4), indicating that Piezo1 is unlikely to be a crucial component of the MET apparatus in the mammalian auditory system.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank MC Holley for commenting on an earlier version of the manuscript.
Corns L. F., Marcotti W.. Piezo1 haploinsufficiency does not alter mechanotransduction in mouse cochlear outer hair cells. Physiol Rep, 4 (3), 2016, e12701, doi: 10.14814/phy2.12701
Funding Information
This work was supported by Wellcome Trust Grant 102892 to W.M.
References
- Askew, C. , Rochat C., Pan B., Asai Y., Ahmed H., and Child E., et al. 2015. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad, J. A. , Hacohen N., and Corey D. P.. 1989. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc. Natl Acad. Sci. USA 86:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, C. , Gnanasambandam R., Nicolai C., Sachs F., and Gottlieb P. A.. 2013. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl Acad. Sci. USA 110:E1162–E1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M. , Fettiplace R., Nam J. H., and Ricci A. J.. 2009. Localization of inner hair cell mechanotransducer channels using high‐speed calcium imaging. Nat. Neurosci. 12:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M. , Kim K. X., and Fettiplace R.. 2014. Conductance and block of hair‐cell mechanotransducer channels in transmembrane channel‐like protein mutants. J. Gen. Physiol. 144:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns, L. F. , Johnson S. L., Kros C. J., and Marcotti W.. 2014. Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc. Natl Acad. Sci. USA 111:14918–14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns, L. F. , Johnson S. L., Kros C. J., and Marcotti W.. 2016. Tmc1 point mutation affects Ca2+ sensitivity and block by dihydrostreptomycin of the mechano‐electrical transducer current of mouse outer hair cells. J. Neurosci. 36: 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste, B. , Mathur J., Schmidt M., Earley T. J., Ranade S., and Petrus M. J., et al. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste, B. , Xiao B., Santos J. S., Syeda R., Grandl J., and Spencer K. S., et al. 2012. Piezo proteins are pore‐forming subunits of mechanically activated channels. Nature 483:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste, B. , Murthy S. E., Mathur J., Schmidt M., Mechioukhi Y., and Delmas P., et al. 2015. Piezo1 ion channel pore properties are dictated by C‐terminal region. Nat. Commun. 6:7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, A. C. , Evans M. G., and Fettiplace R.. 1991. The actions of calcium on the mechano electrical transducer current of turtle hair cells. J. Physiol. 434:369–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace, R. , and Hackney C. M.. 2006. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 7:19–29. [DOI] [PubMed] [Google Scholar]
- Ge, J. , Li W., Zhao Q., Li N., Chen M., and Zhi P., et al. 2015. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527:64–69. [DOI] [PubMed] [Google Scholar]
- Géléoc, G. S. , Lennan G. W., Richardson G. P., and Kros C. J.. 1997. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. Biol. Sci. B 264:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jero, J. , Coling D. E., and Lalwani A. K.. 2001. The use of Preyer's reflex in evaluation of hearing in mice. Acta Otolaryngol. 121:585–589. [PubMed] [Google Scholar]
- Johnson, S. L. , Beurg M., Marcotti W., and Fettiplace R.. 2011. Prestin‐driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70:1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima, Y. , Géléoc G. S., Kurima K., Labay V., Lelli A., and Asai Y., et al. 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel‐like genes. J. Clin. Invest. 121:4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, A. R. 1992. An intracellular medium formulary. J. Neurosci. Methods 44:91–100. [DOI] [PubMed] [Google Scholar]
- Kim, K. X. , and Fettiplace R.. 2013. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel‐like proteins. J. Gen. Physiol. 141:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese, A. B. , Das A., and Hudspeth A. J.. 1989. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear. Res. 37:203–217. [DOI] [PubMed] [Google Scholar]
- Kros, C. J. , Rüsch A., and Richardson G. P.. 1992. Mechano‐electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc. Biol. Sci. B 249:185–193. [DOI] [PubMed] [Google Scholar]
- Kurima, K. , Peters L. M., Yang Y., Riazuddin S., Ahmed Z. M., and Naz S., et al. 2002. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair‐cell function. Nat. Genet. 30:277–284. [DOI] [PubMed] [Google Scholar]
- Kurima, K. , Ebrahim S., Pan B., Sedlacek M., Sengupta P., and Millis B. A., et al. 2015. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep. 12:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Hou B., Tumova S., Muraki K., Bruns A., and Ludlow M. J., et al. 2014. Piezo1 integration of vascular architecture with physiological force. Nature 515:279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Pecka J. L., Zhang Q., Soukup G. A., Beisel K. W., and He D. Z.. 2014. Characterization of transcriptomes of cochlear inner and outer hair cells. J. Neurosci. 34:11085–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti, W. , van Netten S. M., and Kros C. J.. 2005. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters hair cells through the mechano‐electrical transducer channels. J. Physiol. 567:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti, W. , Corns L. F., Desmonds T., Kirkwood N. K., Richardson G. P., and Kros C. J.. 2014. Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano‐electrical transducer channel. J. Neurosci. 34:5505–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, T. , and Beutner D.. 2000. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc. Natl Acad. Sci. USA 97:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius, B. , and Honoré E.. 2012. Sensing pressure with ion channels. Trends Neurosci. 35:477–486. [DOI] [PubMed] [Google Scholar]
- Ohmori, H. 1985. Mechano‐electrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol. 359:189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B. , Géléoc G. S., Asai Y., Horwitz G. C., Kurima K., and Ishikawa K., et al. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles, J. O. , Comis S. D., and Osborne M. P.. 1984. Cross‐links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15:103–112. [DOI] [PubMed] [Google Scholar]
- Ranade, S. S. , Qiu Z., Woo S. H., Hur S. S., Murthy S. E., and Cahalan S. M., et al. 2014a. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl Acad. Sci. USA 111:10347–10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade, S. S. , Woo S. H., Dubin A. E., Moshourab R. A., Wetzel C., and Petrus M., et al. 2014b. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci, A. 2002. Differences in mechano‐transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J. Neurophysiol. 87:1738–1748. [DOI] [PubMed] [Google Scholar]
- Ricci, A. J. , Wu Y. C., and Fettiplace R.. 1998. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J. Neurosci. 18:8261–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander, M. , Kachar B., and Müller U.. 2010. The cell biology of hearing. J. Cell Biol. 190:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer, E. A. , and Holt J. R.. 2007. Sensory transduction and adaptation in inner and outer hair cells of the mouse auditory system. J. Neurophysiol. 98:3360–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, S. H. , Ranade S., Weyer A. D., Dubin A. E., Baba Y., and Qiu Z., et al. 2014. Piezo2 is required for Merkel‐cell mechanotransduction. Nature 509:622–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, W. , Grillet N., Elledge H. M., Wagner T. F., Zhao B., and Johnson K. R., et al. 2012. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151:1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B. , Wu Z., Grillet N., Yan L., Xiong W., and Harkins‐Perry S., et al. 2014. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84:954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]


