Abstract
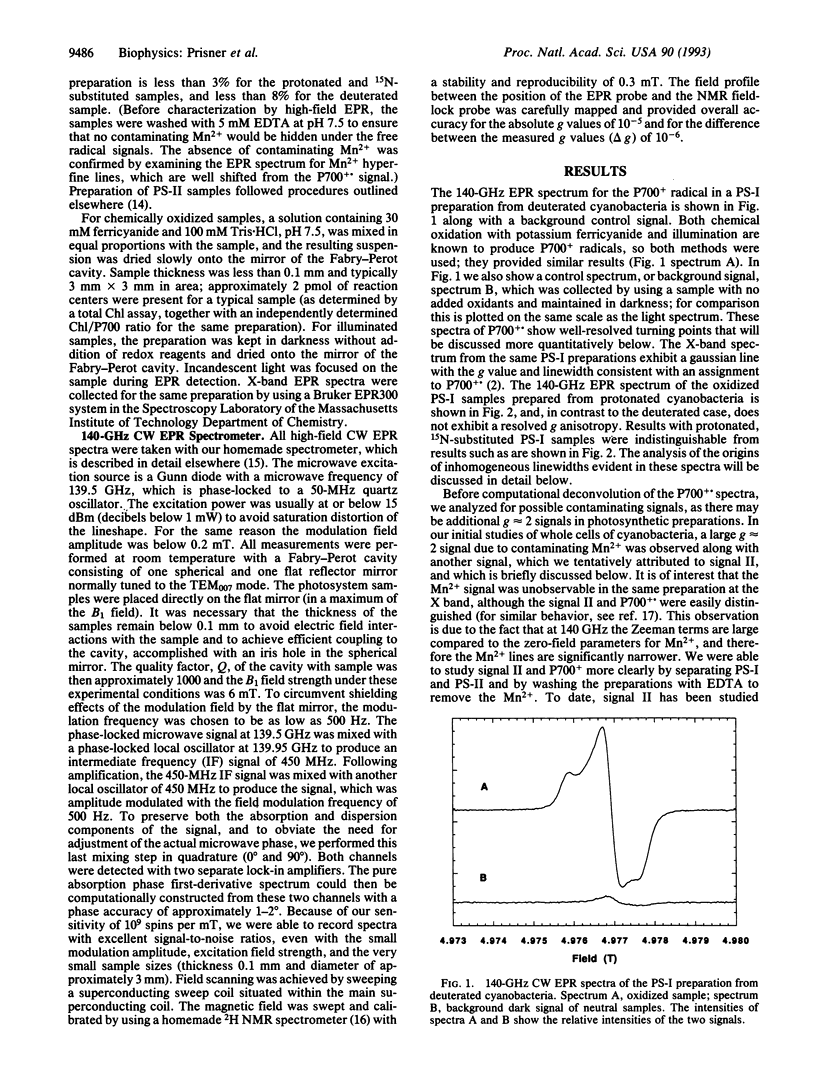
We report high-field continuous wave EPR spectra of P700+. in preparations obtained from deuterated cyanobacteria (Synechococcus lividus). Measurements were performed with photosystem I (PS-I) preparations, whole cells from cyanobacteria grown in 2H2O, and photosystem II (PS-II) preparations, as well as with protonated PS-I preparations. Because of the significantly improved resolution of our 140-GHz spectrometer (as compared with X- or Q-band EPR) the principal values of the g-tensor of the primary donor P700+. could be resolved and measured with high accuracy as g11 = 2.00304, g22 = 2.00262, and g33 = 2.00232. Other signals arising from Mn2+ and a dark signal from PS-II at g approximately 2.00266 are distinguished from the P700+. g-tensor powder pattern. The measured g values are compared with those of several bacterial reaction center donors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Rees D. C., Deisenhofer J., Michel H., Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry B. A., Babcock G. T. Tyrosine radicals are involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7099–7103. doi: 10.1073/pnas.84.20.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Fasanella E. L., Gordy W. Electron spin resonance of an irradiated single crystal of L-tyrosine-HC. Proc Natl Acad Sci U S A. 1969 Feb;62(2):299–304. doi: 10.1073/pnas.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A. E., Yachandra V. K., Guiles R. D., Britt R. D., Dexheimer S. L., Sauer K., Klein M. P. Low-potential iron-sulfur centers in photosystem I: an X-ray absorption spectroscopy study. Biochemistry. 1988 May 31;27(11):4013–4020. doi: 10.1021/bi00411a018. [DOI] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. Characterization of primary reactants in bacterial photosynthesis. I. Comparison of the light-induced EPR signal (g=2.0026) with that of a bacteriochlorophyll radical. Biochim Biophys Acta. 1972 May 25;267(2):363–374. doi: 10.1016/0005-2728(72)90123-5. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. F., Brudvig G. W. A guide to electron paramagnetic resonance spectroscopy of Photosystem II membranes. Biochim Biophys Acta. 1991 Jan 3;1056(1):1–18. doi: 10.1016/s0005-2728(05)80067-2. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'malley P. J., Babcock G. T. Electron nuclear double resonance evidence supporting a monomeric nature for P700 in spinach chloroplasts. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1098–1101. doi: 10.1073/pnas.81.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Sato V. L., Sauer K. Exciton interaction in the photosystem I reaction center from spinach chloroplasts. Absorption and circular dichroism difference spectra. Biochemistry. 1972 Nov 21;11(24):4591–4595. doi: 10.1021/bi00774a027. [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Budil D. E., Freed J. H. Thermodynamics and dynamics of phosphatidylcholine-cholesterol mixed model membranes in the liquid crystalline state: effects of water. Biophys J. 1993 Sep;65(3):1283–1294. doi: 10.1016/S0006-3495(93)81160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


