Abstract
Objectives: Based on recent findings of aromatase and estrogen receptor beta (ERβ) expression in non-small-cell lung cancer, we assessed the clinicopathological and prognostic significance of aromatase and ERβ expression and their relationship to epidermal growth factor receptor (EGFR) mutation in lung adenocarcinoma. Materials and methods: We evaluated 150 resected primary lung adenocarcinoma specimens. Expression of aromatase, ERα, ERβ, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was evaluated by immunostaining, and EGFR and KRAS mutations were analyzed. Overall survival (OS) and recurrence-free survival (RFS) were calculated using the Kaplan-Meier method. Results: Expression of aromatase, ERα, ERβ, PR, and HER2 was detected in 88.0%, 1.3%, 79.3%, 2.7%, and 39.3% of specimens, respectively. In patients with EGFR wild-type lung adenocarcinoma, high aromatase expression was an independent predictor of poor OS (hazard ratio [HR]=2.638; 95% confidence interval [CI], 1.173-5.936; P=.019) and RFS (HR=2.505; 95% CI, 1.154-5.434; P=.020). Positive ERβ expression was also an independent predictor of poor RFS (HR=4.013; 95% CI, 1.219-13.207; P=.022). Furthermore, high aromatase expression was a significant predictor of poor survival only in females (OS, P=.010; RFS, P=.007), whereas positive ERβ expression was an important predictor of poor survival only in males (OS, P=.073; RFS, P=.051). No prognostic significance was observed in patients with EGFR mutations. Conclusions: Our findings suggest that EGFR wild-type lung adenocarcinoma is an estrogen-dependent carcinoma, and aromatase expression and ERβ expression are potent prognostic markers for EGFR wild-type lung adenocarcinoma.
Keywords: Aromatase, estrogen receptor beta (ERβ), estrogen signaling pathway, EGFR mutation, lung adenocarcinoma
Introduction
Lung cancer is one of the most common cancers globally and is currently the leading cause of death in both females and males [1]. Smoking remains the major cause of lung cancer, but ~53% of all females with lung cancer are non-smokers [2]. Interestingly, a gradual increase in the adenocarcinoma subtype of lung cancer has been reported, despite a decline in the smoking population [3-4]. Therefore, etiologic factors other than tobacco may also play a role in the development of lung adenocarcinoma.
Epidermal growth factor receptor (EGFR) is the most frequently mutated proto-oncogene, particularly in lung adenocarcinoma of non-smoker females, and its mutations are thought to play an important role in carcinogenesis [5]. However, recent evidence has suggested that estrogen may also play an important role in the development of non-small cell lung cancer (NSCLC), particularly adenocarcinoma [6]. Several studies have reported that estrogen stimulates the proliferation and progression of lung carcinoma cells, functions that were shown to be significantly suppressed by antiestrogenic agents both in vitro and in vivo [7-10].
Estrogen is converted from androgen by aromatase, a key enzyme in estrogen biosynthesis. In addition to its expression in the ovary and placenta, aromatase is present in male and female extragonadal tissues, including breast and lung [11]. Aromatase expression is elevated in certain malignancies, such as breast carcinomas, suggesting that tumor progression caused by stimulation of estrogen signaling pathway could be enhanced by circulating estrogen as well as by localized autocrine or paracrine production of estrogen by aromatase. Recently, aromatase expression in NSCLC has also been reported [12-14]. Weinberg et al. demonstrated that aromatase was expressed in NSCLC cell lines, and aromatase inhibitor (AI) suppressed tumor growth in vitro and in vivo [12]. Mah et al. reported that low aromatase expression was associated with favorable survival in female NSCLC patients, particularly those older than 65 years [13].
In estrogen signaling pathway, estrogens exert their effects mainly via estrogen receptor (ER) [6]. ER is a hormone receptor, as is progesterone receptor (PR). ER has two isoforms, ERα and ERβ, which are encoded by distinct genes and are expressed in various tissues or at various levels in the same tissue [15]. In the normal lung, ERβ has been reported to be expressed at a higher level than ERα [9]. Although the expression patterns of ERα and ERβ in NSCLC were highly inconsistent among these reports [6], most of the results showed that there were no or a low (under 10%) rate of ERα-positive cases and a higher rate (over 50%) of ERβ-positive cases [16]. Previously, ERα was considered a tumor promoter, whereas ERβ was believed to inhibit tumorigenesis [17]. However, recent studies have demonstrated that ERβ can function as a tumor promoter in the absence of ERα expression [18-22]. The association between ERβ expression and the prognosis of lung cancer patients remains controversial [23-27]. Wu et al. reported that ERβ expression was associated with favorable prognosis in NSCLC [23]. However, Stabile et al. reported that ERβ expression was associated with a poor prognosis in lung cancer [26].
Recent reports have suggested an interaction between EGFR pathway and estrogen signaling pathway in the development of breast and lung cancer; additionally, estrogen signaling pathway is regulated by membrane receptor tyrosine kinases, including EGFR and human epidermal growth factor receptor 2 (HER2) [26,28-32]. The EGFR pathway becomes activated when estrogen is depleted, and ERβ expression is increased following treatment with EGFR tyrosin kinase inhibitors (EGFR-TKIs) in NSCLC cells [33-35]. Nose et al. reported that strong nuclear expression of ERβ is correlated with EGFR mutations in lung adenocarcinoma [31]. However, the role of aromatase and ERβ in estrogen signaling pathway and the association between the expression of these proteins and clinicopathological factors, including EGFR mutation, in lung adenocarcinoma, are not well understood.
The purpose of the present study was to examine the correlation between aromatase/ERβ expression and clinicopathological prognostic factors, including EGFR mutations, and to evaluate the prognostic significance in lung adenocarcinoma.
Materials and methods
Patients and tissue specimens
One hundred and fifty lung adenocarcinoma specimens were obtained from patients who underwent complete surgical resection consecutively from 2004 to 2008 at Gunma University Hospital. The clinicopathological factors of the patients are shown in Table 1. The disease stage was determined according to the seventh edition of the TNM classification for lung and pleural tumors [36]. All of the procedures were approved by the Ethics Committee on Human Research of Gunma University Graduate School of Medicine, and written informed consent was obtained from all of the patients before surgery.
Table 1.
Clinicopathological factors of the patients
| Characteristics | N | % |
|---|---|---|
| No. of patients | 150 | 100.0 |
| Age (year) | ||
| Mean | 66 | |
| Range | 36-84 | |
| Sex | ||
| Male | 63 | 42.0 |
| Female | 87 | 58.0 |
| Menopause | ||
| Premenopausal | 4 | 4.6 |
| Postmenopausal | 81 | 93.1 |
| Unknown | 2 | 2.3 |
| Smoking status | ||
| Ever-smoker | 68 | 45.3 |
| Non-smoker | 82 | 54.7 |
| Tumor diameter (mm) | ||
| Mean | 24 | |
| Range | 6-70 | |
| Pathologic stage | ||
| IA | 85 | 56.7 |
| IB | 26 | 17.3 |
| IIA | 5 | 3.3 |
| IIB | 6 | 4.0 |
| IIIA | 22 | 14.7 |
| IIIB | 6 | 4.0 |
| IV | 0 | 0.0 |
| Pleural invasion | ||
| Absent | 102 | 68.0 |
| Present | 48 | 32.0 |
| Lymphatic invasion | ||
| Absent | 101 | 67.3 |
| Present | 49 | 32.7 |
| Vascular invasion | ||
| Absent | 104 | 69.3 |
| Present | 46 | 30.7 |
Immunohistochemistry
Serial tissue sections of 4-µm thickness sliced from paraffin-embedded specimens were used for immunohistochemistry using the labeled streptavidin-biotin method. Immunostaining for ERα, ERβ, PR, aromatase, Ki-67, and HER2 was performed with the antibodies listed in Table 2. The slides were deparaffinized with xylene and rehydrated with ethanol. For ERα, PR, and Ki-67 analysis, antigen retrieval was performed according to the manufacturer’s instructions. For ERβ, antigen retrieval was carried out by autoclaving the slides in citrate buffer (0.01 mol/L) at 121°C for 5 min. For HER2, immunohistochemical staining was performed using BenchMark XT (Ventana, Tucson, AZ, USA), an automatic immunohistochemical staining system. Nuclear positive immunoreactivity for ERα, ERβ, and PR was counted among 1000 cells per case and was recorded as “positive” for positive results of more than 10% [14]. Immunoreactive intensity of ERβ was scored into four phases (0, negative; 1+, weak; 2+, moderate; and 3+, strong). For Ki-67, 1000 cells were counted per case, and the proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 labeling index; LI) in each sample. Cytoplasmic staining for aromatase in over 10% of the cancer lesion was recorded as “positive”, and immunoreactive intensity was scored into four phases (0, negative; 1+, weak; 2+, moderate; and 3+, strong) [6,13]. HER2 immunoreactivity was evaluated using the DAKO HercepTest scoring system (DakoCytomation), and over 2+ was considered “positive”. Two observers (K.T. and T.O.) who were unaware of the clinical data independently reviewed all pathological slides.
Table 2.
Primary antibodies used in the present study
| Antigen | Clone | Dilution | Source |
|---|---|---|---|
| ERα | 1D5 | 1:50 | DakoCytomation, Glostrup, Denmark |
| ERβ | 14C8 | 1:200 | GeneTex, CA, USA |
| PR | PGR636 | 1:800 | DakoCytomation, Glostrup, Denmark |
| Aromatase | #677/H7 | 1:1000 | Contributed by Dr. Evans DB, Novartis, Basel, Switzerland |
| Ki-67 | MIB1 | 1:150 | DakoCytomation, Glostrup, Denmark |
| HER2 | 4B5 | 1:1 | Ventana, Tucson, AZ, USA |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Gene mutation analysis
We examined EGFR and KRAS mutations in the present study. Genomic DNA was extracted from a 3- to 5-mm cube of tumor tissue using a DNA Mini Kit (Qiagen, Hilden, Germany) and subsequently diluted to 20 ng/µL. KRAS and EGFR mutations were analyzed by sequencing as described previously [37,38].
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM Co., Armonk, NY, USA). Student’s t-test and chi-squared test were used to compare percentages and mean values, respectively. Survival was calculated using the Kaplan-Meier method and confirmed using the log-rank test. Overall survival (OS) was determined as the time from tumor resection to death from any cause. Recurrence-free survival (RFS) was defined as the time between tumor resection and first disease recurrence or death. The median follow up for survivors was 65.5 months (average, 63.7 months; range, 1-117 months). Variables with P value less than .05 after univariate analysis were entered into multivariate analysis using the Cox proportional hazards model. P < .05 was deemed to indicate statistical significance. The midpoint and median intensity (1.5) was used to define low and high aromatase expression as previously described [13]. For univariate and multivariate analyses, each continuous variable (age and Ki-67 LI) was dichotomized at the median value.
Results
Immunohistochemical analysis
Among the 150 cases, ERα, ERβ, PR, aromatase, and HER2 were detected in 2 (1.3%), 119 (79.3%), 4 (2.7%), 132 (88.0%), and 7 (4.7%) cases, respectively. Figure 1 shows representative staining for ERα, ERβ, PR, and aromatase. Regarding the immunoreactive intensity of aromatase, 18 (12.0%) cases were scored as 0, 60 (40.0%) cases were scored as 1+, 51 (34.0%) cases were scored as 2+, and 21 (14.0%) cases were scored as 3+ (Figure 2). Therefore, low expression group comprised 78 (52.0%) cases, and high expression group comprised 72 (48.0%) cases. ERβ staining was seen in both the cytoplasm and nucleus. Regarding the immunoreactive intensity of ERβ, 31 cases were scored as 0 (20.7%), 98 as 1+ (65.3%), 18 as 2+ (12.0%), and 3 as 3+ (2.0%). Additionally, among the tumors that stained positive for ERβ, almost all of the tumor tissue stained positive. Therefore, ERβ immunoreactivity was not scored using scoring system such as the Allred score [39].
Figure 1.
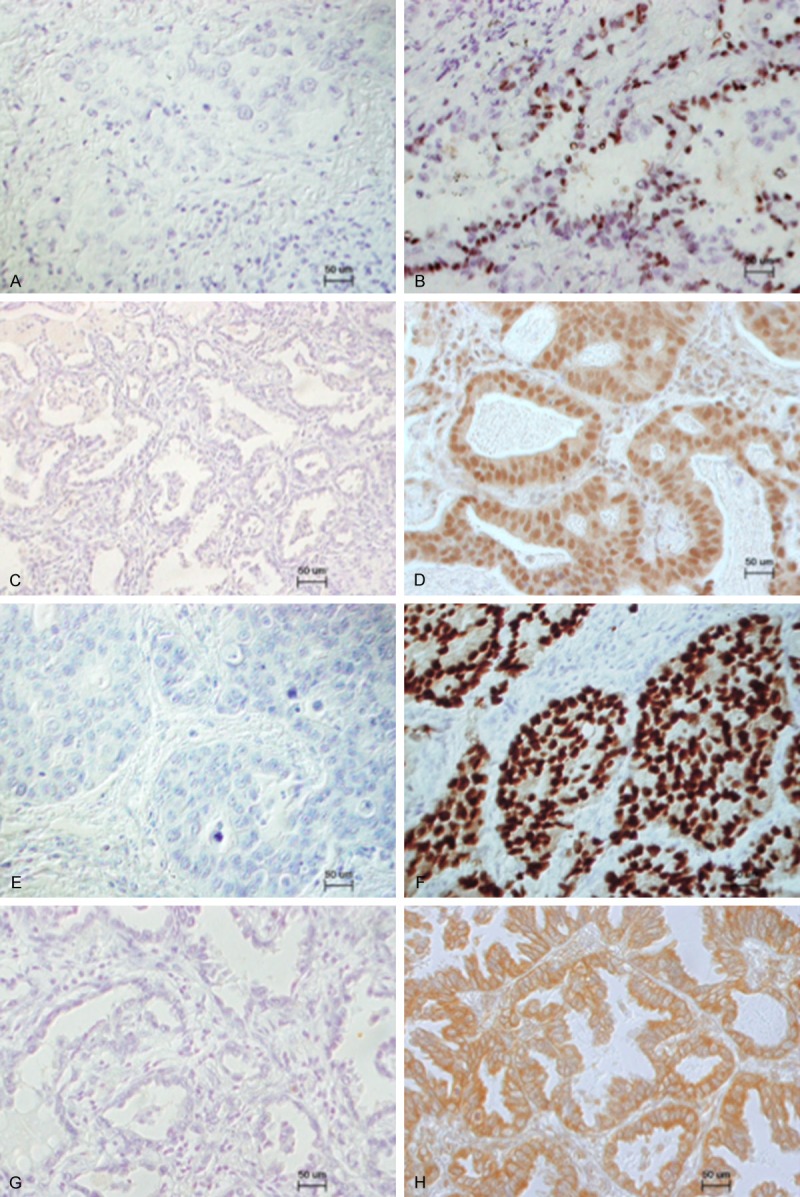
Representative immunohistochemical staining of ERα, ERβ, PR, and aromatase in lung adenocarcinoma. A. Negative staining of ERα; B. Positive staining of ERα; C. Negative staining of ERβ; D. Positive staining of ERβ; E. Negative staining of PR; F. Positive staining of PR; G. Negative staining of aromatase; H. Positive staining of aromatase.
Figure 2.
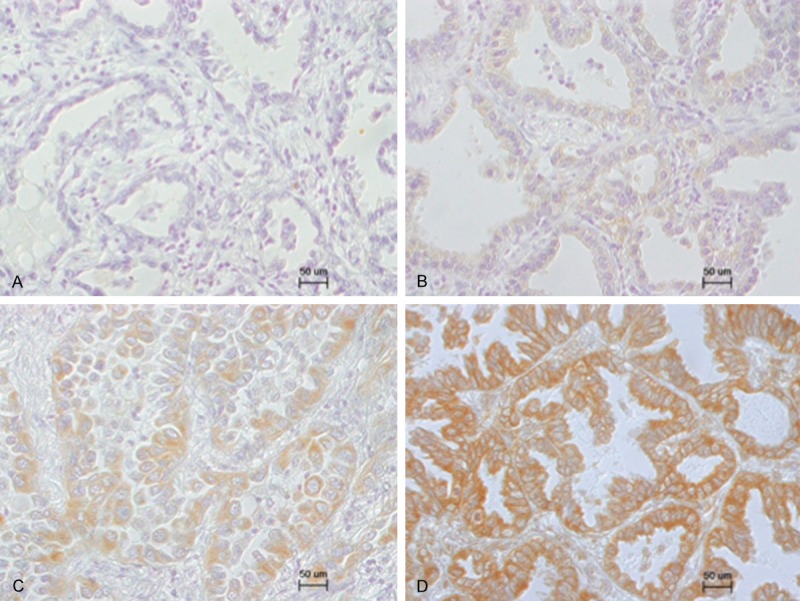
Representative immunohistochemical staining pattern of aromatase in lung adenocarcinoma. Specimens were assigned one of four scores (0, negative; 1+, weak; 2+, moderate; and 3+, strong) according to the intensity of immunoreactivity. A, 0; B, 1+; C, 2+; D, 3+.
Association between aromatase/ERβ expression and clinicopathological factors, including EGFR and KRAS mutation
In 10 of the 150 cases, DNA could not be extracted because the specimen was too small. Among the remaining 140 cases, EGFR and KRAS mutations were found in 62 (44.3%) and 23 (16.4%) cases, respectively. The occurrences of these mutations were mutually exclusive. Aromatase expression status was significantly associated with pleural invasion (P=.037), and ERβ expression was not significantly associated with clinicopathological factors (Table S1).
Survival analysis
On univariate analysis, 10 variables were found to be significantly associated with poor OS. For RFS, eight variables were identified as statistically significant factors (Table 3). Multivariate analysis demonstrated that older age, advanced pathological stage, EGFR wild-type status, high aromatase expression, and high Ki-67 LI score were significant independent predictors of poor OS; additionally, older age, advanced pathological stage, lymphatic invasion, HER2 expression, and high Ki-67 LI score were significant independent predictors of poor RFS (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic factors in all patients
| Variable | No. of patients (%) | Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| 5-year OS (%) | P value | 5-year RFS (%) | P value | OS | RFS | |||||||
|
|
|
|||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |||||||
| All cases | 150 | (100.0) | 73.6 | 63.8 | ||||||||
| Age (years: median 69) | ||||||||||||
| <69 | 72 | (48.0) | 81.6 | 72.1 | 1.000 | - | - | 1.000 | - | - | ||
| ≥69 | 78 | (52.0) | 66.0 | 0.001 | 56.1 | 0.011 | 3.178 | 1.527-6.616 | 0.002 | 1.861 | 1.035-3.348 | 0.038 |
| Sex | ||||||||||||
| Male | 63 | (42.0) | 69.5 | 60.2 | ||||||||
| Female | 87 | (58.0) | 76.6 | 0.106 | 66.3 | 0.150 | ||||||
| Smoking status | ||||||||||||
| Non-smoker | 82 | (54.7) | 81.5 | 71.9 | 1.000 | - | - | 1.000 | - | - | ||
| Ever-smoker | 68 | (45.3) | 63.8 | 0.004 | 54.0 | 0.005 | 0.770 | 0.343-1.729 | 0. 526 | 0.989 | 0.549-1.779 | 0.969 |
| Pathologic stage | ||||||||||||
| I, II | 122 | (81.3) | 84.2 | 76.1 | 1.000 | - | - | 1.000 | - | - | ||
| III | 28 | (18.7) | 28.6 | <0.001 | 10.7 | <0.001 | 2.024 | 1.358-3.016 | 0.001 | 1.697 | 1.223-2.353 | 0.002 |
| Pleural invasion | ||||||||||||
| Absent | 102 | (68.0) | 85.0 | 75.2 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 48 | (32.0) | 50.0 | <0.001 | 39.6 | <0.001 | 0.985 | 0.481-2.019 | 0.968 | 1.201 | 0.676-2.132 | 0.533 |
| Lymphatic invasion | ||||||||||||
| Absent | 101 | (67.3) | 88.9 | 82.0 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 49 | (32.7) | 41.9 | <0.001 | 26.5 | <0.001 | 1.609 | 0.664-3.900 | 0.292 | 2.418 | 1.101-5.309 | 0.028 |
| Vascular invasion | ||||||||||||
| Absent | 104 | (69.3) | 86.4 | 79.6 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 46 | (30.7) | 44.6 | <0.001 | 28.3 | <0.001 | 1.727 | 0.735-4.059 | 0.210 | 1.362 | 0.661-2.808 | 0.402 |
| EGFR mutation | ||||||||||||
| Mutant | 62 | (44.3) | 83.8 | 69.3 | 1.000 | - | - | |||||
| Wild type | 78 | (55.7) | 65.1 | 0.001 | 58.8 | 0.079 | 2.954 | 1.245-7.009 | 0.014 | |||
| KRAS mutation | ||||||||||||
| Mutant | 23 | (16.4) | 52.2 | 47.8 | 1.205 | 0.548-2.651 | 0.643 | |||||
| Wild type | 117 | (83.6) | 77.6 | 0.035 | 66.6 | 0.126 | 1.000 | - | - | |||
| Aromatase | ||||||||||||
| Low expression | 78 | (52.0) | 81.7 | 70.3 | 1.000 | - | - | |||||
| High expression | 72 | (48.0) | 65.1 | 0.039 | 56.9 | 0.101 | 2.235 | 1.142-4.374 | 0.013 | |||
| ERα | ||||||||||||
| Negative | 148 | (98.7) | 73.9 | 64.0 | ||||||||
| Positive | 2 | (1.3) | 50.0 | 0.405 | 50.0 | 0.568 | ||||||
| ERβ | ||||||||||||
| Negative | 31 | (20.7) | 76.9 | 74.2 | ||||||||
| Positive | 119 | (79.3) | 72.7 | 0.447 | 61.0 | 0.145 | ||||||
| PR | ||||||||||||
| Negative | 146 | (97.3) | 73.6 | 63.5 | ||||||||
| Positive | 4 | (2.7) | 75.0 | 0.724 | 75.0 | 0.573 | ||||||
| HER2 | ||||||||||||
| Negative | 143 | (95.3) | 74.5 | 64.9 | 1.000 | - | - | |||||
| Positive | 7 | (4.7) | 57.1 | 0.091 | 42.9 | 0.015 | 3.093 | 1.151-8.311 | 0.025 | |||
| Ki-67 LI (%: median 11.5) | ||||||||||||
| Low score | 75 | (50.0) | 87.7 | 84.0 | 1.000 | - | - | 1.000 | - | - | ||
| High score | 75 | (50.0) | 59.3 | <0.001 | 43.3 | <0.001 | 2.591 | 1.115-6.021 | 0.027 | 2.620 | 1.299-5.284 | 0.007 |
OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; LI, labeling index.
Survival analysis according to EGFR/KRAS mutation status
To clarify the prognostic significance of aromatase/ERβ expression according to EGFR/KRAS mutation status, we examined survival analysis stratified by EGFR/KRAS mutation status. No relationship was found between survival and KRAS mutation status (data not shown); however, a significant relationship was found between survival and EGFR mutation status. On univariate analysis of the EGFR wild-type population, eight variables were significantly associated with poor OS. Regarding RFS, nine variables were identified as statistically significant factors (Table 4). Multivariate analysis showed that older age, advanced pathological stage, vascular invasion, and high aromatase expression were significant independent predictors of poor OS. Concerning RFS, older age, advanced pathological stage, lymphatic invasion, high aromatase expression, and ERβ positive status were significant independent predictors (Table 4).
Table 4.
Univariate and multivariate analyses of prognostic factors in patients with EGFR wild-type lung adenocarcinoma
| Variable | No. of patients (%) | Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| 5-year OS (%) | P value | 5-year RFS (%) | P value | OS | RFS | |||||||
|
|
|
|||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |||||||
| All cases | 78 | (100.0) | 65.1 | 58.8 | ||||||||
| Age (years: median 69) | ||||||||||||
| <69 | 33 | (42.3) | 78.0 | 75.5 | 1.000 | - | - | 1.000 | - | - | ||
| ≥69 | 45 | (57.7) | 55.6 | 0.003 | 46.7 | 0.003 | 3.497 | 1.436-8.514 | 0.006 | 2.775 | 1.193-6.455 | 0.018 |
| Sex | ||||||||||||
| Male | 40 | (51.3) | 59.6 | 54.8 | ||||||||
| Female | 38 | (48.7) | 70.7 | 0.105 | 63.2 | 0.180 | ||||||
| Smoking status | ||||||||||||
| Non-smoker | 34 | (43.6) | 79.1 | 70.6 | 1.000 | - | - | 1.000 | - | - | ||
| Ever-smoker | 44 | (56.4) | 54.1 | 0.009 | 54.8 | 0.021 | 0.788 | 0.248-2.505 | 0.687 | 0.453 | 0.147-1.395 | 0.168 |
| Pathologic stage | ||||||||||||
| I, II | 62 | (79.5) | 77.2 | 71.0 | 1.000 | - | - | 1.000 | - | - | ||
| III | 16 | (20.5) | 18.8 | <0.001 | 12.5 | <0.001 | 1.917 | 1.158-3.175 | 0.011 | 1.715 | 1.037-2.836 | 0.036 |
| Pleural invasion | ||||||||||||
| Absent | 47 | (60.3) | 82.9 | 76.4 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 31 | (39.7) | 38.7 | <0.001 | 32.3 | 0.001 | 1.034 | 0.424-2.524 | 0.941 | 1.247 | 0.527-2.955 | 0.615 |
| Lymphatic invasion | ||||||||||||
| Absent | 47 | (60.3) | 86.9 | 80.7 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 31 | (39.7) | 32.3 | <0.001 | 25.8 | <0.001 | 1.454 | 0.521-4.056 | 0.474 | 3.275 | 1.079-9.941 | 0.036 |
| Vascular invasion | ||||||||||||
| Absent | 49 | (62.8) | 85.6 | 79.5 | 1.000 | - | - | 1.000 | - | - | ||
| Present | 29 | (37.2) | 30.7 | <0.001 | 24.1 | <0.001 | 2.893 | 1.076-7.775 | 0.035 | 1.645 | 0.572-4.734 | 0.356 |
| KRAS mutation | ||||||||||||
| Mutant | 23 | (29.5) | 52.2 | 47.8 | ||||||||
| Wild type | 55 | (70.5) | 70.6 | 0.473 | 63.4 | 0.430 | ||||||
| Aromatase | ||||||||||||
| Low expression | 43 | (55.1) | 78.9 | 72.1 | 1.000 | - | - | 1.000 | - | - | ||
| High expression | 35 | (44.9) | 48.2 | 0.005 | 42.9 | 0.010 | 2.638 | 1.173-5.936 | 0.019 | 2.505 | 1.154-5.434 | 0.020 |
| ERα | ||||||||||||
| Negative | 76 | (97.4) | 65.5 | 59.1 | ||||||||
| Positive | 2 | (2.6) | 50.0 | 0.663 | 50.0 | 0.687 | ||||||
| ERβ | ||||||||||||
| Negative | 19 | (24.4) | 78.9 | 78.9 | 1.000 | - | - | |||||
| Positive | 59 | (75.6) | 60.5 | 0.079 | 52.3 | 0.031 | 4.013 | 1.219-13.207 | 0.022 | |||
| PR | ||||||||||||
| Negative | 75 | (96.2) | 65.0 | 58.5 | ||||||||
| Positive | 3 | (3.8) | 66.7 | 0.638 | 66.7 | 0.637 | ||||||
| HER2 | ||||||||||||
| Negative | 74 | (94.9) | 66.1 | 59.5 | ||||||||
| Positive | 4 | (5.1) | 50.0 | 0.060 | 50.0 | 0.133 | ||||||
| Ki-67 LI (%: median 11.5) | ||||||||||||
| Low score | 36 | (46.2) | 83.0 | 80.6 | 1.000 | - | - | 1.000 | - | - | ||
| High score | 42 | (53.8) | 49.5 | <0.001 | 40.1 | <0.001 | 1.634 | 0.501-5.334 | 0.416 | 2.239 | 0.752-6.666 | 0.148 |
OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; LI, labeling index.
Figure 3 shows the survival curves of 78 patients with EGFR wild-type lung adenocarcinoma according to aromatase and ERβ expression, respectively. Interestingly, differences in survival became clearer when patients were stratified by aromatase and ERβ expression. Patients with high aromatase expression had poor prognosis in both OS (Figure 3A; P=.005) and RFS (Figure 3B; P=.010). Patients with ERβ-positive also had poor prognosis in terms of RFS (Figure 3D; P=.031); an identical tendency was observed for OS (Figure 3C; P=.079). Furthermore, patients with high expression of aromatase and ERβ-positive had a poorer prognosis than patients with low expression of aromatase and ERβ-negative in terms of both OS (Figure 3E; P =.004) and RFS (Figure 3F; P=.002).
Figure 3.
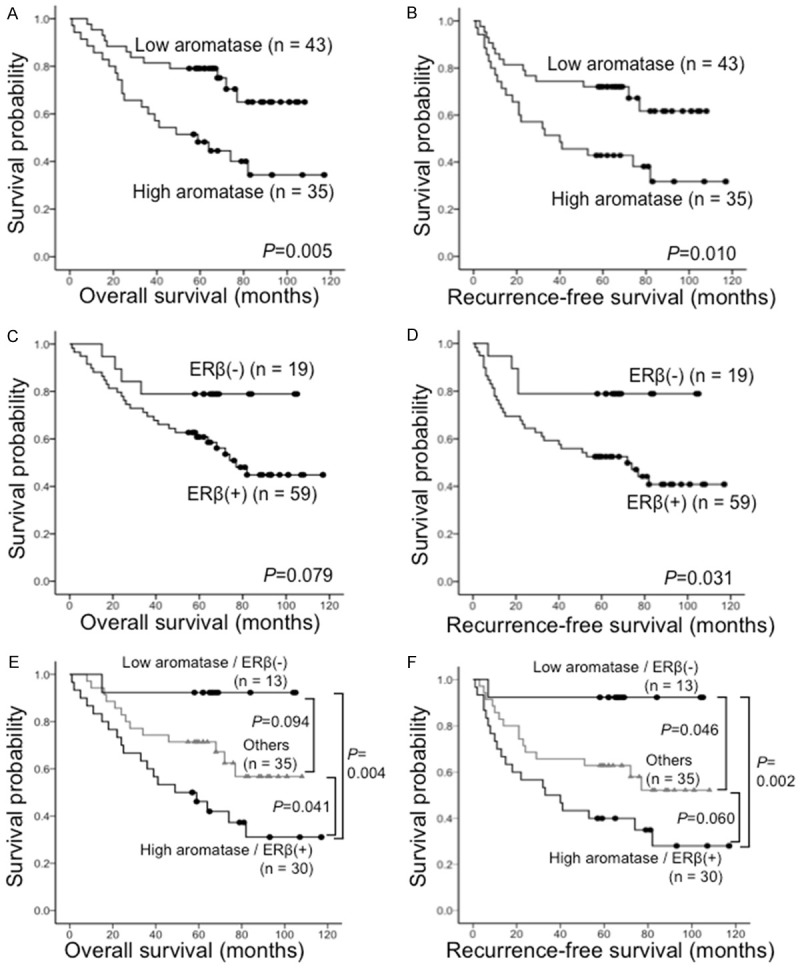
Kaplan-Meier survival curves of 78 patients with EGFR wild-type lung adenocarcinoma according to the immunoreactivity results for aromatase and ERβ. A. Overall survival (OS) stratified by high versus low expression of aromatase. B. Recurrence-free survival (RFS) stratified by high versus low expression of aromatase. C. OS stratified by positive versus negative expression of ERβ. D. RFS stratified by positive versus negative expression of ERβ. E. OS stratified by combined high expression of aromatase/positive expression of ERβ versus combined low expression of aromatase/negative expression of ERβ versus others. F. RFS stratified by combined high expression of aromatase/positive expression of ERβ versus combined low expression of aromatase/negative expression of ERβ versus others.
Conversely, no significant difference was noted in the survival of patients with EGFR mutant lung adenocarcinoma according to aromatase and ERβ expression (Table S2 and Figure S1).
Survival analysis according to sex in patients with EGFR wild-type lung adenocarcinoma
Next, we performed a survival analysis stratified by sex to clarify the prognostic impact of hormonal effect on sex in patients with EGFR wild-type lung adenocarcinoma. Figure 4 shows the RFS curves of males and females with EGFR wild-type lung adenocarcinoma according to aromatase and ERβ expression, respectively. High aromatase expression was significantly associated with poor prognosis only in females (Figure 4B; P=.007), whereas ERβ-positive had a tendency for poor prognosis only in males (Figure 4C; P=.051). Furthermore, patients with high expression of aromatase and ERβ-positive had a poorer prognosis than patients with low expression of aromatase and ERβ-negative, but only among females (Figure 4F; P=.008).
Figure 4.

Kaplan-Meier survival curves of male and female patients with EGFR wild-type lung adenocarcinoma according to the immunoreactivity results for aromatase and ERβ. A. Recurrence-free survival (RFS) of males stratified by high versus low expression of aromatase. B. RFS of females stratified by high versus low expression of aromatase. C. RFS of males stratified by positive versus negative expression of ERβ. D. RFS of females stratified by positive versus negative expression of ERβ. E. RFS of males stratified by combined high expression of aromatase/positive expression of ERβ versus combined low expression of aromatase/negative expression of ERβ versus others. F. RFS of females stratified by combined high expression of aromatase/positive expression of ERβ versus combined low expression of aromatase/negative expression of ERβ versus others.
Discussion
Understanding the role of estrogen and EGFR pathways in lung adenocarcinoma is necessary to develop new preventative and treatment strategies. We report here for the first time that aromatase and ERβ expression are independent, unfavorable prognostic factors in EGFR wild-type lung adenocarcinoma. Furthermore, regarding EGFR wild-type lung adenocarcinoma, we showed that high aromatase expression was a significant predictor of poor survival only in females, whereas ERβ expression was an important predictor of poor survival only in males. These observations indicate that these pathways are important to a different extent in males and females with lung adenocarcinoma.
In our study, PR was detected only in 2.7%. The rate of PR expression in lung tumors varies among reports, ranging from no expression to marginal (22%-35%) or even high expression (39%-63%) [40]. This difference may be related to the antibody used in each study. The antibody we used is a representative PR antibody widely used for breast cancer research and to guide clinicians’ choice of therapy. However, its use remains limited for lung tumors, and the most appropriate antibody for PR staining in lung tumors is yet to be identified.
Recent studies linking ER expression status with EGFR mutation have suggested that considering these signaling pathways together may provide important insight into lung cancer biology [31,41-42]. Thus, we analyzed the association of aromatase/ERβ expression with EGFR mutation status. Interestingly, our subgroup analysis showed a significant difference in OS and RFS according to aromatase expression, and a significant difference in RFS according to ERβ expression, only in EGFR wild-type lung adenocarcinoma.
Mah et al. reported that low aromatase expression was found to be associated with favorable survival in female NSCLC patients in the United States [13]. However, in reports based on Asian populations, aromatase expression had no association with prognosis, although aromatase was expressed in more than 60% of lung cancer patients [14,27]. The result of Mah’s report [13] is consistent with that of ours, although those of later reports are not. However, those previous studies grouped adenocarcinoma and other histological types of NSCLC together. Furthermore, EGFR mutation status was not analyzed in those studies. The precise mechanism underlying the worse survival regarding the association of aromatase expression with EGFR mutation status in our study is unknown. However, the frequency of EGFR mutations in NSCLC varies among races: from 27 to 60% in Asians and from 8 to 16% in Europeans, Africans, and Caucasian Americans [43-44]. Based on these observations and our findings, we speculate that the prognostic discrepancy in relation to the aromatase expression level among previous reports might be attributable to the difference in EGFR mutation status in each study.
To further examine aromatase and ERβ as a predictor of survival, and to assess the importance of sex, we analyzed the association between aromatase/ERβ expression and sex in EGFR wild-type patients according to survival. As expected, our result showed that high aromatase expression was a significant predictor of poor survival only in females, and we found no predictive value for aromatase expression levels in males. By contrast, our result showed that ERβ-positive was a significant predictor of poor survival only in males, and we found no predictive value for ERβ expression levels in females.
The differences in these sex-related results seem to depend on the difference in the status of, and sensitivity to, reproductive hormones according to sex. In postmenopausal females, circulating estrogen levels are decreased due to the decline in estrogen production by the ovaries [45]. Under these conditions, local estrogen production through aromatase might be an important determinant of estrogen levels. In fact, Niikawa et al. demonstrated that the intratumoral estradiol concentration was significantly higher in NSCLC than in nonneoplastic lung tissue and was positively correlated with intratumoral aromatase expression [46]. In our study, we did not distinguish females according to menopause status because only one of the females with EGFR wild-type lung adenocarcinoma was premenopausal. In other words, our result indicated that aromatase expression level is prognostic factor in postmenopausal females with EGFR wild-type lung adenocarcinoma. Therefore, intratumoral aromatase expression is associated with tumor progression via estrogen signaling pathway in postmenopausal females, particularly in EGFR wild-type lung adenocarcinoma.
In contrast to females, circulating estrogen levels in males are almost the same as those in postmenopausal females, and this condition remains relatively constant with age [47]. Martin et al. demonstrated that ER-positive breast cancer cells are hypersensitive to low doses of estrogen with long-term estrogen deprivation [48]. Furthermore, in males, a higher level of androgen, which is a substrate of estrogen synthesis, is present than in females [47]. Based on these observations, at low expression levels, aromatase may produce an amount of estrogen sufficient for proliferation of ER-positive cells. In the present study, 92.1% of lung adenocarcinoma expressed aromatase at lower levels in males. Therefore, these findings support our hypothesis that lung adenocarcinoma in males supplies sufficient levels of estrogen to activate ER for tumor cell maintenance, and that the level of tumor proliferation activity-mediated via estrogen signaling pathway-is more dependent on ERβ than aromatase expression in males.
The role of AIs in lung adenocarcinoma is unclear. However, many studies have reported that AIs demonstrated significant anti-tumor effects in NSCLC expressing aromatase both in vitro and in vivo [12,16,33,46,49]. These observations and our findings revealed that EGFR wild-type lung adenocarcinoma patients with high aromatase expression are a suitable subset for AI treatment, particularly in postmenopausal females. Currently, a phase I clinical trial of the irreversible steroidal AI exemestane in combination with chemotherapy for late-stage lung cancer in postmenopausal females is underway (NCT01664754). We are awaiting the results of this phase I study, which may reveal that AI treatment is effective for lung cancer in postmenopausal females.
Stabile et al. reported that increased EGFR signaling might be caused by depletion of estrogen signals induced by endocrine therapy, and targeting both pathways could be beneficial for therapy [34]. A phase II trial of erlotinib (EGFR-TKI) or erlotinib + fulvestrant in previously treated male and female advanced NSCLC has been completed [Garon EB, Siegfried JM, Dubinett SM, Elashoff RM, Park DJ, Parikh RJ, Patel R, Hu EH, Reckamp KL, Adams B, Martinez D, Wang HJ, Kabbinavar F, Dacic S, Brennan M, Laux I, Márquez-Garban DC, Stabile LP, Slamon DJ, Pietras RJ. Results of TORI-L-03, a randomized, multicenter phase II clinical trial of erlotinib (E) or E + fulvestrant (F) in previously treated advanced non-small cell lung cancer (NSCLC). Presented at the 104th Annual Meeting of the American Association for Cancer Research; Washington DC. PA, April 6-10, 2013. p. Abstract 4664; Unpublished results]. Interestingly, the clinical benefit rate was significantly higher among patients treated with the combination compared with erlotinib alone among patients with EGFR wild-type tumors, although the survival and response rates were similar between the two treatment arms in unselected patients. The latter finding supports our suggestion that estrogen signaling pathway plays an important role in the development of EGFR wild-type lung adenocarcinoma. Thus, endocrine therapy could also be beneficial for EGFR wild-type lung adenocarcinoma as a combination therapy with EGFR-TKI.
The limitations of present study include selection of antibodies, the retrospective design, and relatively small number of patients, all of whom were Japanese. Thus, large, population-based prospective studies with ethnically diverse populations are warranted to elucidate the role of growth factor pathways, including EGFR and/or estrogen signaling pathways, in lung adenocarcinoma.
In conclusion, we demonstrated that aromatase and ERβ expressions are independent negative prognostic factors in EGFR wild-type lung adenocarcinoma and that high aromatase expression is a significant predictor of poor survival only in females, whereas ERβ-positive is an important predictor of poor survival only in males. We suggest that EGFR wild-type lung adenocarcinoma is a hormone-related carcinoma.
Acknowledgements
We thank Toshiaki Hikino (Division of Diagnostic Pathology, Graduate School of Medicine, Gunma University) for skillful technical assistance. The first author was supported by Grant-in-Aid for Young Scientists (B) (24791454) from Japan Society for the Promotion of Science (JSPS).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J. Clin. Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 3.Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H. The increasing incidence of lung adenocarcinoma: reality or artifact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;26:14–23. doi: 10.1093/ije/26.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Lam B, Lam WK, Lam CL, Ooi GC, Ho JCM, Wong MP, Tsang KW. Adenocarcinoma of the lung in Chinese patients: a revisit and some perspectives from the literature. Postgrad Med J. 2001;77:708–712. doi: 10.1136/pmj.77.913.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakegawa S, Shimizu K, Sugano M, Miyamae Y, Kaira K, Araki T, Nakano T, Kamiyoshihara M, Kawashima O, Takeyoshi I. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer. 2011;117:4257–4266. doi: 10.1002/cncr.26010. [DOI] [PubMed] [Google Scholar]
- 6.Verma MK, Miki Y, Sasano H. Aromatase in human lung carcinoma. Steroids. 2011;76:759–764. doi: 10.1016/j.steroids.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor α and β and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 8.Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human nonsmall cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–1605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 9.Omoto Y, Kobayashi Y, Nishida K, Tsuchiya E, Eguchi H, Nakagawa K, Ishikawa Y, Yamori T, Iwase H, Fujii Y, Warner M, Gustafsson JÅ, Hayashi S. Expression, function, and clinical implications of the estrogen receptor β in human lung cancers. Biochem Biophysl Res Commun. 2001;285:340–347. doi: 10.1006/bbrc.2001.5158. [DOI] [PubMed] [Google Scholar]
- 10.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors α and β in human lung tissues and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 11.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–344. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg OK, Márquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 13.Mah V, Seligson DB, Li A, Márquez DC, Wistuba II, Elshimali Y, Fishbein MC, Chia D, Pietras RJ, Goodglick L. Aromatase expression predicts survival in women with early-stage non-small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe K, Miki Y, Ono K, Mori M, Kakinuma H, Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S, Evans DB, Sugawara S, Suzuki T, Sasano H. Highly concordant coexpression of aromatase and estrogen receptor β in non-small cell lung cancer. Hum Patol. 2009;41:190–198. doi: 10.1016/j.humpath.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson JÅ. Estrogen receptor beta - a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- 16.Miki Y, Suzuki T, Abe K, Suzuki S, Niikawa H, Iida S, Hata S, Akahira J, Mori K, Evans DB, Kondo T, Yamada-Okabe H, Sasano H. Intratumoral localization of aromatase and interaction between stromal and parenchymal cells in the non-small cell lung carcinoma microenvironment. Cancer Res. 2010;70:6659–6669. doi: 10.1158/0008-5472.CAN-09-4653. [DOI] [PubMed] [Google Scholar]
- 17.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Retal Cancer. 2004;11:537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skliris GP, Leygue E, Watson PH, Murphy LC. Estrogen receptor α negative breast cancer patients: estrogen receptor β as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109:1–10. doi: 10.1016/j.jsbmb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Edén P, Peterson C, Malmström P, Isola J, Borg Å, Fernö M. Estrogen receptorβ expression is associated with tamoxifen response in ERα-negative breast carcinoma. Clin Cancer Res. 2007;13:1987–1994. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Weihua Z, Warner M, Gustafsson JÅ. Estrogen receptors ERα and ERβ in proliferation in the rodent mammary gland. Proc Natl Acad Sci U S A. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptor β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JÅ, Hayashi S. Estrogen receptor β is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319–324. doi: 10.1007/s00432-002-0336-3. [DOI] [PubMed] [Google Scholar]
- 23.Wu CT, Chang YL, Shih JY, Lee YC. The significance of estrogen receptor β in 301 surgically treated non-small cell lung cancers. J Thoracic Cardiovasc Surg. 2005;130:979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, Brooks S. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 25.Skov BG, Fisher BM, Pappot H. Oestrogen receptor beta over expression in males with nonsmall cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Stabile LP, Dacic S, Land SR, Lenzner DE, Dhir R, Acquafondata M, Landreneau RJ, Grandis JR, Siegfried JM. Combined analysis of estrogen receptor β-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17:154–164. doi: 10.1158/1078-0432.CCR-10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma MK, Miki Y, Abe K, Nagasaki S, Niikawa H, Suzuki S, Kondo T, Sasano H. Co-expression of estrogen receptor beta and aromatase in Japanese lung cancer patients: Gender-dependent clinical outcome. Life Sci. 2012;91:800–808. doi: 10.1016/j.lfs.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 30.Miki Y, Abe K, Suzuki S, Suzuki T, Sasano H. Suppression of estrogen actions in human lung cancer. Mol Cell Endocrinol. 2011;340:168–174. doi: 10.1016/j.mce.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, Onitsuka T, Yasumoto K. Association between estrogen receptor-β expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J. Clin. Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 32.Pietras RJ, Márquez DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 33.Koutras A, Giannopoulou E, Kritikou I, Antonacopoulou A, Evans TR, Papavassiliou AG, Kalofonos H. Antiproliferative effect of exemestane in lung cancer cells. Mol Cancer. 2009;8:109. doi: 10.1186/1476-4598-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 35.Shen H, Yuan Y, Sun J, Gao W, Shu YQ. Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomed Pharmacother. 2010;64:88–92. doi: 10.1016/j.biopha.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Leslie Sobin MG CW, editor. TNM Classification of Malignant Tumors. 7th Ed. Geneva: UICC International Union Against Cancer; 2009. pp. 136–146. [Google Scholar]
- 37.Araki T, Shimizu K, Nakamura T, Baba M, Kawai Y, Nakamura K, Mitani Y, Obayashi K, Aomori T, Fujita Y, Miyamae Y, Kakegawa S, Kaira K, Lezhava A, Hayashizaki Y, Takeyoshi I, Yamamoto K. Clinical screening assay for EGFR exon 19 mutations using PNA-clamp smart amplification process version 2 in lung adenocarcinoma. Oncol Rep. 2011;26:1213–1219. doi: 10.3892/or.2011.1391. [DOI] [PubMed] [Google Scholar]
- 38.Miyamae Y, Shimizu K, Mitani Y, Araki T, Kawai Y, Baba M, Kakegawa S, Sugano M, Kaira K, Lezhava A, Hayashizaki Y, Yamamoto K, Takeyoshi I. Mutation detection of epidermal growth factor receptor and KRAS genes using the smart amplification process version 2 from formalin-fixed, paraffin-embedded lung cancer tissue. J Mol Diagn. 2010;12:257–264. doi: 10.2353/jmoldx.2010.090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 40.Kishi S, Yokohira M, Yamakawa K, Saoo K, Imaida K. Significance of the progesterone receptor and epidermal growth factor receptor, but not the estrogen receptor, in chemically induced lung carcinogenesis in female A/J mice. Oncol Letters. 2014;8:2379–2386. doi: 10.3892/ol.2014.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, Ozburn NC, Woods DM, Tang X, Mehran RJ, Moran C, Lee JJ, Wistuba II. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toh CK, Ahmad B, Soong R, Chuah KL, Tan SH, Hee SW, Leong SS, Tan EH, Lim WT. Correlation between epidermal growth factor receptor mutation and expression of female hormone receptors in East-Asian lung adenocarcinomas. J Thorac Oncol. 2010;5:17–22. doi: 10.1097/JTO.0b013e3181c0a602. [DOI] [PubMed] [Google Scholar]
- 43.Tam IYS, Chung LP, Suen WS, Wang E, Wong MCM, Ho KK, Lam WK, Chiu SW, Girard L, Minna JD, Gazdar AF, Wong MP. Distinct epidermal growth factor receptor and KRAS mutation patterns in nonsmall cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 44.Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure MJ, Sidransky D. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 45.Folkerd EJ, Martin LA, Kendall A, Dowsett M. The relationship between factors affecting endogenous oestradiol levels in postmenopausal women and breast cancer. J Steroid Biochem Mol Biol. 2006;102:250–255. doi: 10.1016/j.jsbmb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 46.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, Honma S, Evans DB, Hayashi S, Kondo T, Sasano H. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–4426. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 48.Martin LA, Farmer I, Johnston SRD, Ali S, Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocrine-Related Cancer. 2005;12:S75–S84. doi: 10.1677/erc.1.01023. [DOI] [PubMed] [Google Scholar]
- 49.Márquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann NY Acad Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


