Abstract
Marine protected areas can prevent over-exploitation, but their effect on marine diseases is less clear. We examined how marine reserves can reduce diseases affecting reef-building corals following acute and chronic disturbances. One year after a severe tropical cyclone, corals inside reserves had sevenfold lower levels of disease than those in non-reserves. Similarly, disease prevalence was threefold lower on reserve reefs following chronic exposure to terrestrial run-off from a degraded river catchment, when exposure duration was below the long-term site average. Examination of 35 predictor variables indicated that lower levels of derelict fishing line and injured corals inside reserves were correlated with lower levels of coral disease in both case studies, signifying that successful disease mitigation occurs when activities that damage reefs are restricted. Conversely, reserves were ineffective in moderating disease when sites were exposed to higher than average levels of run-off, demonstrating that reductions in water quality undermine resilience afforded by reserve protection. In addition to implementing protected areas, we highlight that disease management efforts should also target improving water quality and limiting anthropogenic activities that cause injury.
Keywords: coral disease, marine protected areas, no-take reserve, pollution run-off, resilience, water quality
1. Introduction
Mitigating disease threats is challenging in marine environments [1–4]. Managers confronted with controlling disease outbreaks on land have several tools available, including quarantine and culling to restrict contact between healthy and infected individuals, vaccination, chemical and biological controls, vector elimination or regulation, and genetic breeding for disease resistance or tolerance [5–7]. However, inherent difficulties associated with implementing such disease control methods in natural aquatic environments limit their applicability for marine species [3,4].
Multiple threats have degraded reefs around the world [8], jeopardizing the US$375 billion in goods and services coral reefs provide each year [9]. Degradation of coral reefs worldwide has led to widespread establishment of marine protected areas. Protected areas might influence disease in coral populations, although influences could be either beneficial or detrimental to coral health. For example, protected areas might facilitate the spread of disease by increasing densities or cover of susceptible coral hosts [10,11], or by increasing densities of fishes that are either vectors for coral pathogens or cause feeding injuries that increase coral susceptibility to opportunistic pathogens [12,13]. Conversely, high densities of herbivorous fish within reserves could limit the growth of algae [14], which have been reported to act as reservoirs of pathogens on reefs in the Caribbean and Indo-Pacific [15,16]. Moreover, exclusion of activities that injure corals inside marine reserves, for example, fishing methods and gears that directly damage corals (e.g. [17,18]) and high-intensity tourism [19,20], is likely to mitigate disease by reducing entry points for opportunistic coral pathogens [20–23].
Evidence from studies testing the efficacy of marine reserves as management tools for preventing disease in coral populations varies. For example, no-take marine reserves have been shown to mitigate coral disease by maintaining functionally diverse fish assemblages [13] and by reducing direct damage associated with fishing activities and derelict gear [24]. However, other studies have found little evidence that protected areas mitigate coral disease [25–27], although authors have cautioned that either poor compliance with fishing restrictions or the presence of environmental influences that permeate reserve borders could have negated reserve effectiveness in their studies. In the light of global efforts to increase marine protected areas, evaluation of their potential to enhance the health of reef-building corals and their resilience to major disturbances is required.
The effect of marine reserves on infectious disease depends on whether disease is driven by density-dependent transmission or environmental stressors. Protection from fishing and other extractive activities can benefit targeted populations and enhance biodiversity within marine reserves [28,29]. As a result, some infectious agents can thrive within dense populations of protected species [30] and selective culling of diseased individuals may benefit the fishery [31]. Alternatively, the complex interactions within food webs mean that protection of predators in reserves can decrease prey densities, which can indirectly reduce epidemics in those prey [32]. It is less clear if reserves are effective at alleviating stressors beyond reserve boundaries, such as extreme climate events or pollutants. Proponents have assumed that marine protected areas enhance the resilience of populations to such stressors because of overall improved health of the ecosystem (e.g. [33,34], but see [35]); however, studies testing the ability of protected areas to defend marine populations following acute and chronic environmental disturbances have reported mixed outcomes [34,36–39]. Studies that have reported lower levels of disease inside reserves suggest that protected populations may be more resilient to additional disturbances that transcend reserve boundaries than previously assumed, although this has never been explicitly tested.
Some disease epizootics have been linked to both acute and chronic environmental stressors. For example, the passage of intense tropical storms is associated with subsequent disease in organisms as diverse as plants [40], urchins [41] and reef corals [42]. Furthermore, other marine disease outbreaks are linked to chronic exposure to pollutants, such as sewage [43], terrestrial sediment or agricultural herbicides [44,45], nutrients and fertilizers [46,47], and aquaculture [48]. Given that pressures on marine biodiversity are projected to escalate adjacent to polluting population centres and coastlines [9] and with predicted increases in the number of intense tropical storms [49], it is imperative to further assess the potential role protected areas could play in managing marine diseases subject to environmental disturbances.
Here, we evaluate whether assemblages of coral diseases differ inside versus outside well-established no-take marine reserves in the Great Barrier Reef Marine Park 1 year following a severe tropical cyclone (acute disturbance) and annual flood run-off from a degraded coastal catchment (chronic disturbance).
2. Material and methods
(a). Study locations and protected areas management
We conducted this study on fringing inshore coral reefs in the Palm Islands (18°34′ S, 146°29′ E) and Keppel Islands (23°10′ S, 150°57′ E), both of which are located between approximately 12 and 15 km from the mainland of Australia (figure 1). The islands and surrounding reefs are popular for reef recreation in the Great Barrier Reef Marine Park [50]; thus recreational hook and line fishing pressure on the narrow fringing reef communities is high [51]. In each island group, reefs in two management zones (characterized as reserves or non-reserves) were surveyed to assess the efficacy of reserves as tools for mitigating coral disease (figure 1). Marine National Parks (MNP) are no-take reserves (‘reserves’) where extractive activities, including fishing and collecting, are prohibited. Habitat Protection (HP) zones (non-reserves) are open to hook and line fishing, spear fishing and collecting. Reserves surveyed in this study were zoned in 1987 (25 years of protection at the time of the surveys), while non-reserve study sites have always been open to fishing. Additional comprehensive regulations and protection for each zone can be found in the electronic supplementary material and at www.gbrmpa.gov.au.
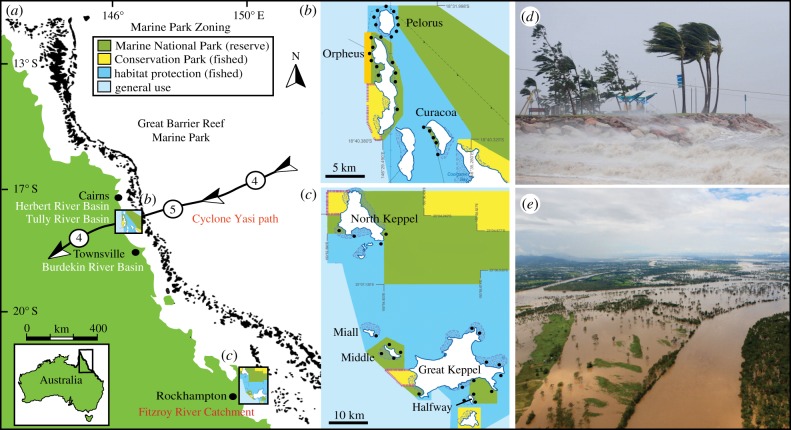
Figure 1.
Locations of 47 reef sites surveyed in two regions of the (a) Great Barrier Reef Marine Park, Australia. Surveys were conducted in the (b) Palm Islands in 2012, 1 year following an acute tropical cyclone; and (c) Keppel Islands in 2013, two months following 14 weeks of chronic exposure to terrestrial flooding from the Fitzroy River Catchment. Areas shaded in green are no-take reserves and represent 33% of the Marine Park; areas shaded in dark blue are open to fishing and comprise 28% of the Marine Park. Activities permitted in each zone are listed in the electronic supplementary material (table S1) and at www.gbrmpa.gov.au. Wind gusts and modelled cyclone path (arrow direction) obtained from the Australian Bureau of Meteorology (www.bom.gov.au), with tropical cyclone intensity category indicated at three time points in the open circles between arrows highlighting the cyclone path. Photographs illustrate (d) coastal wind and wave intensity during Cyclone Yasi in February 2011 and (e) severe flooding of the Fitzroy River Basin in January 2011. Photos: Jonathan Wood and Ian Hitchcock (Getty Images).
In the Palm Islands, we surveyed 14 long-term survey sites within MNP reserves (42 transects) and 12 long-term HP sites open to fishing (36 transects) during the middle of the austral summer in February 2012 (figure 1b). To aid in distinguishing between disturbance caused by the cyclone and longer term disturbances caused by recreational activities, sites were further categorized as either windward sites that were exposed to cyclonic winds or leeward sites that were sheltered from wave damage (see [52]). There were eight leeward and six windward MNP sites and six HP sites in each leeward and windward location. These surveys were conducted approximately 12 months after the passage of a severe category 5 cyclone in early February 2011 (see [52,53] for specific details of Cyclone Yasi and figure 1d).
In the Keppel Islands, we surveyed 11 long-term monitoring sites within MNP reserves (33 transects) and 10 long-term HP sites open to fishing (22 transects) at the end of the austral autumn in May 2013 (figure 1c). The adjacent Fitzroy River catchment is prone to periodic, large-scale flooding events [54], with flood plumes inundating Keppel Island reefs [36] (figure 1e). These surveys were conducted two months following 14 weeks of exposure to flood plumes and subsequent primary productivity from flooding of the degraded Fitzroy River watershed (137 757 km2). Additional specific details of the flood plumes are described in [36].
(b). Coral disease surveys and visual census of reef fishes
At each of the 47 sites, we surveyed coral health on three 15 × 2 m belt transects. Transects corresponded to the first 15 m of concurrent transects for underwater visual census (UVC) of fish communities (briefly described below). Within each 30 m2 belt transect, we identified each coral colony over 5 cm in diameter to genus and further classified each coral as either healthy (no disease observed) or affected by one or more of six common Indo-Pacific coral diseases: black band disease, skeletal eroding band, brown band disease, white syndromes, atramentous necrosis or growth anomalies [55]. As an estimate of the intensity of site use, we recorded other indicators of coral health, such as physical injury (recently exposed skeleton from breakage), the abundance and health status of corals entangled in derelict monofilament fishing line, apparent tissue death due to sediment accumulation, bleaching, and cuts and scars from predation by crown-of-thorns starfish and corallivorous marine snails [19,20,55]. We determined benthic coral and macroalgal cover using standard line-intercept surveys along each 15 m transect.
We used a modified UVC technique to survey 261 species of diurnal, non-cryptic reef fish, from 17 families, according to methods described in [24,39] and the electronic supplemental methods. Briefly, we deployed five replicate belt transects at each site on reef slopes, parallel to the reef crest and within a depth range of 3–10 m, depending on the reef slope topography at each site. We conducted fish community UVC surveys on SCUBA, using two observers who swam in close proximity to each other. The first three transect tapes deployed at each site at the completion of the fish UVC were left in place for the coral community and disease surveys, described above.
(c). Water quality exposure frequency
In both the Palm Islands and Keppel Island region, we mapped water types and flood plumes using Moderate Resolution Imaging Spectroradiometer (MODIS) true colour satellite imagery [56]. We acquired daily MODIS Level-0 data from the NASA Ocean Color website (http://oceancolor.gsfc.nasa.gov) and converted them into true colour images with a spatial resolution of 500 × 500 m, using the SeaWiFS Data Analysis System (SeaDAS) [57]. We created 22 weekly composite images from daily images covering the period 1 December to 30 April in wet seasons for each of 8 years (2006–2013) to minimize the amount of area without data due to masking by clouds [56]. We spectrally enhanced the true colour images (from red-green-blue to hue-saturation-intensity colour system), and classified them into plume water types corresponding to one of three GBR water types (primary, secondary and tertiary) through a supervised classification using spectral signatures from GBR river plume waters [54,58].
For the purposes of this study, we applied the ‘primary’ and ‘secondary’ water type classifications to quantify the frequency at which monitoring sites were exposed to highly turbid water from flood plumes and subsequent re-suspension during the 2006–2013 wet seasons (December–April inclusive). The primary water type represents high turbidity [54], as typically found close to river outflows, with high levels of coloured dissolved organic matter and total suspended sediments (TSS) [59]. TSS and the diffuse attenuation coefficient for photosynthetically active radiation (kdPAR) in the primary water type are typically approximately 36.8 ± 5.5 mg l−1 and 0.73 ± 0.54 m−2, respectively [56]. The secondary water type is typically found more distant to river outflows and is characterized by elevated chlorophyll-a concentrations, with TSS concentrations reduced due to sedimentation closer to coastlines. We assigned each of the 22 weekly composite images per wet season (2006–2013) a presence/absence (0/1) value for the primary and secondary water types in each pixel (500 × 500 m resolution). We then calculated a z-score to capture the frequency of exposure to primary and secondary water during surveys in 2013, relative to the mean primary and secondary water exposure each site had experienced in the years 2006–2013. A z-score value above zero means that exposure for the 14 weeks (wet season) leading up to surveys in 2013 was greater than the 2006–2013 mean for the site, while a z-score below zero means that exposure was lower than the 2006–2013 mean for the site.
(d). Statistical modelling
We used a multivariate distance-based linear regression model [60] to identify the strength and significance of the relationships between the prevalence and patterns of coral disease types and 35 predictor variables (electronic supplementary material, table S2). This model is robust to zero-inflated datasets and makes no assumptions about the distribution of the response variable. We calculated coral disease prevalence (response variable) for each 30 m2 belt transect by dividing the number of colonies with disease or other signs of compromised health by the total number of colonies present. Biodiversity indices included as predictor variables in the model were calculated according to the lowest taxonomic group, using the total number of individuals surveyed per transect area (coral genera 30 m−2 and fish species 200 m−2). Prior to inclusion in the model, we grouped each fish species into one of 12 broad functional roles in coral reef habitats [39] (electronic supplementary material, table S3). Fish abundance values were down-weighted using a fourth-root transformation to account for clumped distributions of abundant schooling species [61]. As regression-based models can be sensitive to variables that are correlated, variables with correlations greater than 0.70 were identified using draftsman's plots and excluded from the final analysis (excluded variables are specified in electronic supplementary material, table S2 [62]). Individual predictors were transformed on a case-by-case basis to meet test assumptions and then fitted conditionally in a stepwise manner using tests based on 9999 permutations of the residuals under the reduced model [60,61]. Owing to the large number of predictor variables, we based model selection (to obtain the best-fit model while maintaining model parsimony) on Bayesian information criterion (BIC) [63].
To visualize each best-fit model, we used distance-based redundancy analysis (dbRDA) [60] based on the prevalence patterns between independent observations. The optimal predictor variable vector(s) (model base variables) was overlaid on a bi-plot [61]. In addition, variables that might be responsible for any differences detected in the dbRDA plots were investigated by calculating Pearson correlations with redundancy analysis axes. Differences in the structure of coral disease assemblages between reserves and non-reserves were detected using a permutational multivariate analysis of variance [61]. The analysis was based on a type III partial sums of squares, and 999 random permutations of the residuals under the reduced model. Where differences in disease assemblages were detected, the type contributing the most to the dissimilarity was identified using similarity of percentages analyses. Multivariate analyses and modelling were based on zero-adjusted Bray–Curtis similarity or Euclidean distance matrices and analyses performed using Primer v. 6 [61].
Significant variables identified in the multivariate distance-based linear model were compared between reserve and non-reserve sites using a Welch's unequal variance t-test or an exact Poisson test. Differences between reserve status and coral disease prevalence were analysed using generalized linear mixed-effects models with a binomial error distribution and logit link function (glmer function of the lme4 package). Reserve status was treated as a fixed effect, while transect was nested into site and treated as a random effect. We compared each model to the null model using a likelihood ratio test. Pairwise post hoc comparisons between groups were tested using general linear hypothesis tests (glht function in multicomp package). Analyses were conducted using R v. 3.2.3 [64].
3. Results
Surveys of 36 104 corals at 47 sites (4230 m2 reef area) revealed that assemblages of coral diseases (assessed by composition and abundance) differed significantly between reserves and non-reserves, 1 year following the acute disturbance of the tropical cyclone passing over the Palm Islands (mean dissimilarity of disease assemblage = 62.2%, pseudo-F1,24 = 16.4, p < 0.001; figure 2) and two months following a chronic disturbance caused by terrestrial run-off from flooding of the degraded watershed adjacent to the Keppel Islands (mean dissimilarity of disease assemblage = 53.9%, pseudo-F1,19 = 4.0, p = 0.03; figure 3).
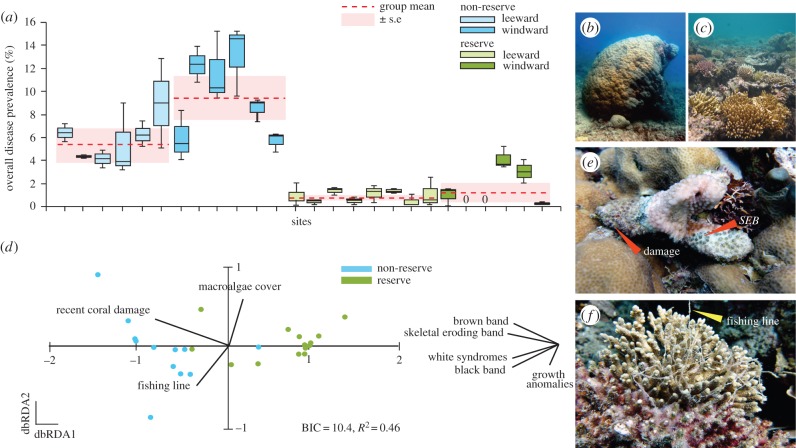
Figure 2.
Comparisons of (a) coral disease prevalence among reserve (light and dark green) and non-reserve (light and dark blue) sites in the Palm Islands 1 year following a severe tropical cyclone. Surveys were conducted on both (b) windward and (c) leeward reef sites. Each box and solid line represents the first, third and second quartiles, respectively, while whiskers indicate data range. Dashed line (red) represents group means ± s.e. (pink). Letters represent significant differences among groups using a generalized linear hypothesis test with pairwise comparisons and Bonferroni correction. (d) Visual representation of similarities among coral disease assemblages between reserve and non-reserve sites using a distance-based redundancy analysis (dbRDA). Vectors depict significant environmental and biological variables (electronic supplementary material, table S2) forming the best-fit model identified using Bayesian information criterion (BIC). Disease vectors to the right of each panel represent coral diseases super-imposed on the ordination as vectors (Pearson correlations with vectors offset for ease of distinguishing them from the main panels). The length and direction of the vectors represent the strength and direction of the relationship. dbRDA1=86.7% fitted and 39.8% total model variation; dbRDA2=11.7% fitted and 5.4% total model variation. Photographs of (e) skeletal eroding band (SEB) disease affecting a damaged coral fragment and (f) a coral colony entangled in derelict fishing line.
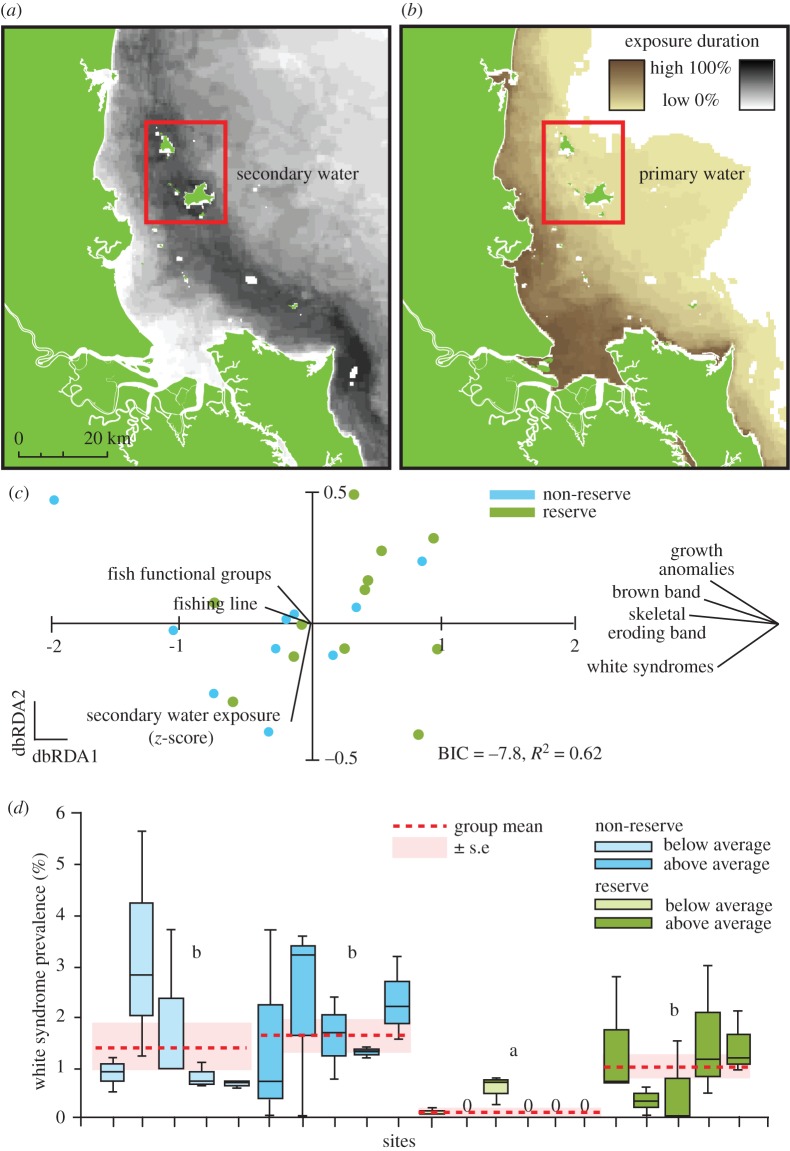
Figure 3.
Frequency of exposure to (a) secondary water and (b) primary water in the Keppel Islands (red boxes) during the 2013 wet season (December–April inclusive). (c) Distance-based redundancy analysis (dbRDA), indicating the similarity in coral disease assemblages among reserve and non-reserve sites two months following chronic exposure to terrestrial water run-off. See figure 2 for model and vector descriptions. dbRDA1 = 82.6% fitted and 48.0% total model variation; dbRDA2 = 13.2% and 7.4% total fitted model variation. Comparisons of (d) coral disease prevalence among reserve (light and dark green) and non-reserve (light and dark blue) sites 1 year following chronic exposure to secondary terrestrial water (calculated as z-score). See figure 2 for graph interpretation. (Note: secondary water z-score is calculated as the frequency of exposure to secondary water in 2013, relative to the secondary exposure annually since 2006. A z-score value above zero means that exposure for the 14 weeks (wet season) leading up to surveys in 2013 was greater than the 2006–2013 mean for the site, while a z-score below zero means that exposure was lower than the 2006–2013 mean for the site.)
(a). Influence of protected areas on coral disease following an acute disturbance
Overall, total coral disease levels at Palm Island sites 1 year following a severe cyclone were sevenfold lower inside reserves (mean ± s.e. = 1.0 ± 0.3%) than in non-reserves (7.4 ± 0.9%, z = −878.5, p < 0.001, sample size = 26 sites, figure 2a). Four of the five diseases recorded had significantly lower levels inside reserves: white syndromes (10-fold), brown band (sevenfold), skeletal eroding band (sixfold) and black band (fivefold lower; electronic supplementary material, table S4). No cases of atramentous necrosis were recorded at Palm Island sites. Within each of the reserve and non-reserve groups, total mean prevalence of the five diseases was not influenced by windward versus leeward location of sites when included as a fixed effect in in the model (Exposure: z = −1.4, p = 0.17; Exposure × Reserve Status: z = −0.56, p = 0.56), although sites located on the windward sides of islands had considerably greater apparent impacts from the cyclone than leeward sites (figure 2c,d). None of the sites were exposed to primary or secondary water run-off during the wet season surveyed (all water quality values = 0).
Three factors explained 45.9% of the variability in the structure of coral disease assemblages among sites in the Palm Islands: prevalence of recently exposed coral skeleton from physical damage, abundance of derelict fishing line and macroalgal cover (best-fit distance-based linear model, BIC = 10.4, R2 = 0.46, d.f. = 22; figure 2b). As visualized using the dbRDA, disease assemblages were clearly demarcated by reserve status, driven by correlations with coral damage and fishing line along axis 1 (Pearson correlations = −0.90 and −0.40, respectively), with significantly higher levels inside non-reserves (mean damage ± s.e. = 3.8 ± 0.7% versus 1.1 ± 0.4%; mean pieces of derelict fishing line ± s.e. = 1.7 ± 0.7 versus 0.6 ± 0.2 m−2; electronic supplementary material, table S7). All five coral disease types were closely associated with non-reserve sites (strong negative correlations with axis 1, Pearson r > 0.5). Partial correlations (ρ) with axis 1 revealed that higher levels of two ciliate diseases, skeletal eroding band disease (ρ = −0.67) and brown band disease (ρ = −0.60), in combination with white syndromes (ρ = −0.61) were predominantly driving the separation between reserve and non-reserve sites. This effect was best explained by higher levels of coral damage and the abundance of derelict fishing line at non-reserve sites (figure 2b,e).
Macroalgal cover did not differ significantly between reserves (1.1 ± 0.5%) and non-reserves (1.8 ± 1.0%, electronic supplementary material, table S7); however, strong correlations with axis 2 suggest that cover of macroalgae (r = 0.7) and the abundance of derelict fishing line (r = −0.6) structures coral disease assemblages across all sites, regardless of reserve status (figure 2d). A partial correlation (ρ) with axis 2 illustrates that black band disease (ρ = −0.33) is most strongly associated with higher levels of macroalgal cover and pieces of discarded fishing line across all sites (figure 2b,f).
(b). Influence of protected areas on coral disease following a chronic disturbance
The spatial extent of the secondary water plume in the Keppel Islands during the 2013 wet season was 4256 km2 (figure 3a), with sites exposed to secondary water for an average of 13.6 weeks out of a possible 22 weeks. The maximum spatial extent of the primary water plume during the 2013 wet season was 2861 km2 (figure 3b), with a mean weekly coverage of primary water of 5.4 weeks over the 8 years. We found no association between primary and secondary water exposure at the sites (r = 0.23, p > 0.05).
Although we recorded 50% fewer cases of disease inside reserves in the Keppel Islands, there was not a clear difference in total disease prevalence inside reserves (mean ± s.e. = 1.4 ± 0.7%) compared with non-reserves (3.4 ± 1.0%, z = −2.3, p = 0.02, sample size = 21 sites) in the Keppel Islands. We did not record any derelict fishing line in the 11 sites we surveyed inside protected areas, and we observed four diseases out of six commonly recorded in the Indo-Pacific (no cases of black band disease or atramentous necrosis). The abundance of derelict fishing line, number of fish functional groups represented, exposure to secondary water (z-score), and density of reef fish explained 61.8% of the variability in disease assemblages (best-fit distance-based linear model, BIC = −7.8, R2 = 0.56, d.f. = 17; figure 3c). Visualized differences in the structure and abundance of coral disease assemblages demonstrated a considerable separation between reserve and non-reserve sites (figure 3c). The differentiation between reserves and non-reserves was strongly linked to correlations with abundance of derelict fishing line and number of fish functional groups along axis 1 (Pearson correlations = −0.71 and −0.61, respectively). Of the two variables, levels of derelict fishing gear outside reserves were elevated compared with inside reserves (1.5 ± 0.4 m−2 versus 0, respectively); however, reserve status did not influence the mean number of fish functional groups represented (9.0 ± 0.4 m−2 versus 8.9 ± 0.3 per 200 m2, electronic supplementary material, table S7). Mirroring results in the Palm Islands, partial correlations (ρ) with axis 1 revealed that higher levels of the two ciliate diseases, skeletal eroding band disease (ρ = −0.82) and brown band disease (ρ = −0.68), in combination with white syndromes (ρ = −0.56) were predominantly driving the separation, which is best explained by higher abundances of derelict fishing line and fewer represented fish functional groups (figure 3c).
Reserve and non-reserve sites were not differentially exposed to secondary water (reserve mean z-score ± s.e. = 0.7 ± 0.2, non-reserve = 0.8 ± 0.2, electronic supplementary material, table S7); however, significant variation among sites within the respective zones (electronic supplementary material, table S4) and strong correlations with axis 2 suggest that secondary water exposure (r = −0.9) structures coral disease assemblages across all sites, regardless of reserve status (figure 3c). A partial correlation (ρ) with axis 2 illustrates that white syndromes (ρ = −0.4) were most strongly associated with reefs that experienced anomalous exposure levels to secondary water (figure 3c). Moreover, white syndromes comprised the dominant diseases observed in the Keppel Islands and were responsible for the greatest dissimilarity in disease assemblages between reserves and non-reserves (41.7%, similarity percentages analysis). We found threefold lower mean prevalence of white syndromes in reserves than in non-reserves for sites exposed to levels of secondary water that were lower than the long-term average (z-score < 0), whereas no difference in white syndromes was found between reserves and non-reserves for sites that experienced higher levels of secondary water exposure (figure 3d; electronic supplementary material, table S8).
4. Discussion
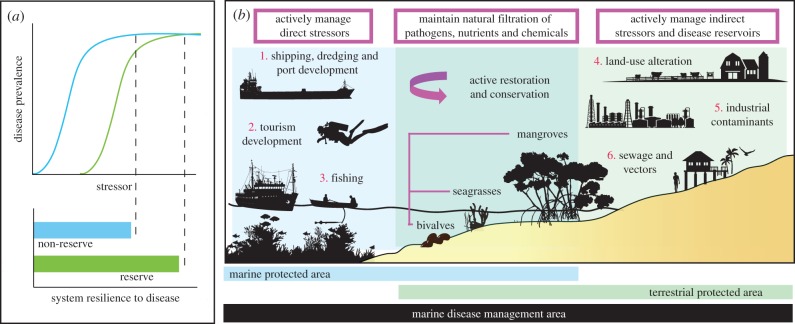
Our results show that no-take marine reserves can reduce coral disease following an acute disturbance from a severe cyclone and also following chronic exposure to terrestrial run-off when exposure duration was lower than the long-term site average. Conversely, reserves were not effective in lowering disease levels when sites were exposed to higher than average levels of run-off, indicating that reductions in water quality undermine the efficacy of protected areas to alleviate disease. Even though large-scale disturbances such as cyclones and run-off cannot be mitigated by reef protection itself, our results suggest that resilience afforded by complete protection from fishing activities can moderate coral reef health compared with areas that remain heavily fished (figure 4a). We suggest effective disease management efforts should target improving water quality and limiting anthropogenic activities that cause injury (figure 4b).
Figure 4.
Conceptual diagram illustrating (a) the results of this study and the potential for protected areas to moderate disease prevalence in marine species following exposure to unbounded environmental stressors (acute and chronic) by enhancing system resilience to disease and (b) active strategies to increase resistance and moderate known drivers of disease (red numbers) by integrating marine and terrestrial management areas.
Our analyses indicate that lower abundances of derelict fishing line and recently injured corals inside reserves were the primary mechanism for lower coral disease prevalence in both island groups. Many studies link fishing with increased coral breakage [18,65]; therefore, moderating activities that damage corals is postulated to reduce disease levels by limiting injuries that facilitate an entry point for coral pathogens (i.e. [20–23]). Tissue abrasions and injuries have been shown to promote disease development by providing a primary site for the invasion of pathogens in a wide variety of taxonomic groups, such as humans and terrestrial mega-fauna [66], plants and trees [67], insects [68], fishes [69] and marine invertebrates like sponges and corals [70]. For corals, ciliate infections causing brown band disease have been associated with tissue injury from tourists [19], as well as predation by the crown-of-thorns starfish [23,71] and a coral-feeding snail [22]. Likewise, lacerations on coral tissues increase susceptibility to colonization by the ciliate that causes skeletal eroding band disease [20,21]. Here, ciliate-mediated tissue loss from both skeletal eroding band and brown band disease accompanied higher levels of derelict fishing line. We hypothesize that fishing line not only causes coral tissue injury and skeleton damage, but it also provides additional surfaces for potential pathogens to colonize, increasing their capacity to infect wounds. The dominance of ciliate diseases outside reserves observed in this study, coupled with parallel conclusions reached by Lamb et al. [24], provide further evidence that protected areas are effective for moderating diseases that are associated with fishing activities.
In many instances, reefs subject to chronic stress from fishing have failed to recover from natural disturbances such as tropical storms [72], which could also suggest that corals located outside reserves in our study may have been slower to recover from cyclone damage. Invertebrate immune responses are known to be depleted during regeneration of wounds, resulting in reduced capacity to develop an immune response following exposure to a foreign substance and reinforcing the likelihood of disease development [70]. Complete regrowth of tissues during wound repair can take several months in normal circumstances; therefore, persistent disturbances from fishing activities in non-reserves could facilitate infections well beyond the cyclone.
Macroalgal cover appears to be a significant driver of coral disease assemblages regardless of reserve status, particularly for black band disease. Benthic algae are common reservoirs for a variety of potential coral pathogens. While physical contact with a certain macroalga can trigger a virulent reef-coral disease [15], initial damage or stress to the coral may also be a prerequisite for transmission of alga-associated microbes [73]. Moreover, black band disease is composed of complex and diverse polymicrobial mats that are capable of infecting injured corals transplanted downstream [74]. This suggests that mechanical damage could increase both the likelihood of dislodgement and transmission of marine pathogens.
Elevated exposure to secondary plume water is an important driver of coral disease assemblages and appears to undermine the potential of protected areas to alleviate tissue loss from white syndromes, a devastating group of diseases [45]. Secondary waters are characterized by finer sediment fractions and higher chlorophyll-a concentrations that reflect enhanced phytoplankton biomass from nutrient enrichment [54]. Nutrient enrichment can lead to blooms of toxic microalgae. While intense algal blooms that cause mortality via anoxia or toxic exposure have obvious immediate effects on marine populations, chronic hypoxia or exposure to algal blooms could be equally detrimental in the development of disease. For instance, nutrient enriched primary productivity is linked with increases in the severity of amoebic gill disease affecting multiple fish species [75], promotion of the debilitating tumour-forming disease fibropapillomatosis in sea turtles [76], and pathogenic infection in amphibians [77]. Furthermore, nutrient enrichment increases the prevalence and severity of multiple coral diseases in controlled laboratory and field settings [47,78].
Because exposure to secondary water did not differ between reserves and non-reserves, it is possible that the combined effects of exposure to finer sediment fractions and mechanical damage, led to more white syndromes outside reserves. Several white disease outbreaks have accompanied mechanical damage. Disease-causing rapid tissue loss was associated with colony fragmentation and physical contact with sediment in multiple coral species following a hurricane [42], inferring a direct link with sediment and injury. Not only are fine sediment fractions the most difficult for corals to expel and remove [79], but fine sediments are also often positively correlated with total organic carbon content [80]. In experimental studies, disease development was enhanced by elevated organic carbon, suggesting that coral pathogens are carbon-limited [81]. The extensive breadth of evidence outlined above suggests that physical disruption of coral tissues by fishing activities, paired with costly energy expenditure required for sediment removal and wound-healing processes, increase the probability of disease.
Protected area location is a key consideration for meeting conservation goals [82]. Satellite-derived water quality data were critical in assessing both the benefits and limitations of reserves to moderate disease in this study, and offer potential for identifying and forecasting locations that are at increased risk of outbreaks [83]. Exposure to disturbances is a determinant of the vulnerability of marine ecosystems [84] and such disturbances are not often spatially uniform [59]. Therefore, it is helpful to consider disturbances when establishing protected areas for the management of marine disease. However, exposure to disturbance is rarely considered in marine protected areas planning [85] (but see [86]) and debate surrounds discussions about whether to protect high- versus low-risk areas [85]. Our study emphasizes additional factors for decision-makers to consider when using protected areas as tools for moderating coral diseases in regions with variable risks of exposure to run-off and cyclonic winds. It is likely that marine organisms will be threatened by multiple disturbances in the future; thus moderating outbreaks of disease using protected areas will require additional pre-emptive management tools and techniques.
Our results indicate that although marine reserves were only partially effective in mitigating disease prevalence following chronic exposure to land-based run-off, further reductions in marine diseases are likely in protected areas that encompass terrestrial, freshwater and marine environments (figure 4b). For example, the restoration or creation of riparian zones and wetlands can increase nutrient and sediment residence time and allow for nutrient cycling (e.g. [87]), and could also reduce levels of disease-causing pollutants that enter coastal reefs. Mangroves and constructed wetlands are often used as bio-filters for natural sewage and fertilizer control [88]. Bivalves could have huge potential for reducing transmission of disease to coral reefs from terrestrial sources by filtering pathogenic microorganisms from the water column [89]. Ecosystem filtration of toxins, nutrients and pathogenic microorganisms provided by coastal mangroves, seagrasses and bivalves has not yet been examined as a tool to alleviate marine diseases. An important area for future research would be to assess the level to which these habitats sequester pollutants and alleviate marine disease impacts. More importantly, marine and terrestrial environments are often regarded as two separate ecosystems, and managed as independent entities [90]. Significant benefits to marine organism health might arise from investing conservation efforts into connecting marine and terrestrial systems [36]. In some areas, this investment has led to improvements in downstream and coastal water quality (e.g. [91]).
Protected areas are likely to be inadequate for alleviating marine disease under several conditions. For example, protected areas may be unsuitable for protecting highly mobile mega-fauna and fishes that traverse boundaries. Displacing impacts outside protected area boundaries commonly occurs in terrestrial and marine environments [92], and could further degrade adjacent ecosystems and override the benefit of locally managed areas. Furthermore, range shifts due to anthropogenic and climate-driven processes may cause both pathogens and hosts to move out of protected areas [93], potentially reducing the relevance of fixed spatial locations as conservation strategies for moderating disease. The benefits and limitations presented here represent only some of the considerations needed to inform the development of spatial management strategies for moderating marine disease.
Overall, protected areas represent a promising conservation tool for reducing diseases promoted by activities that increase physical injuries. Although protected areas have been implemented in many ecosystems throughout the world, this is the first study to demonstrate that the efficacy of protected areas to alleviate coral disease can still occur following both an acute and chronic environmental disturbance. Our understanding of the pathogens that cause most coral diseases is still unclear, especially compared with diseases that occur on land. The openness of the marine environment means that it is extremely difficult to pinpoint the underlying disease-causing agent or agents. Therefore, it is vital to determine which activities lead to elevated disease levels, and moderate impacts that increase susceptibility to infection.
Supplementary Material
Acknowledgements
We thank L. Kelly and F.J. Pollock for their assistance in data collection, and S. Beveridge, S. Harte and M. Hein for technical support in the field. We thank R. Yoshioka for statistical advice. We credit Robert Adrian Hillman, Nebojsa Kontic, Keko-ka, Posscriptum (Shutterstock) and Tracey Saxby (Integration and Application Network, University of Maryland Center for Environmental Science) for vector illustrations used in figure 4. Surveys were conducted under GBRMPA permit G12/35232.1. The invitation to submit this article arose from an NSF-RCN working group on ‘Ecology of Infectious Marine Diseases.’
Data accessibility
The datasets supporting this article are available in the electronic supplementary material.
Authors' contributions
J.B.L. and B.L.W conceived and designed the study. J.B.L., D.M.C. and D.H.W. conducted field surveys. J.B.L. and A.S.W. performed data analyses. A.S.W. and M.J.D. compiled and analysed satellite imagery. All authors wrote the manuscript and gave final approval.
Competing interests
We have no competing interests.
Funding
Funding for this study was provided by an Australian Government's National Environmental Research Program (NERP) Tropical Ecosystems Hub grant to D.M.C., D.H.W. and B.L.W. J.B.L. was supported by the Australian Institute of Marine Science at James Cook University and a NatureNet Fellowship provided by The Nature Conservancy and Cornell University. A.S.W. was supported by the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies at James Cook University. M.J.D. was supported by TropWater at James Cook University.
References
- 1.Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. 2009. Climate change and wildlife diseases: when does the host matter the most? Ecology 90, 912–920. ( 10.1890/08-0616.1) [DOI] [PubMed] [Google Scholar]
- 2.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 3.Bruckner AW. 2002. Priorities for effective management of coral diseases. NOAA Technical Memorandum NMFS-OPR-22.
- 4.McCallum H, Harvell D, Dobson A. 2003. Rates of spread of marine pathogens. Ecol. Lett. 6, 1062–1067. ( 10.1046/j.1461-0248.2003.00545.x) [DOI] [Google Scholar]
- 5.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife---threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 7.Scheffer RP. 1997. The nature of disease in plants. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Ward JR, Lafferty KD. 2004. The elusive baseline of marine disease: are diseases in ocean ecosystems increasing. PLoS Biol. 2, e120 ( 10.1371/journal.pbio.0020120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke LM, Reytar K, Spalding M, Perry A. 2011. Reefs at risk revisited. Washington, DC: World Resources Institute. [Google Scholar]
- 10.Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM. 2007. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5, e124 ( 10.1371/journal.pbio.0050124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCallum H, Gerber L, Jani A. 2005. Does infectious disease influence the efficacy of marine protected areas? A theoretical framework. J. Appl. Ecol. 42, 688–698. ( 10.1111/j.1365-2664.2005.01043.x) [DOI] [Google Scholar]
- 12.Aeby GS, Santavy DL. 2006. Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar. Ecol. Prog. Ser. 318, 103–110. ( 10.3354/meps318103) [DOI] [Google Scholar]
- 13.Raymundo LJ, Halford AR, Maypa AP, Kerr AM. 2009. Functionally diverse reef-fish communities ameliorate coral disease. Proc. Natl Acad. Sci. USA 106, 17 067–17 070. ( 10.1073/pnas.0900365106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellwood DR, Hoey AS, Choat JH. 2003. Limited functional redundancy in high diversity systems: resilience and ecosystem function on coral reefs. Ecol. Lett. 6, 281–285. ( 10.1046/j.1461-0248.2003.00432.x) [DOI] [Google Scholar]
- 15.Nugues MM, Smith GW, Hooidonk RJ, Seabra MI, Bak RP. 2004. Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923. ( 10.1111/j.1461-0248.2004.00651.x) [DOI] [Google Scholar]
- 16.Smith JE, et al. 2006. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 9, 835–845. ( 10.1111/j.1461-0248.2006.00937.x) [DOI] [PubMed] [Google Scholar]
- 17.Asoh K, Yoshikawa T, Kosaki R, Marschall EA. 2004. Damage to cauliflower coral by monofilament fishing lines in Hawaii. Conserv. Biol. 18, 1645–1650. ( 10.1111/j.1523-1739.2004.00122.x) [DOI] [Google Scholar]
- 18.Yoshikawa T, Asoh K. 2004. Entanglement of monofilament fishing lines and coral death. Biol. Conserv. 117, 557–560. ( 10.1016/j.biocon.2003.09.025) [DOI] [Google Scholar]
- 19.Lamb JB, Willis BL. 2011. Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park. Conserv. Biol. 25, 1044–1052. ( 10.1111/j.1523-1739.2011.01724.x) [DOI] [PubMed] [Google Scholar]
- 20.Lamb JB, True JD, Piromvaragorn S, Willis BL. 2014. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 178, 88–96. ( 10.1016/j.biocon.2014.06.027) [DOI] [Google Scholar]
- 21.Page CA, Willis BL. 2008. Epidemiology of skeletal eroding band on the Great Barrier Reef and the role of injury in the initiation of this widespread coral disease. Coral Reefs 27, 257–272. ( 10.1007/s00338-007-0317-8). [DOI] [Google Scholar]
- 22.Nicolet K, Hoogenboom M, Gardiner N, Pratchett M, Willis B. 2013. The corallivorous invertebrate Drupella aids in transmission of brown band disease on the Great Barrier Reef. Coral Reefs 32, 585–595. ( 10.1007/s00338-013-1010-8) [DOI] [Google Scholar]
- 23.Katz SM, Pollock FJ, Bourne DG, Willis BL. 2014. Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs 33, 705–716. ( 10.1007/s00338-014-1153-2) [DOI] [Google Scholar]
- 24.Lamb JB, Williamson DH, Russ GR, Willis BL. 2015. Protected areas mitigate diseases of reef-building corals by reducing damage from fishing. Ecology 96, 2555–2567. ( 10.1890/14-1952.1) [DOI] [PubMed] [Google Scholar]
- 25.Page CA et al. . 2009. Influence of marine reserves on coral disease prevalence. Dis. Aquat. Organ. 87, 135–150. ( 10.3354/dao02112) [DOI] [PubMed] [Google Scholar]
- 26.McClanahan T, Weil E, Maina J. 2009. Strong relationship between coral bleaching and growth anomalies in massive Porites. Glob. Change Biol. 15, 1804–1816. ( 10.1111/j.1365-2486.2008.01799.x) [DOI] [Google Scholar]
- 27.Coelho VR, Manfrino C. 2007. Coral community decline at a remote Caribbean island: marine no-take reserves are not enough. Aquat. Conserv. Mar. Freshwater Ecosyst. 17, 666–685. ( 10.1002/aqc.822) [DOI] [Google Scholar]
- 28.Russ GR, Cheal AJ, Dolman AM, Emslie MJ, Evans RD, Miller I, Sweatman H, Williamson DH. 2008. Rapid increase in fish numbers follows creation of world's largest marine reserve network. Curr. Biol. 18, R514–R515. ( 10.1016/j.cub.2008.04.016) [DOI] [PubMed] [Google Scholar]
- 29.Lester SE, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, Airamé S, Warner RR. 2009. Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Progr. Ser. 384, 33–46. ( 10.3354/meps08029) [DOI] [Google Scholar]
- 30.Stokesbury KD, Harris BP, Marino MC, Nogueira JI. 2007. Sea scallop mass mortality in a marine protected area. Mar. Ecol. Progr. Ser. 349, 151–158. ( 10.3354/meps07113) [DOI] [Google Scholar]
- 31.Ben-Horin T, Lafferty KD, Bidegain G, Lenihan HS. 2016. Fishing diseased abalone to promote yield and conservation. Phil. Trans. R. Soc. B 371, 20150211 ( 10.1098/rstb.2015.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafferty KD. 2004. Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 14, 1566–1573. ( 10.1890/03-5088) [DOI] [Google Scholar]
- 33.Hughes TP, Graham NA, Jackson JB, Mumby PJ, Steneck RS. 2010. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642. ( 10.1016/j.tree.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 34.Selig ER, Bruno JF. 2010. A global analysis of the effectiveness of marine protected areas in preventing coral loss. PLoS ONE 5, e9278 ( 10.1371/journal.pone.0009278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling S, Johnson C, Frusher S, Ridgway K. 2009. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl Acad. Sci. USA 106, 22 341–22 345. ( 10.1073/pnas.0907529106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenger AS, Williamson DH, da Silva ET, Ceccarelli DM, Browne N, Petus C, Devlin MJ. In press. Effects of reduced water quality on coral reefs in and out of no-take marine reserves. Conserv. Biol. ( 10.1111/cobi.12576) [DOI] [PubMed] [Google Scholar]
- 37.Graham NAJ, et al. 2008. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 3, e3039 ( 10.1371/journal.pone.0003039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halpern B, Selkoe K, White C, Albert S, Aswani S, Lauer M. 2013. Marine protected areas and resilience to sedimentation in the Solomon Islands. Coral Reefs 32, 61–69. ( 10.1007/s00338-012-0981-1) [DOI] [Google Scholar]
- 39.Williamson DH, Ceccarelli DM, Evans RD, Jones GP, Russ GR. 2014. Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol. Evol. 4, 337–354. ( 10.1002/ece3.934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irey M, Gottwald TR, Graham JH, Riley TD, Carlton G. 2006. Post-hurricane analysis of citrus canker spread and progress towards the development of a predictive model to estimate disease spread due to catastrophic weather events. Plant Health Progr. 10 ( 10.1094/PHP-2006-0822-01-RS) [DOI] [Google Scholar]
- 41.Scheibling RE, Lauzon-Guay J-S. 2010. Killer storms: North Atlantic hurricanes and disease outbreaks in sea urchins. Limnol. Oceanogr. 55, 2331–2338. ( 10.4319/lo.2010.55.6.2331) [DOI] [Google Scholar]
- 42.Brandt ME, Smith TB, Correa AMS, Vega-Thurber R. 2013. Disturbance driven colony fragmentation as a driver of a coral disease outbreak. PLoS ONE 8, e57164 ( 10.1371/journal.pone.0057164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wear SL, Thurber RV. 2015. Sewage pollution: mitigation is key for coral reef stewardship. Ann. NY Acad. Sci. 1355, 15–30. ( 10.1111/nyas.12785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renault T. 2015. Immunotoxicological effects of environmental contaminants on marine bivalves. Fish Shellfish Immunol. 46, 88–93. ( 10.1016/j.fsi.2015.04.011) [DOI] [PubMed] [Google Scholar]
- 45.Pollock FJ, Lamb JB, Field SN, Heron SF, Schaffelke B, Shedrawi G, Bourne DG, Willis BL. 2014. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 9, e102498 ( 10.1371/journal.pone.0102498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gochfeld D, Easson C, Freeman C, Thacker R, Olson J. 2012. Disease and nutrient enrichment as potential stressors on the Caribbean sponge Aplysina cauliformis and its bacterial symbionts. Mar. Ecol. Progr. Ser. 456, 101–111. ( 10.3354/meps09716) [DOI] [Google Scholar]
- 47.Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, McMinds R, Zaneveld JR. 2014. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Change Biol. 20, 544–554. ( 10.1111/gcb.12450) [DOI] [PubMed] [Google Scholar]
- 48.Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM. 2015. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 7, 471–496. ( 10.1146/annurev-marine-010814-015646) [DOI] [PubMed] [Google Scholar]
- 49.Webster PJ, Holland GJ, Curry JA, Chang H-R. 2005. Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 309, 1844–1846. ( 10.1126/science.1116448) [DOI] [PubMed] [Google Scholar]
- 50.Harriott VJ. 2002. Marine tourism impacts and their management on the Great Barrier Reef. Townsville, Australia: CRC Reef Research Centre. [Google Scholar]
- 51.Higgs J, McInnes K.2001. Biennial recreational fishing survey of Queensland residents. Cairns, Australia: Queensland Fisheries Management Authority.
- 52.Lukoschek V, Cross P, Torda G, Zimmerman R, Willis BL. 2013. The importance of coral larval recruitment for the recovery of reefs impacted by Cyclone Yasi in the central Great Barrier Reef. PLoS ONE 8, e65363 ( 10.1371/journal.pone.0065363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beeden R, Maynard J, Puotinen M, Marshall P, Dryden J, Goldberg J, Williams G. 2015. Impacts and recovery from severe tropical Cyclone Yasi on the Great Barrier Reef. PLos ONE 10, e0121272 ( 10.1371/journal.pone.0121272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devlin M, McKinna L, Alvarez-Romero J, Petus C, Abott B, Harkness P, Brodie J. 2012. Mapping the pollutants in surface riverine flood plume waters in the Great Barrier Reef, Australia. Mar. Pollut. Bull. 65, 224–235. ( 10.1016/j.marpolbul.2012.03.001) [DOI] [PubMed] [Google Scholar]
- 55.Willis BL, Page CA, Dinsdale EA.2004. Coral disease on the Great Barrier Reef. In Coral health and disease (eds E Rosenberg, Y Loya), pp. 69–104. Berlin, Germany: Springer.
- 56.Álvarez-Romero JG, et al. 2013. A novel approach to model exposure of coastal-marine ecosystems to riverine flood plumes based on remote sensing techniques. J. Environ. Manage. 119, 194–207. ( 10.1016/j.jenvman.2013.01.036) [DOI] [PubMed] [Google Scholar]
- 57.Baith K, Lindsay R, Fu G, McClain CR. 2001. Data analysis system developed for ocean color satellite sensors. Eos Trans. Am. Geophys. Union 82, 202 ( 10.1029/01EO00109) [DOI] [Google Scholar]
- 58.Petus C, da Silva ET, Devlin M, Wenger AS, Álvarez-Romero JG. 2014. Using MODIS data for mapping of water types within river plumes in the Great Barrier Reef, Australia: towards the production of river plume risk maps for reef and seagrass ecosystems. J. Environ. Manage. 137, 163–177. ( 10.1016/j.jenvman.2013.11.050) [DOI] [PubMed] [Google Scholar]
- 59.Devlin MJ, Da Silva ET, Petus C, Wenger A, Zeh D, Tracey D, Álvarez-Romero JG, Brodie J. 2013. Combining in-situ water quality and remotely sensed data across spatial and temporal scales to measure variability in wet season chlorophyll-a: Great Barrier Reef lagoon (Queensland, Australia). Ecol. Process. 2, 1–22. ( 10.1186/2192-1709-2-31) [DOI] [Google Scholar]
- 60.McArdle BH, Anderson MJ. 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82, 290–297. ( 10.1890/0012-9658(2001)082%5B0290:FMMTCD%5D2.0.CO;2) [DOI] [Google Scholar]
- 61.Anderson M, Gorley R, Clarke K. 2008. PERMANOVA+for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E. [Google Scholar]
- 62.Dormann CF, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 63.Schwarz G. 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. ( 10.1214/aos/1176344136) [DOI] [Google Scholar]
- 64.R Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Core Team.
- 65.Mangi SC, Roberts C. 2006. Quantifying the environmental impacts of artisanal fishing gear on Kenya's coral reef ecosystems. Mar. Pollut. Bull. 52, 1646–1660. ( 10.1016/j.marpolbul.2006.06.006) [DOI] [PubMed] [Google Scholar]
- 66.Wobeser GA. 2013. Essentials of disease in wild animals. New York, NY: John Wiley & Sons. [Google Scholar]
- 67.Underwood W. 2012. The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 3, 85 ( 10.3389/fpls.2012.00085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrandon D, Imler J-L, Hetru C, Hoffmann JA. 2007. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874. ( 10.1038/nri2194) [DOI] [PubMed] [Google Scholar]
- 69.Austin B, Austin DA. 2007. Bacterial fish pathogens: disease of farmed and wild fish. Berlin, Germany: Springer. [Google Scholar]
- 70.Mydlarz LD, Jones LE, Harvell CD. 2006. Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu. Rev. Ecol. Evol. System. 37, 251–288. ( 10.1146/annurev.ecolsys.37.091305.110103) [DOI] [Google Scholar]
- 71.Nugues M, Bak R. 2009. Brown-band syndrome on feeding scars of the crown-of-thorn starfish Acanthaster planci. Coral Reefs 28, 507–510. ( 10.1007/s00338-009-0468-x) [DOI] [Google Scholar]
- 72.Connell J. 1997. Disturbance and recovery of coral assemblages. Coral Reefs 16, S101–S113. ( 10.1007/s003380050246) [DOI] [Google Scholar]
- 73.Sweet MJ, Bythell JC, Nugues MM. 2013. Algae as reservoirs for coral pathogens PLoS ONE 8, e69717 ( 10.1371/journal.pone.0069717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rutzler K, Santavy DL. 1983. The black band disease of Atlantic reef corals. I. Description of the cyanophyte pathogen. PSZNI. Mar. Ecol. 4, 301–319. ( 10.1111/j.1439-0485.1983.tb00116.x) [DOI] [Google Scholar]
- 75.Nowak B. 2001. Qualitative evaluation of risk factors for amoebic gill disease in Atlantic salmon. In Proc. OIE Intl. Conf. on risk analysis in aquatic animal health, Paris, France, 8–10 February 2000 (ed. CJ Rogers), pp. 148–155. Paris, France: Office International des Epizooties. [Google Scholar]
- 76.Van Houtan KS, Hargrove SK, Balazs GH. 2010. Land use, macroalgae, and a tumor-forming disease in marine turtles. PLoS ONE 5, e12900 ( 10.1371/journal.pone.0012900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson PT, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, Sutherland DR, Carpenter SR. 2007. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl Acad. Sci. USA 104, 15 781–15 786. ( 10.1073/pnas.0707763104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruno JF, Petes LE, Drew Harvell C, Hettinger A. 2003. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061. ( 10.1046/j.1461-0248.2003.00544.x) [DOI] [Google Scholar]
- 79.Weber M, Lott C, Fabricius K. 2006. Sedimentation stress in a scleractinian coral exposed to terrestrial and marine sediments with contrasting physical, organic and geochemical properties. J. Exp. Mar. Biol. Ecol. 336, 18–32. ( 10.1016/j.jembe.2006.04.007) [DOI] [Google Scholar]
- 80.De Falco G, Magni P, Teräsvuori L, Matteucci G. 2004. Sediment grain size and organic carbon distribution in the Cabras lagoon (Sardinia, western Mediterranean). Chem. Ecol. 20, 367–377. ( 10.1080/02757540310001629189) [DOI] [Google Scholar]
- 81.Kline DI, Kuntz NM, Breitbart M, Knowlton N, Rohwer F. 2006. Role of elevated organic carbon levels and microbial activity in coral mortality. Mar. Ecol. Progr. Ser. 314, 119–125. ( 10.3354/meps314119) [DOI] [Google Scholar]
- 82.Halpern BS. 2003. The impact of marine reserves: do reserves work and does reserve size matter? Ecol. Appl. 13, 117–137. ( 10.1890/1051-0761(2003)013%5B0117:TIOMRD%5D2.0.CO;2) [DOI] [Google Scholar]
- 83.Maynard J, et al. 2016. Improving marine disease surveillance through sea temperature monitoring, outlooks and projections. Phil. Trans. R. Soc. B 371, 20150208 ( 10.1098/rstb.2015.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marshall PA, Johnson JE. 2007. The Great Barrier Reef and climate change: vulnerability and management implications. In Climate change and the Great Barrier Reef, pp. 774–801. Townsville, Australia: Great Barrier Reef Marine Park Authority and the Australian Greenhouse Office, Australia. [Google Scholar]
- 85.Game ET, McDonald-Madden E, Puotinen ML, Possingham HP. 2008. Should we protect the strong or the weak? Risk, resilience, and the selection of marine protected areas. Conserv. Biol. 22, 1619–1629. ( 10.1111/j.1523-1739.2008.01037.x) [DOI] [PubMed] [Google Scholar]
- 86.Maynard JA, et al. 2015. Great Barrier Reef no-take areas include a range of disturbance regimes. Conserv. Lett. ( 10.1111/conl.12198) [DOI] [Google Scholar]
- 87.Burt T, Pinay G. 2005. Linking hydrology and biogeochemistry in complex landscapes. Prog. Phys. Geogr. 29, 297–316. ( 10.1191/0309133305pp450ra) [DOI] [Google Scholar]
- 88.Yang Q, Tam N, Wong YS, Luan T, Su W, Lan C, Shin P, Cheung SG. 2008. Potential use of mangroves as constructed wetland for municipal sewage treatment in Futian, Shenzhen, China. Mar. Pollut. Bull. 57, 735–743. ( 10.1016/j.marpolbul.2008.01.037) [DOI] [PubMed] [Google Scholar]
- 89.Faust C, Stallknecht D, Swayne D, Brown J. 2009. Filter-feeding bivalves can remove avian influenza viruses from water and reduce infectivity. Proc. R. Soc. B 276, 3727–3735. ( 10.1098/rspb.2009.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alvarez-Romero JG, Pressey RL, Ban NC, Vance-Borland K, Willer C, Klein CJ, Gaines SD. 2011. Integrated land-sea conservation planning: the missing links. Annu. Rev. Ecol. Evol. System. 42, 381–409. ( 10.1146/annurev-ecolsys-102209-144702) [DOI] [Google Scholar]
- 91.Fennessy M, Cronk J. 1997. The effectiveness and restoration potential of riparian ecotones for the management of nonpoint source pollution, particularly nitrate. Crit. Rev. Environ. Sci. Technol. 27, 285–317. ( 10.1080/10643389709388502) [DOI] [Google Scholar]
- 92.Agardy T, Di Sciara GN, Christie P. 2011. Mind the gap: addressing the shortcomings of marine protected areas through large scale marine spatial planning. Mar. Policy 35, 226–232. ( 10.1016/j.marpol.2010.10.006) [DOI] [Google Scholar]
- 93.Hannah L, Midgley G, Andelman S, Araújo M, Hughes G, Martinez-Meyer E, Pearson R, Williams P. 2007. Protected area needs in a changing climate. Front. Ecol. Environ. 5, 131–138. ( 10.1890/1540-9295(2007)5%5B131:PANIAC%5D2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available in the electronic supplementary material.