Abstract
Ecological communities that experience stable climate conditions have been speculated to preserve more specialized interspecific associations and have higher proportions of smaller ranged species (SRS). Thus, areas with disproportionally large numbers of SRS are expected to coincide geographically with a high degree of community-level ecological specialization, but this suggestion remains poorly supported with empirical evidence. Here, we analysed data for hummingbird resource specialization, range size, contemporary climate, and Late Quaternary climate stability for 46 hummingbird–plant mutualistic networks distributed across the Americas, representing 130 hummingbird species (ca 40% of all hummingbird species). We demonstrate a positive relationship between the proportion of SRS of hummingbirds and community-level specialization, i.e. the division of the floral niche among coexisting hummingbird species. This relationship remained strong even when accounting for climate, furthermore, the effect of SRS on specialization was far stronger than the effect of specialization on SRS, suggesting that climate largely influences specialization through species' range-size dynamics. Irrespective of the exact mechanism involved, our results indicate that communities consisting of higher proportions of SRS may be vulnerable to disturbance not only because of their small geographical ranges, but also because of their high degree of specialization.
Keywords: biogeography, climate gradients, macroecology, mutualistic networks, range size, specialization
1. Introduction
Ecological specialization may facilitate species coexistence and speciation and is therefore hypothesized to structure global patterns of biodiversity [1]. Notably, higher degrees of community-level resource specialization, i.e. the division of local resources, may be associated with reduced interspecific competition and greater local richness [2]. It is therefore debated whether high ecological specialization in the tropics may contribute to the observed continental-scale increase in species richness towards the tropics [3–10]. Likewise, it is speculated that large-scale geographical differences in ecological specialization coincides with patterns of range-size frequency distributions [11].
We address this and the role of extrinsic factors, such as climate, as potential determinants of community-level specialization and range-size distributions. The contemporary climate has been suggested to influence ecological specialization, with communities in productive areas having the highest degree of specialization [12,13]. Similarly, in areas with low contemporary seasonality, where resource availability is supposedly relatively stable throughout the year, communities may have a higher degree of specialization than those found in more seasonal environments [14–16]. Recent studies have also pointed towards historical climate fluctuations as influencing the local degree of specialization, as unstable climatic conditions are hypothesized to disrupt specialized species interactions, either through changes in the phenology of species or through increased dynamics in range-size position [6,11,17–19]. Thus, ecological and historical factors may both shape geographical patterns of specialization. This has been found for hummingbird–plant networks, which have higher community-wide specialization in areas with higher precipitation and temperature, lower seasonality, and more stable climate conditions since the last glacial maximum [6,20].
The contemporary and historical climate may also affect the geographical distribution of species range-sizes [21–23]. For instance, variable climate conditions have traditionally been suggested to select for broad environmental tolerance, which promotes large ranges in seasonal areas [23–27], though see [28,29]. A highly seasonal climate may also force species to migrate in order to track suitable environmental conditions, and as smaller ranged species (SRS) have been suggested to have weaker dispersal ability than larger ranged species [30], they are more likely to be residents in seasonally stable environments. This reasoning may be extended to historical fluctuations in climate, which may have forced species either to adapt to the new conditions, track suitable climatic conditions, or to go locally extinct. As SRS may struggle to track suitable climate conditions [30], these would suffer from an increased probability of local extinction under climate change [27,31]. In accordance with this, Late Quaternary climate-change velocity correlates negatively with the global distribution of proportionally smaller ranged amphibian, mammal, and bird species [31].
Taken together, numerous studies have pointed towards historical climate stability and low contemporary seasonality as being important to support both ecological specialization and high proportions of SRS. Thus, areas with disproportionally large numbers of SRS are expected to coincide geographically with a high degree of community-level ecological specialization, but this hypothesis remains poorly supported [6,11,19]. We test this using a database consisting of 46 quantitative hummingbird–plant networks, i.e. local community studies recording the frequency of visits between all coexisting hummingbird and plant species. The 46 networks are distributed widely across the American mainland [20]. Specifically, we investigate: (i) whether specialization in hummingbird–plant networks is positively related with the proportion of SRS in a community, and (ii) the nature of the causal relationship between climate (contemporary and Late Quaternary), specialization, and SRS. Hummingbirds are well suited for such large-scale comparative studies on the pattern of ecological specialization as they are highly specialized on nectar-feeding, and because hummingbird pollinators and plants are mutually dependent [32–34]. Moreover, hummingbirds are highly successful, being the second most species-rich family of birds, and able to thrive in an array of environments across most of the Americas [35]. Hence, hummingbird–plant communities have long served as a model system for examining ecological and evolutionary processes as determinants of ecological specialization at the community level [32,33]. Our study advances the current understanding of how geographical patterns of range size and specialization are shaped, and has additional implications for the conservation of species communities with specialized associations.
2. Material and methods
(a). Hummingbird–plant network data
We used a database consisting of 46 hummingbird–plant networks [20], from which we constructed weighted interaction networks for the hummingbirds and their associated nectar plants (figure 1). Taking a network approach allows detailed information about the interaction frequencies between all hummingbird and plant species within a given community to be summarized as a metric value (see below). We applied a network approach (as opposed to a species-level approach) as we aimed to assess the processes operating at the community level, notably whether areas with disproportionally large numbers of SRS coincide geographically with communities with a high degree of ecological specialization. For this study, mutualistic networks were presented as matrices consisting of P (number of plant species) × H (number of hummingbird species) with matrix entries indicating the frequency of each interaction (i.e. the number of visitations recorded for a given hummingbird–plant species pair). Known incidents of nectar robbing, for instance if a hummingbird pierced the flower corolla without contacting the floral reproductive organs, were not considered since they represent antagonistic rather than mutualistic interactions [36]. We included a network in the study if it fulfilled the following criteria: (i) each study must have a community approach, i.e. aiming to include all hummingbird and hummingbird-visited plant species within the given community over the sampled period, (ii) networks need to consist of weighted data, i.e. include frequency of interactions, since binary networks are more sensitive to differences in sampling effort [37], and (iii) island networks were not included since species from islands are naturally constrained in their geographical distribution by the hard range boundaries imposed by the sea. Measuring species range-size solely as the number of occupied grid cells would therefore contain less biological and mostly geographical information and, hence, is not comparable to range sizes on the continent.
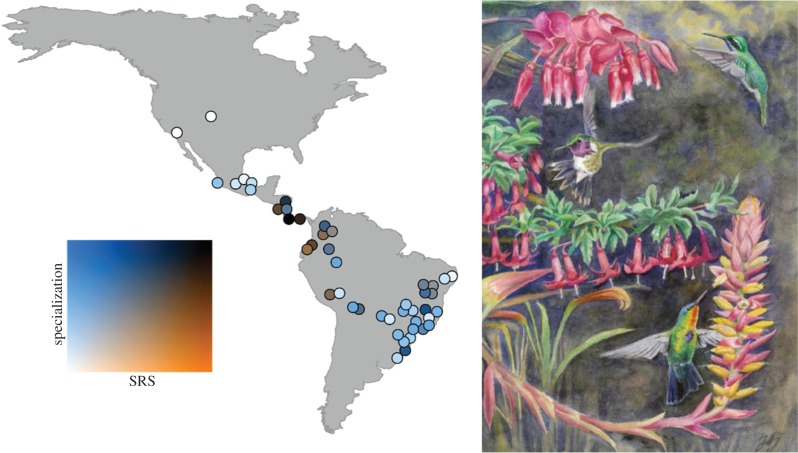
Figure 1.
Geographical pattern of specialization and the proportion of smaller ranged species (SRS) for 46 hummingbird communities across the mainland Americas. The colouration of each circle on the map indicates the degree of specialization in relation to the proportion of SRS; black indicates both high SRS and specialization, white conversely indicates both low SRS and degree of specialization. Orange and blue indicate poorer correlation through either high SRS or specialization, respectively. Note that some points have been slightly moved to avoid overlap. SRS was arcsine square-root transformed to improve normality. The painted illustration shows three hummingbird species from the Costa Rican highlands, where the network with the highest degree of specialization and SRS is found in the dataset (specialization = 0.782, SRS = 0.6). From the top: volcano hummingbird (Selasphorus flammula), white-bellied mountain-gem (Lampornis hemileucus), and fiery-throated hummingbird (Panterpe insignis). Painting by Jon Fjeldså.
(b). Measuring hummingbird range-size proportions
The geographical range-size of each hummingbird species was extracted from an updated database previously presented in Rahbek & Graves [38]—see [39,40] for details on method and data sources. As an estimate of hummingbird geographical range-size, we used the total number of occupied 1° × 1° latitude–longitude grid cells. Following Jetz & Rahbek [41], in order to determine the community-level proportion of smallest ranging species, we divided the total number of species (n = 130) into quartiles according to range size, i.e. the first quartile consists of 25% of species with the smallest ranges (n = 33) and the fourth quartile consists of the 25% with the largest range sizes. For each network, we calculated the proportion of first quartile species. This measure is better suited to quantify the relative importance of smaller ranged hummingbird species in a given community than the mean or median range size of co-occurring species, as wide-ranging species contribute with more distributional records than narrow-ranging species [41]. Specifically for our dataset, since larger ranging species occurred in many of the studied communities, the mean and median range size in a given community was mostly determined by the range sizes of these species (Pearson correlation between mean and median range size and the proportion of large-ranging species (fourth quartile): n = 46, r = 0.86, p < 0.001; r = 0.83, p < 0.001, respectively).
It should be noted, however, that the range-size frequency distribution (RSFD) of our data is skewed towards larger ranges than the RSFD of all hummingbird species of the world (electronic supplementary material, figure S1). This is why we refer to first quartile species as ‘smaller ranged’ species rather than using the term ‘restricted-range’ species as in Jetz & Rahbek [41] and others using continental data on all species (see also ‘Statistical Analyses'). The community-level proportion of SRS was arcsine square-root transformed before further analysis.
(c). Specialization, richness, environmental variables, and sampling intensity
Following Blüthgen et al. [42] ecological specialization 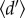 for each hummingbird community was calculated as the weighted mean of the normalized Kullback–Leibler distance for all coexisting hummingbird species [43]. The estimate is based on the number of visits recorded between each animal and plant species (here hummingbirds and their nectar plants) and has been shown to be relatively insensitive to sampling intensity and network size (see electronic supplementary material, S2, for details regarding the calculation [42,44,45]). Conceptually,
for each hummingbird community was calculated as the weighted mean of the normalized Kullback–Leibler distance for all coexisting hummingbird species [43]. The estimate is based on the number of visits recorded between each animal and plant species (here hummingbirds and their nectar plants) and has been shown to be relatively insensitive to sampling intensity and network size (see electronic supplementary material, S2, for details regarding the calculation [42,44,45]). Conceptually, 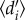 quantifies the uniqueness of the realized Eltonian niche of species [46], relative to the other species in the community, accounting for differences in species availability. Since independent estimates of species abundance were not available for most networks, we followed Blüthgen et al. and used the total number of interactions of each hummingbird and plant species in each respective network as measures of species availability [42,47]. Hummingbird specialization
quantifies the uniqueness of the realized Eltonian niche of species [46], relative to the other species in the community, accounting for differences in species availability. Since independent estimates of species abundance were not available for most networks, we followed Blüthgen et al. and used the total number of interactions of each hummingbird and plant species in each respective network as measures of species availability [42,47]. Hummingbird specialization 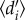 was related to network size [6], i.e. the richness of hummingbird and plant species in the network (correlation coefficient; r = 0.38, p = 0.009), and network asymmetry [48], i.e. the ratio between the richness of hummingbird and plant species (r = 0.43, p = 0.003). Conversely, variation in sampling intensity, calculated for each network by dividing the total number of observed interactions (square-root transformed) with the richness for plants and hummingbirds [49], showed no significant associations to specialization (r = −0.28, p > 0.05).
was related to network size [6], i.e. the richness of hummingbird and plant species in the network (correlation coefficient; r = 0.38, p = 0.009), and network asymmetry [48], i.e. the ratio between the richness of hummingbird and plant species (r = 0.43, p = 0.003). Conversely, variation in sampling intensity, calculated for each network by dividing the total number of observed interactions (square-root transformed) with the richness for plants and hummingbirds [49], showed no significant associations to specialization (r = −0.28, p > 0.05).
The contemporary climate variables hypothesized to predict specialization and SRS, i.e. mean annual temperature (MAT), mean annual precipitation (MAP), temperature seasonality (i.e. standard deviation in annual temperature; TS), and precipitation seasonality (i.e. coefficient of variation for annual precipitation: PS), were extracted from the WorldClim database with a resolution of 1 × 1 km (http://www.worldclim.org; [50]). We estimated variables reflecting historical climate change as the absolute difference in temperature and precipitation between pre-industrial time and the last glacial maximum (21 000 years ago), i.e. temperature and precipitation anomalies (AnomT and AnomP). To generate projections of climate anomaly, we used the Hadley Centre Model v. 3 (HadCM3) at 3.75 × 2.5 arc degrees resolution and subsequently statistically downscaled to 0.1 × 0.1 arc degrees [51]. We also included a measure of topographic heterogeneity (i.e. range in elevation; TH), as predictors of both specialization and SRS [52,53]. Historical climate and topography are known to have interactive effects [31], so, in supplementary analyses, we included estimates of temperature and precipitation velocity (VelT and VelP), which capture the buffering effect in mountain areas where species can track their original climate zone by migrating a short distance up or down slope [54]. For each community, TH and estimates of historical and contemporary climate were calculated as the average of values within a radius of 10 km from the sampled location. Given the large geographical extent of the data, we assume that the regional downscaled climate estimates are good indicators of the variation of local climate among communities. To meet statistical assumptions of normality, MAT was squared and MAP, TS, AnomT, AnomP, VelT, and VelP were log-transformed prior to further analyses. All variables were scaled to zero mean and unit variance.
(d). Statistical analyses
Structural equation models (SEMs) are statistical tools used to evaluate multivariate hypotheses. Compared to multiple regression models, the main advantage is that they seek to account for both direct and indirect effects among predictor and response variables while allowing multiple dependent variables to be tested simultaneously. We constructed two SEMs based on a priori hypotheses, considering different causal paths among the response variables. First, we considered a link from SRS to specialization, corresponding to a scenario where a high proportion of SRS (e.g. through lowered range-size dynamics) affects the degree of hummingbird specialization in the local plant community. Second, we considered the opposite link from specialization to SRS, corresponding to a scenario where the degree of hummingbird specialization in a local community influences hummingbirds’ range-size. Due to the relatively low sample size (n = 46) in comparison to the number of predictor variables, the two models were simplified through an a priori variable selection based on the Akaike information criterion (AIC). Specifically, for each response variable, we fitted linear models including all possible combinations of potential predictor variables and only included those predictors in the initial SEM that were represented in at least one of the best-fit models, i.e. ΔAIC < 2 in relation to the model with the lowest AIC ([55]; electronic supplementary material, figure S2). The two SEMs were evaluated through the χ2 test, comparative fit index (CFI), and the Root Mean Square Error of Approximation (RMSA) [56]. The χ2 value indicates the divergence between the sample and the fitted structures in the data; a non-significant result (p > 0.05) indicates good model fit. The CFI compares the χ2 of the model with the χ2 value of an independent model assuming no correlation among all variables while accounting for sample size. With a range from 0 to 1, we accepted models with CFIs > 0.09 [57]. Lastly, the RMSA was considered because of its sensitivity to the number of estimated parameters in the model. Here, values below 0.07 were used as an indication of good model fit [57]. We expected some degree of correlation among the included climate predictors. In order to obtain reliable model fit according to the three above-mentioned indices, we identified and added this covariance based on high modification indices and large residual correlations [58,59]. By stepwise refitting, we simplified the SEMs, removing non-significant links conditional on the model fit, i.e. assessed by the χ2 test, CFI, and RMSA, being satisfied [60,61]. As the two final SEMs differ in the number of climate variables included (see Results; figure 2), we cannot use the χ2 test, CFI, and RMSA measures of fit for comparison of model fit among the final SEM models. Hence, the contribution of each predictor variable was evaluated through the standardized path coefficients. All SEMs were constructed and analysed with the R package lavaan [62].
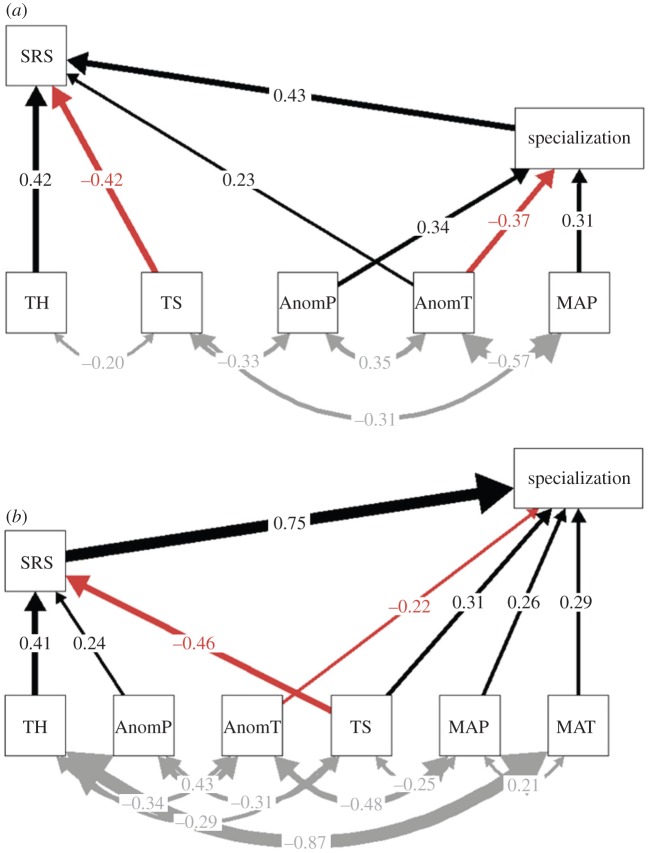
Figure 2.
Results from the two final structural equation models showing the direct and indirect links of topography, contemporary climate, and Quaternary climate velocity on specialization and the proportion of SRS (SRS; n = 46). (a) The path structure when specialization is hypothesized to predict SRS. (b) The paths for the possible opposite scenario where SRS is hypothesized to predict specialization. Black arrows indicate positive relationships, red arrows indicate negative relationships; the thickness of each arrow illustrates the strength, i.e. standardized path coefficients. The double-headed grey arrows indicate covariance links. MAT, mean annual temperature; MAP, mean annual precipitation; TS, temperature seasonality; PS, precipitation seasonality; AnomT, temperature anomaly; AnomP, precipitation anomaly; TH, topographic heterogeneity.
Supplementary analyses showed negligible effects of residual spatial autocorrelation on the SEM results (electronic supplementary material, S4). Other supplementary analyses were conducted to first account for the potential biases of network size and asymmetry on specialization. Here, instead of using raw values of specialization, we fitted SEMs including residuals of a linear regression predicting specialization by network size and network asymmetry (electronic supplementary material, figure S5). Secondly, to test if our results are sensitive to the used historical climate metric, we fitted SEMs using climate velocities rather than anomalies (electronic supplementary material, figure S6). Thirdly, we evaluated the robustness of the results with use of different range-size cut-offs to define SRS (i.e. ranging from 20% to 30% of species having the smallest range sizes; electronic supplementary material, figure S7). Fourthly, because the RSFD of our data is skewed towards larger ranges than the RSFD of all hummingbird species of the world (electronic supplementary material, figure S1), analyses were conducted using a redefined measure of the proportion of SRS based on the first quartile species of the global pool of mainland hummingbird species (n = 318), as in Jetz et al. [63], rather than the one in our dataset (n = 130; electronic supplementary material, figure S8). Finally, the latitudinal variation in continental or biome width may constitute hard boundaries to the range size of species [28,64], which could also influence the association between SRS and specialization. To account for this, we corrected the empirical SRS values by a null model, which uses geographical dispersion fields to sample species randomly and thereby generate null SRS values for each community [65]. The null model integrates data of the presence–absence of all 318 hummingbird species across mainland Americas at a 1° × 1° latitude–longitude resolution. The probability of sampling species from grid cells is weighted by the similarity in species composition with the focal network. For each community, this creates a regional source pool of species based on the concept that dispersal of species is most likely to occur among biogeographically similar regions [66,65]. Thus, we defined the regional source pool of each focal community based on the idea that species living in communities with more similar species compositions constitute its source pool by higher probability (see electronic supplementary material, S9, for algorithmic details). Deviations between the observed SRS values and the density curve of the null-generated SRS values were standardized as a z-score:
3. Results
We found a positive correlation between specialization and SRS (r = 0.39, p < 0.001, n = 46). For the SEM containing a hypothesized predictive effect from specialization to SRS, a positive association was found between the two (standardized coefficient; β = 0.43, figure 2a). Here, we found that SRS was negatively associated with TS (β = −0.42) and positively associated with topographical heterogeneity (TH; β = 0.42) and temperature anomaly (AnomT; β = 0.23), whereas specialization was negatively related to temperature anomaly (AnomT; β = −0.37) and positively related to both MAP (β = 0.31) and precipitation anomaly (AnomP; β = 0.34). In the SEM considering the scenario where specialization is affected by SRS (figure 2b), there was a strong positive link from SRS to specialization (β = 0.75). In comparison to the first SEM, we here found additional links between specialization and MAT (β = 0.29), TS (β = 0.31) and a positive influence of AnomP on SRS instead of on specialization (AnomP; β = 0.24).
The strong association between SRS and specialization was insensitive to specialization estimates when correcting for network size and asymmetry (electronic supplementary material, figure S5). The results from the SEM pairs including interactive effects of TH and historical climate through estimates of climate-change velocities also showed similar results (electronic supplementary material, figure S6).
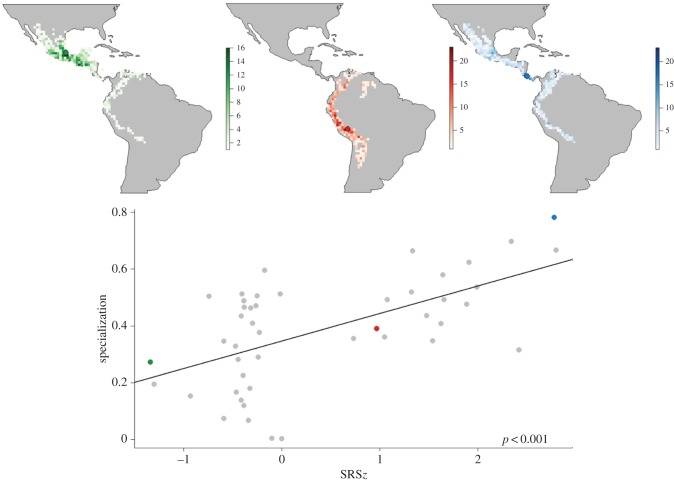
Linear regression including SRS variables calculated using different range-size cut-offs to define SRS (ranging from 20% to 30% of species having the smallest range sizes) demonstrated a robust association between the degree of specialization and SRS (electronic supplementary material, figure S7). Likewise, when using the first quartile of the global mainland species pool of hummingbirds rather than the first quartile of our dataset as a threshold to define SRS, the positive association to specialization was maintained (electronic supplementary material, figure S8). Finally, the null-model-corrected SRS remained significantly positively associated with specialization (R2 = 0.357, p < 0.001, n = 46; figure 3), indicating that the influence of biome or continental width on the range size of species is negligible in respect to the association between SRS and specialization.
Figure 3.
Scatterplot showing the correlation between specialization and a geographical null model correction of SRS (see Material and methods for details). Deviations between the observed SRS values and the density curve of the null-generated SRS values were standardized as z-scores. Maps show examples of the sampling frequency of grid cells for 1 000 randomizations within the hummingbird dispersion fields associated with three coloured (green, red, and blue) example networks spanning the spectra of SRSz-scores.
4. Discussion
For hummingbird–plant networks across mainland Americas, we found that communities with high proportions of SRS also have a high degree of ecological specialization (figures 1 and 2). The association between SRS and specialization was insensitive to the definition used for SRS (electronic supplementary material, figures S7 and S8), to the influence of biome or continental width as accounted for by a null model (figure 3), and to the influence of species richness and network asymmetry on specialization (electronic supplementary material, figure S5). Also, although topography was an important predictor of SRS, and contemporary and historical climate were important for the prediction of both SRS and specialization, it did not affect the strong association between specialization and SRS (figure 2; electronic supplementary material, figure S6). The question is then which mechanism causes SRS and specialization to be interrelated.
In respect to current climate, precipitation was positively correlated with hummingbird specialization, possibly due to either increased productivity and thus greater opportunities for specialization, or a lower importance of insect pollinators in comparison to hummingbirds as pollinators in more rainy conditions, thereby favouring hummingbird–plant specialization [5,67,68]. Most interestingly for our study, we found a strong consistent negative association between TS and SRS (figure 2) and, when accounting for the indirect effects of climate on specialization via SRS, a direct positive association of TS on specialization appeared (figure 2b). A similar positive association between modularity and TS has been documented for frugivorous bird–plant networks [49], which was argued to derive from a higher annual turnover in species composition and interactions in more seasonal environments. The combined effects of topography and climate seasonality on SRS (figure 2), together with the much stronger effect of SRS on specialization than vice versa (β = 0.75 versus β = 0.43), is in accordance with the hypothesis that climate stability may increase specialization through reduced annual species range dynamics [30,69], facilitating adaptation to local foraging niches. This may be caused by lower population variability in climatically stable areas, however, the direct association between niche breadth and climatically induced population dynamics still lacks sufficient support by empirical evidence [70]. Also, as a positive direct link from specialization to SRS remained present in all SEM models, we note that we cannot rule out the opposing hypothesis, i.e. that less specific adaptations to local food resources may extend the range over which species can occur, resulting in proportionally fewer SRS in the more generalized communities. Thus, although we are able to confirm the hypothesized interrelatedness between SRS and specialization, we are unable to identify firmly the underlying mechanism causing this association or their causal relationships with the present data.
In addition to the contemporary climate, we found correlations with the estimates of historical climate anomaly. However, their effects were less consistent in the follow-up analyses (electronic supplementary material, figures S3 and S4) than those of the contemporary climate, which in our models showed greater and more consistent importance in predicting SRS and specialization. This indicates that Late Quaternary climate stability may play a role, but a minor one compared to contemporary climate. Notably, in contrast to the suggested importance of historical climate changes for species range dynamics in previous studies [11,31], our results indicate that for hummingbirds, contemporary seasonality is more important for the preservation of SRS [69]. The observed positive link between precipitation anomaly and specialization could derive from historical high productivity, which ultimately facilitates specialization. In contrast, the positive association with SRS could be explained by recent speciation events following the onset of glacial cycles during the Late Pleistocene, where species repeatedly disperse, become isolated, and possibly speciate in a heterogeneous environment—e.g. on mountain tops [71]—see Garcia-Moreno et al. [72] for an explicit example with hummingbirds. Mechanisms, such as the latter, related to the evolutionary history of species also operate on timescales beyond the last glacial maximum [11,69] and may influence the inter-correlation of richness of SRS, high levels of specialization, and local high speciation-low extinction dynamics. Therefore, in order to understand what causes communities consisting of mainly SRS to be more specialized, one could test the hypothesis that specialized hummingbirds and their nectar-food plants have concerted population dynamics in more stable environments, ranking from current seasonality to climates at deep evolutionary timescales [73], or whether communities with high SRS and specialization reflect ongoing speciation [71]. This could potentially identify the main mechanism and temporal scale facilitating specialization in communities consisting of mainly SRS, which have lower dispersal ability and may thus depend more on nectar-food plants from the local community.
Irrespective of the exact mechanism involved, the detected relationship between SRS and specialization has relevance for ecological and evolutionary theory regarding their respective geographical patterns. Specifically, it illustrates that interspecific interactions are of great importance to consider when studying biological patterns on large spatial scales, at least for highly specialized systems such as hummingbird–plant communities. Our results also have implications for conservation of species engaged in mutualistic associations, especially as anthropogenic activity may impact mutualistic interactions [74], and cause pollinator and linked plant extinctions [75,76]. For instance, the strong link between SRS and specialization indicates that some communities may be fragile in multiple ways, both by having SRS that are slow in tracking ongoing climate changes and by having specialized species less prone to switching their interactions and at higher risk of secondary extinctions [30,31,77].
Supplementary Material
Supplementary Material
Acknowledgements
We thank Louis A. Hansen for extracting hummingbird range sizes and Bjørn Hermansen for map construction and help with GIS-related issues. We also thank Ivan Olsen and Grete Sonne for comments on early stages of the manuscript. S.W. thanks the National Institute of Natural Resources (INRENA) for research permits in Perú and L.R.L., the Ministerio del Medio Ambiente de Colombia for permission to enter Chiribiquete.
Data accessibility
Location, network characteristics, and SRS (the proportion of SRS) values of each hummingbird–plant network is presented in the electronic supplementary material, table S1. The same dataset has also been used and described for the analyses in Martin González et al. [20].
Authors' contributions
J.S. wrote the manuscript, carried out all statistical analyses, participated in the design and coordination of the study; A.M.M.G. assembled the hummingbird–plant database; J.V.B. and P.K.M. collected field data and participated in the design of the study and drafted the manuscript; B.S. and M.Sc. advised on the statistical analyses; A.C.A., F.P.A., S.M.A., A.C.B., P.C.A., T.T.I., G.K., C.L., C.A., F.M.G.L.C., A.O.M., C.G.M., M.A.M., A.C.M., D.N.B., G.M.O., P.E.O., J.F.O., L.C.R., L.R.L., A.M.R., M.S., A.T., I.G.V., Z.W., and S.W. collected data; J.F. participated in the design of the study and made the painted illustrations; J.C.S. and C.R. participated in the design of the study and helped draft the manuscript; B.D. participated in the design and coordination of the study and helped draft the manuscript. All authors critically revised and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Financial support was provided by the Frimodt-Heineke Foundation, Svend G. Fiedler and wife's Foundation, and the PICA scholarship to J.S. A.M.M.G. was supported by the Spanish Ministry of Education; P.K.M. and J.V.B. were supported by a CAPES-PDSE scholarship (99999.012341/2013-04 and 99999.008012/2014-08, respectively); A.C.B. by the OTICON Fonden; P.A.C. by the David Lack studentship from the British Ornithologists’ Union and Wolfson College, University of Oxford; C.L. by the CACyPI-Uatx-2013; M.A.M. by the Universidad Estatal a distancia (UNED), Costa Rica, and the Senckenberg Biodiversity and Climate Research Centre (BIK-F), Frankfurt, Germany; L.R.L. by FAEP and Unicamp; T.T.I. by the Frimodt-Heineke Foundation, the Knud Højgaard Foundation, and Faculty of Natural Sciences, University of Aarhus. FAPESP supported A.C.A. and M.S.; FAPEMIG supported P.K.M. and P.E.O.; CNPq supported A.C.A., G.M.O., L.C.R., L.R.L., M.S., P.K.M., P.E.O., I.G.V., and J.V.B.; CAPES supported F.P.A., F.M.G.L.C., L.R.L., L.C.R., and J.V.B.; and FUNDECT supported A.C.A. J.S., A.M.M.G., P.K.M., D.N.B., Z.W.; C.R. and B.D. thank the Danish National Research Foundation for its support of the Center for Macroecology, Evolution and Climate. J.C.S. was supported by the European Research Council (ERC-2012-StG-310886-HISTFUNC).
References
- 1.Bascompte J, Jordano P. 2007. Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Syst. 38, 567–593. ( 10.1146/annurev.ecolsys.38.091206.095818) [DOI] [Google Scholar]
- 2.Miller TE, Burns JH, Munguia P, Walters EL, Kneitel JM, Richards PM, Mouquet N, Buckley HL. 2005. A critical review of twenty years’ use of the resource-ratio theory. Am. Nat. 165, 439–448. ( 10.1086/428681) [DOI] [PubMed] [Google Scholar]
- 3.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269. ( 10.1146/annurev.ecolsys.39.110707.173430) [DOI] [Google Scholar]
- 4.Dyer LA, et al. 2007. Host specificity of Lepidoptera in tropical and temperate forests. Nature 448, 696–699. ( 10.1038/nature05884) [DOI] [PubMed] [Google Scholar]
- 5.Schleuning M, et al. 2012. Specialization of mutualistic interaction networks decreases toward tropical latitudes. Curr. Biol. 22, 1925–1931. ( 10.1016/j.cub.2012.08.015) [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard B, et al. 2011. Specialization in plant–hummingbird networks is associated with species richness, contemporary precipitation and quaternary climate-change velocity. PLoS ONE 6, e25891 ( 10.1371/journal.pone.0025891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen JM, Jordano P. 2002. Geographic patterns in plant-pollinator mutualistic networks. Ecology 83, 2416–2424. ( 10.1890/0012-9658) [DOI] [Google Scholar]
- 8.Ollerton J, Cranmer L. 2002. Latitudinal trends in plant-pollinator interactions: are tropical plants more specialised? Oikos 98, 340–350. ( 10.1034/j.1600-0706.2002.980215.x) [DOI] [Google Scholar]
- 9.Dobzhansky T. 1950. Evolution in the tropics. Am. Sci. 38, 209–221. [Google Scholar]
- 10.MacArthur RH. 1969. Patterns of communities in the tropics. Biol. J. Linn. Soc. 1, 19–30. ( 10.1111/j.1095-8312.1969.tb01809.x) [DOI] [Google Scholar]
- 11.Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120. ( 10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schemske DW. 2002. Ecological and evolutionary perspectives on the origins of tropical diversity. In Foundations of tropical forest biology (eds RL Chazdon, TC Whitmore), pp. 163–173. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 13.Srivastava DS, Lawton JH. 1998. Why more productive sites have more species: an experimental test of theory using tree-hole communities. Am. Nat. 152, 510–529. ( 10.1086/286187) [DOI] [PubMed] [Google Scholar]
- 14.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.2307/2458977) [DOI] [Google Scholar]
- 15.Abrahamczyk S, Kessler M. 2010. Hummingbird diversity, food niche characters, and assemblage composition along a latitudinal precipitation gradient in the Bolivian lowlands. J. Ornithol. 151, 615–625. ( 10.1007/s10336-010-0496-x) [DOI] [Google Scholar]
- 16.Maruyama PK, Oliveira GM, Ferreira C, Dalsgaard B, Oliveira PE. 2013. Pollination syndromes ignored: importance of non-ornithophilous flowers to neotropical savanna hummingbirds. Naturwissenschaften 100, 1061–1068. ( 10.1007/s00114-013-1111-9) [DOI] [PubMed] [Google Scholar]
- 17.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 18.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 19.Dalsgaard B, et al. 2013. Historical climate-change influences modularity and nestedness of pollination networks. Ecography 36, 1331–1340. ( 10.1111/j.1600-0587.2013.00201.x) [DOI] [Google Scholar]
- 20.González AM, et al. 2015. The macroecology of phylogenetically structured hummingbird–plant networks. Glob. Ecol. Biogeogr. 24, 1212–1224. ( 10.1111/geb.12355) [DOI] [Google Scholar]
- 21.Svenning J-C. 2003. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol. Lett. 6, 646–653. ( 10.1046/j.1461-0248.2003.00477.x) [DOI] [Google Scholar]
- 22.Araújo MB, Nogués-Bravo D, Diniz-Filho JAF, Haywood AM, Valdes PJ, Rahbek C. 2008. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 31, 8–15. ( 10.1111/j.2007.0906-7590.05318.x) [DOI] [Google Scholar]
- 23.Araújo MB, Pearson RG. 2005. Equilibrium of species’ distributions with climate. Ecography 28, 693–695. ( 10.1111/j.2005.0906-7590.04253.x) [DOI] [Google Scholar]
- 24.Stevens G. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256. ( 10.1086/284913) [DOI] [Google Scholar]
- 25.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927. ( 10.1086/285580) [DOI] [PubMed] [Google Scholar]
- 28.Colwell RK, Hurtt GC. 1994. Nonbiological gradients in species richness and a spurious rapoport effect. Am. Nat. 144, 570–595. ( 10.2307/2462939) [DOI] [Google Scholar]
- 29.Gaston KJ, Blackburn TM, Spicer JI. 1998. Rapoport's rule: time for an epitaph? Trends Ecol. Evol. 13, 70–74. ( 10.1016/S0169-5347(97)01236-6) [DOI] [PubMed] [Google Scholar]
- 30.Laube I, Korntheuer H, Schwager M, Trautmann S, Rahbek C, Böhning-Gaese K. 2013. Towards a more mechanistic understanding of traits and range sizes. Glob. Ecol. Biogeogr. 22, 233–241. ( 10.1111/j.1466-8238.2012.00798.x) [DOI] [Google Scholar]
- 31.Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, Svenning J-C. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664. ( 10.1126/science.1210173) [DOI] [PubMed] [Google Scholar]
- 32.Stiles FG. 1981. Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann. Missouri Bot. Gard. 68, 323–351. ( 10.2307/2398801) [DOI] [Google Scholar]
- 33.Stiles FG. 1978. Ecological and evolutionary implications of bird pollination. Am. Zool. 18, 715–727. ( 10.2307/3882531) [DOI] [Google Scholar]
- 34.Temeles EJ, Kress WJ. 2003. Adaptation in a plant–hummingbird association. Science 300, 630–633. ( 10.1126/science.1080003) [DOI] [PubMed] [Google Scholar]
- 35.McGuire JA, Witt CC, Remsen JV Jr, Corl A, Rabosky DL, Altshuler DL, Dudley R. 2014. Molecular phylogenetics and the diversification of hummingbirds. Curr. Biol. 24, 910–916. ( 10.1016/j.cub.2014.03.016) [DOI] [PubMed] [Google Scholar]
- 36.Maruyama P, Vizentin-Bugoni J, Dalsgaard B, Sazima I, Sazima M. 2015. Nectar robbery by a hermit hummingbird: association to floral phenotype and its influence on flowers and network structure. Oecologia 178, 783–793. ( 10.1007/s00442-015-3275-9) [DOI] [PubMed] [Google Scholar]
- 37.Banašek-Richter C, Cattin MF, Bersier LF. 2004. Sampling effects and the robustness of quantitative and qualitative food-web descriptors. J. Theor. Biol. 226, 23–32. ( 10.1016/S0022-5193(03)00305-9) [DOI] [PubMed] [Google Scholar]
- 38.Rahbek C, Graves GR. 2000. Detection of macro-ecological patterns in South American hummingbirds is affected by spatial scale. Proc. R. Soc. Lond. B 267, 2259–2265. ( 10.1098/rspb.2000.1277) [DOI] [Google Scholar]
- 39.Rahbek C, Gotelli NJ, Colwell RK, Entsminger GL, Rangel TF, Graves GR. 2007. Predicting continental-scale patterns of bird species richness with spatially explicit models. Proc. R. Soc. B 274, 165–174. ( 10.1098/rspb.2006.3700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holt BG, et al. 2013. An update of Wallace's zoogeographic regions of the world. Science 339, 74–78. ( 10.1126/science.1228282) [DOI] [PubMed] [Google Scholar]
- 41.Jetz W, Rahbek C. 2002. Geographic range size and determinants of avian species richness. Science 297, 1548–1551. ( 10.1126/science.1072779) [DOI] [PubMed] [Google Scholar]
- 42.Blüthgen N, Menzel F, Blüthgen N. 2006. Measuring specialization in species interaction networks. BMC Ecol. 6, 9 ( 10.1038/nature03450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dormann C, Gruber B, Fründ J. 2008. Introducing the bipartite package: analysing ecological networks. R News 8, 8–11. [Google Scholar]
- 44.Fründ J, McCann KS, Williams NM. 2015. Sampling bias is a challenge for quantifying specialization and network structure: lessons from a quantitative niche model. Oikos ( 10.1111/oik.02256) [DOI] [Google Scholar]
- 45.Vizentin-Bugoni J, Maruyama PK, Debastiani VJ, Duarte L, Dalsgaard B, Sazima M. 2016. Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant–hummingbird network. J. Anim. Ecol. 85, 262–272. ( 10.1111/1365-2656.12459) [DOI] [PubMed] [Google Scholar]
- 46.Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N. 2010. Defining and measuring ecological specialization. J. Appl. Ecol. 47, 15–25. ( 10.1111/j.1365-2664.2009.01744.x) [DOI] [Google Scholar]
- 47.Vázquez DP, Aizen MA. 2003. Null model analyses of specialization in plant–pollinator interactions. Ecology 84, 2493–2501. ( 10.1890/02-0587) [DOI] [Google Scholar]
- 48.Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N. 2007. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346. ( 10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- 49.Schleuning M, et al. 2014. Ecological, historical and evolutionary determinants of modularity in weighted seed-dispersal networks. Ecol. Lett. 17, 454–463. ( 10.1111/ele.12245) [DOI] [PubMed] [Google Scholar]
- 50.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 51.Singarayer JS, Valdes PJ. 2010. High-latitude climate sensitivity to ice-sheet forcing over the last 120kyr. Quat. Sci. Rev. 29, 43–55. ( 10.1016/j.quascirev.2009.10.011) [DOI] [Google Scholar]
- 52.Terborgh J. 1971. Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology 52, 23–40. ( 10.2307/1934735) [DOI] [Google Scholar]
- 53.Feinsinger P, Colwell R, Terborgh J, Chaplin S. 1979. Elevation and the morphology, flight energetics, and foraging ecology of tropical hummingbirds. Am. Nat. 113, 481–497. ( 10.1086/283408) [DOI] [Google Scholar]
- 54.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055. ( 10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 55.Burnham K, Anderson D. 2002. Model selection and multimodel inference: a practical information-theoretic approach. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 56.Shipley B. 2002. Cause and correlation in biology: a user‘s guide to path analysis, structural equations and causal inference Cambridge, UK: Cambridge University Press. [Google Scholar]
- 57.Hooper D, Coughlan J, Mullen M. 2008. Structural equation modelling: guidelines for determining model fit. Journal of Business Research Methods 6, 53–60. [Google Scholar]
- 58.Grace JB, Schoolmaster DR, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. 2012. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, 1–44. ( 10.1890/ES12-00048.1) [DOI] [Google Scholar]
- 59.Sandom C, Dalby L, Fløjgaard C, Kissling WD, Lenoir J, Sandel B, Trøjelsgaard K, Ejrnæs R, Svenning J-C. 2013. Mammal predator and prey species richness are strongly linked at macroscales. Ecology 94, 1112–1122. ( 10.1890/12-1342.1) [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Kissling WD, He F. 2013. Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. J. Biogeogr. 40, 1131–1142. ( 10.1111/jbi.12063) [DOI] [Google Scholar]
- 61.Ferger SW, Schleuning M, Hemp A, Howell KM, Böhning-Gaese K. 2014. Food resources and vegetation structure mediate climatic effects on species richness of birds. Glob. Ecol. Biogeogr. 23, 541–549. ( 10.1111/geb.12151) [DOI] [Google Scholar]
- 62.Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. ( 10.18637/jss.vo48.i02) [DOI] [Google Scholar]
- 63.Jetz W, Rahbek C, Colwell RK. 2004. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol. Lett. 7, 1180–1191. ( 10.1111/j.1461-0248.2004.00678.x) [DOI] [Google Scholar]
- 64.Colwell RK, Lees DC. 2000. The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol. Evol. 15, 70–76. ( 10.1016/S0169-5347(99)01767-X) [DOI] [PubMed] [Google Scholar]
- 65.Lessard J-P, Borregaard MK, Fordyce JA, Rahbek C, Weiser MD, Dunn RR, Sanders NJ. 2012. Strong influence of regional species pools on continent-wide structuring of local communities. Proc. R. Soc. B 279, 266–274. ( 10.1098/rspb.2011.0552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carstensen DW, Lessard J-P, Holt BG, Borregaard MK. 2013. Introducing the biogeographic species pool. Ecography 36, 1–9. ( 10.1111/j.1600-0587.2013.00329.x) [DOI] [Google Scholar]
- 67.Dalsgaard B, González AMM, Olesen JM, Ollerton J, Timmermann A, Andersen LH, Tossas AG. 2009. Plant-hummingbird interactions in the West Indies: floral specialisation gradients associated with environment and hummingbird size. Oecologia 159, 757–766. ( 10.2307/40309943) [DOI] [PubMed] [Google Scholar]
- 68.Cruden RW. 1972. Pollinators in high-elevation ecosystems: relative effectiveness of birds and bees. Science 176, 1439–1440. ( 10.2307/1734592) [DOI] [PubMed] [Google Scholar]
- 69.Fjeldså J, Lambin E, Mertens B. 1999. Correlation between endemism and local ecoclimatic stability documented by comparing Andean bird distributions and remotely sensed land surface data. Ecography 22, 63–78. ( 10.2307/3683208) [DOI] [Google Scholar]
- 70.Vázquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 71.Weir JT. 2006. Divergent timing and patterns of species accumulation in lowland and highland neotropical birds. Evolution 60, 842–855. ( 10.1554/05-272.1) [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Moreno J, Arctander P, Fjeldså J. 1999. Strong diversification at the treeline among Metallura hummingbirds. Auk, 116, 702–711. ( 10.2307/4089331) [DOI] [Google Scholar]
- 73.Marske KA, Rahbek C, Nogués-Bravo D. 2013. Phylogeography: spanning the ecology-evolution continuum. Ecography 36, 1169–1181. ( 10.1111/j.1600-0587.2013.00244.x) [DOI] [Google Scholar]
- 74.Sebastián-González E, Dalsgaard B, Sandel B, Guimarães PR. 2015. Macroecological trends in nestedness and modularity of seed-dispersal networks: human impact matters. Glob. Ecol. Biogeogr. 24, 293–303. ( 10.1111/geb.12270) [DOI] [Google Scholar]
- 75.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 76.Ollerton J, Erenler H, Edwards M, Crockett R. 2014. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. ( 10.1126/science.1257259) [DOI] [PubMed] [Google Scholar]
- 77.Blüthgen N. 2010. Why network analysis is often disconnected from community ecology: a critique and an ecologist's guide. Basic Appl. Ecol. 11, 185–195. ( 10.1016/j.baae.2010.01.001) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Location, network characteristics, and SRS (the proportion of SRS) values of each hummingbird–plant network is presented in the electronic supplementary material, table S1. The same dataset has also been used and described for the analyses in Martin González et al. [20].