ABSTRACT
Cytotoxic T cells are considered crucial for antitumor immunity and their induction is the aim of various immunotherapeutic strategies. High frequencies of tumor-specific CD8+ T cells alone, however, are no guarantee for long-term tumor control. Here, we analyzed the functionality of tumor-specific CD8+ T cells in melanoma patients upon dendritic cell vaccination by measuring multiple T cell effector functions considered crucial for anticancer immunity, including the expression of pro-inflammatory cytokines, chemokines and cytotoxic markers (IFNγ, TNFα, IL-2, CCL4, CD107a). We identified small numbers of multifunctional (polyfunctional) tumor-specific CD8+ T cells in several patients and dendritic cell therapy was able to improve the functionality of these pre-existing tumor-specific CD8+ T cells. Generated multifunctional CD8+ T cell responses could persist for up to ten years and within the same patient functionality could vary greatly for the different vaccination antigens. Importantly, after one cycle of DC vaccination highly functional CD8+ T cells were only detected in patients displaying prolonged overall survival. Our results shed light on the dynamics of multifunctional tumor-specific CD8+ T cells during metastatic melanoma and reveal a new feature of dendritic cell vaccination in vivo.
KEYWORDS: Cancer, dendritic cell vaccination, immunotherapy, melanoma, multifunctional T cells, polyfunctional T cells, T cell function
Introduction
T cells are crucial mediators of anticancer immunity and well-established in vitro and in vivo experiments proved their ability to destroy tumor cells.1,2 The presence of circulating, tumor-infiltrating lymphocytes in cancer patients is linked to improved survival.3,4 Monoclonal antibodies counteracting T cell suppression by inhibiting CTLA-4 (Ipilimumab) and PD-1 (Pembrolizumab) signaling, provoke unprecedented response rates in metastatic melanoma patients.5,6 Interestingly, in these, as well as in previous immunotherapeutic strategies aiming at the induction of cancer-specific T cell responses, a subset of patients experienced remarkably long lasting responses, suggesting a link between T cell activation and long-term tumor control.7,8
To improve clinical efficacy of cancer immunotherapies, and our understanding of the mechanisms underlying favorable responses, it is essential to find biomarkers that distinguish responders from non-responders. Recent studies in the field of HIV highlight T cell functionality as an important indicator for an effective immune response, as T cell lymphocytes that simultaneously expressed more than three effector functions correlated with disease control.9,10 Recent studies also report on the presence of polyfunctional T cells in melanoma patients and show that adoptively transferred T cells can maintain a multifunctional phenotype.11,12 Little, however, is known about the in vivo induction of multifunctional T cells by immunotherapeutic intervention, their persistence and their impact on tumor control and survival.
To date, various T cell functions have been considered important for long-term tumor control. Whereas cytotoxicity is necessary for the induction of apoptosis – a hallmark of anticancer immunity – secretion of proinflammatory cytokines, such as TNFα, CCL4 and IFNγ, were shown to play an important role as well.1,13,14 Tumor cells treated with IFNγ upregulate antigen processing and presentation pathways leading to increased immunogenicity and IFNγ signaling during priming of T cells polarizes responses toward the favorable TH1 type.13 Combined with TNFα, IFNγ is able to induce permanent senescence in cancer cells.14,15 CCL4, induces the secretion of IL-12 by dendritic cells (DCs), and together with TNFα, enhances the recruitment of DC precursors to peripheral tissues.16 Finally, IL-2 allows cytotoxic CD8+ T cell lymphocytes to expand independently from CD4+ helper T cells and enhances NK cell activity.15
In this study we retrospectively analyzed the tumor-specific CD8+ T cell responses in peripheral blood from metastatic melanoma patients enrolled in ongoing dendritic cell vaccination trials. Patients were followed during the course of their disease and functional CD8+ T cell responses were assessed using a flow cytometry-based assay to simultaneously measure the production of the proinflammatory cytokines IFNγ, TNFα, CCL4, as well as IL-2, and the expression of CD107a as surrogate marker for cytotoxicity.
Our findings show that metastatic melanoma patients can harbor naturally-induced, tumor-specific multifunctional T cells, that dendritic cell based vaccination enhances the functionality of these cells and that induced responses can last for several years. Finally, in our patient cohort highly multifunctional T cell responses seem to preferentially appear in patients with prolonged survival.
Results
Study design and patient characteristics
The primary aim of this retrospective study was to explore the functional composition of the tumor-specific CD8+ T cell responses in late-stage melanoma patients and to investigate the presence of multifunctional T cells. For this purposes we identified a set of 19 melanoma patients that previously displayed tumor-specific CD8+ T cell responses in blood or skin after DC vaccination at the Radboud university medical centre (Table 1). As only few patients develop detectable tumor-specific CD8+ T cell responses during the therapy, the cohort was rather limited and due to the retrospective nature of the study sample availability was suboptimal. Patients were vaccinated in our institute from 1999 to 2013 according to various protocols using autologous, antigen-loaded DCs (Table S1 for protocol details). In short, patients were injected with ex-vivo generated, mature DCs, loaded with peptides derived from the gp100- or tyrosinase-proteins. Patients were injected three times per vaccination cycle. After vaccination, the induction of antigen-specific T cell responses was assessed using both a DTH-skin test and a tetramer-staining assay for peripheral blood T cells. Of 19 patients tested in this study, six did not display detectable, functional T cell responses and are therefore excluded from analysis of functionality. In some patients we observed a disparity between the detection of responding tetramer-positive CD8+ T cells in peripheral blood as assessed by routine immunomonitoring during the original clinical trials (Table 1). This could be based on different TCR affinities necessary to trigger an effector response or to strongly bind a tetramer as described by several studies.17,18 Overall survival of included patients ranged from 5 to 179 mo and the median survival time in this cohort of long-term and intermediate tumor controllers was 29 mo.
Table 1.
Patient characteristics. Daclizumab (0.5 mg/kg) was administered 4 or 8 d before the first DC vaccination to deplete regulatory T cells. DTH – Delayed-type hypersensitivity reaction induced by injection of antigen-loaded dendritic cells. Skin-test infiltrating lymphocytes were analyzed for antigen-specificity and functionality 40 – no specific T cells found, + tetramer-specific T cells, +++ functional, specific T cells. TT – Targeted therapy with BRAF inhibitor. I – Immunotherapy with anti-CTLA4 antibodies. RT – Radiotherapy. TMZ – Chemotherapy with Temozolomide.
| Tumor-specific CD8+ T cells |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | Stage at baseline | AJCC stage | Sites of disease | Systemic pretreatment | Pre Blood | Post blood | Post DTH | Functional response blood4 | PFS | OS | Response | Salvage treatment |
| I-B-04 | 31 | f | M1c | IV | Liver | - | NA | +++ | + | 162+ | 162+ | CR | None | |
| I-C-04a | 66 | m | M1a | IV | LN | - | + | +++ | + | 13 | 43 | SD | None | |
| I-C-09 | 33 | f | M1a | IV | LN | - | - | +++ | + | 139 | 180+ | SD1 | Surgery | |
| II-E-08a | 56 | m | N3 | IIIc | LN | - | + | + | +++ | + | 9 | 51 | NED | DTIC |
| III-A-04a | 28 | f | M1c | IV | LN, retroperitoneal | Daclizumab 4 | + | - | + | <4 | 13 | PD | Unknown | |
| III-A-06 | 68 | f | M1c | IV | Lung, LN, skin | Daclizumab 4 | - | + | - | 5 | 25 | PD | DTIC | |
| III-A-07 | 25 | m | M1c | IV | Liver, lung, LN, skin | Daclizumab 4 | - | + | - | <4 | 7 | PD | DTIC | |
| III-B-01 | 53 | f | M1b | IV | Lung, LN, skin | Daclizumab 8 | - | + | - | <4 | 10 | PD | DTIC | |
| IV-A-01a | 69 | m | M1a | IV | LN, skin | DTIC | + | +++ | + | 39 | 128 | MR | Surgery, I | |
| IV-C-05a | 31 | f | M1c | IV | Lung, skin, breast | - | - | + | + | + | 16 | 90+ | SD2 | Surgery |
| IV-C-12a | 38 | f | N1b | IIIb | LN | - | + | + | +++ | + | 8 | 16 | NED | DTIC |
| IV-D-03a | 42 | f | M1c | IV | Liver, lung | DTIC | - | + | - | + | 6 | 26 | SD | TMZ |
| IV-D-06a | 61 | f | M1a | IV | LN | - | + | + | +++ | + | 15 | 24 | SD | None |
| VI-B-01a | 58 | m | M1a | IV | Skin3 | - | - | + | +++ | + | 18 | 22 | SD | Surgery, TT |
| VI-B-03 | 48 | f | M1a | IV | LN | - | - | - | - | - | 7 | 40 | SD | TT, I |
| VI-B-08 | 54 | f | M1c | IV | Skin, muscle, intestine | - | + | + | +++ | + | 15 | 29 | MR | I, RT, TT, Surgery |
| VI-B-10 | 66 | f | M1c | IV | Lung, skin, mesentery | - | - | - | - | - | <4 | 32+ | PD | TT, I |
| VI-B-11 | 48 | m | M1c | IV | Lung, mesentery, intestine | - | + | + | - | - | <4 | 6 | PD | None |
| VI-B-13 | 46 | m | M1b | IV | LN, lung | - | - | + | +++ | + | 29+ | 29+ | CR | None |
aSamples were stained with tetrameric MHC complexes.
1Not evaluable for clinical response because no target lesion at start of vaccination
2Partial remission of a distant LN metastasis, CR after surgery for >10y
3The term ‘skin’ both includes subcutaneous and/or cutaneous metastasis.
4A functional response is determined positive when after background-subtraction more than 0.2% of CD8+ T cells express a functional marker.
Few multifunctional, tumor-specific CD8+ T cells are detected in metastatic melanoma patients
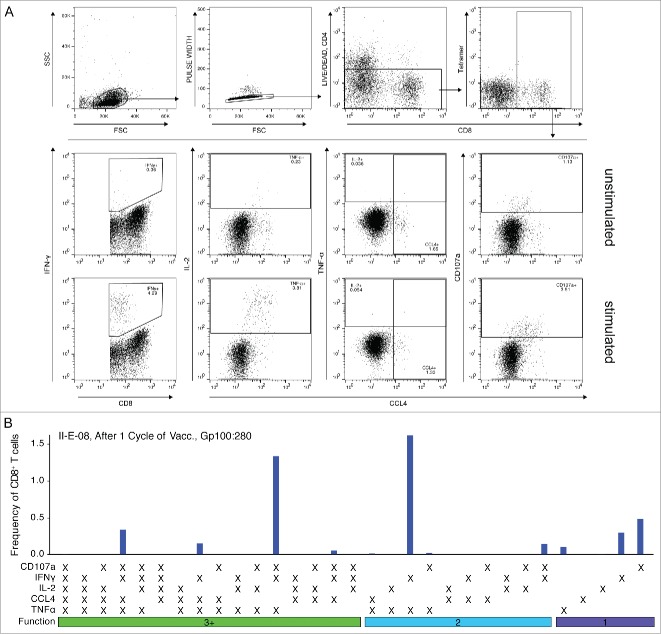
To standardize monitoring, we based our initial analysis on the period after one cycle of dendritic cell vaccination, thus 1 to 8 mo after apheresis, where samples were available for all patients (Fig. 1). Functionality of tumor-specific CD8+ T cells was assessed by measuring the simultaneous expression of IFNγ, TNFα, CCL4, IL-2 and CD107a upon antigenic stimulation using a flow cytometry-based assay (Fig. 2A). As tetramer-staining diminished upon stimulation with specific peptide or SEB in most tested samples and remaining tetramer-positive T cells were skewed in some cases toward a highly multifunctional phenotype we gated on the total CD8+ T cell population for further analysis (Fig. S1). Fig. 2 displays the results of a representative response showing the expression of the functional markers by CD8+ T cells when stimulated with the specific peptide. To analyze the complexity of the detected T cell response we employed a Boolean gating algorithm that revealed the functional response patterns as represented by the 31 possible combinatorial phenotypes (Fig. 2B). All samples were background-subtracted using the CD28/CD49d control.
Figure 1.
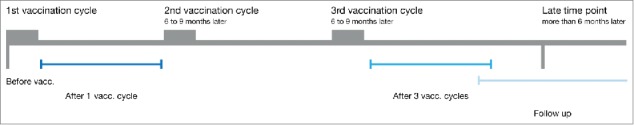
Overview of the DC vaccination therapy treatment schedule. Melanoma patients underwent leukapharesis at the beginning of each vaccination cycle. The leukapharesis samples of the first DC vaccination cycle are referred to as “before vaccination” samples. After the first cycle of three biweekly DC injections, blood was drawn at several time points. These samples are referred to as “after one cycle of DC vaccination” samples. If the patient showed no progressive disease, he/she was qualified for a second and third cycle of DC vaccination. Blood drawn within 8 mo after the third cycle of DC vaccination therapy are referred to as “after three cycles of DC vaccination”. Samples drawn after this time point are referred to as “follow-up”.
Figure 2.
Gating strategy to measure the functional CD8+ T cell response using flow cytometry and response pattern. CD8+ T cells were stimulated as described using αCD28/αCD49-coated beads with or without specific peptide (unstimulated, stimulated) and the expression of IFNγ, TNFα, CCL4, IL-2 and CD107a was measured. (A) Shown is the gating strategy for one representative sample. Expression of functional markers was determined using single gates. (B) Stimulated samples were background-subtracted using the unstimulated control and functional combinations were computed using the Boolean gating algorithm included in FlowJo. Displayed is the frequency of CD8+ T cells showing each of the 31 possible functional patterns. Non-responding cells are not shown.
After one cycle of DC vaccination, the frequency of tumor-specific, responding CD8+ T cells was low in most tested individuals. Nevertheless, several patients showed strong responses against various vaccination antigens with up to 5% responding cells (Fig. 3A). In total, functional CD8+ T cell responses were detected in 10 of 19 patients after one cycle of DC vaccination and CD8+ T cells expressing CD107a clearly dominated the immune response against all tested antigens (Fig. 3B). Expression of TNFα was measured in ca. twenty percent of responding cells and high fractions of CCL4 expressing cells were found within the tyrosinase-specific responses. In contrast, IFNγ expression contributed only marginally to most detected T cell responses. Likewise, only few CD8+ T cells expressed IL-2 possibly indicating a predominance of effector memory T cells.15 Interestingly, none of the measured markers was consistently expressed by all CD8+ T cells emphasizing the importance of measuring several effector functions to assess anticancer immune responses comprehensively.
Figure 3.
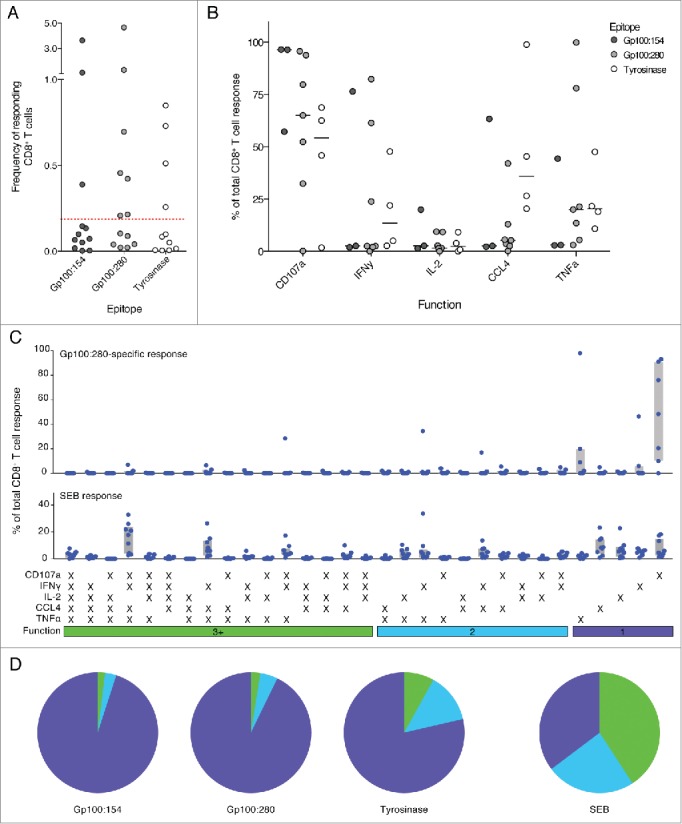
Few tumor-specific, multifunctional CD8+ T cells were detected in melanoma patients after one cycle of DC vaccination. (A) Responding CD8+ T cells were detected for all tested peptides. Most responses did however not reach the detection threshold of 0.2% responding cells. (Red dotted line) (B) The relative contribution of each functional marker to the total response was measured. Most tumor-specific CD8+ T cells expressed CD107a. Only few cells expressed IL-2. Lines indicate the median. (C) and (D) Normalized distribution of functional T cell response patterns. Combinational analysis of expressed markers revealed small fractions of highly functional CD8+ T cells in a number of patients; most responding cells, however, were monofunctional. SEB-specific CD8+ T cells in contrast show high fractions of T cells with three and more functions and are mainly multifunctional. Gray bars depict the interquartile range.
Next we analyzed the functionality of the detected tumor-specific CD8+ T cells. Comparing responses between different individuals required normalization. For this purpose, the total frequency of responding CD8+ T cells was set to 100% and the contribution of each functional pattern was displayed as fraction of the total response. gp100:280-specific CD8+ T cells appeared to be mainly monofunctional as sole expression of CD107a was the most prevalent response pattern (Fig. 3C). Nevertheless, a subgroup of patients consistently harbored CD8+ T cells co-expressing three or more functional markers (multifunctional T cells). The combinatorial expression of all five functional markers, in contrast, was not observed. Interestingly, gp100:280-specific CD8+ T cells acquired only a limited set of functional response patterns of which the most predominant functional phenotypes consisted of CD8+ T cells expressing IFNγ, CCL4 and/or TNFα with or without CD107a (Fig. 3C). Immune responses against the other vaccination epitopes gp100:154 and tyrosinase were similarly dominated by monofunctional, CD107a expressing cells (Fig. 3D). CD8+ T cells specific for tyrosinase appeared to show enhanced fractions of multifunctional T cells when compared to cells specific for the gp100 epitopes.
To investigate whether the tested melanoma patients were generally able to generate potent functional responses, CD8+ T cells were stimulated with Staphylococcal enterotoxin B (SEB). SEB crosslinks MHC molecules and T cell receptor complexes (TCR) leading to strong activation of T cells with various specificities. SEB-specific responses could be detected in all analyzed patients and multifunctional T cells dominated these responses (Fig. 3C, D).
In summary, in metastatic melanoma patients, despite intact CD8+ T cell responses to SEB, only low numbers of multifunctional tumor-specific CD8+ T cells could be detected after one cycle of DC vaccination.
Dendritic cell-vaccination therapy improves CD8+ T cell functionality
Next, we explored whether naturally induced multifunctional CD8+ T cells were present already before DC vaccination and if DC vaccination was able to boost the functionality of these cells. Interestingly, we identified naturally occurring tumor-specific T cell responses in various patients with magnitudes of up to 2.7% responding CD8+ T cells (Fig. 4). In general, CD107a-expressing CD8+ T cells dominated the spontaneously induced antitumor immune response and most cells were monofunctional.
Figure 4.
The functional CD8+ T cell response before DC vaccination was dominated by monofunctional cells. (A) Naturally occurring tumor-specific CD8+ T cell responses above detection threshold were detected for all tested peptides. (B) The relative contribution of each functional marker to the total response was similar to after one cycle of DC vaccination. Most tumor-specific CD8+ T cells expressed CD107a. Only few cells expressed IL-2. Lines indicate the median. (C) Combinational analysis of expressed markers revealed mainly monofunctional tumor-specific CD8+ T responses. SEB-specific CD8+ T cells in contrast displayed high fractions of T cells with three and more functions similar to the time point after one cycle of DC vaccination.
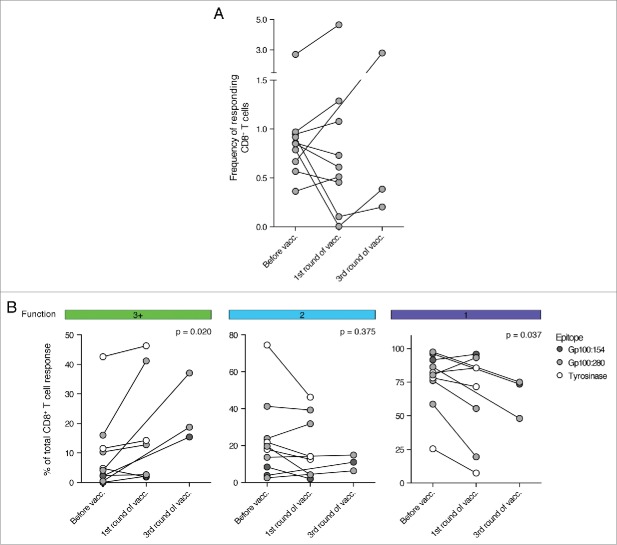
To analyze whether the functionality of these pre-existing T cell responses could be generally increased by DC vaccination, we matched each pre-vaccination response with the first post-vaccination response. For three patients we could not detect a tumor-specific CD8+ T cell response after one cycle of DC vaccination, despite the presence of such a response before vaccination. In these cases we analyzed peripheral blood mononuclear cells (PBMCs) at a later time point and could identify tumor-specific CD8+ T cell responses directly after the third cycle of vaccination. Interestingly, whereas no apparent change in the total frequency of responding CD8+ T cells could be detected (Fig. 5A), we could measure an increase in CD8+ T cell functionality after vaccination (Fig. 5B). Tumor-specific CD8+ T cell responses at 1 or 3 cycles of DC vaccination showed a significant increase in the fraction of multifunctional T cells. Consequently, the relative contribution of monofunctional CD8+ T cells to the overall tumor-specific T cell response declined.
Figure 5.
DC vaccination improved the functionality of pre-existing, tumor-specific CD8+ T cell responses. The functionality of tumor-specific CD8+ T cells was measured before and at the first available time point after DC vaccination. (A) Shown is the development of the frequency of responding CD8+ T cells during the course of DC therapy. No significant trend could be observed. (B) The fraction of multifunctional CD8+ T cells increased during DC vaccination therapy whereas the contribution of monofunctional T cells to the tumor-specific CD8+ T cell response declined. (n = 10, Wilcoxon matched-pairs signed rank test, samples after 1 and 3 cycles of DC vaccination are analyzed together as one “after vaccination” time point).
These data, thus, demonstrate that DC-based immunotherapy was able to enhance the functionality of pre-existing CD8+ T cells in the analyzed patient cohort.
Highly functional tumor-specific CD8+ T cells are enriched in long-term tumor controllers
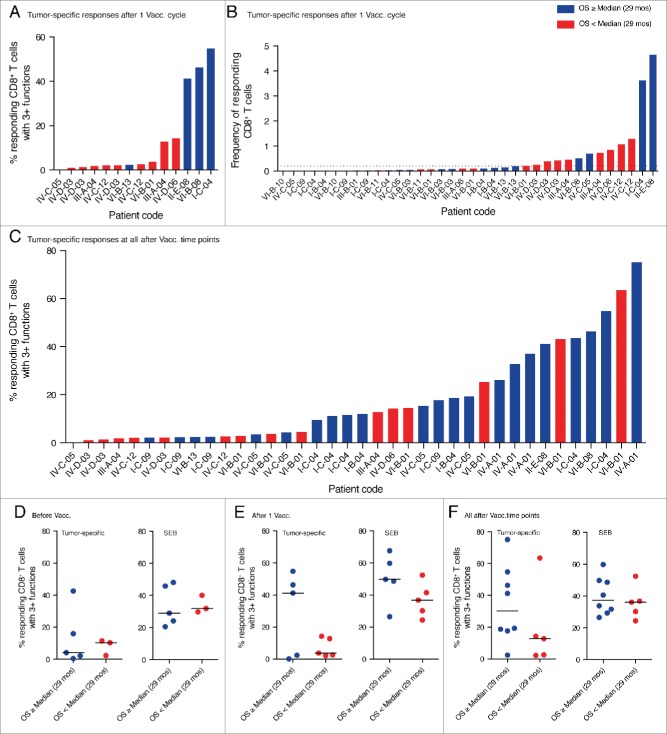
Next we investigated the generation of multifunctional CD8+ T cells in relation to patient survival. Interestingly, we found that after one cycle of dendritic cell vaccination tumor-specific, highly functional CD8+ T cell responses were only present in patients with overall survival above or equal to the median of analyzed patients (29 mo) (Fig. 6A). Monofunctional CD8+ T cell responses, in contrast, were detected in all patients. The highest magnitude of responding CD8+ T cells (frequency of total responding CD8+ T cells) was found in patients with above median overall survival, but strong responses could also be observed in patients with overall survival below median (Fig. 6B).
Figure 6.
Highly functional CD8+ T cell responses preferentially appeared in patients with prolonged survival. (A) Shown is the percentage of multifunctional T cells within the tumor-specific CD8+ T cell responses detected after one cycle of DC vaccination. High fractions of multifunctional T cells only appeared in responses of patients with above median overall survival. (B) Depicted is the total frequency of responding, tumor-specific CD8+ T cells after one cycle of DC vaccination. The strongest responses were observed in patients with prolonged survival but also patients with below median survival displayed high frequencies of responding CD8+ T cells. The dotted line indicates the cut-off value for positivity of 0.2% specifically responding cells. (C) Shown is the percentage of multifunctional T cells within the tumor-specific CD8+ T cell responses detected after any cycle of DC vaccination including follow up. Highly functional T cell responses preferentially appeared in patients with prolonged overall survival. Multiple bars for the same patient represent responses to different tumor antigens (A and B) and/or at different time points (C). (D, E, F) To account for responses against multiple tested antigens by the same individual, for each patient only the one response with the highest fraction of multifunctional T cells is shown. Highly functional T cell responses preferentially developed in patients with prolonged survival whereas there is no difference in the SEB-specific response.
We previously recognized that some patients only develop detectable CD8+ T cell responses after several cycles of DC vaccination. For this reason, we decided to extend our analysis to time points beyond one cycle of DC vaccination if material was available. We observed that patients without detectable T cell response after one cycle of DC vaccination could develop such a response after two or three cycles. Interestingly, over the entire observation period highly multifunctional T cell responses were almost exclusively observed in patients with overall survival above median (Fig. 6C).
To account for responses against multiple tested antigens by the same individual, we then compared for each patient only the one response with the highest fraction of multifunctional T cells. After one cycle of vaccination high fractions of tumor-specific, multifunctional T cells only appeared in patients with prolonged survival (Fig. 6E) and over the entire observation period we could observe a trend toward enrichment of highly multifunctional T cells in patients with prolonged survival (Fig. 6F). Before vaccination, highly multifunctional CD8+ T cells were absent in most patients with the exception of one patient with above median overall survival (Fig. 6D). Interestingly, we could not observe such a trend for the SEB-induced responses, which contained high fractions of multifunctional T cells in all patients and at all time points.
Together, our data indicate that highly multifunctional tumor-specific CD8+ T cells appear predominantly in patients with above median overall survival, whereas poorly functional responses are observed in patients of both survival groups.
Multifunctional T cells show enhanced effector function as compared to monofunctional T cells
We assessed whether multifunctional T cells display enhanced effector functions. For this purpose, the median fluorescence intensity (MFI) of a set of detected CD8+ T cell responses was measured to estimate the relative cytokine expression by multifunctional versus monofunctional CD8+ T cells. Strikingly, in multifunctional CD8+ T cells the median expression of IFNγ and TNFα was four and five times higher, respectively, demonstrating significantly increased effector function (Fig. 7). In the few cases where multifunctional CD8+ T cells expressed IL-2, we could likewise see a strong increase in the MFI of these cells as compared to monofunctional cells.
Figure 7.
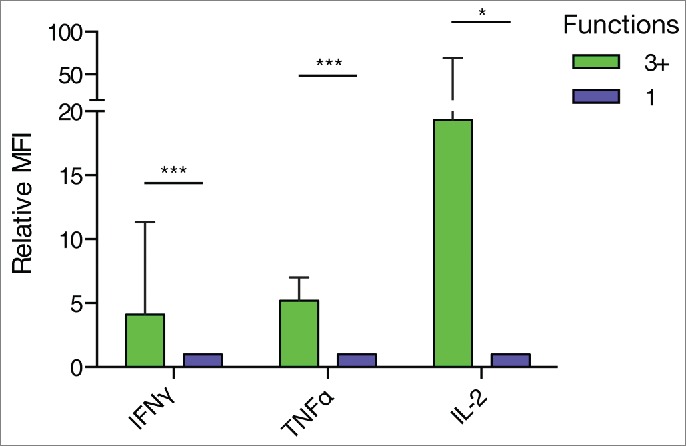
Multifunctional CD8+ T cells displayed elevated effector functions compared to monofunctional cells. The median fluorescence intensity of CD8+ T cells expressing three or more functions (3+) relative to CD8+ T cells expressing one function was analyzed. T cells expressing 3+ functions showed increased relative expression of IL-2, IFNγ and TNFα. (*** P ≤ 0.001, ** P ≤ 0.01, * P ≤ 0.05, Wilcoxon Signed Rank Test, IFNγ: n = 10, TNFα: n = 9; IL-2: n = 6).
Though rare in numbers, tumor-specific multifunctional CD8+ T cells with enhanced effector functions might thus be able to contribute significantly to the anticancer immune response.
Persistence of multifunctional CD8+ T cells in long-term tumor controllers
To analyze the dynamics of multifunctional T cells and their persistence in melanoma patients, we decided to follow four individuals during the entire course of their disease.
We could repeatedly detect multifunctional T cell responses in these patients over a time period of up to 128 mo (Fig. 8). Importantly, after the last cycle of vaccination, induced multifunctional CD8+ T cell responses persisted for several years, indicating the induction of long-lasting memory responses. In three of the four patients followed the highest fraction of multifunctional T cells was detectable in follow-up samples taken at later time points in life (up to 106 mo after the last cycle of DC vaccination).
Figure 8.

Multifunctional, tumor-specific CD8+ T cells persist for several years. Patients with satisfactory sample availability were followed during the course of their disease. Dotted lines indicate the frequency of responding, tumor-specific CD8+ T cells (right y-axis) and filled lines indicate the percentage of multifunctional T cells within these responses (left y-axis). Blue lines indicate the last administration of DC vaccination therapy. Orange lines indicate the time point of disease progression, red lines indicate the death of the patient. The functionality of DC vaccination-induced CD8+ T cell responses increased for most patients during the period of vaccination and remained steady afterwards. The functionality of T cell responses against different tumor antigens within the same patients developed differently. Induced multifunctional T cells persisted in some cases for more than 100 mo after last administration of DC vaccination.
T cell functionality appeared to be regulated on an antigen- or epitope-level as the fraction of multifunctional T cells developed differently for gp100:154 and gp100:280-specific T cells in patients VI-B-01 and I-C-04. Furthermore, the functionality of vaccine-induced CD8+ T cells developed independently from the SEB-induced CD8+ T cell responses (Fig. S2) and analysis of all samples with CD8+ T cell responses against more than one tumor peptide revealed that in most cases the functionality differed between antigens (Fig. S3). Furthermore, the fraction of multifunctional T cells developed independently from the magnitude of the T cell response and high fractions of multifunctional CD8+ T cells could be detected in responses of high and low magnitude (Fig. 8). To investigate whether the antigen-specific reduction of functionality was depending on TCR affinity or intrinsic defects in effector functions, selected samples with high numbers of Tetramer+ T cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycine (Iono) activating the TCR signaling cascade downstream of the TCR resulting in a strong avidity-independent stimulation. Despite TCR-independent stimulation, we observed a reduction in tetramer signal, although less pronounced as upon TCR-specific stimulation (Fig. S4A). Whereas the frequency of responding cells did not differ between total CD8+ and Tetramer+CD8+ T cells (Fig. S4B), functionality and functional composition did (Fig. S4C, D). We observed a total lack of IL-2 production in Tetramer+CD8+ T cells but not total CD8+ T cells stimulated with SEB or PMA/Iono. Likewise, peptide stimulated CD8+ T cells were not able to secrete IL-2. Furthermore, the functionality of Tetramer+CD8+ T cells as compared to total CD8+ T cells stimulated with PMA/Iono was reduced in all tested samples. Moreover, in two of the three samples CD8+ T cells stimulated with TAA-peptide showed further reduced functionality.
These data demonstrate that induced multifunctional CD8+ T cells are persisting for several years and that within the same patient the multifunctionality of CD8+ T cells appears to develop differently for each tested tumor antigens.
Discussion
Despite their established role in anticancer immunity, high frequencies of tumor-specific T cells in melanoma patients as measured by multimer-staining or IFNγ secretion have not been shown to guarantee tumor regression or survival benefit.4,19 A study by Weide et al. demonstrated a positive correlation between naturally occurring tumor-specific T cells in melanoma, as measured by secretion of one out of six cytokines.4 However, that study focused on monofunctional CD8+ T cells without an in-depth analysis of the combinatorial secretion of cytokines. Interestingly, studies on HIV, a disease with similar dissonance between T cell frequency and clinical outcome, identified CD8+ T cells exhibiting multiple functions as a prognostic biomarker.9,10 Here, we show that naturally occurring tumor-specific T cells in end-stage melanoma patients were predominantly monofunctional. Subsequent DC vaccination enhanced their functionality and induced multifunctional CD8+ T cells that persisted for up to 10 years. These results indicate a long-lasting memory protection, which is in line with our finding that high levels of multifunctional CD8+ T cells are enriched in patients with above median survival.
Especially this latter finding suggests an important role for multifunctional T cells in antitumor immunity. Distinct mechanisms may explain the extended tumor control: first, the concerted action of several anticancer functions allows a more comprehensive attack on the tumor. IFNγ and TNFα were shown to synergistically mediate cytotoxicity, and indeed, most long-term tumor controllers showed increased fractions of IFNγ+TNFα+CD107a+ CD8+ T cells.14,15 Alternatively, highly functional T cells as major producers of IFNγ and TNFα (Fig. 7) may boost local effector cell production. According to the “spark”-theory, vaccine-induced T cells might generate local conditions that favor in vivo priming of antitumor T cells, which in turn reject the tumor.19 In this respect, potent production of TH1 cytokines by vaccine-induced T cells might be more important than cytolytic activity.12,19 Furthermore, as the analysis of the MFI indicated, multifunctional T cells produce disproportionally high concentrations of effector cytokines, which could partly compensate for their low numbers. Despite their relatively low frequency, highly functional T cells thus appear to be a pivotal population of CD8+ T cells equipped with strong effector functions.
In this study, the majority of tumor-specific monofunctional CD8+ T cells displayed a CD107a+ phenotype and overall CD107a was the most abundantly expressed molecule. In contrast, antigen-specific CD8+ T cell responses in HIV patients, as measured by other groups, were dominated by CCL4 expression and in blood of HIV progressing patients high fractions of monofunctional CCL4+ CD8+ T cells were observed.9,10,20,21 However, high frequencies of CCL4+ monofunctional tumor-specific CD8+ T cells were absent in this study. As CCL4-binding to the CCR5 receptor can inhibit viral entry, production of CCL4 is likely one of the most important effector functions in HIV.22-25 In melanoma this mechanism does not play a role and cytotoxicity is more important hence CD107a expression. The expression of effector functions by antigen-specific CD8+ T cells can differ between diseases and adapt to generate the most effective CD8+ T cell response for a particular threat as previously shown for CD4+ T cells.26
Strikingly, we found that DC vaccination enhances the functionality of pre-existing tumor-specific CD8+ T cell responses. As this effect was restricted to a limited number of patients further improvements of the treatment regiment are necessary. Studies suggest that repeated, but limited exposure to high levels of antigen are important to maintain highly functional CD4+ T cells.15 In this respect, earlier and continued administration of booster vaccines, as practiced by some groups, might be beneficial for the persistent maintenance of multifunctional T cells. Our findings, that some patients only developed detectable T cell responses after two or three cycles of DC vaccination might further support this theory. Unfortunately, longitudinal analysis in our study was hampered by the limited number of patients with sufficient material available at all time points, making it difficult to deduce further trends or common dynamic patterns from these data.
Interestingly, the fraction of multifunctional T cells differed between the employed vaccination antigens in the same patient. Patient VI-B-01, for instance, developed strong CD8+ T cell responses against both the gp100:154 and the gp100:280 peptide with more than 3% and 2% responding CD8+ T cells, respectively. Nevertheless, the CD8+ T cell response against the gp100:154 peptide developed highly multifunctional T cells already after two cycles of vaccination, the response against gp100:280 in contrast remained monofunctional until late after the third cycle of vaccination. Similarly, the functionality of the gp100:154 and gp100:280 response in patient I-C-04 was unlinked. Furthermore, our data indicate differences in the functional composition of the CD8+ T cell responses against different antigens. Together, these results indicate that the fraction of multifunctional CD8+ T cells might be regulated on the clonal or antigen level. Several possible reasons come into account for this observation: dendritic cells are pulsed separately with the different peptides before injection into the patients and there could be differences in the expression of immunomodulatory factors. As all DCs were activated together, it is however more likely that differences in the T cell repertoire of the melanoma patients might be causative for the observed differences. The presence or absence of high avidity T cell clones before vaccination for example could be responsible for differences in functionality and Harari et al. showed that low-avidity clones are more multifunctional than high-avidity clones.27,28 Our data indicate that impaired signaling via the TCR – e.g. due to sub-optimal avidity between TCR and MHC:peptide complex – could be in part responsible for the reduced functionality observed in some patients. Stimulation of CD8+ T cells with PMA/Iono restored secretion of IFNγ, TNFα or CD107a, which was reduced when CD8+ T cells were stimulated with the relevant peptide. Furthermore, a recent study by Chiu et al. describes the influence of the antigen-concentration on the ability of T cells to perform multiple effector functions simultaneously.29 Despite the apparent influence of TCR signaling on CD8+ T cell functionality, we could observe a lack of IL-2 production by tumor-specific CD8+ T cells, which was independent of direct TCR engagement. Tetramer-specific CD8+ T cells activated with SEB or PMA/Iono as well as tumor peptide-stimulated CD8+ T cells were unable to secrete IL-2, whereas SEB- or PMA/Iono-stimulated total CD8+ T cells produced high amounts of this T cell growth factor. These results indicate an intrinsic defect of the analyzed tumor-specific CD8+ T cells to produce IL-2 independent of suboptimal TCR avidity and possibly due to a (terminal) differentiation or exhaustion state.15,30 Finally, selective induction of tolerance or exhaustion via e.g. regulatory T cells or myeloid-derived suppressor cells could account for the observed differences in functionality. Myeloid-derived suppressor cells, for example, are equipped with a variety of mechanism to suppress CD8+ T cells in an antigen-specific and non-specific way, e.g. via altering the specificity of TCRs by nitration of tyrosine residues.31-33
The five reported markers of functionality described here are likely not the only mediators of tumor control, could, however, represent surrogate markers for a complex and successful antitumor immune response. In a recent study, Ulloa-Montoya et al., employing an unbiased gene-expression analysis approach, came to similar conclusions and showed that the activation of interferon (IFN)-stimulated genes, specific chemokine genes (including CCL4), and the activation of immune effector function genes (cytotoxic pathways) is characteristic for regressing metastatic melanoma lesions after immunotherapy.34
Our data show that highly functional CD8+ T cells primarily appear in patients with extended overall survival. After one cycle of DC vaccination, high fractions of multifunctional T cells were only observed in patients with above median survival. When extending the analysis to time points after two and three cycles of vaccination similar results could be observed. Markedly, of the below-median survivors only patient VI-B-01 developed highly functional CD8+ T cell responses in this extended analysis. However, with 22 mo VI-B-01 displayed already prolonged overall survival compared to metastatic melanoma patients treated with e.g. chemotherapy. Although the retrospective study design and low patient numbers do not allow conclusive data on the causal relation it is tempting to speculate that multifunctional CD8+ T cells contribute to improved overall survival. Standardized studies, with large, diversified patient cohorts and fixed time points are needed to extend our findings and validate T cell functionality for routine immunomonitoring. Testing the five reported features is feasible with routine laboratory techniques allowing quick translation to local laboratories.
To summarize, in this study we could observe multifunctional, tumor-specific CD8+ T cell responses in metastatic melanoma patients that were preferably detected in individuals with extended overall survival. Furthermore, we showed that DC vaccination is able to improve CD8+ T cell functionality generating long-lasting, multifunctional CD8+ T cell responses.
Methods
Patients
PBMCs or peripheral blood lymphocytes (PBLs) were collected from melanoma patients with loco regional resectable disease (stage III) as well as irresectable loco regional or distant metastatic disease (stage IV) at different time points during the course of their disease (n = 19) (Table 1 and Table S1 for patient details). All patients underwent immunotherapy in our department as described in the original studies and indicated in Table S1.35-38 In short, patients underwent leukapheresis and either myeloid DCs or monocytes were isolated and the latter were differentiated to monocyte-derived DCs by incubating with IL-4 and GM-CSF. Subsequently, DCs were activated and loaded with antigen either by pulsing with gp100- or tyrosinase-derived peptides or by electroporation with TAA-coding mRNA (gp100, tyrosinase full protein). Finally, the prepared DC vaccination was administered to patients intravenously, intradermally or intranodally three times following a biweekly injection schedule. Patients were confirmed to be HLA*02:01 and, in some cases, HLA-DR4 positive and World Health Organization performance status 0 or 1. Primary tumors or metastases were tested for tumor-associated antigen expression (gp100 and tyrosinase). The original studies were approved by the Regional Institutional Review Board and patients signed informed consent.
Sample acquisition
Various amounts of whole blood were obtained by venipuncture or leukapheresis using vacutainer collection tubes at the time points indicated in (Fig. 1). In order to achieve processing within 6 h, vacutainer tubes were transported within the Radboudumc at room temperature to the Department of Tumor Immunology. PBMCs were isolated via gradient centrifugation using Lymphoprep (Axis-Shield) following the manufacturer's instructions. Cells were frozen in Xvivo 15 (Lonza) supplemented with 40% Albuman 200 g/L (Sanquin) and 10% CryoSure-DMSO (WAK Chemie Medical GMBH) in Cryo.S vials (greiner bio-one) at 10 to 100 × 106 cells per vial using Cryo 1°C Freezing Container (NALGENE) following the manufacturer's instructions. The freezing container was placed at −80°C, and vials were transferred into the vapor phase of liquid nitrogen after about 72 h for long-term storage.
For intracellular cytokine secretion testing, 1 to 2 vials per sample with varying cell numbers depending on experimental setup and availability were thawed. In case of several time points measured for a single patient, we attempted to include all samples in one experiment which was however not always possible.
T cell stimulation and staining
Cryopreserved PBMCs and PBLs were thawed, washed, and suspended at concentrations between 2 and 5·106 cells/mL in Iscove's Modified Dulbecco's Medium (IMDM) (Gibco) supplemented with 10% human serum (Sigma Aldrich, H1513), antibiotic-antimycotic (Gibco), and Alexa700- or APC-labeled anti-CD107a (H4A3, BD PharMingen, 5 μL). The serum lot was not pretested for performance. T cells were stimulated using CD28/49c coated beads (BD Bioscience), either alone or in combination with 1 μg/mL Staphylococcus enterotoxin B (Sigma Aldrich), PMA/Ionomycin (50 ng/mL, 1 μg/mL or 10 ng/ml, 0.5 g/mL) or 10 μg/mL peptide gp100154–167, gp100280–288 and wild-type tyrosinase369–376. Peptides were generated in our research facility according to GMP standards. Brefeldine A (Sigma Aldrich, 10 μg/mL) and Monensine (eBioscience, 1:1000 [2 μM]) were added and cells were incubated for at least 5.5 h but not more than 6 h at 37°C and 5% CO2.
After incubation, T cells were washed 3x with PBS and dead cells were stained using LIVE/DEAD Fixable Far Red Dead Cell Stain Kit (Life Technologies, 1:2000 in PBS, 100 μL) or Fixable Viability Dye eFluor® 780 (eBioscience, 1:2000 in PBS, 100 μL) on ice for 30 min. Cells were washed 2x with PBS and 1x with PBS supplemented with 1% bovine serum albumin and 0,05% NaN3 (PBA). T cells were stained for surface marker expression in 50 μL PBA supplemented with APC-Cy7- or PE-Cy7-labeled anti-CD4+ (RPA-T4, BD PharMingen, 1:200; SK3, eBioscience, 1:500) and PE-CF594- or BV510-conjugated anti-CD8+ (RPA-T8, BD Horizon, 1:200; 1:16) at 4°C for 10 min. In some cases (Table 1), cells were also stained with APC-labeled tetrameric MHC complexes containing the gp100154–167, gp100280–288, or tyrosinase369–376 peptide (Sanquin). Several samples treated only with co-stimulatory beads, were incubated with HIV77–85-HLA-A2.1-tetramers specific for the irrelevant HIV-peptide (SLYNTVATL) or CEA-peptide (YLSGANLNL) to account for background staining.
Subsequently, the cells were 2x washed using PBA and then PBS, and fixed using PBS supplemented with 4% paraformaldehyde (Merck) for 4 min on ice. After that, cells were permeabilized by washing 2x with 0.5% Saponin-supplemented PBA (Riedel-de Haën). The cells were stained for cytokines in 50 μL PBA supplemented with 0.5% Saponin and PerCP-Cy5.5- or BV421-conjugated anti-IFNγ (B27, BD PharMingen, 1:10; 1:10), PE-labeled anti-IL-2 (MQ1–17H12, eBioscience, 1:100), FITC-labeled anti-CCL4 (24006, R&D systems, 1:5) and PE-Cy7- or PerCP-Cy5.5-labled anti-TNFα (MAb11, eBioscience, 1:100; 1:10) at 4°C for 30 min. Cells were washed 2x using PBA supplemented with 0.5% Saponin and PBA. Finally, cells were resuspended in PBA, kept at 4°C and measured within 2 h after staining.
Flow cytometry and gating strategy
Fluorescence was acquired using a CyAn ADP Analyzer (Beckman Coulter), a FACSVerse or a FACSAria (both Becton Dickinson) and analyzed with FlowJo software (Tree Star). For SEB- and peptide-stimulated samples at least 10,000 and 25,000 viable CD8+ T cells were recorded. For each patient and time point 1 to 4 samples treated only with co-stimulatory beads were analyzed and at least 18,000 viable CD8+ T cells were recorded for each replicate sample. Compensation was calculated using single stained samples. Using forward and sideward scatter (FSC, SSC) we first gated on the lymphocyte population. Subsequently, single cells were selected plotting FSC area against pulse width or SSC area against SSC width and subsequently FSC area against FSC height. Living, CD4− lymphocytes were selected by plotting APC-Cy7 against CD8+ and gating on APC-Cy7− cells (Fig. 2A). Finally, we selected CD8+ cells by plotting CD8+ against tetramers. Since the tetramer signal vanished in samples with strong cytokine-secretion, tetramer-positivity was not taken into account (Fig. S1). Alternatively, live, CD8+CD4− T cells were selected by first gating on APC-Cy7− cells and later on CD4−, CD8+ cells. Cytokine-secretion was determined using single gates defined for the CD8+ lymphocyte population. To analyze the functional diversity of CD8+ T cells combination gates were created using the built-in Boolean gating algorithm of FlowJo. To account for unspecific activation, samples were background subtracted using the average values of 1 to 4 corresponding replicate samples stimulated only with co-stimulatory beads. To account for the different magnitude of total vaccine- and SEB-specific CD8+ T cell responses, the frequencies of cytokine combinations were normalized and given as the percentage of total vaccine- or SEB-specific response. For this purpose the frequency of CD8+ T cells expressing a particular functional combination was divided by the total frequency of responding CD8+ T cells in that sample. Positive reactivity to an experimental antigen was defined as 0.2% of responding CD8+ T cells after background-subtraction corresponding to 90% of CD28/CD49d-stimulated samples being below this threshold. Responses were not considered positive if the antigen-specific reactivity level was below this threshold. Raw data can be provided upon request.
Data analysis and statistics
Analysis and presentation of distributions was performed using SPICE version 5.3 (downloaded from http://exon.niaid.nih.gov),39 and PRISM for windows version 5.03 (GraphPad). For statistical analysis, the Wilcoxon matched-pairs test (all PRISM) was used to compare matched groups and the Wilcoxon signed rank test was used when comparing groups and constant values.
General lab operation
These studies were conducted in a laboratory that operates under exploratory research principles. The study was performed using established laboratory protocols.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Harm Westdorp for data management.
Author's contribution
Conception and design: E. H. J. G. Aarntzen, C. G. Figdor, I. J. M. de Vries
Development of methodology: E. J. H. G. Aarntzen, T. Duiveman-deBoer
Acquisition of data: T. Duiveman-deBoer, F. Wimmers
Analysis and interpretation of data: E. H. J. G. Aarntzen, J. F. M. Jacobs, J. Tel, F. Wimmers
Writing, review, and/or revision of the manuscript: E. H. J. G. Aarntzen, C. G. Figdor, J. F. M. Jacobs, J. Tel, I. J. M. de Vries, F. Wimmers
Administrative, technical, or material support: F. Wimmers, T. Duiveman-deBoer
Study supervision: J. F. M. Jacobs, J. Tel, C. G. Figdor, I. J. M. de Vries
Funding
This work was supported by a Radboud University Medical Center PhD grant. J. J. and J. T. are supported by grants NWO-Veni-016.136.101 and NWO-Veni-863.130.24 from the Netherlands Organization for Scientific Research. J. d. V. received an NWO-Vici award 918.146.55. C. F. received the NWO Spinoza award and the ERC Advanced Grant PATHFINDER (269019).
References
- 1.Vanderbruggen P, Traversari C, Chomez P, Lurquin C, Deplaen E, Vandeneynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic lymphocytes-T on a human-melanoma. Science 1991; 254:1643-7; PMID:1840703; http://dx.doi.org/ 10.1126/science.1840703 [DOI] [PubMed] [Google Scholar]
- 2.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000; 6:1018-23; PMID:10973322; http://dx.doi.org/ 10.1038/79526 [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 4.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A et al.. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 2012; 30:1835-41; PMID:22529253; http://dx.doi.org/ 10.1200/JCO.2011.40.2271 [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Hodi FS, Kefford R, Hamid O, Daud A, Wolchok JD, Hwu W-J, Gangadhar TC, Patnaik A, Joshua AM et al.. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). ASCO Meeting Abstracts 2014; 32:LBA9000 [Google Scholar]
- 7.Ribas A, Flaherty KT. Gauging the long-term benefits of ipilimumab in melanoma. J Clin Oncol 2015; 33(17):1865-66; PMID:25667273; http://dx.doi.org/ 10.1200/JCO.2014.59.5041 [DOI] [PubMed] [Google Scholar]
- 8.Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT et al.. Five-year survival rates for treatment-naive patients with advanced melanoma who received Ipilimumab plus Dacarbazine in a phase III trial. J Clin Oncol 2015; 33:1191-6; PMID:25713437; http://dx.doi.org/ 10.1200/JCO.2014.56.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M et al.. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8(+) T cells. Blood 2006; 107:4781-9; PMID:16467198; http://dx.doi.org/ 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 2008; 38:350-63; PMID:18200635; http://dx.doi.org/ 10.1002/eji.200737768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan JD, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS et al.. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. P Natl Acad Sci USA 2008; 105:20410-5; PMID:19074257; http://dx.doi.org/23519018 10.1073/pnas.0810114105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma C, Cheung AF, Chodon T, Koya RC, Wu ZQ, Ng C, Avramis E, Cochran AJ, Witte ON, Baltimore D et al.. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov 2013; 3:418-29; PMID:23519018; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836-48; PMID:17063185; http://dx.doi.org/ 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- 14.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al.. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:23376950; http://dx.doi.org/ 10.1038/nature11824 [DOI] [PubMed] [Google Scholar]
- 15.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247-58; PMID:18323851; http://dx.doi.org/ 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 16.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol 2000; 1:311-6; PMID:11017102; http://dx.doi.org/ 10.1038/79758 [DOI] [PubMed] [Google Scholar]
- 17.Rubio-Godoy V, Dutoit V, Rimoldi D, Lienard D, Lejeune F, Speiser D, Guillaume P, Cerottini JC, Romero P, Valmori D. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci U S A 2001; 98:10302-7; PMID:11517329; http://dx.doi.org/ 10.1073/pnas.181348898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons GE, Roszkowski JJ, Man S, Yee C, Kast WM, Nishimura MI. T-cell receptor tetramer binding or the lack there of does not necessitate antigen reactivity in T-cell receptor transduced T cells. Cancer Immunol, Immunother 2006; 55:1142-50; PMID:16374636; http://dx.doi.org/ 10.1007/s00262-005-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulie PG, Connerotte T. Human tumor-specific T lymphocytes: does function matter more than number? Curr Opin Immunol 2005; 17:320-5; PMID:15886124; http://dx.doi.org/ 10.1016/j.coi.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Ndhlovu ZM, Oelke M, Schneck JP, Griffin DE. Dynamic regulation of functionally distinct virus-specific T cells. Proc Natl Acad Sci U S A 2010; 107:3669-74; PMID:20133680; http://dx.doi.org/ 10.1073/pnas.0915168107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I et al.. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 2007; 204:2473-85; PMID:17893201; http://dx.doi.org/ 10.1084/jem.20070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995; 270:1811-5; PMID:8525373; http://dx.doi.org/ 10.1126/science.270.5243.1811 [DOI] [PubMed] [Google Scholar]
- 23.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med 1997; 186:139-46; PMID:9207008; http://dx.doi.org/ 10.1084/jem.186.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, Hoxie JA, Marsh M. Differential regulation of CXCR4 and CCR5 endocytosis. J Cell Sci 1998; 111 (Pt 18):2819-30; PMID:9718374 [DOI] [PubMed] [Google Scholar]
- 25.Vila-Coro AJ, Mellado M, Martin de Ana A, Lucas P, del Real G, Martinez AC, Rodriguez-Frade JM. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc Natl Acad Sci U S A 2000; 97:3388-93; PMID:10725362; http://dx.doi.org/ 10.1073/pnas.97.7.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 2006; 203:2865-77; PMID:17158960; http://dx.doi.org/ 10.1084/jem.20052246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harari A, Cellerai C, Bellutti Enders F, Kostler J, Codarri L, Tapia G, Boyman O, Castro E, Gaudieri S, James I et al.. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc Natl Acad Sci USA 2007; 104:16233-8; PMID:17911249; http://dx.doi.org/ 10.1073/pnas.0707570104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J Exp Med 1997; 186:757-66; PMID:9271591; http://dx.doi.org/ 10.1084/jem.186.5.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu YL, Shan L, Huang H, Haupt C, Bessell C, Canaday DH, Zhang H, Ho YC, Powell JD, Oelke M et al.. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J Clin Invest 2014; 124:198-208; PMID:24292711; http://dx.doi.org/ 10.1172/JCI70510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77:4911-27; PMID:12663797; http://dx.doi.org/ 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13:828-35; PMID:17603493; http://dx.doi.org/ 10.1038/nm1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004; 172:989-99; PMID:14707072; http://dx.doi.org/ 10.4049/jimmunol.172.2.989 [DOI] [PubMed] [Google Scholar]
- 34.Wang E, Bedognetti D, Marincola FM. Prediction of response to anticancer immunotherapy using gene signatures. J Clin Oncol 2013; 31:2369-71; PMID:23715576; http://dx.doi.org/ 10.1200/JCO.2013.49.2157 [DOI] [PubMed] [Google Scholar]
- 35.Lesterhuis WJ, Schreibelt G, Scharenborg NM, Brouwer HM, Gerritsen MJ, Croockewit S, Coulie PG, Torensma R, Adema GJ, Figdor CG et al.. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol, Immunother 2011; 60:249-60; PMID:21069321; http://dx.doi.org/ 10.1007/s00262-010-0942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al.. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1127 [DOI] [PubMed] [Google Scholar]
- 37.Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T et al.. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res 2012; 18:5460-70; PMID:22896657; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3368 [DOI] [PubMed] [Google Scholar]
- 38.Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, Klasen IS, Hilbrands LB, Figdor CG, de Vries IJ et al.. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res 2010; 16:5067-78; PMID:20736326; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1757 [DOI] [PubMed] [Google Scholar]
- 39.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79:167-74; PMID:21265010; http://dx.doi.org/ 10.1002/cyto.a.21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, Van Rossum MM, Adema GJ, Figdor CG, Punt CJ, De Vries IJ. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res 2012; 72:6102-10; PMID:23010076; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.