Abstract
The rise in gonococcal antibiotic resistance and the threat of untreatable infection are focusing attention on strategies to limit the spread of drug-resistant gonorrhea. Mathematical models provide a framework to link the natural history of infection and patient behavior to epidemiological outcomes and can be used to guide research and enhance the public health impact of interventions. While limited knowledge of key disease parameters and networks of spread has impeded development of operational models of gonococcal transmission, new tools in gonococcal surveillance may provide useful data to aid tracking and modeling. Here, we highlight critical questions in the management of gonorrhea that can be addressed by mathematical models and identify key data needs. Our overarching aim is to articulate a shared agenda across gonococcus-related fields from microbiology to epidemiology that will catalyze a comprehensive evidence-based clinical and public health strategy for management of gonococcal infections and antimicrobial resistance.
Keywords: antibiotic resistance, mathematical modeling, gonorrhea, Neisseria gonorrhoeae, sexually transmitted infections, immunity, vaccine
Gonorrhea was an early priority for antibiotic treatment, such as for military personnel during World War II [1]. Now, the emergence of ceftriaxone-resistant Neisseria gonorrhoeae and the threat of potentially untreatable gonococcal infections is a call to action to improve strategies and tools for prevention, surveillance, and treatment of gonorrhea [2]. With its high incidence (an estimated 820 000 cases in the United States annually [3] and >105 million cases worldwide [4]), gonorrhea presents a widespread global health risk. However, considering its global burden, gonorrhea has received underinvestment in research relative to other infectious diseases [5]. Efforts to develop vaccines [6] and test old [7] and develop new [2] antibiotics are critically important. However, while we await the breakthroughs that introduce novel therapies and vaccines, and even once these innovations come into use, it is equally important to establish an evidence-based approach to optimizing the public health tool kit for management of gonorrhea, to minimize the overall burden of disease and slow or control the spread of antibiotic-resistant strains. This requires a better understanding of the epidemiology and transmission dynamics of all N. gonorrhoeae strains, including those susceptible to drugs, which are not only precursors of resistant strains but also their competitors.
In fundamental work on gonorrhea, Hethcote and Yorke [8, 9] used mathematical modeling to deduce from epidemiological data (overall incidence and characteristics of the at-risk population) and from data on the biology of infection (duration of infection and lack of protective immunity) that the population must not be homogeneously mixing, and a crucial aspect of gonorrhea epidemiology is circulation in core groups of individuals who are part of a more highly connected sexual network than average. Since then, a number of models of gonococcal transmission have been proposed [10]. These models have varying structures, differing in how they represent sex partnerships, including compartmental models (Figure 1) [11, 12], partnership models [13], and individual-based models [14, 15], and in their assumptions about key parameters in transmission dynamics, as well as in the relative fitness costs of resistance and, more generally, strain competition [16, 17]. While these models have offered valuable inferences about the effects of duration of infection, population size, and sex-partner concurrency and the effects of treatment capacity on disease incidence and persistence, among others the uncertainty surrounding the assumptions inherent in the model structure and parameterization have, so far, limited their applicability.
Figure 1.
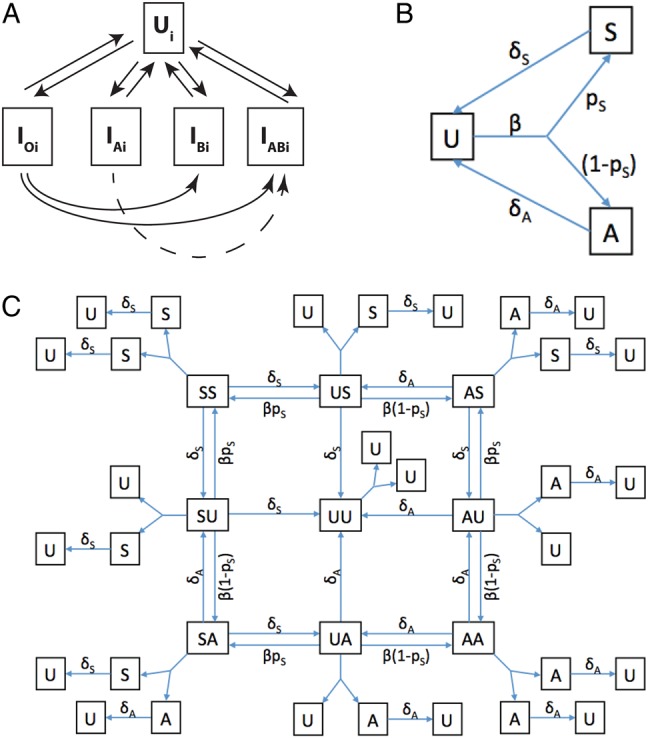
Examples of different structures of transmission models for gonorrhea. A, In the diagram (adapted from [11]) of a model to explore drug resistance, uninfected (U) and infected (I) individuals may be of multiple risk classes (denoted by the subscript “i”), and the infected strain may be susceptible to both drugs (subscript “o”), resistant to drug A (subscript “A”), resistant to drug B (subscript “B”), or resistant to both (subscript “AB”). B, This model considers a single strain and distinguishes symptomatic (S) and asymptomatic (A) infections, with uninfected individuals denoted by “U.” Here, β is the effective contact rate leading to transmission, pS is the probability that infection will be symptomatic, and the rates of recovery from symptomatic and asymptomatic infection are δS and δA, respectively. The models in panels A and B are examples of compartmental models, which track numbers of individuals in the population who are in different states regarding infection and do not explicitly represent sex partnerships. C, This model represents the natural history of gonorrhea in the same way as the model in panel B, but it is a pair model, which explicitly represents the process of formation and dissolution of partnerships. Arrows denoting partnership formation have been omitted for clarity. Boxes with single letters denote singletons, who are uninfected (U), have symptomatic infection (S), or asymptomatic infection (A); boxes with pairs of letters denote partnerships and the status of the 2 partners. The model tracks the numbers of singletons and partnerships of each type. Singletons can have an infection acquired in a previous partnership. If so, then they can recover from it but cannot transmit it, as transmission of infection requires being in partnership with an infected partner. While the pair formulation has greater complexity, it allows explicit representation of processes such as treated index patients becoming reinfected from an infected partner and for partner notification and treatment to prevent this occurrence.
We summarized some of the key questions related to gonorrhea management in Box 1. Given the potential for mathematical models to address those questions and improve control of gonorrhea, it is critical to consider the gaps in knowledge of the natural history and transmission of gonorrhea and identify those that may impact the outcome of the models; that is, what data do we need to acquire and what methods do we need to develop to establish the best-informed strategy to manage gonorrhea and counter the emergence and spread of drug resistance?
Box 1. Research Questions to Guide Public Health Management of Gonorrhea.
Screening, surveillance, and prevention
-
1. In what ways can we improve on and restructure current surveillance programs, particularly when the aim is preventing the spread of antibiotic resistance? More generally, what is the optimal surveillance structure (at local, regional, national, and international levels) to inform timely and appropriate proportionate action to decrease the burden of disease? What are the roles of screening, outbreak surveillance using molecular tools, and identification of target groups (eg, according to sexual behavior and/or travel history) for which extra surveillance efforts are warranted? How can surveillance be optimized to assess the effectiveness of public health interventions when applied at scale in real-world conditions?
2. What are the optimal intervals for routine screening, and to what extent should screening be population dependent, based on demographic or individual behavioral risk factors?
3. Given the use of nucleic acid amplification tests for diagnosis in place of culture, what strategy of testing antibiotic susceptibility would allow for the greatest sensitivity for detecting the emergence of resistance within the population?
4. How should novel molecular diagnostic tests that report antibiotic susceptibilities be incorporated into screening, surveillance, and treatment strategies to maximize their epidemiological impact?
5. How can vaccines best be deployed to combat drug resistance and to decrease the overall burden of disease?
6. What conditions promote severe sequelae of infection, including pelvic inflammatory disease and infertility? Is the risk primarily due to incident infection or long-term prevalent infection (including due to treatment failure)? Should test of cure be recommended for female patients? Do gonococcal strains differ in their propensity to cause sequelae? What diagnostic tests or interventions might improve not only the incidence of infection but also the incidence of severe manifestations of infection?
Diagnostic analysis
-
1. What is the best use and potential impact of a rapid diagnostic test that provides information on antibiotic susceptibility in real-time? How might continuing evolution of the bacterial population in response to such diagnostic tests and the resulting changes in treatment strategies affect their long-term impact, compared to their short-term impact?
2. What are the risk groups for which such diagnostic tests should be used?
3. What is the potential impact of home gonorrhea test kits?
Treatment
-
1. Under what contexts should empirical treatment be guided by local antibiotic resistance, rather than by aggregated nationwide patterns?
2. What are the benefits and downsides of multidrug treatment?
3. What is the impact of dual treatment with ceftriaxone and azithromycin on Neisseria gonorrhoeae specimens resistant to one of the 2 drugs?
4. Can persistent infection due to treatment failure be distinguished from reinfection?
5. What are the impacts, either positive or negative, of expedited partners services, which include giving individuals with a gonorrhea diagnosis antibiotics to provide to their partners for expedited treatment?
Behavioral change
-
1. What role might behavior change play, including spontaneous behavioral change motivated by changing risk perceptions or changes promoted by public health interventions?
2. Would such changes potentiate or reduce the effects of changing antimicrobial use policies or diagnostics?
Below, we list areas of uncertainty and suggest approaches to address them, drawing on current and prospective sources of data and ongoing research efforts. We note that the relative impact of these areas of uncertainty on mathematical models depends on the structure of the models themselves and is difficult to anticipate without construction and calibration of these models. However, it is important to emphasize that one need not definitively address each of the questions below to advance the frontiers of gonorrhea epidemiology and address some of the key questions described in Box 1.
We encourage researchers across microbiology, immunology, epidemiology, and behavioral science to collaborate in multidisciplinary studies to maximize the value of insights gained by obtaining as much information as possible about the epidemiologic and behavioral characteristics of the populations of patients sampled, as well as the characteristics of the N. gonorrhoeae strains infecting them, including anatomical sites of infection. Examples of such studies are given throughout the text.
FITNESS OF N. GONORRHOEAE LINEAGES AND ANTIBIOTIC SELECTION PRESSURE
Epidemiological and clinical data are needed to address the following questions: what is the relationship between antibiotic use and emergence of resistance within individual hosts and communities (Figures 1A and 2) [11]? What is the rate of emergence of resistance, given treatment, and how does this depend on genetic background, minimum inhibitory concentration, and the kinetics of the drug used? Those questions have a bearing on the fitness cost or the selective advantage that resistant strains may carry. For example, results from a study by Goldstein et al [19] suggest that when resistance prevalence becomes common enough, multidrug resistance in men who have sex with men (MSM) is no longer associated with recent travel, suggesting local proliferation. Is this proliferation the result of the biological fitness advantage of multidrug-resistant strain, or is it aided by antibiotic use, including for reasons other than gonorrhea treatment?
Figure 2.
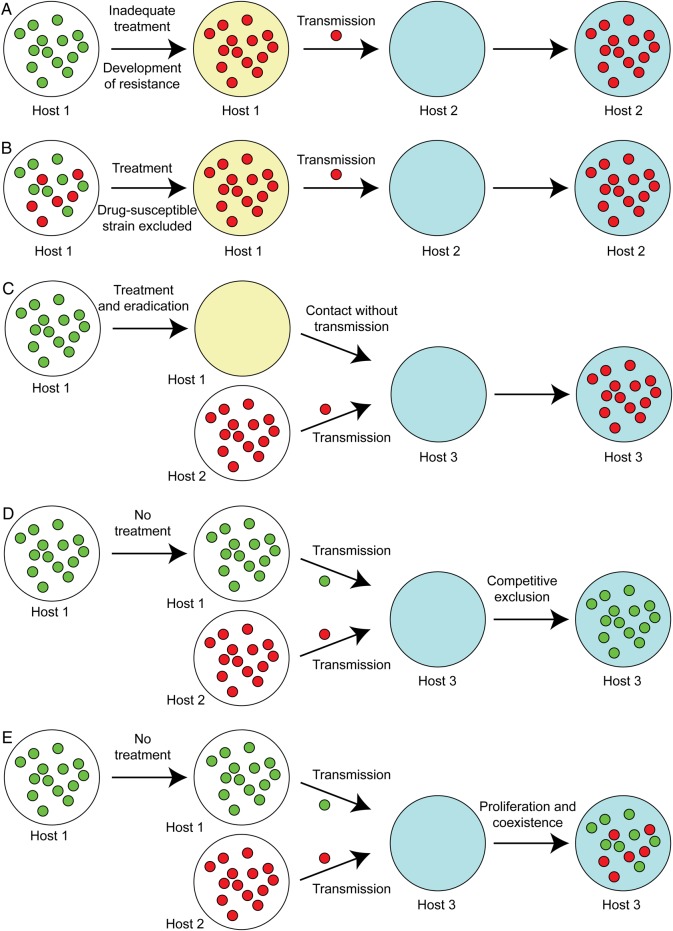
Mechanisms that represent possible relationships between antibiotic treatment and resistance (based on Figure 3 in [18]). Untreated hosts (white large circles) infected with Neisseria gonorrhoeae (small circles [green, antibiotic susceptible; red, antibiotic resistant]) can remain untreated, such as in asymptomatic infection, or receive treatment (yellow large circles). Sex partners/new hosts (blue large circles) can then acquire N. gonorrhoeae through transmission. A, Inadequate treatment (as suspected may occur in pharyngeal infections, which require higher antibiotic concentrations for eradication than at other sites) may result in selection for resistance, which can then be transmitted to uninfected individuals. B, Treatment of an individual infected with a mixed population of resistant and susceptible strains may select for the resistant strains. C, Successful treatment of an individual infected with an antibiotic-susceptible strain prevents the strain from transmitting to other hosts, making those hosts more likely to be infected by resistant strains than they would otherwise have been and shifting the competitive balance toward resistant strains. D, Exposure to an individual with antibiotic-susceptible gonococcus and to an individual with resistant gonococcus may result in infection with antibiotic-susceptible gonococcus, if there is a sufficiently large relative fitness cost to resistance, or in mixed infection. E, Mixed infections then present the opportunity for transformation of antibiotic-susceptible strains into resistant strains through horizontal gene transfer.
Defining the fitness cost of resistance is relevant to predicting the emergence and spread of resistant strains. Epidemiological evidence suggests that these costs might, in some cases, be severe: in the 5 years since the first case of ceftriaxone-resistant gonorrhea emerged in 2009, only a handful of cases have been reported, including cases in France, Spain, and Australia. The reasons for the limited number of cases remain to be elucidated, but suspicion centers on the fitness costs of carrying the antibiotic resistance determinant. Azithromycin resistance has remained at low levels in the United States [20]; population genomics studies will be helpful in estimating the extent to which this is due to the fitness cost of resistance-conferring mutations (which might be seen if resistance emerges independently in multiple locations but resistant populations fail to continue to circulate and expand). In vitro studies have indicated that genetic background can be a key determinant of antibiotic resistance phenotype [21], and in vivo study of quinolone resistance in an estrogenized mouse model showed that quinolone resistance can have either a fitness cost or advantage, depending on the genetic background [22], emphasizing the epidemiological implications of compensatory mutations in antibiotic-resistant strains.
These observations indicate the importance of combining microbiological studies performed in vitro to determine the relative fitness of isolates having different resistance determinants and genetic backgrounds with studies using animal models, such as in the quinolone-resistance experiments [22]. Molecular epidemiological surveys of both resistant and closely related susceptible isolates may also help identify cases in which there is reversion from resistance to susceptibility, suggesting a fitness cost for resistance and providing a set of isolates for direct experimental evaluation. Such data can then be used to help parameterize both single and multistrain mathematical models that incorporate empirical data on fitness, rather than making the simple assumption of a fixed fitness cost for resistance.
THE IMPACT OF IMMUNITY
While immunity to gonorrhea following infection has long been treated as nonexistent or negligible in models, work on development of a gonococcal vaccine eliciting protective immunity [6] raises important questions about the impact of a vaccine both on overall incidence and on antibiotic resistance.
A vaccine that results in reduction in the incidence and prevalence of infection will (1) reduce the frequency of the initial emergence of resistant strains, by reducing the number of existent gonococci in which mutation and genetic exchange can occur; and (2) reduce the incidence of antibiotic treatment, although whether this effect will substantially reduce selective pressure favoring proliferation of gonococcal resistance is unclear, because, at least in simple models, the selective pressure for resistance is proportional to the probability that each infection is treated, not to the number of infections treated [11, 23]. However, a vaccine that reduces the overall infection burden might be expected to reduce resistant infection proportionately.
More subtle effects might also be possible. Vaccine efficacy can be considered in terms of a reduction in the likelihood of acquiring infection per exposure if uninfected and, if infected, a reduction in the severity of symptoms and in infectiousness [24]. In the context of gonorrhea, the bacterial load likely affects severity of symptoms, infectiousness per exposure, and the duration of infection, including through symptom severity affecting rates of care-seeking (the average duration of infection will also be affected by the frequency of testing for infection in the absence of symptoms). As reduction in severity of symptoms could result in asymptomatic infection, the consequences of vaccination may be complicated to predict a priori: whereas reducing likelihood of infection or duration of infectiousness could lower incidence, reduction in symptom severity could prolong infection (and result in more-frequent sex acts while infected, if symptoms deter sexual activity), raising concern for greater transmission or acquisition of resistance. However, if a higher proportion of infections remains asymptomatic, the incidence of treatment per infection may decline, reducing selection pressures. Mathematical modeling studies may be helpful in designing vaccine trials, to measure these different effects, and appropriate monitoring, to better anticipate how to distribute a vaccine optimally.
If a vaccine is equally efficacious against all strains regardless of their antibiotic susceptibility, then vaccination could be cost-effective if targeted at groups at high risk of acquiring infection, especially if these core groups were disproportionately at risk for resistant infections, which are more expensive to treat [25].
If antibiotic-resistant strains are less fit, then such a vaccine might select against resistant strains more strongly than against susceptible ones, amplifying their competitive disadvantage. In this scenario, the less fit strains have a lower effective reproduction number (R), which means that the same proportionate reduction of R for both wild-type and resistant strains yields a value for the resistant strains that is more likely to be below the epidemic threshold value of 1, below which transmission is not sustained [26], and that, if R remains >1 for the resistant strain, then it will be closer to 1 than R for the wild-type strain.
Additionally, if a resistance determinant that is even slightly immunogenic is included in a vaccine, then it could contribute to a reduction of antibiotic resistance [27]. Even a slightly efficacious vaccine may have significant impact on overall incidence of drug-resistant infection [28] and, if targeted to high-risk groups, may be cost-effective [29].
DEMOGRAPHIC, GEOGRAPHIC, AND SEX-PARTNER “SUPPLY AND DEMAND” MODELS OF GONOCOCCAL SEXUAL CONTACT NETWORKS
One of the main questions raised by the remarkable heterogeneity observed in data from surveillance programs (Figure 3) is how gonorrhea is spread geographically and through demographic groups. There is a need to better understand the combination of factors that determines risk of infection in different population groups in different settings [31]. While the existence of a core subgroup within the population maintains gonorrhea as an endemic disease, it is unclear how individuals in the core group are linked across sexual networks (eg, heterosexual and homosexual networks) and from one city to another. Indeed, molecular typing reveals that gonorrhea can spread in different sexual contact networks within the same city, with different behavioral characteristics, although with individuals acting as links between networks [32]. Furthermore, HIV serosorting may be an important behavioral characteristic resulting in highly interconnected networks of high-risk behavior for transmission of sexually transmitted infections (STIs) other than HIV [33]. The belated rise in the rates of ciprofloxacin resistance in the southeastern United States despite its high prevalence of gonorrhea, as compared to the West Coast [34], for example, may stem from the fact that the sexual contact networks in these 2 locations are fairly distinct. A model of demographic and geographic spread of gonorrhea would aid in development of an improved surveillance network, as well as in targeting resources, including screening, monitoring for outbreaks, and behavioral and therapeutic interventions.
Figure 3.
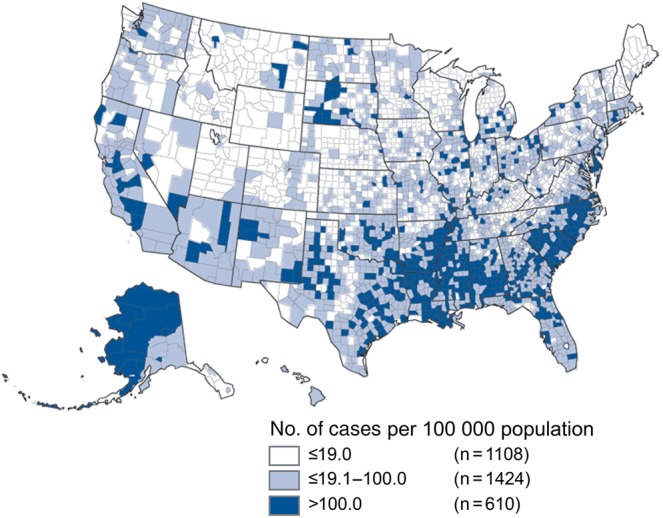
Geographic heterogeneity in gonococcal infection incidence. A major challenge to mathematical modeling of gonococcal disease dynamics and spread is geographic heterogeneity. County-based incidence data (white, ≤19.0 cases/100 000 population; gray, 19.1–100.0 cases/100 000 population; and blue, >100.0 cases/100 000 population). The underlying demographic and geographic sexual contact network structure is unknown [30].
Several new data streams offer opportunities to develop better models of gonorrhea spread. Genome sequences from large numbers of N. gonorrhoeae isolates can be used to reconstruct the phylogenetic relationships among the isolates. Then, incorporating the dates and locations and the sexual orientation of the infected individuals into the phylogenetic model can provide evidence of routes of spread across the population [35]. This approach can be explored for use in identifying outbreaks at the local level, with the hypothesis that outbreaks are identifiable by unexpectedly high prevalence of a single clone, as well as description of contact networks at multiple geographic scales. Similar models, which treat host location and, in this example, sexual preference as discrete traits of a pathogen, have aided in inferring the spread and evolution of various other infectious diseases [36, 37].
Another potential data source to characterize sexual contact networks is social network data, such as from online dating or so-called hook-up sites, which to date have been analyzed as individual-level risk factors for infection, as well as a way to sample individuals [38, 39]. Data obtainable from these sites can include demographic characteristics of users and their preferences for partners, as well as the locations in which they are based and where they search. While the representativeness of these data is not clear—whether it is different for MSM, compared with heterosexuals; how well user demographic characteristics can be defined; how many sexual contacts occur offline, compared with online; how the nature of those contacts might differ; and how well online data from the entire population reflect the behaviors of a subset, such as those at high risk of having gonorrhea—these data may at least provide a scaffold to learn demographic and geographic preferences and help bound the parameters of a model of spread.
Furthermore, by providing information on both members of a sex partnership, these data on sexual networks offer insights into partnership dynamics and the effects of behavior change on those dynamics, which is a long-standing issue in understanding the epidemiology of STIs. For example, in a heterosexual population, the number of sex partnerships involving men must be the same as the number involving women, yet, in surveys, men on average report a higher number of partnerships per unit time than women. One approach is to assume that female sex workers are undersampled and account for the missing partnerships; another is to adjust the estimates for one sex or both, assuming overreporting by men and/or underreporting by women [40]. While an assumption is necessary, there is little evidence which particular assumption is the right one. The issue becomes more complex when considering changes in behavior, which requires understanding of how partnerships form as a function of the availability of partners. Data from online and mobile partnering networks could inform how partnership acquisition depends on the availability and desirability of potential partners. Such data sets could also help to provide evidence on true levels of assortativeness in mixing by demographic and other characteristics, another important set of structural assumptions in transmission models. Another potential information source for addressing these issues is randomized trials of interventions designed to reduce risky sexual activity in a segment of the population [41].
TRANSMISSION PARAMETERS
Models of the spread of gonococcal infection require estimates of a set of parameters representing the mechanistic, biological, and clinical features of gonorrhea. These include the per-sex-act probabilities of transmission to and from each of the mucosal sites of infection, the durations of symptomatic and asymptomatic infection (at each site of infection), and the proportion of infections (again, at each site of infection) that prompts the infected individual to seek evaluation and treatment.
Transmission rates from cervical to urethral infection were estimated in a classic study of Navy sailors on shore leave and the gonorrhea prevalence among the women they visited [42]. Rates of urethral to cervical transmission have also been examined [43], although the estimate was based on only 12 cases. In neither of these studies was it clear whether the infections among women were symptomatic or asymptomatic. Little is known about the rates of transmission from pharyngeal or rectal infection. Recently, transmission probabilities per unprotected sex act were estimated using models with behavioral parameters drawn from surveys [44]. Behavioral surveys in high-risk populations, particularly in populations with repeat infections, together with regular short-interval screening, should provide an opportunity to further explore and refine these estimates and to understand the potential effectiveness behavioral interventions, such as encouraging use of condoms for oral sex.
The course of infection may be characterized in terms of duration and severity, and it is unclear how those factors affect the likelihood of onward transmission. In one study, symptomatic urethral or cervical infection prompted men and women to seek care on average 5 days and 12 days, respectively, after initiation of symptoms [16]. Asymptomatic infection is often assumed to last 6 months on average [8], but there are no clear data on the spontaneous resolution of either asymptomatic or symptomatic infection (such as in the preantibiotic era or in the case of a treatment-resistant strain). In one study, men with asymptomatic infection remained infected until treated [45]. While prospective population monitoring with interval screening could help set the lower limit, estimates of duration of infection in the context of modeling efforts will require thorough sensitivity analyses.
The symptomatic fraction (ie, the proportion of cases at each site of infection that prompts the infected individual to seek evaluation and treatment) is believed to be high for urethral infection and relatively low for cervical, pharyngeal, and rectal infection [46]. In a population of MSM screened for STIs, 64% of gonorrhea cases were nonurethral; most of these were pharyngeal (and asymptomatic), and the rest were rectal, with 84% of the latter being asymptomatic [47]. Retrospective analysis of annual screening of a population of MSM indicated rectal gonococcal prevalence of 24% when diagnosed by nucleic acid amplification testing (the prevalence was 9% when diagnosed by culture) [48]. Further information is needed to assess the roles of symptomatic and asymptomatic infection at different anatomical sites in the transmission dynamics and acquisition of resistance of N. gonorrhoeae.
ADDITIONAL QUESTIONS RELATED TO TREATMENT GUIDELINES
While we have identified a number of questions to improve our understanding of the fundamental epidemiology and the structure of transmission models, there are other questions with potential direct applicability to treatment guidelines. First, what are the target groups requiring extra intervention efforts? Previous work [19] suggests that during the early stages of the rise in rates of resistance to a particular antibiotic in N. gonorrhoeae, history of recent travel to an area in which the prevalence of resistance is higher is associated with carrying a resistant phenotype. What would be required to screen for recent travel in individuals with a diagnosis of gonorrhea? Is it feasible and cost-effective for infected individuals with such recent travel history (including domestic travel) and their sexual contacts to be tested for antimicrobial susceptibility? Second, during later stages, when resistant phenotypes, particularly multidrug-resistant phenotypes, are established in the community, should individuals presenting for treatment of STIs be screened for gonorrhea, including asymptomatic, nonurethral infection, with positive specimens routinely tested for antimicrobial susceptibility? Third, modeling results suggest that extra screening/treatment efforts focusing on high-risk individuals, while initially acting to reduce the prevalence of gonorrhea in the community, may eventually contribute to the proliferation of resistant strains [11]. To what extent could this be balanced by antimicrobial susceptibility testing or use of other antibiotics? Fourth, a recent study suggests that dual treatment of cefixime plus azithromycin yielded better outcomes than treatment with cefixime plus doxycycline [49]. This suggests that when resistance to the primary antimicrobial emerges, recommendation for dual treatment with azithromycin (or, more genrally, an effective combination therapy [50]) may slow the spread of resistant strains. Should reporting of the antibiotic regimen selected for treatment be mandatory, to permit inference about the relationship among community-wide antibiotic pressure, disease incidence, and resistance? Fifth, while models routinely dichotomize the continuous range of MIC into susceptible and resistant isolates and assume that treatment will fail for the latter and succeed for the former (except for a low frequency of resistance emergence), evidence suggests that the situation is more subtle. Elevated MICs are associated with an increased probability of treatment failure [51], with the mucosal site of infection further impacting this probability. How would incorporating this probabilistic framework into transmission models affect guidance for decisions about screening and antibiotic dosing?
CONCLUSIONS
A unified agenda to address the immense burden of gonorrhea and the threat of resistance should ideally incorporate both development of novel technologies and basic science and optimization of public health strategies. Just as it is clear that we need technological and scientific advances—including point-of-care diagnostic testing for resistance, novel therapeutic options, and, ideally, antigonococcal vaccines—we will need better models of gonococcal transmission, which incorporate more of the details of the evolution and interactions of strains, and better understanding of the risk factors associated with antimicrobial resistance, to maximize the effectiveness of our current surveillance and interventions and to best deploy any innovations. As described above, efforts ranging from pharmaceutical advances to behavioral studies can address key unknowns and be combined to establish strong empirical data-driven public health surveillance and intervention strategies to control the spread of antibiotic-resistant gonorrhea and reduce the overall incidence of disease.
Notes
Acknowledgments. We thank 2 anonymous reviewers for their helpful comments.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institutes of Health (NIH), and the United Kingdom (UK) Medical Research Council, the UK National Health Service, the UK National Institute for Health Research, the UK Department of Health, and Public Health England.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (1-K08-AI104767 to Y. H. G.), the National Institute of General Medical Sciences (award U54GM088558 to M. L. and E. G.), the NIH (K01 award 1K01AI101010–01 to E. G.), and the UK National Institute for Health Research Health Protection Research Unit in Modelling Methodology at Imperial College London, in partnership with Public Health England (grant HPRU-2012-10080 to P. J. W.) and the UK Medical Research Council (grant MR/K010174/1 to P. J. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lax E. The mold in Dr. Florey's coat: the story of the penicillin miracle. 1st ed New York: H. Holt, 2004. [Google Scholar]
- 2.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–7. [DOI] [PubMed] [Google Scholar]
- 3.Satterwhite CL, Torrone E, Meites E et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40:187–93. [DOI] [PubMed] [Google Scholar]
- 4.Department of Reproductive Health and Research, World Health Organization (WHO). Global incidence and prevalence of selected curable sexually transmitted infections: 2008. Geneva: WHO, 2012. http://www.who.int/reproductivehealth/publications/rtis/2008_STI_estimates.pdf Accessed 8 February 2013. [Google Scholar]
- 5.Head MG, Fitchett JR, Cooke MK, Wurie FB, Hayward AC, Atun R. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect Dis 2013; 13:55–64. [DOI] [PubMed] [Google Scholar]
- 6.Jerse AE, Bash MC, Russell MW. Vaccines against gonorrhea: current status and future challenges. Vaccine 2014; 32:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkcaldy RD, Weinstock HS, Moore PC et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hethcote HW, Yorke JA.. Gonorrhea transmission dynamics and control. Berlin, New York: Springer-Verlag, 1984. [Google Scholar]
- 9.Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis 1978; 5:51–6. [DOI] [PubMed] [Google Scholar]
- 10.Garnett GP. An introduction to mathematical models in sexually transmitted disease epidemiology. Sex Transm Infect 2002; 78:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CH, McCabe CJ, Fisman DN. Core groups, antimicrobial resistance and rebound in gonorrhoea in North America. Sex Transm Infect 2012; 88:200–4. [DOI] [PubMed] [Google Scholar]
- 12.White PJ, Ward H, Cassell JA, Mercer CH, Garnett GP. Vicious and virtuous circles in the dynamics of infectious disease and the provision of health care: gonorrhea in Britain as an example. J Infect Dis 2005; 192:824–36. [DOI] [PubMed] [Google Scholar]
- 13.Dietz K, Hadeler KP. Epidemiological models for sexually transmitted diseases. J Math Biol 1988; 26:1–25. [DOI] [PubMed] [Google Scholar]
- 14.Kretzschmar M, van Duynhoven YT, Severijnen AJ. Modeling prevention strategies for gonorrhea and Chlamydia using stochastic network simulations. Am J Epidemiol 1996; 144:306–17. [DOI] [PubMed] [Google Scholar]
- 15.Ghani AC, Swinton J, Garnett GP. The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis 1997; 24:45–56. [DOI] [PubMed] [Google Scholar]
- 16.Garnett GP, Mertz KJ, Finelli L, Levine WC, St Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philos Trans R Soc Lond B Biol Sci 1999; 354:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnett GP, Bowden FJ. Epidemiology and control and curable sexually transmitted diseases: opportunities and problems. Sex Transm Dis 2000; 27:588–99. [DOI] [PubMed] [Google Scholar]
- 18.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis 2002; 8:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein E, Kirkcaldy RD, Reshef D et al. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg Infect Dis 2012; 18:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkcaldy RD, Soge O, Papp JR et al. Analysis of Neisseria gonorrhoeae azithromycin susceptibility in the United States by the Gonococcal Isolate Surveillance Project, 2005 to 2013. Antimicrob Agents Chemother 2015; 59:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golparian D, Shafer WM, Ohnishi M, Unemo M. Importance of multidrug efflux pumps in the antimicrobial resistance property of clinical multidrug-resistant isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother 2014; 58:3556–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz AN, Begum AA, Wu H et al. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J Infect Dis 2012; 205:1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonhoeffer S, Lipsitch M, Levin BR. Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci U S A 1997; 94:12106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halloran ME, Longini IM Jr, Struchiner CJ. Design and interpretation of vaccine field studies. Epidemiol Rev 1999; 21:73–88. [DOI] [PubMed] [Google Scholar]
- 25.Roberts RR, Hota B, Ahmad I et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49:1175–84. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RM, May RM.. Infectious diseases of humans dynamics and control. Oxford, New York: Oxford University Press, 1991. [Google Scholar]
- 27.Joice R, Lipsitch M. Targeting imperfect vaccines against drug-resistance determinants: a strategy for countering the rise of drug resistance. PLoS One 2013; 8:e68940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regnier SA, Huels J. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: results from a decision-analysis model. Hum Vaccin Immunother 2014; 10:3737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garnett GP. The theoretical impact and cost-effectiveness of vaccines that protect against sexually transmitted infections and disease. Vaccine 2014; 32:1536–42. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Figure 15. Gonorrhea—rates of reported cases by county, United States, 2013. Updated 16 December 2014. http://www.cdc.gov/std/stats13/figures/15.htm. Accessed 10 October 2015. [Google Scholar]
- 31.Gomez GB, Ward H, Garnett GP. Risk pathways for gonorrhea acquisition in sex workers: can we distinguish confounding from an exposure effect using a priori hypotheses? J Infect Dis 2014; 210(suppl 2):S579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymans R, Am A, Bruisten SM et al. Distinct Neisseria gonorrhoeae transmission networks among men who have sex with men in Amsterdam, The Netherlands. J Infect Dis 2012; 206:596–605. [DOI] [PubMed] [Google Scholar]
- 33.Ronn M, White PJ, Hughes G, Ward H. Developing a conceptual framework of seroadaptive behaviors in HIV-diagnosed men who have sex with men. J Infect Dis 2014; 210(suppl 2):S586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC. Gonococcal Isolate Surveillance Project (GISP) Annual Report 2013. http://www.cdc.gov/std/gisp2013/gisp-2013-all-profiles.pdf. Accessed 14 October 2015.
- 35.Grad YH, Kirkcaldy RD, Trees D et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 2014; 14:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLoS Comput Biol 2009; 5:e1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudas G, Bedford T, Lycett S, Rambaut A. Reassortment between influenza B lineages and the emergence of a coadapted PB1-PB2-HA gene complex. Mol Biol Evol 2015; 32:162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris SR, Knapp JS, Moore DF et al. Using strain typing to characterise a fluoroquinolone-resistant Neisseria gonorrhoeae transmission network in southern California. Sex Transm Infect 2008; 84:290–1. [DOI] [PubMed] [Google Scholar]
- 39.Beymer MR, Weiss RE, Bolan RK et al. Sex on demand: geosocial networking phone apps and risk of sexually transmitted infections among a cross-sectional sample of men who have sex with men in Los Angeles County. Sex Transm Infect 2014; 90:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garnett GP, Anderson RM. Balancing sexual partnerships in an age and activity stratified model of HIV transmission in heterosexual populations. IMA J Math Appl Med Biol 1994; 11:161–92. [DOI] [PubMed] [Google Scholar]
- 41.Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet 2012; 379:1320–9. [DOI] [PubMed] [Google Scholar]
- 42.Hooper RR, Reynolds GH, Jones OG et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol 1978; 108:136–44. [DOI] [PubMed] [Google Scholar]
- 43.Platt R, Rice PA, McCormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA 1983; 250:3205–9. [PubMed] [Google Scholar]
- 44.Hui B, Fairley CK, Chen M et al. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model. Sex Transm Infect 2015; 91:365–9. [DOI] [PubMed] [Google Scholar]
- 45.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med 1974; 290:117–23. [DOI] [PubMed] [Google Scholar]
- 46.Marrazzo JM, Apicella MA.. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed Philadelphia, PA: Elsevier/Saunders, 2015:2446–62. [Google Scholar]
- 47.Kent CK, Chaw JK, Wong W et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41:67–74. [DOI] [PubMed] [Google Scholar]
- 48.Turner AN, Reese PC, Ervin M, Davis JA, Fields KS, Bazan JA. HIV, rectal chlamydia, and rectal gonorrhea in men who have sex with men attending a sexually transmitted disease clinic in a midwestern US city. Sex Transm Dis 2013; 40:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbee LA, Kerani RP, Dombrowski JC, Soge OO, Golden MR. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis 2013; 56:1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiridou M, Soetens LC, Koedijk FD, Van der Sande MA, Wallinga J. Public health measures to control the spread of antimicrobial resistance in Neisseria gonorrhoeae in men who have sex with men. Epidemiol Infect 2015; 143:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen VG, Mitterni L, Seah C et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013; 309:163–70. [DOI] [PubMed] [Google Scholar]