Abstract
To examine the activity of somatosensory cortex (S1) neurons to self-generated shear forces on the index and thumb, two monkeys were trained to grasp a stationary metal tab with a key grip and exert forces without the fingers slipping in one of four orthogonal directions for 1 s. A majority (∼85%) of slowly adapting and rapidly adapting (RA) S1 neurons had activity modulated with shear force direction. The cells were recorded mainly in areas 1 and 2 of the S1, although some area 3b neurons also responded to shear direction or magnitude. The preferred shear vectors were distributed in every direction, with tuning arcs varying from 50° to 170°. Some RA neurons sensitive to dynamic shear force direction also responded to static shear force but within a narrower range, suggesting that the direction of the shear force may influence the adaptation rate. Other neurons were modulated with shear forces in diametrically opposite directions. The directional sensitivity of S1 cortical neurons is consistent with recordings from cutaneous afferents showing that shear direction, even without slip, is a powerful stimulus to S1 neurons.
Keywords: grasping, direction, normal force, tangential force, single-unit recording
the interaction between an object surface and the fingers during dynamic manipulation can be described in terms of grip forces normal to the skin and tangential shearing forces, resulting from gravity and object inertial resistance to acceleration. Successful grasping and lifting require the application of adequate grip forces normal to the fingers before the object weight can be safely transferred to the digit skin as a tangential shear force (Johansson and Westling 1984). In addition, arm movements submit hand-held objects to a range of acceleration changes that impart parallel changes in shear forces to the skin (Flanagan and Wing 1993, 1995; Saels et al. 1999). The blocking of cutaneous feedback with a local anesthetic leads to unstable object handling and excessive grip force (Augurelle et al. 2003; Monzée et al. 2003). The perception of shear forces, as distinct from perpendicular skin indentation, is well documented (Biggs and Srinivasan 2002; Paré et al. 2002; Wheat et al. 2004). Despite the many studies investigating responses in somatosensory cortex (S1) to normal indentation of the skin, little is known about how shear force direction and force magnitude are represented in the S1 cortex.
Natural stimuli encountered during tactile exploration tend to result in both shear and normal forces on the surface of the skin (Goodwin et al. 1997; Lederman and Klatzky 1987). It is well known that the tactile perception of textures is enhanced by movement on the skin (Katz 1989) and recently described more fully by Weber et al. (2013). Variations in both normal and shear forces arising from forces between the hand and the explored surface, no doubt, contribute to this enhancement. Cutaneous mechanoreceptors display directional sensitivity to surfaces scanned over the skin (Edin et al. 1995; Goodwin and Morley 1987; Srinivasan et al. 1990). At the cortical level, slip on the skin evokes robust responses from neurons in S1 (Costanzo and Gardner 1980; DiCarlo and Johnson 2000; Mountcastle and Powell 1959; Warren et al. 1986; Whitsel et al. 1972). However, these studies did not differentiate slip from shear without movement, as for example, what occurs when holding and filling a goblet held between the fingers. In this situation, the grip force increases in proportion to the increase in weight to prevent slip. Salimi et al. (1999b) found that shear force perturbations, delivered as abrupt load increases in a single direction during stationary holding, provoked strong excitation of S1 neurons in the monkey.
The rationale for the present study originates from investigations examining the encoding of shear force direction without slip on cutaneous mechanoreceptors of the hand in both humans and monkeys (Birznieks et al. 2001; Wheat et al. 2010). Birznieks and colleagues (2001) showed that cutaneous mechanoreceptors were tuned to the direction of shear force applied to the finger skin. In addition to the direction of shear, Wheat and colleagues (2010) demonstrated that force magnitude further modulated the activity of cutaneous afferents in the monkey digits. Since the responses of S1 neurons to normal force applications were similar to cutaneous receptors, we hypothesized that the cortical representation of shear forces should also be similar. Consequently, the objective of the present study was to investigate cortical responses to shear forces in different directions, similar to those occurring in a natural sensorimotor manipulation task, in which the subject deliberately applies shear forces in different directions to a stationary hand-held object.
MATERIALS AND METHODS
Task and Apparatus
This study was approved by the Animal Ethics Committee of the Faculty of Medicine of the Université de Montréal. Two Macaca fascicularis monkeys (CR01 and FL02) were trained to perform two grasping tasks using the variant of the precision grip, called the key grip, in which the thumb is adducted against the radial edge of the index (Napier 1960). The first task, in which the monkeys grasped, lifted, and held a flat metal tab for a fruit juice reward, was used to search for modulated neurons but not studied further. The second task was more difficult, although the monkeys used the identical key grip to grasp the same, now immobilized, metal tab as shown in Fig. 1. This second task required the monkey to exert an isometric grip force between 0.5 and 3.0 N and then apply an isometric shear force between 0.5 and 2.0 N in one of four approximately orthogonal directions (proximal, distal, radial, or ulnar) by attempting to pull, push, lift, or press the stationary tab to obtain a fruit juice reward. The metal tab was locked in position, such that no real movement could occur. The position of the hand on the metal tab was similar in both monkeys. However, the magnitude of grip force and tangential shear differed with shear force direction (Fig. 2). The grasping surfaces were covered with a high-resolution pressure-sensitive grid (Tekscan, South Boston, MA) to record the pressure distribution exerted by the index and thumb with a spatial and temporal resolution of 1.02 mm at 100 Hz to insure that the fingers, once in position, did not slip (Fig. 3).
Fig. 1.

A: the apparatus and position of the hand used to exert shear forces. The 3-dimensional (3D) force and torque sensors behind the grasping tab measured shear force magnitude and direction. A load cell mounted in a calliper behind the grasping tab recorded grip force normal to the fingers. (Tekscan, South Boston, MA) pressure-sensitive grids covered the grasping surfaces to detect slip. The entire device could either be displaced vertically during a lift-and-hold task or locked in a stationary position to record shear forces in 1 of 4 directions (lifting, pressing, pulling, and pushing). B: the shear (black trace) and grip (gray trace) forces on a single trial in the radial (lifting) direction.
Fig. 2.
Average shear and grip force profiles for each of the 4 directional conditions. The shear forces are represented by the positive traces above the line and grip force as the traces below the line for the first (CR01; dashed line) and second (FL02; solid line) monkey. Both monkeys exerted somewhat similar forces when lifting and pulling, although monkey CR01 additionally used the webbing between Digits 1 and 2 to push and press on the manipulandum, resulting in a grip force that was less than the shear force. The central polar plot represents the distribution of shear force directions on a logarithmic scale, where 1% represents ∼240 trials.
Fig. 3.
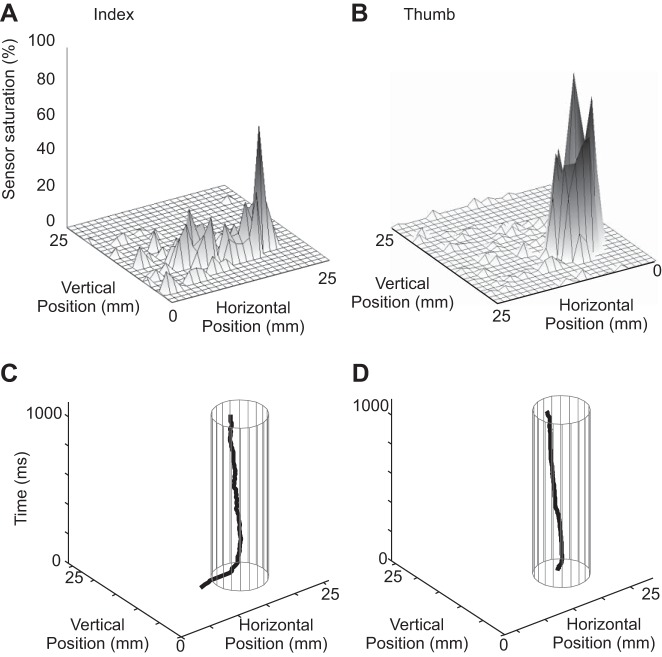
The 2D pressure distributions exerted by the index and thumb are shown (A and B) for the time when the animal exerted a minimum grip and shear force of 0.5 N until successful completion of the trial (∼1.0 s). The pressure grid measures are expressed in percentages of maximal voltage. The pressure is greatest near the geometric center of the skin contact area and least around the perimeter. The absence of slip for the index (C) and thumb (D) is demonstrated by the stability of the center of finger pressure during shear force application. The cylinder indicates the approximate area of maximal finger pressure. At trial onset, the centers of pressure indicated slip, as the fingers were positioned before grasping, but as the fingers grasped the tab and exerted tangential force, the centers of pressure remained stationary.
Data acquisition consisted of blocks of at least 30 trials in each of the 4 shear force directions for a total of >120 trials for each cell. The rewarded direction was then changed to another required vector. No specific cues were provided when the selected vector was changed, although a 1.0-kHz tone signaled that the shear force vector reached the force direction window. Instead, the monkeys identified the new, correct force vector by trial and error for each condition. Correct trials consisted of maintaining the minimal shear force vector within an arc of ±35° of one of the four orthogonal axes (lifting 0°, pulling 90°, pressing down 180°, and pushing 270°) for at least 1.0 s. The monkeys executed the task without visual feedback, relying on a 1.0-kHz tone to indicate that the shear force vector was within the ±35° force window. A three-dimensional force and torque sensor (ATI Industrial Automation, Apex, NC) measured the reactive shear forces parallel to the grasping surface, and a load cell sampled the grip force normal to the skin surface at a frequency of 250 Hz. Regardless of whether the trial was successful, the animal had to release the metal tab and remove its hand from the manipulandum to initiate another trial.
Cell Identification and Receptive Field Mapping
Each neuron was carefully examined to identify and map its cutaneous receptive field. The cutaneous nature of the response was established if the neurons responded to either light punctate pressure with a blunt probe that slightly indented the skin or to stroking the receptive field with a soft camel-hair brush. Cells with proprioceptive fields, identified by joint manipulation of the wrist and fingers, were excluded from this study. The cutaneous responses were classified further as rapidly adapting (RA) if the neuron responded to the application of skin indentation with the probe but not to sustained pressure with the same probe. Although this was not always easily achieved with the hand-held probe, the classification was substantiated further by the neural response to grasping the manipulandum during the lift-and-hold task, similar to the procedures used by Salimi et al. (1999a). That is, RA neurons responded with a brisk activity increase limited to the dynamic application of grip and shear forces, which decreased abruptly during sustained grasping. Neurons were labeled as slowly adapting (SA) neurons if the increased neural discharge continued for the duration of the holding/shear force phase. The location and area of the receptive fields were reproduced on a standard drawing of the hand. All of the cutaneous receptive fields were at least partially on the glabrous skin of the hand, and none of receptive fields were exclusively on the hairy skin. In addition, all of the receptive fields were at least in partial contact with the grasping surfaces. The standard drawings of the hand were subsequently scanned, and the total number of pixels of each receptive field was used to compare the receptive field size with the corresponding cortical areas using an ANOVA.
Surgical Preparation, Unit Recording, and Histology
Under isoflurane anesthesia and sterile conditions, an 18-mm circular recording chamber was surgically implanted over the central sulcus, 18 mm lateral to the midline and 1 mm anterior to stereotaxic interaural zero. After postoperative recovery, a single-unit recording was conducted daily using glass-coated tungsten electrodes with a resistance between 0.5 and 1.0 MΩ, mounted on a Trent Wells hydraulic microdrive for monkey CR01 or a modified Crist Instrument screw-driven double microdrive for monkey FL02. At the conclusion of experimentation, the brains were labeled for sectioning using electrodes coated with Texas Red or India ink before perfusion. The animals were euthanized with an overdose of pentobarbital sodium, and ventricular perfusion was performed with saline, followed by a 10% solution of phosphate-buffered formalin. The brain region of interest was blocked perpendicular to the central and intraparietal sulci, and 40 μm frozen sections were stained with Cresyl violet.
Data Analysis
Activity in a single direction.
Each trial was divided into two phases: a dynamic part involving the increase of grip and shear forces and a static phase, in which the monkey maintained both forces for 1 s. Consistent with the activity recorded during the lift-and-hold task, established in an earlier study (Salimi et al. 1999a), some cortical neurons only responded to the dynamic phase (RAs), whereas others responded during both the dynamic and static phases (SAs). Consequently, the mean firing rate was calculated during the dynamic phase for RA neurons and both the dynamic and static phases for SA neurons.
The first objective was to determine whether S1 neurons have a preferred shear force direction. To address this question, we used a modified version of the Rayleigh test (Fisher 1996) for which firing rate was considered as the distribution parameter. The Rayleigh test was chosen as the primary analysis, since it is commonly used to demonstrate directional tuning for neuronal activity (Fitzgerald et al. 2006; Kalaska et al. 1989; Li et al. 2001). The null hypothesis of the test is that in the absence of a direction effect, the firing rate should be evenly distributed in all angular directions. Since each of the four task conditions had a tolerated arc of ±35°, the Rayleigh test used this variability to interpolate the direction of the theoretical tuning peak by comparing the shear force direction (θi) of each trial according to mean firing rate standard score (ρi), calculated across all trials (Eq. 1)
| (1) |
where n is the number of trials. This test calculates a normalized mean firing rate vector (R0) with a statistical significance (p) at the interpolated resultant vector angle (Eq. 2)
| (2) |
This procedure statistically determined a neuron's bias for a theoretical direction by evaluating a single mode of departure from a uniform random distribution assumed by the prior normalization. To evaluate the modal dispersion, we calculated a circular SD (σ; Eq. 3) using the resultant mean vector length R0 (Zar 1999)
| (3) |
Activity in multiple directions and force magnitude.
A significant limitation of the Rayleigh test is that it evaluates only unimodal tuning directions, and it fails to identify symmetrical bidirectional distributions, in which opposite directions would cancel each other. Moreover, recent studies have indicated that the activity of primary cutaneous afferents can be modulated by both shear force direction and magnitude (Birznieks et al. 2001; Wheat et al. 2010). The objective was then to measure separately the effect of the four tested directions and the effect of force magnitude on the neuronal firing rate. Consequently, we used an analysis of covariance (ANCOVA) to isolate activity changes statistically, due to shear force magnitude from the effect of shear force direction. The Rayleigh test evaluated each trial as a single sample with the mean firing rate as the dependant variable, whereas the ANCOVA examined that the mean shear force applied during the trial and each direction (up, down, pull, or push) was represented as a category in the subsequent analysis. The ANCOVA first provided a correlation between firing rate and force magnitude, and a coefficient >0.25 in at least one direction with P < 0.05 was required to consider a particular neuron as tuned to shear force magnitude.
The ANCOVA calculated the impact of direction over firing rate by removing the effect of force magnitude from the data. The effect of direction was evaluated by the comparison of pairs of directions to determine significant differences using post hoc Tukey tests. On the basis of this analysis, the neurons were sorted into one of five classes. The first group presented a significantly increased firing rate in one direction compared with the other three and was labeled single-direction neurons. Neurons responding to two adjacent target directions were classified as adjacent-direction neurons. Another group of neurons was even more broadly tuned and demonstrated a significant increase in firing for three directions compared with the fourth direction (three-direction neurons). Neurons presenting a significantly higher firing rate in the push-pull or lift-press axes compared with the other axis were labeled opposite-direction neurons. Finally, the remaining neurons were judged to be unaffected by shear force direction.
Comparison of Rayleigh Test with ANCOVA
The Rayleigh test and the ANCOVA both evaluated directional tuning using different methods. The Rayleigh test took into account the unique direction of each trial but neglected the variability of force magnitude. In contrast, the ANCOVA distinguished the force magnitude from the force direction, although only the amplitude could be seen as a parametric variable, whereas the direction was divided into the four arbitrary direction conditions imposed by the task. Accordingly, the ANCOVA determined whether any one direction was different from any other, and we relied on post hoc Tukey tests to determine whether one particular direction was significantly different from the others. Therefore, we consider both tests to be complementary. The Rayleigh test used all of the available directional information to provide an accurate description of directionality with the possible confound of force magnitude. The ANCOVA test removed the directional influence from the force magnitude data but had a greater risk of making type-one (false-negative) errors.
RESULTS
Distribution of Finger Pressure and Shear without Slip
As with any object manipulation task, the monkeys in the present study were required to exert sufficient grip force, normal to the grasping surface, to insure that the fingers did not slip during the application of shear forces. Figure 3, A and B, shows a single time frame of the two-dimensional pressure distributions beneath the index and thumb recorded during shear force application. Figure 3A demonstrates that the finger pressure was unevenly distributed over the contact areas. Both fingers formed pressure profiles, with the greatest pressure exerted near the middle of the fingertip contact area with less pressure about the contact perimeter. Each trial began as the monkey reached for the metal tab and slid the finger over the surface into the grasping position. Once the fingers had established a firm grasp, the pressure-sensor matrix was used to insure that there was no further displacement of the centers of pressure. In a typical trial (Fig. 3, C and D), the centers of finger pressure remained stationary, showing that the fingers did not slip or roll significantly during shear force application.
RA and SA Discharge Patterns
On the basis of their discharge pattern, 77 neurons (53%) were classified as clearly SA, whereas the remaining 67 (47%) neurons were identified as RA. Eight of the SA neurons presented activity modulation during both the dynamic and static phases of the task for a single direction but only responded to the dynamic phase for other directions. These neurons were nevertheless labeled as SAs. Table 1 presents the proportion of actively modulated neurons according to the RA/SA classification.
Table 1.
Classification of recorded neurons
| Target Directions ANCOVA-Tukey |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total RA/SA | Direction by Rayleigh | Single | Adjacent | Three | Opposite | ND | Grip Force Magnitude Only | Direction and Magnitude of Shear and Grip | |
| RA | 67 (47%) | 45 (67%) | 9 (13%) | 6 (9%) | 4 (6%) | 4 (6%) | 44 (66%) | 2 (3%) | 2 (3%) |
| SA | 77 (53%) | 66 (86%) | 29 (38%) | 6 (8%) | 6 (8%) | 8 (10%) | 28 (36%) | 2 (3%) | 19 (25%) |
| Total | 144 | 111 (77%) | 38 (26%) | 12 (8%) | 10 (7%) | 12 (8%) | 72 (50%) | 4 (3%) | 21 (15%) |
Direction tuning, Rayleigh test (P < 0.05), target direction, analysis of covariance (ANCOVA) and Tukey (P < 0.05), and force magnitude correlation with mean firing rate in ANCOVA (r > 0.25 for P < 0.01). RA, rapidly adapting; SA, slowly adapting; ND, nondirectional.
Unidirectional Modulation with Shear Force
A one-way ANCOVA tested whether the mean trial firing rate varied with the direction of shear force, independent of its magnitude. A total of 72 neurons (50%), 49 SAs (34%) and 23 (16%) RAs (see Table 1), had significant activity increases for one, two, or three of the four directions (defined by the matrix of post hoc Tukey pairwise comparison tests at P < 0.05). Shear forces exerted in a single direction excited 29 SA neurons (38%) and 9 RA neurons (13%). The mean SD calculated with the Rayleigh test was 84° for these neurons. Figure 4 shows an example of an SA neuron with a relatively narrow unidirectional tuning and a maximal firing rate in the lifting direction. It should be noted that although this neuron demonstrated greater activity associated with the shear forces in the lifting direction, the tangential forces in the pushing and pressing directions were of larger magnitude. There is also an apparent decrease in activity in the opposite pressing direction, although we cannot determine whether this represents a true inhibition or merely a disfacilitation.
Fig. 4.
Directional activity rasters and histograms for a slowly adapting (SA) neuron in area 1 with a relatively narrow tuning property. The light gray traces over the histograms show the mean grip force, and the darker gray traces show the mean shear force for all trials in each direction with the scale shown on the right. The central polar plot displays the direction and magnitude of each shear force vector. The shaded gray area shows the relative mean firing rate in each of the 4 directions. The heavy black arrow line at 7° from the lifting direction represents the Rayleigh test resultant vector of firing rate activity. This cell has a narrow range of directional sensitivity restricted to tangential forces exerted in the radial (lifting) direction. The size and location of the receptive field are shown in the top left. imp/s, impulses per second.
Shear forces in two adjacent directions significantly excited 12 neurons (8%), 6 SAs (8%) and 6 RAs (9%), suggesting an underlying sensitivity to a force vector in between two of the adjacent orthogonal directions (Table 1). For example, the neuron shown in Fig. 5 had higher firing rates for both pulling and pressing compared with the lifting and pushing directions. This suggests that the preferred shear force vector was located somewhere midway between the proximal (90°) and ulnar (180°) directions. Although the peak activity in the pressing direction occurred before the grip force onset, only the later activity was included in the data analysis. This earlier activity frequently resulted from the skin moving across the grasping surface as the animal repositioned the hand between trials and consequently, was a reaction to slip rather than a response to the shear force alone.
Fig. 5.
Directional activity rasters and histograms for an area 1 rapidly adapting (RA) neuron with a broader tuning scope than the neuron shown in Fig. 4. The conventions are the same as in Fig. 4. The resultant vector line suggests that the cell's preferred direction is between the ulnar and proximal directions and results from a shear force produced by a combination of pulling and pressing down on the grasping surface. Activity occurring before the grip onset reflects contact as the fingers were repositioned between trials. Only activity occurring after the grip onset was used in the force analysis.
A total of 10 neurons (7%), 6 SAs (8%) and 4 RAs (6%), showed significant activity increases in three of the shear force directions (Table 1). These cells were labeled as broadly modulated with a mean SD of 144°. An example is shown in Fig. 6. This neuron, like the neuron shown in Fig. 4, shows a directional preference that differs significantly from the direction of maximum shear force. It is also interesting to note that the neuron in Fig. 6 demonstrated an SA shear force response for the pulling direction in contrast to a dynamic RA-like response in the lifting and pressing directions. Seven additional SA neurons (9%) in both monkeys presented similar, more broadly tuned dynamic (RA) responses compared with their narrower static (SA) response. This observation raised the question as to whether RA neurons are tuned to a wider range of shear directions than SA neurons. To assess the directional tuning of the RA and SA neurons, we applied a Rayleigh test to the normalized mean firing rate of each neuron. With this less-restrictive analysis, 111 neurons (77%) showed significant activity increases for a specific direction. The Rayleigh test, in agreement with the ANCOVA, showed that more SA (66 neurons, 86%) than RA (45 neurons, 67%) neurons (Table 1) had a significant directional preference. Figure 7A shows that the calculated vectors were relatively evenly distributed across all angular directions for both RA and SA neurons, except for the pulling-pressing (proximal-ulnar) quadrant, which was clearly under-,represented (Fig. 7A). Nevertheless, the maximal neuronal firing rate was not significantly affected by their direction (Fig. 7B). Figure 7C displays the width of excitatory tuning for each of the cells represented by the calculated Rayleigh SDs, which ranged from 54 to 171°. The tuning of RA neurons was slightly broader than for SA neurons (92° for RAs vs. 86° for SAs, P < 0.05).
Fig. 6.
Directional activity rasters and histograms for an area 2 neuron with a broader tuning range than the neurons shown in Figs. 4 and 5. The conventions are the same as in Fig. 4. It should be noted that the SA discharge behavior is related to the static phase only in the pulling direction.
Fig. 7.
A: distribution of preferred directions of RA (gray) and SA (black) neurons, according to the Rayleigh test for all unidirectional cells. The vector length corresponds to the mean firing rate of the modulation. Zero degrees (0°) corresponds to the radial direction, 90° to the proximal, 180° to the ulnar, and 270° to the distal direction of shear force. Vectors generated in the pressing-pulling directions are notably absent. B: the mean peak firing rate for RA (light gray) and SA (dark gray) neurons shown according to their preferred direction. The vertical bars stand for SD for each 45° category. C: the width of RA (gray) and SA (black) tuning, sorted according to cortical area. The gray zone represents the mean SD for each subcategory.
Bidirectional Modulation with Shear Force
A further 12 neurons [8 SA (10%) and 4 RA (6%)] had increased activity for shear force vectors in diametrically opposite directions, demonstrating an activity modulation for the orientation of the shear (indicated as opposite in Table 1). An example of a bidirectional neuron modulated to the pushing-pulling axis is shown in Fig. 8. These neurons were observed in both monkeys and appeared to respond to shear forces that both stretched and compressed the receptive field skin along a particular axis.
Fig. 8.
Directional activity rasters and histograms for an SA neuron recorded in area 1 and excited by shear forces in diametrically opposite directions. The conventions are the same as in Fig. 4. Since the Rayleigh test is not significant for this type of bidirectional tuning, no resultant vector is shown.
Rayleigh and ANCOVA Comparison
Although the two statistical techniques considered different aspects of the data, the two tests were congruent for all neurons, except for the bidirectional neurons, which were excluded from the Rayleigh test. That is, all of the remaining neurons with a significant ANCOVA and post hoc Tukey tests were also significant with the Rayleigh test. However, 50 neurons (45%) with significant Rayleigh scores had inconsistent post hoc Tukey tests in the ANCOVA and were excluded.
Anatomical Distribution of Recorded Neurons
The neuronal recordings were obtained entirely from the thumb and index regions of S1, which included areas 3b, 1, and 2. Microstimulation (300 ms, 300 Hz, maximum 30 μA) at the recording site of each neuron was used to exclude neurons of the motor cortex (M1), recorded deep in the precentral sulcus. Specifically, neurons with cutaneous receptive fields recorded at sites from which finger and wrist movements were evoked were eliminated in the present study. Examination of the histological reconstruction of the recording sites failed to reveal any specific pattern of activity associated with a particular cytoarchitectonic area of S1. Although more cells were recorded in area 2 [61 neurons (42%)] and area 1 [56 neurons (39%)], 16 neurons of the 27 neurons recorded in area 3b (59%) also responded to force direction and magnitude. Although area 2 might be considered by some to be far along the processing pathway for touch, it has strong somatotopically organized connections with M1 (Stepniewska et al. 1993), and the importance of these connections for object manipulation is well established (Brochier et al. 1999; Hikosaka et al. 1985). Further analysis failed to demonstrate significantly different distribution patterns for either the maximal response angle or the breadth of tuning (see Fig. 7C). The receptive field areas, as reflected by the total number of pixels on the digitized standard drawings, were compared with an ANOVA. In contrast to the report by Iwamura et al. (1993), our analysis failed to reveal any difference in the size of receptive fields in areas 1 and 2 compared with area 3b.
Relation of Receptive Fields to Directional Tuning Properties
To assess the possible relationship between the size and location of the cutaneous receptive fields to shear force direction, we superimposed the standard drawings of the receptive fields and compared these with the preferred tuning direction. All of the receptive fields were on the index and thumb and were, at least partially, in contact with the grasping surfaces. Figure 9 shows the superimposed receptive fields and direction tuning vectors on standard figurines of the hand. The receptive field analysis failed to reveal any difference in size between neurons with receptive fields on the index compared with the thumb. Despite the considerable overlap among the neuronal fields, we were unable to discern any pattern that would suggest a mechanical anisotropy of the skin, such that the receptor type and/or the location and size of the receptive field might influence the preferred direction.
Fig. 9.

Receptive fields and preferred directions of unidirectional neurons. A: size and location of RA receptive fields are displayed beside their preferred direction, represented as arrowheads at the center of the receptive fields. B: SA neurons showing the diversity of preferred directions within similar receptive fields. Six neurons with double and discontinuous receptive fields, including 1 on digit 3, are also indicated.
Normal and Tangential Shear Forces
Virtually all of the neurons recorded in this study were excited by the minute normal forces associated with the initial contact of the fingers with the device. However, our force measurements and data analyses focused on shear and normal forces >0.05 N. In general, the shear forces were more variable than the normal forces. This is probably because the more powerful muscles of the elbow and shoulder were involved in pushing and pulling, whereas lifting and pressing primarily involved muscles of the wrist, although no electromyographies were recorded to substantiate this hypothesis. Regardless of the muscles involved, greater shear forces require greater grip forces to prevent the fingers from slipping, consequently limiting the magnitude of the shear forces. Table 2 shows the mean normal and shear forces in each direction for each monkey. A postrecording analysis revealed that monkey CR01 exerted a shear force that was greater than the grip force when pushing or pressing the tab. A shear force of this magnitude should have caused the fingers to slip on the grasping surface. However, the Tekscan matrices failed to indicate any evidence of slip, indicating that monkey CR01 used the webbing between the thumb and index to exert an additional tangential force by pushing isometrically in the distal direction. For monkey CR01, the tangential force did not fully reflect the total shear force on the skin for the pressing and pushing directions. Consequently, one might have expected fewer neurons modulated in these directions. Instead, the population of neurons with receptive fields on the fingers responded to shear forces distributed in all angular directions for both monkeys. Table 2 shows that this possibility was eliminated for the second monkey (FL02), because the grip forces exceeded the shear forces in all four directions.
Table 2.
Distribution of forces according to direction of grasping for each monkey
| Mean Grip Force |
Mean Shear Force |
|||
|---|---|---|---|---|
| CR01 | FL02 | CR01 | FL02 | |
| Lifting (0°) | 1.9 ± 0.5 N | 2.9 ± 1.1 N | 1.3 ± 0.5 N | 0.9 ± 0.3 N |
| Pulling (90°) | 2.2 ± 0.6 N | 2.8 ± 1.2 N | 1.9 ± 0.5 N | 1.0 ± 0.3 N |
| Pressing (180°) | 1.1 ± 0.4 N | 2.3 ± 0.7 N | 3.5 ± 0.7 N | 1.1 ± 0.4 N |
| Pushing (270°) | 1.1 ± 0.5 N | 2.8 ± 0.9 N | 2.8 ± 0.9 N | 1.3 ± 0.5 N |
Means ± SD.
To investigate the relative impact of shear force magnitude on neuronal activity, we used an ANCOVA to remove statistically the effect of shear force direction to reveal the residual effect of shear force magnitude. A normal force can exist alone, whereas shear forces are always accompanied by a certain amount of normal force. Despite this reliance of shear forces upon normal force, there was nevertheless sufficient normal force variation to compute correlation coefficients for both parameters. For the range of forces tested in this experiment, only four neurons (2%) responded to normal force alone without significant modulation for either shear direction or shear force magnitude. In spite of the paucity of neurons related exclusively to either shear direction or magnitude, a substantial number of cells, 19 SA (25%) and 2 RA neurons (3%), had significantly increased activity related to normal and shear forces in combination with shear force direction. This suggests that normal and shear forces and their resultants are not represented by separate independent cell populations, but instead, these parameters are represented, to varying degrees, in the activity of the same S1 neurons.
DISCUSSION
Encoding of Tangential Force Direction and Magnitude
In general, our results are very similar to those of Birznieks et al. (2001) and Wheat et al. (2010) on peripheral afferents. The S1 cortical neurons were strongly tuned to shear force direction, and like the peripheral afferents, a slightly higher proportion (86%) of SA S1 neurons was directionally tuned compared with 67% of RA neurons. However, the breadth of tuning directions appeared roughly the same.
There is considerable literature suggesting the existence of a mechanical bias or anisotropies favoring shear force, in particular, directions on the fingertips. Hägar-Ross et al. (1996) found that skin mechanoreceptors responded at shorter latencies to forces applied in the direction of gravity regardless of hand position, and although they suggested that this might be the result of central processing, we were unable to find evidence of this. In contrast, Jones and Hunter (1992) found that the pinch force responses to applied shearing loads were higher for loads in the distal direction away from the palm. More recently, Birznieks et al. (2001) found that the directional biases were different depending on receptor type: a distal bias for SA type I, a proximal bias for SA type II, and proximal and radial bias for RA type I. Delhaye et al. (2014), using a computer-controlled application of tangential forces to the fingertip, concluded that the shape and amplitude of the skin deformations were highly dependent on the direction of force application. Unfortunately, our data on S1 neuronal activity did not reveal any particular directional bias related to either cortical location or adaptation rate. However, as shown by the radial diagram in Fig. 2, the shear forces in the present study varied widely, because they were self-generated and rather poorly controlled. Therefore, the results of the present study are not precise enough to be compared with the externally and exactly applied forces in the studies by Birznieks et al. (2001), Delhaye et al. (2014), and Wheat et al. (2010).
Response to Normal Skin Indentations
Virtually all of the neurons in the present study discharged on initial contact with the metal tab, since most cutaneous receptors are very sensitive to minute skin indentations (Iggo and Muir 1969; Johansson et al. 1980; Talbot et al. 1968; Werner and Mountcastle 1965). However, our instrumentation was unable to measure these very low (<2 mN) normal forces associated with simply contacting the grasping surface with the fingers. Had we been able to measure these minute contact forces, we might have found a larger percentage of neurons modulated with normal force. As it happened, 25 (18%) neurons demonstrated significant correlations with normal grip forces in the range of 0.05–1.0 N, but of these, only 4 neurons (3%) had activity correlated with normal grip force without significant modulation with either shear force direction or magnitude. In the present study, 60 (41%) S1 neurons responded to the shear force direction, but the activity did not scale with the shear force magnitude. Furthermore, one-fourth of SA neurons presented activity modulation related to some combination of direction, grip, and shear force. These data are also congruent with our observations on the combination of shear force and slip involved in tactile exploration (P. Fortier-Poisson and A. M. Smith, doi:10.1152/jn.00747.2014). It would appear that shear force magnitude and direction are not encoded by separate neuronal populations. Instead, single S1 neurons respond to both shear force and direction to various degrees.
Table 2 revealed a particular problem presented by monkey CR01. The tangential forces generated by pushing and pressing exceeded the normal force, although the pressure-sensitive surfaces showed no indication of slip. We concluded that in addition to grasping with the fingertips, this monkey was pushing and pressing the manipulandum with the webbing between the index and thumb, and therefore, for these directions, the total tangential force was greater than the amount of shear force actually exerted on the fingers for the pushing and pressing directions. Despite this uncertainty about the shear force in these pushing and pressing directions for this monkey, neurons with receptive fields on the fingers responded to shear forces evenly in the same angular directions for both monkeys. Otherwise, this behavior could have introduced a bias in the Rayleigh statistical test, since it did not take into account the magnitude of exerted forces. However, since the ANCOVA correlation between force magnitude and firing rate was calculated for each direction individually, the number of neurons modulated with force amplitude might have been underestimated.
Grip and Shear Force Variations with Direction
Although the monkeys were only required to exert a minimum grip and shear force of 0.5 N for successful completion of the task, the actual grip and shear force magnitudes varied considerably with direction. In general, the pushing and pressing directions elicited greater shear forces, probably because the more powerful proximal muscles of the elbow and shoulder were involved. Nevertheless, the grip forces had to increase in conjunction with the shear forces to prevent the fingers from slipping on the manipulandum. In spite of these shear force differences, many neurons, such as those shown in Figs. 4 and 6, had maximal activity for orientations that were different from the direction of maximal shear force. We conclude from this that the possible confounding of shear force directions and shear force magnitude did not significantly influence the preferred force vector of individual neurons.
Contribution of Efferent Copy to S1 Discharge
The present task was selected because of its similarity to many natural manipulative behaviors and because it offered an opportunity to study cortical sensorimotor integration in a functionally realistic environment. However, the task raises the issue as to the degree to which changes in S1 spike frequency are a combination of central motor commands (by way of an efferent copy signal) as opposed to pure cutaneous feedback from shear forces applied to the receptive field. This issue was succinctly stated by Evarts (1981), as it applied to both S1 and M1 neurons with cutaneous receptive fields. Movement-related increases in discharge in S1 before stimulation of cutaneous receptive fields have been reported previously by many investigators (Evarts and Fromm 1981; Fetz et al. 1980; Nelson 1987; Salimi et al. 1999a), and their data emphasize the close reciprocal connections with M1. Although we cannot determine the exact degree to which the responses in the present study are due to purely sensory feedback, several additional observations are worth considering. The first is that Costanzo and Gardner (1980) have shown that many S1 neurons are sensitive to the direction of slip on the skin, which would provide physiological support for the accurate perception of shear reported by several investigators (Biggs and Srinivasan 2002; Paré et al. 2002; Srinivasan et al. 1990; Wheat et al. 2004). The second is that in the present study, some of the shear forces were driven by muscular activity at the elbow and shoulder but were applied by the grasping tab directly to the finger skin. Consequently, the command to the muscles of the shoulder, elbow, and wrist generating the tangential forces was only remotely related to shear on the skin. Third, the S1 tuning properties are very similar to the tuning of cutaneous afferents recorded in the fingers of anesthetized monkeys (Wheat et al. 2010). Taken together, these observations provide strong circumstantial evidence that the activity recorded in the present study was, in large part, if not entirely, the result of cutaneous feedback rather than a feed-forward motor command. Ultimately, experiments recording cortical responses to the passive application of controlled shear forces in the unanesthetized monkey will be needed to confirm this hypothesis.
Range of Unidirectional Tuning
The monkeys were required to generate blocks of shear forces within an arc of ±35° for one of the four orthogonal axes. All of the cells with modulated activity appeared to have either unimodal or bimodal responses to direction (Fig. 7). However, the absence of modulated neurons for the pulling-pressing quadrant (proximal-ulnar) for both monkeys was unexpected. We are reluctant to attach any particular meaning to this observation other than to suggest that it probably reflects some biomechanical constraint on the monkeys' wrist and hand or possibly from using a lift-and-hold task to search for modulated neurons. Nevertheless, the majority of S1 neurons was maximally excited with a unimodal relation to direction, although the width of the tuning excitation varied considerably. Regardless as to whether the ANCOVA indicated that the neuronal activity was modulated with one, two adjacent, or three target directions, the only feature that seemed to distinguish these neurons was the width of their directional modulations. The neurons responding to two contiguous targets suggested a single preferred direction interpolated between the two targets. The neurons responding to three targets suggest an even broader tuning for direction.
Bidirectional Tuning
To date, no bidirectional shear force responses have been reported for peripheral cutaneous afferents (Birznieks et al. 2001; Wheat et al. 2010). However, the statistical tests used in these papers evaluated only a single tuning direction and would not have identified symmetrical activity in opposite directions. Salimi et al. (1999b) demonstrated a bidirectional response in an S1 neuron as a hand-held object was accelerated and then decelerated in a lift-and-hold task. The bidirectional modulation might suggest a response to shear forces that both stretched and compressed the receptive field skin, or it might be an emergent property based on multiple converging peripheral afferents, as suggested for other features, such as roughness and orientation (Bensmaia et al. 2008; Dodson et al. 1998). Ultimately, whether these bidirectional responses reflect an emergent property of cortical neurons or instead, result from receptors sensitive to both compression and stretch requires further investigation.
Adaptation Properties and Directional Tuning
RA skin receptors discharge only during the dynamic component of skin deformation, whereas SA receptors continue to discharge during the static phase (Werner and Mountcastle 1965), and since S1 neurons presented similar responses, they were organized into similar categories (Mountcastle et al. 1969; Sur et al. 1984). In a grasp, lift-and-hold task performed by monkeys (Salimi et al. 1999a), we also observed that some S1 neurons responded only at the grip onset and adapted quickly to static finger pressure, whereas other S1 neurons continued firing during the maintained grasping. However, the generally accepted assumption that the RA and SA properties of S1 neurons are conferred by the excitation of peripheral receptors with similar properties has been questioned by Pei and colleagues (2009), who suggested that RA and SA afferents converge on the majority of S1 neurons. In the present study, we found eight SA neurons that demonstrated RA responses to a wider range of tangential force directions compared with a narrower static directional response (see Fig. 6). These observations suggest that a difference in threshold between dynamic and static shear forces may be determined by the direction of the shear force stimulation. That is, modulation to shear force in the optimal direction may explain how some neurons show SA properties in their preferred direction and only RA properties over the remainder of their directional tuning range.
General Conclusion and Limitations
The present results are consistent with behavioral studies showing that human subjects can readily perceive and discriminate sensations arising from tangential shear forces applied to the skin (Biggs and Srinivasan 2002; Goodwin and Wheat 2004; Paré et al. 2002). Furthermore, single fiber microneurographic recordings (Birznieks et al. 2001; Wheat et al. 2010) indicate that nearly all afferents in the hand have unimodal responses to tangential force vectors applied to the skin. Admittedly, the shear forces in the present study were self-generated and were not systematically controlled applications to the passive skin to truly test the neuronal responses to all shear angles with controlled force magnitudes. However, the study of self-generated shear forces more accurately reveals the activity of S1 neurons during object manipulation. Despite this important limitation, the activity of single cells in S1 was, in general, very similar to the cutaneous afferents responding to shear forces applied passively to the finger. Furthermore, a new activity pattern emerged in S1, as shown by the neurons with bimodal activity. In addition, some neurons showed an SA-type response in only one direction, but in other directions, they behaved as RA-type neurons. Most S1 cortical neurons, such as primary afferents, were not exclusively sensitive to either normal or tangential forces. Instead, each unit responded, to varying degrees, to force direction and force magnitude. Ultimately, these responses suggest a population coding process starting in the peripheral afferents with further processing within S1.
GRANTS
Support for this research was provided by individual and group grants from Canadian Institutes of Health Research and Fonds de la Recherche en Santé du Québec for the Groupe de Recherche du Système Nerveux Central of the Université de Montréal.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J-S.L. and A.M.S. conception and design of research; P.F-P., J-S.L., and A.M.S. performed experiments; P.F-P., J-S.L., and A.M.S. analyzed data; P.F-P. and A.M.S. interpreted results of experiments; P.F-P. and A.M.S. prepared figures; P.F-P. and A.M.S. drafted manuscript; P.F-P., J-S.L., and A.M.S. edited and revised manuscript; P.F-P., J-S.L., and A.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical assistance of L. Lessard and C. Valiquette is gratefully acknowledged. The authors also thank Drs. N. Dancause and J. F. Kalaska for their helpful critique of the manuscript.
REFERENCES
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining the safety margin during the manipulation of hand-held objects. J Neurophysiol 89: 665–671, 2003. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Denchev PV, Dammann JF III, Craig JC, Hsiao SS. The representation of stimulus orientation in the early stages of somatosensory processing. J Neurosci 28: 776–786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs J, Srinivasan MA. Tangential versus normal displacements of skin: relative effectiveness for producing tactile sensations. In: Proceedings 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems. HAPTICS 2002, p. 121–128. [Google Scholar]
- Birznieks I, Jenmalm P, Goodwin AW, Johansson RS. Encoding of direction of fingertip forces by human tactile afferents. J Neurosci 21: 8222–8237, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochier T, Boudreau M-J, Paré M, Smith AM. The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res 128: 31–40, 1999. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Gardner EP. A quantitative analysis of responses of direction-sensitive neurons in somatosensory cortex of awake monkeys. J Neurophysiol 43: 1319–1341, 1980. [DOI] [PubMed] [Google Scholar]
- Delhaye B, Lefevre P, Thonnard JL. Dynamics of fingertip contact during the onset of tangential slip. J R Soc Interface 11: 20140698, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JJ, Johnson KO. Spatial and temporal structure of receptive fields in primate somatosensory area 3b: effects of stimulus scanning direction and orientation. J Neurosci 20: 495–510, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson MJ, Goodwin AW, Browning AS, Gehring HM. Peripheral neural mechanisms determining the orientation of cylinders grasped by the digits. J Neurosci 18: 521–530, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Essick GK, Trulsson M, Olsson KA. Receptor encoding of moving tactile stimuli in humans. I. Temporal pattern of discharge of individual low-threshold mechanoreceptors. J Neurosci 15: 830–847, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Role of motor cortex in voluntary movements in primates. In: Handbook of Physiology, The Nervous System, Motor Control. Bethesda, MD: American Physiological Society, 1981, sect. 1, vol. II, part 2, p. 1083–1120. [Google Scholar]
- Evarts EV, Fromm C. Transcortical and servo control of movement. Can J Physiol Pharmacol 59: 757–775, 1981. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Finocchio DV, Baker MA, Soso MJ. Sensory and motor responses of precentral cortex cells during comparable passive and active joint movements. J Neurophysiol 43: 1070–1089, 1980. [DOI] [PubMed] [Google Scholar]
- Fisher N. Statistical Analysis for Circular Data. Cambridge, UK: Cambridge University Press, 1996. [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: representation of orientation on different finger pads. J Neurosci 26: 6473–6484, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. Modulation of grip force with load force during point-to-point arm movements. Exp Brain Res 95: 131–143, 1993. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The stability of precision grip forces during cyclic arm movements with a hand-held load. Exp Brain Res 105: 455–464, 1995. [DOI] [PubMed] [Google Scholar]
- Fortier-Poisson P, Smith AM. Neuronal activity in somatosensory cortex related to tactile exploration. J Neurophysiol. doi: 10.1152/jn.00747.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AW, Macefield VG, Bisley JW. Encoding of object curvature by tactile afferents from human fingers. J Neurophysiol 78: 2881–2888, 1997. [DOI] [PubMed] [Google Scholar]
- Goodwin AW, Morley JW. Sinusoidal movement of a grating across the monkey's fingerpad: effect of contact angle and force of the grating on afferent fiber responses. J Neurosci 7: 2192–2202, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AW, Wheat HE. Sensory signals in neural populations underlying tactile perception and manipulation. Annu Rev Neurosci 27: 53–77, 2004. [DOI] [PubMed] [Google Scholar]
- Häger-Ross C, Cole KJ, Johansson RS. Grip-force responses to unanticipated object loading: load direction reveals body- and gravity-referenced intrinsic task variables. Exp Brain Res 110: 142–150, 1996. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Tanaka M, Sakamoto M, Iwamura Y. Deficits in manipulative behaviors induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Res 325: 375–380, 1985. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir R. The structure and function of a slowly adapting touch corpuscule in hairy skin. J Physiol 200: 763–796, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O. Rostrocaudal gradiants in the neuronal receptive field complexity in the finger region of the alert monkey's postcentral gyrus. Exp Brain Res 92: 360–368, 1993. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB, Westling G. Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Res 184: 343–351, 1980. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Influences of cutaneous sensory input on the motor coordination during precision manipulation. In: Somatosensory Mechanisms, edited by von Euler C, Franzen O, Lindblom U, and Oteson D. London: Macmillan, 1984, p. 249–260. [Google Scholar]
- Jones LA, Hunter IW. Changes in pinch force with bidirectional loads. J Mot Behav 24: 157–164, 1992. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud'Homme MJ. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D, Kruger LE. The World of Touch. Hillsdale, NJ: Lawrence Erlbaum, 1989. [Google Scholar]
- Lederman SJ, Klatzky RL. Hand movements: a window into haptic object recognition. Cogn Psychol 19: 342–368, 1987. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Taylor MM. Fingertip force, surface geometry and the perception of roughness by active touch. Percept Psychophys 12: 401–408, 1972. [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30: 593–607, 2001. [DOI] [PubMed] [Google Scholar]
- Monzée J, Lamarre Y, Smith AM. The effects of digital anesthesia on force control in a precision grip. J Neurophysiol 89: 672–683, 2003. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Powell TP. Central nervous mechanisms subserving position sense and kinesthesia. Bull Johns Hopkins Hosp 105: 173–200, 1959. [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neural mechanisms in flutter-vibration studied in unanesthetized monkeys. Neural periodicity and frequency discrimination. J Neurophysiol 32: 452–484, 1969. [DOI] [PubMed] [Google Scholar]
- Napier JR. Studies of the hands of living primates. Proc Zool Soc Lond 134: 647–657, 1960. [Google Scholar]
- Nelson RJ. Activity of monkey primary somatosensory cortical neuron changes prior to active movement. Brain Res 406: 402–407, 1987. [DOI] [PubMed] [Google Scholar]
- Paré M, Carnahan H, Smith AM. Magnitude estimation of tangential force applied to the fingerpad. Exp Brain Res 142: 342–348, 2002. [DOI] [PubMed] [Google Scholar]
- Pei YC, Denchev PV, Hsiao SS, Craig JC, Bensmaia SJ. Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J Neurophysiol 102: 1843–1853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saels P, Thonnard JL, Detrembleur C, Smith AM. Impact of the surface slipperiness of grasped objects on their subsequent acceleration. Neuropsychologia 37: 751–756, 1999. [DOI] [PubMed] [Google Scholar]
- Salimi I, Brochier T, Smith AM. Neuronal activity in somatosensory cortex of monkeys using a precision grip. I. Receptive fields and discharge patterns. J Neurophysiol 81: 825–834, 1999a. [DOI] [PubMed] [Google Scholar]
- Salimi I, Brochier T, Smith AM. Neuronal activity in somatosensory cortex of monkeys using a precision grip. III. Responses to altered friction and perturbations. J Neurophysiol 81: 845–857, 1999b. [DOI] [PubMed] [Google Scholar]
- Srinivasan MA, Whitehouse JM, Lamotte RH. Tactile detection of slip: surface microgeometry and peripheral neural codes. J Neurophysiol 63: 1323–1332, 1990. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol 330: 238–271, 1993. [DOI] [PubMed] [Google Scholar]
- Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol 51: 724–744, 1984. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanorecptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- Warren S, Hämäläinen HA, Gardner EP. Objective classification of motion- and direction-sensitive neurons in primary somatosensory cortex of awake monkeys. J Neurophysiol 56: 598–622, 1986. [DOI] [PubMed] [Google Scholar]
- Weber AI, Saal HP, Lieber JD, Cheng JW, Manfredi LR, Dammann JF III, Bensmaia SJ. Spatial and temporal codes mediate the tactile perception of natural textures. Proc Natl Acad Sci USA 110: 17107–17112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner G, Mountcastle VB. Neural activity in mechanoreceptive cutaneous afferents: stimulus-response relations, Weber functions and information transmission. J Neurophysiol 28: 359–397, 1965. [DOI] [PubMed] [Google Scholar]
- Wheat HE, Salo LM, Goodwin AW. Cutaneous afferents from the monkeys fingers: responses to tangential and normal forces. J Neurophysiol 103: 950–961, 2010. [DOI] [PubMed] [Google Scholar]
- Wheat HE, Salo LM, Goodwin AW. Human ability to scale and discriminate forces typical of those occurring during grasp and manipulation. J Neurosci 24: 3394–3401, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Roppolo JR, Werner G. Cortical information processing of stimulus motion on primate skin. J Neurophysiol 35: 691–717, 1972. [DOI] [PubMed] [Google Scholar]
- Zar JK. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1999. [Google Scholar]