Significance
The immunoglobulin heavy chain (IgH) 3′regulatory region (3′RR) fine-tunes IgH gene expression during B cell development. One singularity of this region is its quasi-palindromic structure conserved in the 3′RR of other species. By comparing previous mouse knockout (KO) models (3′RR- and hs3b-4 KO) to a novel mutant devoid of the quasi-palindrome (3′PAL KO), we highlighted common features and differences that specify two distinct regulatory entities: (i) the distal module (hs4) is sufficient for normal IgH expression up to the naïve B cell stage; (ii) during B-cell activation, the proximal module (quasi-palindrome) is important for both class switch recombination and somatic hypermutation; and (iii) in plasma cells, the quasi-palindrome is required for robust transcription of the IgH locus.
Keywords: immunoglobulin gene regulation, enhancers, B-cell development
Abstract
As a master regulator of functional Ig heavy chain (IgH) expression, the IgH 3′ regulatory region (3′RR) controls multiple transcription events at various stages of B-cell ontogeny, from newly formed B cells until the ultimate plasma cell stage. The IgH 3′RR plays a pivotal role in early B-cell receptor expression, germ-line transcription preceding class switch recombination, interactions between targeted switch (S) regions, variable region transcription before somatic hypermutation, and antibody heavy chain production, but the functional ranking of its different elements is still inaccurate, especially that of its evolutionarily conserved quasi-palindromic structure. By comparing relevant previous knockout (KO) mouse models (3′RR KO and hs3b-4 KO) to a novel mutant devoid of the 3′RR quasi-palindromic region (3′PAL KO), we pinpointed common features and differences that specify two distinct regulatory entities acting sequentially during B-cell ontogeny. Independently of exogenous antigens, the 3′RR distal part, including hs4, fine-tuned B-cell receptor expression in newly formed and naïve B-cell subsets. At mature stages, the 3′RR portion including the quasi-palindrome dictated antigen-dependent locus remodeling (global somatic hypermutation and class switch recombination to major isotypes) in activated B cells and antibody production in plasma cells.
Immunoglobulin heavy chain (IgH) expression is critical for B-cell development and survival. In developing B-lineage cells, accessibility to the major remodeling events [VDJ recombination, somatic hypermutation (SHM), class switch recombination (CSR), and locus suicide recombination] depends on epigenetic changes and germ-line transcription of many regions, including VH promoters, I/switch region promoters, cis-regulatory region enhancers, and chromatin insulators (1–3). A focus on knockout (KO) models for IgH cis-regulatory regions (enhancers and chromatin insulators) is a means to simplify the regulation picture. At the preproB stage, the IgH locus undergoes long-range looping in a “rosette-like” structure that brings into close proximity major IgH regulatory regions, such as the VH to DH intergenic control regions (IGCR1 and -2), the Eµ intronic enhancer, the 3′ regulatory region (3′RR), and the 3′IgH CTCF-binding elements (CBEs) (4–6). Initiation of VDJ recombination is assisted by the Eµ enhancer, which provides efficient transcription and accessibility to initiate DH to JH rearrangements (7–10), as well as the IGCR1 and -2 elements that ordinate the VH to DJH second recombination step (5, 11–13). Devoid of enhancer activity (14, 15), 3′CBE (hs5 to hs8) likely participate in IgH folding before VDJ recombination because deletion of hs5 to -7 only impacts use of proximal VH regions (16). In pre-B cells, once a functional H chain is expressed as a component of the pre–B-cell recptor (BCR), the Eµ enhancer function switches from DJH region accessibility to Igµ chain expression, and consequently modulates pre-BCR expression and expansion of the pre–B-cell compartment (17, 18). The activity of Eµ even extends to the newly formed (NF)/immature stage, where it tunes BCR expression and influences B-cell fate (18). The 3′RR has been proven to be dispensable for locus contraction and VDJ recombination (19, 20). Its transcriptional activity starts after the pre-B stage and continues throughout B-cell development (21). The large window of activity of the 3′RR implies that its regulatory function shifts sequentially to modulate the expression of functional H chains (in BCR-expressing cells or plasma cells), the production of germ-line regulatory transcripts correlated with Ag-dependent remodeling events, such as CSR, SHM (for review, see ref. 1), or even suicide recombination (3). The multiple KO and transgenic models developed to study 3′RR function (21) have brought considerable information, although quite puzzling, given that models have been mostly studied individually. Transgenic models carrying bacterial artificial chromosomes prohibit B-cell development and chromatin studies but provided information on CSR and SHM (22). Taking CSR into account, bacterial artificial chromosome transgene studies point out a cumulative activity of IgH 3′ enhancer elements, with special activities for some of them, such as hs1-2, hs4 alone or combined with hs3b (23, 24), and on the other hand, exonerate any effect of the hs3 homologs (25). Transgenic models contradict endogenous deletion studies with regards to BCR expression and antibody secretion (23). From endogenous deletion models, we learned that 3′RR enhancers share redundant functions because individual KOs had no significant consequences on B-cell remodeling events (26–28), whereas combined deletion of hs3b and hs4 decreased CSR to all isotypes, except for IgG1 (29). The entire 3′RR deletion demonstrates the potency of the region at all steps: deficient mice cumulate BCR-expression defects (30), global SHM defects (31), abrogated CSR, and failure to secrete Igs (32).
Another singularity of the 3′RR is its quasi-palindromic structure centered around hs1-2, composed of inverted repeats for about 25 kb and terminating by virtually identical hs3a and hs3b enhancers in the mouse (33, 34). A similar quasi-palindromic organization is conserved in the 3′RR of other species, including humans and apes (3, 35, 36). Strikingly, evolution did not conserve virtual homology of 3′RR inverted regions but preserved its global structure. Such a selection implies a dedicated function for the 3′RR quasi-palindrome that has not yet been elucidated. Our present study describes and compares a new KO mouse model devoid of the quasi-palindromic 3′RR proximal module (3′PAL KO) to relevant models (Fig. 1) lacking the distal module (hs3b-4 KO) (29) or the entire region (3′RR KO) (32). Common features and differences raised by this side-by-side comparison reveal that the 3′RR is composed of two functional entities that activate sequentially during B-cell development.
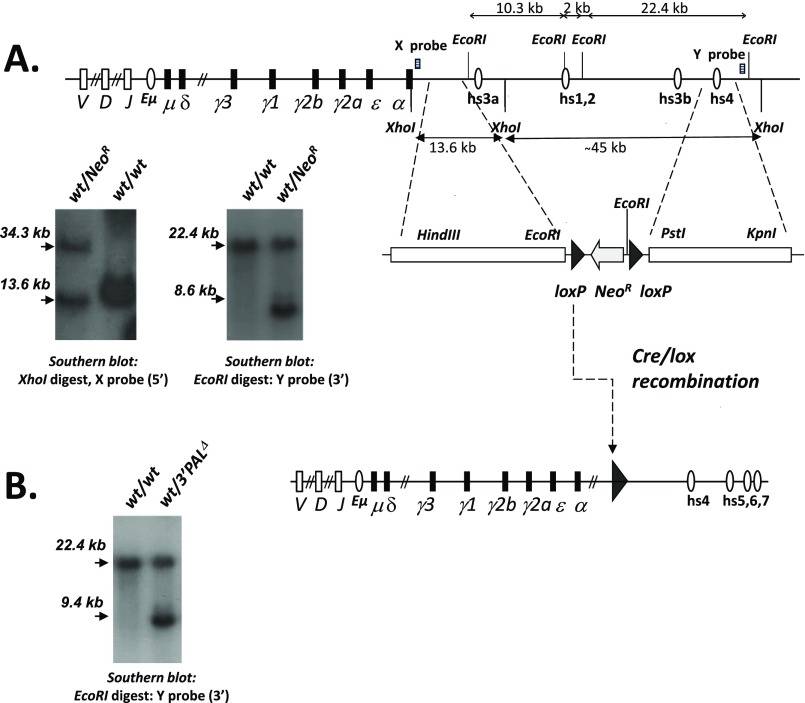
Fig. 1.
The IgH 3′RR: structure and KO mouse models. Mouse IgH locus showing details of genomic regions across the 3′RR. Horizontal triangles represent inverted repeated regions forming a quasi-palindrome. Deletions carried out in the mouse models used for the study are indicated (see introductory paragraphs for details).
Results
Deletion of the Proximal IgH 3′RR Module in the Mouse.
To determine the role of the “proximal 3′RR structural module” (Fig. 1) (called “3′PAL” for IgH 3′ quasi-palindromic region), we introduced a loxP-pTK-neoR-loxP cassette in place of the 26.4-kb region that includes hs3a, hs1-2, and hs3b enhancers by homologous recombination into 129/Ola ES cells (Fig. S1A). Once introduced into the mouse germ line, the selection cassette was deleted, as described previously (18), to get the 3′PALΔ model (Fig. S1B). Our study used either homozygous mutant mice (of mixed background, predominantly 129) or F1 heterozygous mice obtained after breeding to wt C57BL/6 mice. We compared 3′PAL deletion to previous models devoid of the two distal 3′IgH enhancers (hs3b-4Δ) (29) or the entire 3′RR (3′RRΔ) (32).
Fig. S1.
Targeting the IgH 3′RR proximal (quasi-palindromic) module in the mouse. (A) ES cell targeting strategy used to generate the 3′PAL KO mouse model in which the hs3a to hs3b region (so called 3′RR proximal module) was disrupted by insertion of a loxP site. Map of the mouse wt IgH 3′RR. Closed circles stand for transcriptional enhancers. Targeting construct and Southern blots of knockout ES cells or animals carrying NeoR insertion. The 5′ probe (X, 0.8-kb EcoRI-HindIII fragment) detects genomic 13.6-kb and 34.3-kb XhoI bands after homozygous recombination. The 3′ probe (Y, 0.6-kb XhoI-HindIII fragment) detects genomic 22.4-kb and 8.6-kb EcoRI bands in the targeted locus. (B) Map of the cre-deleted targeted locus. Southern blot on mouse tail DNA with 3′ Y probe detects genomic 22.4-kb and 9.4-kb EcoRI bands in the targeted locus.
The Distal 3′RR Module (hs4) Is Sufficient for Antigen-Independent B-Cell Ontogeny (from PreproB to Naïve B Cells).
Because the hs4 enhancer remains upon our 3′PAL deletion, this mouse KO strain is a model of choice to evaluate the function of the 3′RR distal module in developing B cells. To avoid any disparity linked to the murine genetic background (30, 37), the antigen-independent phase of B-cell development was assessed in mouse models carrying IgH alleles of distinct allotypes. In such models, IgM-allotype expression in developing B-lineage cells reflects competition between the “b” allotype IgH wt allele (from C57BL/6 mice) and the “a” allotype IgH mutant allele (from 129 mice). We compared bone marrow and spleen B-lineage cell subsets of wt F1 mice (IgHwta/wtb) to models carrying heterozygous deletions of the IgH 3′RR modules: proximal module (this study, 3′PALΔa/wtb), distal hs3b and hs4 elements (hs3b-4Δa/wtb), and the complete 3′RR (3′RRΔa/wtb). We combined surface staining with intracellular IgM-allotype staining to precisely determine, in each relevant cell subset, both the proportion (and the resulting ratio) of IgMa or IgMb-expressing cells and the mean fluorescence of each IgM-allotype (and its ratio). Disruption of part or the entire 3′RR did not compromise early B-cell development because all four models displayed similar numbers and proportions of IgMa- or IgMb-expressing cells in the bone marrow NF/immature B-cell subset (Fig. 2, Fig. S2, and Table S1). Even though a slight decrease in expression of the mutated allele (IgMa) had already been seen in NF B cells from 3′RRΔa/wtb and hs3b-4Δa/wtb animals (Fig. 2), this had no consequence on the inflow of NF B cells. Deletion of the proximal 3′RR module had no effect on developing B-lineage cells: 3′PALΔa/wtb animals displayed normal numbers and proportions of all bone marrow and spleen B-cell subsets (Fig. S2), most likely the consequence of normal IgMa heavy chain expression in these cells (Fig. 2). This theory was confirmed in homozygous 3′PALΔ/Δ mice harboring B-cell compartments comparable to wt mice (Table S2).
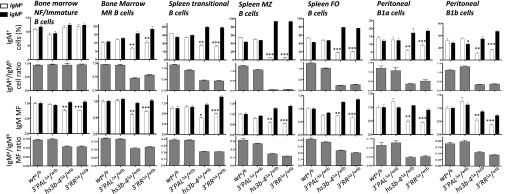
Fig. 2.
Normal IgM-expressing naïve B-cell subsets require the 3′RR distal module. B-lineage cell subsets in heterozygous models: wta/b, 3′PALΔa/wtb, hs3b-4Δa/wtb, and 3′RRΔa/wtb. Bone marrow NF/immature and mature recirculating (MR) B cells; spleen transitional, MZ, and FO B cells; peritoneal cavity B1a and B1b subsets were analyzed by flow cytometry as described in Fig. S2. Top histograms display percentages of IgMa (white) or IgMb (black) expressing cells; gray histograms below reported the corresponding ratio. Bottom histograms give intracellular IgMa or IgMb allotype mean fluorescence (MF), gray histograms below report the corresponding MF ratio. Cell numbers were collected from five to seven animals of each genotype. Significant differences were indicated by P values: *P < 0.05, **P < 0.01, ***P < 0.001 according to the Mann–Whitney u test.
Fig. S2.
B-cell subsets analysis by flow cytometry in a/b heterozygous mutant models. Freshly collected cells from 8- to 10-wk-old F1 mice (expressing IgH alleles of a and b allotypes) of each genotype (wt a/b, 3′PALΔa/wtb, hs3b-4Δa/wtb, and 3′RRΔa/wtb) were stained with fluorescent antibodies and analyzed by FACS. For Bone marrow B220+-gated cells, IgMa- and IgMb-expressing cells were separated: percentages of B220int/IgM+ NF/immature B cells and B220high/IgM+ mature recirculating (MR) B cells are indicated. Splenic populations: dot plots show percentage of AA4.1+-gated transitional B cells expressing intracellular IgMa or IgMb allotypes; percentage of cells expressing intracellular IgMa or IgMb allotypes in MZ B cells (gated on CD19+ B220+ CD21high CD23low) and FO B cells (gated on CD19+ B220+ CD21low CD23high). For B220+-gated peritoneal cavity B-lineage cells, IgMa- and IgMb-expressing cells were separated: percentage of CD5−/IgM+ B1b B cells and CD5+/IgM+ B1a B cells are indicated.
Table S1.
Absolute numbers of leukocytes and B-lineage cells in bone marrow, spleen and Peyer’s patches of 8- to 12-wk-old mice carrying IgH alleles of different haplotypes: wta/wtb (129-C57BL/6 F1 mice), 3′PALΔa/wtb, hs3b‐4Δa/wtb, and 3′RRΔa/wtb
| Location | wta/wtb | IgMa/IgMb ratio | 3′PALΔa/wtb | IgMa/IgMb ratio | hs3b-4Δa/wtb | IgMa/IgMb ratio | 3′RRΔa/wtb | IgMa/IgMb ratio |
| Bone marrow | ||||||||
| Leukocytes | 83.7 ± 2.5 n = 11 | — | 79.8 ± 9.7 n = 9 | — | 66.4 ± 6.4 n = 4 | — | 71.1 ± 3.0 n = 11 | — |
| Total B cells (B220+) | 25.7 ± 1.7 n = 11 | — | 24.2 ± 3.7 n = 9 | — | 17.8 ± 1.8 n = 4 | — | 22.3 ± 0.9 n = 11 | — |
| IgMa immature B cells (B220int IgMa+) | 8.8 ± 0.2 n = 7 | 0.93 | 6.2 ± 0.7 n = 5 | 0.95 | 7.6 ± 0.4 n = 4 | 0.93 | 9.1 ± 0.5 n = 11 | 0.96 |
| IgMb immature B cells (B220int IgMb+) | 9.5 ± 0.3 n = 7 | 6.5 ± 0.8 n = 5 | 8.2 ± 0.8 n = 4 | 9.5 ± 0.6 n = 11 | ||||
| IgMa mature B cells (B220high IgMa+) | 8.3 ± 0.4 n = 7 | 0.94 | 8.1 ± 0.5 n = 5 | 0.93 | 4.6 ± 0.6 n = 4 | 0.43 | 7.9 ± 0.5 n = 11 | 0.57 |
| IgMb mature B cells (B220high IgMb+) | 8.8 ± 0.4 n = 7 | 8.7 ± 0.5 n = 5 | 10.6 ± 1.8 n = 4 | 13.9 ± 0.9 n = 11 | ||||
| Spleen | ||||||||
| Leukocytes | 149.2 ± 13.6 n = 19 | — | 172.3 ± 10.4 n = 14 | — | 179.7 ± 12.6 n = 9 | — | 156.6 ± 6.6 n = 16 | — |
| Total B cells (B220+ CD19+) | 44.9 ± 4.7 n = 11 | — | 51.3 ± 3.3 n = 9 | — | 50.7 ± 5.2 n = 4 | — | 54.8 ± 2.4 n = 11 | — |
| IgMa follicular B cells (CD23high CD21low IgMa+) | 18.0 ± 2.3 n = 7 | 1.09 | 16.1 ± 0.8 n = 5 | 0.89 | 6.1 ± 0.8 n = 4 | 0.21 | 8.5 ± 0.6 n = 7 | 0.25 |
| IgMb follicular B cells (CD23high CD21low IgMb+) | 16.5 ± 2.2 n = 7 | 18.1 ± 1.0 n = 5 | 29.1 ± 2.2 n = 4 | 34.1 ± 2.0 n = 7 | ||||
| IgMa marginal zone B cells (CD23low CD21high IgMa+) | 2.2 ± 0.2 n = 7 | 1.16 | 1.6 ± 0.2 n = 5 | 0.94 | 0.3 ± 0.04 n = 4 | 0.05 | 0.3 ± 0.03 n = 7 | 0.06 |
| IgMb marginal zone B cells (CD23low CD21high IgMb+) | 1.9 ± 0.1 n = 7 | 1.7 ± 0.2 n = 5 | 5.7 ± 0.4 n = 4 | 4.6 ± 0.3 n = 7 | ||||
| IgMa transitional B cells (AA4.1+ IgMa+) | 8.0 ± 0.6 n = 7 | 1.13 | 5.2 ± 0.8 n = 5 | 0.93 | 4.2 ± 0.5 n = 4 | 0.44 | 3.4 ± 0.5 n = 7 | 0.44 |
| IgMb transitional B cells (AA4.1+ IgMb+) | 7.1 ± 0.6 n = 7 | 5.6 ± 0.8 n = 5 | 9.5 ± 1.0 n = 4 | 7.7 ± 1.1 n = 7 | ||||
| Peyer's patches | ||||||||
| Leukocytes | 15.0 ± 1.3 n = 11 | — | 20.6 ± 3.7 n = 9 | — | 14.2 ± 3.3 n = 4 | — | 11.0 ± 2.4 n = 7 | — |
| Total B cells (B220+) | 9.8 ± 0.9 n = 11 | — | 14.6 ± 2.8 n = 9 | — | 10.9 ± 2.4 n = 4 | — | 8.3 ± 1.9 n = 7 | — |
| IgMa B cells | 3.9 ± 0.3 n = 11 | 1.11 | 5.2 ± 0.7 n = 9 | 1.21 | 2.2 ± 0.5 n = 4 | 0.30 | 2.0 ± 0.5 n = 7 | 0.37 |
| IgMb B cells | 3.5 ± 0.3 n = 11 | 4.3 ± 0.6 n = 9 | 7.2 ± 1.6 n = 4 | 5.4 ± 1.3 n = 7 |
Means ± SEM are reported; number of animals is reported; within a given B-cell subset expressing a- or b- allele, the a/b ratio is reported.
Table S2.
Absolute numbers of leukocytes and B-lineage cells in bone marrow, spleen, peritoneal cavity, and Peyer’s patches of 8-wk-old wt and 3′PALΔ/Δ mice
| Location | WT | 3′PALΔ/Δ | P value |
| Bone marrow | |||
| Leukocytes (×106) | 71.89 ± 3.96 n = 9 | 61.93 ± 2.96 n = 11 | NS |
| B220+ cells (×106) | 25.75 ± 1.89 n = 9 | 21.03 ± 1.37 n = 11 | P = 0.0227 |
| IgM−/B220+/CD43low cells (×106) | 11.39 ± 0.89 n = 9 | 9.07 ± 0.26 n = 11 | P = 0.0111 |
| IgM−/B220+/CD43high cells (×106) | 4.52 ± 0.72 n = 9 | 3.48 ± 0.30 n = 11 | NS |
| B220+/CD117+ cells (×106) | 1.69 ± 0.10 n = 9 | 1.68 ± 0.13 n = 11 | NS |
| B220+/CD117− cells (×106) | 23.45 ± 1.68 n = 9 | 19.25 ± 1.25 n = 11 | P = 0.04 |
| Spleen | |||
| Leukocytes (×106) | 174.82 ± 16.34 n = 9 | 138.6 ± 10.23 n = 11 | NS |
| B220+ cells (x106) | 72.22 ± 8.40 n = 9 | 56.50 ± 4.42 n = 11 | NS |
| B220+/IgM+/IgD+ cells (×106) | 61.80 ± 8.62 n = 9 | 45.47 ± 4.18 n = 11 | NS |
| Marginal zone B cells (×106) | 7.21 ± 0.72 n = 9 | 9.64 ± 1.06 n = 11 | NS |
| Follicular B cells (×106) | 45.44 ± 4.72 n = 16 | 34.935 ± 3.66 n = 9 | NS |
| Transitional B cells (×106) | 12.31 ± 0.87 n = 15 | 11.54 ± 1.22 n = 15 | NS |
| Peritoneal cavity | |||
| Leukocytes (×106) | 2.85 ± 0.42 N= 9 | 1.91 ± 0.42 n = 11 | NS |
| B220+ cells (×106) | 1.38 ± 0.25 n = 9 | 0.83 ± 0.24 n = 11 | P = 0.0448 |
| CD5+/IgM+ cells (×106) | 0.39 ± 0.09 n = 9 | 0.24 ± 0.07 n = 11 | NS |
| CD5−/IgM+ cells (×106) | 0.75 ± 0.18 n = 9 | 0.48 ± 0.15 n = 11 | P = 0.0378 |
| Peyer's patches | |||
| Leukocytes (×106) | 7.53 ± 1.10 n = 6 | 10.67 ± 1.57 n = 6 | NS |
| B220+ cells (×106) | 4.76 ± 0.91 n = 6 | 5.84 ± 0.96 n = 6 | NS |
| B220+/IgA+ cells (×106) | 0.56 ± 0.11 n = 6 | 0.88 ± 0.18 n = 6 | NS |
| B220+/IgM+ cells (×106) | 2.83 ± 0.43 n = 6 | 3.78 ± 0.68 n = 6 | NS |
| B220+/PNAlow/FASlow cells (×106) | 3.97 ± 0.30 n = 8 | 3.56 ± 0.45 n = 5 | NS |
| B220+/PNAHigh/FASHigh cells (×106) | 2.51 ± 0.32 n = 8 | 1.61 ± 0.25 n = 5 | NS |
Means ± SEM are reported; number of animals is reported; significance (P value) between the two genotypes is assessed by a Student t test.
The Distal 3′RR Module (hs4) Is Required for Efficient BCR Expression in Transitional B Cells.
Deletion of the proximal 3′RR module strongly contrasted with 3′IgH-deletions that encompass the distal hs4 element: in the spleen of 3′RRΔa/wtb and hs3b-4Δa/wtb animals, numbers and proportions of splenic transitional B cells expressing IgMa allele was decreased (Fig. 2, Fig. S2, and Table S1). This disadvantage in the generation of transitional B cells was correlated with decreased expression (about twofold compared with wt cells, as evaluated by mean fluorescence intensity) of the IgMa mutated allele (Fig. 2). This result was associated with a decrease in numbers and proportions of IgMa-expressing mature B-lineage cells: marginal zone (MZ) and follicular (FO) B cells from the spleen, mature recirculating B cells in the bone marrow, and B1 cell subsets in the peritoneal cavity in 3′RRΔa/wtb and hs3b-4Δa/wtb animals (Fig. 2, Fig. S2, and Table S1). Deletion of either proximal, distal, or the entire 3′RR showed marked differences on Ig heavy chain expression in developing B cells. A locus devoid of the distal module (3′RRΔ and hs3b-4Δ) failed to express a normal amount of surface Igµ heavy chain (already observed in NF B cells) with drastic consequences on the capacity to generate mature naïve B-cell subsets. On the other hand, our 3′PAL KO model proved that the hs4 enhancer was per se sufficient to drive efficient expression of µ heavy chain in developing B cells.
The Proximal 3′RR Module Drives Germ-Line Transcription and CSR to γ1, γ3, and γ2a Genes.
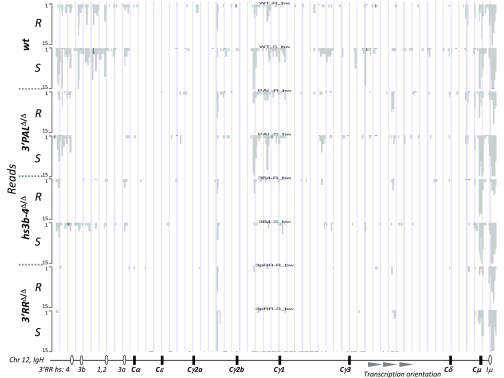
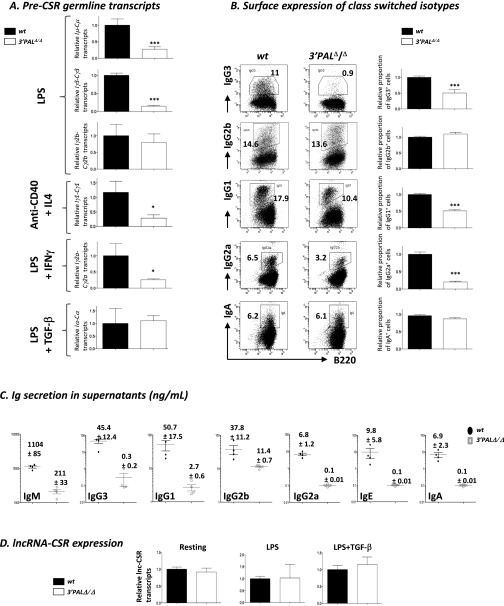
Global IgH locus transcriptional activation was assessed by comparing RNA-seq data obtained from resting and in vitro LPS-activated splenic B cells from wt and homozygous 3′PAL, hs3b-4, and 3′RR KO animals. If sense and antisense germ-line transcripts (GLT) for the whole locus were above background in 3′RR KO B cells, deletions of distal and proximal 3′RR modules led to intermediate levels of IgH GLT upon LPS activation (Fig. 3 and Fig. S3). Compared with wt cells, induction of sense GLT in the γ3 region was decreased in all KO models, whereas GLT of the μ region was not affected by hs3b-4 deletion. In contrast, γ2b region GLT were still normally induced in 3′PAL-deficient B cells (Fig. 3). To more precisely evaluate the function of the proximal 3′RR on CSR, splenic B cells from wt and homozygous 3′PAL KO mice were subjected to appropriate in vitro stimulations and tested for switched BCR expression and GLT (Fig. S4 A and B). B cells deficient for the quasi-palindrome displayed a significant CSR defect to IgG3, IgG1, and IgG2a isotypes (reduced by two- to threefold). This finding was correlated with a consistent defect in GLT of both donor and acceptor switch regions (Fig. S4A) in 3′PALΔ/Δ: Iγ3-Cγ3 GLT was reduced by fivefold; Iµ-Cµ, Iγ1-Cγ1, and Iγ2a-Cγ2a GLT were reduced by threefold. In contrast to complete 3′RR deletion (32), in vitro CSR to IgG2b and IgA was not significantly reduced by the deletion of the proximal 3′RR region (Fig. S4B) and their corresponding germ-line transcripts were detected in almost normal proportions in stimulated 3′PALΔ/Δ B cells (Fig. S4 A and B). Decreased Ig secretion of all isotypes in supernatants of 3′PALΔ/Δ-stimulated cells (Fig. S4C) was a feature shared with the 3′RR deletion, suggesting, beyond an isotype-specific CSR defect, a global Ig secretion defect (Discussion). As potential targets of the IgH 3′RR, we determined expression of the recently described long-noncoding RNA (lncRNA) associated with CSR (38) and found, based on RNA-seq, no variation in all models in resting or LPS-activated B cells (Fig. 3). This finding was confirmed by quantitative RT-PCR (qRT-PCR) experiments showing that wt and 3′PAL-deficient B cells (resting or stimulated) displayed same amounts of lncRNA-CSR transcripts (Fig. S4D).
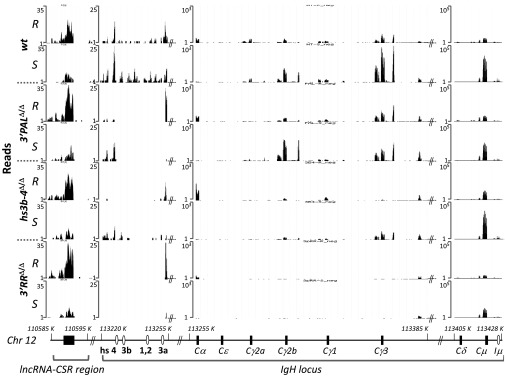
Fig. 3.
Varigated induction of IgH-associated transcripts upon 3′RR deletions. Expression of sense transcripts at the IgH locus based on RNA-seq read distribution in resting B cells sorted from the spleen (R) and LPS-activated B cells for 48 h (S) from wt, 3′PALΔ/Δ, hs3b-4Δ/Δ, and 3′RRΔ/Δ mice. Each RNA-seq experiment was performed on a pool of equivalent numbers of B cells sorted from four age-matched animals of the same genotype. Reads were aligned on mouse GRCm38/mm10 assembly using University of California, Santa Cruz Genome Browser (49), base position on mouse Chr12 are reported. A map of the IgH locus (not to scale), including constant genes, regulatory elements, and orientation of the reported transcripts is indicated.
Fig. S3.
Activation-induced IgH antisense transcripts. Expression of antisense transcripts at the IgH locus based on RNA-seq read distribution in CD43-sorted resting B cells from the spleen (R) and LPS-activated B cells for 48 h (S) from WT, 3′PALΔ/Δ, hs3b-4Δ/Δ and 3′RRΔ/Δ mice. Each RNA-seq experiment was performed on a pool of equivalent numbers of B cells from four animals of each genotype. Reads were aligned on mouse GRCm38/mm10 assembly using University of California, Santa Cruz Genome Browser (genome.ucsc.edu) (49).
Fig. S4.
Impaired CSR in in vitro-stimulated B cells lacking the IgH 3′RR proximal module. Resting splenic B cells (CD43− fraction) from wt and 3′PALΔ/Δ mice were stimulated in vitro to induce CSR with LPS (IgG2b and IgG3), LPS + TGF-β (for IgA), LPS + IFN-γ (for IgG2a) or anti-CD40 + IL-4 (for IgG1 and IgE). (A) For each constant gene, germ-line transcription (Ix-Cx) was quantified by qRT-PCR and normalized to Gapdh expression after 48–72 h stimulation. Mean and SEM are reported, significant differences were indicated by P values: *P < 0.05, ***P < 0.001 according to the Mann–Whitney u test (n = 4–11 animals per genotype). (B) At day 4, percentage of isotype switched cells was analyzed by flow cytometry for surface Ig isotype expression: Center shows one representative experiment, Right displays statistical analysis of the proportion in vitro-induced isotype switched B cells for each condition. Mean and SEM are reported, significant differences are indicated by P values: *P < 0.05, ***P < 0.001 according to the Mann–Whitney u test (n = 12–28 animals per genotype). (C) Antibody isotype secretion was quantified by ELISA in stimulated B-cell supernatants at day 4. (D) Transcription of lncRNA for CSR was quantified by qRT-PCR (38) and normalized to Gapdh expression in wt and 3′PALΔ/Δ resting splenic and activated B cells for 48 (LPS) to 72 h (LPS+TGF-β). Mean and SEM are indicated (n = 6 animals).
The Proximal 3′RR Module Controls SHM in a Transcription-Dependent Manner.
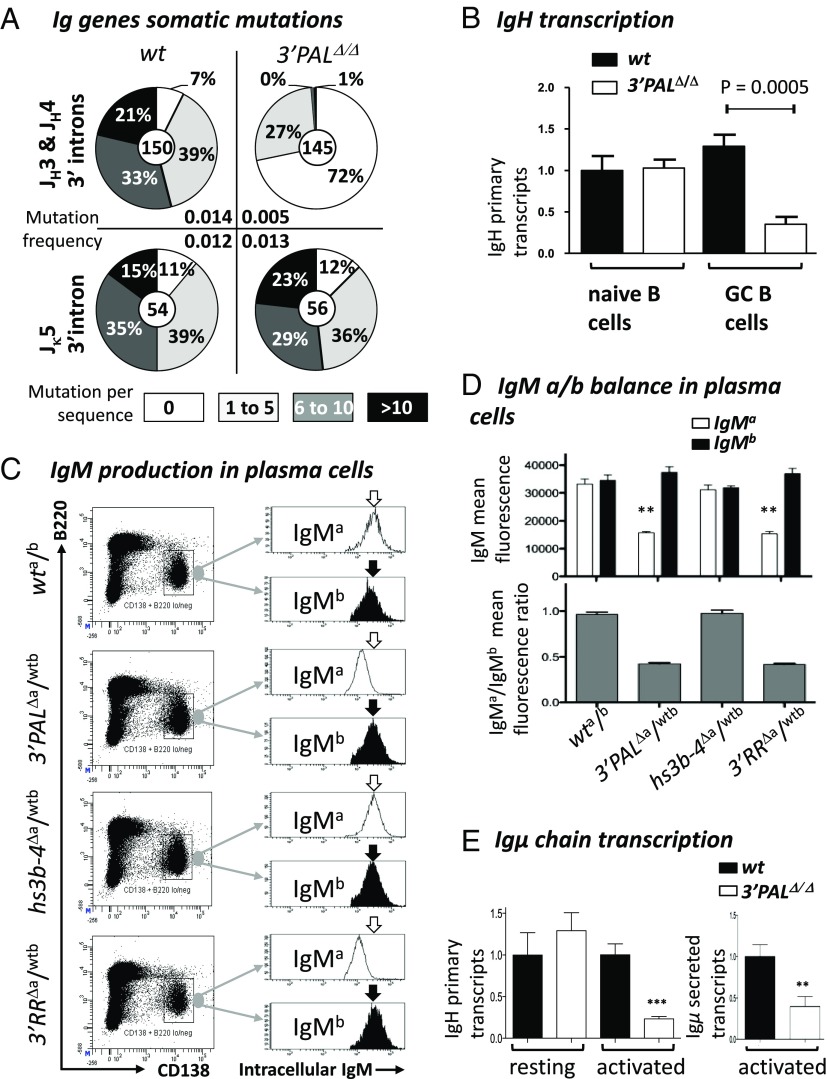
SHM was assayed in GC B cells (B220+/PNAHigh/FAS+) sorted from Peyer’s patches of wt and homozygous 3′PALΔ/Δ animals. At the IgH locus, SHM frequency in the ∼500-bp intronic regions downstream from rearranged-JH3 or -JH4 segments was reduced by ∼threefold in 3′PALΔ/Δ GC B cells (0.005 mutations per base pair) compared with wt (0.014 mutations per base pair) (Fig. 4A). When comparing the distribution of mutated sequences, we found that 3′PALΔ/Δ GC B cells were largely unmutated (72%), whereas wt control had only a small proportion (7%) of unmutated cells (Fig. 4A). Mutated IgH alleles from GC B cells devoid of the proximal module contained only few mutations (27% of sequences, with fewer than six substitutions). Similarly to the whole 3′RR deletion (31), highly mutated IgH alleles (>six mutations) were almost absent in our 3′PAL mutants (Fig. 4A). As a control experiment, SHM was also quantified in the intron downstream from the Jκ5 segment and found both the mutation frequency and the percentage of mutated sequences to be comparable with that in wt (Fig. 4A). We finally quantified IgH primary transcription by qRT-PCR with a probe downstream from the JH4 intron and found a two- to threefold reduction in CG B cells lacking the proximal module (Fig. 4B). In naïve B cells (B220+/PNAlow/FAS−) sorted from Peyer’s patches, IgH primary transcription levels were similar in wt and 3′PALΔ/Δ mice, confirming that the 3′RR proximal module did not modulate heavy chain expression in resting B cells.
Fig. 4.
Proximal 3′RR module controls IgH somatic mutations in GC B cells and Ig production in plasma cells. (A) GC B-cell DNA was isolated from B220+ PNAhigh/FAShigh Peyer’s patch cells from unimmunized 8-wk-old wt and 3′PAL KO mice. Pie plots showed the percentage of mutated clones for each group (proportional to the area in each slice).Total number of clones analyzed for each genotype was noted in the center. Mutation frequency (mutation per base pair, indicated for each group) was calculated from sequences that contained at least one mutation; clonally related sequences were excluded. (B) Relative IgH primary transcripts was estimated by qRT-PCR, performed using IgHwt TaqMan probe and normalized to Cd79a transcripts (see Table S3 for details). (C) Intracellular IgM-allotype expression in plasma cells from heterozygous mouse models (wta/b; 3′PALΔa/wtb, hs3b-4Δa/wtb, and 3′RRΔa/wtb) was measured by FACS analysis. After sheep red blood cell challenge, plasma cells from the spleen were gated based on surface expression of B220low and CD138+. This specific population was assayed for intracellular expression of IgMa or IgMb allotypes. One representative experiment is shown. (D) Upper histogram shows mean fluorescence of intracellular IgMa (white) or IgMb (black) allotype expression (estimated by flow cytometry as described above) in plasma cells collected from at least five animals of each genotype. Gray histograms below report the corresponding MF IgMa/IgMb ratio. (E) IgH primary and secreted-Igµ transcripts in wt and 3′PALΔ/Δ B cells. (Left) Primary transcription of rearranged IgVH regions (normalized to Gapdh transcripts) was quantified by qRT-PCR in CD43− resting B cells from wt and 3′PALΔ/Δ animals). (Right) Transcription of the secreted form of Ig µ heavy chain was quantified by qRT-PCR (see Table S3 for details). Significant differences were indicated by P values: **P < 0.01, ***P < 0.001 according to the Mann–Whitney u test.
Plasma Cell Antibody Production and the Antigen-Specific Responses Rely on the 3′RR Proximal Module.
The first evidence of Ig secretion defects in animals lacking the 3′RR distal module was the drastic reduction of all circulating Ig, including IgM, in the serum of 3′PALΔ/Δ mice (Fig. S5A). This hypogammaglobulinemia was similar to that previously observed in 3′RR KO mice (32). When challenged with ovalbumin, heterozygous 3′PALΔa/wtb mutants proved that a deficient allele was unable to support efficient antibody production (Fig. S5B). The IgMa-specific response was consistently decreased (Fig. S5B), whereas IgG1a and IgG2aa responses were completely abrogated (Fig. S5B), probably the consequence of combined CSR and Ig secretion defects. Plasma cell differentiation and ability to produce Ig was then evaluated in heterozygous mice after intraperitoneal challenge with sheep red blood cells. All models showed efficient generation of plasma cells expressing both IgMa and IgMb allotypes in the spleen 6 d after challenge (Fig. 4C). Remarkably, plasma cells carrying IgH alleles devoid of the proximal module (3′PALΔ and 3′RRΔ alleles) exhibited a strong defect in intracellular IgM expression (Fig. 4D). To look for the origin of the defect, IgH transcription was assayed in resting and in vitro-activated B cells from homozygous 3′PAL KO mice. In this plasmablast-enriched population, both the IgH primary and the secreted form of the µ chain transcripts were drastically reduced in the absence of the proximal module (Fig. 4E).
Fig. S5.
The 3′RR quasi-palindrome controls serum antibody production and antigen-specific immune response. (A) Serum Ig isotype levels quantified by ELISA in 8-wk-old wt and 3′PALΔ/Δ mice. Mean ± SEM are shown and significant differences indicated with a P value, according to the Student t test. (B) ELISA quantification of ovalbumin-specific Ig isotype in serum from immunized 8-wk-old wta/b and 3′PALΔa/wtb mice. Ab levels are indicated on the y axis in arbitrary units by comparison with a control serum. Days postimmunization are indicated on the x axis; arrows indicate first and second antigen injections. Each point is the mean of serum quantification for five animals. (Upper) “a” allotype responses for IgM, IgG1 and IgG2a isotypes; (Lower) “b” allotype responses for the same Ig classes. One significant experiment is shown.
Discussion
There is no doubt that the 3′RR plays a key role in IgH locus regulation (39) but the role of the conserved quasi-palindrome and the hierarchy between the modules remained poorly understood. By comparing relevant KO mouse models, our study demonstrated that the 3′RR includes at least two functional modules: (i) the distal module, which includes the hs4 enhancer element; and (ii) the proximal module, defined as the 3′RR quasi-palindrome. First, independently of antigen stimuli, we confirmed that VDJ recombination does not require any of the 3′RR enhancers. In all models, early B-cell development was preserved, with normal pro– and pre–B-cell compartments. This finding correlates with studies proving normal VDJ rearrangements and VH use in such models (19, 40). Second, our study identifies the critical role of the distal 3′RR module for Ig heavy chain expression in a defined window starting as soon as a complete BCR is expressed (NF B cells) and extending until the naïve B-cell stage. In B-lineage precursors, a first contribution was assigned to CBEs, which have been proposed to facilitate interactions between locus distant regions (14, 20, 41, 42). Deletion of hs5-7 CBEs downstream from the mouse 3′RR resulted in only a slight modification of D and VH use (16), suggesting a modest contribution of 3′ CBEs in VDJ joining, although it is not excluded that full deletion (including hs8) might have a more drastic effect (39). Once VDJ recombination is completed, the 3′RR and the promoter of the VDJ-rearranged segment continue to interact and then starts the actual 3′RR stepwise transcriptional activation by its distal module. Indeed, when developing B cells were driven by IgH alleles lacking the hs4 distal enhancer (hs3b-4Δ or 3′RRΔ), a progressive decrease in µH-chain expression was already observed in NF B cells (about 30% decrease at this stage compared with wt, based on IgMa/IgMb mean fluorescence ratio) and rose to a 50% decrease in transitional B cells. Such “low BCR-expressing” NF and transitional B cells displayed a differentiation disadvantage (observed in both models devoid of hs4) toward mature MZ and FO B-cell subsets. In the 3′PAL KO model, normal Igµ expression in bone marrow NF B cells and spleen mature B subsets clearly shows the proximal 3′RR module as dispensable at these stages. These findings pinpoint the window of activity for the distal module and support a stepwise activation of the IgH 3′RR: hs4 is required to maintain optimal µ heavy chain transcription from the NF bone marrow to the mature MZ and FO splenic B cells. This window of activity clearly overlaps with that of Eµ, recently described as active on µH-chain transcription from pre-B to transitional B-cell stages (18).
When mature B cells encounter antigen and engage in an immune response, the 3′RR proximal module takes over most of IgH locus regulatory mechanisms: SHM, CSR, and antibody production. First, SHM seems strongly dependent on the proximal module. The drastic reduction in SHM frequency in germinal center (GC) B cells from 3′PALΔ/Δ mice (0.005 mutations per base pair downstream from the JH introns, excluding unmutated sequences) was similar to that observed in 3′RR-deficient animals (31), a model described as deficient for recruiting activation-induced cytidine deaminase (AID) in IgH variable regions (43). In our 3′PALΔ mice, the SHM defect was also correlated with a decrease (at least twofold) in IgH primary transcription, a defect comparable to that seen in 3′RR-deficient mice (31). The IgH transcription defect in 3′PAL-deficient GC B cells also provides further evidence that hs4 does not influence H-chain expression once B cells are activated. Even if such a twofold transcription decrease appears modest, it is certainly significant. Indeed, it is admitted that the level of BCR expression modulates B-cell fate and that a basal level of H-chain transcription is necessary for B-cell survival (44). Besides a similar transcription defect in both models, the proportion of unmutated sequences (72%) in 3′PAL-deficient GC B cells was, however, lower than in 3′RR-KO mice (95%) (31). Even if hs4 does not impact transcription at this stage, its partnership with the 3′PAL module for AID recruitment or targeting can be suspected in light of the higher proportion of unmutated IgH alleles in 3′RR KO than in 3′PAL-deficient mice. Second, germ-line transcription of major switch regions is regulated by the proximal 3′RR module. Transcription of the donor Sµ region is decreased by at least threefold in 3′PAL-deficient B cells induced for CSR in vitro (32, 45); the same is true for GLT of Sγ3, Sγ1, and Sγ2a acceptor switch regions, two features shared with the 3′RR-KO model (32). The failure of a 3′PAL-deficient allele to support IgG1- or IgG2a-specific responses is also consistent with a CSR defect. Interestingly, the 3′PALΔ/Δ phenotype preserved normal GLT and CSR to IgG2b and IgA, whereas these processes were affected in animals lacking both hs3b and hs4 enhancers or the entire 3′RR (29, 32). Specifying that hs4 is by itself sufficient for normal GLT and CSR to Cγ2b and Cα, our study underlined the complexity of constant gene transcriptional regulation for CSR. Among potential regulatory mechanisms involving the 3′RR, the lncRNA for CSR, recently described in the CH12 cell line undergoing CSR to IgA, promotes the CSR-stimulating activity of the 3′RR via a long-distance interaction with the hs4 region (38). It is possible that lncRNA-CSR (normally expressed in 3′PAL-deficient B cells), promotes efficient CSR to IgA and IgG2b in this model that conserved the hs4 module. Third, in plasma cells, the common feature shared by both 3′RR and 3′PAL deletions proved that Ig heavy chain transcription and antibody production are directly under the control of the 3′RR proximal module.
We hypothesize that IgH 3′RR modules respond sequentially maybe independently to external stimuli: (i) the distal (hs4) module being responsible for efficient heavy chain and BCR expression in NF and naïve B-cell subsets, and (ii) the proximal quasi-palindromic module being later activated by exogenous antigen stimulation to support both SHM, CSR (with some help from hs4 in the situation of Sγ2b and Sα CSR), and prolonged IgH overexpression in antibody secreting cells.
Materials and Methods
Flow Cytometry.
Once isolated from mouse organs, single-cell suspensions of bone marrow, spleen, peritoneal cavity, and Peyer’s patches were labeled with various fluorescent antibodies, as detailed in SI Materials and Methods. To collect plasma cells, mice were injected with 200 μL sheep red blood cell suspension (bioMérieux) in the peritoneal cavity. At day 6, mice were killed and splenic plasma cells analyzed by flow cytometry as described in SI Materials and Methods. All animal experiments were performed according to the guidelines of the Comité Régional d’Ethique du Limousin (CREAAL 7-07-2012 approved protocol).
RNA Isolation and qRT-PCR.
Total RNA was isolated and RT-PCR was performed as described previously (18). TaqMan probes and primers, previously described in (9, 31, 38, 46–48), are listed in Table S3. Relative mRNA levels were normalized to Gapdh or Cd79a transcripts.
Table S3.
Primers for real-time qRT-PCR and SHM used in this study
| qRT-PCR or SHM | Primer/probe | Source |
| Real-time qRT-PCR | ||
| Cd79a | Mm00432423_m1 | Applied Biosystems |
| Gapdh | Mm99999915_g1 | Applied Biosystems |
| IgH primary transcripts | ||
| IgHWT for | 5′TTCTGAGCATTGCAGACTAATCTTG3′ | (31) |
| IgHWT rev | 5′CCTAGACAGTTTATTTCCCAACTTCTC3′ | (31) |
| IgHWT probe | 5′CCCTGAGGGAGCCG3′ | (31) |
| Germ-line transcripts | ||
| Iμ-Cμ for | 5′ACCTGGGAATGTATGGTTGTGGCTT3′ | (46) |
| Iμ-Cμ rev | 5′TCTGAACCTTCAAGGATGCTCTTG3′ | (46) |
| Iγ3-Cγ3 for | 5′AACTACTGCTACCACCACCACCAG3′ | (46) |
| Iγ3-Cγ3 rev | 5′ACCAAGGGATAGACAGATGGGG3′ | (46) |
| Iγ1-Cγ1 for | 5′GGCCCTTCCAGATCTTTGAG3′ | (46) |
| Iγ1-Cγ1 rev | 5′ATGGAGTTAGTTTGGGCAGCA3′ | (46) |
| Iγ2b-Cγ2b for | 5′CCAACCAGGAAGAGTCCAGAG3′ | (48) |
| Iγ2b-Cγ2b rev | 5′ACAGGGATCCAGAGTTCCAAGT3′ | (48) |
| Iγ2a-Cγ2a for | 5′GCTGATGTACCTACCGAGAGA3′ | (46) |
| Iγ2a-Cγ2a rev | 5′GCTGGGCCAGGTGCTCGAGGTT3′ | (46) |
| Iα-Cα for | 5′CTACCATAGGGAAGATAGCCT3′ | (47) |
| Iα-Cα rev | 5′TAATCGTGAATCAGGCAG3′ | (47) |
| LNC-RNA for CSR transcripts | ||
| Lnc-RNA-CSR for | 5′-CACATTTGATTGCCTCTCCACTTG-3′ | (38) |
| Lnc-RNA-CSR rev | 5′-AAAACATCCCTCCAACCAGTCAC-3′ | (38) |
| SHM | ||
| IgH locus | ||
| VHJ558 family for | 5′GCGAAGCTTARGCCTGGGRCTTCAGTGAAG3′ | (9) |
| 5′MAREμ rev | 5′CAGCAACTACCCTTTTGAGACCGA3′ | Present study |
| Igκ locus | ||
| Igκ for | 5′GGCTGCAGSTTCAGTGGCAGTGGRTCWGGRAC3′ | (31) |
| Igκ rev | 5′AGCGAATTCAACTTAGGAGACAAAAGAGAGAAC3′ | (31) |
RNA-Seq Analysis.
RNA-seq libraries were prepared from a pool of equivalent numbers of B-purified B cells, either resting B cells from the spleen or in vitro-activated B cells for 48 h with LPS, from four animals of each genotype. Sequencing and analysis are described in SI Materials and Methods.
SHM.
The experimental procedures for Peyer’s patch GC and naïve B cells sorting, genomic extraction, amplification, and cloning have been previously reported (9). Amplification of VDJH- or VJκ-rearranged DNA fragments was performed with the appropriate primers (Table S3). Mutational analysis was performed in either the 500-bp intronic region just downstream from JH3 and JH4 segments (for IgH locus) or in the 554-bp intronic region located downstream from the Jκ5 segment (for Igκ locus).
SI Materials and Methods
Mouse Models.
Heterozygous mice (3′PALΔa/wtb, 3′RRΔa/wtb and hs3b-4Δa/wtb were generated by crossing homozygous mice with C57BL/6 mice. Mixed Sv/129 x C57BL/6 mice were used as controls. Mice were bred and housed in specific pathogen-free conditions at 21–23 °C with a 12-h light/dark cycle. All experiments were performed according to the guidelines of the Comité Régional d’Ethique de l’Expérimentation Animale du Limousin and were approved as part of the protocol registered under no. CREAAL 7-07-2012.
Southern Blots.
Genomic Southern blots were performed as follows: 20 µg genomic DNA was digested by XhoI or SacI. DNA was transferred to nylon membranes (MP Biomedicals) by capillarity. Blots were hybridized with [32P]-labeled probes generated by random priming. “X” probe located upstream from the 5′ homology arm was a 0.8-kpb HindIII-EcoRI fragment; “Y” probe located downstream from the 3′ homology arm was a, 0.6-kpb XhoI-HindIII fragment, as indicated in Fig. S1.
Flow Cytometry.
For bone marrow, cells were labeled with B220-BV510, CD117-PE, CD43-PE-Cy7, IgM-FITC, CD19-APC-H7 and CD93-APC (clone AA4.1, eBioscience), IgMa-FITC (clone DS-1, BD Pharmingen), IgMb-PE (clone AF6-78, BD Pharmingen). Splenocytes were stained with B220-BV510, CD21-BV605, CD23-PE-Cy7, CD19-APC-H7, CD93-APC, IgD-FITC. For the peritoneal cavity, Abs used were B220-BV510, CD5-BV605, IgMa-FITC, IgMb-PE. For Peyer’s patches, cells were labeled with B220-BV510 or B220-V450, IgA-FITC, IgM-PE, peanut agglutinin (PNA)-FITC, FAS-PE (SouthernBiotech, BD Biosciences). Flow cytometry analysis was performed on a BD LSR Fortessa cell analyzer (BD Biosciences). FACS data were analyzed by FACSDIVA software (BD Biosciences). For intracellular staining, cells were first stained for surface markers. After washing, cells were treated with a Cytofix/Cytoperm kit and intracellular staining was performed with IgMa-FITC, IgMb-PE Abs.
For plasma cell analysis, single cells from spleens were washed with 2% (vol/vol) FCS-PBS. Cell surface staining was performed with the following Abs: B220-BV510, CD138-allophycocyanin. For intracellular staining, 5 × 106 cells were first stained for surface proteins. Then, cells were treated with a Cytofix/Cytoperm kit (BD Biosciences) and intracellular staining was performed with IgMa-FITC, IgMb-PE Abs.
RNA-Seq Analysis.
Total RNA was extracted using miRNeasy kit (Qiagen) from either freshly purified splenic B cells, obtained after gradient separation using Lympholyte Mammal (Cedarlane) followed by CD43− selection (Miltenyi Biotec), or from purified splenic B cells stimulated in vitro (106 cells m/L) for 48 h in RMPI 1640 medium (Lonza) supplemented with 10% FCS (Lonza) and with 5 µg/mL LPS (Cayla Invivogen), separated on a lympholyte mammal gradient. For each genotype (wt, 3′PALΔ/Δ, hs3b-4Δ/Δ, 3′RRΔ/Δ), RNA sample was prepared from a pool of equivalent numbers of purified B cells from four animals. For RNA-seq library, 1 µg of total RNA was depleted from ribosomal RNA with Ribo-Zero Gold (Illumina), ligated, reverse-transcribed, and amplified (14 cycles) with the reagents from the TruSeq Stranded Total RNA kit (Illumina). Libraries were quantified with the Bioanalyzer DNA 1000 Kit (Agilent). Four nanomolars of libraries were then diluted and denatured according to the Illumina recommendations. Paired-end 150-bp reads were sequenced on an Illumina NextsEq. 500 sequencer, using NextSeq 500/550 High Output Kit (Illumina). Illumina NextSeq 500 paired-end 2 × 150-nt reads were mapped with STAR release v2.4.0a versus mm10 with gene model from ensembl release 77, with default parameters. Quantification of genes was then performed using featureCounts release subread-1.4.6-p1-Linux-x86_64 with “–primary -g gene_name -p -s 1 -M” options based on Ensembl GTF release 77 annotations.
Secreted Ig in Plasma and Supernatants.
Supernatants from in vitro-stimulated B cells, harvested after 4-d culture, and sera from wt or homozygous mutant mice were tested for the presence and concentration of different Ig isotypes by ELISA, as previously described (29).
Specific Ig Isotype Response upon Ag Stimulation.
Animal cohorts, including wta/wtb and 3′PALΔa/wtb, were analyzed for the presence of ovalbumin-specific Ig isotypes in sera by ELISA. Allotype-specific IgM, IgG1, and IgG2a responses to OVA were determined as previously described (28).
Acknowledgments
We thank all members of the Service Commun d’Animalerie and Plateforme de Transgenose of Limoges University for mouse work; Catherine Ouk-Martin and the FACS sorting facility of Limoges University; Drs. Laurent Delpy, Christophe Sirac, and Brice Laffleur for discussions and helpful comments; and Virginie Magnone, Alexandra Popa, and Pascal Barbry for help and fruitful discussions. This work was developed in close collaboration with the functional genomics platform of Nice Sophia Antipolis, a partner of the National Infrastructure France Génomique, thanks to support by the Cancéropôle PACA and Commissariat aux Grands Investissements (PB: ANR-10-INBS-09-03 and ANR-10-INBS-09-02). This work was supported in part by a PhD joint scholarship from Centre National de la Recherche Scientifique and Région Limousin (to M.M.); a PhD scholarship from the Région Limousin (to A.G., P.R., and A.-G.B.); a PhD scholarship from the Ministère de l’Enseignement Supérieur et de la Recherche (to A.S.); Grant DOC20150602943 from Fondation ARC (to A.S.); post doc Fellowship Grant ANR-11-BSV302701 (to S.L.N.); and grants from ARC (#SL220100601332), Conseil Régional du Limousin, Ligue Contre le Cancer, Comités de la Région Limousin, ANR-11-BSV30270, and Comité d’Orientation Recherche Cancer en Limousin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE76217).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514090113/-/DCSupplemental.
References
- 1.Perlot T, Alt FW. Cis-regulatory elements and epigenetic changes control genomic rearrangements of the IgH locus. Adv Immunol. 2008;99:1–32. doi: 10.1016/S0065-2776(08)00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sleckman BP, Oltz EM. Preparing targets for V(D)J recombinase: Transcription paves the way. J Immunol. 2012;188(1):7–9. doi: 10.4049/jimmunol.1103195. [DOI] [PubMed] [Google Scholar]
- 3.Péron S, et al. AID-driven deletion causes immunoglobulin heavy chain locus suicide recombination in B cells. Science. 2012;336(6083):931–934. doi: 10.1126/science.1218692. [DOI] [PubMed] [Google Scholar]
- 4.Jhunjhunwala S, et al. The 3D structure of the immunoglobulin heavy-chain locus: Implications for long-range genomic interactions. Cell. 2008;133(2):265–279. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477(7365):424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi NM, Feeney AJ. CTCF and ncRNA regulate the three-dimensional structure of antigen receptor loci to facilitate V(D)J recombination. Front Immunol. 2014;5:49. doi: 10.3389/fimmu.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar R, Pierce S, Bolland DJ, Corcoran A, Oltz EM. Regulation of IgH gene assembly: Role of the intronic enhancer and 5'DQ52 region in targeting DHJH recombination. J Immunol. 2006;176(4):2439–2447. doi: 10.4049/jimmunol.176.4.2439. [DOI] [PubMed] [Google Scholar]
- 8.Bolland DJ, et al. Antisense intergenic transcription precedes IgH D-to-J recombination and is controlled by the intronic enhancer Emu. Mol Cell Biol. 2007;27(15):5523–5533. doi: 10.1128/MCB.02407-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlot T, Alt FW, Bassing CH, Suh H, Pinaud E. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci USA. 2005;102(40):14362–14367. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai E, Bottaro A, Davidson L, Sleckman BP, Alt FW. Recombination and transcription of the endogenous Ig heavy chain locus is effected by the Ig heavy chain intronic enhancer core region in the absence of the matrix attachment regions. Proc Natl Acad Sci USA. 1999;96(4):1526–1531. doi: 10.1073/pnas.96.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem. 2010;285(13):9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giallourakis CC, et al. Elements between the IgH variable (V) and diversity (D) clusters influence antisense transcription and lineage-specific V(D)J recombination. Proc Natl Acad Sci USA. 2010;107(51):22207–22212. doi: 10.1073/pnas.1015954107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SG, Guo C, Su A, Zhang Y, Alt FW. CTCF-binding elements 1 and 2 in the Igh intergenic control region cooperatively regulate V(D)J recombination. Proc Natl Acad Sci USA. 2015;112(6):1815–1820. doi: 10.1073/pnas.1424936112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolás L, Chaudhuri J. 4C-ing the Igh Landscape. Immunity. 2013;39(2):199–201. doi: 10.1016/j.immuni.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett FE, et al. Chromatin architecture near a potential 3′ end of the igh locus involves modular regulation of histone modifications during B-cell development and in vivo occupancy at CTCF sites. Mol Cell Biol. 2005;25(4):1511–1525. doi: 10.1128/MCB.25.4.1511-1525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpi SA, et al. Germline deletion of Igh 3′ regulatory region elements hs 5, 6, 7 (hs5-7) affects B cell-specific regulation, rearrangement, and insulation of the Igh locus. J Immunol. 2012;188(6):2556–2566. doi: 10.4049/jimmunol.1102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Eckhardt LA. A role for the IgH intronic enhancer E mu in enforcing allelic exclusion. J Exp Med. 2009;206(1):153–167. doi: 10.1084/jem.20081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquet M, et al. The Eμ enhancer region influences H chain expression and B cell fate without impacting IgVH repertoire and immune response in vivo. J Immunol. 2014;193(3):1171–1183. doi: 10.4049/jimmunol.1302868. [DOI] [PubMed] [Google Scholar]
- 19.Rouaud P, et al. Enhancers located in heavy chain regulatory region (hs3a, hs1,2, hs3b, and hs4) are dispensable for diversity of VDJ recombination. J Biol Chem. 2012;287(11):8356–8360. doi: 10.1074/jbc.M112.341024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medvedovic J, et al. Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity. 2013;39(2):229–244. doi: 10.1016/j.immuni.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinaud E, et al. The IgH locus 3′ regulatory region: Pulling the strings from behind. Adv Immunol. 2011;110:27–70. doi: 10.1016/B978-0-12-387663-8.00002-8. [DOI] [PubMed] [Google Scholar]
- 22.Dunnick WA, et al. Switch recombination and somatic hypermutation are controlled by the heavy chain 3′ enhancer region. J Exp Med. 2009;206(12):2613–2623. doi: 10.1084/jem.20091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunnick WA, Shi J, Zerbato JM, Fontaine CA, Collins JT. Enhancement of antibody class-switch recombination by the cumulative activity of four separate elements. J Immunol. 2011;187(9):4733–4743. doi: 10.4049/jimmunol.1101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunnick WA, Shi J, Fontaine C, Collins JT. Transgenes of the mouse immunoglobulin heavy chain locus, lacking distal elements in the 3′ regulatory region, are impaired for class switch recombination. PLoS One. 2013;8(2):e55842. doi: 10.1371/journal.pone.0055842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y, et al. Homologous elements hs3a and hs3b in the 3′ regulatory region of the murine immunoglobulin heavy chain (IgH) locus are both dispensable for class-switch recombination. J Biol Chem. 2011;286(31):27123–27131. doi: 10.1074/jbc.M111.230995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manis JP, et al. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J Exp Med. 1998;188(8):1421–1431. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent-Fabert C, et al. Ig synthesis and class switching do not require the presence of the hs4 enhancer in the 3′ IgH regulatory region. J Immunol. 2009;182(11):6926–6932. doi: 10.4049/jimmunol.0900214. [DOI] [PubMed] [Google Scholar]
- 28.Bébin A-G, et al. In vivo redundant function of the 3′ IgH regulatory element HS3b in the mouse. J Immunol. 2010;184(7):3710–3717. doi: 10.4049/jimmunol.0901978. [DOI] [PubMed] [Google Scholar]
- 29.Pinaud E, et al. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 2001;15(2):187–199. doi: 10.1016/s1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 30.Saintamand A, et al. The IgH 3′ regulatory region governs μ chain transcription in mature B lymphocytes and the B cell fate. Oncotarget. 2015;6(7):4845–4852. doi: 10.18632/oncotarget.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouaud P, et al. The IgH 3′ regulatory region controls somatic hypermutation in germinal center B cells. J Exp Med. 2013;210(8):1501–1507. doi: 10.1084/jem.20130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent-Fabert C, et al. Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood. 2010;116(11):1895–1898. doi: 10.1182/blood-2010-01-264689. [DOI] [PubMed] [Google Scholar]
- 33.Chauveau C, Cogné M. Palindromic structure of the IgH 3′locus control region. Nat Genet. 1996;14(1):15–16. doi: 10.1038/ng0996-15. [DOI] [PubMed] [Google Scholar]
- 34.Saleque S, et al. Dyad symmetry within the mouse 3′ IgH regulatory region includes two virtually identical enhancers (C alpha3′E and hs3) J Immunol. 1997;158(10):4780–4787. [PubMed] [Google Scholar]
- 35.Sepulveda MA, Garrett FE, Price-Whelan A, Birshtein BK. Comparative analysis of human and mouse 3′ Igh regulatory regions identifies distinctive structural features. Mol Immunol. 2005;42(5):605–615. doi: 10.1016/j.molimm.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.D’Addabbo P, Scascitelli M, Giambra V, Rocchi M, Frezza D. Position and sequence conservation in amniota of polymorphic enhancer HS1.2 within the palindrome of IgH 3′regulatory region. BMC Evol Biol. 2011;11:71. doi: 10.1186/1471-2148-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminski DA, Stavnezer J. Antibody class switching differs among SJL, C57BL/6 and 129 mice. Int Immunol. 2007;19(4):545–556. doi: 10.1093/intimm/dxm020. [DOI] [PubMed] [Google Scholar]
- 38.Pefanis E, et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161(4):774–789. doi: 10.1016/j.cell.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birshtein BK. Epigenetic regulation of individual modules of the immunoglobulin heavy chain locus 3′ regulatory region. Front Immunol. 2014;5:163. doi: 10.3389/fimmu.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morvan CL, Pinaud E, Decourt C, Cuvillier A, Cogné M. The immunoglobulin heavy-chain locus hs3b and hs4 3′ enhancers are dispensable for VDJ assembly and somatic hypermutation. Blood. 2003;102(4):1421–1427. doi: 10.1182/blood-2002-12-3827. [DOI] [PubMed] [Google Scholar]
- 41.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci USA. 2011;108(23):9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degner SC, Wong TP, Jankevicius G, Feeney AJ. Cutting edge: Developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 2009;182(1):44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maul RW, et al. Spt5 accumulation at variable genes distinguishes somatic hypermutation in germinal center B cells from ex vivo-activated cells. J Exp Med. 2014;211(11):2297–2306. doi: 10.1084/jem.20131512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 45.Saintamand A, et al. Elucidation of IgH 3′ region regulatory role during class switch recombination via germline deletion. Nat Commun. 2015;6:7084. doi: 10.1038/ncomms8084. [DOI] [PubMed] [Google Scholar]
- 46.Park S-R, et al. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10(5):540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S-R, Seo G-Y, Choi A-J, Stavnezer J, Kim P-H. Analysis of transforming growth factor-beta1-induced Ig germ-line gamma2b transcription and its implication for IgA isotype switching. Eur J Immunol. 2005;35(3):946–956. doi: 10.1002/eji.200425848. [DOI] [PubMed] [Google Scholar]
- 48.Sellars M, Reina-San-Martin B, Kastner P, Chan S. Ikaros controls isotype selection during immunoglobulin class switch recombination. J Exp Med. 2009;206(5):1073–1087. doi: 10.1084/jem.20082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent WJ, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]