Abstract
Glyceollins are phytoalexins produced in soybeans under stressful growth conditions. Based on prior evaluations, they show potential to treat multiple diseases, including certain cancers, Type 2 diabetes and cardiovascular conditions. The aim of the present studies was to expand on recent studies designed to initially characterize the intestinal disposition of glyceollins. Specifically, studies were undertaken in Caco-2 cells to evaluate glyceollins' effects on apical efflux transporters, namely MRP2 and BCRP, which are the locus of several intestinal drug-drug and drug-food interactions. 5 (and 6)-Carboxy-2′,7′-dichloroflourescein (CDF) was used to provide a readout on MRP2 activity; whereas, BODIPY-prazosin, provided an indication of BCRP alteration. Glyceollins were shown to reverse MRP2-mediated CDF transport asymmetry in a concentration dependent manner, with activity similar to the MRP2 inhibitor, MK-571. Likewise, they demonstrated concentration dependent inhibition of BCRP-mediated efflux of BODIPY-prazosin with a potency similar to that of Ko143. Glyceollin did not appreciably alter MRP2 or BCRP expression following 24 hours of continuous exposure. The possibility that glyceollin-mediated inhibition of genistein metabolite efflux by either transporter was evaluated. However, results demonstrated an interaction at the level of glyceollin inhibition of genistein metabolism rather than inhibition of metabolite transport.
Keywords: Drug Interaction, Flavonoids, Genistein, Glyceollin, MRP2, BCRP, Isoflavone, Phytoestrogen
Introduction
Polyphenolic phytochemicals are produced in abundance in soy-beans, and are present in soy-derived food products (1). Investigation of their potential to reduce the incidence and severity of a multitude of diseases associated with oxidative stress (cancer, cardiovascular, inflammatory, diabetes, and neurodegenerative), has created widespread interest in their intentional use to promote human health (2). Associated with this effort, pharmacokinetic studies have revealed poor bioavailability of these phytochemicals due to extensive metabolism (3). As an example, systematic investigations of genistein intestinal and hepatic disposition have revealed an elaborate integration of intestinal metabolism, and subsequent carrier-mediated apically-directed efflux of metabolites that severely limit genistein systemic bioavailability following oral ingestion (4).
When soybeans are exposed to stressful conditions, such as fungal or bacterial pathogens, or UV light, they have been shown to produce glyceollins (5). Initial studies of glyceollins' biological effects indicated that they were estrogen receptor antagonists (6), exhibited anti-proliferative effects in estrogen-dependent breast cancer cells (7), and inhibited cell proliferation in mouse xenograft breast cancer models (8). More recently, glyceollins demonstrated improvement in glucose homeostasis in response to a glucose challenge in a prediabetic rat model of type 2 diabetes (9) and lowered cholesterol in a hamster model of human cholesterol metabolism (10). Overall, these pharmacologic effects have generated interest in the use of glyceollin-enriched soy as a nutri-pharmaceutical.
Previous studies designed to evaluate the transport and metabolism of glyceollins revealed high permeability in Caco-2, and metabolism primarily via direct glucuronidation and sulfation in Caco-2 (11) and in rats following oral administration (12). These findings parallel those observed with other phytochemicals, for example with genistein (13) and resveratrol (14). Glyceollins were also shown not to alter Pgp activity or induce Pgp expression in Caco-2 (11), thereby anticipating no alteration of the absorption of drugs or nutrients that are Pgp substrates. Two other ABC transporters in the small intestine that participate in the absorption of drugs and nutrients, and are implicated in drug-drug and drug-food interactions, are MRP2 (ABCC2) and BCRP (ABCG2) (15). The purpose of the studies reported herein was to evaluate the potential for glyceollins to alter the function and expression of these two transporters, again using Caco-2 as an in vitro model of the small intestine. Results from these studies provided stimulus for an evaluation of the potential for glyceollins to alter genistein absorption; accordingly, an initial glyceollin-genistein interaction study was conducted in Caco-2.
Experimental
Materials
A mixture of glyceollins I, II, and III was isolated using a procedure developed at the Southern Regional Research Center (ARS, USDA, New Orleans, LA) (6, 8) and used in recent studies to evaluate effects on glucose disposition in fat cells (9), serum cholesterol in hamsters (10), and metabolism in rat plasma (12). UV-Vis spectrophotometry at 285 nm allowed an estimation of the proportions of the three isomers used in all experiments: glyceollin I (68%), glyceollin II (21%), and glyceollin III (11%). Hereafter, this mixture will be referred to as “glyceollin.” Genistein was purchased from Indofine Chemical Company (Hillsborough, NJ). BODIPY-prazosin was purchased from Life Technologies, Grand Island, NY. MK-571, sodium salt, was purchased from AdipoGen, Inc. (San Diego, CA). Lucifer yellow (di-lithium salt) was purchased from ThermoFisher Scientific Co. (Waltham, MA). Except where noted in detail below, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Methods
Cell Culture
Caco-2 cells were obtained from ATCC (Manassas, VA) at passage 27. Cells were maintained in stock cultures in Dulbecco's Modified Eagle's Medium (Life Technologies Inc.) supplemented with 10% fetal bovine serum (HyClone, Thermo Scientific), and 1% MEM non-essential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine and 1% antibiotic/antimycotic solution (Mediatech, Inc. Manassas, VA) at 37°C in a humidified incubator with 5% CO2. Stock cultures were fed 3-times per week and passed weekly. For CDF and BODIPY-prazosin uptake assays, cells were seeded at 2 × 105 cells/mL. For RT-qPCR and western blot analyses, cells were seeded onto 10 cm plates at a density of 2 × 105 cells/mL. Cells were seeded onto collagen coated 0.4 μm-PTFE Transwell-COL® permeable supports (12 mm/12-well/1.12 cm2 for CDF transport studies, or 24 mm/6-well/4.67 cm2 for genistein absorption studies) at a seeding density of 1.2 × 105 cells/mL and cultured for 2.5 to 3.5 weeks before use. For all assays, cells were used between passages 35 – 55.
Effects on CDF Transport and Uptake
The AB and BA directional transport of 5 μM CDF, an MRP2 and MRP3 substrate (16), in the absence vs. presence of 10, 30 or 100 μM MK-571 (MRP2 inhibitor positive control (17)), or glyceollin was evaluated. A 5 mM stock solution of CDFA, the non-fluorescent di-acetate ester prodrug of CDF, was prepared in 1:1 DMSO:ethanol and diluted to 50 μM in pH 7.4 transport buffer (25 mM HEPES, 5 mM glucose, 1 mM NaH2PO4, 145 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2). Prior to CDFA incubation, cells on Transwell-COL® supports were washed twice with 37°C transport buffer, incubated for 30 minutes at 37° C with an inhibitor or, in the case of controls an equivalent concentration of co-solvent (0.1% DMSO/0.1% ethanol), on both sides of the filter, and their transepithelial electrical resistance (TEER) measured (EVOM2 meter, World Precision Instruments, Sarasota, FL). Filters with TEER values < 200 Ohm-cm2 were not used in an incubation. Following addition of CDFA to the donor compartment, filters were placed on an orbital shaker (150 rpm) set at 37° C. Receiver compartment samples (0.4 mL) were taken every 30 min with volume replacement for 120 minutes for analysis of cumulative CDF mass transport in either the apical to basolateral (AB) or basolateral to apical (BA) transport directions. Initial and final donor samples were taken for determination of mass balance, which was always > 95%. Chemical hydrolysis of CDFA to CDF at 37° C in the pH 7.4 transport buffer was determined to be < 1% in 120 minutes (details of methodology found below in Effects on CDFA hydrolysis by Caco-2 cells). Calculation of CDF permeability coefficient was based on the following equation, where P = permeability in cm/s, dm/dt is flux (the slope of the cumulative amount transported vs. time), A is the Transwell® support surface area (1.12 cm2) and C is the average of the initial and final donor concentrations (nmoles/mL) of CDF in the form of CDFA.
At the conclusion of the 2-hour incubation, cells were washed three times with 0.5 mL of cold transport buffer, after which 200 μL of methanol was added. After 15 minutes at room temperature, the methanol extract was centrifuged for 5 minutes at 4,000 rpm and the supernatant collected. CDF concentration in donor, receiver and cell extracts was based on fluorescence measured in a microplate fluorescence reader (Biotek Synergy 4, Winooski, VT) set at 485 nm/530 nm for excitation and emission wavelengths, respectively, with comparison to standards, which ranged from 7.8 to 1,000 nM with r2 > 0.999. Estimation of the potency of the positive control, MK-571 and glyceollin to reduce the B/A ratio was evaluated using the following equation: Effect (E) = E0 * 1 - C/(IC50 + C), where E is the ratio normalized to that observed in the absence of either MK-571 or glyceollin (control value), E0 is the ratio in the absence of either compound (100%), and IC50 is the concentration (C) of MK-571 or glyceollin that reduces the ratio to 50%.
Net uptake of CDF following exposure to 5 μM CDFA into cells grown on 24-well plates was also used to determine if glyceollin altered MRP2 and/or MRP3 activity. Following a 30 min pre-incubation in the presence of a test compound, Caco-2 cells between 75 – 95% confluency were exposed to CDFA for 30 minutes at 37° C. The concentration range of MK-571 and glyceollin was 1 to 100 μM. At the conclusion of an incubation, cells were washed 3 times in ice-cold transport buffer and then lysed in 0.1% triton X-100 (15 min at 37° C), centrifuged at 4,000 rpm for 5 minutes and the supernatant collected for analysis of CDF flouresence and protein content. Fluorescence was measured as described in the preceding transport experiments and was normalized to protein content based on the BCA protein assay (ThermoScientific). Bovine serum albumin was used as a reference protein and standards were prepared over the concentration range 50 to 1600 μg/mL (r2 > 0.99 for the absorbance vs. albumin standard concentration relationship).
Effects on BODIPY-prazosin uptake
Uptake of BODIPY-prazosin, a BCRP substrate (18) into cells grown on 24-well plates was also used to determine glyceollin effects on BCRP. Following three washes with 37°C transport buffer and a subsequent 30 min pre-incubation in the presence of a test compound, Caco-2 cells between 80% – 95% confluency were exposed to 5 μM CAM for 30 minutes at 37° C. The concentration range of the three compounds evaluated, Ko143, the positive control for BCRP inhibition (19), glyceollin, and verapamil, a P-glycoprotein inhibitor (19), was 1 to 100 μM. At the conclusion of the incubation, cells were washed 3 times in ice-cold transport buffer and then lysed in 0.1% triton X-100 (15 min at 37° C). Subsequently, fluorescence was measured in a microplate fluorescence reader at 485nm/530 nm (excitation/emission wavelengths). Measured fluorescence was normalized to protein content, as described for the CDF uptake experiments. For Ko143 and glyceollin, protein normalized net fluorescence as a function of compound concentration was fit to the following equation to estimate EC50 (the concentration (C) of Ko143 or glyceollin that achieves 50% of the maximum effect), Emax (the maximum estimated effect), and E0 is the effect observed in the absence of compound, which was set to 100%: E = E0 + Emax * C/(EC50 + C).
Alteration of MRP2 and BCRP mRNA Expression
Caco-2 cells were exposed to solvent (negative control, 0.1% DMSO/0.1% ethanol), 1, 10 or 100 μM glyceollin for 24 hrs at 80 – 90% confluency. There were three wells for each treatment condition, and each condition was repeated a total of three times with cells from consecutive passage numbers 33 – 35. Total RNA samples were collected using RNAeasy Plus kits (QIAGEN). Samples were stored at -70°C until time of analysis. The concentration of total RNA in the samples was measured by a ND-1000 NanoDrop® spectrophotometer (ThermoScientific). The A260/A280 purity of all samples was in the range of 2.0-2.1, and the A260/A230 ratio was 1.8-2.3. The integrity of all samples was confirmed by the Experion Automated Electrophoresis System (BioRad, Inc., Hercules, CA), with the 18S/28S ratio being higher than 1.6. Five μg of each RNA sample was reverse transcribed in a 20 μL reaction volume by a commercial first-strand cDNA synthesis kit (SABiosciences-QIAGEN). ABCC2 and ABCG2 primer, and those of three housekeeping genes: beta-2-microglobulin (B2M), hypoxanthine phosphoribosyltransferase (HPRT1) and ribosomal protein L13A (RPL13A) were purchased from SABiosciences-QIAGEN, as well as RT SYBR Green Fluor qPCR Mastermix. Two microliters (2 μL) of cDNA was used in 25 μL of qPCR reaction mix using a standard 2-step temperature protocol and a Bio-Rad iQ5 Thermocycler. Each 96-well plate contained non-template control, calibrator sample for each gene, and all experimental samples, which were each run in duplicate. Melting curve analysis was performed for each PCR plate. Delta delta Ct method was used to calculate the fold change in MDR1 gene expression (20).
Alteration of MRP2 and BCRP Expression by Western Blot Analysis
Caco-2 cells were seeded in 10 cm2 plates at a density of 60 to 70% confluence. The cells were treated with the indicated concentrations for 24 hrs. The media was then removed and the cells were scraped into 1 mL of phosphate buffered saline (PBS) plus 3 mM EDTA. Cell suspensions were spun for five minutes at 2,000 × g and the supernatant was aspirated. Pellets were lysed by vortexing in 200 μL of M-PER mammalian protein extraction buffer (Pierce, cat. # 78501) containing protease and phosphatase inhibitors (Sigma, cat. #'s P1860-1ML, P0044, and P5726). The samples were then spun in a microcentrifuge for five minutes at 12,000 × g and the supernatants collected. Protein concentrations were determined using a nanodrop spectrophotometer (Thermo Life Sciences) and 100 μg of total protein was loaded into each well and run on a 4 to 12% polyacrylamide gel (Life Technologies). Subsequently, gels were blotted onto nitrocellulose using the iblot transfer system (Life Technologies). Blots were blocked for one hour at room temperature in 1 × PBST (PBS with tween 20, Cell Signaling, Danvers, MA) containing 5% non-fat milk, washed in 1 × PBST and incubated overnight at 4°C in 5 mL of primary antibody (M2III-6 for MRP2, Covance, Dedham, MA, and BXP-21 for BCRP, Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:500 dilution in 5% BSA/PBST (Sigma cat # A7906-1 KG). Blots were then washed in 1 × PBST and incubated with fluorescently-labeled secondary antibodies (Alexa-Fluor 488, Life Technologies) for 60 minutes at room temperature. The blots were then washed in 1 × PBST and scanned using a Versadoc 4000 scanner and (BioRad, Hercules, CA). Bands were quantified using Quantity One software (BioRad, Hercules, CA) and normalized to bands corresponding to the housekeeping Rho-GDI protein. Three independent samples were prepared for each treatment condition.
Alteration of MRP2 and BCRP Function
Caco-2 cells grown on Transwell® permeable supports for 24 days were exposed to 1, 10 or 100 μM glyceollin for 24 hours to determine if such exposure altered MRP2 activity. After 24 hours, both sides of the filters were washed 3 times with 37°C transport buffer. Potential effects on MRP2 activity were then measured using the CDF assay described above with comparison to CDF permeability in control incubations. CDF permeability was measured in the BA direction only, on the basis of the greater sensitivity of MK-571 mediated inhibition of permeability in this direction observed in the MRP2 inhibition assay. This approach was used previously to measure alteration of Pgp activity in Caco-2 cells (11). With respect to assessment of BCRP function, Caco-2 cells were grown on 24-well plates for 2 days, at which time they were 80 – 95% confluent. Subsequently, they were exposed to 1, 10 or 100 μM glyceollin for 24 hours. Following this exposure, cells were washed with 37° C transport buffer and then subjected to the BODIPY-prazosin uptake assay described previously.
Genistein Transport and Metabolism
50 mM stock solutions of genistein or glyceollin were prepared in equal volumes of DMSO and ethanol, and subsequently diluted to 2 μM (genistein) or 30 μM (glyceollin) in pH 7.4 transport buffer. Prior to genistein and glyceollin exposure, cells were washed twice with 37°C transport buffer, incubated for 30 minutes at 37° C. Apical side volume was 1.5 mL and basolateral side volume was 2.6 mL. Following addition of genistein without (control) vs. with 30 μM glyceollin to the donor compartment, filters were placed on an orbital shaker (150 rpm) set at 37° C. Final DMSO and ethanol concentrations in controls and glyceollin-present incubations were 0.03% (v/v). Donor and receiver compartment samples (0.5 mL) were taken initially, and every 60 min thereafter for a total of five samples. An equivalent volume of either genistein solution (donor side) or blank transport buffer (receiver side) was added after each sample to maintain a constant volume. All samples were stored at -80° C until time of analysis. At the end of the incubation, cells were harvested for determination of intracellular genistein. Accordingly, cells on filters were washed three times with 1 mL of 4°C HEPES transport buffer and removed from the Transwell® filter cup apparatus and transferred to a blank 6-well culture plate to which subsequently 0.5 mL of methanol was added. After 30 minutes at room temperature, filters were stored at -80 °C until time of analysis. On the day of analysis, filters were thawed and the methanol evaporated using a dry-heat oven set at 37°C. Subsequently, 0.5 mL of internal standard solution (2.0 μM prazosin in HEPES transport buffer) was added to all samples and standards. After 30 minutes at 37°C, samples were transferred to microcentrifuge tubes and centrifuged at room temperature for 5 minutes at 4,000 rpm.
Genistein concentration in all samples (donors, receivers and cells) was measured using HPLC with UV detection at 259 nm. A Waters 600 controller equipped with a 717 plus autosampler and 996 photodiode array detector were used (Waters Corporation, Milford, MA). A gradient-based separation was performed on Waters X-Bridge C18 column (2.1 × 100 mm, 5 μm particle size). Mobile phase A was 10 mM potassium phosphate, pH 2.7:acetonitrile:methanol in the ratio of 8:1:1. Mobile phase B was methanol:water in the ratio of 9:1. The gradient elution profile consisted of mobile phase A: mobile phase B of 90%:10% from 0 – 4 minutes; 50%:50% from 4 – 5 minutes; 10%:90% from 5 – 8 minutes; 50%:50% from 8 – 10 minutes; and return to initial conditions (90%:10%) for 2 minutes prior to the next injection. Column temperature was 30°C and flow rate was 0.4 mL/min. Standards were prepared fresh for each run and ranged from 0.1 to 1.6 μM for donor and receiver compartment samples, and from 8 to 12.8 nM for cell extract samples. For all samples and standards, 2 × 200 μL aliquots were transferred to separate HPLC vials. To each aliquot, 300 μL of 50 mM sodium phosphate buffer, pH 6 was added and incubated for 3 hours at 37°C. For one of the aliquots, the buffer contained glucuronidase/sulfatase from H. pomatia (β-glucuronidase Type HP-2, Sigma-Aldrich) at a final concentration of 50,000 units/mL of β-glucuronidase and 3750 units/mL of sulfatase. The difference in genistein concentration between the two aliquots was taken to represent total glucuronide and sulfate conjugates concentration in a sample.
Essentially the same equation as described for the CDF transport studies was used to calculate genistein aglycone absorptive permeability. The fraction of genistein metabolized (Fm) was calculated using the following equation, where total genistein is the sum of the amounts of genistein aglycone and conjugates measured in donor, receiver and cells at the end of the 4-hour incubation (samples incubated with H. pomatia), and genistein aglycone is the sum of the amount of aglycone measured in donor, receiver and cells at the end of the incubation:
Excretion clearance of the conjugate metabolites into the apical and basolateral compartments was calculated using the following equation, where Jmet represents metabolite excretion rate (nmoles/sec), Acell represents the amount (nmoles) of conjugates in the cells (Acell,total genistein – Acell,aglycone), and Vmonolayer represents the volume (mL) of cells on a filter. The monolayer volume was calculated as the product of the height of a typical Caco-2 cell (25 μm) and Transwell® insert surface area (4.67 cm2):
Data Analysis
IC50 estimates for alteration of CDF permeability, or EC50 estimates for alteration of BODIPY-prazosin uptake, were calculated using Phoenix WinNonlin 6.3 (Pharsight Corporation, Mountain View, CA). Statistical analyses were conducted using GraphPad Prism (Prism 6 for Windows, GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± standard error of the mean (SEM). An unpaired two-tailed Student's t-test was used to compare two means. One-way analysis of variance followed by, if appropriate, multiple comparison testing (Tukey's) were used to determine if there was statistically significant differences (defined as p < 0.05) between multiple groups in an experiment.
Results
Effects on MRP2 Activity
In transport experiments conducted with Caco-2 cells grown on Transwell® supports, under control conditions, CDF permeability was on average 3.7 ± 0.36 fold greater in the BA direction vs. the AB direction (Figure 1A). As shown in Figure 1A, this BA/AB permeability ratio was significantly reduced (p < 0.05) when both sides of the filters were exposed to MK-571 (100 μM), an MRP2 inhibitor (17), 30 minutes prior to and during the 120 minute incubation, but were not altered in the presence of the same concentration of verapamil, a Pgp inhibitor (19), in which the ratio was 3.6 (from a single experiment with n = 3 filters in each transport direction). In the case of 100 μM glyceollin, the ratio was reduced to a similar extent as was observed with MK-571 (0.9 ± 0.10 and 0.8 ± 0.16, respectively). For both compounds, the cause of the reduced ratio was a statistically significant increase in AB directional transport coupled with a statistically significant reduction in BA directional transport (Figure 1B).
Figure 1.
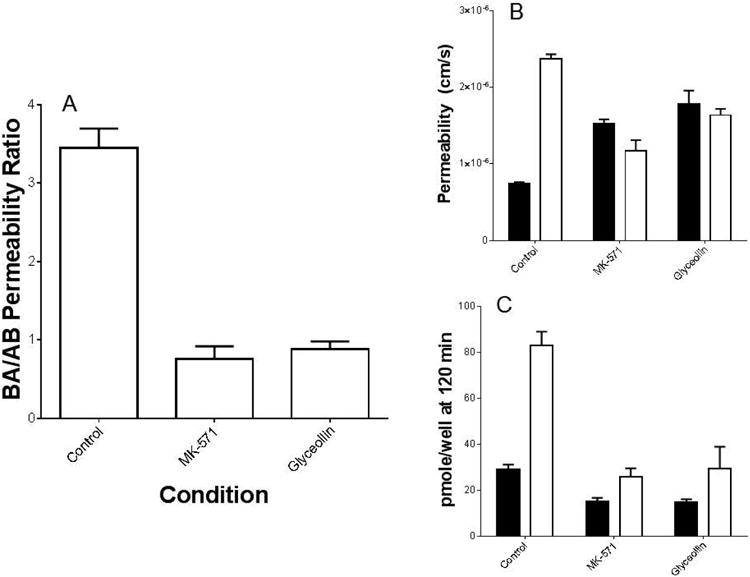
CDF permeability in Caco-2 cells grown on Transwell® filters. (A) Average ratios of CDF permeability in the Basolateral-to-Apical (BA) direction relative to that in the Apical-to-Basolateral (AB) direction. Each value represents the average of multiple experiments (n = 7 for control “no inhibitor” and n = 3 for each compound) with there being 3 filters in each transport direction per experiment. MK-571 and glyceollin ratios were significantly reduced relative to the control (p < 0.05), and were not different from one another. (B) Mean CDF permeability results in the absence (control) vs. presence of MK-571 or glyceollin, and (C) mean pmole CDF remaining in cells at 120 minutes. Open bars represent transport in the AB direction, and closed bars in the BA direction. For both (B) and (C), MK-571 and glyceollin results were significantly different from the corresponding control/transport direction (p < 0.05). MK-571 and glyceollin concentration was 100 μM.
Evaluation of the effect of concentration of MK-571 and glyceollin on CDF BA/AB permeability ratio (Figure 2) resulted in IC50 estimates of 22 μM (CV = 0.09%) and 53 μM (CV = 0.22%), respectively, indicating a potency of glyceollin to reduce CDF directional transport within 3-fold of MK-571.
Figure 2.
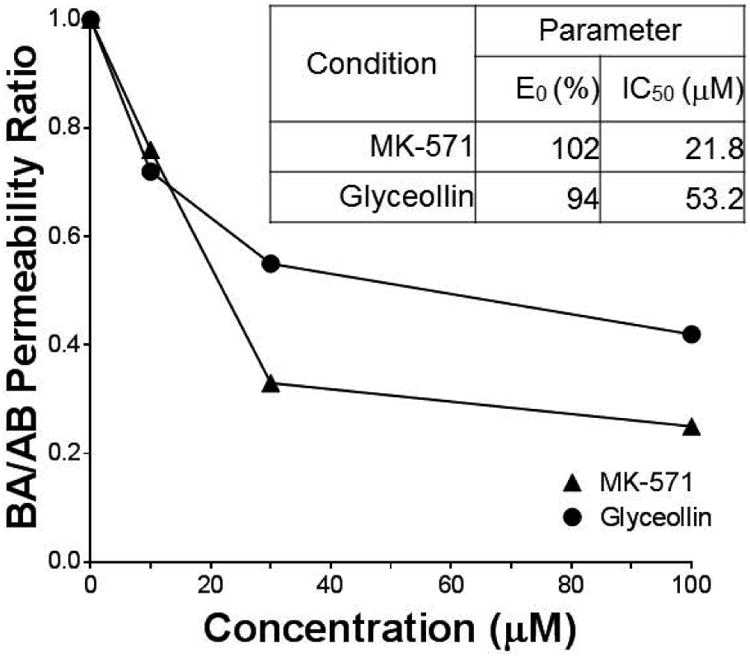
Influence of compound concentration on CDF permeability in Caco-2. MK-571 and glyceollin concentration effects on BA/AB fractional permeability ratio are presented relative to control (0 μM compound, Ratio = 1.0). There were three filters in each transport direction at each concentration.
Results of studies designed to evaluate concentration dependent uptake of CDF into Caco-2 cells grown on plastic when exposed 30 minutes to 5 μM CDFA are summarized in Figure 3A. CDF permeability results for MK-571 and glyceollin (Figures 1 and 2) provided an expectation that uptake would have increased as a result of either compound inhibiting MRP2-mediated efflux of CDF; however, this was clearly not the case. In fact, just the opposite was observed for both compounds, there being a significant reduction in uptake at 100 μM. Evaluation of CDFA hydrolysis by Caco-2 cells indicated that the presence of 100 μM MK-571 or glyceollin did not alter the rate of esterase-mediated conversion to CDF (Figure 3B).
Figure 3.
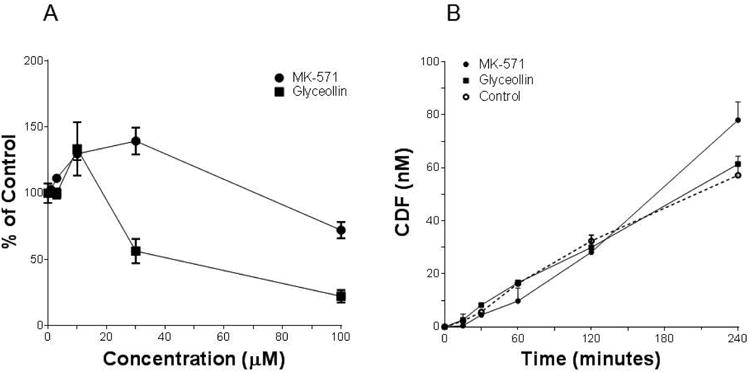
CDF uptake and CDFA hydrolysis by Caco-2. (A) Net CDF fluorescence normalized to cell protein was measured following a 30 minute incubation with 5 μM CDFA in the presence of varying concentrations of MK-571 or glyceollin. Results represent the average of 2 (glyceollin) or 4 (MK-571) separate experiments, with n = 4 for each concentration per experiment. (B) CDF concentration as a function of time following incubation with 5 μM CDFA for up to 4 hours in the absence (Control, dashed line) or presence of 100 μM of either MK-571 or glyceollin. N = 3 for each condition. Specific activities based on initial rate conditions were 6.0, 6.2 and 8.5 nM/min/mg protein for control, glyceollin and MK-571, respectively.
Effects on BCRP Activity
Glyceollin effects on BCRP activity were examined by evaluating the ability to alter BODIPY-prazosin uptake following a 30 minute incubation with 5 μM of this fluorescent BCRP substrate. Results of these uptake evaluations are summarized in Figure 4. Both Ko143 and glyceollin exposure resulted in concentration-dependent enhanced uptake of BODIPY-prazosin. As summarized in the accompanying table, the potency of glyceollin to produce this inhibitory effect on BODIPY-prazosin efflux by BCRP was similar to that of Ko143.
Figure 4.
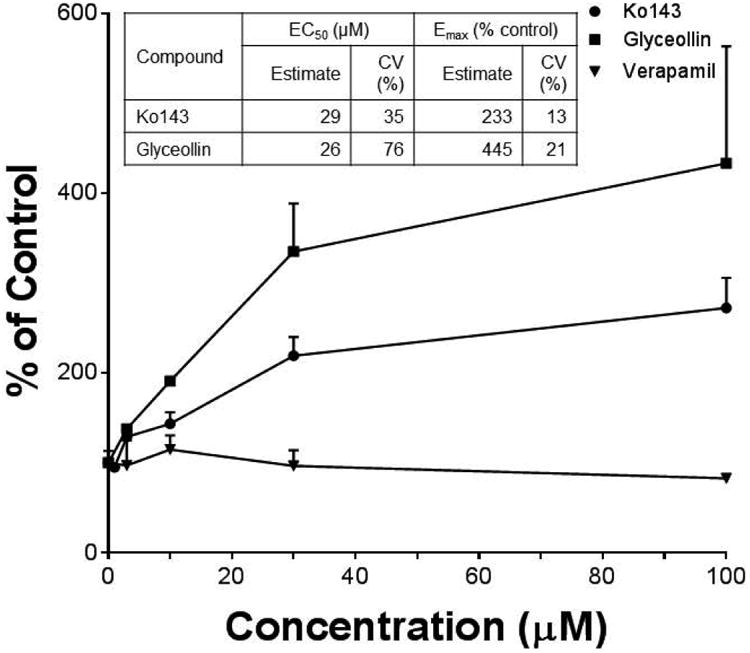
BODIPY-prazosin uptake in Caco-2. Average net fluorescence normalized to cell protein was measured following a 30 minute incubation with 5 μM BODIPY-prazosin in the presence of varying concentrations of the indicated compounds. Results are based on 2 separate experiments with n = 4 for each concentration per experiment. Table summarizes the model-derived estimates of EC50 and Emax with associated coefficients of variation (% coefficient of variation) of the estimates.
Alteration of MRP2 and BCRP mRNA, protein and functional expression
The effect of glyceollin exposure time and concentration on Caco-2 viability was reported in a previous study (11), and demonstrated that cell viability was not altered following 24 hours of exposure to 100 μM glyceollin; however, exposure for 72 hours at this concentration resulted in appreciable loss of cell viability. Based on these findings, the effects of glyceollin exposure for 24 hours at 1, 10 and 100 μM on mRNA and protein levels of MRP2 and BCRP were determined. In addition, the effects of these concentrations on CDF permeability in the BA direction, and on BODIPY-prazosin uptake were also evaluated. The results of these studies are summarized in Figure 5.
Figure 5.
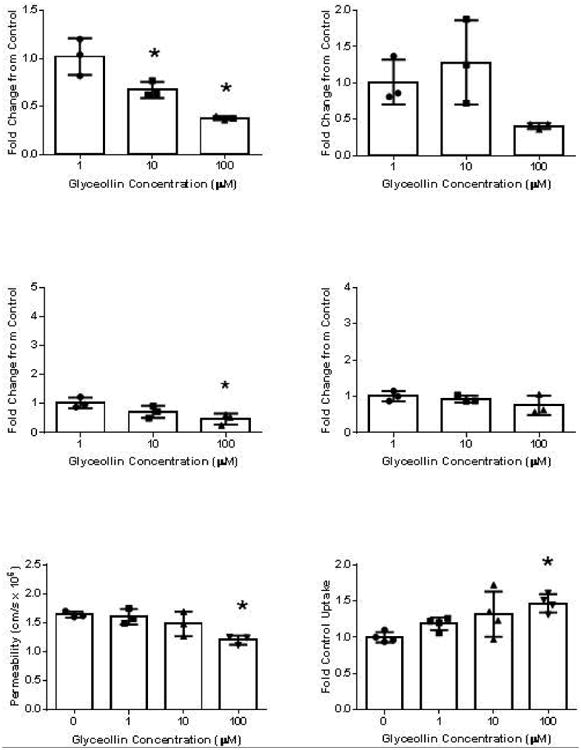
Influence of glyceollin exposure on ABCC2 (left panel) and ABCG2 (right panel) mRNA (top row) and protein (middle row) expression, and on MRP2-mediated CDF BA permeability (bottom left) or BCRP-mediated BODIPY-prazosin uptake (bottom right) in Caco-2. For mRNA and protein studies, cells were exposed to the indicated concentrations for 24 hours before analysis. For CDF permeability, cells grown on filters for 3 weeks and subsequently exposed to the indicated concentrations for 24 hours were used. For mRNA and protein, bar height represents the mean % of control from 3 experiments (individual points) with each point representing the average of four replicates. For CDF permeability, n = 3 for each concentration. For BODIPY-prazosin, n = 4 for each concentration. “*” represents significantly different from control (p < 0.05).
With respect to ABCC2/MRP2, exposure to glyceollin reduced ABCC2 mRNA expression at 100 μM to 38% of control, and to 47% of control with respect to MRP2 specific protein expression. Functionally, CDF BA directional permeability following 24 hours of exposure to 100 μM glyceollin was reduced to 73% of control.
With respect to ABCG2/BCRP, exposure to glyceollin up 100 μM glyceollin for 24 hours did not alter mRNA or protein expression. At 100 μM glyceollin, BODIPY-prazosin uptake was increased by 47% relative to control (p < 0.05), indicative of a decline in BCRP function, but much smaller than the change observed when uptake was measured in the presence of this concentration (433% on average, Figure 4).
Influence of glyceollin on genistein disposition in Caco-2
As summarized in Table 1, absorptive (AB) directional permeability of 2 μM genistein was 1.3 ± 0.30 × 10-5 cm/s. In the presence of 30 μM glyceollin, genistein permeability increased to 2.2 ± 0.06 × 10-5 cm/s (a 69% increase over control). These results are the averages of two separate experiments, each with n = 3 filters for both control and 30 μM glyceollin conditions. The results suggest that glyceollin enhanced genistein aglycone permeability; however, some of this enhancement may have been due to glyceollin non specific increase in Caco-2 permeability, as in companion experiments, lucifer yellow permeability increased from 0.13 ± 0.035 × 10-5 cm/s to 0.59 ± 0.021 × 10-5 cm/s in the presence of 50 μM glyceollin.
Table 1.
Genistein absorptive permeability and fraction metabolized (Fm) estimates, and conjugate metabolite excretion parameters.
| Geniste in Permeability (cm/s) | Fm | Metabolite Excretion Rate, Jmet (nmole/s) | Metabolite Clearance, Clexcretion (mL/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Apical | Basolateral | Apical | Basolateral | |||||||||
|
|
||||||||||||
| Condition1 | Mean | % CV | Mean2 | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV |
| Control | 1.3 × 10-5 | 32.7 | 0.47 | 31.3 | 10.5 × 10-5 | 21.8 | 3.7 × 10-5 ** | 15.9 | 2.4 × 10-3 | 11.4 | 0.9 × 10-3 ** | 41.0 |
| Glyceollin | 2.2 × 10-5 * | 3.8 | 0.13* (0.03and 0.23) | 109.1 | 6.5 × 10-5 * | 16.4 | 2.2 × 10-5 | 71.1 | 5.9 × 10-3 * | 60.0 | 0.8 × 10-3 ** | 13.1 |
For Control and Glyceollin conditions, genistein permeability and Fm are the averages of 6 filters from two experiments of 3 filters each. Metabolite excretion parameters are also the average of 6 filters from the two experiments for the Controls. For the Glyceollin condition, excretion parameters are the average of 3 filters from the second experiment.
In the case of Glyceollin condition, the means for each of the two experiments are also presented in parentheses.
Significantly different from Control (p < 0.05).
Significantly different from Apical (p < 0.05).
Measurement of genistein concentration in apical, basolateral and cell compartments before and after incubation of samples with β-glucuronidase and sulfatase to convert conjugate metabolites back to the aglycone provided a measure of glyceollin's effects on genistein conjugative metabolism in Caco-2. In the absence of glyceollin, the fraction of genistein metabolized (Fm in Table 1) to these conjugates was on average 47% ± 10.5%. In the presence of glyceollin, Fm was reduced to 3% ± 0.7% in Experiment 1 and to 23% ± 1.1% in Experiment 2 (p < 0.05 for both experiments). In the absence of glyceollin, total genistein recovery was 83% and 99% in the two experiments. In the presence of glyceollin, this recovery was 89% and 90% respectively for the two experiments. In both cases (Control and Glyceollin treatments), recoveries around 90% indicate that the majority of genistein metabolism was accounted for by conjugation (Figure 6), which is a finding reported by others (22). In control incubations, conjugate metabolite excretion rates (Jmet) into the apical compartment were on average 2.8-fold higher compared to rates into the basolateral compartment (Table 1). The difference was statistically significant in both experiments (p < 0.05, two-way ANOVA followed by Tukey's test for multiple comparisons), and is consistent with the reported preferential apical excretion of genistein sulfate conjugates in Caco-2, which were shown to be more abundant relative to the glucuronide metabolites (22). In the presence of glyceollin, apical metabolite excretion rate was significantly reduced relative to the control: 6.5 ± 0.61 × 10-5 nmoles/sec from Experiment 2 (n = 3) vs 10.5 ± 0.93 × 10-5 nmoles/sec in controls (n = 6). These metabolite excretion data are also summarized in Figure 7. Metabolite excretion could not be reliably determined in Experiment 1 in the presence of glyceollin, due to the lower level of metabolism observed in this experiment, and which resulted in metabolite levels below the quantitation limit (0.1 μM). Statistical analysis indicated no difference in metabolite excretion data for the controls between the two experiments. Given that glyceollin inhibited conjugative metabolism of genistein in these experiments, intracellular conjugates were lower in the glyceollin treated condition: 9 ± 2.7 pmoles/filter (n = 3, Experiment 2), versus 31 ± 1.2 pmoles/filter for controls (n = 6). Correcting the apical excretion rates for this difference, resulted in an apical excretion clearance that was higher in the presence of glyceollin (Table 1). Basolateral conjugate excretion clearance was not different between the Control and Glyceollin treatments.
Figure 6.
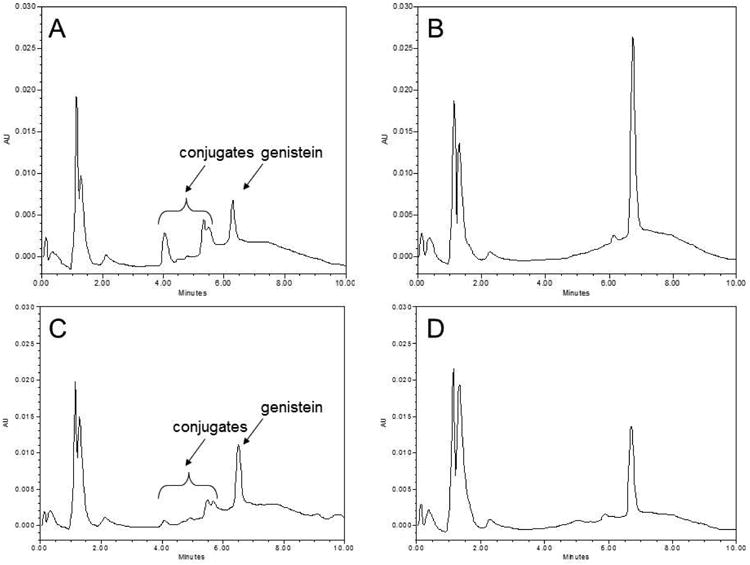
Genistein and conjugate metabolite chromatograms. (A; no H. pomatia present) and (B; H. pomatia present) represent paired aliquots taken from a 4 hour donor sample from the control (no glyceollin) genistein transport condition. (C) and (D) represent respective paired aliquots obtained from a 4 hour donor sample collected following genistein transport in the presence of 30 μM glyceollin.
Figure 7.
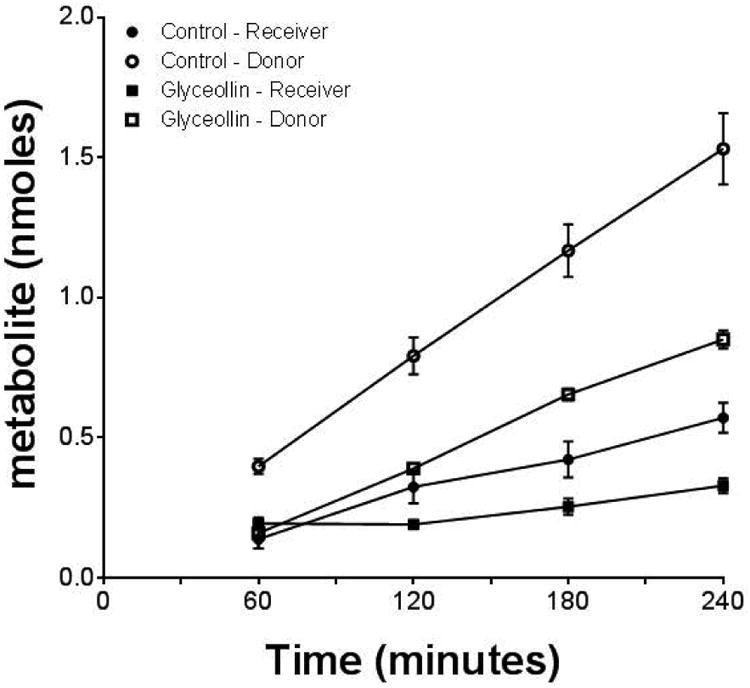
Genistein conjugate metabolite excretion. Average cumulative amount of conjugate metabolite observed in apical (donor) and basolateral (receiver) compartments in the absence (Control) or presence of 30 μM glyceollin on the donor side. N = 6 for Controls and n = 3 for Glyceollin (2nd experiment only).
Discussion
Research aimed at understanding the intestinal transport and metabolism of dietary phytochemicals is driven by the desire to maximize their health benefits. Glyceollin, a soy-derived phytoalexin, has demonstrated pre-clinical effects in vitro and in animal models of breast cancer (8), diabetes (9), and hypercholesterolemia (10). Glyceollin was shown to be highly permeable across Caco-2 monolayers, and to be directly metabolized to sulfate and glucuronide conjugates in this intestinal model system (11), and in rat plasma and feces (12). These findings are similar to those reported for another soy-derived phytochemical, genistein (3, 22-26), which is understood to have poor systemic availability as a result of primarily glucuronidation and sulfation.
In addition to understanding the intestinal pharmacokinetics of phytochemicals, studies have also been conducted to understand the effects these agents may have on intestinal epithelial physiology, and their potential to alter the intestinal disposition of other phytochemicals or drugs (15). There are several studies reporting the effects of genistein in this regard (27-29). Glyceollin was found to have no effect on Pgp activity, or to induce or repress Pgp expression following 24 hours of exposure to concentrations up to 100 μM in Caco-2 (11). The objective of the studies conducted in the present report was to expand evaluation of glyceollin's potential to alter intestinal transporter activity and expression, specifically on the two other major apically expressed efflux transporters in the intestine: ABCC2 (MRP2) and ABCG2 (BCRP).
CDF is an established MRP2 and MRP3 substrate (16). However, it is also an OATP substrate, and may be a specific substrate of OATP2B1 that is expressed on intestinal brush border membranes, including Caco-2 cells (30). In order to isolate it as a MRP2/3 probe substrate, its di-acetate ester prodrug, CDFA, which is permeable via passive diffusion (16), can be used. Subsequent hydrolysis by cellular esterases converts the non-fluorescent CDFA to the fluorescent MRP2/3 probe substrate. Existence of a 3.7-fold asymmetry in BA relative to AB permeability, indicates that MRP2-mediated CDF efflux on the apical side was more influential than MRP3-mediated efflux on the basolateral side in determining net CDF transport in control experiments. Similar asymmetry in CDF cell concentration measured at the conclusion of the 2-hour transport experiment was also observed (Figure 1C). In the presence of 100 μM MK-571, a selective MRP1/2 inhibitor (17), or the same concentration of glyceollin, net secretory CDF directional permeability was lost as a result of an increase in AB permeability concomitant with a decrease in BA permeability. Importantly, control studies indicated that neither MK-571 nor glyceollin altered the rate of CDFA hydrolysis by Caco-2 cells (Figure 3B). Connecting these results with the changes in directional permeability, confirms that MK-571 is an MRP2 inhibitor in these studies, and by extension support that glyceollin inhibited MRP2.
CDF cell concentration measured at the conclusion of the 2-hour incubation was reduced in the presence of MK-571 in both transport directions. Reduction in cell concentration in the AB direction, within the context of enhanced permeability in this direction, is consistent with enhanced MRP3 function when MRP2 is inhibited, as reported to occur in hepatocytes (16). Consistent with this interpretation, CDF retention in 30 minute uptake studies was reduced in the presence of 100 μM MK-571 relative to control (Figure 3A). In the case of glyceollin, patterns of alteration in CDF cell concentration in the presence of 100 μM glyceollin in the transport studies (Figure 1), and CDF retention in uptake studies (Figure 3A) were similar to those observed with MK-571, suggesting that glyceollin inhibits MRP2, and not MRP3. As shown in Figure 2, both MK-571 and glyceollin altered CDF directional permeability in a concentration dependent manner. Estimated potency of this presumed MRP2 inhibition was similar for the two compounds.
BODIPY-prazosin uptake studies demonstrated that glyceollin promoted retention of this BCRP substrate in a concentration dependent manner, with an EC50 similar to the known BCRP inhibitor, Ko143 (19). In contrast to these similar effects, verapamil, a well-known Pgp inhibitor, had no effect, thus supporting that BODIPY-prazosin uptake was specific for BCRP.
In addition to comparing the effects of glyceollin on MRP2 and BCRP function in real time, comparison was also made of their ability to induce the expression of these two efflux transporters. A very modest concentration-related decrease in MRP2 mRNA, protein and function was observed at following 24 hours of exposure to 100 μM glyceollin, the highest concentration tested. Effects of glyceollin on BCRP mRNA and protein expression were absent over the 1 to 100 μM range, and a slight increase in BODIPY-prazosin uptake, indicative of reduced BCRP function, was observed. Overall, similar to our previous findings with Pgp (11), glyceollin does not appear to induce the expression of key intestinal efflux transporters in an in vitro model of the human small intestine.
Several studies evaluating genistein intestinal disposition, including in Caco-2, have determined that it is metabolized primarily to sulfate and glucuronide conjugates, and that these conjugates are substrates for MRP2 and BCRP (22-26). Based on findings reported herein, that glyceollin inhibited both transporters, and that genistein and glyceollin could be co-administered in a soy-derived product, a preliminary evaluation of the potential for glyceollin to alter genistein metabolite disposition in Caco-2 was made. Specifically, it was hypothesized that glyceollin could enhance the transcellular absorption of the metabolites by inhibiting their apical excretion, and possibly even enhancing their basolateral efflux, thereby increasing systemic delivery of total genistein, and subsequently exposure to the biologically active aglycone due to post-absorptive reconversion (14). Importantly, this hypothesis of a pharmacokinetic interaction resulting in enhanced genistein biological potency stands separate from the reported in vivo effects of glyceollin administered apart from genistein (9-10), and provides an alternative explanation vis-à-vis a glyceollin-direct effect regarding altered transcriptional profiles in monkeys fed a glyceollin-soy diet vs. a soy diet alone (31). In addition to applying to genistein intestinal disposition, this type of pharmacokinetic interaction has been suggested recently for luteolin (32) and for (-)-epicatechin (33). To initially test this hypothesis for glyceollin-mediated alteration of genistein metabolite disposition, absorptive transport of 2 μM genistein was evaluated in order to be consistent with a reported study also conducted in Caco-2 at this concentration, which demonstrated that 1) the apical excretion of several genistein conjugates was reduced, and 2) the basolateral excretion of these metabolites was enhanced in the presence of the BCRP inhibitor, Ko143 (19). The concentration of glyceollin was 30 μM, and chosen since this was similar to the IC50 for inhibition of MRP-2 mediated CDF transport (Figure 2), as well as to the EC50 for the glyceollin effect on BCRP-mediated uptake of BODIPY-prazosin (Figure 4). The principal finding in these studies, aside from replicating in the Control the asymmetric (predominantly apical) excretion of the metabolites observed in the Yang et al study (22), was that glyceollin reduced genistein conjugative metabolism to an average of 13% compared to 47% observed in controls (Table 1). Associated with this reduction, genistein permeability was enhanced. This finding is similar to luteolin and querceitin inhibition of (-)-epicatechin metabolism in Caco-2, and implicated increased transport of the parent polyphenol (33). Based on our previous work demonstrating that both glucuronidation and sulfation of glyceollin are primary metabolic routes in rats (12) and in Caco-2 (11), this metabolic interaction is not unexpected. Further studies will be needed to determine the mechanism and potency of glyceollin inhibition of genistein conjugation in intestinal tissue, and if this in vitro effect of enhanced genistein transport translates to whole animals.
Although apical metabolite excretion rate was reduced in the presence glyceollin (Figure 7 and Table 1), which is consistent with the hypothesis of glyceollin inhibition of metabolite efflux via MRP2 and/or BCRP, this difference was actually reversed upon correcting for the lower intracellular genistein conjugate concentrations observed in the Glyceollin condition. With respect to basolateral metabolite excretion rate and clearance, there was no evidence that glyceollin enhanced basolateral delivery of metabolites, in contrast to what was observed in the Yang, et. al. study via Ko143-mediated inhibition of BCRP (22). As stated, this genistein/glyceollin interaction evaluation in Caco-2 was an initial study to determine the potential for glyceollin to increase the bioavailability of genistein through a transporter-mediated interaction. Clearly more studies (such as in situ intestinal perfusion and in vivo exposure), and analytical methods that detect individual conjugates, which may have different transport characterisitcs (22, 32-33), are needed to determine if glyceollin can positively influence genistein intestinal absorption and systemic bioavailability, and, if so, the relative contributions of altered metabolism and metabolite transport.
In conclusion, glyceollin demonstrated strong evidence of inhibition of both MRP2 and BCRP in Caco-2; whereas, there was little to no alteration in the expression of these two ABC transporters following 24 hours of exposure up to 100 μM. Evaluation of the potential for glyceollin to improve total genistein transport (aglycone + conjugative metabolites) through a transport-mediated interaction actually revealed a metabolic interaction, via presumptively glyceollin inhibition of genistein metabolism, and which appeared to translate to improved transport of genistein aglycone.
Acknowledgments
This work was funded by a Louisiana Cancer Research Consortium Seed Grant (OSP-7011-003) and in part by Grant Number 2G12MD007595 from the National Institutes on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD or NIH.
The advice of Richard Graves and Grace Ledet in the conduct of the prazosin and genistein analyses by HPLC is gratefully acknowledged.
Footnotes
Author Disclosure Statement: No competing financial interests exist.
Contributor Information
Chukwuemezie Chimezie, Email: cchurx818@gmail.com.
Adina Ewing, Email: aewing89@icloud.com.
Chandler Schexnayder, Email: cschexn2@xula.edu.
Melyssa Bratton, Email: mbratton@xula.edu.
Elena Glotser, Email: eglotser@xula.edu.
Elena Skripnikova, Email: eskripni@xula.edu.
Pedro Sá, Email: pedro_cdb@hotmail.com.
Stephen Boué, Email: steve.boue@ars.usda.gov.
References
- 1.Horn-Ross PL, Barnes S, Lee M, Coward L, Mandel JE, Koo J, John EM, Smith M. Assessing phytoestrogen exposure in epidemiologic studies: development of a data base (United States) Cancer Causes Control. 2000;11:289–298. doi: 10.1023/a:1008995606699. [DOI] [PubMed] [Google Scholar]
- 2.Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. 2001;49:1069–1086. doi: 10.1021/jf0009246. [DOI] [PubMed] [Google Scholar]
- 3.Hu M. Commentary: bioavailability of flavonoids and polyphenols: call to arms. Mol Pharm. 2007;4:803–806. doi: 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai P, Zhu L, Luo F, Lu L, Li Q, Wang L, Wang Y, Wang X, Hu M, Liu Z. Triple recycling processes impact systemic and local bioavailability of orally administered flavonoids. AAPS J. 2015;17:723–736. doi: 10.1208/s12248-015-9732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonhoff A, Grisebach H. Elicitor-induced accumulation of glyceollin and callose in soybean roots and localized resistance against Phytophthora megasperma F sp. Glycinea. Plant Sci. 1988;54:203–209. [Google Scholar]
- 6.Burow ME, Boue SM, Collins-Burow BM, Melnik LI, Duong BN, Carter-Wientjes CH, Li S, Wiese TE, Cleveland TE, McLachlan JA. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann MC, Tilghman SL, Boue' SM, Salvo VA, Elliott S, Williams KY, Skripnikova EV, Ashe H, Payton-Stewart F, Vanhoy-Rhodes L, Fonseca JP, Corbitt C, Collins-Burow BM, Howell MH, Lacey M, Shih BY, Carter-Wientjes C, Cleveland TE, McLachlan JA, Wiese TE, Beckman BS, Burow ME. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J Pharmacol Exp Therap. 2010;332:35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 9.Boué SM, Isakova IA, Burow ME, Cao H, Bhatnagar D, Sarver JG, Shinde KV, Erhardt PW, Heiman ML. Glyceollins, soy isoflavone phytoalexins, improve oral glucose disposal by stimulating basal and insulin-mediated uptake mediated by glucose transporters in adipocytes. J Agricul Food Chem. 2012;60:6376–6382. doi: 10.1021/jf301057d. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Xie Z, Boué SM, Bhatnagar D, Yokoyama W, Yu L, Wang TTY. Cholesterol-lowering activity of soy-derived glyceollins in the golden Syrian hamster model. J Agricul Food Chem. 2013;61:5772–5782. doi: 10.1021/jf400557p. [DOI] [PubMed] [Google Scholar]
- 11.Chimezie C, Ewing AC, Quadri SS, Cole RB, Boué SM, Omari CF, Bratton M, Glotser E, Skripnikova E, Townley I, Stratford RE. Glyceollin transport, metabolism, and effects on P-glycoprotein function in caco-2 cells. J Med Food. 2014;17:462–471. doi: 10.1089/jmf.2013.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quadri SS, Stratford RE, Boué SM, Cole RB. Identification of glyceollin metabolites derived from conjugation with glutathione and glucuronic acid in male ZDSD rats by online liquid chromatography-electrospray ionization tandem mass spectrometry. J Agricul Food Chem. 2014;62:2692–2700. doi: 10.1021/jf403498f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and in a perfused rat intestinal model. Drug Metab Dispos. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 14.Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, Brown VA, Brenner DE, Singh R, Steward WP, Gescher AJ, Brown K. Sulfate metabolites provide and intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Trans Med. 2013;5:205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez AI, Real R, Pérez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 16.Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KLR. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Therap. 2003;304:801–809. doi: 10.1124/jpet.102.044107. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Volpe DA, Wang Y, Zhang W, Bode C, Owen A, Hidalgo IJ. Use of transporter knockdown caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab Dispos. 2011;39:1196–1202. doi: 10.1124/dmd.111.038075. [DOI] [PubMed] [Google Scholar]
- 18.Bakhsheshian J, Hall MD, Robey RW, Herrmann MA, Chen JQ, Bates SE, Gottesman MM. Overlapping substrate and inhibitor specificity of human and murine ABCG2. Drug Metab Dispos. 2013;41:1805–1812. doi: 10.1124/dmd.113.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JA, Haslam IS, Coleman T, Simmons NL. Breast cancer resistance protein BCRP (ABCG2)-mediated transepithelial nitrofurantoin secretion and its regulation in human intestinal epithelial (Caco-2) layers. Eur J Pharmacol. 2011;672:70–76. doi: 10.1016/j.ejphar.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Dahan A, Amidon GL. MRP2 mediated drug-drug interaction: indomethacin increases sulfasalazine absorption in the small intestine, potentially decreasing its colonic targeting. Int J Pharmaceut. 2010;386:216–220. doi: 10.1016/j.ijpharm.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Zhu W, Gao S, Yin T, Jiang W, Hu M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the role of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:1883–1893. doi: 10.1124/dmd.111.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Zanden JJ, Worteboer HM, Bijlsma S, Punt A, Usta M, van Bladeren PJ, Rietjens IMCM, Cnubben NHP. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69:699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J Pharmacol Exp Therap. 2003;304:1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Xu H, Wang SWJ, Hu M. Breast cancer resistance protein (BCRP) and sulfotransferase contribute significantly to the disposition of genistein in the mouse intestine. AAPS J. 2010;12:525–536. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55:159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 27.Jäger W, Winter O, Halper B, Salamon A, Sartori M, Gajdzik L, Hamilton G, Theyer G, Graf J, Thalhammer T. Modulation of liver cannalicular transport processes by the tyrosine-kinase inhibitor genistein: implications of genistein metabolism in the rat. Hepatology. 1997;26:1467–1476. doi: 10.1002/hep.510260613. [DOI] [PubMed] [Google Scholar]
- 28.Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther. 2006;9:279–287. doi: 10.1111/j.1365-2885.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 30.Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artusson P, Tsuji A. Predominant contribution of organic anion transporting polypeptide OATP=B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal caco-2 cells. Drug Metab Dispos. 2006;34:1423–1431. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- 31.Wood CE, Boué SM, Collins-Burow BM, Rhodes LV, Register TC, cline JM, Dewi FN, Burow ME. Glyceollin-elicited soy protein consumption induces distinct transcriptional effects as compared to standard soy protein. J Agric Food Chem. 2012;60:81–86. doi: 10.1021/jf2034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L, Li Y, Chen WY, Zeng S, Dong LN, Peng XJ, Jiang W, Hu M, Liu ZQ. Breast cancer resistance protein-mediated efflux of luteolin glucuronides in HeLa cells overexpressing UDP-glucuronosyltransferase IA9. Pharm Res. 2014;31:847–860. doi: 10.1007/s11095-013-1207-0. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Bridge B, Lévèques A, Li H, Bertschy E, Patin A, Actis-Goretta L. Modulation of (-)-epicatechin metabolism by coadministration with other polyphenols in caco-2 cell model. Drug Metab Dispos. 2015;43:9–16. doi: 10.1124/dmd.114.060590. [DOI] [PubMed] [Google Scholar]


