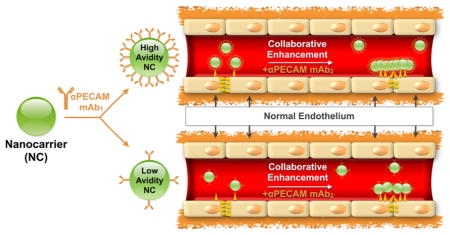
Abstract
Nanocarriers (NCs) coated with antibodies (Abs) to extracellular epitopes of the transmembrane glycoprotein PECAM (platelet endothelial cell adhesion molecule-1/CD31) enable targeted drug delivery to vascular endothelial cells. Recent studies revealed that paired Abs directed to adjacent, yet distinct epitopes of PECAM stimulate each other’s binding to endothelial cells in vitro and in vivo (“collaborative enhancement”). This phenomenon improves targeting of therapeutic fusion proteins, yet its potential role in targeting multivalent NCs has not been addressed. Herein, we studied the effects of Ab-mediated collaborative enhancement on multivalent NC spheres coated with PECAM Abs (Ab/NC, ~180 nm diameter). We found that PECAM Abs do mutually enhance endothelial cell binding of Ab/NC coated by paired, but not “self” Ab. In vitro, collaborative enhancement of endothelial binding of Ab/NC by paired Abs is modulated by Ab/NC avidity, epitope selection, and flow. Cell fixation, but not blocking of endocytosis, obliterated collaborative enhancement of Ab/NC binding, indicating that the effect is mediated by molecular reorganization of PECAM molecules in the endothelial plasmalemma. The collaborative enhancement of Ab/NC binding was affirmed in vivo. Intravascular injection of paired Abs enhanced targeting of Ab/NC to pulmonary vasculature in mice by an order of magnitude. This stimulatory effect greatly exceeded enhancement of Ab targeting by paired Abs, indicating that ‘“collaborative enhancement”‘ effect is even more pronounced for relatively large multivalent carriers versus free Abs, likely due to more profound consequences of positive alteration of epitope accessibility. This phenomenon provides a potential paradigm for optimizing the endothelial-targeted nanocarrier delivery of therapeutic agents.
Keywords: nanocarrier, targeted drug delivery, PECAM-1, multivalent interactions, collaborative enhancement
GRAPHIC ABSTRACT
Targeting drugs using nanocarriers (NCs) is a promising yet challenging area of nanomedicine.1–4 Carriers offer numerous advantages including high payload and its isolation en route, multivalent anchoring, and intracellular delivery, but also face difficult translational issues, necessitating mechanistic studies of their targeting. Binding to target cells is different for free affinity ligands (e.g., antibodies (Abs) or their fragments) versus carriers coated with the same ligand molecules.5–7 Complex dynamics of such affinity interactions are governed by specific features of both carrier design such as ligand surface density and of the target cell and molecule such as its accessibility and conformation in plasma membrane.8
Targeting drugs to endothelial cells (ECs) has the potential to improve management of diseases involving ischemia, inflammation, thrombosis, and tumor growth.9–16 In particular, the surface of carriers coated with Abs to the cell adhesion molecule PECAM (platelet endothelial cell adhesion molecule 1, PECAM-1, CD31) enables endothelial delivery of NCs, improving therapeutic effects of their drug cargoes in animal models.17–21 These encouraging results justify further optimization of endothelial targeting and effect of anti-PECAM/NC (indicated as Ab/NC unless indicated otherwise). PECAM, a 130 kDa transmembrane glycoprotein of the Ig-superfamily, is stably expressed on the surface of ECs, preferentially at cell–cell borders,22,23 and mediates interactions of leukocytes with endothelium.24–26
Targeting to PECAM is modulated by selection of the anchoring epitopes. For example, anchoring of nanocarriers coated by anti-PECAM Abs to distinct PECAM epitopes results in different rates of endothelial endocytosis and intracellular trafficking of Ab/NC.27 Furthermore, functional effects of Abs to distinct PECAM epitopes differ in terms of the influence of homologous PECAM–PECAM interactions between adjacent endothelial cells or/and adhering leukocytes.26,28
We recently discovered that paired anti-PECAM Abs to adjacent yet distinct epitopes on PECAM increased each other’s binding to endothelium,29 a counterintuitive finding since steric hindrance might be expected to inhibit binding. Alternatively, this “collaborative enhancement” of endothelial targeting by the paired Abs may be attributable to increased accessibility of the epitopes for the stimulated antibody due to conformational changes in the target molecule induced by the stimulating antibody. Collaborative enhancement of targeting may also have important practical biomedical implications. For example, paired antibodies boost endothelial targeting29 and the therapeutic effect30,31 of a protein drug fused with a scFv directed to an adjacent PECAM epitope.
Here we have investigated the effect of the paired anti-PECAM Ab on targeting Ab/NC and report that paired antibodies stimulate the endothelial targeting of Ab/NC directed to the first and second Ig domains of PECAM (i.e., IgD1 and IgD2).
RESULTS AND DISCUSSION
Collaborative Enhancement of Endothelial Binding of Ab/NC by Paired Ab: Effects of Flow, Epitope Selection, Ab/NC Avidity, and Cellular Status
Employing a live-cell system of human umbilical vein endothelial cells (HUVECs), we studied mutual effects of paired anti-PECAM Abs on endothelial binding of Ab/NC variably coated with approximately 50 or 200 Ab molecules per spherical particle (final diameter of ~180 nm). Binding characteristics of monoclonal Abs to distinct epitopes in IgD1 in human PECAM, Ab62 and Ab37 (Ab1h and Ab2h, respectively), and in IgD2 in mouse PECAM, Ab 390 and Ab Mec13 (Ab1m and Ab2m) are shown in Table S1.29
Ab1h/NC, but not control IgG/NC, bound to static EC proportionally to low and high Ab surface density (Figure 1), consistent with previous studies.32,33 Preincubation with an excess of the same soluble Ab1h (i.e., “self”) inhibited endothelial binding of Ab1h/NC, whereas pretreatment with control IgG (“solo”) had no effect. In contrast, pretreatment with Ab2h to a second distinct epitope (“paired”) enhanced binding of Ab1h/NC most significantly for high density Ab/NC (Figure 1B; P < 0.001).
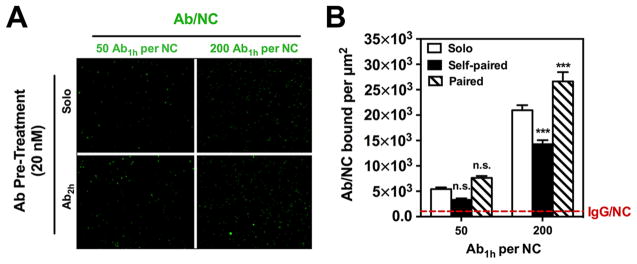
Figure 1.
Free paired Ab2h preincubation specifically enhances endothelial cell (EC) binding of anti-PECAM Ab1h/NCs at low and high Ab densities. (A) Representative fluorescence images showing that HUVEC binding of green fluorescent NCs having 25% surface coating with Ab1h (i.e., 50 Ab/particle) or fully coated with Ab1h (i.e., 200 Ab/particle) was increased when EC were preincubated with free paired Ab2h (20 nM) for 30 min compared with solo IgG pretreatment (20 nM). (B) EC binding of Ab1h/ NC at low and high Ab density over particle surface (50 Ab/NC and 200 Ab/NC, respectively) with solo IgG (open bars) was inhibited by self-paired free Ab1h (filled bars) and significantly enhanced by paired free Ab2h (hashed bars) at high Ab density. The EC binding of fluorescent particles in each image field (104 μm2), as quantified by fluorescence microscopy, was represented as Ab/NC bound per μm2. Dashed line represents total binding of nontargeted IgG-coated NCs. Data are mean ± SE (n = 8). n.s., P > 0.05; ***, P < 0.001.
Therefore, “paired” Ab2h enhances binding of Ab1h/NC, similarly to its effect on binding of free Ab1h, likely via increasing accessibility of the Ab1h epitope.29 However, unlike “collaborative binding” of free Ab1h and Ab2h, no enhancement was observed at simultaneous coincubation of “paired” Ab2h with Ab1h/NC; pretreatment with paired Ab2h is required to stimulate Ab1h/NC binding (Supporting Information, Figure S1). Apparently, Ab1h/NCs have faster binding kinetics than relatively small free Ab1h; hence, Ab2h-mediated exposure of the secondary Ab1h PECAM epitope must precede the administration of Ab/NCs. This is not necessary for free Abs.
To increase the physiological relevance of the model, we studied effects of Ab2h on the binding of Ab1h/NC to endothelial cells under acute flow. We found that flow (i) stimulates “solo” Ab1h/NC binding and (ii) potentiates the collaborative enhancement of Ab1h/NC binding by paired Ab2h pretreatment (Figure 2). The latter was a somewhat unexpected finding, the specific mechanisms of which merit separate investigation. Here we explored two potential means by which flow might influence the collaborative enhancement of NC binding. First, the distribution of PECAM on HUVEC was examined under static conditions, after short-term (30 min) exposure to flow, and following overnight flow adaption. In contrast to a prominent change in the distribution of filamentous actin (F-actin), no effect was seen on the localization of PECAM, which remained predominantly at cell–cell junctions (Supporting Information, Figure S3). Second, we investigated endocytosis of Ab1h/NC bound to PECAM, a process recently shown to be modulated by flow.32–35 We found that collaborative enhancement of Ab1h/NC binding to endothelial cells under flow was unaffected when internalization was blocked via ATP-depletion with sodium azide/2-deoxyglucose (NaN3/2-DG) (Figure 3A,B). In contrast, paraformaldehyde (PFA)-fixation of cells completely abrogated the effect of pretreatment with paired Ab2h (Figure 3C). Since both methods effectively block endocytosis of Ab1h/NC (Figure S3), these results suggest that it may be conformational flexibility and lateral diffusion36,37 of PECAM molecules, eliminated by PFA but not NaN3/2-DG, which may be critical for the collaborative enhancement effect.
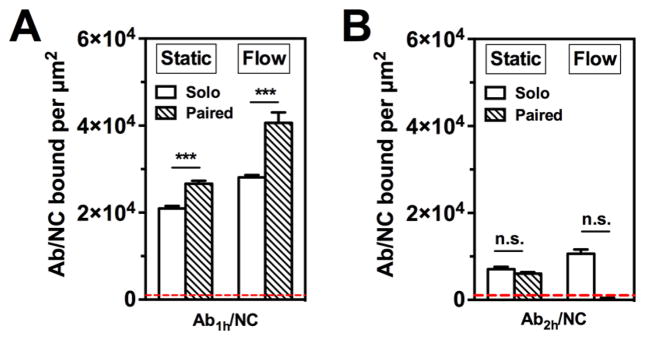
Figure 2.
Fluid shear stress potentiates endothelial binding of anti-PECAM Ab1h/NC induced by paired Ab2h incubation. Under static and flow conditions (4 dyn/cm2), paired Ab2h preincubation (20 nM) significantly enhanced endothelial binding of Ab1h/NCs (200 Abs/NC) (A), whereas paired Ab1h had no effect on endothelial binding of Ab2h/NCs (200 Abs/ NC) (B). The number of EC bound Ab1h/NCs was quantified in each fluorescence image field (104 μm2); dashed line indicates nonspecific binding of fully coated control IgG/NC. Data are shown as mean±SE (n=8). ***, P < 0.001; n.s., P > 0.05. Open bars show NC binding with solo treatment; hashed bars show NC binding with paired free Ab treatment.
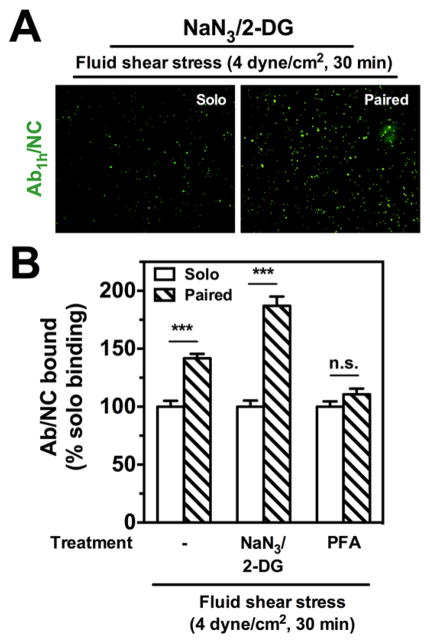
Figure 3.
Blocking internalization does not abrogate stimulated endothelial binding of anti-PECAMAb1h/NCs. (A) Under flow conditions of acute fluid shear stress (4 dyn/cm2, 30 min), fluorescence images show endothelial HUVEC binding of Ab1h/NCs (green) with paired Ab2h pretreatment (20 nM) is increased compared to solo IgG pretreatment with NaN3/2-DG (5 mM). (B) Despite blocking of internalization with NaN3/2-DG, Ab1h/NC binding is significantly increased by 87% over solo binding under flow. For cells prefixed with paraformaldehyde (PFA, 1%, 10 min) and then subjected to flow, the enhanced endothelial binding of Ab1h/ NCs (200 Abs/NC) with free Ab2h preincubation (20 nM) is completely abolished to control IgG levels. The number of EC bound fluorescent particles in each image filed (104 μm2) was quantified by fluorescence microscopy under flow conditions and presented as % solo binding. Data are mean±SE (n = 8). n.s., P > 0.05. Open bars show NC binding with solo treatment; hashed bars show NC binding with paired free Ab treatment.
Under physiologic conditions, individual factors such as flow and nanocarrier avidity, are likely to both influence the magnitude of the collaborative enhancement effect in complex ways. For example, under flow conditions, pretreatment with Ab2h resulted in a significant increase in Ab1h/NC binding only at higher density of targeted anti-PECAM antibody (Supporting Information, Figure S4). As mentioned above, the details of these interactions and their underlying mechanisms deserve separate investigation; in the context of this study, it suffices to say that the observed effects of flow were encouraging in terms of the potential physiological relevance of the collaborative enhancement effect and motivated the animal studies shown in the following sections.
It is important to note that the paired enhancement effect is not necessarily mutual. In our initial report of the collaborative enhancement effect, we found that while Ab2h significantly enhanced the binding of Ab1h, Ab1h had no effect on the binding of free Ab2h. This was different from the paired mouse anti-PECAM antibodies (Ab1m and Ab2m), where the effect was mutual; that is, each antibody enhanced the binding of the other.29 Consistent with this previous result, we found here that Ab1h had no effect on binding of Ab2h/NC, regardless of the presence of flow (Figure 2B). While these results are somewhat counterintuitive, since Ab2h/NC generally displays less effective solo binding than Ab1h/NC (thereby providing “more room” for stimulation), clearly the stimulatory effects of paired Abs are epitope dependent and, for the time being, must be determined empirically.
In Vivo Ab/NC Targeting to Mouse Pulmonary Vasculature Is Enhanced with Paired Antibody
To examine the ultimate physiological relevance and potential medical utility of these in vitro findings we recapitulated them in vivo. The pulmonary vasculature, with its privileged perfusion, extended endothelial surface area,38 and high PECAM expression levels (up to 106 copies per cell),39,40 is the preferential site of accumulation of PECAM-targeted agents.41–44 Accordingly, we studied effects of preadministration of self-and paired PECAM antibodies on the pulmonary accumulation of radiolabeled Ab/NC injected in naïve mice, by determining percent injected dose per gram of lung tissue (%ID/g) following intravenous (IV) injection.
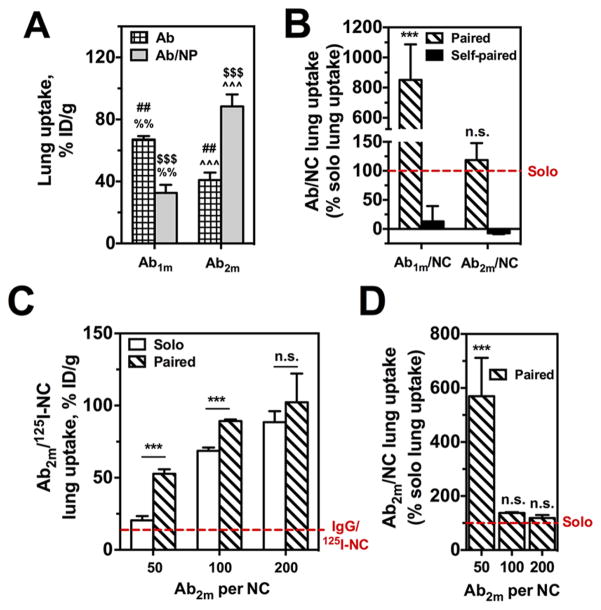
Ab1m/125I-NC injected “solo” accumulated in the pulmonary vasculature due to specific PECAM-targeting, in contrast with control IgG/NC (Figure 4A). Injection of excess free “self” Ab1m obliterated pulmonary accumulation of Ab1m/125I-NC to the level of nonspecific uptake of IgG/NC via the classical competitive inhibition paradigm. In stark contrast, paired Ab2m enhanced the pulmonary uptake of Ab1m/125I-NC more than 5.4-fold relative to solo Ab/NC administration (Figure 5A, P < 0.001). Concomitantly, the blood level of Ab/NC was decreased in mice preinjected with the paired Ab relative to solo Ab/NC delivery, likely depleted in the blood pool due to more effective binding to the endothelium. This result establishes that collaborative enhancement of Ab/NC by paired antibodies does operate in vivo and enables almost an order of magnitude improvement in targeting. In fact, the effect exceeds that for free antibodies: Ab2m caused markedly more modest, approximately 2-fold stimulation in the pulmonary uptake of 125I-Ab1m (Supporting Information, Figure S5A).29 When lung uptake is corrected for nonspecific IgG/NC uptake, there is more than a 8.5-fold enhancement in Ab1m/NC lung uptake following paired Ab2m pretreatment versus solo treatment (Figure 5B).
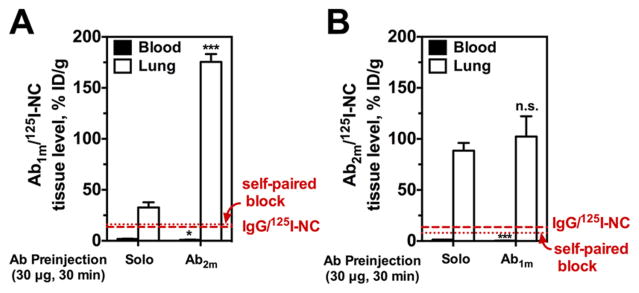
Figure 4.
In vivo endothelial targeting of Ab/125I-NC to muPECAM is enhanced by paired muPECAM Ab. (A) Lung tissue activity of Ab1m/125I-NC (200 μg NC per mouse) at 30 min p.i. is enhanced significantly with paired Ab2m (30 μg for 30 min) as compared to IgG preinjection. Blood levels of Ab1m/125I-NC following paired Ab treatment are lower than blood in IgG pretreated mice. (B) Lung tissue activity of Ab2m/125I-NC (200 μg NC per mouse) is increased non-significantly with paired Ab1m (30 μg for 30 min), whereas blood levels following paired Ab treatment are significantly lower than blood levels in IgG pretreated mice. Dashed line indicates lung uptake of nonspecific IgG/125I-NC and dotted line is Ab1m/125I-NC or Ab2m/125I-NC lung uptake blocked with respective self-paired Ab pretreatment. Data represented as mean ± SE (n = 3–9 mice per group). ***, P < 0.001; n.s., P > 0.05.
Figure 5.
Paired Ab enhancement of anti-PECAM nanocarrier lung targeting is dependent on epitope accessibility and nanocarrier avidity. (A) Pulmonary uptake of PECAM-targeted free anti-PECAM 125I-Abs (Ab1m and Ab2m) versus fully coated Ab/125I-NC show differential binding depending on muPECAM epitope accessibility once Ab is immobilized on NC. Crossed and open bars show vascular-targeted binding with solo treatment for free Ab or NC, respectively. (#) Comparison of free Ab2m to free Ab1m; (%) comparison of free Ab1m to Ab1m/NC; ($) comparison of Ab1m/NC to Ab2m/ NC; (^) comparison of free Ab2m to Ab2m/NC. ##, %%, P < 0.01; $$$, ^^^, P < 0.001. (B) Preinjection of paired Abs differentially stimulated lung uptake of fully coated Ab/NCs depending on epitope targeted as compared to solo control IgG or self-paired Ab preinjection. (C) Preadministration of paired Ab1m (20 nM) enhances lung uptake of Ab2m/NCs with different targeted antibody density on the particle surface (i.e., no. of Abs per particle) as compared to solo IgG. (D) Collaborative enhancement of Ab2m/NC lung uptake with paired Abs, presented as percent solo lung uptake, decreases with increased NC avidity. Data are reported as the mean ± SE (n = 3–9 mice per group). ***, P < 0.001, n.s., P > 0.05. Hashed, filled, and open bars show NC binding with paired Ab, self-paired Ab, or solo IgG treatment, respectively.
Vice versa, paired Ab1m similarly enhanced 125I-Ab2m lung uptake about 2-fold, whereas targeting was obliterated by unlabeled self-paired Ab injection to the level of nonspecific uptake of control IgG (Supporting Information, Figure S5B).29 In this case, however, the nanocarrier effects did not mirror what was seen with the free antibody. Specifically, lung uptake of Ab2m/ 125I-NC was not significantly affected by paired Ab1m when compared to solo binding (Figure 4B, Figure 5B).
Pulmonary accumulation of radiolabeled free Ab1m was considerably higher than that of Ab2m, which is likely due to the fact that Ab1m has an order of magnitude higher affinity in contrast to Ab2m (Supporting Information, Table S1). Notwithstanding this, Ab2m/NC has higher lung uptake versus Ab1m/NC; in other words, Ab2m/NC accumulates in the lungs better, while Ab1m/NC has worse lung uptake compared to their respective free antibodies. This opposing and unpredictable outcome can be attributed speculatively to elevated avidity of multivalent Ab2m/NC and reduced accessibility of PECAM epitope to large Ab1m/NC versus free antibody counterparts.
The innocuous effect of paired Ab1m on particles carrying Ab2m at high surface density is perhaps because the high avidity Ab2m/NC engages enough PECAM copies to achieve maximal binding, even if the presence of paired Ab1m exposes additional epitopes. To explore this, pulmonary uptake of Ab2m/125I-NC coated with 50 and 100 Abs per particle with solo and Ab1m pretreatment was examined. As expected, the absolute amplitude of targeting efficiency decreased with reducing Ab coating surface density (Figure 5C), reflecting reduced avidity of the Ab/NC toward the Ab2m-targeted PECAM epitope. However, reduction of Ab2m/NC avidity indeed unveils significant collaborative enhancement by free paired Ab1m. When corrected for IgG/NC lung uptake, lower avidity nanocarriers with 50 Ab per NC have greater than 5.5-fold increase in lung targeting specificity with paired Ab pretreatment compared to solo binding (Figure 5D). Paired Ab1m presumably exposes more of the Ab2m epitope, and this effect of collaborative enhancement stimulated lung uptake of Ab2m/NC, which was only significantly enhanced when antibody coverage for Ab2m/NC was <100 Abs per NC (i.e., avidity was suboptimal).
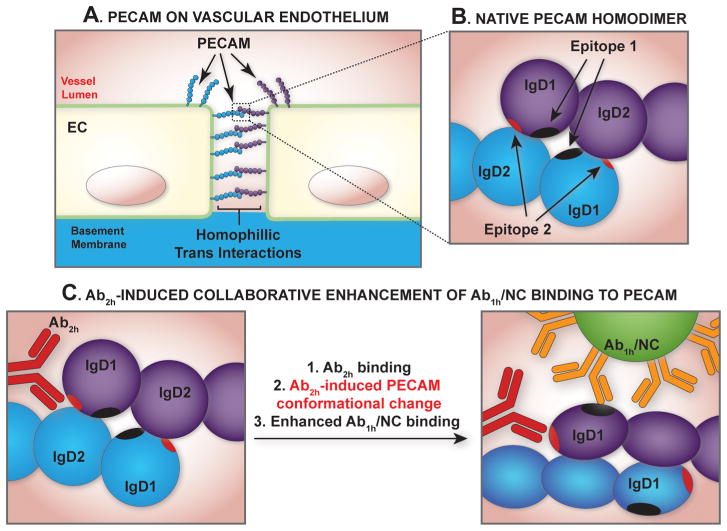
The current report demonstrates for the first time the enhancement of nanoparticle binding to surface motifs by modulating the target using paired antibodies to adjacent epitopes at the membrane surface. Figure 6 illustrates our hypothetical model of collaborative enhancement with PECAM-targeted reagents. The model, which borrows conceptually from protein allosteric modulation by ligand-induced protein conformational changes, takes into account the observation that a modulating antibody, assigned here as Ab2h accessible to epitope 2, when incubated with live cells in vitro or injected in vivo, increases the accessibility of a second distinct but paired PECAM epitope 1, such that Ab1h/NC binding has increased. Vascular PECAM exists in monomeric form in sensor domains in the plasmalemma and as is concentrated at EC intercellular junctions in trans–trans homophilic dimers (Figure 6A,B). The binding of a paired ligand may cause conformational changes in PECAM making the second epitope more accessible, which may be especially important for improving the binding of Ab/NC—large entities that require multivalent anchoring (Figure 6C).
Figure 6.
Hypothetical model of collaborative enhancement of anti-PECAM/NC binding with paired Ab. (A) PECAM expression on the vascular endothelium exists both in monomeric and trans-endothelial homodimeric forms. (B) Homodimeric binding is mediated by IgG domains 1 and 2 (IgD1 and IgD2), which bear epitopes of variable accessibility and affinity for respective Ab ligands, as depicted by the spherical and ellipsoidal shapes of IgD1 and IgD2. (C) The paired antibody Ab2h increases the accessibility of a second distinct but paired PECAM epitope, such that Ab1h/NC binding has increased.
While our findings are clearly relevant for pulmonary drug delivery, it is important to distinguish what specific molecular mechanisms underlay the phenomenon of collaborative enhancement revealed for PECAM targeting, and whether or not it has generalizable features that extend to other targetable endothelial cell surface adhesion molecules. In this context, a systematic approach to mathematical modeling could be developed not only to describe the differential kinetics of targeted nanocarrier binding that result from induction of an augmentation of epitope accessibility but also to provide new physical insights for advancing modeling of multiparameter optimization in order to achieve safe and efficacious targeting in drug delivery applications. We have extensive experience in developing and implementing computational models of nanocarrier targeting to endothelium.36,37,45 However, even the simplest modeling approaches intended to incorporate obvious factors impacting the design of functionalized therapeutic agents such as binding affinity, multivalancy, and in vivo targeting to endothelial cells require an extensive array of in vitro and/or in vivo experimental results for validation or data fitting. For example, to investigate the kinetic rate constants of nanocarrier attachment and detachment, critical information regarding receptor density, ligand density on the nanocarrier surface, and shear stress conditions were required before a temporal relationship between nanocarrier detachment rate and multivalent binding could be discerned.46 Although the data from the present study, as well as those from our previous experiments,29 are not sufficiently replete to enable us to populate a kinetic-based model describing the interplay between free and nanocarrier-bound antibodies, the findings we report are certainly consistent with the occurrence of molecular events through which binding of a particular antibody mediates the augmentation of subsequent binding of nanocarriers bearing another antibody. The facilitated exposure of the epitope participating in such collaboratively enhanced nanocarrier binding, as has been demonstrated herein both in vitro and in vivo, may occur through alterations in receptor conformation or orientation, emergence from sequestration within the glycocalyx or molecular spacing that confers a favorable thermodynamic profile for binding to occur. The challenge will be to demonstrate experimentally which of these, or other effects, is responsible for causing the collaborative enhancement we have described, in order to promote drug delivery applications to other potentially valuable targeting epitopes as well as to yield new modeling considerations for in silico discovery guided by careful investigational observation.
Interestingly, recent studies from other laboratories focused on mechanisms of PECAM signaling and adhesive function in leukocyte transmigration revealed that disruption of homophilic interactions between PECAM molecules in the endothelium by an Fab to PECAM IgD1 improved the binding of a second Fab to PECAM IgD6.47 It is tempting to speculate that ligand-induced stepwise disassembly of PECAM “bunches” leading to exposure of more and more binding sites is involved in trans-cellular trafficking of leukocytes. In this context, Ab/NC of the present study might be viewed as an oversimplified model of a leukocyte. Unclear at this time is if the effect described herein is due to Ab-mediated disruption of homopohilic PECAM–PECAM interaction exposing more PECAM binding sites to the nanocarrier. This may not necessarily be the case since some of the stimulatory paired PECAM Abs (i.e., Ab1h and Ab2m) employed in these studies are known to have no effect on trans-homophilic PECAM interactions. It is widely accepted that long-range conformational change is the mechanism of affinity modulation for members of the integrin,48 cadherin,49 and selectin50,51 families, but there are only a few examples of this within Ig superfamily CAMs, most notably carcinoembryonic antigen (CEA)-related CAM 1 (CEACAM1),52 Axonin 153 and the neural cell adhesion molecule L1.54 Future structural studies are planned to examine the detailed conformational state of PECAM during antibody-mediated collaborative enhancement.
CONCLUSION
This paper represents the first attempt to define the role of the “collaborative enhancement” phenomenon in the vascular immunotargeting of nanocarriers via the cell adhesion molecule PECAM. Previously described with free anti-PECAM Abs and therapeutic fusion proteins, binding of a paired “stimulatory” ligand to one epitope can also lead to increased binding events of PECAM-targeted nanocarriers directed to a second distinct epitope. This study also showed the importance of epitope accessibility and avidity on endothelial targeting of nanocarriers. This paradigm offers a new approach for optimizing and enhancing the endothelial-targeted delivery of NC platforms carrying diverse therapeutic cargoes, from antithrombotic agents to antioxidants. Investigations are ongoing to improve understanding of the phenomena being reported.
METHODS
Materials
Fluorescein isothiocyanate (FITC)-labeled polystyrene spheres (~125 nm effective diameter) were purchased from Polysciences (Warrington, PA). 125I-radiolabled poly(4- vinylphenol) nanoparticle spheres (~145 nm effective diameter) were prepared as previously described.55,56 Purified monoclonal antibodies (mAbs) to human PECAM (huPECAM), clone 62 (mouse IgG2a, referred to as Ab1h), and clone 37 (mouse IgG1, referred to as Ab2h), were generously provided by Dr. M. Nakada (Centocor, Malvern, PA).26 The antimouse PECAM (muPECAM) monoclonal antibody clones 390 (rat IgG2a, referred to as Ab1m)57 and MEC13.3 (rat IgG2a, referred to as Ab2m)58 were purchased from BD Bioscience (Chicago, IL) and BioLegend (San Diego, CA), respectively. Nontargeted IgG Ab was an isotype mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Fluorescent secondary antibody to antihuPECAM were purchased from Invitrogen (Carlsbad, CA). ActinRed 555 reagent was purchased from Life Technologies (Norwalk, CT). Human umbilical vein endothelial cells (HUVECs) endogenously expressing native huPECAM were purchased from Lonza (Walkersville, MD) and maintained in EGM-2 media (Lonza) supplemented with 10% (v/v) fetal bovine serum (FBS).
Ab Coating of Nanocarriers
The polystyrene-based nanocarriers (NC or 125I-NC) were coated with Abs using established adsorption techniques based on interactions between the hydrophobic domains on the surface of mAbs and the relatively hydrophobic NC. Abs (in aqueous buffered solution containing ≤0.09% sodium azide (NaN3)) were added to a NC suspension in PBS, vigorously vortexed for 1 min, and then placed on a rotating shaker for 1 h at room temperature. Briefly, the Ab/NC mixture was centrifuged at 12000g for 3 min and the supernatant (unbound Abs) was separated from the NC pellet (with Abs bound). A saturating antibody density of the NC surface was ~200 Ab molecules/NC (~8000 Ab molecules/μm2). For variable anti-PECAM coating densities, total antibody density was balanced to saturation with nontargeted IgG. Ab/NC formulations had hydrodynamic diameters ranging from 180 to 210 nm as measured by dynamic light scattering (DLS, BI-90 Plus Particle Sizer, Brookhaven Instruments, Brookhaven, NY).
Live-Cell Anti-PECAM/NC Binding Studies
Asix-channel μ-microslide (Ibidi, Germany) connected to a variable-speed peristaltic pump (Rainin RP-1, Columbus, OH) with a media reservoir, was used to subject HUVEC monolayers to treatments under static incubation conditions or under acute fluid shear stress (4 dyn/cm2, 30min).32 HUVECs were maintained in an incubation chamber at 37 °C in 5% CO2/95% air, and starved overnight in endothelial basal medium containing 0.5% fetal bovine serum without supplements prior to experiments. Cells were preincubated with an Ab solution(paired, self-paired, or solo control IgGin Hank’s Balanced Salt Solution (HBSS)) for 30 min. The pump reservoir was loaded with Ab/NC (2.0 – 109 NC/mL in HBSS), and cells then incubated or perfused for 30min. Thereafter, cells were washed extensively with HBSS to remove unbound particles, and cells were fixed with 1% paraformaldehyde (PFA) for 10 min for subsequent fluorescent staining and analysis. In studies examining the effect of endocytosis blockade on Ab/NC binding, HUVECs were preincubated with 5 mM sodium azide (NaN3)/5 mM 2-deoxyglucose (2-DG) for 30 min or prefixed with 1% paraformaldehyde (PFA) for 10 min before incubating with free Abs or perfusing with Ab/NCs.
Microscopy and Quantification of Cell-Bound Anti-PECAM/NCs
PFA-fixed cells were treated and analyzed as previously described.32 Briefly, washed cells were mounted with ProLong Antifade reagent with DAPI (Molecular Probes, Eugene OR) for subsequent fluorescence microscopy. Slides were imaged using an Olympus IX70 inverted fluorescence microscope, 40– PlanApo objectives and filters optimized for green fluorescence. Images were acquired using a Hamamatsu Orca-1 CCD camera and analyzed with ImagePro 3.0 imaging software (Media Cybernetics, Silver Spring, MD). For quantification, endothelial bound-immunobeads are scored automatically for total green fluorescent particles within an image field size of 104 μm2. The data are shown as the mean of ≥6 images ± standard error (SE). Ab/NC binding with paired, self-paired, or solo control IgG pretreatment are shown as hashed bars, filled bars, or open bars, respectively, unless otherwise noted.
PECAM Distribution Studies
HUVEC were seeded in a poly-dimethylsiloxane flow chamber coated with fibronectin and grown until confluent. Once confluent, cells were exposed to either static conditions or fluid shear stress (5 dyn/cm2) for 30 min or 12 h. Following cessation of flow, cells were fixed with 1% PFA, washed, and blocked with 3% BSA in HBSS prior to staining. PECAM staining was performed using Ab1h at 1 μg/mL for 1 h at RT, followed by fluorescent antimouse secondary antibody. For F-actin staining, cells were permeabilized with 0.1% Triton X-100 for 5 min, washed, and treated with ActinRed 555 reagent per manufacturer protocol. Images were acquired using a Zeiss AxioObserver inverted microscope and analyzed using an open-source version of the ImageJ platform.
Biodistribution Studies
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the US National Institutes of Health and approved by the University of Pennsylvania IACUC. C57BL/6 female mice (18–22 g) were anesthetized and injected intravenously (IV) via jugular vein with unlabeled Ab (anti-PECAM or IgG, 30 μg/mouse). Following 30 min Ab pretreatment, the contralateral jugular vein was injected IV with approximately 2 μCi 125I-Ab (0.2 μg) or Ab/125I-NCs (0.2 mg) coated with anti- PECAM formulations or control IgG. All formulations were sterilized by passing through a 0.2 μM filter prior to injection. At 30 min postinjection (p.i.) of the radioligand, and tissues and organs (blood, lungs, heart, kidneys, liver, spleen, brain, and thyroid) were collected and weighed. Tissue radioactivity was measured in a γ-counter, and targeting parameters including percent of injected dose per gram of tissue (% ID/g) were calculated as previously described.29 Data are reported as the mean ± SE of n = 3–9 mice per group.
Statistical Analysis
Results are expressed as mean ±SE unless otherwise noted. Significant differences between group means were determined using two-way ANOVA followed by posthoc Holm–Šídák multiple comparison test, with alpha = 5.0%.
Supplementary Material
Acknowledgments
This work was supported by NIH R01- HL073940 (V.R.M.) and R01-HL087036 (V.R.M.), and U01- EB016027 (D.M.E./V.R.M.), and the support of NIH NRSA fellowship HL07954 (J.H.) through the Institute for Medicine and Engineering (P.F.D.).
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Supporting Information Available: Supplemental figures and tables. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/nn505672x.
REFERENCES AND NOTES
- 1.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard M, Zern BJ, Anselmo AC, Shuvaev VV, Mitragotri S, Muzykantov V. Vascular Targeting of Nanocarriers: Perplexing Aspects of the Seemingly Straightforward Paradigm. ACS Nano. 2014;8:4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 4.Langer R. Drug Delivery and Targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 5.Mane V, Muro S. Biodistribution and Endocytosis of ICAM-1-Targeting Antibodies Versus Nanocarriers in the Gastrointestinal Tract in Mice. Int J Nanomed. 2012;7:4223–4237. doi: 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho K, Lapitsky Y, Shi M, Shoichet MS. Tunable Immunonanoparticle Binding to Cancer Cells: Thermodynamic Analysis of Targeted Drug Delivery Vehicles. Soft Matter. 2009;5:1074–1080. [Google Scholar]
- 7.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr, Banaszak Holl MM. The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chem Biol. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Simone E, Ding BS, Muzykantov V. Targeted Delivery of Therapeutics to Endothelium. Cell Tissue Res. 2009;335:283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aird WC. Endothelium as a Therapeutic Target in Sepsis. Curr Drug Targets. 2007;8:501–507. doi: 10.2174/138945007780362782. [DOI] [PubMed] [Google Scholar]
- 10.Ogawara K, Rots MG, Kok RJ, Moorlag HE, Van Loenen AM, Meijer DK, Haisma HJ, Molema G. A Novel Strategy to Modify Adenovirus Tropism and Enhance Transgene Delivery to Activated Vascular Endothelial Cells In Vitro and In Vivo. Hum Gene Ther. 2004;15:433–443. doi: 10.1089/10430340460745766. [DOI] [PubMed] [Google Scholar]
- 11.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. Prophylactic Thrombolysis by Thrombin-Activated Latent Prourokinase Targeted to PECAM-1 in the Pulmonary Vasculature. Blood. 2008;111:1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC, 3rd, Restel BH, Ozawa MG, Moya CA, Rangel R, et al. A Hybrid Vector for Ligand-Directed Tumor Targeting and Molecular Imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 13.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive Proteomic Mapping of the Endothelial Surface in Lung and Solid Tumours for Tissue-Specific Therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Dean DA. Gene Therapy for ALI/ARDS. Crit Care Clin. 2011;27:705–718. doi: 10.1016/j.ccc.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kee PH, Kim H, Huang S, Laing ST, Moody MR, Vela D, Klegerman ME, McPherson DD. Nitric Oxide Pretreatment Enhances Atheroma Component Highlighting In Vivo with Intercellular Adhesion Molecule-1-Targeted Echogenic Liposomes. Ultrasound Med Biol. 2014;40:1167–1176. doi: 10.1016/j.ultrasmedbio.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripathy S, Vinokour E, McMahon KM, Volpert OV, Thaxton CS. High-Density Lipoprotein Nanoparticles Deliver RNAi to Endothelial Cells to Inhibit Angiogenesis. Part Part Syst Char. 2014;13:1141–1150. doi: 10.1002/ppsc.201400036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzykantov VR. Biomedical Aspects of Targeted Delivery of Drugs to Pulmonary Endothelium. Expert Opin Drug Delivery. 2005;2:909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 18.Hood ED, Chorny M, Greineder CF, ISA, Levy RJ, Muzykantov VR. Endothelial Targeting of Nanocarriers Loaded with Antioxidant Enzymes for Protection against Vascular Oxidative Stress and Inflammation. Biomaterials. 2014;35:3708–3715. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard MD, Greineder CF, Hood ED, Muzykantov VR. Endothelial Targeting of Liposomes Encapsulating SOD/CatalaseMimetic EUK-134 Alleviates Acute Pulmonary Inflammation. J Controlled Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard MD, Hood ED, Greineder CF, Alferiev IS, Chorny M, Muzykantov V. Targeting to Endothelial Cells Augments the Protective Effect of Novel Dual Bioactive Antioxidant/Anti-Inflammatory Nanoparticles. Mol Pharmaceutics. 2014;11:2262–2270. doi: 10.1021/mp400677y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preissler G, Loehe F, Huff IV, Ebersberger U, Shuvaev VV, Bittmann I, Hermanns I, Kirkpatrick JC, Fischer K, Eichhorn ME, et al. Targeted Endothelial Delivery of Nanosized Catalase Immunoconjugates Protects Lung Grafts Donated after Cardiac Death. Transplantation. 2011;92:380–387. doi: 10.1097/TP.0b013e318226bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: A Novel Endothelial Cell-Cell Adhesion Molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albelda SM. Endothelial and Epithelial Cell Adhesion Molecules. Am J Respir Cell Mol Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 24.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 Is Required for Transendothelial Migration of Leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman PJ. The Biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakada MT, Amin K, Christofidou-Solomidou M, O’Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, et al. Antibodies against the First Ig-Like Domain of Human Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) That Inhibit PECAM-1-Dependent Homophilic Adhesion Block In Vivo Neutrophil Recruitment. J Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- 27.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential Intra-Endothelial Delivery of Polymer Nanocarriers Targeted to Distinct PECAM-1 Epitopes. J Controlled Release. 2008;130:226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually Distinct Ig Homology Domains in PECAM-1 Regulate Homophilic Binding and Modulate Receptor Affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 29.Chacko AM, Nayak M, Greineder CF, Delisser HM, Muzykantov VR. Collaborative Enhancement of Antibody Binding to Distinct PECAM-1 Epitopes Modulates Endothelial Targeting. PLoS One. 2012;7:e34958. doi: 10.1371/journal.pone.0034958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greineder CF, Chacko AM, Zaytsev S, Zern BJ, Carnemolla R, Hood ED, Han J, Ding BS, Esmon CT, Muzykantov VR. Vascular Immunotargeting to Endothelial Determinant ICAM-1 Enables Optimal Partnering of Recombinant scFv-Thrombomodulin Fusion with Endogenous Cofactor. PLoS One. 2013;8:e80110. doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greineder CF, Brenza JB, Carnemolla R, Zaitsev S, Hood ED, Pan DC, Ding BS, Esmon CT, Chacko AM, Muzykantov VR. Dual Targeting of Therapeutics to Endothelial Cells: Collaborative Enhancement of Delivery and Effect. FASEB J. 2015 doi: 10.1096/fj.15-271213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and Chronic Shear Stress Differently Regulate Endothelial Internalization of Nanocarriers Targeted to Platelet-Endothelial Cell Adhesion Molecule-1. ACS Nano. 2012;6:8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderon AJ, Muzykantov V, Muro S, Eckmann DM. Flow Dynamics, Binding and Detachment of Spherical Carriers Targeted to ICAM-1 on Endothelial Cells. Biorheology. 2009;46:323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muro S, Koval M, Muzykantov V. Endothelial Endocytic Pathways: Gates for Vascular Drug Delivery. Curr Vasc Pharmacol. 2004;2:281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 35.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A Novel Endocytic Pathway Induced by Clustering Endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Weller GE, Zern B, Ayyaswamy PS, Eckmann DM, Muzykantov VR, Radhakrishnan R. Computational Model for Nanocarrier Binding to Endothelium Validated Using In Vivo, In Vitro, and Atomic Force Microscopy Experiments. Proc Natl Acad Sci U S A. 2010;107:16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Agrawal NJ, Calderon A, Ayyaswamy PS, Eckmann DM, Radhakrishnan R. Multivalent Binding of Nanocarrier to Endothelial Cells under Shear Flow. Biophys J. 2011;101:319–326. doi: 10.1016/j.bpj.2011.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stan RV. Anatomy of the Pulmonary Endothelium. In: Voelkel NF, Rounds S, editors. The Pulmonary Endothelium: Function in Health and Disease. John Wiley & Sons, Ltd; Chichester, UK: 2009. pp. 25–32. [Google Scholar]
- 39.Newman PJ. The Role of PECAM-1 in Vascular Cell Biology. Ann N Y Acad Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- 40.Muro S, Muzykantov VR. Targeting of Antioxidant and Anti-Thrombotic Drugs to Endothelial Cell Adhesion Molecules. Curr Pharm Des. 2005;11:2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 41.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin Facilitates Internalization and Pulmonary Targeting of an Anti-Endothelial Cell Antibody (Platelet- Endothelial Cell Adhesion Molecule 1): A Strategy for Vascular Immunotargeting of Drugs. Proc Natl Acad Sci U S A. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Tan Y, Viroonchatapan E, Pitt BR, Huang L. Targeted Gene Delivery to Pulmonary Endothelium by Anti-PECAM Antibody. Am J Physiol Lung Cell Mol Physiol. 2000;278:L504–L511. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 43.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial Targeting of a Recombinant Construct Fusing a PECAM-1 Single-Chain Variable Antibody Fragment (scFv) with Prourokinase Facilitates Prophylactic Thrombolysis in the Pulmonary Vasculature. Blood. 2005;106:4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, et al. Platelet- Endothelial Cell Adhesion Molecule-1-Directed Immunotargeting to Cardiopulmonary Vasculature. J Pharmacol Exp Ther. 2002;300:777–786. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Bradley R, Eckmann DM, Ayyaswamy PS, Radhakrishnan R. Multiscale Modeling of Functionalized Nanocarriers in Targeted Drug Delivery. Curr Nanosci. 2011;7:727–735. doi: 10.2174/157341311797483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haun JB, Hammer DA. Quantifying Nanoparticle Adhesion Mediated by Specific Molecular Interactions. Langmuir. 2008;24:8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 47.Mei H, Campbell JM, Paddock CM, Lertkiatmongkol P, Mosesson MW, Albrecht R, Newman PJ. Regulation of Endothelial Cell Barrier Function by Antibody-Driven Affinity Modulation of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) J Biol Chem. 2014;289:20836–20844. doi: 10.1074/jbc.M114.557454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo BH, Carman CV, Springer TA. Structural Basis of Integrin Regulation and Signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Q, Maruthamuthu V, Li F, Leckband D. Allosteric Cross Talk between Cadherin Extracellular Domains. Biophys J. 2010;99:95–104. doi: 10.1016/j.bpj.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldron TT, Springer TA. Transmission of Allostery through the Lectin Domain in Selectin-Mediated Cell Adhesion. Proc Natl Acad Sci U S A. 2009;106:85–90. doi: 10.1073/pnas.0810620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu S, Zhang Y, Long M. Visualization of Allostery in P-Selectin Lectin Domain Using MD Simulations. PLoS One. 2010;5:e15417. doi: 10.1371/journal.pone.0015417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klaile E, Vorontsova O, Sigmundsson K, Muller MM, Singer BB, Ofverstedt LG, Svensson S, Skoglund U, Obrink B. The CEACAM1 N-Terminal Ig Domain Mediates Cis- and Trans-Binding and Is Essential for Allosteric Rearrangements of CEACAM1 Microclusters. J Cell Biol. 2009;187:553–567. doi: 10.1083/jcb.200904149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The Crystal Structure of the Ligand Binding Module of Axonin-1/TAG-1 Suggests a Zipper Mechanism for Neural Cell Adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 54.Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, Charness ME, Miller KW. An Alcohol Binding Site on the Neural Cell Adhesion Molecule L1. Proc Natl Acad Sci U S A. 2008;105:371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zern BJ, Chacko AM, Liu J, Greineder CF, Blankemeyer ER, Radhakrishnan R, Muzykantov V. Reduction of Nanoparticle Avidity Enhances the Selectivity of Vascular Targeting and PET Detection of Pulmonary Inflammation. ACS Nano. 2013;7:2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simone EA, Zern BJ, Chacko AM, Mikitsh JL, Blankemeyer ER, Muro S, Stan RV, Muzykantov VR. Endothelial Targeting of Polymeric Nanoparticles Stably Labeled with the PET Imaging Radioisotope Iodine-124. Biomaterials. 2012;33:5406–5413. doi: 10.1016/j.biomaterials.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christofidou-Solomidou M, Nakada MT, Williams J, Muller WA, DeLisser HM. Neutrophil Platelet Endothelial Cell Adhesion Molecule-1 Participates in Neutrophil Recruitment at Inflammatory Sites and Is Down-Regulated after Leukocyte Extravasation. J Immunol. 1997;158:4872–4878. [PubMed] [Google Scholar]
- 58.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, et al. Monoclonal Antibodies Specific for Endothelial Cells of Mouse Blood Vessels. Their Application in the Identification of Adult and Embryonic Endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.