Abstract
HIV-1 latently infected cells in vivo can be found in extremely low frequencies. Therefore, in vitro cell culture models have been used extensively for the study of HIV-1 latency. Often, these in vitro systems utilize defective viruses. Defective viruses allow for synchronized infections and circumvent the use of antiretrovirals. In addition, replication-defective viruses cause minimal cytopathicity because they fail to spread and usually do not encode env or accessory genes. On the other hand, replication-competent viruses encode all or most viral genes and better recapitulate the nuances of the viral replication cycle. The study of latency with replication-competent viruses requires the use of antiretroviral drugs in culture, and this mirrors the use of antiretroviral treatment (ART) in vivo. We describe a model that utilizes cultured central memory CD4+ T cells and replication-competent HIV-1. This method generates latently infected cells that can be reactivated using latency reversing agents in the presence of antiretroviral drugs. We also describe a method for the removal of productively infected cells prior to viral reactivation, which takes advantage of the downregulation of CD4 by HIV-1, and the use of a GFP-encoding virus for increased throughput.
Introduction
The existence of cellular reservoirs where HIV-1 resides in a latent state constitutes a formidable barrier toward eradication of viral infection despite the ability of combination antiretroviral therapy (ART) to durably suppress viral replication and restore the circulating CD4+ T cell population.1–3 One of the major known cellular reservoirs is established in quiescent central memory CD4+ T cells.4,5 Reactivation of latent viruses followed by killing (“shock and kill”) of the infected cells has been proposed as a possible strategy to purge the latent reservoir.6 The interest in discovering signals that will induce latent proviruses through the introduction of latency-reversing agents (LRAs) has prompted the development of in vitro cellular models.7–19
In an effort to recapitulate latency in the CD4+ central memory T cell subset (TCM), we previously developed a latency model in which naive cells from the peripheral blood of healthy donors are activated and polarized in vitro to direct differentiation into TCM.13,20 In vitro culture of these cells in the presence of interleukin (IL)-2 leads to the acquisition of a quiescent phenotype.20 We initially utilized an envelope-defective proviral construct that was pseudotyped with a second plasmid encoding a full-length HIV-1 envelope glycoprotein gene. This system was designed to circumvent the use of antiretrovirals because the virus was engineered to be replication defective.
However, two reasons prompted us to explore the use of replication-competent viruses. First, we wished to create an in vitro model that would more closely resemble the in vivo environment in which replication-competent, full-length HIV-1 is present and viral replication is suppressed by the presence of ART. This will allow for more accurate predictions of the efficacy of candidate LRAs to support future HIV-1 eradication clinical trials. Second, as we recently reported,21 we have documented a recombination event between the proviral construct and the envelope glycoprotein construct, leading to the production of an unexpected replication-competent virus in culture that, if ignored, can complicate the interpretation of results.
Materials and Methods
Reagents
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: nelfinavir, raltegravir (Cat. #11680) from Merck & Company, Inc., human rIL-2 from Dr. Maurice Gately, Hoffmann-La Roche Inc.,22 HIV-1NL4-3 from Dr. Malcolm Martin,23 MT-2 cells from Dr. Douglas Richman,24,25 and ACH-2 cells from Dr. Thomas Folks.7,26 HIV-1 NLENG1-IRES was a kind gift from Dr. David Levy.27 The VQA plasmid was a kind gift from Dr. Greg Laird and Dr. Robert Siliciano.
Generation of infected cultured TCM cells
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors following protocols outlined in IRB #67637 (University of Utah Institutional Review Board approved) or obtained from the Gulf Coast Regional Blood Center (Houston, TX). Naive cells were isolated and cultured TCM cells were generated and infected as previously described.13,20,28 Briefly, naive CD4 T cells were magnetically isolated from healthy donor blood samples using a commercial kit (either Miltenyi Biotec, Cat. #130-094-131 or Stemcell Technologies, Cat. #19155). Naive CD4 T cells were activated using human αCD3/αCD28-coated magnetic beads (one bead per cell, Life Technologies, Cat. #11131D) in the presence of human αIL-4 (2 μg/106, Peprotech, Cat. #500-p24), αIL-12 (4 μg/106, Peprotech, Cat. #500-p154g), and tumor growth factor (TGF)-β1 (0.8 μg/106, Peprotech, Cat. #100-21) for 3 days. After 3 days, cells were maintained at a concentration of 106 cells/ml in media containing 30 IU of human IL-2. HIV-1NL4-3 and HIV-1 NLENG1-IRES viruses were generated in HEK293FT cells using calcium phosphate transfection as previously described.13 To titrate virus stocks, SupT1 cells were infected by spinoculation using a concentration of 1–100 μl virus stock/2.5×106 cells/0.5 ml and centrifugated for 2 h at 37°C and 1620×g. For infection of cultured TCM cells with HIV-1NL4-3 or HIV-1 NLENG1-IRES, cells were infected by spinoculation at a multiplicity of infection (MOI) of 0.1 using a concentration of 106 cells/0.5 ml and centrifugated for 2 h at 37°C and 162×g. Prior to infection of cells with HIV-1NL4-3, cells were cultured in standard tissue culture flasks. Following infection of cells with HIV-1NL4-3, cells were cultured in either standard tissue culture flasks at a cell density of 106 cells/ml or in 96-well round bottom plates using a density of 105 cells/100 μl/well. Prior to infection of cells with HIV-1 NLENG1-IRES, cells were cultured in 96-well flat bottom plates using a density of 2×105 cells/200 μl/well. After spinoculation, NLENG1-IRES infected cells were cultured in this same condition.
Removal of productively infected cells using CD4-positive isolation
Magnetic isolation of CD4-positive cells was achieved using a Dynabeads CD4-positive isolation kit as described by the manufacturer (Life Technologies, Cat. #11551D) with the exception that 75 μl of the αCD4 magnetic bead suspension was added per 107 cells instead of 25 μl.
Viral reactivation
Then 1–3×105 cells were left untreated, stimulated with Dynabeads Human T-Activator CD3/CD28 (1 bead/cell, Life Technologies, Cat. #11132D), 100 nM bryostatin-1 (National Cancer Institute, CARE pharmacological core), ingenol 3,20-dibenzoate (Santa Cruz Biotechnology, CARE pharmacological core), 330 nM SAHA (Merck, CARE pharmacological core), or 10 μg/mL PAM3CSK4 (Invivogen), for 48 h.
Flow cytometry analysis
For analysis of HIV-1NL4-3-infected cells, samples were first stained with cell viability dye (Fixable Viability Dye eFluor 450, affymetrix, eBioscience, San Diego, CA) at 0.1 μl/1–3×105 cells for 15 min at 4°C and then stained, intracellularly, with a conjugated ICp24-FITC antibody (KC57, Coulter) as previously described.13 For the detection of surface CD4 expression, cells were stained with mouse antihuman CD4-APC (clone S3.5, Invitrogen). Flow cytometry was performed with a BD FacsCanto II flow cytometer using FACSDiva acquisition software (Becton Dickinson, Mountain View, CA). Data were analyzed with Flow Jo (TreeStar Inc, Ashland, OR).
Assay for infection of indicator cells
On day 17, 100-μl aliquots of cell culture supernatents were added to 400 μl of MT2 cells (2.5×105). MT-2 culture ART concentrations were matched with those of the innoculating cell culture supernatents. Cells were centrifuged for 2 h at 2,900 rpm at 37°C. Following spinoculation, MT-2 cells were cultured for an additional 48 h in 500 ml of fresh RPMI. ICp24 was measured by flow cytometry.
PCR analysis
Quantitative polymerase chain reaction (qPCR) for cell-associated and supernatant HIV-1 mRNA was carried out according to a recently published protocol.29 Briefly, cultured cells were counted and pelleted by centrifugation. Aliquots of 105 cells underwent RNA extraction and purification using a commercial viral RNA isolation kit according to the manufacturer's protocol (Zymo Research). DNase treatment was performed (Quanta Biosciences) followed by cDNA synthesis using qScript cDNA Supermix containing oligo(dT) primers and random hexamers according to the manufacturer's protocol (Quanta Biosciences). RNA aliquots that did not contain reverse transcriptase (no RT controls) were run in parallel for every sample. Real-time quantitative PCR was subsequently performed in triplicate on cDNA and RNA (no RT control) samples using TaqMan Universal Master Mix II (Applied Biosystems) on a Roche LC480 Real-Time PCR instrument. Primers and probe used were as follows: forward primer (5′ to 3′) CAGATCCTGCATATAAGCAGCTG, reverse primer (5′ to 3′) TTTTTTTTTTTTTTTTTTTTTTTTGAAGCAC, and probe (5′ to 3′) FAM-CCTGTACTGGGTCTCTCTGG-BHQ1. Cycling conditions were as follows: 50°C for 2 min followed by 95°C for 10 min for polymerase activation, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Serial 10-fold dilutions of a plasmid containing the HIV-1 3′-LTR (VQA plasmid; obtained from Greg Laird and Robert Siliciano) from 106 to 1 copy per well were amplified in triplicate along with unknowns in order to provide a standard curve and quantify cell-associated viral mRNA.
Results
Generation of latently infected cells in the context of a spreading HIV-1 infection and ART
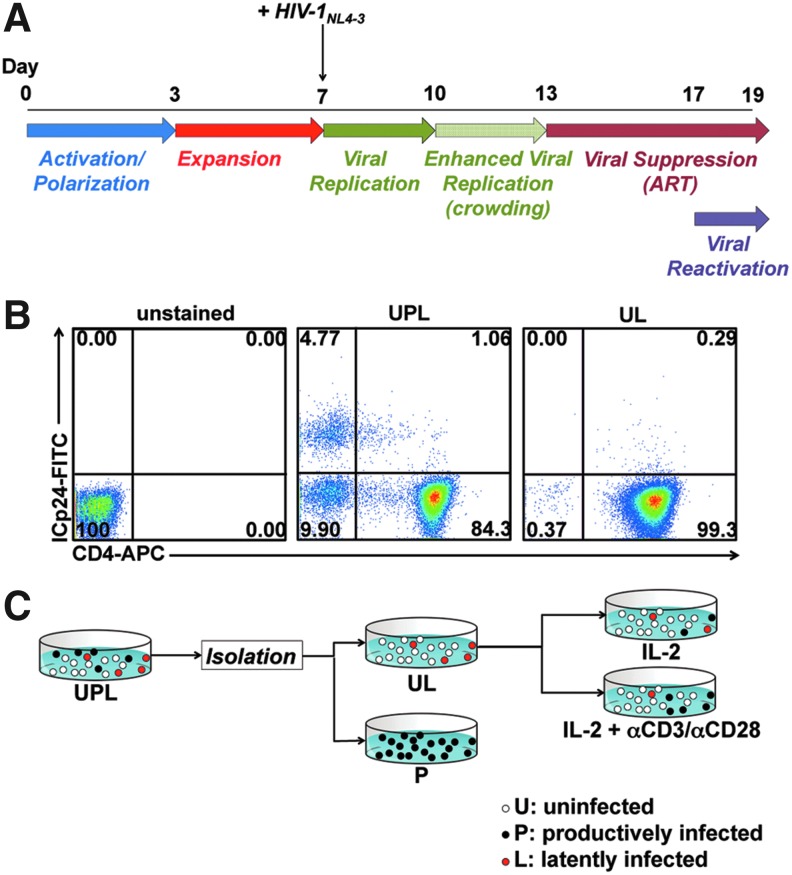
To generate relevant target cells to study viral latency, we utilized a previously described cell culture method to induce in vitro differentiation of TCM cells by activating peripheral blood naive CD4+ T cells under conditions that block polarization to Th1 or Th2 cells.13,20,28 These in vitro differentiated cells are phenotypically very similar to freshly isolated TCM cells and are referred to as cultured TCM cells.13 We then exposed cultured TCM cells to replication-competent HIV-1NL4-3, an X4-tropic virus that encodes a complete HIV-1 genome.23 Following inoculation of the culture with HIV-1NL4-3 at day 7 postisolation, viral spread in culture was allowed for 6 days. Because cell-to-cell transmission of HIV-1 is highly efficient in vitro,30 we incorporated a “cell crowding” step as described in Materials and Methods. In this method, cells are cultured in round-bottom wells, which allows them to cluster by gravity, in contrast to culture in flasks, in which cells are not confined to a small surface area.
ART was introduced to the cultures, starting at day 13, and maintained for the remainder of the experiment. ART consisted of either 1 μM raltegravir and 0.5 μM nelfinavir or 1 μM nelfinavir alone. The effectiveness of ART treatment to block viral spread was confirmed by exposing MT2 cells,31 used as indicators, to supernatants from the above cultures and verifying lack of infection (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid).
HIV-1NL4-3-infected cultures contained significant numbers of productively infected cells (i.e., ICp24+) on day 17 (Fig. 1B middle panel). We then wished to ascertain whether latently infected cells could be detected. To that end, we removed productively infected cells based on their ability to potently downregulate the CD4 receptor,32–34 as described by Davis et al.35 We, therefore, isolated cells expressing high levels of cell surface CD4, which would presumably contain both uninfected (“U”) and latently infected (“L”) cells (Fig. 1B). The magnetic beads were detached from these cells to prevent any interference with downstream analyses. This procedure rendered a purity of 98.5±1.5% of CD4+ICp24(−) cells (Fig. 1A shows data for a representative donor out of nine tested). Latently infected cells and uninfected cells constitute the positive fraction (“UL”) binding to the magnetic beads as they express high levels of CD4, whereas productively infected cells (“P”) express low levels of CD4 (Fig. 1B).
FIG. 1.
Generation of cultured TCM cells latently infected with HIV-1NL4-3. (A) Protocol used for the generation of latently infected cultured central memory T cell subset (TCM) cells using HIV-1NL4-3. From days 0 to 3, naive CD4 T cells were activated with αCD3/αCD28 beads in the presence of αIL-4, αIL-12, and tumor growth factor (TGF)-β1. From days 3 to 7, cultured TCM cells proliferated rapidly and were maintained from 1–3×106 cells/ml. On day 7, cells were infected with HIV-1NL4-3 and from days 7–10, cells were cultured in standard tissue culture flasks. From days 10 to 13, a crowded condition was imposed by culturing in U-bottom 96-well plates. From days 13 to 17, cells were cultured in standard tissue culture flasks in the presence of antiretroviral treatment (ART). (B) Cells from a single blood donor (Donor 5) were cultured and infected with HIV-1NL4-3 following Protocol B. On day 17, CD4+ cells were isolated using positive magnetic selection. Cells before isolation are denoted UPL and purified cells are denoted UL. Cells were stained with a cell viability dye followed by cell-surface staining with a CD4-APC antibody then stained intracellularly with a p24-FITC antibody. Dot plots of the viable fraction are shown. (C) Cultured TCM cells latently infected with HIV-1NL4-3 were generated as indicated in (A). On day 17 HIV-1-infected cells containing uninfected, productively infected, and latently infected cells (UPL) were subjected to magnetic isolation based on cell-surface CD4 expression. CD4+ cells contain uninfected and latently infected cells (UL) and CD4− cells contain productively infected cells (P). The UL fraction was treated with either interleukin (IL)-2 alone or IL-2+αCD3/αCD28 for 48 h.
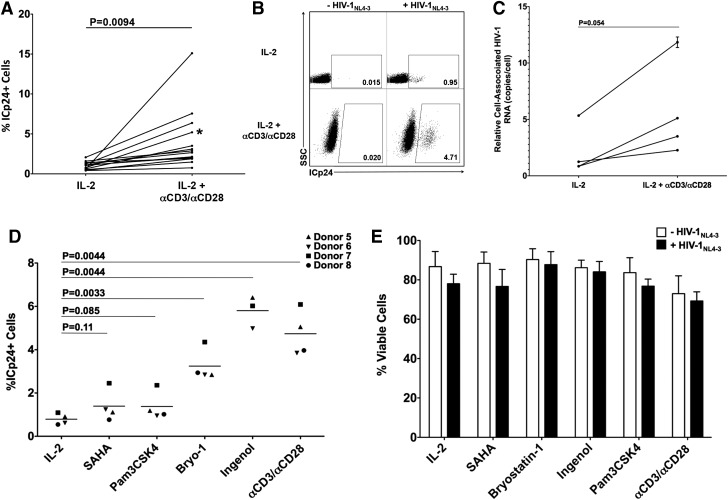
We then treated cells in the UL fraction with αCD3/αCD28 beads+IL-2 or with IL-2 alone (baseline) for 48 h in order to reactivate latent viruses that may be present in this population. After reactivation, cells were collected and analyzed for ICp24 expresion (Fig. 2A and B) and cell-associated HIV-1 RNA (Fig. 2C). Upon stimulation with αCD3/αCD28, ICp24+ cells consistently increased relative to IL-2 alone (Fig. 2A). The increase in the protein levels was concomitant with increased levels of cell-associated HIV-1 RNA (Fig. 2C). We also detected HIV-1 RNA from two culture supernatants and found 17.5-fold and 3.5-fold inceases in HIV-1 RNA upon stimulation with αCD3/αCD28 (Supplementary Fig. S2A). This indicates that viral reactivation leads to release of virions into the medium.
FIG. 2.
Reactivation of HIV-1 from latently infected cultured TCM cells. (A) Fourteen CD4+ purified samples were treated with IL-2 alone or IL-2+αCD3/αCD28 for 48 h. ICp24 was analyzed by flow cytometry. Significance was calculated using a two-tailed paired t-test analysis (p values provided). Asterisk indicates data corresponding to dot plot figures in (B). (B) Representative dot plots of IL-2 and IL-2+αCD3/αCD28-stimulated UL fractions. (C) Four CD4+ purified samples were treated with IL-2 alone or IL-2+αCD3/αCD28 for 48 h. CA HIV-1 RNA copies were measured by quantitative polymerase chain reaction (qPCR) in triplicate samples. Normalization of cell-associated HIV-1 RNA to a cellular RNA would not be feasible for the comparison of HIV-1 transcripts produced from quiescent cells to those generated from cells treated with a strong cell activation stimulus. We, therefore, report HIV-1 RNA values normalized to input cell number. Mean values are plotted and error bars denote standard deviations. (D) UL fractions were stimulated with 330 nM SAHA, 10 μg/ml PAM3CSK4, 100 nM bryostatin-1, 100 nM ingenol 3,20-dibenzoate and ICp24 and viability from Donor 5-7 (E) were measured using flow cytometry. Significance was calculated using a two-tailed paired t-test analysis (p values provided).
In a comparison of 16 independent infections we noted a strong linear correlation between the levels of productive and latent infections. The slope of the graph generated with these data (Supplementary Fig. S3) is 0.19, which means that in a typical experiment the ratio between productive and latent infection frequencies is 6-fold. Therefore, given a certain level of productive infection, the frequency of latent infection that will ensue in this model is predictable.
Viral reactivation with αCD3/αCD28 in the absence of ART led to viral spread in the culture (Supplementary Fig. S4). Levels of ICp24+ cells (Supplementary Fig. S4A) and cell-associated HIV-1 RNA (Supplementary Fig. S4B) increased after αCD3/αCD28 treatment with levels that were dramatically higher than those observed for samples cultured in ART.
To test the potential of this latency model to detect reactivation by other LRAs commonly used in the field, we assembled a small panel of LRAs that included SAHA,36 PAM3CSK4,37 bryostatin-1,38,39 and ingenol 3,20-dibenzoate40 and used them to stimulate UL fractions from three or four blood donor samples (Fig. 2D). We observed increased levels, although to varying degrees, of ICp24+ cells for all LRAs tested. Treatment with the PKC agonists bryostatin-1 and ingenol 3,20-dibenzoate resulted in strong reactivation of latent HIV-1 (62% and 127% average increases relative to αCD3/αCD28, respectively). However, treatment with PAM3CSK4 and SAHA resulted in weak stimulation (15% and 22% average increases relative to αCD3/αCD28, respectively). Therefore, the relative abilities of known LRAs to reactivate latent viruses generated through infection by a replication-competent virus closely resembled those previously reported in a system using env-defective virus.13,38
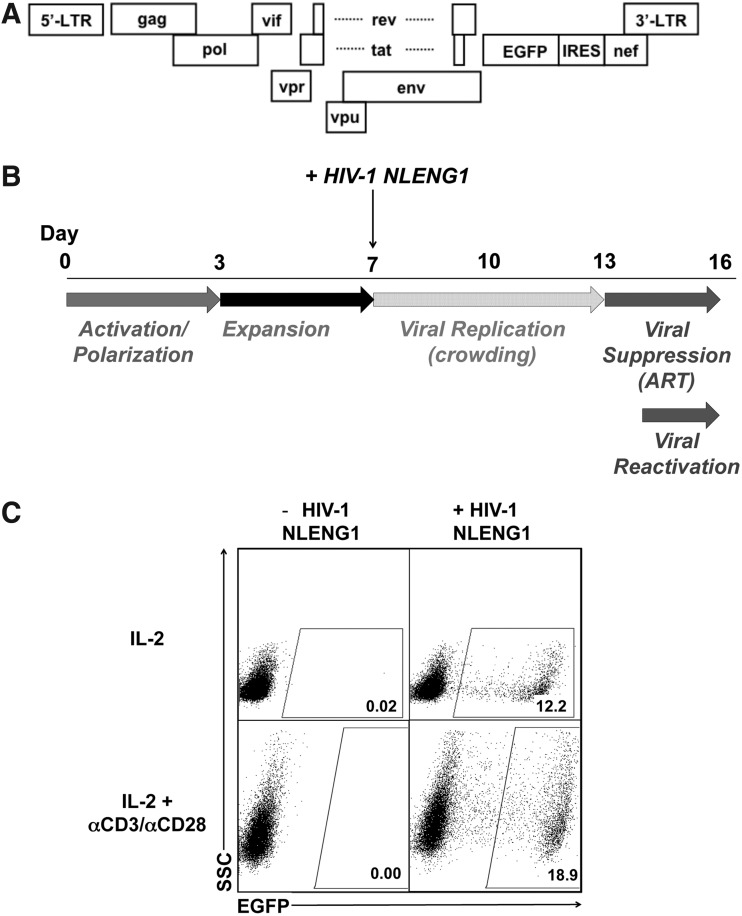
Establishment of viral latency by a reporter virus that is replication competent
To facilitate drug discovery efforts, for which higher cell numbers and more rapid assessment of latency reversal are desired, we modified the above protocol as follows. We used the HIV-1NL4-3-derived construct, HIV-1 NLENG1-IRES,27 in which the EGFP coding sequence followed by an IRES element was inserted between the env and nef genes (Fig. 3A). Cells infected with HIV-1 NLENG1-IRES were crowded for the entire 6-day viral spread period to compensate for the slower replication of this virus (Fig. 3B). On day 13, 2 μM raltegravir was added to cell cultures. On day 14, cells were stimulated with IL-2 or IL-2+αCD3/αCD28 beads for 48 h and EGFP expression was measured by flow cytometry as a measure of viral reactivation (Fig. 3C). Treatment with αCD3/αCD28 beads resulted in increased production of EGFP+ cells in all nine samples. PHA treatment resulted in higher levels of EGFP+ cells in approximately half of the donor samples tested (data not shown).
FIG. 3.
Generation of cultured TCM cells latently infected with HIV-1 NLENG1-IRES and reactivation of latent HIV-1. (A) Plasmid used for the generation of latently infected cells with an EGFP reporter. (B) Protocol for the generation of cultured TCM cells latently infected with HIV-1 NLENG1-IRES. (C) Cells were cultured and infected with HIV-1 NLENG1-IRES. On day 14, cultures were treated with IL-2 alone or IL-2+αCD3/αCD28 or IL-2+PHA. EGFP expression was measured using flow cytometry on day 16. Representative dot plots are shown for IL-2 and IL-2+αCD3/αCD28-treated cultures.
Discussion
We describe here a primary T cell in vitro model for studying HIV-1 latency using replication-competent virus whose spread in culture is suppressed by the addition of ART. In contrast to previous models used for the study of HIV-1 latency that employ single-round, pseudotyped viruses, this assay permits the use of full-length HIV-1. It likely that HIV-1 accessory genes influence the establishment of or the reactivation from latency. For example, we expect that upon reactivation, HIV-1 accessory genes will downregulate cell surface markers, such as CD4, tetherin, MHC I, NTBA, CCR7, and CD1d,32–34,41–45 and in this manner HIV-1 may hinder recognition by effector cells. Since it is now clear that reactivation of latent HIV-1 is not always followed by recognition by immune surveillance mechanisms,46 the actions of accessory proteins during the process of reactivation must be taken into consideration when testing for CTL and NK killing. For example, HIV-1-infected cells can escape CTL killing due, at least in part, to downregulation of MHC-1 by nef.47 Additionally, HIV-1-infected cells may also avoid killing by natural killer cells through active downregulation of NTBA by vpu.45 Therefore, the model we present here can be adapted for testing of immune effector mechanisms, with active participation of the accessory genes.
As previously reported,13 cells that are induced to differentiate in the TCM lineage express abundant CXCR4. Therefore, they should be infectable with any X4-tropic or dual-tropic HIV-1 strains. In contrast, CCR5 levels in these cells are extremely low. If a high level infection with an R5-tropic virus is desired, it would be preferable to induce a TH1 differentiation pathway (in lieu of TCM) as previously shown,13 which induces CCR5 expression.
To facilitate drug discovery efforts, we also describe the use of a replication-competent HIV-1 virus that expresses EGFP.27 Detection of HIV-1 reactivation with this virus does not require cell fixation or HIV-1 marker staining, but simply direct flow cytometric analysis. Therefore, the use of the HIV-1 NLENG1-IRES virus would be ideal for medium- or high-throughput screening in search of novel LRAs.
One disadvantage of using replication-competent viruses for the study of latency is that, as we report here, there is a background of productively infected cells. We show that this can be overcome by the removal of CD4(-) cells via magnetic bead isolation. The result is a population containing both uninfected and latently infected cells, which is largely devoid of productively infected ones. Therefore, studies aimed at documenting the presence or absence of transcription factors and coactivators at the HIV-1 LTR, as well as studies on the cellular transcription profiles of latently infected cells, can be undertaken with minimal contamination from productively infected cells.
The inability of any single in vitro model of HIV-1 latency to recapitulate all aspects of in vivo latency38 has spurred our efforts to develop a system that more closely resembles HIV-1 latency in vivo. To accomplish that, we introduced conditions that would allow the establishment of latency by a replication-competent virus whose replication is suppressed by the presence of ART. This configuration of the assay offers advantages, such as exclusion of productively infected cells, the presence of all viral genes, and cell-to-cell transmission of HIV-1. However, implementation of these features results in a more laborious assay with increased material costs. A second variation of the assay uses a virus encoding EGFP, which allows for a less laborious and less costly method. These methodologies represent valuable tools for preclinical discovery and characteriztion of novel LRAs.
Supplementary Material
Acknowledgments
This work was supported by NIAID Grants R21 AI106438-01 and R21 AI116212-01 to A.B. and R01 087508 to V.P. L.J.M. was supported by NIH 5UO1TW006671-10. P.B. is supported by the Agency for Innovation by Science and Technology in Flanders (IWT; Grant 111393) and L.V. is supported by the Research Foundation Flanders (FWO; Grant 1.8.020.09.N.00). This work was also supported by the Collaboratory of AIDS Researchers for Eradication (CARE; NIH Grant U19AI096113, V.P., Project Leader), by a European ERANET, Grant HIV-ERA/SBO-IWT (EURECA: Grant 130442), and by the Bill & Melinda Gates Foundation, Grant ID OPP1035848 to L.V.
We thank Dr. Greg Laird and Dr. Robert Siliciano for providing the VQA plasmid and Dr. David N. Levy for providing the HIV-1 NLENG1-IRES plasmid. We are greatful to assistance provided by James Marvin at the University of Utah Flow Cytometry Core facility. We thank the CARE Pharmacology Core Facility at the University of North Carolina at Chapel Hill for providing SAHA (Merck), Bryostatin-1 (National Cancer Institute), and ingenol 3,20-dibenzoate (Santa Cruz Biotechnology). We are grateful to Angela Presson for reviewing our statistical analyses.
Author Disclosure Statement
A.B. and V.P. are inventors on Patent Application US2010/0291067 describing a previously published HIV-1 latency model.
References
- 1.Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278(5341):1295–1300 [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Carruth L, Finzi D, et al. : Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997;387(6629):183–188 [DOI] [PubMed] [Google Scholar]
- 3.Wong JK, Hezareh M, Gunthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278(5341):1291–1295 [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Hill BJ, Ambrozak DR, et al. : T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: Implications for HIV pathogenesis. J Virol 2004;78(3):1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomont N, El-Far M, Ancuta P, et al. : HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009;15(8):893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG: HIV: Shock and kill. Nature 2012;487(7408):439–440 [DOI] [PubMed] [Google Scholar]
- 7.Folks TM, Clouse KA, Justement J, et al. : Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Sci Acad USA 1989;86(7):2365–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomerantz RJ, Trono D, Feinberg MB, and Baltimore D: Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: A molecular model for latency. Cell 1990;61(7):1271–1276 [DOI] [PubMed] [Google Scholar]
- 9.Antoni BA, Rabson AB, Kinter A, et al. : NF-kappa B-dependent and -independent pathways of HIV activation in a chronically infected T cell line. Virology 1994;202(2):684–694 [DOI] [PubMed] [Google Scholar]
- 10.Jordan A, Bisgrove D, and Verdin E: HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003;22(8):1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micheva-Viteva S, Kobayashi Y, Edelstein LC, et al. : High-throughput screening uncovers a compound that activates latent HIV-1 and acts cooperatively with a histone deacetylase (HDAC) inhibitor. J Biol Chem 2011;286(24):21083–21091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duverger A, Jones J, May J, et al. : Determinants of the establishment of human immunodeficiency virus type 1 latency. J Virol 2009;83(7):3078–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosque A. and Planelles V: Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood 2009;113(1):58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini A, Harper JM, and Romerio F: An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol 2008;181(11):7713–7720 [DOI] [PubMed] [Google Scholar]
- 15.Yang HC, Xing S, Shan L, et al. : Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest 2009;119(11):3473–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi M, Pearson RJ, and Karn J: Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol 2010;84(13):6425–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras X, Barboric M, Lenasi T, and Peterlin BM: HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Path 2007;3(10):1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swiggard WJ, Baytop C, Yu JJ, et al. : Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol 2005;79(22):14179–14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh S, Solomon A, Wightman F, et al. : CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: A novel model of HIV-1 latency. Blood 2007;110(13):4161–4164 [DOI] [PubMed] [Google Scholar]
- 20.Bosque A. and Planelles V: Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods 2011;53(1):54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonczkowski P, De Spiegelaere W, Bosque A, et al. : Replication competent virus as an important source of bias in HIV latency models utilizing single round viral constructs. Retrovirology 2014;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahm HW. and Stein S: Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr 1985;326:357–361 [DOI] [PubMed] [Google Scholar]
- 23.Adachi A, Gendelman HE, Koenig S, et al. : Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 1986;59(2):284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada S, Koyanagi Y, and Yamamoto N: Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 1985;229(4713):563–566 [DOI] [PubMed] [Google Scholar]
- 25.Haertle T, Carrera CJ, Wasson DB, et al. : Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2',3'-dideoxyadenosine derivatives. J Biol Chem 1988;263(12):5870–5875 [PubMed] [Google Scholar]
- 26.Clouse KA, Powell D, Washington I, et al. : Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 1989;142(2):431–438 [PubMed] [Google Scholar]
- 27.Trinite B, Chan CN, Lee CS, et al. : Suppression of Foxo1 activity and down-modulation of CD62L (L-selectin) in HIV-1 infected resting CD4 T cells. PloS One 2014;9(10):e110719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messi M, Giacchetto I, Nagata K, et al. : Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol 2003;4(1):78–86 [DOI] [PubMed] [Google Scholar]
- 29.Shan L, Rabi SA, Laird GM, et al. : A novel PCR assay for quantification of HIV-1 RNA. J Virol 2013;87(11):6521–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dale BM, Alvarez RA, and Chen BK: Mechanisms of enhanced HIV spread through T-cell virological synapses. Immunol Rev 2013;251(1):113–124 [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi I, Kubonishi I, Yoshimoto S, and Shiraishi Y: A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gan 1981;72(6):978–981 [PubMed] [Google Scholar]
- 32.Lama J: The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr HIV Res 2003;1(2):167–184 [DOI] [PubMed] [Google Scholar]
- 33.Levesque K, Finzi A, Binette J, and Cohen EA: Role of CD4 receptor down-regulation during HIV-1 infection. Curr HIV Res 2004;2(1):51–59 [DOI] [PubMed] [Google Scholar]
- 34.Lindwasser OW, Chaudhuri R, and Bonifacino JS: Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr Mol Med 2007;7(2):171–184 [DOI] [PubMed] [Google Scholar]
- 35.Davis ZB, Ward JP, and Barker E: Preparation and use of HIV-1 infected primary CD4+ T-cells as target cells in natural killer cell cytotoxic assays. J Visual Exp: JoVE 2011(49):2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archin NM, Liberty AL, Kashuba AD, et al. : Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012;487(7408):482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novis CL, Archin NM, Buzon MJ, et al. : Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology 2013;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spina CA, Anderson J, Archin NM, et al. : An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Path 2013;9(12):e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehla R, Bivalkar-Mehla S, Zhang R, et al. : Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PloS One 2010;5(6):e11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandelo Jose D, Bartholomeeusen K, da Cunha RD, et al. : Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology 2014;462–463:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neil SJ, Zang T, and Bieniasz PD: Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008;451(7177):425–430 [DOI] [PubMed] [Google Scholar]
- 42.Van Damme N, Goff D, Katsura C, et al. : The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008;3(4):245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramirez PW, Famiglietti M, Sowrirajan B, et al. : Downmodulation of CCR7 by HIV-1 Vpu results in impaired migration and chemotactic signaling within CD4(+) T cells. Cell Rep 2014;7(6):2019–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy N, Pacini G, Berlioz-Torrent C, and Janvier K: Mechanisms underlying HIV-1 Vpu-mediated viral egress. Front Microbiol 2014;5:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah AH, Sowrirajan B, Davis ZB, et al. : Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 2010;8(5):397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36(3):491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins KL, Chen BK, Kalams SA, et al. : HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 1998;391(6665):397–401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.