Abstract
Dysfunction of the endothelial lining of lesion-prone areas of the arterial vasculature is an important contributor to the pathobiology of atherosclerotic cardiovascular disease. Endothelial cell dysfunction (ECD), in its broadest sense, encompasses a constellation of various non-adaptive alterations in functional phenotype, which have important implications for the regulation of hemostasis and thrombosis, local vascular tone and redox balance, and the orchestration of acute and chronic inflammatory reactions within the arterial wall. In this review, we trace the evolution of the concept of endothelial cell dysfunction, focusing on recent insights into the cellular and molecular mechanisms that underlie its pivotal roles in atherosclerotic lesion initiation and progression; explore its relationship to classic, as well as more recently defined, clinical risk factors for atherosclerotic cardiovascular disease; consider current approaches to the clinical assessment of endothelial cell dysfunction; and outline some promising new directions for its early detection and treatment.
Keywords: Atherosclerosis, Endothelial Cell Dysfunction, Vascular Homeostasis
Subject Terms: Vascular Disease, Atherosclerosis, Endothelium/Vascular Type/Nitric Oide, Pathophysiology
Introduction and Overview
Homeostasis in the cardiovascular system is maintained by a hierarchy of interacting genetic and epigenetic programs, the disequilibrium of which contributes to the pathogenesis of complex disease processes such as atherosclerosis and its life-threatening complications, myocardial infarction and stroke. Vascular endothelium, the continuous cellular lining of the cardiovascular system, “Nature’s container for blood”1, is an important locus of critical regulatory nodes in this homeostatic network. In health, this distributed organ supports the normal functioning of the various tissues and organs of the body; in disease, its dysfunction within the walls of large arteries is an important contributor to the local and systemic manifestations of atherosclerotic cardiovascular disease (ACVD)2.
Endothelial cell dysfunction (ECD) [Dialogue Box], manifested in lesion-prone areas of the arterial vasculature, results in the earliest detectable changes in the life history of an atherosclerotic lesion3, 4 — the focal permeation, trapping and physicochemical modification of circulating lipoprotein particles in the sub-endothelial space5. This sets into motion a complex pathogenic sequence4–11, initially involving the selective recruitment of circulating monocytes from the blood into the intima, where they differentiate into macrophages and internalize modified lipoproteins to become foam cells (the hallmark of early fatty streak lesions); multiple chemokines and growth factors elaborated by activated endothelium and macrophages then act on neighboring smooth muscle cells (or their precursors12) to induce their proliferation and synthesis of extracellular matrix components within the intimal compartment, thus generating a fibromuscular plaque. Progressive structural remodeling of developing lesions results in the formation of a fibrous cap, overlying a lipid-rich, necrotic core consisting of oxidized lipoproteins, cholesterol crystals and cellular debris, and is accompanied by varying degrees of matrix remodeling and calcification. The lateral edges of these complicated plaques contain a rich population of inflammatory cells (activated macrophages and T-cells, natural killer T-cells, dendritic cells), which further modulate the endothelial proinflammatory phenotype, and contribute to structural instability of the plaque through the proteolytic modification of its extracellular matrix components13–15. In unstable or vulnerable plaques, this may result in a catastrophic transition in the life history of an atherosclerotic lesion—frank plaque rupture, with luminal release of the highly thrombogenic contents of the necrotic core, triggering an atherothrombotic occlusion16–19. Alternatively, significant clinical sequelae also can result from superficial intimal erosions, without evidence of plaque rupture19. The latter critical transition appears to be a consequence of endothelial cell apoptosis, with localized endothelial denudation and the triggering of thrombus formation20. These superficial erosions typically occur on the surface of lesions containing abundant smooth muscle cells and proteoglycans, but few macrophages, and characteristically are associated with regions of disturbed blood flow. Other stable lesions, characterized by a thick fibrous cap, and less lipid and inflammatory cell content, can progressively encroach on the vessel lumen causing ischemic symptoms, but typically do not precipitate atherothrombotic events. In a given individual, multiple atherosclerotic plaques can co-exist—each at its own stage of pathobiologic evolution17, 19.
Dialogue Box: Endothelial Cell Dysfunction (ECD).
In the lexicon of modern Cardiovascular Medicine, the term “endothelial dysfunction” typically is used to refer to abnormalities in the production or bioavailability of endothelial-derived nitric oxide and resultant deleterious changes in vascular reactivity. For the purpose of this Compendium on Atherosclerosis, we use the term “endothelial cell dysfunction (ECD)” to encompass all of the maladaptive changes (multifactorial, localized and systemic, acute and chronic) in the functional phenotype of endothelial cells, which are associated with atherosclerotic cardiovascular disease.
Given its multiple, mechanistically important roles throughout this complex series of events, endothelial cell dysfunction thus appears to constitute a pathogenic sine qua non for atherosclerotic cardiovascular disease. In this review, we will trace the evolution of the concept of endothelial cell dysfunction, focusing on recent insights into the cellular and molecular mechanisms that underlie its pivotal roles in atherosclerotic lesion formation and progression; explore its relationship to classic, as well as more recently defined, clinical risk factors for ACVD; consider approaches to the detection of ECD; and outline some promising new directions for its treatment.
Understanding Endothelial Cell Dysfunction
The evolution of our understanding of the vital functions of the vascular endothelium and the various manifestations of its dysfunction in the context of ACVD resembles the parable of “The Blind Men and the Elephant”21. Probed with the tools of classic cell biology and physiology, the endothelium initially was characterized as a vast, selectively permeable interface22, separating the vascular and interstitial compartments of the body and serving as a gateman, regulating the transport of fluid and macromolecules via an elaborate system of transcellular vesicles and intercellular junctional complexes23–26. Localized loss of this selective barrier function (manifested as edema), coupled with the emigration of white blood cells (pus formation), have been recognized since antiquity as cardinal signs of inflammation-- the body’s primal reaction to tissue injury or infection27. Examined from the perspective of classic biochemistry and pharmacology28, and aided by the development of reliable methods for the in vitro culture of endothelial cells29, a diverse repertoire of metabolic functions of the endothelium became apparent--- including the biosynthesis and degradation of vasoactive mediators, the enzymatic buffering of reactive oxygen species, the transport and metabolism of lipoproteins, the secretion and enzymatic remodeling of extracellular matrix components, the elaboration of various growth factors, cytokines and hormone-like substances, and the biosynthesis of prostaglandins (in particular prostacyclin) and other potent autocoids30. From the perspective of hemostasis and thrombosis, the intact, normally functioning endothelial lining provides an ideal container for blood; its luminal surface does not activate the intrinsic coagulation cascade or promote platelet adhesion, and actually exhibits anticoagulant and fibrinolytic properties31. Examined from a bioengineering perspective, the individual endothelial cells comprising the lining of various parts of the cardiovascular system are seen to function as local biomechanical transducers, sensing and transducing the diverse forces imparted by the pulsatile flow of blood into biological responses32, 33. Finally, when challenged with certain pro-inflammatory cytokines or bacterial products (e.g., endotoxins), endothelial cells undergo a coordinated program of gene activation, which (reversibly) alters many of these vital functional properties, presumably serving as an adaptive response to potentially noxious stimuli30. Thus, when viewed from these different perspectives, the vascular endothelium can be variously characterized as a distributed organ, a dynamically adaptable interface, and, at the individual cell level, an integrator of the local pathophysiological milieu. And, dysfunction of the endothelium, in the broadest sense, would encompass various non-adaptive alterations in its normal functional phenotype, with important implications for the regulation of hemostasis and thrombosis31, local vascular tone30 and redox balance34, and the orchestration of acute and chronic inflammation30.
While the term “endothelial dysfunction” has clearly found its place in the lexicon of modern Cardiovascular Medicine35–37, the evolution of this working concept has a rich history dating from the early practice of Anatomical Pathology, in the 1850’s, and continuing through the development of the modern field of Vascular Cell Biology, in the latter half of the 20th century. This conceptual evolution has contributed in important ways to our present day understanding of the cellular and molecular mechanisms of atherosclerotic cardiovascular disease. Its origins can be traced to the writings of Rudolph Virchow38, who called attention to the localized accumulation of circulating lipids and other macromolecular components of plasma at sites of early lesion formation (lipid insudation), which was detectable at autopsy and presumably reflected a localized change in endothelial permeability in life. Subsequently, the focal, non-random distribution of this permeability change was graphically illustrated by the mapping of blue and white areas, en face, in the aortas of experimental animals following injection of the plasma protein-binding azo dye Evans Blue39, 40. Blue areas were also associated with an increased rate of endothelial cell turn-over in normal animals 40, suggesting that they represented areas of chronic intimal injury, which, in animals made hypercholesterolemic by dietary alterations, were correlated with developing atherosclerotic lesions41. When examined ultrastructurally at the pre-lesional stage, these lesion-prone areas exhibited alterations in endothelial morphology, striking changes in the amount and composition of extracellular matrix, and the accumulation of extracellular liposomes, derived from plasma lipoproteins which had become trapped in the subendothelial space and undergone complex biochemical and physicochemical alterations5, 42. While these detailed morphologic descriptions of early intimal changes strongly supported the notion of localized dysfunction of the endothelial lining, the pathophysiological stimuli, underlying mechanisms and consequences for atherogenesis remained to be established.
Early characterizations of endothelial cell dysfunction, in the context of atherogenesis, focused on a loss of anatomical integrity of the intima, as exemplified by the seminal “Response-to-Injury Hypothesis” of Ross and Glomset7, which stimulated much fruitful activity in the field. As originally formulated, the Response-to-Injury Hypothesis postulated that the initiating event in the atherogenic process was some form of overt injury to the intimal endothelial lining, induced by noxious substances (e.g., oxidized cholesterol, constituents of cigarette smoke, hyperhomocystemia, hyperglycemia, etc.) or altered hemodynamic forces (e.g., blood flow disturbances generated by hypertension). In particular, focal endothelial desquamation was envisioned as an inciting stimulus for platelet adhesion and the localized release of platelet-derived growth factors, which then would elicit the migration, proliferation and phenotypic modulation of medial smooth muscle cells, thus generating a fibromuscular plaque6. The experimental demonstration that this sequence of events could be induced simply by balloon catheter denudation of the intima lent support to this hypothesis43, and catalyzed an abundance of studies of the potential roles of growth factors and injury-repair mechanisms in the atherogenic process. However, the direct relevance of this form of endothelial injury to the development of natural atherosclerosis was unclear, given that careful morphologic examination of the earliest fatty streak lesions in diet-induced animal models failed to demonstrate overt intimal injury or platelet adhesion44, 45. With the expanded awareness of the repertoire of vital functions of endothelial cells and their capacity for dynamic phenotypic modulation, the term “endothelial (cell) dysfunction” was introduced into the mainstream of atherosclerosis research46, 47. Various pathophysiologic stimuli of endothelial cell dysfunction (TABLE 1) soon became the focus of fruitful investigation by multiple groups, and the molecular manifestations of ECD began to be characterized in detail11, 15, 19, 30, 35.
TABLE 1.
Pathophysiological Stimuli of Endothelial Cell Dysfunction in Atherogenesis
|
Nitric Oxide and Endothelial Cell Dysfunction
A major conceptual advance in the field of Vascular Cell Biology was the demonstration that the endothelial lining is a source of various autocoid factors that can influence the behavior of other types of cells within the vessel wall (pericytes, smooth muscle) and in the circulating blood (platelets and leukocytes). Using cultured vascular cells, as well as bioassays with isolated organs and vascular strips, several endothelial-derived autocrine and paracrine mediators were identified48–53. In particular, endothelial-derived prostacyclin and platelet-derived thromboxane A2 were characterized as mutually antagonistic components of a dynamic “hemostatic/thrombotic balance” at the vessel-blood interface30, 31, and thus as potential targets for therapeutic modulation. In fact, pharmacologic manipulation of this aspect of arachidonic acid metabolism remains an important clinical strategy in the treatment and prevention of ACVD52. Collectively, these studies documented an active role of the vascular endothelium in the maintenance of blood fluidity and the regulation of vascular tone, and provided the experimental tools and conceptual framework to further define the roles of endothelium in cardiovascular physiology and pathophysiology.
In 1980, Furchgott and Zawadzki demonstrated that vasodilation induced by acetylcholine was dependent on the presence of an intact endothelium and appeared to be mediated by a potent humoral factor54, later known as endothelium-derived relaxing factor (EDRF). Several studies documented that EDRF was a labile substance with a half-life of only seconds in oxygenated physiological media55. Release of EDRF from blood vessels was observed under basal conditions, as well as after stimulation with acetylcholine56. The biological effects of EDRF were shown to be inhibited by hemoglobin57, and to be mediated by stimulation of a soluble guanylate cyclase with the consequent elevation of intracellular cyclic GMP levels in vascular smooth muscle cells58. Based on the similarities in the pharmacological properties of EDRF and NO, EDRF subsequently was identified as NO or a labile nitroso species59, 60. A detailed comparison of the biological actions of EDRF and NO on vascular smooth muscle strips61 and platelets62 also showed that EDRF and NO were indistinguishable. In 1988, L-Arginine was shown to be the precursor for the synthesis of NO by vascular endothelial cells63.
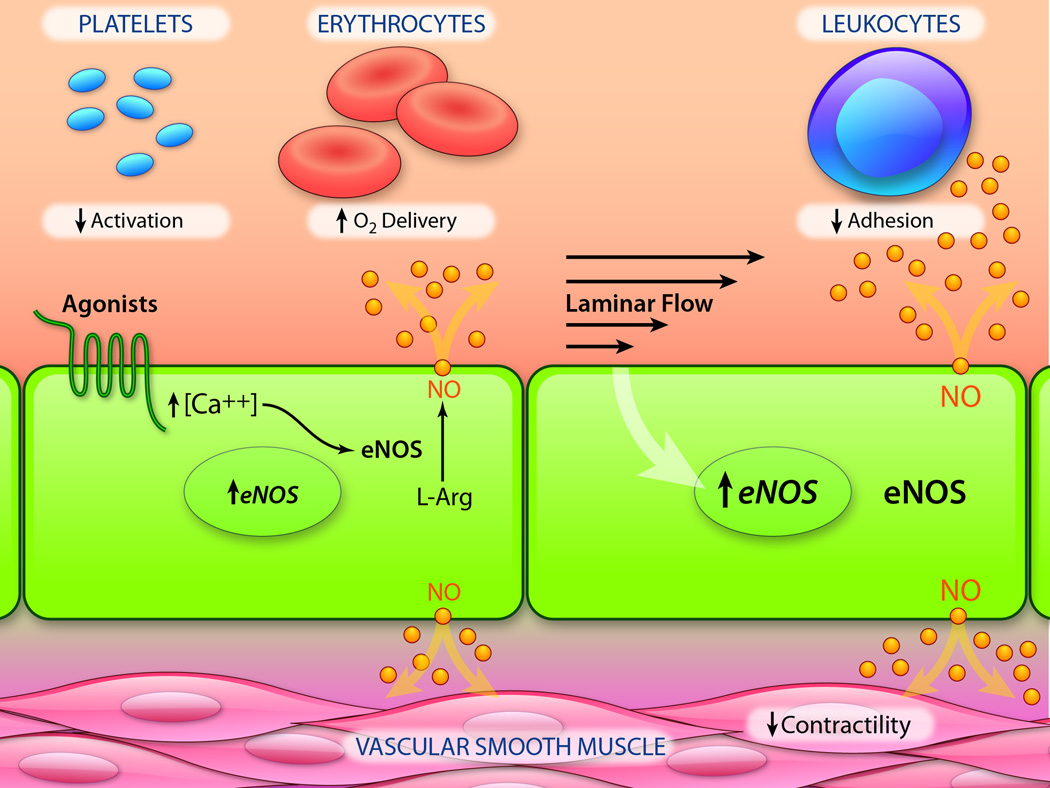
Endothelial cells metabolize L-arginine via the endothelial isoform of nitric oxide synthase (eNOS)64, 65 to form NO, with L-citrulline as a byproduct [Figure 1]. This overall process is subject to both transcriptional and complex post-translational regulation66, 67. A basal level production of NO by endothelial cells contributes to regulating vasomotor tone and preserving the non-thrombogenic behavior of the vascular lining. The synthesis of NO can be stimulated by receptor-dependent agonists (acetylcholine, bradykinin), non-receptor-dependent agonists (calcium ionophores), and also fluctuations in blood flow66, 68. Additionally, transcription of the eNOS gene is differentially regulated by fluid mechanical forces, such that endothelial cells in arterial geometries exposed to undisturbed laminar flow exhibit enhanced NO-forming capacity2, 33. Once NO is produced by eNOS, it can rapidly diffuse across cell membranes to act as a potent paracrine mediator, but it can also react with superoxide to form peroxynitrite anion, which leads to its inactivation. Particularly relevant for vascular homeostasis are the actions of NO on adjacent smooth muscle cells and circulating blood platelets and leukocytes. Many of these physiological actions are mediated through activation of soluble guanylate cyclase (sGC), which catalyzes the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). Other actions of NO are mediated by S-nitrosylation, the addition of an NO group to a Cys thiol to form an S-nitrosoprotein69. S-nitrosylation has been shown to modify the function of proteins in a reversible manner, which is analogous to phosphorylation70. In the cardiovascular system, S-nitrosylation of various target proteins has been demonstrated to modulate important cellular physiological processes including cell proliferation60, apoptosis71, 72, exocytosis73, and ion channel activity74, as well as blood flow and systemic oxygen delivery75, 76.
Figure 1. Endothelial-derived Nitric Oxide: Production and Biological Actions.
Endothelial cells rapidly produce nitric oxide (NO) via a unique isoform of nitric oxide synthase (eNOS) in response to agonists (e.g., acetylcholine, bradykinin) and fluctuations in blood flow. Once generated, NO rapidly diffuses through the endothelial plasma membrane to activate guanylate cyclase in several cell types present in the blood (platelets, leukocytes) and also within the vessel wall (smooth muscle). Activation of guanylate cyclase in platelets results in inhibition of activation, adhesion and aggregation; in leukocytes, decreased adhesivity; in smooth muscle cells, dephosphorylation of myosin light chain and vasorelaxation. NO also reacts with hemoglobin in erythrocytes, enhancing oxygen delivery to tissues. Chronic exposure of endothelial cells to laminar flow results in transcriptional upregulation of eNOS, thus increasing their NO-forming capacity. (Illustration Credit: Ben Smith)
The importance of endothelial-derived NO in the regulation of systemic blood pressure was first demonstrated in animal models, and then in humans, by using L-arginine-based competitive inhibitors. These inhibitors increase vascular resistance and blood pressure, and decrease blood flow77. Subsequent studies using genetically modified mice documented that eNOS-deficient mice are hypertensive, and that aortic rings isolated from these mice respond to acetylcholine with paradoxical vasoconstriction, but do relax in response to sodium nitroprusside or papaverine78, 79. Furthermore, these eNOS-deficient mice were found to display increased endothelial-leukocyte interactions, platelet aggregation and thrombosis80, 81, and, when crossed with ApoE-deficient (hypercholersterolemic) mice, developed accelerated atherosclerosis82.
In a classic study, the clinical significance of EDRF for human atherosclerotic cardiovascular disease was first demonstrated by Ganz and colleagues, using coronary angiography83. The coronary arteries of individuals with advanced atherosclerotic disease showed a dose-dependent, paradoxical vasoconstriction in response to graded concentrations of acetylcholine, in contrast to the vasodilation observed in individuals with angiographically normal coronary arteries. Importantly, coronary vessels with minimal disease also constricted in response to acetylcholine, while coronary vessels from all three patient groups dilated in response to nitroglycerin. Hypercholesterolemia-dependent alterations in endothelium-dependent vasodilation were also documented in animal models of atherosclerosis84–86. Taken together, these observations suggested that a deficiency in endothelial NO production or its bioavailability, in both humans and animals, might actually precede the formation of clinically significant atherosclerotic lesions. Impairment of flow-mediated vasodilation, measured non-invasively in peripheral vessels such as the brachial artery, appears to correlate with direct measurements of altered vasoreactivity in the coronary circulation87. Peripheral manifestations of endothelial dysfunction have also been shown to be an independent predictor of ACVD events36. This has stimulated the development and application of various FDA-approved devices to measure flow-mediated arterial dilation as a clinically useful index in the assessment of cardiovascular disease. However, as considered in greater detail below, the pathogenesis of endothelial cell dysfunction and its pathophysiological consequences extend beyond endothelial nitric oxide metabolism.
Endothelial Proinflammatory Activation and Endothelial Cell Dysfunction in Atherogenesis
The involvement of the endothelial lining of small blood vessels (e.g., post-capillary venules) in acute inflammatory responses, induced by injury or infection, has long been appreciated25, 26. Indeed, the cardinal signs of inflammation (rubor, calor, tumor, dolor) elicited by the action of histamine and other phlogistic agents can be mechanistically related to their effects on microvascular tone, permeability and leukocyte diapedesis. These acute responses, which have been referred to as “endothelial stimulation”, or “Type I Activation”88, are rapid in onset, self-limited in nature, and typically do not result in sustained morphological or functional changes. In contrast, endothelial cells can undergo a dramatic modulation in their functional phenotype in response to certain bacterial products, such as Gram-negative endotoxins, and other pathogen-associated molecular patterns (PAMPs), modified lipoproteins, and other damage-associated molecular patterns (DAMPs), or cytokines, such as Interleukin-1 (IL-1), Tumor Necrosis Factor (TNF) and Interferon-gamma89. The hallmark of this form of endothelial response, termed “Type II Activation” 88–90, is the activation of pleiotropic transcription factors, such as Nuclear factor-kappa-B (NFκB), resulting in the expression of various effector proteins with important pathophysiologic implications. Originally described as “endothelial activation antigens”91, detectable on the surface of cultured human endothelial cells in vitro following their incubation with cytokines or endotoxin, these effector proteins include Class-II major histocompatibility antigens, involved in antigen presentation; inducible endothelial-leukocyte adhesion molecules, such as ELAM-1 (E-selectin) and VCAM-1; procoagulant molecules, such as Tissue Factor, and secreted chemokines, such as Interleukin-8 (IL-8) and Monocyte Chemotractant Protein-1 (MCP-1)92–95. Together this activation program confers a localized, temporally coordinated, “proinflammatory endothelial phenotype” [Figure 2], which is detectable in vivo, at sites of inflammation, in the microvasculature of humans and experimental animals96, 97, and has become a keystone in our understanding of the active role that endothelial cells play in acute and chronic inflammatory responses, and related disease processes30.
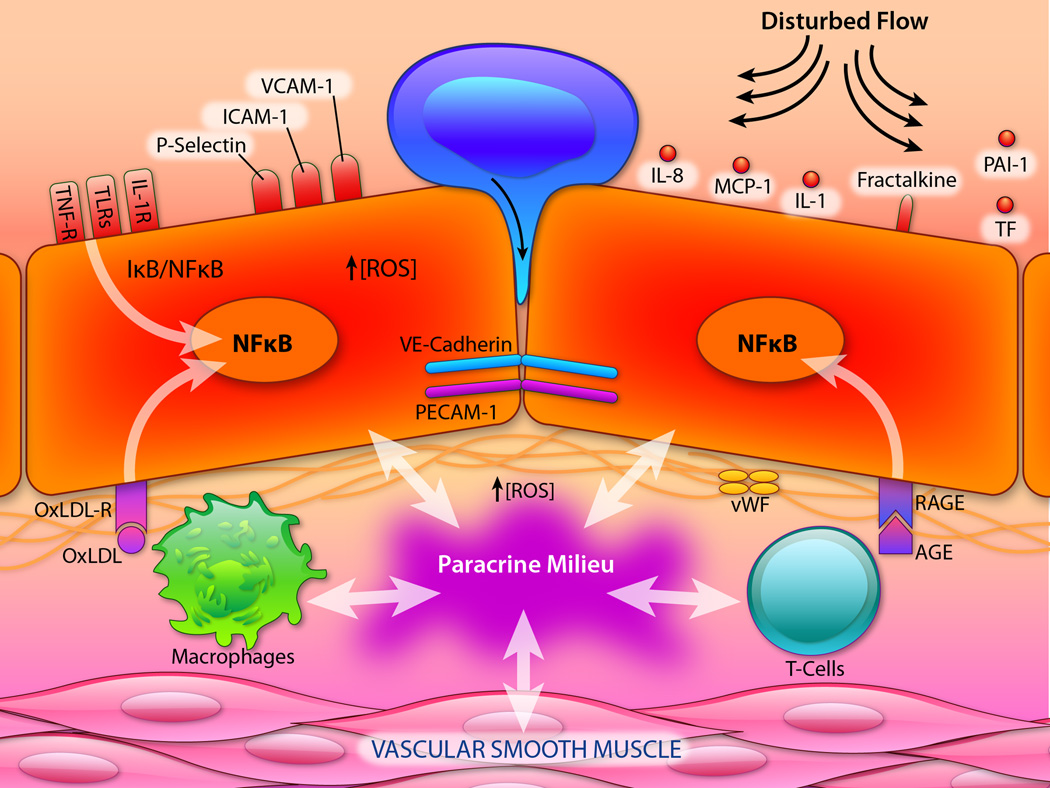
Figure 2. Endothelial Pro-Inflammatory Activation.
In lesion-prone regions of the arterial vasculature, the actions of pro-inflammatory agonists (e.g., IL-1, TNF, endotoxin), oxidized lipoproteins (oxLDL) and advanced glycation end products (AGE), as well as biomechanical stimulation by disturbed blood flow, leads to endothelial activation. These biochemical and biomechanical stimuli signal predominantly via the pleiotropic transcription factor NFκB, resulting in a coordinated program of genetic regulation within the endothelial cell. This includes the cell surface expression of adhesion molecules (e.g., VCAM-1), secreted and membrane-associated chemokines (e.g., MCP-1, Fractalkine), and pro-thrombotic mediators (e.g., TF, vWF, PAI-1). These events foster the selective recruitment of monocytes and various types of T-lymphocytes, which become resident in the subendothelial space. The concerted actions of activated endothelial cells, smooth muscle cells, monocyte/macrophages and lymphocytes result in the production of a complex paracrine milieu of cytokines, growth factors and reactive oxygen species (ROS) within the vessel wall, which perpetuates a chronic pro-inflammatory state and fosters atherosclerotic lesion progression. (vWF, vonWillebrand Factor; PAI-1, Plasminogen Activator Inhibitor-1; RAGE, Receptor for AGE; OxLDL-R, Receptor for Oxidized LDL; IL-R, TNF-R, Receptor(s) for IL-1, TNF; and TLRs, Toll-like Receptors). (Illustration Credit: Ben Smith).
A critical link between the activation program manifested by microvascular endothelium during inflammatory reactions and arterial endothelial cell dysfunction in atherogenesis came with the discovery of the Athero-ELAM (VCAM-1)98. Initially characterized as a cytokine-inducible activation antigen in cultured endothelial cells which exhibited a sustained pattern of expression (in contrast to “acute ELAMs” such as E-selectin), VCAM-1 was found to have selective adhesivity for mononuclear leukocytes and lymphocytes, via their expression of its counterreceptor VLA-499–101. Importantly, its expression in vivo, in human coronary atherosclerotic plaques and in both dietary and genetic animal models of hypercholesterolemia is localized to the intact endothelium overlying atherosclerotic lesions, and actually precedes the earliest recruitment of mononuclear leukocytes to nascent lesions102–105. Further studies implicated components of oxidized lipoproteins, such as lysophosphatidylcholine, as potent stimuli for its expression, thus mechanistically linking VCAM-1 induction to the atherogenic process106. Additionally, its spatial pattern of expression in vivo in various animal models of atherosclerosis is localized to those regions of the arterial vasculature that are most susceptible to lesion formation103, 104, and the detection of its soluble isoform in circulating plasma is correlated clinically with disease severity (lesion burden) in patients with atherosclerosis107. Finally, its genetic deficiency in mouse models of atherosclerosis results in a reduction in lesion formation108. These findings thus highlighted the pathogenic significance of VCAM-1 in the context of atherogenesis, and also implicated it as a molecular marker of endothelial cell dysfunction in the clinical setting35.
A second important link between atherogenesis and proinflammatory endothelial activation is the intrinsic capacity of activated vascular cells (endothelium, smooth muscle) to secrete chemokines, such as IL-1, MCP-1, granulocyte-monocyte stimulating factor (GM-CSF) and IL-8, thus generating localized, intercellular autocrine and paracrine signaling loops within the vessel wall53, 90. Coupled with the influx of various classes of T-lymphocytes and monocytes/macrophages109, each contributing their particular repertoire of cytokines (in part triggered by the recognition of antigens on native and oxidized lipoproteins), a complex immuno-regulatory network is thus established within the developing atherosclerotic lesion, which has both local and systemic consequencies15, 110–112. Locally, the balance of pro- and anti-inflammatory mediators (e.g., IL-1; IL-11), as well as agents that promote the resolution of inflammation (e.g., Resolvins)113 can contribute to lesion progression or regression, while systemic manifestations, such as the induction of acute-phase reactants (e.g., C-reactive protein) by endothelial-derived Interleukin-6 (IL-6), can serve as useful diagnostic and prognostic indices114.
It is noteworthy that NFκB appears to play a central role in the proinflammatory activation of endothelium in atherogenesis. Various of the pathophysiologic stimuli of ECD have been implicated in NFκB signaling in arterial endothelium115; expression of many of the effector molecules associated with ECD in atherogenesis (e.g., VCAM-1, MCP-1) are under NFκB control; and certain flow-sensitive endothelial genes (e.g., platelet-derived growth factor-B) have non-canonical NFκB binding sites (“shear-stress-response elements”) in their promoters116. Interestingly, in atherosclerosis-prone arterial geometries, endothelial NFκB appears to be primed for enhanced activation in response to systemic stimuli [Figure 2], such as bacterial endotoxin or hypercholesterolemia117. Recent studies suggest that NFκB activation may also result in fundamental changes in endothelial chromatin structure, through the formation of super-enhancer complexes, which can confer an epigenetic level of regulation to the proinflammatory endothelial phenotype in atherogenesis118.
This expanded appreciation of the roles of inflammatory and immuno-regulatory mediators and cellular processes in atherogenesis has led to a reformulation of the original “Response-to-Injury Hypothesis” into what is now termed the “Inflammatory Hypothesis of Atherothrombosis”11, 13–15. In addition to providing a unifying view of the pathophysiologic mechanisms involved in lesion initiation, progression and critical transitions, which are linked to many of the known risk factors for ACVD, the basic premise that atherosclerosis is a chronic inflammatory disease has important and timely clinical implications. These include the recent revision of the traditional clinical risk factors119 to include systemic indices of inflammatory status120, 121, and, as considered below, the initiation of randomized placebo-controlled clinical trials of anti-inflammatory treatments for ACVD122. Importantly, the evolution of the concept of endothelial cell dysfunction has provided the conceptual framework for these paradigm-shifting, basic to translational advances.
Hemodynamics, Endothelial Cell Dysfunction and the Focal Nature of Atherosclerosis
An intriguing aspect of the pathobiology of atherosclerosis is the observation that the earliest lesions, in both humans and various experimental animal models, characteristically develop in a distinctive, non-random pattern, the geometry of which correlates with arterial branch points and other regions of altered hemodynamics123–125. Detailed studies of the blood flow in these lesion-prone regions has revealed complex, disturbed laminar flow patterns (flow separation, recirculation, reattachment), which create significant temporal and spatial gradients over relatively short distances, resulting in a high oscillatory index and a low time-averaged wall shear stress32, 33. Initial hypotheses suggested that these conditions might result in longer dwell times for lipoproteins, favoring their increased permeation into the vessel wall126, or that disturbed flow might physically disrupt endothelial integrity, creating foci of injury/repair reminiscent of the Response-to-Injury Hypothesis of Atherosclerosis7. As discussed above, however, the latter explanation was challenged by detailed morphologic studies documenting the presence of an intact endothelial lining in atherosclerosis-susceptible regions during early lesion formation44, 45, 127. The subsequent development of experimental fluid mechanical devices that could reproduce defined laminar, turbulent and disturbed flows over the surface of cultured endothelial cell monolayers in vitro33, 128–131 revealed that the fluid mechanical forces generated by arterial blood flow could also act directly on endothelial cells to alter their morphological and functional properties. For example, exposure of cultured endothelial cells to undisturbed laminar shear stresses induces changes in cell shape, alignment, surface glycocalyx and cytoskeletal organization that mimic the morphology of aortic endothelium in vivo in lesion-resistant geometries132–137. In contrast, exposure to disturbed flows induce endothelial cell turnover and senescence 138, 139, and increased oxidative stress34, as well as alterations in cell shape and the organization of cytoskeletal and intercellular junctional proteins changes140–143 similar to those seen in lesion-prone areas of the arterial vasculature in vivo33. These observations suggested that distinct hemodynamic forces might constitute a local risk factor for endothelial cell dysfunction in atherogenesis144.
A fundamental link between hemodynamic forces and atherogenesis came with the demonstration that the expression of various endothelial genes important in hemostasis and thrombosis, growth regulation and proinflammatory activation were transcriptionally regulated by defined fluid mechanical stimuli145–149. Since several of these genes were directly involved in endothelial adhesion biology (e.g., ICAM-1), these observations suggested a novel paradigm linking biomechanical stimulation with endothelial activation150. Further mechanistic analyses revealed the existence of shear stress response elements (SSREs) in the promoters of pathophysiologically relevant genes such as the platelet-derived growth factor, eNOS and VCAM-1, that acted to up- or down-regulate gene transcription116, 151, 152. Interestingly, clusters of multiple genes appeared to be differentially responsive to the spatial and temporal properties of the applied shear stresses, thus suggesting a complex, dynamic system of biomechanical endothelial gene regulation. In early studies, Topper and co-workers utilized high-throughput molecular biological techniques to define the effects of undisturbed laminar shear stresses, similar to those present in lesion-resistant arterial geometries, on the expression of endothelial genes that might have special relevance for atherogenesis153. Notably, several pathophysiologically important endothelial genes showed a sustained, differential upregulation with undisturbed laminar flow, compared with turbulent flow. These included COX-2 (the inducible isoform of cyclo-oxygenase), eNOS, and manganese-dependent superoxide dismutase--- enzymes that exert potent anti-thrombotic, anti-adhesive, anti-inflammatory and anti-oxidant effects, both within the vascular lining and in interacting cells, such as platelets, leukocytes and vascular smooth muscle. These findings, together with the observation that, in the face of potent systemic drivers of cardiovascular risk (e.g., hypercholesterolemia)12, 111, 154, certain regions of the arterial vasculature remain relatively resistant to the development of atherosclerotic lesions, led to the formulation of an alternative hypothesis for the selective distribution of atherosclerotic lesions, the “Atheroprotective Gene Hypothesis” 155–157. This hypothesis postulated that undisturbed laminar shear stresses upregulate the coordinated expression of a set of atheroprotective genes in endothelial cells, which then act locally in lesion-resistant areas to offset the effects of systemic risk factors in ACVD. The critical testing of this hypothesis entailed a four-step experimental approach—first, a detailed characterization of the actual near-wall shear stress profiles in atheroprone and atheroprotected arterial geometries; second, the recreation of these complex flows, with spatial and temporal fidelity, over cultured human endothelial monolayers; third, a genome-wide comparative analysis of the resultant transcriptomes and their correlation with known pathogenic mechanisms in atherosclerosis; and fourth, the validation of these genetic expression programs in endothelial cells in atherosclerosis-resistant and atherosclerosis-susceptible vascular geometries in vivo.
The combined efforts of several groups subsequently produced a robust body of experimental observations linking specific patterns of hemodynamic stimulation with the transcriptional regulation of functional phenotypes in endothelium in vitro and in vivo2, 130, 158–163. In in vitro model systems, individual genes with special pathophysiologic significance for atherogenesis (e.g., IL-8, VCAM-1) were found to show a differential expression pattern that correlated with atheroprone waveforms, while clusters of genes associated with vasoprotection were coordinately upregulated by atheroprotective waveforms161 [Figure 3]. Characterization of mRNAs expressed in vivo in endothelial cells selectively isolated from lesion-prone geometries in experimental animals provided important validation of these in vitro results163. Taken together, these studies led to the recognition that a major cause of endothelial cell dysfunction in lesion-prone arterial geometries appeared to be the absence of undisturbed laminar shear stresses, which normally serve as a positive regulator of a vasoprotective endothelial phenotype in vascular homeostasis [Figure 4]2, 33, 164. This realization motivated the comparative bioinformatic analysis of the endothelial transcriptomes elicited by atheroprotective versus atheroprone fluid mechanical stimulation, with special attention focused on transcriptional regulators161, 165, 166. Among the transcription factors most responsive to hemodynamic stimuli, the zinc finger transcription factor, Kruppel-like Factor 2 (KLF2) appeared to show the strongest differential upregulation by atheroprotective waveform stimulation166. Expression of KLF2 had been previously demonstrated in the endothelium of atheroresistant regions of human arteries specimens, using in situ hybridization167. When experimentally introduced into cultured human endothelial monolayers, via viral vectors, KLF2 coordinately upregulated a large number of the genes that where associated with atheroprotective hemodynamic stimulation166, 168. Taken together these observations focused attention on KLF2 as a potential critical regulatory node in the endothelial homeostatic network2. Several studies documented that expression of KLF2 in endothelial cells promotes an anti-inflammatory, anti-thrombotic endothelial phenotype, which is explained, at least in part, by its antagonism of the NFκB pathway169. In addition, the expression of KLF2 was found to regulate other endothelial cell functions important for atherogenesis, including endothelial barrier function170, metabolism171, and the release of microRNAs via the shedding of endothelial microvesicles172. Endothelial KLF2 expression also stimulates the production of several autocoids, including NO and natriuretic-peptide C (CNP), which have been shown to be deficient in dysfunctional endothelium in vivo173. Furthermore, mice genetically deficient in KLF2 display enhanced atherosclerotic plaque formation when compared to wild type controls174.
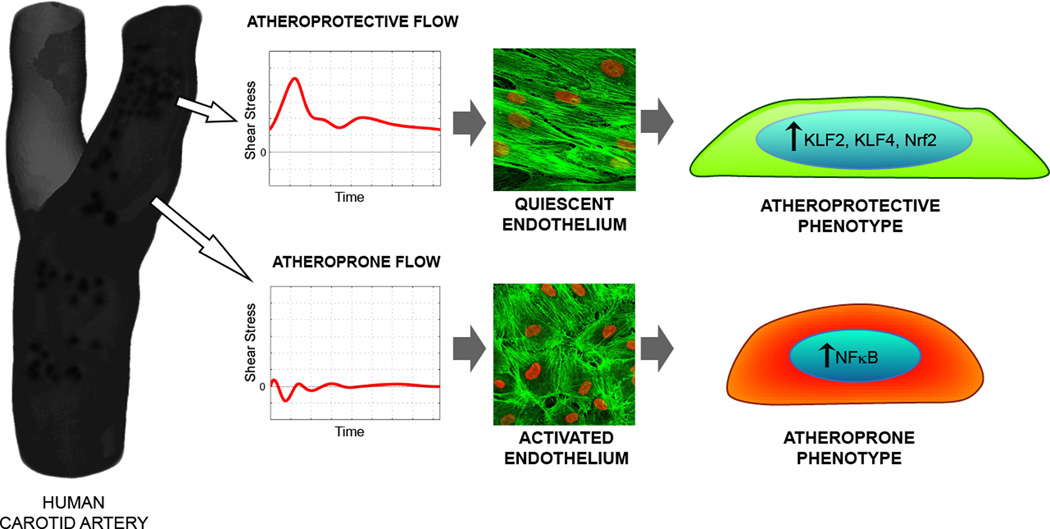
Figure 3. Hemodynamics and Endothelial Phenotypes.
Computational analyses of in vivo blood flow patterns in the carotid artery bifurcation, in normal human subjects, yielded representative near-wall shear stress waveforms from two hemodynamically distinct, clinically relevant locations: the distal internal carotid (an atherosclerosis-resistant region) and the carotid sinus (an atherosclerosis-susceptible region). Exposure of cultured human endothelial monolayers to these two distinct biomechanical stimuli, resulted in markedly different cell morphologies (visualized here by cytoskeletal actin staining) and functional phenotypes. Pulsatile (unidirectional) laminar flow induced upregulation of key transcription factors (KLF2, KLF4, Nrf2), which orchestrated a multifunctional atheroprotective phenotype; in contrast, disturbed (oscillatory) flow resulted in enhanced expression of the pleiotropic transcription factor NFκB, resulting in a proinflammatory, atheroprone phenotype. (See Ref. 161 for details.)
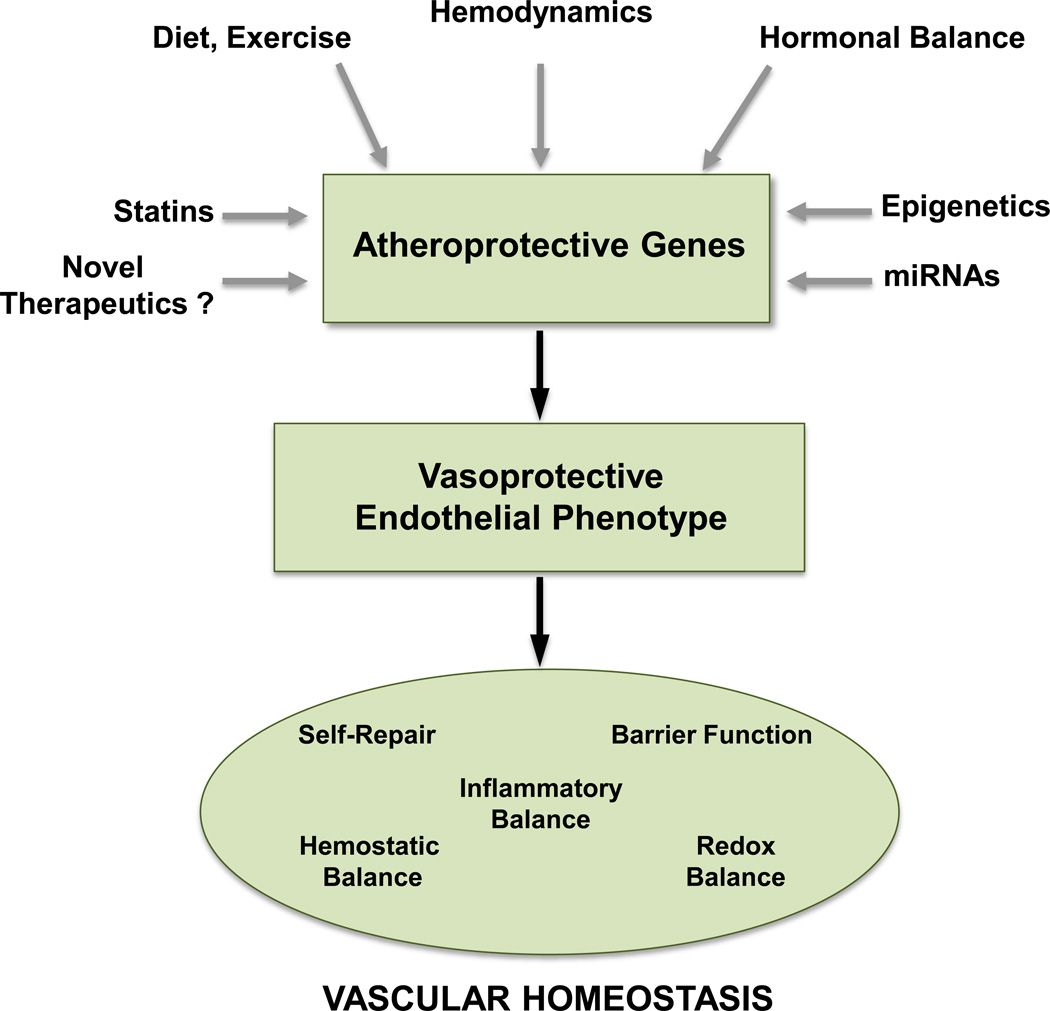
Figure 4. Endothelial Atheroprotective Genes and Vascular Homeostasis.
The expression of atheroprotective genes in vascular endothelium is regulated by key transcriptional factors (e.g., KLF2, KLF4, Nrf2) in response to hemodynamic, hormonal and environmental stimuli. The coordination of this genetic program is further influenced by miRNAs, epigenetic modifications, and pharmacologic agents. The resultant vasoprotective endothelial phenotype supports a spectrum of functions critical to the maintenance of vascular homeostasis.
Importantly, the statins, a widely prescribed class of cardio-protective drugs, were found to upregulate KLF2 expression in cultured human endothelial cells at pharmacologically relevant doses175, 176. This class of HMG CoA reductase inhibitors was originally designed to reduce the biosynthesis of endogenous cholesterol, and thus treat hypercholesterolemia—a major risk factor in ACVD. Biochemically they block production of mevalonate, which forms two major downstream products: farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Statin-mediated upregulation of KLF2 in human endothelial cells is dependent on the depletion of GGPP175–177, which is known to prenylate several members of the Rho superfamily. Notably, upregulation of KLF2 is critical for many of the statin-dependent transcriptional changes in endothelial cells, thus implicating KLF2 in the so-called pleiotropic, non-lipid-lowering, beneficial cardiovascular effects of this class of cardiovascular drugs178, 179.
The atheroprotective flow-mediated expression of KLF2 in endothelial cells is dependent of the activation of the MEKK3/MEK5/ERK5/MEF2 signaling pathway, and several characterized modifiers including AMPK, SIRT1, PKCζ, SUMO specific protease 2 and histone deacetylase 5 2, 180. Activation of MEK5 and/or ERK5 per se leads to endothelial vasoprotection in both a KLF2-dependent and KLF2-independent manner, suggesting that additional targets of these kinases may be involved in mediating the beneficial effects of flow181, 182. One such target is the transcription factor Kruppel-like factor 4 (KLF4). KLF4 also is upregulated by atheroprotective flow in cultured endothelial cells via MEK5/ERK5/MEF2 signaling181, 183. KLF4 expression promotes vasoprotective gene expression in cultured endothelial cells, via several downstream transcriptional targets that are also activated by KLF2181, 183, 184. Using endothelial-selective gain-of-function and loss-of- function approaches, an important role for KLF4 in atheroprotection has been documented in a mouse model of atherosclerosis184.
Another noteworthy flow-mediated transcriptional regulator of atheroprotection is NF-E2-related factor-2 (Nrf2). Nrf2 is activated by atheroprotective flow in cultured endothelial cells, via the phosphoinositol 3-kinase/Akt and Erk5 pathways and controls a series of downstream target genes that play a role in the regulation of intracellular redox balance, as well as resistance to extracellular oxidant stresses185–187. In vivo, Nrf2 is differentially expressed in those regions of the vasculature that are relatively atherosclerosis-resistant, thus further suggesting its pathophysiological relevance185, 188. Recent studies have documented that KLF2 and Nrf2 act independently in the activation of flow-mediated gene expression, but suggest a requirement for KLF2 expression for full activity of Nrf2 in mediating antioxidant vasoprotection189, 190. Together, these two transcription factors account for the activation of ~70% of the atheroprotective flow-induced endothelial transcriptome190, thus pointing to their critical role as master regulators of the vasoprotective endothelial phenotype [Figures 3 and 4].
Beyond their major effects at a transcriptional level, atheroprotective and atheroprone flow stimulation appear to influence endothelial gene expression via two additional mechanisms: microRNAs (miRNAs) and epigenetic modifications191, 192[Figure 4]. Initial in vivo miRNA profiling studies, comparing arterial regions in normal adult swine, identified high expression of miR10a in atheroprotective regions193. In cultured endothelial cells, a major action of miR10a is to down-regulate the NFκB pro-inflammatory pathway. Atheroprotective flow also has been shown to upregulate the expression of miR19-a194, miR-23b195, and miR101196 in cultured endothelial cells, leading to the suppression of endothelial cell proliferation. In contrast, expression of miR92a197, miR663198, miR712199 and miR34a200 is downregulated by atheroprotective flow and upregulated by atheroprone flow in cultured endothelial cells. The atheroprotective flow-dependent suppression of miR92a expression results in the upregulation of KLF2 and KLF4 and certain of their downstream transcriptional targets in vitro and in vivo197, 201. Inhibition of miRNA-92a in endothelial cells modulates endothelial activation in response to shear stress and oxidized LDL, and limits the development of atherosclerosis in LDL receptor-deficient mice at least in part by increasing the expression of KLF2 and KLF4, thus suppressing endothelial activation202. Inhibition of the expression of miR663 suppresses atheroprone flow-mediated endothelial activation198. miR712 downregulates the expression of tissue inhibitor of metalloproteinase 3 (TIMP3) leading to the stimulation of endothelial inflammation, permeability and atherosclerotic lesion formation in mice199. Similarly, the downregulation of miR34a contributes to the atheroprotective flow-mediated suppression of endothelial inflammation by downregulating NFκB signaling200. Finally, atheroprotective flow also induces the secretion of the miR143–145 cluster via a KLF2-dependent pathway. These secreted miRNAs can act on neighboring vascular smooth muscle cells to regulate their turnover and phenotype, and reduce atherosclerotic lesion size in ApoE-deficient mice172. The broad reaching implications of miRNAs for the diagnosis and treatment of ACVD are the subject of another review in this Compendium on Atherosclerosis series.
Recently, it has become apparent that hemodynamic forces can modulate endothelial gene expression at an epigenetic level. For example, two independent studies have shown that atheroprone flow significantly modulates endothelial DNA methylation patterns via alterations in DNA methyltransferase (DNMT) activity, in particular DNMT1203, 204. In addition, at the single gene level, disturbed flow was shown to increase methylation of the proximal promoter of KLF4 in endothelial cells leading to the suppression of its expression by blocking a MEF2 binding site, and by regulating DNMT3A. This MEF2 site was also hypermethylated in swine aortic endothelium isolated from atherosclerosis susceptible regions of the aorta where KLF4 expression is low205. In light of the recent awareness of the global role of epigenetic changes in pro-inflammatory endothelial cell activation118, this new area of flow-mediated gene regulation may offer interesting opportunities for targeted pharmacological interventions.
In addition to the basic role that the anatomical configuration of large arteries plays in defining distinct hemodynamic environments, physiological factors that alter systemic and/or local blood flow also can significantly influence endothelial functional phenotype. For example, aerobic exercise appears to exert some of its well described beneficial cardiovascular effects by modifying flow patterns in the vicinity of arterial branch points, thus altering the wall shear stresses experienced by the endothelial lining206. A transition to more undisturbed laminar flow in lesion-prone geometries should favor the increased expression of certain atheroprotective genes (e.g., eNOS), the inhibition of arterial stiffness, and the restoration of a vasoprotective endothelial phenotype207, 208.
Multiple atherosclerotic lesions in a given human subject can progress, regress, or remain stable, each behaving in an autonomous fashion, thus suggesting that local factors are important determinants of their pathobiology 209. Utilizing a combination of intravascular ultrasound, biplane angiography and blood flow measurements, Stone and coworkers undertook a detailed assessment of endothelial wall shear stresses and local arterial wall morphology in the coronary arteries of patients with acute coronary syndromes-- at the time of intravascular intervention (stenting) and 6 months later210. In addition to providing fresh insights into the natural history of coronary atherosclerotic disease, these and other vascular profiling studies suggested that endothelial shear stresses were a potentially useful predictor of subsequent pathobiological behavior of a given lesion210–212. These observations were then extended in the Prediction of Progression of Coronary Artery Disease and Clinical Outcome Using Vascular Profiling of Shear Stress and Wall Morphology (PREDICTION) Study, which demonstrated a strong correlation between localized, low (disturbed) endothelial shear stresses and subsequent lesion progression to a clinical event213. Taken together, these studies suggest that vascular profiling, combined with biological imaging techniques, potentially could be used to guide more targeted interventional therapies in acute coronary syndromes214–216.
CLINICAL ASSESSMENT OF ENDOTHELIAL CELL DYSFUNCTION AND ITS RELATIONSHIP TO CARDIOVASCULAR RISK
Given its involvement at all stages of ACVD progression, and its predictive significance for cardiovascular events36, there has been considerable interest in the detection and monitoring of ECD in the clinical setting. While direct measurement of impaired endothelial-dependent dilation during coronary angiography remains the historical “gold-standard”83, various indirect approaches, such as the measurement of brachial artery diameter via non-invasive ultrasound imaging in response to flow-mediated reactive hyperemia, have found their way into general practice36. The concordance of these measurements of peripheral arterial vasoreactivity with coronary artery endothelial-dependent vasodilation has been established in controlled comparative studies87, lending further support to their use for cardiovascular risk evaluation in a given patient. Since endothelial cell dysfunction also has been implicated in the pathophysiology of various disease states in addition to ACVD, e.g., hypertension, diabetes, chronic heart and renal failure217, these indirect indices of ECD may have broader clinical significance.
In light of the expanded appreciation of the multiple roles that endothelium plays in cardiovascular homeostasis, the search for clinically useful measures of ECD, which more directly reflect the pathobiology of ACVD at the cellular and molecular level, has burgeoned218. These efforts encompass a broad spectrum of activity—including the validation of circulating plasma biomarkers that reflect pathogenic events occurring within developing lesions per se (e.g., the secretion of inflammation-associated cytokines, such as IL-1 and IL-6, or the shedding of induced cell surface adhesion receptors, such as sVCAM, sE-selectin), as well as the measurement of systemic indices of the inflammatory state, such as C-reactive protein. Efforts in biomarker discovery now extend well beyond the validation of individual candidate molecules of interest, and are employing the power of high-throughput genomics, proteomics and metabolomics, coupled with bioinformatic analyses. Complementary to these blood-based analytical approaches has been the emergence of novel imaging strategies to assess the clinical significance of individual atherosclerotic lesions, at a given moment, in a patient at risk219, 220. By employing state-of-the-art molecular imaging technologies to localize pathogenic events in arterial endothelium (e.g., expression of VCAM-1), this approach ideally would enable the assessment of the natural history of lesions, in real-time, thus potentially allowing the identification of a unstable plaque before its transition into a clinical event, or the evaluation of novel therapeutic strategies designed to target ECD per se221. If implemented in a cost-effective fashion, these approaches could represent a significant advance over the current use of traditional imaging techniques, which assess segmental arterial stenosis or vascular calcification, to guide interventional therapies. A separate review in this Compendium on Atherosclerosis series is devoted to this topic.
At the present time, considerable attention is focused on the application of high-sensitivity C-reactive protein (hsCRP) as a robust, clinical useful biomarker in the assessment of ACVD in individual patients and populations at risk222. C-reactive protein is one of a class of acute phase reactants elaborated by the liver in response to systemic inflammatory stimuli such as IL-6. In the context of atherogenesis, the proinflammatory activation of endothelial cells would be an important source of IL-6 generation. Significantly, in prospective epidemiological studies of initially healthy men and women, hsCRP has been shown to predict future risk for heart attack and stroke, independent of plasma LDL cholesterol associated risk223, 224. Multiple studies also have shown that statins reduce hsCRP in a manner largely independent of their action in reducing blood LDL cholesterol levels225, thus suggesting that these widely prescribed agents are exerting their potent cardiovascular protective effects via both lipid-lowering and anti-inflammatory actions226. The demonstration that statins can act directly on endothelial cells to activate KLF2, resulting in a vasoprotective endothelial phenotype [Figure 4], provides an important mechanistic insight into the interrelationships of statin therapy, hsCRP measurements and ACVD risk. The ability of arterial endothelial cells, in a lesion-susceptible geometry, to resist multifactorial pathophysiologic stimuli of ECD [Table 1], through the coordinated expression of multiple atheroprotective genes, in effect reflects their reduced proinflammatory set-point. Thus, hsCRP might be viewed as a systemic index of this local pathophysiologic state. This awareness has led to the development and validation of improved algorithms for the assessment of cardiovascular risk--The Reynolds Risk Scores for Women and Men120, 121, which incorporate indices of systemic proinflammatory reactivity.
Taken together, these considerations provide cogent epidemiological support for the hypothesis that atherosclerosis is indeed a chronic inflammatory process. The critical testing of this hypothesis is the goal of two ongoing prospective clinical trials assessing different anti-inflammatory approaches to ACVD prevention—CIRT (Cardiovascular Inflammation Reduction Trial), which is evaluating low-dose methotrexate, and CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study), which is evaluating IL-1β inhibition122.
OPPORTUNITIES AND FUTURE DIRECTIONS
What does the future hold for our understanding of the role(s) of endothelial cell dysfunction in the pathogenesis of ACVD, and, importantly, its more effective detection, treatment and prevention in the clinical setting? At present, three areas of opportunity warrant consideration: first, understanding the various roles of endothelial metabolism in health and disease; second, characterizing the clinical consequences of genetic variations in atheroprotective genes; and third, targeting endothelial cell dysfunction via novel therapeutic strategies.
Endothelium is both the largest distributed organ in the human body (with an aggregate mass comparable to other vital organs such as the kidney), and an integral part, anatomically and functionally, of each of the body’s composite tissues and organs227. As we have considered in detail in this review, endothelial cell dysfunction in response to specific pathophysiological stimuli (e.g., hypercholesterolemia and other dyslipidemias, diabetes, obesity, hypertension, aging) has important local manifestations within the walls of arteries in lesion-susceptible regions, but also can be detected in non-lesion regions of the vasculature36. A systemic context for ECD becomes especially relevant when considering the interrelationships of type 2 diabetes, insulin resistance, the Metabolic Syndrome and ACVD risk228, 229. One important mechanistic link appears to be the capacity of lipid-laden, dysfunctional adipocytes to generate a variety of proinflammatory “adipokines” (e.g., TNF), which can act locally within adipose tissue to activate macrophages and microvascular endothelium, and also systemically to stimulate NFκB-dependent pathways in other target tissues, including the NFκB-primed arterial endothelial cells in lesion-susceptible regions14, 230, 231. Within the endothelium, per se, the nuclear receptor PPARγ, an important transcriptional regulator of lipid metabolism, appears to play an central role in controlling metabolic and vascular responses to a high fat diet, as well as the pharmacologic effects of certain anti-diabetic agents232. Further, there is increasing evidence that a broad spectrum of basic metabolic pathways in endothelium are modulated by specific pathophysiologic stimuli associated with ECD171, 233. In contrast, relatively little effort has been given to applying state-of-the-art metabolomic approaches to characterize endothelial metabolism in the context of ACVD. Such studies offer much potential for enriching our understanding of the role endothelial metabolism in maintaining vascular homeostasis, and pointing the way to biomarkers useful for the diagnosis of early ECD in individuals at risk234.
Currently, there is a growing awareness that Cardiovascular Medicine is poised to become more “personalized” and “precise” through the translation of genome-based discoveries into clinical practice235. Indeed, several of the reviews in this Compendium on Atherosclerosis series are related to this theme. These efforts are deeply rooted in the fundamental insights into ACVD pathogenesis that the study of familial hypercholesterolemias and other dyslipidemias have provided. Recent translational activities centered on the association of mutations in the gene encoding proprotein convertase subtilisin/kexin type 9 (PCSK9) with alterations in LDL receptor intracellular trafficking and hypercholesterolemia provide a vivid example of continued progress in this realm221. Given the state of our current understanding of the endothelial expression of atheroprotective genes and their critical roles in the generation of a vasoprotective phenotype [Figure 4], detailed analyses of the genetics of ECD in ACVD would appear to be a timely undertaking. The ACVD-association of specific alterations in the genes encoding key transcriptional regulators, such as KLF2, or major downstream effectors, of the vasoprotective endothelial phenotype, would not only further substantiate the Atheroprotective Gene Hypothesis, but would enable better definition of cardiovascular risk in a given patient or population.
Endothelial cell dysfunction appears to be a reversible process. However, to date, treatment of ECD has been focused largely on ameliorating known risk factors for ACVD rather than specifically targeting endothelial-based mechanisms36. Looking to the future, the development of pharmacomimetics of the natural, flow-mediated vasoprotective endothelial phenotype would appear to be a potentially fruitful strategy. One can envision such drugs acting on endothelial cells in atheroprone regions of the arterial vasculature to re-program their expression of a vasoprotective phenotype, thus offsetting the effects of systemic risk factors, such as hypercholesterolemia, and slowing the progression of atherosclerotic lesion development. Novel biomarkers of ECD, directly related to intrinsic, vessel wall processes, could serve as a guide in the application of these endothelial-targeted agents. As detailed above, the statins may indeed be exerting some of their well-recognized pleiotropic (i.e., non-lipid lower) beneficial effects via endothelial KLF2 activation175, 176. However, members of this class of HMG-CoA reductase inhibitors were not optimized around this endothelial-targeted action, thus highlighting the opportunity for the future development of selective therapies for endothelial cell dysfunction in atherogenesis [Figure 4].
In a monograph entitled “Endothelium: Its Development, Morphology, Function, and Pathology”, published in 1954 and totaling 155 pages, Dr. Rudolf Altschul, Professor of Histology at the University of Saskatchewan began his state-of-the-art review with the statement-- “While working on problems of arteriosclerosis, I have realized not only how little I knew about endothelium, but also how much I ought to know for the proper understanding of arteriosclerosis.” 236. Several decades later, this insightful comment continues to motivate and illuminate our understanding of ACVD.
Supplementary Material
Acknowledgments
The authors wish to acknowledge past and present members of our laboratories in The Center for Excellence in Vascular Biology (Vascular Research Division, Department of Pathology) and our colleagues in the Cardiovascular Research Division at the Brigham and Women’s Hospital, as well as longtime collaborators in the Fluid Mechanics Laboratory at the Massachusetts Institute of Technology.
GRANT SUPPORT: The research activities of the authors are supported by grants from the National Institutes of Health [AG32443 (MAG, GGC); HL007627 (MAG); HL130624, HL118826 (GGC), as well as institutional academic resources (Department of Pathology, Brigham and Women’s Hospital, Boston, MA).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- ACVD
Atherosclerotic Cardiovascular Disease
- ApoE
Apolipoprotein E
- Athero-ELAM
Atherosclerosis-associated Endothelial-Leukocyte Adhesion Molecule
- CNP
C-type Natriuretic Peptide
- DAMP
Damage-Associated Molecular Patterns
- DNMT1
DNA Methyl Transferase-1
- ECD
Endothelial Cell Dysfunction
- EDRF
Endothelial-Derived Relaxing Factor
- eNOS
endothelial Nitric Oxide Synthase
- ELAM
Endothelial-Leukocyte Adhesion Molecule
- ERK5
Extracellular signal-Regulated protein Kinase 5
- hs-CRP
high-sensitivity C-Reactive Protein
- ICAM-1
Intercellular Cell Adhesion Molecule-1
- KLF2
Kruppel-Like Factor-2
- KLF4
Kruppel-Like Factor-4
- MEF2
Myocyte-specific Enhancer Binding Factor-2
- MEK5
Mitogen/Extracellular signal-regulated Kinase 5
- MEKK3
Mitogen-activated protein kinase/Extracellular-regulated kinase Kinase Kinase-3
- miRNA
microRNA
- Nrf2
Nuclear Factor Erythroid 2-related factor-2
- PAMP
Pathogen-Associated Molecular Patterns
- sELAM
soluble Endothelial-Leukocyte Adhesion Molecule
- SSRE
Shear Stress Response Element
- sVCAM-1
soluble Vascular Cell Adhesion Molecule-1
- TIMP3
Tissue Inhibitor of Metalloproteinase-3
- TNF
Tumor Necrosis Factor
- VCAM-1
Vascular Cell Adhesion Molecule-1
- VLA-4
Very Late Antigen-4
Footnotes
DISCLOSURES: The authors have no conflicts of interest, financial or otherwise, related to the contents of this Review article.
REFERENCES
- 1.Gimbrone MA., Jr Vascular endothelium: nature's blood-compatible container. Ann N Y Acad Sci. 1987;516:5–11. doi: 10.1111/j.1749-6632.1987.tb33025.x. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone MA, García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 5.Simionescu N, Vasile E, Lupu F, Popescu G, Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. The American journal of pathology. 1986;123:109–125. [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts) N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981;1:293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 12.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 14.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature reviews Immunology. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996;94:2013–2020. doi: 10.1161/01.cir.94.8.2013. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27:705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 18.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Mechanisms of acute coronary syndromes. N Engl J Med. 2013;369:883–884. doi: 10.1056/NEJMc1307806. [DOI] [PubMed] [Google Scholar]
- 20.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GM S. The Udana: The solemn utterances of the Buddha: Forgotten Books. 1902. [Google Scholar]
- 22.Krogh A. The anatomy and physiology of capillaries. Rev. and enl. ed ed: New Haven : Yale university press. London: H. Milford, Oxford university press; 1929. [Google Scholar]
- 23.Palade GE. Fine structure of blood capillaries. JAppl Physiol. 1953;24 [Google Scholar]
- 24.Karnovsky MJ. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967;35:213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majno G. Ultrastructure of the vascular membrane. Washington DC: American Physiological Society; 1965. Section 2, Circulation. [Google Scholar]
- 26.Cotran R. The fine structure of the microvasculature in relation to normal and altered permeability. Philadelphia: WB Saunders Co; 1967. [Google Scholar]
- 27.Majno G. The Healing Hand. Man and wound in the ancient world. Cambridge: University Press; 1975. [Google Scholar]
- 28.Fishman AP. Endothelium: a distributed organ of diverse capabilities. Ann N Y Acad Sci. 1982;401:1–8. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- 29.Gimbrone MA., Jr Culture of vascular endothelium. Progress in hemostasis and thrombosis. 1976;3:1–28. [PubMed] [Google Scholar]
- 30.Gimbrone MA., Jr Vascular Endothelium in Health & Disease. Haber (ed) Molecular Cardiovascular Medicine. ed. 1994:49–62. [Google Scholar]
- 31.Gimbrone MA., Jr . Vascular Endothelium in Hemostasis & Thrombosis. Edinburgh: Churchill Livingstone; 1986. [Google Scholar]
- 32.Davies PF. Flow-mediated endothelial mechanotransduction. Physiological reviews. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7a–11a. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 35.Gimbrone MA. Atherogenesis: Current Concepts. In: Schoen FG Jr, editor. Cardiovascular Pathology Clincopathologic Correlations and Pathogenic Mechanisms. Baltimore: William & Wilkens; 1995. pp. 1–11. MA. [Google Scholar]
- 36.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 37.De Caterina R, Libby P, editors. Endothelial dysfunctions and vascular disease. Oxford: Blackwell Futura; 2007. [Google Scholar]
- 38.Virchow R. Der Ateromatose Prozess der Arterien. Wien Med Wochenschr. 1856:825–827. [Google Scholar]
- 39.Caplan BA, Gerrity RG, Schwartz CJ. Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta I. Quantitative light microscopic findings. Exp Mol Pathol. 1974;21:102–117. doi: 10.1016/0014-4800(74)90082-3. [DOI] [PubMed] [Google Scholar]
- 40.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 41.Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. The American journal of pathology. 1979;95:775–792. [PMC free article] [PubMed] [Google Scholar]
- 42.Simionescu N, Mora R, Vasile E, Lupu F, Filip DA, Simionescu M. Prelesional modifications of the vessel wall in hyperlipidemic atherogenesis. Extracellular accumulation of modified and reassembled lipoproteins. Ann N Y Acad Sci. 1990;598:1–16. doi: 10.1111/j.1749-6632.1990.tb42271.x. [DOI] [PubMed] [Google Scholar]
- 43.Spaet TH, Stemerman MB, Veith FJ, Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circulation research. 1975;36:58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- 44.Davies PF, Bowyer DE. Scanning electron microscopy: arterial endothelial integrity after fixation at physiological pressure. Atherosclerosis. 1975;21:463–49. doi: 10.1016/0021-9150(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 45.Silkworth JB, McLean B, Stehbens WE. The effect of hypercholesterolemia on aortic endothelium studied en face. Atherosclerosis. 1975;22:335–348. doi: 10.1016/0021-9150(75)90015-5. [DOI] [PubMed] [Google Scholar]
- 46.Gimbrone MAJ. Endothelial Dysfunction and the Pathogenesis of Atherosclerosis, Proceedings of the Fifth International Symposium. Springer-Verlag New York: Springer US; 1980. [Google Scholar]
- 47.Gimbrone MAJ. Vascular Endothelium and atherosclerosis. In: IMs, editor. Vascular Injury and Atherosclerosis. New York: Marcel Dekker; 1981. pp. 25–52. [Google Scholar]
- 48.Gimbrone MA, Jr, Alexander RW. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science. 1975;189:219–220. doi: 10.1126/science.1138377. [DOI] [PubMed] [Google Scholar]
- 49.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977;74:3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thrombosis research. 1977;11:323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- 51.Vane JR. The Croonian Lecture, 1993. The endothelium: maestro of the blood circulation. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1994;343:225–246. doi: 10.1098/rstb.1994.0023. [DOI] [PubMed] [Google Scholar]
- 52.Weitz J. Hemostasis, thrombosis, fibrinolysis and cardiovascular disease. In: Mann Z, Libby, Bonow, editors. Braunwald’s Heart Disease. 2. Philadelphia: Elsevier Saunders; 2015. pp. 1809–1833. [Google Scholar]
- 53.Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. The American journal of pathology. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- 54.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 55.Griffith TM, Edwards DH, Lewis MJ, Newby AC, Henderson AH. The nature of endothelium-derived vascular relaxant factor. Nature. 1984;308:645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- 56.Rubanyi GM, Lorenz RR, Vanhoutte PM. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. The American journal of physiology. 1985;249:H95–H101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- 57.Martin W, Villani GM, Jothianandan D, Furchgott RF. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. The Journal of pharmacology and experimental therapeutics. 1985;233:679–685. [PubMed] [Google Scholar]
- 58.Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circulation research. 1983;52:352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- 59.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutchinson PJ, Palmer RM, Moncada S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. European journal of pharmacology. 1987;141:445–451. doi: 10.1016/0014-2999(87)90563-2. [DOI] [PubMed] [Google Scholar]
- 62.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. British journal of pharmacology. 1987;92:181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 64.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D'Angelo DD, Lynch KR, Peach MJ. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- 65.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sessa WC. eNOS at a glance. Journal of cell science. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 67.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. The American journal of physiology. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 69.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. The Journal of experimental medicine. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang-Decker N, Cao S, Chatterjee S, Yao J, Egan LJ, Semela D, Mukhopadhyay D, Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. Journal of cell science. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 73.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 75.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 76.Haldar SM, Stamler JS. S-nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest. 2013;123:101–110. doi: 10.1172/JCI62854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annual review of pharmacology and toxicology. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 78.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 79.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA, Jr, Ertl G, Huang PL. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. American journal of physiology Cell physiology. 2004;286:C1195–C1202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 81.Huang PL. Lessons learned from nitric oxide synthase knockout animals. Seminars in perinatology. 2000;24:87–90. doi: 10.1016/s0146-0005(00)80064-6. [DOI] [PubMed] [Google Scholar]
- 82.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 83.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 84.Freiman PC, Mitchell GG, Heistad DD, Armstrong ML, Harrison DG. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circulation research. 1986;58:783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- 85.Bossaller C, Habib GB, Yamamoto H, Williams C, Wells S, Henry PD. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein e-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- 87.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 88.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 89.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiological reviews. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 90.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 91.Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. Journal of immunology. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 92.Pober JS, Gimbrone MA., Jr Expression of Ia-like antigens by human vascular endothelial cells is inducible in vitro: demonstration by monoclonal antibody binding and immunoprecipitation. Proc Natl Acad Sci U S A. 1982;79:6641–6645. doi: 10.1073/pnas.79.21.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gimbrone MA, Jr, Obin MS, Brock AF, Luis EA, Hass PE, Hebert CA, Yip YK, Leung DW, Lowe DG, Kohr WJ, et al. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989;246:1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- 95.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. The American journal of pathology. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- 96.Cotran RS, Gimbrone MA, Jr, Bevilacqua MP, Mendrick DL, Pober JS. Induction and detection of a human endothelial activation antigen in vivo. The Journal of experimental medicine. 1986;164:661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munro JM, Pober JS, Cotran RS. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. The American journal of pathology. 1989;135:121–133. [PMC free article] [PubMed] [Google Scholar]
- 98.Cybulsky MI, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 99.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 100.Rice GE, Munro JM, Bevilacqua MP. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. The Journal of experimental medicine. 1990;171:1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 102.O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circulation research. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 104.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 105.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 106.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Caterina R, Basta G, Lazzerini G, Dell'Omo G, Petrucci R, Morale M, Carmassi F, Pedrinelli R. Soluble vascular cell adhesion molecule-1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2646–2654. doi: 10.1161/01.atv.17.11.2646. [DOI] [PubMed] [Google Scholar]
- 108.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 110.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. Journal of lipid research. 2009;50(Suppl):S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 112.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Libby P, Ridker P. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006:A33–A46. [Google Scholar]
- 115.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown JD, Lin CY, Duan Q, Griffin G, Federation AJ, Paranal RM, Bair S, Newton G, Lichtman AH, Kung AL, Yang T, Wang H, Luscinskas FW, Croce KJ, Bradner JE, Plutzky J. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Molecular cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 120.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 121.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 123.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 124.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Archives of pathology & laboratory medicine. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 125.Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. Journal of biomechanical engineering. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 126.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proceedings of the Royal Society of London Series B, Biological sciences. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 127.Stehbens WE. The role of hemodynamics in the pathogenesis of atherosclerosis. Progress in cardiovascular diseases. 1975;18:89–103. doi: 10.1016/0033-0620(75)90009-2. [DOI] [PubMed] [Google Scholar]
- 128.Bussolari SR, Dewey CF, Jr, Gimbrone MA., Jr Apparatus for subjecting living cells to fluid shear stress. The Review of scientific instruments. 1982;53:1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 129.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. Journal of biomechanical engineering. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 130.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. Journal of biomechanical engineering. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 131.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 132.Remuzzi A, Dewey CF, Jr, Davies PF, Gimbrone MA., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21:617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 133.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. Journal of biomechanical engineering. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 134.Zand T, Nunnari JJ, Hoffman AH, Savilonis BJ, MacWilliams B, Majno G, Joris I. Endothelial adaptations in aortic stenosis. Correlation with flow parameters. The American journal of pathology. 1988;133:407–418. [PMC free article] [PubMed] [Google Scholar]
- 135.Koo A, Dewey CF, Jr, Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. American journal of physiology Cell physiology. 2013;304:C137–C146. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wong AJ, Pollard TD, Herman IM. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983;219:867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- 137.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annual review of biomedical engineering. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 138.Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S, White A, Biswas S, Khamis R, Chong CK, Cheung WM, Sherwin SJ, Bennett MR, Gil J, Mason JC, Haskard DO, Evans PC. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler Thromb Vasc Biol. 2014;34:985–995. doi: 10.1161/ATVBAHA.114.303415. [DOI] [PubMed] [Google Scholar]
- 139.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circulation research. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 141.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci U S A. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 143.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol. 2013;33:2130–2136. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gimbrone MA, Jr, Cybulsky MI, Kume N, Collins T, Resnick N. Vascular endothelium. An integrator of pathophysiological stimuli in atherogenesis. Ann N Y Acad Sci. 1995;748:122–131. discussion 131-2. [PubMed] [Google Scholar]
- 145.Diamond SL, Sharefkin JB, Dieffenbach C, Frasier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. Journal of cellular physiology. 1990;143:364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 146.Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, Korenaga R, Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205:1345–1352. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 147.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin MC, Almus-Jacobs F, Chen HH, Parry GC, Mackman N, Shyy JY, Chien S. Shear stress induction of the tissue factor gene. J Clin Invest. 1997;99:737–744. doi: 10.1172/JCI119219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gimbrone MA, Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;99:1809–1813. doi: 10.1172/JCI119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279:163–168. doi: 10.1074/jbc.M307528200. [DOI] [PubMed] [Google Scholar]
- 152.Korenaga R, Ando J, Kosaki K, Isshiki M, Takada Y, Kamiya A. Negative transcriptional regulation of the VCAM-1 gene by fluid shear stress in murine endothelial cells. The American journal of physiology. 1997;273:C1506–C1515. doi: 10.1152/ajpcell.1997.273.5.c1506. [DOI] [PubMed] [Google Scholar]
- 153.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Topper JN, Gimbrone MA. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–46. doi: 10.1016/s1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 156.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 157.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 158.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 161.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Annals of biomedical engineering. 2008;36:563–570. doi: 10.1007/s10439-007-9400-0. [DOI] [PubMed] [Google Scholar]
- 164.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 166.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. The American journal of pathology. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 169.Atkins GB, Simon DI. Interplay between NF-kappaB and Kruppel-like factors in vascular inflammation and atherosclerosis: location, location, location. Journal of the American Heart Association. 2013;2:e000290. doi: 10.1161/JAHA.113.000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M, Dimmeler S, Boon RA. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol. 2015;35:137–145. doi: 10.1161/ATVBAHA.114.304277. [DOI] [PubMed] [Google Scholar]
- 172.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature cell biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 173.Moyes AJ, Khambata RS, Villar I, Bubb KJ, Baliga RS, Lumsden NG, Xiao F, Gane PJ, Rebstock AS, Worthington RJ, Simone MI, Mota F, Rivilla F, Vallejo S, Peiro C, Sanchez Ferrer CF, Djordjevic S, Caulfield MJ, MacAllister RJ, Selwood DL, Ahluwalia A, Hobbs AJ. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. doi: 10.1172/JCI74281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circulation research. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 176.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 177.Chu UB, Duellman T, Weaver SJ, Tao Y, Yang J. Endothelial protective genes induced by statin are mimicked by ERK5 activation as triggered by a drug combination of FTI-277 and GGTI-298. Biochimica et biophysica acta. 2015;1850:1415–1425. doi: 10.1016/j.bbagen.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liao JK, Laufs U. Pleiotropic effects of statins. Annual review of pharmacology and toxicology. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 180.Abe J, Berk BC. Novel mechanisms of endothelial mechanotransduction. Arterioscler Thromb Vasc Biol. 2014;34:2378–2386. doi: 10.1161/ATVBAHA.114.303428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Le NT, Takei Y, Izawa-Ishizawa Y, Heo KS, Lee H, Smrcka AV, Miller BL, Ko KA, Ture S, Morrell C, Fujiwara K, Akaike M, Abe J. Identification of activators of ERK5 transcriptional activity by high-throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. Journal of immunology. 2014;193:3803–3815. doi: 10.4049/jimmunol.1400571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, Ludwig S, Roth J, Goebeler M, Schmidt M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4) J Biol Chem. 2010;285:26199–26210. doi: 10.1074/jbc.M110.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, Jain MK. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122:4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circulation research. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 186.Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai YC, Chao YJ, Wang DL, Hsieh HJ. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. Journal of biomedical science. 2009;16:12. doi: 10.1186/1423-0127-16-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blagovic K, Kim LY, Voldman J. Microfluidic perfusion for regulating diffusible signaling in stem cells. PloS one. 2011;6:e22892. doi: 10.1371/journal.pone.0022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 189.Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 190.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40. 41-3. [PubMed] [Google Scholar]
- 191.Schober A, Nazari-Jahantigh M, Weber C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nature reviews Cardiology. 2015;12:361–374. doi: 10.1038/nrcardio.2015.38. [DOI] [PubMed] [Google Scholar]
- 192.Yan MS, Marsden PA. Epigenetics in the Vascular Endothelium: Looking From a Different Perspective in the Epigenomics Era. Arterioscler Thromb Vasc Biol. 2015;35:2297–2306. doi: 10.1161/ATVBAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang KC, Nguyen P, Weiss A, Yeh YT, Chien HS, Lee A, Teng D, Subramaniam S, Li YS, Chien S. MicroRNA-23b regulates cyclin-dependent kinase-activating kinase complex through cyclin H repression to modulate endothelial transcription and growth under flow. Arterioscler Thromb Vasc Biol. 2014;34:1437–1445. doi: 10.1161/ATVBAHA.114.303473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen K, Fan W, Wang X, Ke X, Wu G, Hu C. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun. 2012;427:138–142. doi: 10.1016/j.bbrc.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 197.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. American journal of physiology Heart and circulatory physiology. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nature communications. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fan W, Fang R, Wu X, Liu J, Feng M, Dai G, Chen G, Wu G. Shear-sensitive microRNA-34a modulates flow-dependent regulation of endothelial inflammation. Journal of cell science. 2015;128:70–80. doi: 10.1242/jcs.154252. [DOI] [PubMed] [Google Scholar]
- 201.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circulation research. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 203.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–3199. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou J, Li YS, Wang KC, Chien S. Epigenetic Mechanism in Regulation of Endothelial Function by Disturbed Flow: Induction of DNA Hypermethylation by DNMT1. Cellular and molecular bioengineering. 2014;7:218–224. doi: 10.1007/s12195-014-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jiang YZ, Jimenez JM, Ou K, McCormick ME, Zhang LD, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circulation research. 2014;115:32–43. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Taylor CA, Hughes TJ, Zarins CK. Effect of exercise on hemodynamic conditions in the abdominal aorta. Journal of vascular surgery. 1999;29:1077–1089. doi: 10.1016/s0741-5214(99)70249-1. [DOI] [PubMed] [Google Scholar]
- 207.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circulation research. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 208.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 210.Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 211.Stone PH, Coskun AU, Kinlay S, Popma JJ, Sonka M, Wahle A, Yeghiazarians Y, Maynard C, Kuntz RE, Feldman CL. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J. 2007;28:705–710. doi: 10.1093/eurheartj/ehl575. [DOI] [PubMed] [Google Scholar]
- 212.Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, Timmins LH, Quyyumi AA, Giddens DP. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 213.Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Nakamura S, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimachi F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–181. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 214.Wentzel JJ, Chatzizisis YS, Gijsen FJ, Giannoglou GD, Feldman CL, Stone PH. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res. 2012;96:234–243. doi: 10.1093/cvr/cvs217. [DOI] [PubMed] [Google Scholar]
- 215.Papafaklis MI, Takahashi S, Antoniadis AP, Coskun AU, Tsuda M, Mizuno S, Andreou I, Nakamura S, Makita Y, Hirohata A, Saito S, Feldman CL, Stone PH. Effect of the local hemodynamic environment on the de novo development and progression of eccentric coronary atherosclerosis in humans: insights from PREDICTION. Atherosclerosis. 2015;240:205–211. doi: 10.1016/j.atherosclerosis.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 216.Corban MT, Eshtehardi P, Suo J, McDaniel MC, Timmins LH, Rassoul-Arzrumly E, Maynard C, Mekonnen G, King S, 3rd, Quyyumi AA, Giddens DP, Samady H. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis. 2014;232:271–276. doi: 10.1016/j.atherosclerosis.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 217.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vascular health and risk management. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 218.Libby PREG, Ridker P. Biomarkers, Proteomics, Metabolomics, and Personalized Medicine Braunwald’s Heart Disease. 10th. 1. Saunders, Philadelphia: Elsevier; 2015. pp. 84–93. [Google Scholar]
- 219.Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nuclear Medicine. 2010:33S–37S. doi: 10.2967/jnumed.109.069633. [DOI] [PubMed] [Google Scholar]
- 220.Fernandez-Friera L, Ibanez B, Fuster V. Imaging subclinical atherosclerosis: is it ready for prime time? A review. Journal of cardiovascular translational research. 2014;7:623–634. doi: 10.1007/s12265-014-9582-4. [DOI] [PubMed] [Google Scholar]
- 221.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 222.Ridker PM, Kastelein JJ, Genest J, Koenig W. C-reactive protein and cholesterol are equally strong predictors of cardiovascular risk and both are important for quality clinical care. Eur Heart J. 2013;34:1258–12561. doi: 10.1093/eurheartj/eht022. [DOI] [PubMed] [Google Scholar]
- 223.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 224.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 225.Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 226.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. Pravastatin or Atorvastatin E and Infection Therapy-Thrombolysis in Myocardial Infarction I. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 227.Endothelial Biomedicine. New York: Cambridge University Press; 2007. [Google Scholar]
- 228.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ridker P, Libby P, Buring J. Braunwald’s Heart Disease. 10th. 1 Saunders, Philadelphia: Elsevier; 2015. Risk Markers and the Primary Prevention of Cardiovascular Disease. [Google Scholar]
- 230.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Reviews in endocrine & metabolic disorders. 2014;15:277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 231.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell metabolism. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circulation research. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO molecular medicine. 2014;6:1105–1120. doi: 10.15252/emmm.201404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ginsburg G. Braunwald’s Heart Disease. 10th. 1. Philadelphia: Elsevier, Saunders; 2015. Personalized and Precision Cardiovascular Medicine; pp. 57–63. [Google Scholar]
- 236.Altschul R and Co. NYTM. Endothelium: Its Development. Morphology, Function and Pathology. 1954 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.