Abstract
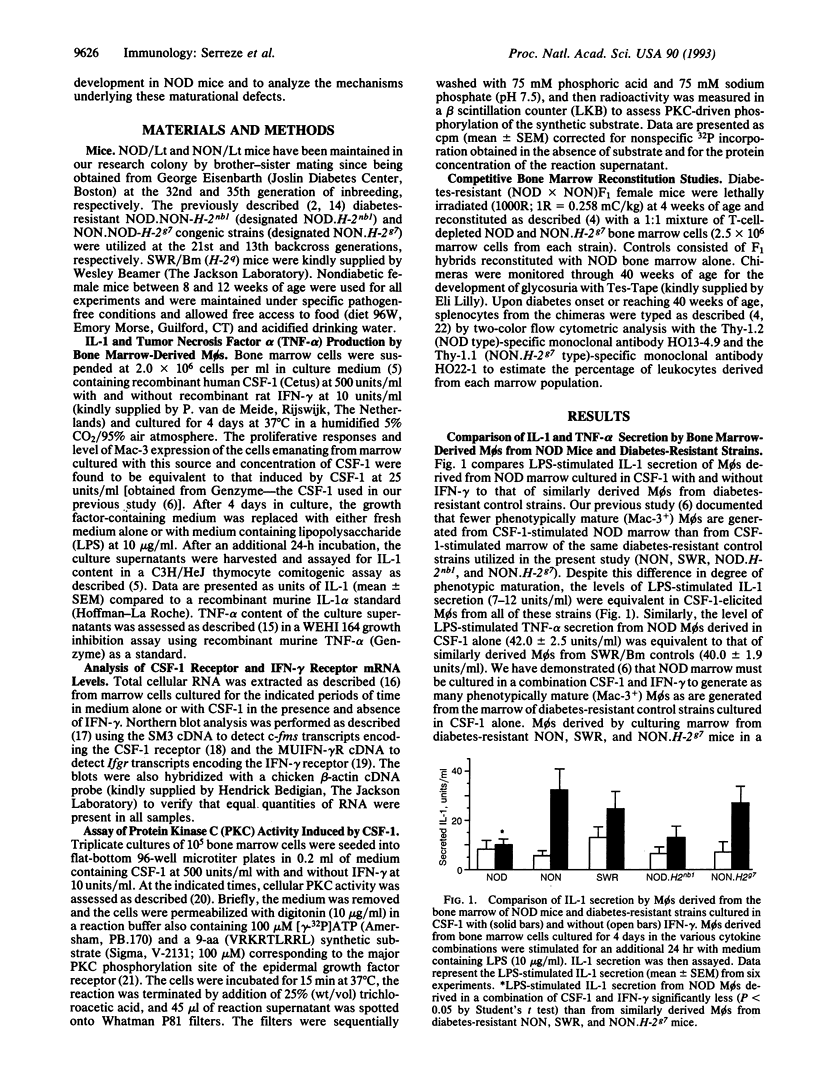
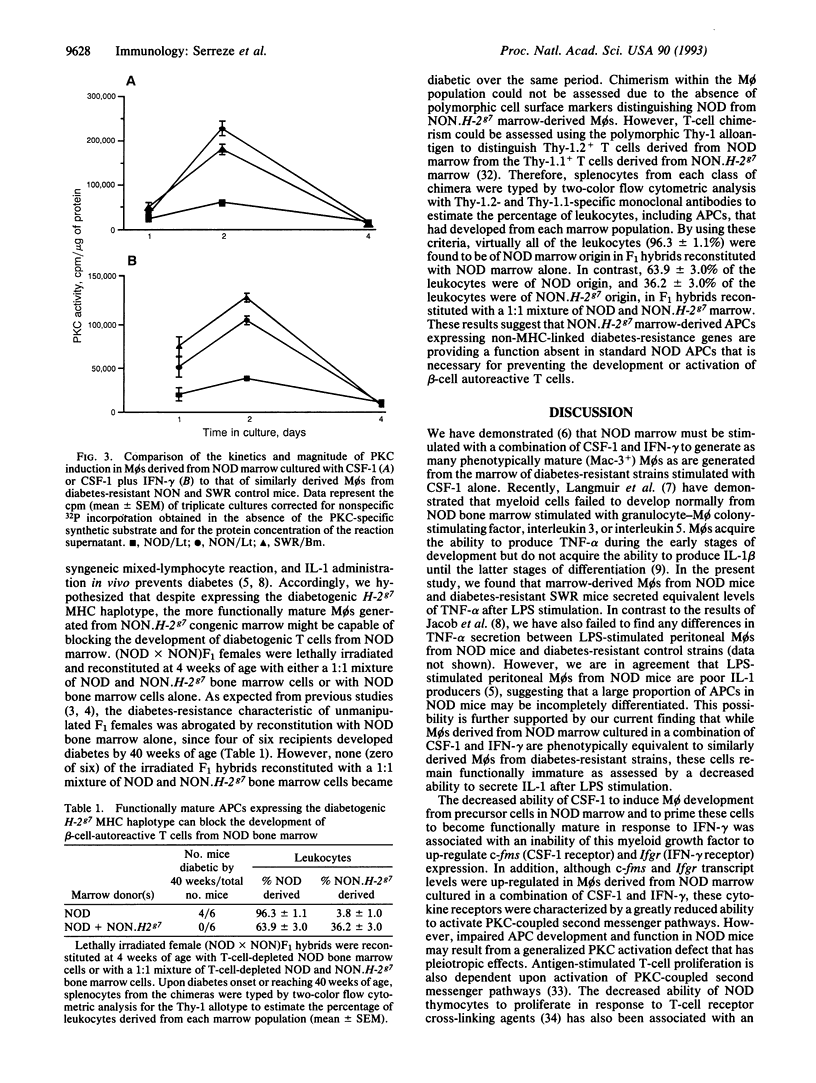
The immunopathogenesis of autoimmune insulin-dependent diabetes in NOD mice entails defects in the development of macrophages (M phi s) from hematopoietic precursors. The present study analyzes the cellular and molecular basis underlying our previous finding that the Mø growth factor colony-stimulating factor 1 (CSF-1) promotes a reduced level of promonocyte proliferation and M phi development from NOD bone marrow. CSF-1 stimulation of NOD marrow induced Møs to differentiate to the point that they secreted levels of tumor necrosis factor alpha equivalent to that of controls. However, CSF-1 failed to prime NOD M phi s to completely differentiate in response to gamma-interferon, as shown by their decreased lipopolysaccharide-stimulated interleukin 1 secretion. These defects, in turn, were associated with an inability of CSF-1 to up-regulate c-fms (CSF-1 receptor) and Ifgr (gamma-interferon receptor) expression. Even though the combination of CSF-1 and gamma-interferon up-regulated c-fms and Ifgr transcript levels in NOD M phi s to levels induced in control M phi s by CSF-1 alone, the protein kinase C activities coupled to these receptors remained 4-fold lower in NOD M phi s than in M phi s derived from the marrow of diabetes-resistant NON and SWR control mice. Despite expressing the diabetogenic H-2g7 haplotype, M phi s derived from cytokine-stimulated marrow of the NON.H-2g7 congenic stock were functionally more mature than similarly derived M phi s from NOD mice. Whereas diabetes resistance was abrogated in 67% of irradiated (NOD x NON)F1 females reconstituted with NOD marrow, no recipients became diabetic after reconstitution with a 1:1 mixture of marrow from NOD and the congenic stock. Thus, failure to develop functionally mature monocytes may be of pathogenic significance in NOD mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brach M. A., Henschler R., Mertelsmann R. H., Herrmann F. Regulation of M-CSF expression by M-CSF: role of protein kinase C and transcription factor NF kappa B. Pathobiology. 1991;59(4):284–288. doi: 10.1159/000163664. [DOI] [PubMed] [Google Scholar]
- Chen B. D., Chou T. H., Ratanatharathorn V. Expression of gamma-interferon receptor in murine bone marrow-derived macrophages associated with macrophage differentiation: evidence of gamma-interferon receptors in the regulation of macrophage proliferation. J Cell Physiol. 1987 Nov;133(2):313–320. doi: 10.1002/jcp.1041330215. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christianson S. W., Shultz L. D., Leiter E. H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993 Jan;42(1):44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- Clare-Salzler M. J., Brooks J., Chai A., Van Herle K., Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992 Sep;90(3):741–748. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Kamdar S. J., Duffy T. M., Fuller J. Synergistic interaction of bacterial lipopolysaccharide and the monocyte-macrophage colony-stimulating factor: potential quantitative and qualitative changes in macrophage-produced cytokine bioactivity. J Leukoc Biol. 1992 Jan;51(1):93–96. doi: 10.1002/jlb.51.1.93. [DOI] [PubMed] [Google Scholar]
- Fan X. D., Goldberg M., Bloom B. R. Interferon-gamma-induced transcriptional activation is mediated by protein kinase C. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5122–5125. doi: 10.1073/pnas.85.14.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando L. P., LeClaire R. D., Obici S., Zavodny P. J., Russell S. W., Pace J. L. Stable expression of a secreted form of the mouse IFN-gamma receptor by rat cells. J Immunol. 1991 Jul 15;147(2):541–547. [PubMed] [Google Scholar]
- Gerling I. C., Serreze D. V., Christianson S. W., Leiter E. H. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992 Dec;41(12):1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K., Leiter E. H. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes. 1990 Apr;39(4):415–425. doi: 10.2337/diab.39.4.415. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Hoggan M. D., Halden N. F., Buckler C. E., Kozak C. A. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol. 1988 Mar;62(3):1055–1056. doi: 10.1128/jvi.62.3.1055-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H., Jayaraman S., Kuchroo V., Diamond B., Saito S., Dorf M. E. Functional analysis of cloned macrophage hybridomas. VII. Modulation of suppressor T cell-inducing activity. J Immunol. 1989 Jul 15;143(2):414–419. [PubMed] [Google Scholar]
- Jacob C. O., Aiso S., Michie S. A., McDevitt H. O., Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990 Feb;87(3):968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Peyser M., Krall M., Mariano T. M., Kumar C. S., Pestka S., Mock B. A. Molecular genetic markers spanning mouse chromosome 10. Genomics. 1990 Nov;8(3):519–524. doi: 10.1016/0888-7543(90)90039-w. [DOI] [PubMed] [Google Scholar]
- Langmuir P. B., Bridgett M. M., Bothwell A. L., Crispe I. N. Bone marrow abnormalities in the non-obese diabetic mouse. Int Immunol. 1993 Feb;5(2):169–177. doi: 10.1093/intimm/5.2.169. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Christianson G. J., Serreze D. V., Ting A. T., Worthen S. M. MHC antigen induction by interferon gamma on cultured mouse pancreatic beta cells and macrophages. Genetic analysis of strain differences and discovery of an "occult" class I-like antigen in NOD/Lt mice. J Exp Med. 1989 Oct 1;170(4):1243–1262. doi: 10.1084/jem.170.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E. H., Serreze D. V. Antigen presenting cells and the immunogenetics of autoimmune diabetes in NOD mice. Reg Immunol. 1992 Sep-Oct;4(5):263–273. [PubMed] [Google Scholar]
- Mamula M. J. The inability to process a self-peptide allows autoreactive T cells to escape tolerance. J Exp Med. 1993 Feb 1;177(2):567–571. doi: 10.1084/jem.177.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., McLachlan A., Houghten R., Thornton G. B., Hughes J. L. Distinction between immunogenicity and tolerogenicity among HBcAg T cell determinants. Influence of peptide-MHC interaction. J Immunol. 1989 Nov 15;143(10):3148–3156. [PubMed] [Google Scholar]
- Prochazka M., Leiter E. H., Serreze D. V., Coleman D. L. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science. 1987 Jul 17;237(4812):286–289. doi: 10.1126/science.2885918. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Worthen S. M., Leiter E. H. Genetic control of diabetogenesis in NOD/Lt mice. Development and analysis of congenic stocks. Diabetes. 1989 Nov;38(11):1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- Sadelain M. W., Qin H. Y., Lauzon J., Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990 May;39(5):583–589. doi: 10.2337/diab.39.5.583. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Gaskins H. R., Leiter E. H. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993 Mar 15;150(6):2534–2543. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988 Jun 1;140(11):3801–3807. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H. Development of diabetogenic T cells from NOD/Lt marrow is blocked when an allo-H-2 haplotype is expressed on cells of hemopoietic origin, but not on thymic epithelium. J Immunol. 1991 Aug 15;147(4):1222–1229. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Worthen S. M., Shultz L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD x NON)F1 mice. Diabetes. 1988 Feb;37(2):252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Sprent J., Webb S. R. Function and specificity of T cell subsets in the mouse. Adv Immunol. 1987;41:39–133. doi: 10.1016/s0065-2776(08)60030-9. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Meyers J., Obermiller P. S. Activation-driven T cell death. II. Quantitative differences alone distinguish stimuli triggering nontransformed T cell proliferation or death. J Immunol. 1992 Sep 1;149(5):1583–1592. [PubMed] [Google Scholar]
- Veis N., Hamilton J. A. Colony stimulating factor-1 stimulates diacylglycerol generation in murine bone marrow-derived macrophages, but not in resident peritoneal macrophages. J Cell Physiol. 1991 May;147(2):298–305. doi: 10.1002/jcp.1041470215. [DOI] [PubMed] [Google Scholar]
- Williams B., Schrier R. W. Characterization of glucose-induced in situ protein kinase C activity in cultured vascular smooth muscle cells. Diabetes. 1992 Nov;41(11):1464–1472. doi: 10.2337/diab.41.11.1464. [DOI] [PubMed] [Google Scholar]
- Witsell A. L., Schook L. B. Macrophage heterogeneity occurs through a developmental mechanism. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1963–1967. doi: 10.1073/pnas.88.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipris D., Lazarus A. H., Crow A. R., Hadzija M., Delovitch T. L. Defective thymic T cell activation by concanavalin A and anti-CD3 in autoimmune nonobese diabetic mice. Evidence for thymic T cell anergy that correlates with the onset of insulitis. J Immunol. 1991 Jun 1;146(11):3763–3771. [PubMed] [Google Scholar]



