Abstract
Objective:
We examined clinical and imaging findings of suspected idiopathic normal pressure hydrocephalus (iNPH) in relation to vascular risk factors and white matter lesions (WMLs), using a nested case-control design in a representative, population-based sample.
Methods:
From a population-based sample, 1,235 persons aged 70 years or older were examined with CT of the brain between 1986 and 2000. We identified 55 persons with hydrocephalic ventricular enlargement, i.e., radiologic findings consistent with iNPH. Among these, 26 had clinical signs that fulfilled international guideline criteria for probable iNPH. These cases were labeled suspected iNPH. Each case was matched to 5 controls from the same sample, based on age, sex, and study cohort. Data on risk factors were obtained from clinical examinations and the Swedish Hospital Discharge Register. History of hypertension, diabetes mellitus (DM), smoking, overweight, history of coronary artery disease, stroke/TIA, and WMLs on CT were examined. Risk factors associated with iNPH with a p value <0.1 in χ2 tests were included in conditional logistic regression models.
Results:
In the regression analyses, suspected iNPH was related to moderate to severe WMLs (odds ratio [OR] 5.2; 95% confidence interval [CI]: 1.5–17.6), while hydrocephalic ventricular enlargement was related to hypertension (OR 2.7; 95% CI: 1.1–6.8), moderate to severe WMLs (OR 6.5; 95% CI: 2.1–20.3), and DM (OR 4.3; 95% CI: 1.1–16.3).
Conclusions:
Hypertension, WMLs, and DM were related to clinical and imaging features of iNPH, suggesting that vascular mechanisms are involved in the pathophysiology. These findings might have implications for understanding disease mechanisms in iNPH and possibly prevention.
Idiopathic normal pressure hydrocephalus (iNPH) is probably more common than previously supposed. We have recently reported that 5.9% of persons older than 80 years have this disorder.1 Treatment with shunt surgery improves symptoms in more than 80% of patients.2 Despite this, iNPH is underdiagnosed and undertreated.1,3 Furthermore, little is known about the underlying disease mechanisms. It is important to learn more about risk factors for the disease in order to understand pathophysiologic mechanisms and suggest preventive measures. Vascular risk factors, such as hypertension, diabetes, and ischemic heart disease, have previously been associated with iNPH in hospital-based, case-control studies.4–9 Also, cerebral white matter lesions (WMLs), which are associated with small vessel disease and white matter ischemia,10 are common in iNPH.11,12 It has therefore been suggested that vascular mechanisms may be related to the development of iNPH. However, previous studies on risk factors have not been population-based. Furthermore, there are no previous epidemiologic investigations regarding WMLs in iNPH.
We aimed to examine vascular risk factors and WMLs in relation to clinical and radiologic findings of suspected iNPH using a nested case-control analysis in a large, representative, population-based sample. The following exposure variables were examined: history of hypertension, diabetes mellitus (DM), smoking, overweight, history of stroke or TIA, coronary artery disease (CAD), and WMLs.
METHODS
Study population.
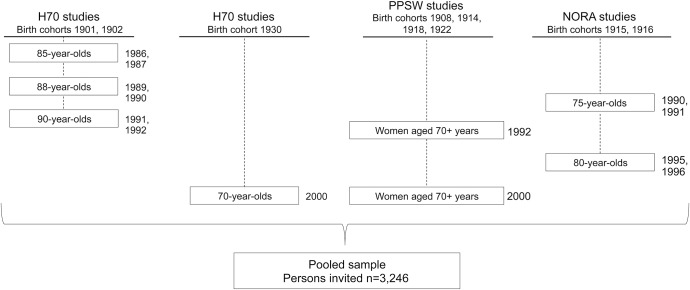
The study population has been described in detail previously.1 Between 1986 and 2000, studies on representative elderly populations in Gothenburg, Sweden, were conducted using identical examinations (including neuropsychiatric examinations and key informant interviews) at each occasion.13 All participants were systematically obtained from the Swedish population register based on birth dates and included people living in private households and in residential care. Subsamples were examined with CT of the brain. The studies included the H85 studies,14–16 the Nordic Research on Ageing studies,17 the Prospective Population Study of Women,18 and the Gerontological and Geriatric Population Studies (H70) in Gothenburg, Sweden19,20 (figure 1). These samples have been described in detail previously and were examined with identical methods by the same research team.
Figure 1. Gothenburg population studies: Overview of cohorts included in the present study.
The sample comprised data from 4 prospective cohort studies. Pooled sample included all persons invited. H70 = Longitudinal Gerontological and Geriatric Population Studies in Gothenburg, Sweden; NORA = Nordic Research on Ageing; PPSW = Prospective Population Study of Women.
In total, 3,246 individuals were invited and 2,179 accepted to take part in a clinical examination (response rate 67%), with no significant difference in response rate between men and women (65% vs 68%; p = 0.107). Of those who took part in the clinical examinations, 1,235 accepted a CT scan of the head (response rate 58.1%; 60% for men and 56% for women; p = 0.067). Participants in the CT study were on average slightly younger and performed better on the Mini-Mental State Examination. There were no significant differences in the prevalence of dementia or major depression between the CT group and non-CT group (table e-1 on the Neurology® Web site at Neurology.org).
The examinations included comprehensive somatic and neuropsychiatric assessments. These comprised medical history, use of medications, ratings of signs and symptoms of psychiatric and somatic disorders, laboratory analyses, and key informant interviews. The neuropsychiatric examinations were performed by psychiatrists between 1986 and 2000 and by experienced psychiatric nurses in 2000.
Standard protocol approvals, registrations, and patient consents.
The Ethics Committee for Medical Research at Gothenburg University approved all studies. Informed consent was obtained from all participants, close relatives, or both.
Diagnosis of clinical and radiologic signs of iNPH.
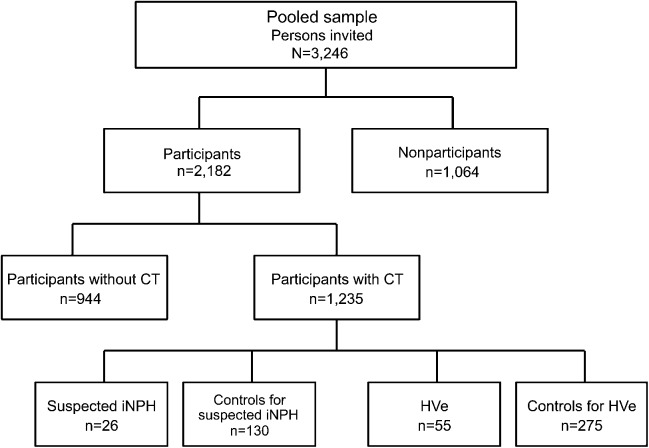
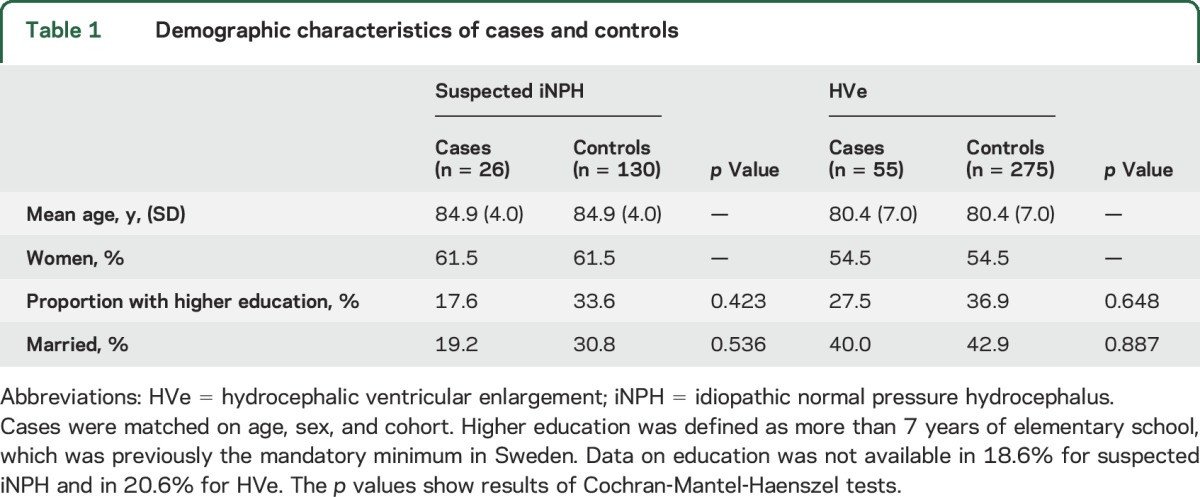
Clinical and radiologic signs of iNPH were evaluated using CT images of the brain and data from clinical examinations. This has previously been described in detail.1 We evaluated all scans for hydrocephalic ventricular enlargement (HVe), i.e., radiologic findings consistent with iNPH. HVe was diagnosed based on previous descriptions of radiologic signs in the literature21–23 and defined as follows: a general enlargement of all 4 ventricles without atrophy or concomitant widening of cortical sulci. Persons with obstruction of CSF flow or other pathology influencing the ventricular system were excluded. Absence or presence of WMLs was not considered for the diagnosis. A consultant neuroradiologist, unaware of the clinical data, made the final diagnosis of HVe in all cases. In total, 55 persons had HVe (mean Evans index 0.36, SD 0.04). Of these, 26 persons had clinical signs and symptoms that fulfilled clinical criteria for probable iNPH in accordance with the American–European iNPH guidelines.21 These cases were labeled suspected iNPH and comprised persons who, in addition to having radiologic findings of iNPH, had gait disturbance according to examination or self-report, and either a Mini-Mental State Examination score of ≤25 or urinary incontinence. Among cases with HVe that did not fulfill criteria for suspected iNPH, 17 had gait disturbance, cognitive impairment, or urinary incontinence, and 12 did not have any symptoms of iNPH. History of severe head trauma, meningitis, or subarachnoid bleeding was used as exclusion criteria for suspected iNPH. Demographic characteristics are shown in table 1.
Table 1.
Demographic characteristics of cases and controls
Clinical assessments.
We used data from the clinical examinations and the Swedish Hospital Discharge Register to determine the occurrence of vascular risk factors. The Swedish Hospital Discharge Register is a validated national health care register that currently records more than 99% of all somatic and psychiatric hospital discharges in Sweden.24 We classified any of the risk factors as present if a previous hospital diagnosis had been made.
Risk factors and WMLs.
We assessed history of hypertension, DM, stroke/TIA, and CAD using data from the Swedish Hospital Discharge Register and self-reported diagnosis as told by a physician. History of hypertension was defined as a previous diagnosis of hypertension or use of antihypertensive medication. DM was defined as previous diagnosis of DM type 1 or type 2, or pharmacologic treatment. Pharmacologic treatment of hypertension and DM was assessed by self-report. Stroke/TIA was defined as a previous diagnosis of ischemic or hemorrhagic stroke or TIA. CAD was defined as previous diagnosis of myocardial infarction or angina pectoris told by a physician. Table e-2 shows the ICD codes in the Swedish Hospital Discharge Register that were used. In addition, we used data from interviews and clinical examinations to assess smoking and overweight. Smoking was classified as past or present cigarette smoking vs never having smoked. Overweight was defined as body mass index >25 kg/m2.
WMLs were examined by experienced radiologists unaware of clinical data. These were made independently of the evaluation for HVe, i.e., WMLs and HVe were not examined by the same persons. The rating of WMLs has previously been described in detail.19,20,25 WMLs were defined as low-density areas in the periventricular and subcortical white matter. Decreased density was rated in relation to the attenuation of normal white matter and was defined as no, mild, moderate, or severe.25 We classified WMLs as moderate to severe vs none or only mild.
Statistical analysis.
We used a nested case-control design. Two groups of cases were defined: those with HVe and those with suspected iNPH. For each case, 5 controls were randomly selected from the total sample matched for age, sex, and cohort (figure 2). The control groups comprised CT participants without radiologic signs of iNPH (275 controls for HVe, and 130 controls for suspected iNPH). We tested frequency and unadjusted odds for categorical data using Pearson χ2 test. Because analyses were performed on a matched sample, the Cochran-Mantel-Haenszel test was also performed. As predetermined, variables with an initial p value <0.1 were further examined in multivariable regression models. Because the sample was matched, we used conditional logistic regression analysis. Separate models were made for HVe and suspected iNPH. Statistical tests were 2-sided and results considered significant at p < 0.05. Analyses were performed using SPSS Statistics 22.0 (IBM Corp., Armonk, NY).
Figure 2. Overview of study sample.
Of those with HVe (n = 55), 26 had suspected iNPH (i.e., had clinical signs that fulfilled guideline criteria for probable iNPH). Among those with HVe that did not fulfill guideline criteria for suspected iNPH (n = 29), 17 had gait disturbance, cognitive impairment, or urinary incontinence, and 12 persons did not have symptoms of iNPH. Both control groups were randomly selected from CT participants and comprised persons without radiologic findings consistent with iNPH. Among persons in the pooled sample, 2 were excluded because of complete lack of data. One person was found to be younger than 70 years and was therefore also excluded. HVe = hydrocephalic ventricular enlargement; iNPH = idiopathic normal pressure hydrocephalus.
RESULTS
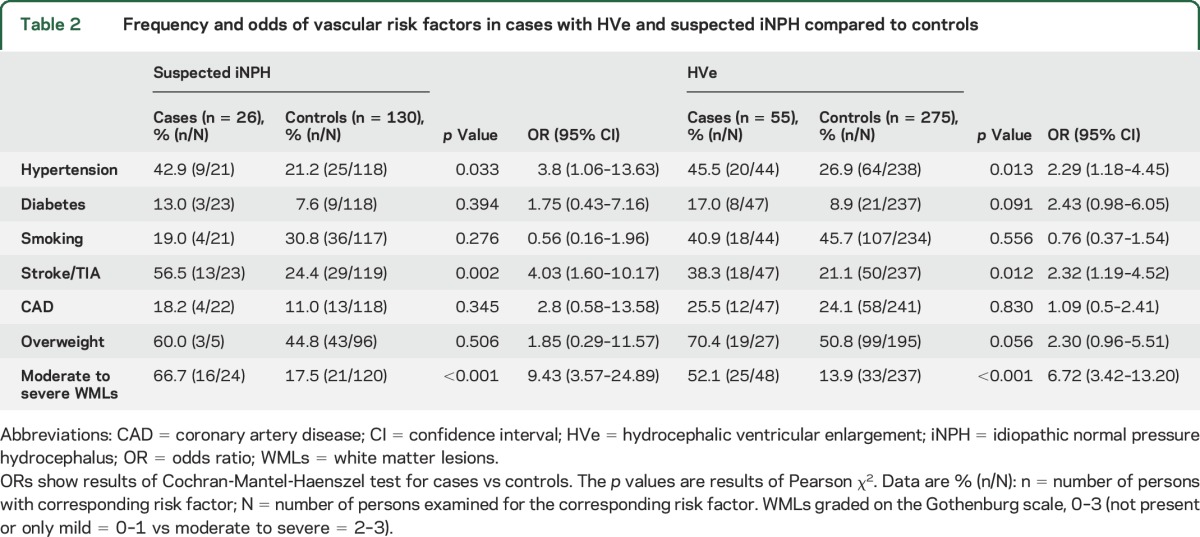
Frequency of risk factors is shown in table 2. History of hypertension and stroke/TIA and moderate to severe WMLs were associated with suspected iNPH compared to matched controls. Similarly, history of hypertension and stroke/TIA and moderate to severe WMLs were associated with HVe compared to matched controls. In addition, the frequency of DM was 17% in those with HVe and 8.9% among controls (p = 0.091). The frequency of overweight was 70.4% in those with HVe compared to 50.8% among controls (p = 0.056).
Table 2.
Frequency and odds of vascular risk factors in cases with HVe and suspected iNPH compared to controls
In the conditional logistic regression models, suspected iNPH was related to moderate to severe WMLs, while HVe was related to history of hypertension, moderate to severe WMLs, and DM. These results are shown in table 3.
Table 3.
Multivariable analysis of vascular risk factors in HVe and suspected iNPH
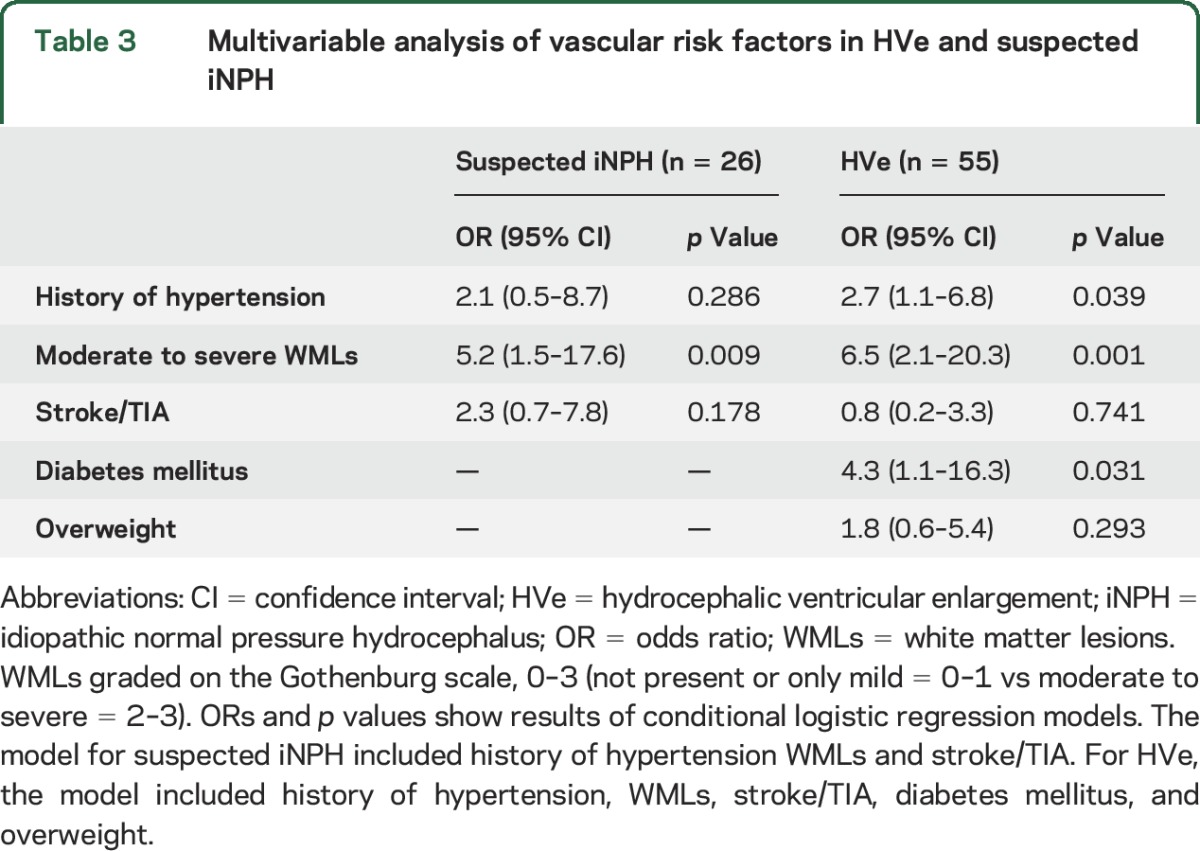
Among cases with HVe, all but 4 persons (51/55, 93%) had history of hypertension, DM, or WMLs on CT. Of those with suspected iNPH, all but 2 persons (24/26, 92%) had history of hypertension, DM, or WMLs on CT.
DISCUSSION
In this population-based, nested case-control study, we examined clinical and imaging findings of suspected iNPH in relation to WMLs and vascular risk factors. We found that WMLs were strongly related to both HVe and suspected iNPH. In addition, history of hypertension and DM were associated with HVe independent of WMLs. Our results suggest that vascular factors are involved in the disease mechanism of this disorder. Our findings may also have implications for prevention, as these risk factors are modifiable. Clinicians should be aware of the high occurrence of vascular risk factors in iNPH.
Previous case-control studies on risk factors for iNPH were hospital-based,4–8,11 and cases and controls were selected separately, thus having a potential risk of selection bias. Also, hospital-based samples probably include more severe cases. These studies reported associations between iNPH, WMLs, and various vascular risk factors, mainly hypertension and DM.4–8,11,12 Our study adds to existing knowledge by being population-based. We examined a large representative sample and found that almost all cases had history of hypertension, DM, or WMLs. Despite having used different methods and diagnostic criteria than previous studies, our results are in line with earlier findings.
The strong association between iNPH and WMLs might indicate that microvascular disturbances in the white matter are part of the disease process in iNPH. Although other explanations might also exist, WMLs have previously been found to give rise to similar subcortical symptoms as iNPH, such as gait disturbance, urinary incontinence, and cognitive impairment.26 Of note, patients with iNPH have also been found to have reduced blood flow in the periventricular white matter compared to controls.27 Previous studies have shown that cerebral blood flow increases after CSF removal, i.e., following lumbar puncture or shunt surgery.28–30 Furthermore, postoperative reduction of WMLs in iNPH correlates with clinical improvement.31,32 It is thus possible that clinical improvement after shunt surgery is attributable to reversal of white matter changes.
Our findings, and those from previous studies, suggest that hypertension and DM are involved in the pathophysiology of iNPH. Both of these conditions are associated with small vessel disease and impaired blood flow with reduced metabolism.10,33 However, we found that hypertension and DM increased the risk of iNPH independently of WMLs. It is thus possible that hypertension and DM increase risk of iNPH through other mechanisms than the development of WMLs. One explanation may be that hypertension has a direct mechanical effect on ventricular size. A relation between hypertension and increased ventricular volume was reported in a prospective cohort study.34 Also, progressive ventricular dilation has been found to occur in spontaneously hypertensive rats.35 In another study, hydrocephalus developed rapidly in sheep after balloons were inserted into the ventricles and set to inflate during systole and deflate during diastole, thus increasing the pulse pressure without concomitant increase in mean CSF pressure.36 It might be that hypertension and DM contribute to stiffening of the large vessels leading to a decrease in windkessel effect, i.e., the ability of elastic arteries to expand during systole.37 As a consequence, pulse pressure is increased resulting in higher mechanical force on the brain parenchyma. This could ultimately lead to distension of the ventricles. Our findings should be seen in light of reports that patients with iNPH and concurrent hypertension or DM have more severe symptoms than those without.38
Among the strengths of this study are the size and representativeness of the population-based sample. All participants were systematically selected from the general population, i.e., participants were not scanned because of symptoms or suspicions of iNPH, and we identified cases with HVe without considering clinical data. Nevertheless, similar to earlier studies, we found significant associations between vascular factors and clinical and imaging features of iNPH.
Another strength is that data were obtained from comprehensive clinical examinations complemented with data from the Swedish Hospital Discharge Register. We used a nested case-control design, which has several advantages compared to standard case-control studies that use hospital-based samples. For example, both cases and controls were obtained from the same population, thus reducing the risk of selection bias. In addition, exposure data were collected prospectively, and the risk of recall bias was minimized. The Hospital Discharge Register included information dating back several decades before the examinations.
There are several important limitations that should be discussed. One main limitation is the cross-sectional design, which precludes the possibility of determining the causal pathway between WMLs and symptoms and signs of iNPH. Several mechanisms for the association might exist and we cannot exclude that iNPH itself causes white matter changes due to edema or CSF stagnation. It might also be that the causal pathway between WMLs and iNPH is bidirectional. Nevertheless, the main finding in this study is the overlap in vascular risk factors, white matter changes, and radiologic and clinical signs of iNPH. Considering the population-based design, our findings might be important for understanding possible disease mechanisms.
Both WMLs and iNPH are associated with cognitive impairment and gait difficulty. Thus, the classic symptoms of iNPH might be due to concomitant white matter disease, although the reverse may also be true. This could possibly explain the overlap between vascular risk factors, white matter changes, and symptoms and signs of iNPH. It has been shown that shunt surgery improves symptoms in those with coexisting iNPH and dementia due to extensive white matter disease,39 and white matter changes are reduced following CSF drainage.31,32,39
Another main limitation was the use of CT. WMLs are better visualized with MRI and were rated in a rather crude way. Several of the CT scans were made using imaging techniques that are inferior to modern standards and we were thus unable to quantify volume of WMLs or analyze blood flow. Also, we cannot exclude that some persons with normal-appearing white matter on CT had a substantially disrupted anatomy that was not detected but might have been visualized by diffusion tensor imaging. This could be one reason why hypertension, DM, and WMLs were independently related to iNPH. However, it might be that CT is more specific for severe WMLs and thus detects changes that are more clinically relevant.40 Suboptimal imaging of WMLs could have reduced our chances of finding significant results. From this standpoint, the associations between iNPH and WMLs were surprisingly strong.
An additional limitation was the lack of precision in diagnosing iNPH. Cases were diagnosed retrospectively based on CT and data from clinical examinations. MRI would have been better for the diagnosis of iNPH. Also, supportive diagnostic tests and response to shunt were not evaluated. However, shunt responsiveness and supplementary testing are not mandatory for the diagnosis of iNPH.21 Although the diagnosis was made in accordance with international guideline criteria, several symptoms of iNPH are common among elderly persons and often nonspecific. Thus, we cannot rule out the possibility of misdiagnosis or that some cases had concomitant disorders that confounded the results. However, it is important to note that the associations were similar or even stronger for HVe. The group with HVe was selected only on the basis of imaging criteria and should therefore be subject to less potential bias due to uncertainty regarding the classification of clinical symptoms.
Another limitation is that the number of cases was relatively small. We cannot exclude that some of the results might have been falsely negative because of lack of statistical power, e.g., risk factors for suspected iNPH. However, it can be difficult to obtain a large number of cases using longitudinally followed cohorts from the general population. Also, in this study, we assessed all persons identified in a large representative sample obtained by merging data from several cohorts. Although the examinations were similar or identical between studies and were conducted by the same research group, we cannot exclude the possibility that procedures might have changed slightly over the years. The matching by cohort should reduce this possible bias.
Several variables were assessed using self-report. A certain risk of reporting error might thus have been entailed, especially in those with suspected iNPH. However, the concomitant use of the Swedish Hospital Discharge Register as well as key informant interviews probably increased accuracy of exposure data. Regarding the assessment of hypertension, some of the participants were treated with antihypertensive medications. It is possible that treatment would influence the relation with iNPH. However, if antihypertensive medications affected the risk of iNPH, our chances of getting significant results would probably have been reduced.
Another limitation is that our sample only included persons aged 70 years or older. It might be that disease mechanisms differ in younger persons with iNPH.
We found that history of hypertension, DM, and WMLs was related to clinical and imaging features of iNPH. Vascular mechanisms are probably involved in the pathophysiology of iNPH. The results of this study might have importance for prevention. Clinicians treating patients with iNPH should be aware of the high occurrence of vascular risk factors.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Tina Jacobsson and Cecilia Mellqvist for helping with the planning and coordination of the study and Mats Tullberg for helping with the initial assessments of the CT images. The authors also thank Kristoffer Bäckman for providing insight and advice regarding statistical analysis.
GLOSSARY
- CAD
coronary artery disease
- CI
confidence interval
- DM
diabetes mellitus
- HVe
hydrocephalic ventricular enlargement
- ICD
International Classification of Diseases
- iNPH
idiopathic normal pressure hydrocephalus
- OR
odds ratio
- WML
white matter lesion
Footnotes
Supplemental data at Neurology.org
Editorial, page 588
AUTHOR CONTRIBUTIONS
Daniel Jaraj made the screening assessment of the CT images, participated in the design of the study, performed statistical analyses, helped coordinate the study as well as process and interpret the data, and drafted the manuscript. Simon Agerskov participated in the design of the study, performed statistical analyses, helped process and interpret the data, and drafted the manuscript. Katrin Rabiei made the screening assessment of the CT images and helped revise the manuscript. Thomas Marlow participated in the design of the study, performed statistical analyses, helped process and interpret the data, and helped revise the manuscript. Christer Jensen made the final radiologic assessment of the CT images and helped revise the manuscript. Xinxin Guo participated in collection of clinical data and helped revise the manuscript. Silke Kern participated in collection of clinical data and helped revise the manuscript. Carsten Wikkelsø conceived the study, participated in the design of the study, participated in the screening assessment of the CT images, helped coordinate the study, and helped revise the manuscript. Ingmar Skoog was principal investigator of the population studies, interpreted the data, participated in the design of the study, and helped revise the manuscript. All authors have read and approved the final manuscript.
STUDY FUNDING
The Swedish Research Council (11267, 2005-8460, 825-2007-7462, 825-2012-5041, 2013-8717), Swedish Research Council for Health, Working Life and Welfare (2001-2646, 2003-0234, 2004-0150, 2006-0020, 2008-1229, 2004-0145, 2006-0596, 2008-1111, 2010-0870, AGECAP 2013-2300, 2013-2496, Epilife 2006-1506), Swedish Brain Power, The Alzheimer's Association Zenith Award (ZEN-01-3151), The Alzheimer's Association Stephanie B. Overstreet Scholars (IIRG-00-2159), The Bank of Sweden Tercentenary Foundation, Hjärnfonden, Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Swedish Alzheimerfonden, Alma och Anna Yhlen's Foundation, Sahlgrenska University Hospital (ALF).
DISCLOSURE
D. Jaraj has received funding from the University of Gothenburg. S. Agerskov has received funding from ISNF (Insamlingsstiftelsen för Neurologisk Forskning). K. Rabiei, T. Marlow, C. Jensen, X. Guo, S. Kern, and C. Wikkelsø report no disclosures relevant to the manuscript. I. Skoog reports grants from the Swedish Research Council, grants from the Swedish Research Council for Health, Working Life and Welfare, during the conduct of the study, and personal fees from Takeda, outside the submitted work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 2014;82:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klinge P, Hellstrom P, Tans J, Wikkelso C. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand 2012;126:145–153. [DOI] [PubMed] [Google Scholar]
- 3.Iseki C, Takahashi Y, Wada M, Kawanami T, Adachi M, Kato T. Incidence of idiopathic normal pressure hydrocephalus (iNPH): a 10-year follow-up study of a rural community in Japan. J Neurol Sci 2014;339:108–112. [DOI] [PubMed] [Google Scholar]
- 4.Graff-Radford NR, Godersky JC. Idiopathic normal pressure hydrocephalus and systemic hypertension. Neurology 1987;37:868–871. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs L. Diabetes mellitus in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 1977;40:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauss JK, Regel JP, Vach W, Droste DW, Borremans JJ, Mergner T. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke 1996;27:24–29. [DOI] [PubMed] [Google Scholar]
- 7.Casmiro M, D'Alessandro R, Cacciatore FM, Daidone R, Calbucci F, Lugaresi E. Risk factors for the syndrome of ventricular enlargement with gait apraxia (idiopathic normal pressure hydrocephalus): a case-control study. J Neurol Neurosurg Psychiatry 1989;52:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eide PK, Pripp AH. Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barriers CNS 2014;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus: research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 11.Bradley WG, Jr, Whittemore AR, Watanabe AS, Davis SJ, Teresi LM, Homyak M. Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible origin of normal-pressure hydrocephalus. AJNR Am J Neuroradiol 1991;12:31–39. [PMC free article] [PubMed] [Google Scholar]
- 12.Krauss JK, Regel JP, Vach W, et al. White matter lesions in patients with idiopathic normal pressure hydrocephalus and in an age-matched control group: a comparative study. Neurosurgery 1997;40:491–495; discussion 495–496. [DOI] [PubMed] [Google Scholar]
- 13.Skoog I. Psychiatric epidemiology of old age: the H70 study—the NAPE lecture 2003. Acta Psychiatr Scand 2004;109:4–18. [DOI] [PubMed] [Google Scholar]
- 14.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med 1993;328:153–158. [DOI] [PubMed] [Google Scholar]
- 15.Skoog I, Olesen PJ, Blennow K, Palmertz B, Johnson SC, Bigler ED. Head size may modify the impact of white matter lesions on dementia. Neurobiol Aging 2012;33:1186–1193. [DOI] [PubMed] [Google Scholar]
- 16.Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia: population study of 85-year-olds. Br J Psychiatry 1999;174:249–253. [DOI] [PubMed] [Google Scholar]
- 17.Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Göteborg, Sweden. Nutrition 2009;25:613–619. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968–1969: a population study. General design, purpose and sampling results. Acta Med Scand 1973;193:311–318. [DOI] [PubMed] [Google Scholar]
- 19.Simoni M, Pantoni L, Pracucci G, et al. Prevalence of CT-detected cerebral abnormalities in an elderly Swedish population sample. Acta Neurol Scand 2008;118:260–267. [DOI] [PubMed] [Google Scholar]
- 20.Olesen PJ, Gustafson DR, Simoni M, et al. Temporal lobe atrophy and white matter lesions are related to major depression over 5 years in the elderly. Neuropsychopharmacology 2010;35:2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:S4–S16; discussion ii–v. [DOI] [PubMed] [Google Scholar]
- 22.Evans WA., Jr An encephalographic ratio for estimating the size of the cerebral ventricles: further experience with serial observations. Am J Dis Child 1942;64:820–830. [Google Scholar]
- 23.Hashimoto M, Ishikawa M, Mori E, Kuwana N. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olesen PJ, Guo X, Gustafson D, et al. A population-based study on the influence of brain atrophy on 20-year survival after age 85. Neurology 2011;76:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- 27.Momjian S, Owler BK, Czosnyka Z, Czosnyka M, Pena A, Pickard JD. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004;127:965–972. [DOI] [PubMed] [Google Scholar]
- 28.Ziegelitz D, Starck G, Kristiansen D, et al. Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J Magn Reson Imaging 2014;39:1533–1542. [DOI] [PubMed] [Google Scholar]
- 29.Kimura M, Tanaka A, Yoshinaga S. Significance of periventricular hemodynamics in normal pressure hydrocephalus. Neurosurgery 1992;30:701–704; discussion 704–705. [PubMed] [Google Scholar]
- 30.Hertel F, Walter C, Schmitt M, et al. Is a combination of Tc-SPECT or perfusion weighted magnetic resonance imaging with spinal tap test helpful in the diagnosis of normal pressure hydrocephalus? J Neurol Neurosurg Psychiatry 2003;74:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tullberg M, Hultin L, Ekholm S, Mansson JE, Fredman P, Wikkelso C. White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand 2002;105:417–426. [DOI] [PubMed] [Google Scholar]
- 32.Tullberg M, Jensen C, Ekholm S, Wikkelso C. Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. AJNR Am J Neuroradiol 2001;22:1665–1673. [PMC free article] [PubMed] [Google Scholar]
- 33.Novak V, Last D, Alsop DC, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 2006;29:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graff-Radford NR, Knopman DS, Penman AD, Coker LH, Mosley TH. Do systolic BP and pulse pressure relate to ventricular enlargement? Eur J Neurol 2013;20:720–724. [DOI] [PubMed] [Google Scholar]
- 35.Ritter S, Dinh TT. Progressive postnatal dilation of brain ventricles in spontaneously hypertensive rats. Brain Res 1986;370:327–332. [DOI] [PubMed] [Google Scholar]
- 36.Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. Experimental hydrocephalus following mechanical increment of intraventricular pulse pressure. Experientia 1977;33:1470–1472. [DOI] [PubMed] [Google Scholar]
- 37.Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther 1995;9:73–83. [DOI] [PubMed] [Google Scholar]
- 38.Hellstrom P, Edsbagge M, Archer T, Tisell M, Tullberg M, Wikkelso C. The neuropsychology of patients with clinically diagnosed idiopathic normal pressure hydrocephalus. Neurosurgery 2007;61:1219–1226; discussion 1227–1228. [DOI] [PubMed] [Google Scholar]
- 39.Tisell M, Tullberg M, Hellstrom P, Edsbagge M, Hogfeldt M, Wikkelso C. Shunt surgery in patients with hydrocephalus and white matter changes. J Neurosurg 2011;114:1432–1438. [DOI] [PubMed] [Google Scholar]
- 40.Lopez OL, Becker JT, Jungreis CA, et al. Computed tomography—but not magnetic resonance imaging—identified periventricular white-matter lesions predict symptomatic cerebrovascular disease in probable Alzheimer's disease. Arch Neurol 1995;52:659–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.