Abstract
Scientists, public health and school officials are paying growing attention to the mechanism underlying the delayed sleep patterns common in human adolescents. Data suggest that a propensity towards evening chronotype develops during puberty, and may be caused by developmental alterations in internal daily timekeeping. New support for this theory has emerged from recent studies which show that pubertal changes in chronotype occur in many laboratory species similar to human adolescents. Using these species as models, we find that pubertal changes in chronotype differ by sex, are internally generated, and driven by reproductive hormones. These chronotype changes are accompanied by alterations in the fundamental properties of the circadian timekeeping system, including endogenous rhythm period and sensitivity to environmental time cues. After comparing the developmental progression of chronotype in different species, we propose a theory regarding the ecological relevance of adolescent chronotype, and provide suggestions for improving the sleep of human adolescents.
Keywords: biological rhythm, reproductive hormone, comparative endocrinology, sleep, puberty, sex, suprachiasmatic nucleus, temporal niche, entrainment, period
Introduction
“Each morning, few of the country’s 17 million high school students are awake enough to get much out of their first class, particularly if it starts before 8 a.m. Sure, many of them stayed up too late the night before, but not because they wanted to. Research shows that teenagers’ body clocks are set to a schedule that is different from that of younger children or adults.” (excerpt from the New York Times [87])
Recently, there has been a growing public discussion regarding the delayed sleep patterns, or evening chronotype, of human adolescents and their influence on health and school performance. The cause of these “night owl tendencies” is theorized to be a pubertal hormonally-driven change in the body’s internal clock. What research supports this claim? If adolescents truly do have a “biological schedule” that is different from that of adults, why might this be? In order to address these questions, our laboratory has examined the rest and activity patterns of two laboratory species during the adolescent period, and then performed an intensive review of the existing developmental sleep and circadian literature to determine how well our conclusions can be generalized to other mammalian species, including human children. We review these data with the goal of drawing out themes that can be used to inform the policy decisions affecting the health of adolescents as well as theories regarding the role of within species “temporal niche” changes in ecology.
Overview of Adolescent Sleep Patterns in Humans: Evidence Suggesting Physiological Underpinnings
Sleep deprivation amongst adolescents is epidemic. Recent studies show that many adolescents maintain schedules during the school year that result in insufficient and ill-timed sleep. Unlike adults, growing adolescents require on average as much as 9-10 hours of sleep per night [29, 33]. Despite this fact, over 45% of adolescents in the United States report obtaining less than 8 hours of sleep on school nights [137]. Similar trends have been observed in other modern societies, including Korea, Brazil, and Italy [11, 59, 62, 209]. This chronic sleep deprivation interferes with daytime functioning. In Iceland over 70% of individuals between the ages of 16-21 reported frequent daytime sleepiness [188]. Other surveys indicate that 15-52% of adolescents have problems with excessive sleepiness, including oversleeping, inadvertent napping, and sleeping during class [62].
At the root of this chronic sleep deprivation is the adolescent tendency to stay up late. Many studies indicate that teenagers maintain later (delayed) bedtimes than younger children, even when wake times are constrained by school or work (Figure 1, [45, 62, 188]). This tendency to stay up late has been attributed to many external influences, ranging from evening work schedules and increased academic responsibilities to late night television and social opportunities [24, 35, 191]. Current evidence, however, demonstrates that social factors do not completely account for the adolescent shift towards evening chronotype. Indeed, some of the social changes may result from the physiological changes.
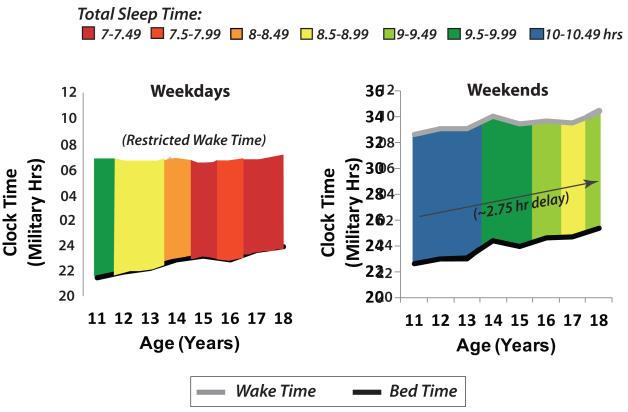
Figure 1. The Timing of Sleep Delays During Adolescence.
This figure was created by averaging data from 13 studies in 9 countries reported in a recent metanalysis of adolescent sleep patterns [62]. The average bed times (black line) and wake times (grey line) are shown for both weekdays (left graph) and weekends (right graph). In general, the timing of sleep becomes progressively later (y-axis = clock time in military hrs) over the course of puberty and adolescence (x-axis=age in years). Note that this change is most striking during weekends, when wake times are relatively unconstrained. On weekdays, when wake times are restricted due to school or work, bedtimes still grow later during adolescence so that total sleep time becomes increasingly restricted (total sleep time is indicated by color, with a key at the top of the figure). Adolescents are recommended to obtain 9-10 hrs of sleep each night, thus anything shown in red (7-7.99 hrs of sleep) can be considered to be severely sleep-restricted.
First, this phenomenon is widespread and cross-cultural: a delay in the timing of sleep during the second decade of life has been observed in over 20 countries on 6 continents, in cultures ranging from pre-industrial to modern (as reviewed in [28, 62]). A recent metanalysis of 13 studies from 9 countries indicated that weekend bedtimes delayed by ~2.75 hrs between the ages of 11-18, even though overall bedtimes and wake times varied by culture [62]. This phenomenon is also not restricted to modern times: although most studies have been cross-sectional, retrospective longitudinal measures indicate that teenagers exhibited later sleep times than children or adults before the advent of computers, MTV, internet, and cell phones [28, 160].
The developmental timing of this transition parallels pubertal development. Girls begin to show a delay in the timing of sleep one year earlier than boys, mimicking their younger pubertal onset (Figure 2A). Similarly, girls show maximum delay at the age of 19.5 years, and boys show maximum delay later at 20.9 years [160]. In other cultures, similar developmental timing is observed, although the peak delay may occur as early as 15-16 years of age [45, 163, 188, 209]. In addition to there being a sex difference in the timing of the adolescent transition into evening chronotype, there is also a sex difference in the magnitude of the delay, with boys showing more extreme changes in chronotype across the adolescent period [160]. Altogether, this evidence suggests that the adolescent transition into a more evening chronotype is driven by pubertal hormones.
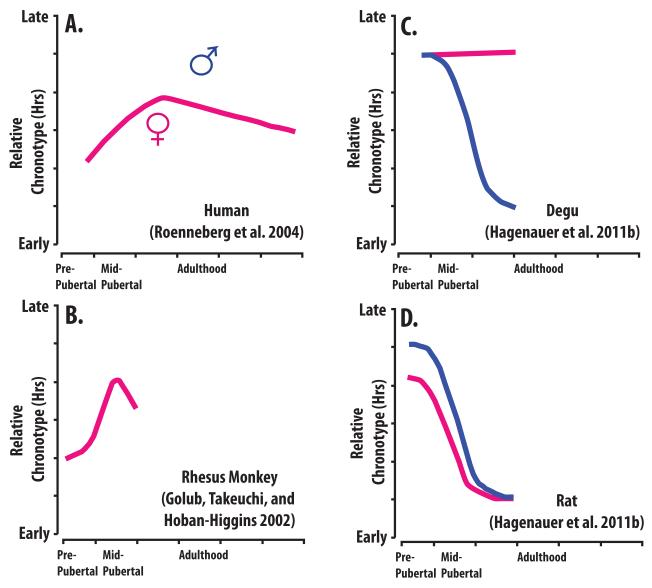
Figure 2. A Comparison of Chronotype Changes During Puberty in Four Well-Studied Species.
Data from males and females are shown in blue and pink, respectively. Chronotype was approximated from the phase variables measured in each experiment [60, 66, 67, 160] and is depicted in terms of relative change in hours. For example, panel C summarizes the results from a study in which pubertal rats showed approximately a 3-4 hr phase advance in multiple phase markers (e.g., peak activity, activity offset [66]). However, as these markers occurred at different hours of the day, what is graphed is a relative phase advance across puberty in general. Pubertal stage was assigned with respect to secondary sex development in the same manner as discussed in [70]. Note that all four species exhibit a shift in chronotype between pre-puberty and puberty, with the two primate species (humans and macaques) exhibiting a phase-delay, and the two rodent species (degus and rats) exhibiting a phase-advance. In general, males show a larger shift during puberty than females. Following maturation, humans (and potentially macaques) exhibit a reversal of this shift, with chronotype growing progressively earlier.
Another indication that the adolescent shift towards a “night-owl” behavior has physiological underpinnings is the presence of this shift even under conditions where external influences are controlled. For example, adolescents continue to show a delayed circadian (or “internal clock”) phase as indicated by daily endocrine rhythms even after several weeks of regulated schedules that allow for sufficient sleep. This delay is also maintained under controlled laboratory conditions in which there is limited possibility for social influence [31, 32]. Both home-based and laboratory studies of adolescents show that delayed circadian phase correlates with secondary-sex development [31, 32, 33, 164], and this correlation holds true for subjective ratings of chronotype and puberty even when grade level in school is held constant [36]. If we assume that teenagers attending the same grade in school are exposed to a similar social environment, this evidence suggests that a biological component drives adolescent sleep patterns.
Physiological Determinants of the Timing of Sleep and Activity
What biological mechanisms might cause adolescents to maintain a different chronotype from children or adults? Traditionally, the timing of sleep is thought to derive from three primary components: an internal (endogenous) daily circadian timekeeping system, a homeostatic drive for sleep (“sleep pressure”) that builds up over the course of being awake and decreases during sleep, and other external constraints (which are often referred to as “masking”). Both internal components are known to be sensitive to sex (gonadal) hormones, such as estrogen and testosterone [123], and thus could be affected by puberty. This review specifically focuses on adolescent changes in the circadian timekeeping system, but there is growing evidence that the homeostatic drive to sleep also changes during adolescence (see recent reviews by Carskadon [27] and Hagenauer et al. [67]).
Introduction to Circadian Rhythms
The circadian timekeeping system plays an important role in determining the timing of sleeping and waking, but that is not its only, or even primary, physiological purpose. Circadian timekeeping is believed to have evolved billions of years ago to aid in efficient photosynthesis and unicellular replication in some of the very first organisms on earth. This physiological role grew as life diversified and multiplied and there became a constant competition for existence. Species began to make sophisticated use of their time-keeping capabilities to adapt to a specific temporal niche (also called chronotype) such as diurnality (day activity) or nocturnality (night activity). The timing of hunting, breeding, and hatching became just as important as their location [50]. In complex organisms the body’s internal clock also became a mechanism that could coordinate activity in tissues in disparate locations, and regulate such essential functions as cell division, hormone release, growth, metabolism, and reproduction [91, 165, 190]. Even on the cellular level, circadian rhythms coordinate and organize a remarkable variety of rhythmic processes. For example, within one large microarray study of the mouse liver, over 8% of the mRNA transcripts examined were found to be rhythmically expressed. Similar percentages have been found in other mammalian tissues, ranging from connective tissue to the cortex [5, 210].
In the laboratory, circadian rhythms are often modeled as sinusoidal oscillations (Figure 3). As mentioned earlier, these rhythms are endogenously-generated. Thus, under conditions in which there are no time cues from the outside world (also referred to as constant or “free-running” conditions), the circadian system will continue to generate daily rhythms. These rhythms will appear to “drift” a little each day, so that for any particular individual the rhythm will start either progressively earlier each day or progressively later because the rhythm’s period (or cycle length) is not exactly 24 hours (typically ranging from 23–25 hrs [150]). Under normal conditions, the endogenous rhythm must be entrained by external time cues (or “zeitgebers,” such as sunlight) to maintain a periodicity that matches environmental rhythms, such as the 24-hr solar day [151]. To allow entrainment, the circadian system is most sensitive to zeitgeber time cues at times of the day when they are not expected to be present. For example, light exposure in the evening causes circadian rhythms to shift later, and light exposure in the early morning causes circadian rhythms to shift earlier, but in most species light exposure during the midday has little effect because it confirms that the circadian system is already aligned properly with the solar day [83, 159].
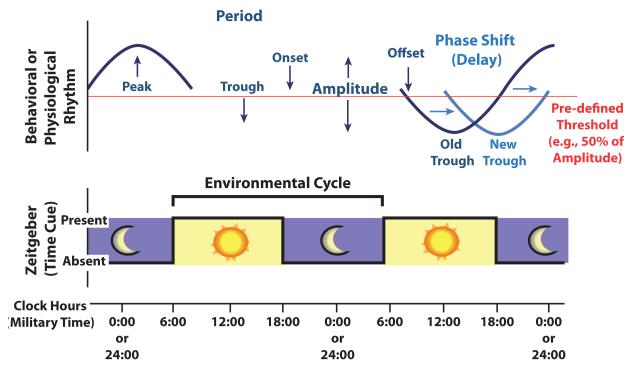
Figure 3. An Illustration of Commonly-Used Circadian Terminology.
Depicted on top is a simple sinusoidal model of a subject’s behavioral or physiological rhythm (e.g., daily activity), and depicted below is the environmental cycle to which the subject was exposed (e.g., a light-dark cycle).
After a rhythm is entrained, there is a stable phase relationship between phase markers for the rhythm (e.g., rhythm onset, peak, offset, and trough) and the zeitgeber time cue. Light is the dominant environmental zeitgeber, and under laboratory conditions the phase of biological and physiological rhythms is traditionally characterized by the relationship of phase markers to the solar day or laboratory light-dark (LD) cycle. If the phase of the rhythm shifts so that phase markers are occurring at a relatively earlier time, this is known as a phase-advance, whereas a shift that causes phase-markers to occur at a later time is known as a phase-delay [125]. Phase can also be compared between individuals or groups, for example the daily sleep rhythms of human adolescents are described as being delayed in phase relative to those of pre-pubertal children or adults [160].
Despite the traditional modeling of circadian rhythms as sinusoidal waves, most rhythms also contain components (or harmonics) with shorter periodicities referred to as ultradian rhythms. The most common ultradian rhythm is bimodal (a rhythm with two peaks, or 12-hr harmonic). This is because most organisms, whether diurnal or nocturnal, increase activity around the transition times of sunrise and sunset (or lights-on and lights- off in the laboratory, [15]). Animals that exhibit activity predominantly at these transition times are referred to as having a crepuscular chronotype [50]).
The Physiology of the Circadian System
Circadian rhythms in mammals are generated endogenously via a molecular negative feedback loop within individual pacemaker cells throughout the brain and body. Within this feedback loop, proteins produced by particular “clock genes” inhibit their own transcription, leading to daily cellular oscillations [18, 71, 211]. The core feedback loop consists of a positive arm, which contains proteins (BMAL1 and CLOCK) that drive transcription, and a negative arm, which contains proteins (PER, CRY) that inhibit transcription. To initiate the loop, a heterodimer of BMAL1 and CLOCK proteins drives the transcription of Per and Cry. The PER and CRY proteins (PER1, PER2, PER3, CRY1, CRY2) then form heterodimers that inhibit their own transcription [18, 211, for review see 71].
There is a particularly dense collection of pacemaker cells within the suprachiasmatic nucleus (SCN) of the hypothalamus in the brain [13]. This region is essential for driving and coordinating circadian rhythms throughout the body [155]. For this reason, the SCN is frequently referred to as the master circadian regulator (or “master clock”, [125]). Within the SCN, pacemaker cells use reciprocal communication to amplify and perpetuate oscillation and to maintain a specific alignment between rhythmic events, a function referred to as oscillator coupling [116, 205]. Molecular oscillations are translated into oscillations of membrane potential and firing rate. Coupling then occurs via electrical gap junctions between SCN cells and synaptic communication (especially involving the neurotransmitters GABA and vasoactive intestinal polypeptide [VIP] [16, 99]. Some non-neuronal glia cells in the SCN also appear to play an important role in this coupling by modulating intercellular communication [116].
Light can influence oscillations in the SCN via three known pathways: a direct pathway between the retina and ventrolateral SCN (vlSCN) called the retinohypothalamic tract (RHT) and two indirect pathways via the intergeniculate leaflet (IGL) and raphe nuclei. The two indirect pathways have distinct neurochemical signatures: projections from the IGL to the SCN contain neuropeptide Y (NPY), and projections from the raphe nuclei to the SCN contain serotonin (5-HT, [127]). The RHT, which is mostly glutamatergic, is the primary pathway mediating photic entrainment. Entrainment occurs when light exposure on the retina activates a special subset ganglion cells, called melanopsin ganglion cells, which are photosensitive to blue green light. This activation causes a rapid release of glutamate in the vlSCN, which binds to NMDA receptors, initiating a series of molecular events previously associated with long-term memory systems, including an intracellular cascade of second messenger signaling and synaptic remodeling. These signaling pathways induce the transcription of immediate early genes, such as cFos [115], as well as two components of the clock gene feedback loop, Per1 and Per2 [121, 173].
If this light exposure occurs during the evening and early morning hours, when Per1 and Per2 transcript levels are low, induction of Per1 and Per2 causes an overall phase shift (or realignment) of the molecular feedback loop ([173]; Figure 1.6) as well as rhythms in membrane potential [99]. This phase-shift propagates to the endogenously-rhythmic dorsomedial (dm) SCN [13, 133, 208] and eventually to rhythmic oscillators throughout the rest of the body [207]. Consequently, phase-shifts in the molecular feedback loop are thought to underlie the photic phase-shift of behavioral rhythms [173, 207] and it has become common to examine the phasing of Per1 and Per2 rhythms as a manner of characterizing the entrainment of the central circadian pacemaker and periphery (e.g., [2, 206, 207]).
It should be noted that the entrainment of the SCN is not the only determinant of the final phase of circadian output. For example, substantial evidence now indicates that adult diurnal and nocturnal species have a similar phasing of many aspects of SCN physiology [68, 180]. Therefore, the downstream coupling (or phase relationship) between the circadian pacemaker and central or peripheral systems plays an important role in determining the phase of behavioral and endocrine rhythms [180]. As mentioned earlier, many areas of the brain outside of the SCN (e.g,. cortex, striatum, hippocampus, amygdala) as well as other tissues in the body (e.g., liver, heart, adrenals, ovaries) contain daily rhythms in clock gene expression. These semi-autonomous or “slave oscillators” are thought to be entrained by output from the SCN as well as by non-photic zeigebers, such as daily rhythms in food intake [64]. Previous work indicates that the phase relationship between the central SCN oscillator and slave oscillators in other regions of the brain closely relates to the phase of behavioral activity rhythms [1, 110, 132, 193, 195], suggesting that these regions may be important for expressed chronotype.
A Bidirectional Relationship Exists Between the Circadian System and Reproductive Axis
One of the primary reasons for hypothesizing that the adolescent shift towards “night-owl” behavior represents the influence of pubertal hormones on the circadian timekeeping system is the large body of evidence indicating that the circadian system regulates the reproductive axis and vice-versa. This evidence is presented below.
Circadian Rhythms Regulate the Reproductive Axis
Circadian rhythms regulate many aspects of reproduction, including the timing of hormone release, ovulation, mating, and parturition [91, 190]. The circadian system also mediates the effects of photoperiod (day length) on seasonal reproduction [190]. In turn, reproductive hormones feedback on the circadian system [89].
In mammals, the reproductive system is controlled by hormones within the hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamus releases pulsatile gonadotropin-releasing hormone (GnRH). GnRH then drives the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) by the anterior pituitary, which then stimulate a hormonal environment conducive to reproduction [84]. The primary steroid hormones produced by the testes are androgens (e.g., testosterone, dihydrotestosterone), and the primary steroid hormones produced by the ovaries are estrogens (e.g., estradiol), and progestins (e.g., progesterone; [84]). However, both sexes produce all three hormone classes and their steroidal precursors, as well as other non-steroidal hormones such as the inhibins [138]. These hormones feed back on the hypothalamus and pituitary to regulate their own production [84].
In adult females, hormone production varies over the course of the reproductive cycle. In humans, this cycle lasts for around 28 days and contains two major phases: follicular and luteal. During the early follicular stage, estrogen and progesterone levels are low and the uterine lining is shed in preparation for a new cycle (menstruation). As follicular development proceeds, estrogen levels increase. When estrogen levels reach peak production, they cause positive feedback on the GnRH system, driving a surge of LH and, consequently, ovulation. Following ovulation, the follicle converts into a secretory luteal body and begins to produce progesterone as well as estrogen. Progesterone levels remain high for most of the luteal phase [84, 112].
In laboratory animals, the female reproductive cycle follows a different progression. With the exception of primates, menstruation does not occur, and therefore the cycles are referred to as estrous cycles instead of menstrual cycles. For some species (e.g., the degu), the estrous cycle contains both a follicular and luteal phase [108]. However, in most traditional laboratory rodents (rats and mice), estrous cycles are short (4-5 days) and do not contain a true luteal phase, except following vaginocervical stimulation (pseudopregnancy). In these species, estrogen peaks on the day of proestrus around 12 hours prior to ovulation, and is followed several hours later by a peak in progesterone. Ovulation occurs that night, and is followed by a period of sexual receptivity (estrus), despite a concurrent drop in steroid hormone levels [112].
In traditional laboratory rodents (rats, mice, hamsters), circadian regulation of the HPG axis has received particular attention because lesions of the SCN eliminate ovulation and produce a state of persistent estrus [39, 154]. Further investigation revealed that the SCN sends a signal daily to GnRH neurons. When estrogen levels are high, this signal helps drive the GnRH/LH surge that leads to ovulation [39]. Therefore, arguably in these species the SCN is actually an integrated component of the HPG axis. Recently, researchers have also found that clock genes are expressed at all levels of the HPG axis, including the GnRH neurons, the pituitary, and the ovaries [171]. These clock genes seem to play an important role in the generation of synchronized pulsatile hormone release [40], and in the preparation of the ovary for ovulation [171].
Circadian Rhythms are Sensitive to Reproductive Hormones
Due to the circadian regulation of ovulation in traditional laboratory rodents, there has been substantial interest in the influence of gonadal hormones on the circadian system. Gonadal hormones can influence the brain in several ways. The activational effects of hormones are direct and transient. If a hormone is functioning activationally, then at any time an experimenter should be able to remove the hormone from the system or block its receptor and the effect will disappear. Activational effects can occur due to hormones binding to their traditional receptors [113], or due to hormonal modulation of the efficacy of neurotransmitter receptors (e.g., progesterone metabolites can alter the function of GABA or NMDA receptors, [101, 162]). Organizational (long term/permanent) effects can also occur in response to steroid hormones. These effects are due to steroid hormones binding at nuclear receptors to produce long-term changes in the transcription of particular genes [113]. Organizational effects typically occur during a sensitive period of development, known as a critical period. Although some organizational changes cause direct functional effects, others may alter hormone receptor distribution and sensitivity. In this case, the organizational effects will not be observed unless hormones are present [179].
Gonadal hormones can transiently affect activity and permanently organize the circadian system. One of the best-known activational effects is on activity rhythms during the female reproductive cycle in rodents. On the day of estrus, following elevated estrogen, female rodents exhibit increased activity and begin their activity earlier in the day. The next day, after hormone levels drop, the females phase-delay activity onset and decrease overall activity levels (Figure 4, [17]). During the human menstrual cycle circadian rhythms may also shift their phasing, but results are contradictory [109, 143, 144, 167]. Indeed, in adult laboratory rodents a wide variety of gonadal hormones can affect the phase of circadian rhythms, including estrogens, progestins, androgens, and non-traditional neuroactive steroids (e.g., rats: [8, 17], hamsters: [49, 51, 129], mice: [23, 47, 78, 90], degus: [80, 100]).
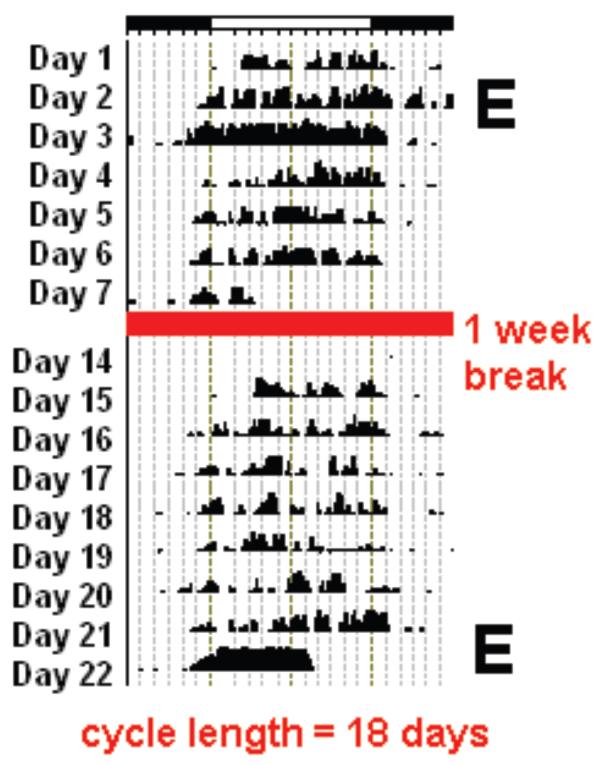
Figure 4. Reproductive Cycles Affect Daily Rest/Activity Rhythms.
In many rodent species, females show large changes in their rest/activity rhythms over the course of their reproductive cycle. Following elevated estrogen, on the day of estrus females exhibit increased activity and begin their activity earlier in the day (phase-advance). Shown are two examples of estrus-typical wheel-running activity from a degu, with the putative day of estrus marked with an “E.” The activity is graphed as an actogram, with each horizontal line sequentially representing a day of activity (# wheel turns/10 min bin) and the environmental light-dark cycle indicated by the bar at the top of the figure. The red line in the middle of the actogram represents a one-week break in recording, and the estrous cycle length for the female is noted at the bottom [108].
Gonadal hormones can influence other circadian parameters as well, including endogenous period [7, 23, 47, 49, 91, 129, 212], rhythm amplitude [8, 17, 100], range of entrainment [49], zeitgeber sensitivity [23, 51, 79, 81], and oscillator coupling [126, 128, 187]. Many of these parameters also exhibit sex differences. For example, hamsters, degus, and mice all show sex differences in their phase-response to light [23, 49, T.M. Lee unpublished data]. Although some of these sex differences are activated by the different hormonal milieu of adult males and females, some sex differences also arise organizationally during development [23]. The sensitivity of circadian parameters to steroidal hormones also exhibits sex differences. These sex differences are determined in some species by the organizational effects of gonadal hormones during the perinatal period (rat: [7], hamster: [212]).
Circadian Physiology is Altered by Gonadal Hormones
Some of the effects of gonadal hormones on circadian rhythms are due to modulation of the circadian pacemaker in the SCN. In laboratory rodents, gonadal hormones have been shown to alter key aspects of SCN physiology, including those necessary for circadian rhythm generation, entrainment, and coupling (Figure 5). For example, both androgens and estrogens can activationally alter photic sensitivity within the entrainment pathway, as measured by the induction of immediate early gene expression (FOS) and clock genes (Per1, Per2, [3, 88, 90]). Photic sensitivity in the SCN is also enhanced during early pregnancy, although the specific hormones involved are unknown [169].
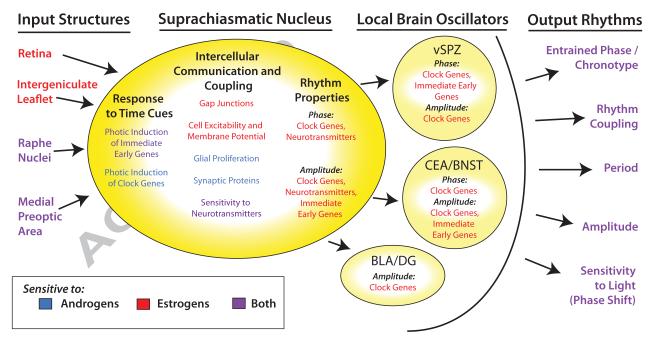
Figure 5. Growing Evidence Suggests That Reproductive Hormones Influence the Functioning of the Circadian System in Adult Rodents.
Color is used to indicate whether a property of the circadian system is known to be modulated by hormone exposure (blue=androgen sensitive; red=estrogen sensitive; purple=sensitive to both androgens and estrogens). Depicted are several major input structures to the suprachiasmatic nucleus (SCN), SCN properties, and output structures that are known to be hormone sensitive (vSPZ = ventral subparaventricular zone, CEA=central amygdala, BNST= oval nucleus of the bed nucleus of the stria terminalis, BLA= basolateral amygdala, DG= dentate gyrus). Please note that that we do not mean to imply that all output rhythms directly derive from pathways traversing the local brain oscillators depicted, nor that these structures receive output directly from the SCN. Finally, on the far right, hormonal effects on circadian output rhythm properties are summarized.
The effect of gonadal hormones on of photic sensitivity could be due to alterations in glutamate signaling from the RHT, as NMDA receptors can be directly modulated by the neuroactive metabolites of progesterone [124]. However, it is just as likely that gonadal hormones alter photic sensitivity by modulating the indirect pathways by which light influences SCN function. In females, estrogen increases the sensitivity of SCN neurons to the neurotransmitter 5-HT [96], which conveys photic information from the raphe nuclei. In males, castration increases NPY content in the SCN and IGL [140]. In females gonadal hormones can also increase the sensitivity of the SCN to cholinergic inputs (ACh; [96, 117, 118]), which are thought to potentially mediate the influence of cognition (sustained attention) on chronotype [63].
These hormonal effects on circadian entrainment pathways may explain why the phasing of many rhythmic components in the SCN is altered by gonadal hormones. For example, the phasing and amplitude of clock gene rhythms (Per2, Cry2) are sensitive to estrogen in females [133, 134]. Rhythms for the clock protein, PER2, are phase-advanced by gonadectomy in males [147], and by early pregnancy in females [169], but not by the estrous cycle [147]. Estrogen can also alter the phasing of neurotransmitter rhythms in the SCN [41, 97, 107]. In the case of VIP, which is a neurotransmitter that is important for oscillator coupling, ovariectomy causes a remarkable 8 hour delay, so that the phasing of the transcript rhythm resembles that of intact males [97, 107]. In male gerbils, VIP levels in the SCN are increased by both castration and testosterone treatment, depending on the season [40], but it is unknown if VIP rhythmicity is also affected as samples were only made at one time point. During early pregnancy, the daily rhythm in FOS changes so dramatically that its short peak extends to encompass most of the daytime [169].
These phase changes may also be caused by gonadal hormones affecting the intercellular communication and coupling in the SCN. Estrogen enhances electrical intercellular coupling by increasing the number of gap junctions in the SCN, [175, 176] in a manner that is opposed by progesterone [176]. In males, estrogen alters the overall excitability of SCN neurons by depolarizing resting membrane potential and increasing spontaneous firing rate [54]. Hormonal manipulations in males (castration, androgen treatment) also change the number of glia and synaptic proteins in the SCN [88, 166], both of which are likely to alter intercellular coupling and communication. It is also worth noting that neuroactive metabolites of progesterone are well-known for their ability to modulate GABAA receptors [101, 162], and GABA is the primary neurotransmitter used both for communication between cells within the SCN and projections from the SCN [127]. This enhanced intercellular communication and coupling could affect the phase of rhythms by enhancing the response of the SCN to photic and non-photic time cues. It is also likely to affect the precision and amplitude of output rhythms.
Outside of the SCN, gonadal hormones alter the amplitude and phasing of circadian rhythms in multiple brain regions in a robust and complex manner. For example, the ventral subparaventricular zone (vSPZ) is located just dorsal to the SCN, and is necessary for the generation of activity rhythms in rats. During early pregnancy, daily FOS rhythms in the vSPZ lose their bimodality, and daily rhythms in PER2 dampen and shift by 4-8 hours [169]. In brain regions important for emotional processing, the central amygdala (CEA) and the oval nucleus of the bed nucleus of the stria terminalis (BNSTov), the peak in PER2 rhythms shifts by as much as 12 hrs over the course of the estrous cycle. This shift is accompanied by a dampening of rhythmicity, and appears to be mediated by a slow (48-72 hr) response to transient estrogen exposure. Due to this slow response, the rhythms of females during proestrus and estrus are high amplitude and similar to those of females that are ovariectomized [147]. In contrast, gonadectomized males have significantly dampened PER2 rhythms [147], and early pregnancy increases the amplitude of both PER2 and FOS rhythms. Early pregnancy also produces a large 8-12 hr phase shift in PER2 rhythms in the BNST [170]. Other brain regions show more subtle changes in response to gonadal hormone fluctuation. In the basolateral amygdala (BLA) and dentate gyrus of the hippocampus (DG), there is little change in PER2 rhythms over the estrous cycle, and no obvious effect of male gonadectomy, but a small increase in rhythm amplitude in ovariectomized females in response to estrogen treatment [147]. Estrogen treatment also produces a non-significant phase-advance of clock gene rhythms (Per1, Per2) in the cortex [133]. Thus, it seems that the effects of gonadal hormones on circadian physiology are diverse, and likely to be dependent on the nature and duration of hormone exposure, the surrounding hormonal milieu, and the sex of the individual, suggesting that the function of gonadal effects on the circadian system may be similarly diverse.
Puberty and Adolescence: A Time for Development of the Circadian System?
Puberty is a time of intense hormonal change. Since reproductive hormones alter circadian rhythms and circadian physiology during early development and adulthood, we expect that puberty should also be a time of circadian rhythm development. What evidence supports this hypothesis? Can these hormonal effects explain the phenomenon of adolescent chronotype in humans?
Puberty and Adolescence
Although colloquially the terms “puberty” and “adolescence” are used interchangeably, scientifically they refer to separate concepts [179]. Traditionally, puberty is defined as the process leading to the attainment of sexual maturation [181], beginning with the activation of the HPG axis, and ending with reproductive competency [153, 178]. Reproductive competency is rarely used as a developmental marker in neuroscience research, however, because it is unreasonable to run mating and pregnancy tests in the middle of an experimental procedure. Therefore, most researchers measure the physiological correlates of sexual maturation – the development of the HPG axis, increases in circulating gonadal hormones (e.g., testosterone, estrogen), and the maturation of the testes, ovaries, uterus, and external genitalia [158].
Adolescence, on the other hand, is defined as the period of social, emotional and cognitive transition between childhood and adulthood [178, 179]. Adolescence encompasses puberty, and typically human neuroscience studies will discuss the period of “adolescence” instead of “puberty,” since human subjects remain embedded in their social environment. In animal studies, the term “adolescence” is traditionally used specifically to refer to research focusing on the neural and behavioral changes accompanying the transition from juvenile dependence into the relative independence of adulthood [181]. This transition includes both the hormone-dependent and hormone-independent remodeling of cortical and limbic circuitry necessary for adult decision-making, cognition and social interaction [179]. As this review focuses primarily on interactions between pubertal hormones and the circadian system, a regulator of the HPG axis, we will henceforth only use the term “adolescent” when referring to human studies.
It should be noted that the progression of puberty in laboratory species is not necessarily analogous to that of humans. To begin with, human puberty is commonly preceded by eight or more years of gonadal “quiescence” following infancy. During this time, gonadotropin releasing hormone (GnRH) pulsatility is suppressed and gonadal steroidogenesis is nearly absent. Puberty is initiated when the HPG axis is released from juvenile inhibition [153]. Some mammalian species, such as the rhesus macaque, show a similar developmental pattern [153], but rodents typically do not [139]. Most rodent species show low levels of steroidogenesis and secondary sex development throughout the juvenile period that then accelerates near the time that reproductive competence develops [139]. Another key difference between the progression of puberty in humans and other species is the role of seasonality. Season plays a crucial role for determining the timing and rapidity of secondary sex development in many species. Photoperiod is typically the environmental signal that indicates season physiologically [61], thus care must be taken when interpreting the results of circadian developmental studies that incorporate manipulations of the daily light-dark cycle.
Pubertal Changes in Chronotype Occur in Animals
In order to better understand the mechanisms underlying changes in chronotype during adolescence in humans, we recently examined the phasing of sleep and activity rhythms during puberty in other mammalian species. To measure changes in chronotype during puberty, we monitored secondary sex development and daily wheel-running activity rhythms in two species of laboratory rodent, the nocturnal rat and the diurnal degu (Octodon degus; [102, 103]), under varying hormonal conditions. We also monitored sleep electrophysiology in pubertal degus, and then performed an intensive review of the existing developmental sleep and circadian literature to determine how well our conclusions could be generalized to other mammalian species.
In both laboratory rodent species we found evidence for a large shift in the timing of daily rest and activity during the pubertal period. The daily rest/activity rhythms of juveniles and early pubertal rodents were characterized by a crepuscular pattern, with the majority of activity occurring near the end of the typical active phase for the species (the end of the night for rats, and the early evening for degus). As puberty progressed, their daily activity began to shift earlier (phase-advance), so that by adulthood most of their activity had consolidated at the beginning of the active phase for the species (Figure 6A, [66-67]). This shift in the timing of daily rest and activity occurred whether we were observing wheel-running activity or general sleep patterns using sleep electrophysiology (degus: [148]; similar data are presented for a small sample size of rats in [76]). This meant that, overall, both rodent species showed a large (2-4) hr shift in the phasing of their rest/activity rhythms during pubertal development, similar to human adolescents (Figure 2C-D, [66-67, 75, 114, 185]). We also found evidence in the literature that there might be a pubertal shift in the timing of daily rhythms in other species, including rhesus macaques (Figure 2B), laboratory mice, and fat sand rats [60, 137, 197, 199, further reviewed in 70]. Overall, these results suggest that chronotype changes during puberty are likely to be a common phenomenon in mammals. The reversal of this shift following maturation, as observed in humans and macaques (Figure 2A-B), has not been as well studied, and it is unclear whether it exists in laboratory rodent species (see p. 35 for a more in depth discussion).
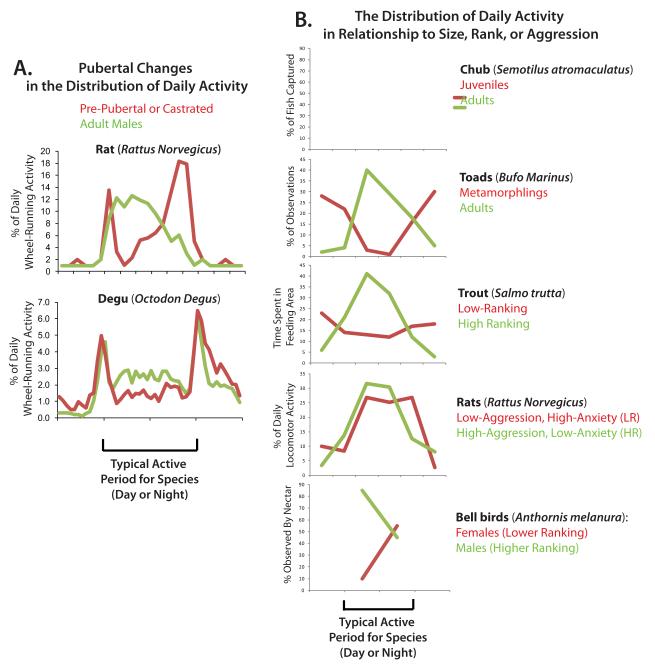
Figure 6. Does the Adolescent Shift in Chronotype Represent A Temporal Social Niche?
The shift in chronotype observed during puberty in rodent species resembles shifts seen in other species related to development and dominance status. On the left is depicted the shift in chronotype seen in laboratory rodents, with the percent of daily activity occurring during each time bin plotted relative to time of day (24 hrs). Time of day is divided into four 6 hr bins, and is presented in terms of the typical active period for adult males of the species (green), with the central 12 hours representing nighttime for the nocturnal rat and daytime for the diurnal degu. Prepubertal animals and castrates (red) are more crepuscular, and have more of their activity occur at the end of the active period [66, 67], when they are less likely to have to compete for resources with adult males. On the right are other examples of younger, weaker, or subordinate individuals maintaining activity at a less preferred time of day (red). Each plot again shows a 24 hr day, divided into four 6 hr bins and centered around the typical active period for the larger or more aggressive/dominant individuals in the species (green). The y-axis depicts the relative amount of time spent engaging in activity (in some graphs this is feeding activity, in others general activity) (chub: [106], trout: [6], toads: [57], bellbirds: [42], rats: [92], but also see [25] for more naturalistic examples).
Similar to humans, the manner that this chronotype shift occurred during puberty differed by sex in rodents (Figure 2C-D). We observed a large sex difference in the magnitude of the chronotype shift during puberty in both species that we examined. For rats, males showed a larger shift in the phasing of rest/activity rhythms during puberty than females [66]. For degus, the sex difference was absolute: females did not seem to shift their activity rhythms at all during puberty, despite the fact that males showed large changes in chronotype [67]. However, this conclusion remains tentative, because a previous study observed changes during chronotype in both males and female degus during puberty [75]. Similar to humans, the developmental timing for the chronotype changes in rats also differed by sex, paralleling sex differences in the progression through puberty [66]. A similar correlation was found in rhesus macaques: a study comparing the activity rhythms of rhesus macaques with normal or delayed pubertal development reported a strong correlation between pubertal timing and a shift in the timing of rest/activity rhythms [60].
Pubertal Changes in Chronotype are Driven by Gonadal Hormones
The presence of sex differences suggested that pubertal hormones might drive the chronotype shift. To test this hypothesis, we removed the gonads (ovaries, testes) from juvenile rats and degus to prevent the onset of puberty. We found that gonadectomized animals either completely lacked a shift in chronotype during the normal pubertal period (degus: [67, 75]) or showed a much diminished shift in chronotype (rats: [66]). The activity patterns of the animals gonadectomized before puberty resembled those of prepubertal rodents, as well as those of animals that were gonadectomized during adulthood in previous studies. For example, castrated adult male rodents under entrained and free-running conditions had activity that was more dispersed across the active period [128], such that activity at the beginning of their active period in the early night was less cohesive, diminished, lost, or delayed [47, 49, 78, 90, 128, although see 23 and 80]. This was also true of ovariectomized adult female rodents: they lost the phase advance and increase in wheel running activity that typically accompanies estrus [49, 78, 100, 129], and in general exhibited a more dispersed activity distribution and delayed activity rhythm phase [187, 203, although see 23].
In these previous studies, the administration of testosterone or dihydrotestosterone to castrated adult males was able to restore the normal circadian activity patterns [47, 78, 90, 128] and estrogen treatment was able to reconsolidate rhythms in ovariectomized rats [187, 203]. Thus, it seemed likely that the pubertal chronotype shift and consolidation of activity rhythms might be driven by either androgens or estrogens. To further clarify the hormonal mechanism responsible, we began a series of hormone replacement studies in rats that had been gonadectomized before puberty. So far, these data indicate that it is likely that the mechanism driving the pubertal chronotype shift in male rats is an activational effect of pubertal testosterone. This pubertal testosterone is then converted into estrogen locally in the brain by the aromatase enzyme (M.H. Hagenauer, C. L. Fournier, A.M. Wehner, J. Eisman, B. Ajegba, and T.M. Lee, unpublished data). Thus, the shift in chronotype that occurs during puberty in male rats may be similar to the shift in the rest/activity rhythm that occurs due to high estrogen during the estrus cycle in adult females [8, 17]. This story is consistent with recent data from adult men demonstrating a positive correlation between circulating testosterone levels and evening chronotype [156]. However, the story may be more complicated in other species. In the degu, previous studies suggest that adult castration does not cause activity rhythms to revert to a delayed, crepuscular distribution, but instead causes the rhythms to phase-advance [80]. Thus, in this species it is unlikely that the hormonal effects on chronotype are purely activational, but may instead represent a permanent, organizational change to the sensitive pubertal brain (e.g., [75]).
Pubertal Changes in Chronotype Are Internally-Generated and Not the Product of Masking
As discussed earlier, the timing of rest and activity rhythms is thought to derive from three primary components: an endogenous daily circadian timekeeping system, a homeostatic drive for sleep, and other external constraints (which are often referred to as “masking”). One of the first questions that arose after observing pubertal changes in chronotype was whether these changes might represent a change in the sensitivity of the animals to the masking properties of light. Since the animals appeared to be more crepuscular during the juvenile period and early puberty, was it possible that they were simply more reactive to the light transition times? To test this hypothesis, we placed juvenile rats into constant lighting conditions to determine if they would still exhibit a crepuscular-like activity distribution, with greater activity occurring at the end of their active period. To our surprise, the crepuscular-like activity distribution not only persisted under constant conditions, it was immediately exaggerated, with even more activity occurring at the end of the active period. Then, in the same manner as under a normal light-dark cycle, their activity shifted so that it gradually concentrated at the beginning of the active period as the animals progressed through puberty under constant conditions. These data suggested that pubertal changes in the distribution of activity were internally-generated and not a change in the passive response of the animals to the light cycle [66].
Do Pubertal Changes in Chronotype Result from Circadian System Development?
If pubertal changes in chronotype are internally-generated, is it possible that they result from hormonally-driven circadian system development? There is growing evidence from multiple species that circadian rhythm properties change during puberty (Table 1). In addition to circadian rhythm phasing and chronotype, these properties include the endogenous period of the rhythms under constant conditions, the sensitivity of the rhythms to resetting by environmental time cues (zeitgebers) such as light, the strength of rhythm coupling, and physical properties of the suprachiasmatic nucleus.
Table 1. Demonstrated Changes in Circadian Properties During Puberty, Adolescence, or Young Adulthood.
| Species: | ||||||
|---|---|---|---|---|---|---|
| Circadian Property: | Human | Degu | Rat | Mouse | Macaque | Fat Sand Rat |
| Chronotype/Phase | X | X | X | X | X | X |
| Free-Running Period | X | X | X? | |||
|
Effect of Light on Period (Aftereffects) |
X | X? | ||||
| Phase Response to Light | X? | X | ||||
|
Crepuscularity, Rhythm Consolidation |
X? | X | X | |||
| Rhythm Amplitude | X | X | X | |||
| SCN Anatomy | X? | X |
red = developmental change known to be dependent on gonadal hormones
Circadian Free-Running Period Changes Around the Time of Puberty and Adolescence
One circadian property that changes around the time of puberty in multiple species is the endogenous free-running period. As mentioned earlier, under conditions in which there are no time cues from the outside world, the circadian system will continue to generate daily rhythms but these rhythms will appear to “drift” a little each day, because the period (or cycle length) of the rhythms only approximates 24 hours (ranging from 23-25 hrs). The actual period of the free-running rhythm is a relatively stable property that varies by species, sex, and occasionally hormonal environment [7, 23, 47, 49, 90, 129, 212]. A few years ago, we discovered that the free-running period of male degus shortens several months after puberty is traditionally considered to have ended (e.g., after reproductive competence and full body size have been attained). The development of this sex difference was dependent on the organizational effects of gonadal hormones [75], and, in particular, estrogen [74]. This finding was provocative because it indicated that hormone-dependent brain reorganization might continue much later into the “adolescent” or “young adult” stage of development than previously expected. It was also interesting because the changes in chronotype observed during the “adolescent” period in humans extends into the early 20’s – well beyond the traditional end of puberty and sexual maturation [160]. In an effort to determine whether changes in circadian period might accompany adolescent changes in chronotype in humans, one study used a forced desynchrony protocol that caused the circadian rhythms of human subjects to free-run by placing the subjects in laboratory housing with an unnatural environmental light cycle that was beyond the range of entrainment (28 hrs instead of the normal 24 hrs). They found that human adolescents have an endogenous period of 24.27 h, significantly longer than the period length found in adults (24.12 h) using similar protocols [28, 34]. Two studies in laboratory rats also revealed a change in free-running period around the time of puberty [66, 114].
Changes in free-running period around the time of puberty and young adulthood are noteworthy because they imply that the circadian system continues to mature late into development. A change in free-running period could also cause an adolescent change in chronotype or entrained phase, because it would alter the amount of daily resetting that is necessary for circadian rhythms to entrain to a normal 24 hr day. In that case, a lengthening of free-running period during adolescence, as is seen in human adolescents, would be expected to cause a delay in circadian chronotype, whereas a shortening of free-running period, as is seen in young male degus, would be expected to produce an advance in chronotype. Although these predictions seem to align well with the chronotype changes that actually occur during puberty and adolescence in these species, there are several reasons to believe that changes in free-running period cannot explain pubertal changes in chronotype in our rodent models. First, the changes in free-running period that are observed in male degus occur several months after the changes in chronotype and entrained phase. Male degus show a shift in their chronotype around the time of puberty, whereas their free-running period shortens several months later around the time that would naturally be their first breeding season in the wild [75]. In rats, both males and females show changes in free-running period around the time of puberty, but these changes differ by sex, with males showing a lengthening of free-running period and females showing a shortening. Thus, these changes in free-running period do not predict the pubertal change in chronotype, which takes the form of a phase-advance in both sexes [66].
To complicate matters, the changes that we observed in the free-running period of male rats during puberty in our study are actually the opposite of what was found in an earlier study by a collaborator [114]. One possible explanation for this discrepancy is the differing duration of time that the rats were placed in constant conditions. In an earlier study in pubertal degus, younger animals were found to maintain a free-running period close to 24 h for almost 2 weeks after placement in constant conditions. These pronounced “aftereffects” of the former light/dark cycle on free-running period did not occur after the animals matured and were placed back into constant conditions again [75]. Recent data from mice also indicates that animals with low levels of reproductive hormones (castrates) are especially sensitive to the effects of light on free-running period (M.P. Butler and R. Silver, unpublished data discussed in [123]). More pronounced “aftereffects” in young animals may explain the contradictions present in the rat studies. In both of the rat studies [66, 114], the free-running period of the male and female rats was closest to 24 h during the youngest developmental time point, immediately after placement into constant conditions. Thus, it may be that the developmental changes in free-running period that we observed in rats reflect developmental changes in the sensitivity of the circadian system to the “aftereffects” of the light/dark cycle. A developmental change of this variety would still be provocative, as it similarly indicates that the circadian system continues to mature late into adolescence.
Adolescents Have a Different Circadian Sensitivity to Light
Another possible mechanism that could underlie adolescent chronotype is a change in the circadian system’s sensitivity to light during puberty and adolescence. For example, one study constructed a “phase response curve” for the sensitivity of the circadian system to light in pubertal mice (49 days of age, [136]), late/post-pubertal mice (63 days of age, [136]) and adults [198]. They constructed the curve by administering brief (15 min) pulses of light at different times of the day while the mice were housed in constant darkness, and then examined the effect of the light pulses on the phasing of the animals’ activity rhythms. In our previous review, we compared the data for the 49-day-old (P49) pubertal mice to the older animals, and found that the pubertal animals were much more sensitive to light. As is typical, all mice were most responsive to light that was administered at a time of day that they perceived to be nighttime (subjective night) based on their own internal rhythms. Light that was administered in the subjective evening caused the circadian rhythms to shift later (delay), and light that was administered in the subjective morning caused the circadian rhythms to shift earlier (advance). For pubertal mice, this tendency was exaggerated, with the animals being clearly more sensitive to evening light than adults [70, 198]. An earlier study also showed that pubertal mice (42 days of age) adjusted to artificial “jet lag” (a phase delay of the light-dark cycle) much faster than adult mice [197].
In humans, there is moderate support for the hypothesis that the circadian system is especially sensitive to evening light in adolescents, and less sensitive to morning light. For example, the chronotype of adolescents and young adults (ages 13-29) was less responsive to latitude-related differences in sunrise time than that of older adults (ages 30-97) [22]. Another study found that during the transition from winter to spring, adolescents became more evening type, and this change in chronotype correlated with increased light exposure in the evening but not the morning [55]. Other evidence suggesting altered light sensitivity during adolescence comes from an intervention study that attempted to determine how best to allow sleep-deprived adolescents to recover sleep on weekends [46]. This study focused on the phenomenon of weekend “social jet lag” in adolescents and young adults. Typically, during the week adolescents follow a schedule in which they stay up late, but then wake up early for school, producing chronic sleep restriction. On the weekends, when they have the opportunity to recover sleep, adolescents and young adults continue to stay up late, exposing themselves to evening light, and then they sleep in and lose several hours of exposure to morning light. This weekend light schedule drives their circadian rhythms to become even more delayed, so that by Monday morning they are completely out of synchrony with the school schedule. In an attempt to alleviate this shift in a realistic manner, the authors realized that adolescents were likely to stay up later on the weekends regardless of the health consequences. Therefore, they attempted an intervention that increased the exposure of adolescents (ages 15–17 years) to bright, short-wavelength light in the morning on the weekends in an attempt to produce a phase advance that would counterbalance the delaying effects of evening light exposure, so that the social “jet lag” on Monday would be less extreme. To their surprise, these interventions had little effect, implying that the circadian systems of the adolescents were relatively insensitive to morning light [46]. These data align well with a study in young adults (aged 21 years on average), which found that bright, short-wavelength light exposure in the morning had no effect on circadian phase, as measured via melatonin rhythms [172]. However, it should be noted that both of these studies lacked a child or older adult control group, and thus it is unclear whether morning light treatment under these particular conditions advances circadian phase in individuals of any age.
Other support for the hypothesis that human adolescents are less sensitive to morning light comes from a preliminary study examining the suppression of melatonin secretion by 1hr light pulses, which is mediated by the same pathways in the SCN as the resetting of circadian rhythms [30]. This study found that late adolescents were significantly less sensitive to dim light exposure (15 lx) in the morning (03:00– 04:00 h) than early adolescents. However, it is clear that morning light exposure still matters to some degree for the circadian entrainment of adolescents, because another study found that preventing adolescents from receiving short-wavelength light exposure later in the morning during school hours caused the students’ circadian rhythms to become even more delayed [56].
We believe that a change in the sensitivity of the circadian system to light cannot be solely responsible for the shift in chronotype observed in our animals, because we observed a reorganization of rest/activity rhythms during puberty even under conditions of constant darkness [66]. However, this evidence does suggest that altered circadian light sensitivity may play a role in the development of chronotype changes during puberty. Even more importantly, it implies that adolescents may be particularly sensitive to circadian disruption by the nighttime electrical lighting that pervades our modern living. Indeed, a recent study found that adolescents in Brazil who did not have electrical lighting at home had significantly earlier sleep onsets and less sleep deprivation than their peers [146].
Other Circadian Properties May Change During Puberty and Adolescence
In addition to pubertal changes in free-running period and the circadian sensitivity to light, there is also some evidence for other forms of circadian system development. For example, multiple authors have commented that there is a prevalence of ultradian components in the rhythms of young rodents under both entrained and free-running conditions which disappears in the post-weaning and pubertal periods [26, 38, 52, 76, 85, 93]. This disappearance may represent an increase in oscillator coupling in the suprachiasmatic nucleus due to pubertal hormones, or simply reflect disruption of the rest period by the high metabolic demands of young, fast-growing animals, which would diminish with age regardless of hormonal environment [9]. Accompanying this decrease in ultradian components is an increase in rhythm amplitude. In rats, the increase in the amplitude of rest/activity rhythms during puberty is particularly notable and strongly related to pubertal hormones: prepubertal gonadectomy prevents activity levels from increasing much beyond prepubertal levels in both males and females [66]. In the degu, there is a brief increase in activity levels during puberty that appears only to be related to gonadal hormones in females, and which then disappears as the animals mature [67], so the increase in activity level cannot explain the development of more consolidated, phase-advanced rest/activity rhythms during puberty.
Other forms of pubertal circadian development have merely been suggested by previous studies. One study observed that sex differences in the range of entrainment of the circadian system in hamsters seemed to depend on gonadal hormone exposure during puberty [49]. In the SCN, there are indications of anatomical changes (growth in nuclear size and nucleoli size) around mid-puberty in rats [10, 130, 131]. There may also be an increased number of cells expressing a neuropeptide (VIP) that is important for photic entrainment and oscillator coupling during late adolescence in humans [183]. However, the correlation between these anatomical changes, secondary sex development and pubertal hormones has not been explicitly tested.
Discussion
This review began with the concept that we could use animal models to address fundamental questions regarding the delayed sleep patterns of human adolescents, while avoiding the technical and ethical issues associated with using human subjects. We presented data indicating that multiple mammalian species showed dramatic changes in the timing and organization of daily rest/activity rhythms during puberty, not unlike human adolescents. These changes exhibited sex differences and are, at least partially, dependent on gonadal hormones. We also demonstrated that other fundamental circadian properties change during puberty and adolescence, including free-running period and circadian sensitivity to light. We conclude that it is likely that multiple aspects of the circadian timekeeping system are affected by pubertal hormones, suggesting that adolescent changes in chronotype may derive from the effects of hormones on the circadian timekeeping system.
It is important to note that there are still multiple experiments that need to be completed to solidify this argument. For example, it would be useful to know whether the hormonal mechanism driving pubertal changes in chronotype is similar across species. In laboratory animals, gonadectomy and hormone replacement experiments could be done in a manner similar to those previously discussed for rats. For humans, it would be useful to know whether chronotype correlates with circulating levels of testosterone and estrogen. As mentioned earlier, a recent study by Randler et al. [156] suggests that there is a positive correlation between evening chronotype and circulating testosterone levels in adult men (ages 19-37). These data suggests that the slow decrease in testosterone observed in adulthood [86] may play a role in the reversal of adolescent chronotype in men. However, to provide a strong conclusion, a follow-up study will need to be performed in which blood is collected at more than one time point to control for potential differences in the phasing of testosterone rhythms in individuals with different chronotypes. Also, in order to generalize these results to the shift towards evening chronotype observed during adolescence, a similar correlation would need to be observed in an adolescent population that is dissociable from age-related changes.
It would also be extremely useful to determine whether these findings can be expanded to estrogen levels in women.
Substantial work still needs to be done to clarify the relationship between adolescent chronotype and pubertal changes in the circadian system. As discussed earlier, we know that gonadal hormones can affect everything from the sensitivity of the circadian system to time cues, to the phasing and coupling of oscillators within the SCN, as well as the strength of SCN output and phasing of downstream oscillators. Based on these data, we would expect that pubertal increases in gonadal hormones might enhance the sensitivity of the SCN to time cues (both photic and non-photic), as well as the coupling and amplitude of neurotransmitter and electrical rhythms. However, to alter chronotype, we believe that each of these changes would need to alter the phasing of SCN rhythms. Thus, in order to best determine the location of pubertal hormone effects within the circadian system, we can first examine whether there is a change in the phasing of circadian rhythms in the suprachiasmatic nucleus and then either look upstream or downstream from those rhythms based on our results. We are currently examining this question using measurements of clock gene expression, but so far have found little physiological evidence for altered circadian phasing (M.H. Hagenauer, D.B. Altshuler, S.S. Wang, J.M. Mossner and T.M. Lee, unpublished data), suggesting that pubertal hormones may influence some aspect of circadian physiology downstream from the entrainment of clock gene rhythms in the SCN.
In light of recent evidence suggesting that estrogen may enhance the influence of the circadian system on the sleep/wake cycle (M.D. Schwartz and J.A. Mong, unpublished data discussed in [123]), it may also be productive to measure the strength of rhythmic output from the SCN during puberty. Since estrogen has the ability to depolarize SCN cells and enhance firing rate [54], we would hypothesize that aromatized testosterone or estrogen during puberty might shift behavioral rhythms by increasing the firing rate of SCN cells. This increased output from the SCN might better outcompete the homeostatic drive to sleep, allowing animals to consolidate their activity into one strong bout at the beginning of the evening, or freeing human adolescents to stay awake later into the evening (when the circadian influence on the sleep/wake cycle peaks in humans, according to the classic two-process model [4]).
Similarly, we know that gonadal hormones can alter properties of the suprachiasmatic nucleus related to oscillator coupling (e.g., the number of electrical gap junctions connecting cells, glial cell proliferation, VIP signaling). Does the consolidation of rest/activity rhythms during puberty in animals relate to an increase in coupling between cellular oscillators?
Finally, in rats, we have learned that the hormone responsible for pubertal chronotype change is testosterone, which is converted locally into estrogen. Therefore, is it possible that we can determine where pubertal hormones are acting in the brain to cause chronotype development using local presentation of receptor antagonists? Does the estrogen bind in the suprachiasmatic nucleus itself, where receptors are relatively rare but estrogenic effects are observed in vitro, or in hormonally-sensitive structures that project to the nucleus, such as the raphe nuclei, or in local downstream oscillators?
Adolescent Chronotype: Are We Observing the Same Phenomenon Across Species?
Before moving on to a discussion of the relevancy of these results, it seems important to first answer a fundamental question: Are we observing the same phenomenon in these species? How generalizable are the conclusions from each experiment? In 1979, Beach laid out two cardinal rules regarding the construction of animal models for human behavior as well as for behavioral comparisons between species. One rule was that any “significant comparison of a particular type of behavior in two different species is impossible unless and until the behavior has been adequately analyzed in each species by itself” [20]. In this sense, the additional data presented here has expanded upon several conclusions from our initial cross-species comparison [70]. First, detailed analyses of activity rhythms across postnatal development supported the assertion that pubertal phase changes in animals show sex differences in their timing and magnitude, with males showing larger changes than females. Since the timing of adolescent phase changes in humans also displays sex differences and human males show larger phase changes than females [160], these findings support the hypothesis that the phenomenon observed in each species shares some common origins. The magnitude of phase change in male degus and rats (3-5 hrs) also resembled that observed in studies of human males [160].
Second, the pubertal phase changes occurring in these rodent species are directly related to a consolidation of ultradian components in the rhythms. This conclusion is consistent with previous data indicating that both androgens and estrogens can consolidate rhythms at the beginning of the active period in rodents [78, 128, 187, 203], but has not been discussed in previous studies of pubertal phase change [60, 75, 185]. Earlier research on the post-weaning period in altricial rodents, such as rats and mice, discussed this rhythm consolidation in terms of the switch from maternal to photic entrainment and in terms of the development of circadian output systems (e.g.,[196]), because altricial species are born at a relatively earlier stage of brain development and have their eyes closed during the early postnatal period. Therefore, these species initially rely on the dam to provide entraining time cues [196]. The reviewed data are the first to note that a similar consolidation of rhythms can occur in precocial species, which open their eyes soon after birth. These species presumably rely on photic entrainment almost immediately during the postnatal period and would have emerged from the burrow in the wild many weeks before the age of the initial measurements in the reviewed studies. Thus, consolidation of rhythms at the beginning of the active period cannot be exclusively attributed to pups developing independence from the dam (or dams and sire, in the case of communally-nesting degus; [82]). The reviewed data are also the first to demonstrate that the developmental decrease in ultradian rhythms during post-weaning development is related to pubertal hormones.
The relationship between rhythm consolidation and phase change in rats and degus is one formal characteristic that differentiates the pubertal phase changes in these species from those occurring in human adolescents. A second characteristic that appears to differ between our rodent species and other species that have been studied in detail (human, macaque) was the developmental timing and the direction of the phase changes (Figure 2). The newer reviewed data did not provide evidence that circadian phase was more advanced in the pre-pubertal animals than the pubertal animals, unlike previous studies in the degu and rat that briefly examined one or two pubertal time points [9, 75, 185]. However, the observation was clearly replicated that circadian phase is more delayed during puberty than adulthood [75, 114] as it is in humans and macaques [60, 160]. Thus, it is unclear whether the changes in laboratory rodents (rats, degus, mice?) are more analogous to the circadian phase advance that occurs following sexual maturity in humans [160] or to the phase delay that occurs between pre-puberty and sexual maturity in humans and macaques [60, 160]. The relationship between phase changes and pubertal timing in each species, as well as the dependency on pubertal hormones, suggests that the latter comparison may be more appropriate, although the direction of phase change during puberty clearly differs between these species.
Consequently, Beach’s second rule of comparative studies pertains, which is that meaningful comparisons should not be based “upon the formal characteristics of behavior, but upon its causal mechanisms and functional outcomes” [20]. The causal mechanisms of the phenomenon, as suggested by the relationship between circadian changes and pubertal timing, sex, and gonadal dependence, indicate that the phenomenon that we are observing is analogous between species, whereas the formal characteristics may differ in the ways discussed above.
There is…great diversity in the ways in which hormones can affect an animal’s nervous system and behavior. To the student, this diversity might seem like an unwanted complexity, something else to complicate the story.”
- Crews (2002, [44])
Indeed, in some ways it seems like it would be surprising if we actually did find an identical phenomenon occurring during puberty in each of these species because there are several striking differences in the progression of puberty between laboratory rodents, such as the degu and rat, and primates, such as macaques and humans. For example, in fast-developing, altricial rodent species, such as rats and mice, puberty rapidly follows weaning, a life transition best associated with infancy in humans. For this reason, there are several hormone-independent circadian changes that occur around the time of puberty, independent of the gonadal hormone-dependent changes in chronotype discussed above. For example, when young rats are allowed to remain with the dam they will continue to show nursing behavior until postnatal ages P28–P40 [25, 43], which overlaps with the beginning of puberty. The dam nurses predominantly during the day, and this nursing pattern has a large influence on the phasing of activity rhythms in the pups [174, 182, 186]. The pups will exhibit a diurnal activity pattern until around age P18 when they begin to consume significant amounts of solid food [21, 186]. Even after this point, the dam continues to serve as an effective zeitgeber until the 4th or 5th week of life (approximately P28–P35, [104, 184]. Thus, the earliest part of the data regarding adolescent chronotype in rats (P21–P30; [66]) overlapped with the typical time of transition from maternal to photic entrainment (for review see [196]), as well as from dependent, diurnal activity to independent, exploratory nocturnal activity. In contrast, in humans and other primates, for whom weaning and puberty are distinct events, circadian system development is likely to progress quite differently.
Likewise, as discussed in the introduction, puberty in primates is characterized by several years of preceding gonadal quiescence [153], whereas puberty in rodents follows low-level steroidogenesis throughout the infantile and juvenile periods [139]. Thus, it is possible that we do not observe a period of relatively advanced phase during pre-puberty in rats and degus because they have already been exposed to gonadal hormones during juvenile development [75 139]. However, it seems unlikely that early-pubertal or juvenile hormone production in the degu and rat delayed circadian phase before our recording period in a manner analogous to the delay observed in human adolescents and pubertal rhesus macaques, as the activity rhythms of rodents gonadectomized prior to puberty appeared similar to those of prepubertal rodents. Since long-term hormone withdrawal is known to alter hormone receptor levels [122, 161], it seems more likely that following quiescence in primates, hormones transiently have different effects on the circadian system than those observed in species with low-level steroidogenesis throughout the juvenile period.
In general, in the realm of reproduction, diversity is certainly the rule and not the exception [44]. Therefore, alternatively, it could simply be that pubertal hormones have different effects on the circadian system of primates and rodents. This would not be an unprecedented conclusion, as gonadal hormones are already known to have species-specific effects during adulthood. For example, testosterone shortens free-running periodin adult mice [90], but not in adult hamsters [128]. Estrogen (17β-Estradiol) shortens free-running periodin adult female hamsters [129, 212] and adult female rats [7] but not adult female degus [100]. Testosterone treatment consolidates rhythms in adult male mice [47, 78], whereas it induces rhythm splitting in starlings [65]. Indeed, since there are pronounced species differences in the degree of circadian regulation of the hypothalamic pituitary gonadal axis [39], it makes sense that gonadal hormone feedback should be similarly diverse.
Finally, the role of artificial lighting in prolonging or exaggerating delayed phase in human adolescents should not be underestimated. During the earliest human circadian experiments, unbeknownst to researchers at the time, subjects lengthened their own free-running period by controlling their daily light exposure [14]. Now it is widely-acknowledged that behavioral habits in humans can lead to altered zeitgeber exposure and atypical circadian phase (e.g., [19, 95]). Individuals that are regularly awake later in the evening and expose themselves to evening light can delay their circadian phase even more. Likewise, individuals that are regularly up early, and expose themselves to morning light, can shift their phase earlier. As discussed above, recent evidence from both human and animal studies suggests that adolescents may be particularly susceptible to circadian disruption by evening light. It seems to be a reasonable hypothesis then that bad lighting and sleeping habits developed during adolescence in humans could prolong the presence of delayed chronotype into young adulthood.
What Does It All Mean? Moving Beyond the Use of Pubertal Laboratory Rodents as Models for Human Adolescents
How about the functional outcomes of phase changes during puberty? Beach’s second rule argues that meaningful comparisons of behavior between species can also be made based upon the “functional outcomes” of that behavior [20]. However, the role for gonadal hormone effects on circadian rhythms has been a source of mystery for years. On this front, the new data that we reviewed have the ability to inform two pre-existing theories.
According to the gonadal hormone “feedback” theory, the circadian timekeeping system is sensitive to gonadal hormones because it plays an integral role in the HPG axis (as reviewed earlier; [89]). In support of this theory, many physiological systems that are regulated by circadian rhythms are also known to influence circadian function. For example, the circadian system drives rhythms in pineal melatonin, and melatonin is an effective zeitgeber [105]. Rhythms in food consumption can also feedback on circadian function [119], as well as rhythms in behavioral arousal [111]. In each of these cases, it seems that the feedback effects of these systems serve a similar purpose across species to align output rhythms with appropriate cues from the internal and external environment. In this case, the diversity of effects of gonadal hormones on circadian rhythms sets them apart from other known feedback effects. Also, the feedback model seems to make the most sense when applied to traditional laboratory rodents (rats, mice, hamsters) that have strong circadian regulation of ovulation and mating [39]. In contrast, the reproduction of many primates, including humans, as well as the degu, is not strictly tied to the circadian cycle [91, 108, 190, M.M. Mahoney, personal communication]. Thus, it is unclear why pubertal hormones would have a similar magnitude of effect on the circadian system of these species.
Another theory that is regularly discussed regarding the influence of gonadal hormones on circadian timekeeping is the “early bird gets the worm” theory. According to this theory, when females are most receptive during their cycle they become hyperactive and active earlier in the day to increase their chance of encountering a mate. In this case, it is in the best interest of reproductively-active males to be active earlier in the day, to increase their probability of gaining first access to females during their receptive period. Although this theory seems to have some explanatory power regarding the pubertal advances in circadian phase observed in males in our two rodent models, it does not seem to explain the pubertal delays in phase observed in humans and macaques. There is a tendency for humans to be more sexually active in the evening, which could provide incentive for individuals to stay up later. However, there is also increased sexual activity on weekends, which suggests that the daily rhythm in sexual activity is an artifact of the modern work week [142]. On weekends, there is a secondary peak in sexual activity that develops in the morning [142], which occurs around the time that testosterone peaks in men [190], again suggesting that the weekday evening peak may be an artifact of modern conditions. Rhesus macaques are known to be regularly sexually active during the morning hours following dawn, although this is unlikely to be their only time of sexual activity [200], so it is unclear why pubertal hormones would drive them to stay up later.
Without an obvious reproductive function, is it possible that changes in circadian rhythms during puberty could serve a purpose that is not strictly reproductive? Outside of the mammalian class, there are numerous examples of developmental shifts in preferred activity times around the time of maturity (Figure 6). For example, juvenile chubs (a variety of fish) are diurnal, whereas adult chubs are nocturnal. These chronotype differences are likely to be related to either risk of predation or optimal hunting time, since juvenile chubs have smaller mouths and consume different prey [106]. Amongst toad species, it is extremely common for younger toads (metamorphlings) to be diurnal, whereas juveniles are crepuscular, and adults are active early in the night. The reason for this chronotype shift appears to be related to the risk of predation for the young toads as well as the need to avoid dehydration. The need to avoid dehydration restricts their habitat to wetter regions, and the high density of the metamorphlings, as well as their small size, increases the risk of predation. During the day the small toads can make use of their adaptive coloring to advertise toxicity and discourage predators (Bufo bufo: [57]). Diurnal behavior can also allow young toads to avoid cannibalistic older toads (Bufo marinus: [152]).
Although, clearly, most human teenagers are not at high risk of being consumed by their parents, there is an argument to be made that the risks, needs, and social interactions of mammalian species, including humans, change during adolescent development. Similarly, there is growing evidence that within-species competition can influence the timing of an individual’s activity within the species’ normal active period (called “Temporal Partitioning”; [98]). This temporal partitioning regularly results in the dominant individual gaining primary access to the early part of the active period, whereas lower-ranking individuals are shunted to either a more unconsolidated or delayed activity pattern (bellbirds: [42], trout: [6], rats: [25]), in a manner that also resembles the division of time between younger and older individuals in the examples presented above (toads: [57, 152]). As a result, this temporal partitioning allows dominant individuals to forage during times when there are higher rewards (bellbirds: [42], trout: [6]), whereas lower-ranking individuals can gain access to resources during a time when there is less personal risk of intimidation and attack (bellbirds: [42]). Therefore, the pubertal shift that we observed in degus and rats from an activity pattern that is more crepuscular/delayed to one that is consolidated earlier during the active period resembles circadian changes associated with a shift in dominance status.
Gonadal hormones correlate with competitive status during adulthood in many species [73] as well as during puberty [12]. For example, testosterone levels are lower in individuals that are frequent recipients of aggressive behavior, and higher in individuals that are frequent initiators (chimpanzees: [12]). During puberty in primates, individuals become increasingly more involved in the adult social hierarchy, engaging in more affiliative behaviors and associating with same sex adults. Pubertal animals also start to be treated competitively by adults, and aggressive interactions can peak during the pubertal period in some species [181]. Amongst rodent species, puberty overlaps with a play period [21, 181], but late puberty is also a time of intense competition [21], as many individuals are eventually evicted or emigrate from the colony or living group (especially males, [53]). Thus, potentially having a circadian system that is sensitive to signals regarding dominance status may be particularly important during the pubertal period. In that case, it is possible that when a temperamental adolescent avoids his parents while staying up late socializing with peers he might actually be responding to a hormonal drive to establish an independent life at a time of day that is not dominated by older individuals.
There are several interesting future experiments that could be done to test the hypothesis that pubertal hormones influence circadian rhythms in a manner that signals dominance status. First, we would expect that pubertal hormones might produce a consolidation and phase advance of activity rhythms in rodents under conditions of low competition (e.g. individual laboratory housing) but not under conditions of high competition (e.g. social housing with older, dominant individuals; [25]). One might also predict that the degree of phase shift and rhythm consolidation would correlate with plasma testosterone levels. Recently the technology to run circadian experiments in socially-housed individuals became available [145], so such experiments are now feasible.
The Societal Relevance of Hormone-Driven Circadian Development
The most important conclusion of our review is that hormonally-driven changes in circadian activity during puberty are common across mammalian species, and therefore likely to be found in humans as well. What do these results mean for society?
For the research community, these results have two important implications. First, the circadian mechanisms in the SCN cannot be assumed to be static after prenatal/infantile development. These data suggest that a number of components of the circadian system change during puberty, including free-running period, circadian light sensitivity, rhythm consolidation, and rhythm amplitude. On a practical level, this means that the results of electrophysiological studies performed on young animals cannot be immediately generalized to adults. This point is particularly significant, because pre-pubertal and pubertal rodents are frequently used for voltage clamp studies (e.g., [94, 157, 204]) due to the greater ease of creating an electrode seal (or “patch”) on younger cell membranes (A.M. Vosko, personal communication). Likewise, college freshman are commonly the subject of human psychological studies, but current evidence suggests that they exhibit rhythms atypical of the rest of the population and exist in a perpetual state of sleep deprivation.
For clinicians, these results stress the need for differential diagnostic considerations for sleep and circadian disorders in adolescents. This is especially important for the diagnosis of circadian phase disorders, such as delayed (DSPD) or advanced (ASPD) sleep phase disorders, as well as for insomnia and narcolepsy. Indeed, an earlier study of human adolescents indicated that 10th grade students maintaining their typical sleep schedule could fall asleep within 5 minutes during a Multiple Sleep Latency Test (MSLT) administered during the morning (8:30 a.m.). Furthermore, 48% of these teens showed at least one episode of sleep onset REM sleep [37]. These data from a clinical screening are hallmarks of narcolepsy [120]. Such findings highlight the importance of revising clinical assumptions in the face of the prevalence of sleep deprivation amongst teens asked to be awake at the “wrong” circadian phase. Recent data also suggest that later bedtimes and chronically shortened sleep place teens at risk for depression and suicidal ideation [58]. Thus, interpretations of teenagers’ complaints of malaise, fatigue, and sadness also need to take into account adolescent sleep and circadian issues [29, 31].
Finally, for policy makers, teachers and parents, these results provide a clear mandate. The effects of sleep deprivation on grades, car accident risk, and mood are indisputable [27, 201, 202]. It is important to determine how we as a society can help adolescents obtain more sleep. One possible intervention is parent-set earlier bedtimes, which not only provide a regular schedule and encourage teenagers to spend sufficient time in bed, but may actually advance circadian rhythm phase by reducing light exposure in the evenings and increasing light exposure in the morning. Two recent studies indicate that earlier parent-set bedtimes can help teenagers adjust to early school start times, leading to greater total sleep time, improved daytime wakefulness, reduced fatigue, and better emotional health [58, 177]. However, despite the superior health outcomes, parent-set bedtimes were associated with rather small increases in total sleep time (19 minutes on average, [177]). In another study, earlier parental-set bedtimes (10 p.m. or earlier) were associated with 40 minutes more sleep per night than later parental-set bedtimes (midnight or later). However, even the adolescents with earliest parental-set bedtimes only received 8 hrs 10 min of sleep per night, on average [58], far less than the recommended 9-10 hours [33, 29].
Further improvements have been observed in studies examining school districts that have delayed middle and high school start times with the goal of accommodating adolescent chronotype and improving teens’ sleep-wake patterns by easing the pressure to wake in the early morning [48, 141, 194]. Moving school start times modestly later (30 min-1 hr) was found to increase total sleep time on weekdays by 15-45 minutes [48, 141], leading to a 20-30% decrease in the number of students reporting daytime sleepiness, difficulty staying awake during class, and tiredness that interfered with completing homework. There was also around a 20% decrease in the number of students reporting feeling unhappy, depressed, irritated or annoyed [141]. Overall, later school start times were associated with better academic performance, including decreased truancy, tardiness, and drop-out rate, and better grades in first hour classes [141, 194]. Perhaps most striking were the results regarding car accident risk: in one study, the adolescent car accident rate dropped by 16% following a 1 hr delay in school start time [48]. Similarly, another study comparing two cities of similar size and demographics found that the city with the later school start time (75-80 minutes later) had 16-19% fewer teenage car accidents per year per capita [192].
For this reason, we advocate for a combination of parental and school-based interventions to improve teenage circadian entrainment and resulting daily sleep. In addition to changing start times, schools can help teenagers gain control over their own sleep patterns by teaching sleep and circadian principles in middle and high school health education. In particular, it appears that teenagers may be more sensitive to the phase-delaying properties of evening light exposure than young children or adults. Therefore, it may be important to stress the fact that reducing light at night, including exposure to TV screens and computers before bedtime, can naturally advance circadian phase [24]. Similarly, incorporating outdoor morning activity into an adolescent schedule might also produce a phase advance. A Japanese study found that adolescents who were exposed to greater light exposure during the day were less likely to have trouble falling asleep at night [72]. In high or low latitudes, daylight exposure can also produce the added benefit of reducing seasonal affective disorder (SAD), a mood disorder that affects more adolescents than any other age group [77, 189]. Overall, it is clear that encouragement, pronouncements, and advocacy from the medical/scientific and public health community is essential for driving such societal changes and curricular enhancements.
Highlights for: The Neuroendocrine Control of the Circadian System: Adolescent Chronotype.
-
-
Delayed sleep patterns, or later chronotype, develop during adolescence in humans
-
-
Pubertal changes in chronotype occur in many other mammalian species
-
-
These changes in chronotype differ by sex and are driven by reproductive hormones
-
-
Fundamental properties of the circadian timekeeping system change during adolescence
-
-
Despite physiological causes, basic interventions can help adolescent sleep problems
Acknowledgements
This research was supported by a grant from the National Science Foundation (TML, MHH - IBN-0952046) as well as an Endocrinology training grant from the National Institute of Health through the Department of Pediatrics at University of Michigan (MHH - T32-DK071212). Additionally, the authors would like to thank Dr. Tammy Jechura, Dr. Megan Mahoney, Dr. Daniel Hummer, and Dr. Mary Carskadon for their earlier work on this topic. The authors are indebted to Kassia Zalewkas, for her work compiling Figure 1, and to Stephanie Stout, Sofya Rozenblat, Jessica Koren, Kassia Zalewkas, Mary Ellen Hagenauer, Dr. Megan Mahoney, Dr. Bob Thompson, Dr. Jimo Borjigin, Dr. Daniel Forger, and Dr. Jill Becker for their feedback on earlier versions of the text.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- [1].Abe H, Honma S, Namihira M, Masubuchi S, Honma K. Behavioural rhythm splitting in the CS mouse is related to clock gene expression outside the suprachiasmatic nucleus. Eur J Neurosci. 2001;14:1121–1128. doi: 10.1046/j.0953-816x.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- [2].Abe H, Honma S, Honma K. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R607–R615. doi: 10.1152/ajpregu.00331.2006. [DOI] [PubMed] [Google Scholar]
- [3].Abizaid A, Mezei G, Horvath TL. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004;1010:35–44. doi: 10.1016/j.brainres.2004.01.089. [DOI] [PubMed] [Google Scholar]
- [4].Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:S683–93. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- [5].Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–50. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- [6].Alanara A, Burns MD. Intraspecific resource partitioning in brown trout: the temporal distribution of foraging is determined by social rank. J Anim Ecol. 2001;70:980–986. [Google Scholar]
- [7].Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am. J. Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- [8].Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26:21–25. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- [9].Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am J Physiol. 1990;258:R634–R644. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- [10].Anderson CH. Nucleolus: Changes at puberty in neurons of the suprachiasmatic nucleus and the preoptic area. Exp Neurol. 1981;74:780–786. doi: 10.1016/0014-4886(81)90251-x. [DOI] [PubMed] [Google Scholar]
- [11].Andrade M, Menna-Barreto L. Sleep Patterns of High School Students Living in Sao Paulo, Brazil. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 118–131. [Google Scholar]
- [12].Anestis SF. Testosterone in juvenile and adolescent male chimpanzees (Pan troglodytes): Effects of dominance, rank, aggression, and behavioral style. Am J Phys Anthropol. 2006;130:536–545. doi: 10.1002/ajpa.20387. [DOI] [PubMed] [Google Scholar]
- [13].Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [14].Aschoff J. Circadian rhythms in man: A self-sustained oscillator with an inherent frequency underlies human 24-hr periodicity. Science. 1965;148:1427–1432. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- [15].Aschoff J. Circadian Activity Patterns with Two Peaks. Ecology. 1966;47:657–662. [Google Scholar]
- [16].Aton SJ, Herzog ED. Come Together, Right…Now: Synchronization of Rhythms in a Mammalian Circadian Clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26:631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- [18].Bae K K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- [19].Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–577. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beach FA. Animal models for human sexuality. In: Potter R, Whelan J, editors. Sex, Hormones and Behavior; Ciba Foundation Symposium 62, Excerpta Medica; Amsterdam. 1979; pp. 113–143. [DOI] [PubMed] [Google Scholar]
- [21].Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Anim Behav. 1964;12:427–441. [Google Scholar]
- [22].Borisenkov MF. The pattern of entrainment of the human sleep-wake rhythm by the natural photoperiod of the north. Chronobiol Int. 2011;28:921–929. doi: 10.3109/07420528.2011.623978. [DOI] [PubMed] [Google Scholar]
- [23].Brockman R, Bunick D, Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm Behav. 2011;60:439–447. doi: 10.1016/j.yhbeh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- [24].Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- [25].Calhoun JB. The Ecology and Sociology of the Norway Rat. US Department of Health, Education, and Welfare; Bethesda MD: 1962. [Google Scholar]
- [26].Cambras T, Diez-Noguera A. Changes in motor activity during the development of the circadian rhythm in the rat. J Interdisciplinary Cycle Res. 1988;19:65–74. [Google Scholar]
- [27].Carskadon MA. Risks of driving while sleepy in adolescents and young adults. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 148–158. [Google Scholar]
- [28].Carskadon MA. Maturation of processes regulating sleep in adolescents. In: Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep in Children: Developmental Changes in Sleep Patterns. ed 2 Informa Healthcare; 2008. pp. 95–109. [Google Scholar]
- [29].Carskadon MA. Sleep in adolescents: The perfect storm. Pediatr Clin N Amer. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carskadon MA, Acebo C, Arnedt JT. Failure to identify pubertally-mediated melatonin sensitivity to light in adolescents. Sleep. 2002;25:A191. [Google Scholar]
- [31].Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- [32].Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms in adolescents. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- [33].Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- [34].Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- [35].Carskadon MA, Mancuso J, Rosekind MR. Impact of Part-Time Employment on Adolescent Sleep Patterns. Sleep Res. 1989;18:114. [Google Scholar]
- [36].Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- [37].Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- [38].Castro ECV, Andrade MMM. Longitudinal study of the spectral composition of behavioral rhythms in the rat. Biol Rhythm Res. 2005;36:131–140. [Google Scholar]
- [39].Chappell PE. Clocks and the Black Box: Circadian influences on gonadotropin releasing hormone secretion. J Neuroendocrinol. 2005;17:119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- [40].Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotrophin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-sectreting GT-17 cell line. J Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cohen IR, Wise PM. Effects of estradiol on the diurnal rhythm of serotonin activity in microdissected brain areas of ovariectomized rats. Endocrinology. 1988;122:2619–25. doi: 10.1210/endo-122-6-2619. [DOI] [PubMed] [Google Scholar]
- [42].Craig JL, Douglas ME. Temporal partitioning of a nectar resource in relation to competitive asymmetries. Anim Behav. 1984;32:624–629. [Google Scholar]
- [43].Cramer CP, Thiels E, Alberts JR. Weaning in rats: maternal behavior. Dev Psychobiol. 1990;23:479–493. doi: 10.1002/dev.420230604. [DOI] [PubMed] [Google Scholar]
- [44].Crews D. Diversity of hormone-behavior relations in reproductive behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd ed MIT Press; 2002. pp. 223–287. [Google Scholar]
- [45].Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [46].Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1669–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on the circadian pacemaker in mice. Proc Natl Acad Sci U.S.A. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. J Clin Sleep Med. 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- [49].Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- [50].DeCoursey PJ. In: Chronobiology: Biological Timekeeping. Dunlap JC, Loros JJ, DeCoursey PJ, editors. Sinauer Associates, MA; 2004. pp. 27–66. [Google Scholar]
- [51].De Tezanos Pinto FT, Golombek DA. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Sci. 1999;65:2497–2504. doi: 10.1016/s0024-3205(99)00516-0. [DOI] [PubMed] [Google Scholar]
- [52].Diez-Noguera A, Cambras T. Sex differences in the development of the motor activity circadian rhythm in rats under constant light. Physiol Behav. 1990;47:889–894. doi: 10.1016/0031-9384(90)90014-u. [DOI] [PubMed] [Google Scholar]
- [53].Ebensperger LA, Chesh AS, Castro RA, Tolhuysen LO, Quirici V, Burger JR, Hayes LD. Instability rules social groups in the communal breeder rodent Octodon degus. Ethol. 2009;115:540–554. [Google Scholar]
- [54].Fatehi M, Fatehi-Hassanabad Z. Effects of 17B-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of the rat. Neuropsychopharmacology. 2008;33:1354–1364. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- [55].Figueiro MG, Rea MS. Evening daylight may cause adolescents to sleep less in spring thatn in winter. Chronobiol Int. 2010;27:1242–1258. doi: 10.3109/07420528.2010.487965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuroendocrinol Lett. 2010;31:92–96. [PMC free article] [PubMed] [Google Scholar]
- [57].Freeland WJ, Kerin SH. Ontogenetic alteration of activity and habitat selection by Bufo marinus. Wildl Res. 1991;18:431–437. [Google Scholar]
- [58].Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep. 2010;33:97–106. doi: 10.1093/sleep/33.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Giannotti F, Cortesi F. Sleep Patterns and Daytime Function in Adolescence: An Epidemiological Survey of an Italian High School Student Sample. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 132–147. [Google Scholar]
- [60].Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 50–68. [Google Scholar]
- [61].Gorman MR, Lee TM. Hormones and biological rhythms. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd ed MIT Press; 2002. pp. 451–494. [Google Scholar]
- [62].Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolesence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [63].Gritton H. Dissertation in fulfillment of a Doctor of Philosophy in Neuroscience. University of Michigan; Ann Arbor: 2011. Bi-directional interactions between cognition and biological rhythms. [Google Scholar]
- [64].Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- [65].Gwinner E. Testosterone induces splitting of circadian locomotor activity in birds. Science. 1974;185:72–74. doi: 10.1126/science.185.4145.72. [DOI] [PubMed] [Google Scholar]
- [66].Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, Lee TM. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm Behav. 2011;60:46–57. doi: 10.1016/j.yhbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm Behav. 2011;60:37–45. doi: 10.1016/j.yhbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hagenauer MH, Lee TM. Circadian organization of the diurnal caviomorph rodent, Octodon degus. Biol Rhythm Res. 2008;39:269–289. [Google Scholar]
- [69].Hagenauer MH, Lee TM. Time for Testosterone: The Suprachiasmatic Nucleus Gets Sexy. Endocrinology. 2011;152:1727–1730. doi: 10.1210/en.2011-0198. [DOI] [PubMed] [Google Scholar]
- [70].Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hastings MH, Herzog ED. Clock Genes, Oscillators, and Cellular Networks in the Suprachiasmatic Nuclei. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- [72].Harada T, Morisane H, Takeuchi H. Effect of Daytime Light Conditions on Sleep Habits and Morningness-Eveningness Preference of Japanese Students Aged 12-15 Years. Psychiatry Clin Neurosci. 2002;56:225–226. doi: 10.1046/j.1440-1819.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- [73].Hirshenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta analysis of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- [74].Hummer DL. Dissertation in fulfillment of a Doctor of Philosophy in Psychology. University of Michigan; Ann Arbor: 2006. Development and sexual differentiation of the circadian system of Octodon degus. [Google Scholar]
- [75].Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running rhythms in the developing diurnal rodent, Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R586–R597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- [76].Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res. 1984;11:185–196. doi: 10.1016/0166-4328(84)90210-9. [DOI] [PubMed] [Google Scholar]
- [77].Imai M, Kayukawa Y, Ohta T, Li L, Nakagawa T. Cross-regional survey of seasonal affective disorders in adults and high school students in Japan. J Affect Disord. 2003;77:127–133. doi: 10.1016/s0165-0327(02)00110-6. [DOI] [PubMed] [Google Scholar]
- [78].Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jechura TJ, Lee TM. Ovarian hormones influence olfactory cue effects on reentrainment in the diurnal rodent, Octodon degus. Horm Behav. 2004;46:349–355. doi: 10.1016/j.yhbeh.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [80].Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav. 2000;38:243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- [81].Jechura TJ, Walsh JM, Lee TM. Testosterone surpresses circadian responsiveness to social cues in the diurnal rodent, Octodon degus. J Biol Rhythms. 2003;18:43–50. doi: 10.1177/0748730402239675. [DOI] [PubMed] [Google Scholar]
- [82].Jesseau SA. Dissertation in fulfillment of a Doctor of Philosophy degree in Psychology. University of Michigan; Ann Arbor: 2004. Kin discrimination and social behavior in communally-nesting degus (Octodon degus) [Google Scholar]
- [83].Johnson CH. Phase Response Curves: What Can They Tell Us About Circadian Clocks? In: Hiroshige T, Honma K, editors. Circadian Clocks from Cell to Human. Hokkaido University Press; Sapporo: 1992. pp. 209–246. [Google Scholar]
- [84].Johnson MH, Everitt BJ. Essential Reproduction, Blackwell Science. Oxford; 2000. The regulation of gonadal function; pp. 88–118. [Google Scholar]
- [85].Joutsiniemi SL, Leinonen L, Laakso ML. Continuous recording of locomotor activity in groups of rats: postweaning maturation. Physiol Behav. 1991;50:649–654. doi: 10.1016/0031-9384(91)90562-3. [DOI] [PubMed] [Google Scholar]
- [86].Juul A, Skakkebaek NE. Androgens and the ageing male. Human Reprod Update. 2002;8:423–433. doi: 10.1093/humupd/8.5.423. [DOI] [PubMed] [Google Scholar]
- [87].Kalish N. The early bird gets the bad grade. The New York Times; Jan 14, 2008. [Google Scholar]
- [88].Karatsoreos IN, Butler MP, LeSauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152:1970–1978. doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Karatsoreos IN, Silver R. Minireview: The Neuroendocrinology of the Suprachiasmatic Nucleus as a Conductor of Body Time in Mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11:91–101. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- [92].Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrin. 2012;37:256–269. doi: 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kittrell EMW, Satinoff E. Development of the circadian rhythm of body temperature in rats. Physiol Behav. 1986;38:99–104. doi: 10.1016/0031-9384(86)90138-1. [DOI] [PubMed] [Google Scholar]
- [94].Klisch C, Inyushkin A, Mordel J, Karnas D, Pevet P, Meissl H. Orexin A modulates neuronal activity of the rodent suprachiasmatic nucleus in vitro. Eur J Neurosci. 2009;30:65–75. doi: 10.1111/j.1460-9568.2009.06794.x. [DOI] [PubMed] [Google Scholar]
- [95].Kohyama J. A newly proposed disease condition produced by light exposure during night: Asynchronization. Brain Dev. 2008 doi: 10.1016/j.braindev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [96].Kow LM, Pfaff DW. Suprachiasmatic neurons in tissue slices from ovariectomized rats: electrophysiological and neuropharmacological characterization and the effects of estrogen treatment. Brain Res. 1984;297:275–286. doi: 10.1016/0006-8993(84)90568-7. [DOI] [PubMed] [Google Scholar]
- [97].Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998;139:4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- [98].Kronfeld-Shor N, Dayan T. Partitioning of time as an ecological resource. Annu Rev Ecol Evol Syst. 2003;34:153–181. [Google Scholar]
- [99].Kuhlman SJ. Biological Rhythms Workshop IB: Neurophysiology of SCN Pacemaker Function. Cold Spring Harbor Symposium on Quantitative Biology. 2007;72:21–33. doi: 10.1101/sqb.2007.72.061. [DOI] [PubMed] [Google Scholar]
- [100].Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58:573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- [101].Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:68–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [102].Lee TM. Octodon degus: A diurnal, social, and long-lived rodent. ILAR Journal. 2004;45:14–24. doi: 10.1093/ilar.45.1.14. [DOI] [PubMed] [Google Scholar]
- [103].Lee TM, Hummer DL, Jechura TJ, Mahoney MM. Pubertal development of sex differences in circadian function: An animal model. In: Dahl RE, Spear LP, editors. Adolescent Brain Development: Vulnerabilities and Opportunities. Vol. 1021. Ann NY Acad Sci; 2004. pp. 262–275. [DOI] [PubMed] [Google Scholar]
- [104].Levin R, Stern JM. Maternal influences on ontogeny of suckling and feeding rhythms in the rat. J Comp Physiol Psychol. 1975;89:711–721. doi: 10.1037/h0077038. [DOI] [PubMed] [Google Scholar]
- [105].Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- [106].Magnan P, FitzGerald GJ. Ontogenetic changes in diel activity, food habits, and spatial distribution of juvenile and adult creek chub. Env Biol Fish. 1984;11:301–307. [Google Scholar]
- [107].Mahoney MM, Ramanathan C, Hagenauer MH, Lee TM, Smale L. Daily Rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci. 2009;30:1537–1543. doi: 10.1111/j.1460-9568.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mahoney MM, Rossi BV, Hagenauer MH, Lee TM. Characterization of the estrous cycle in Octodon degus. Biol Reprod. 2010;84:664–671. doi: 10.1095/biolreprod.110.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209–214. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- [110].Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- [111].Maywood ES, Mrosovsky N. A molecular explanation of interactions between photic and non-photic circadian clock-resetting stimuli. Brain Res Gene Expr Patterns. 2001;1:27–31. doi: 10.1016/s1567-133x(01)00005-9. [DOI] [PubMed] [Google Scholar]
- [112].McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd ed MIT Press; 2002. pp. 117–149. [Google Scholar]
- [113].McCarthy MM, Crews D. Molecular Approaches to Behavioral Neuroendocrinology. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd ed MIT Press; 2002. pp. 39–74. [Google Scholar]
- [114].McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Meijer JH, Schwartz WJ. In search of the pathways for light induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- [116].Miche S, Colwell CS. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol Int. 2001;18:579–600. doi: 10.1081/cbi-100106074. [DOI] [PubMed] [Google Scholar]
- [117].Miller MM, Silver J, Billiar RB. Effects of ovariectomy on the binding of [I-125]-α bungarotoxin (2.2 and 3.3) to the suprachiasmatic nucleus of the hypothalamus: An in vivo autoradiographic analysis. Brain Res. 1982;247:355–364. doi: 10.1016/0006-8993(82)91261-6. [DOI] [PubMed] [Google Scholar]
- [118].Miller MM, Silver J, Billiar RB. Effects of gonadal steroids on the in vivo binding of [I-125]-α bungarotoxin to the suprachiasmatic nucleus. Brain Res. 1984;290:67–75. doi: 10.1016/0006-8993(84)90736-4. [DOI] [PubMed] [Google Scholar]
- [119].Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kasbeek A, Pevet P, Shibata S. Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore food anticipatory rhythms in Bmal1 −/− mice. J Circadian Rhythms. 2009;7:1–13. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mitler MM, Van den Hoed J, Carskadon MA, Richardson G, Park R, Guilleminault C, Dement WC. REM sleep episodes during the Multiple Sleep Latency Test in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1979;46:479–481. doi: 10.1016/0013-4694(79)90149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, Okamura H. Phase-dependent responses of Per1 and Per2 genes to a light stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett. 2000;294:41–44. doi: 10.1016/s0304-3940(00)01545-7. [DOI] [PubMed] [Google Scholar]
- [122].Mohamed MK, Abdel-Rahman AA. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur J Endocrinol. 2000;142:307–314. doi: 10.1530/eje.0.1420307. [DOI] [PubMed] [Google Scholar]
- [123].Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, Rhythms, and the Endocrine Brain: Influence of Sex and Gonadal Hormones. J Neurosci. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Monnet FP, Mahe V, Robel P, Boulieu EE. Neurosteroids, via σ receptors, modulate the H3 norepinephrine release evoked by N-methyl-D-asparatate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Moore-Ede MC, Sulzman FM, Fuller CA. Clocks That Time Us; Characteristics of Circadian Clocks. Harvard University Press; 1982. pp. 30–112. [Google Scholar]
- [126].Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav. 1980;24:741–749. doi: 10.1016/0031-9384(80)90406-0. [DOI] [PubMed] [Google Scholar]
- [127].Morin LP, Allen CN. The Circadian Visual System, 2005. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [128].Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement, or pinealectomy on male hamster running rhythmicity. Physiol Behav. 1981;26:825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- [129].Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- [130].Morishita H, Kawamoto M, Masuda Y, Higuchi K, Tomioka M, Nagamachi N, Mitani H, Osaza T, Adachi H. Quantitative histological changes in the hypothalamic nuclei in the prepuberal, puberal, and postpuberal female rat. Brain Res. 1974;76:41–47. doi: 10.1016/0006-8993(74)90512-5. [DOI] [PubMed] [Google Scholar]
- [131].Morishita H, Nagamachi N, Kawamoto M, Tomioka M, Higuchi K, Hashimoto T, Tanaka T, Kuroiwa S, Nakago K, Mitani H, Miyauchi Y, Ozasa T, Adachi H. The effects of prepuberal castration on the development of the nuclear sizes of the neurons in the hypothalamic nuclei of female rats. Brain Res. 1978;146:388–391. doi: 10.1016/0006-8993(78)90985-x. [DOI] [PubMed] [Google Scholar]
- [132].Mrosovsky N, Edelstein K, Hastings MH, Maywood ES. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. J Biol Rhythms. 2001;16:471–478. doi: 10.1177/074873001129002141. [DOI] [PubMed] [Google Scholar]
- [133].Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–630. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- [134].Nakamura TJ, Shinohara K, Funabashi T, Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res. 2001;41:251–255. doi: 10.1016/s0168-0102(01)00285-1. [DOI] [PubMed] [Google Scholar]
- [135].National Sleep Foundation Sleep and Teens Task Force . Adolescent Sleep Needs and Patterns: Research Report and Resource Guide. National Sleep Foundation; Washington D.C.: 2000. pp. 1–26. http://www.sleepfoundation.org/atf/cf/%7BF6BF2668-A1B4-4FE8-8D1A-A5D39340D9CB%7D/sleep_and_teens_report1.pdf. [Google Scholar]
- [136].Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- [137].Neuman A, Gothilf Y, Haim A, Ben-Aharon G, Zisapel N. Nocturnal patterns and up-regulated excretion of the melatonin metabolite 6-sulfatoxymelatonin in the diurnal rodent Psammomys obesus post-weaning under a short photoperiod. Comp Biochem Physiol. 2005;A 142:297–307. doi: 10.1016/j.cbpa.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [138].Nottelmann ED, Susman EJ, Dorn LD, Inoff-Germain G, Loriaux DL, Cutler GB, Chrousos GP. Developmental Processes in Early Adolescence: Relations among chronologic age, pubertal stage, height, weight, and serum levels of gonadotropins, sex steroids, and adrenal androgens. J Adolesc Health Care. 1987;8:246–260. doi: 10.1016/0197-0070(87)90428-1. [DOI] [PubMed] [Google Scholar]
- [139].Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven; 1994. pp. 363–409. [Google Scholar]
- [140].Oukouchoud R, Vivien-Roels B, Pevet P, Lakhdar-Ghazal N. Hormone-dependent and –independent mechanisms involved in the photoperiodic control of neuropeptide levels in the brain of the jerboa (Jaculus orientalis) Brain Res. 2003;967:63–72. doi: 10.1016/s0006-8993(02)04213-0. [DOI] [PubMed] [Google Scholar]
- [141].Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med. 2010;164:608–614. doi: 10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- [142].Palmer JD, Udry JR, Morris NM. Diurnal and weekly but no lunar rhythms in human copulation. Hum Biol. 1982;54:111–121. [PubMed] [Google Scholar]
- [143].Parry BL, Hauger R, Lin E, Le Veau B, Mostofi N, Clopton PL, Gillin JC. Neuroendocrine effects of light therapy in late luteal phase dysphoric disorder. Biol Psychiatry. 1994;36:356–364. doi: 10.1016/0006-3223(94)91210-6. [DOI] [PubMed] [Google Scholar]
- [144].Parry BL, Javeed S, Laughlin GA, Hauger R, Clopton P. Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry. 2000;48:920–931. doi: 10.1016/s0006-3223(00)00876-3. [DOI] [PubMed] [Google Scholar]
- [145].Paul MJ, Schwartz WJ. On the chronobiology of cohabitation. Cold Spring Harbor Symposia on Quant Biol. 2007;72:615–621. doi: 10.1101/sqb.2007.72.042. [DOI] [PubMed] [Google Scholar]
- [146].Peixoto CAT, da Silva AGT, Carskadon MA, Louzada FM. Adolescents living in homes without electrical lighting have earlier sleep times. Behav Sleep Med. 2009;7:73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- [147].Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci U.S.A. 2006;103:5591–5596. doi: 10.1073/pnas.0601310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Perryman JI. Dissertation in fulfillment of a Doctor of Philosophy in Neuroscience. University of Michigan; Ann Arbor: 2010. Circadian and homeostatic components of sleep across sex and development in a diurnal rodent, Octodon degus. [Google Scholar]
- [149].Pinto FT, Golombek DA. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Sci. 1999;65:2497–2504. doi: 10.1016/s0024-3205(99)00516-0. [DOI] [PubMed] [Google Scholar]
- [150].Pittendrigh CS, Daan S. Functional Analysis of Circadian Pacemakers in Nocturnal Rodents I: The Stability and Lability of Spontaneous Frequency. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- [151].Pittendrigh CS, Daan S. Functional Analysis of Circadian Pacemakers in Nocturnal Rodents IV: Entrainment: Pacemaker as Clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- [152].Pizatto L, Child T, Shine R. Why be Diurnal? Shifts in activity time enable young cane toads to evade cannibalistic conspecifics. Behav Ecol. 2008;19:990–997. [Google Scholar]
- [153].Plant TM. Puberty in primates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven; 1994. pp. 453–485. [Google Scholar]
- [154].Raisman G, Brown-Grant K. The “Suprachiasmatic Syndrome”: Endocrine and Behavioral Abnormalities Following Leisons of the Suprachiasmatic Nuclei in the Female Rat. Proc R Soc B. 2006;198:297–314. doi: 10.1098/rspb.1977.0099. [DOI] [PubMed] [Google Scholar]
- [155].Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- [156].Randler C, Ebenhöh N, Fischer A, Hoechel S, Schroff C, Stoll JC, Vollmer C. Chronotype but not to sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2012.02.008. in press. [DOI] [PubMed] [Google Scholar]
- [157].Rash JE, Olson CO, Pouliot WA, Davidson KGV, Yasamura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36 vs. Connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007;149:350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Richardson GS, Tate BA. Endocrine changes associated with puberty and adolescence. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 27–39. [Google Scholar]
- [159].Roenneberg T, Daan S, Merrow M. The Art of Entrainment. J Biol Rhythms. 2003;18:183–194. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- [160].Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- [161].Rose’Meyer RB, Mellick AS, Garnham BG, Harrison GJ, Massa HM, Griffiths LR. The measurement of adenosine and estrogen receptor expression in rat brains following ovariectomy using quantitative PCR analysis. Brain Res Brain Res Protoc. 2003;11:9–18. doi: 10.1016/s1385-299x(02)00219-2. [DOI] [PubMed] [Google Scholar]
- [162].Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychophamacological properties. Psychoneuroendocrinology. 2002;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- [163].Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep Habits and Circadian Preference in Italian Children and Adolescents. J Sleep Res. 2007;16:163–169. doi: 10.1111/j.1365-2869.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- [164].Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32:1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nature Reviews. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- [166].Satriotomo I, Miki T, Gonzalez D, Matsumoto Y, Li H, Gu H, Takeuchi Y. Excessive testosterone treatment and castration induce reactive astrocytes and fos immunoreactivity in suprachiasmatic nucleus of mice. Brain Res. 2004;1020:130–139. doi: 10.1016/j.brainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- [167].Schechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. International Journal of EndInt J Endocrinol. 2010 doi: 10.1155/2010/259345. DOI: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Schechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Schrader JA, Nunez AA, Smale L. Changes in and dorsal to the rat suprachiasmatic nucleus during early pregnancy. Neuroscience. 2010;171:513–523. doi: 10.1016/j.neuroscience.2010.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Schrader JA, Nunez AA, Smale L. Site-specific changes in brain extra-SCN oscillators during early pregnancy in the rat. J Biol Rhythms. 2011;26:363–367. doi: 10.1177/0748730411409714. [DOI] [PubMed] [Google Scholar]
- [171].Sellix MT, Menaker M. Circadian clocks in the ovary. Trends in Endocrinology and Metabolism. 2010;21:628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-Induced Resetting of a Mammalian Circadian Clock is Associated with Rapid Induction of the mPer1 Transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- [174].Shimoda K, Hanada K, Yamada N, Takahashi K, Takahashi S. Periodic exposure to mother is potent zeitgeber of rat pups’ rhythm. Physiol. Behav. 1985;36:723–730. doi: 10.1016/0031-9384(86)90360-4. [DOI] [PubMed] [Google Scholar]
- [175].Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of estrogen on the expression of connexin32 and connexin43 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Lett. 2000;286:107–110. doi: 10.1016/s0304-3940(00)01102-2. [DOI] [PubMed] [Google Scholar]
- [176].Shinohara K, Funabashi T, Nakamura TJ, Kimura F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett. 2001;309:37–40. doi: 10.1016/s0304-3940(01)02022-5. [DOI] [PubMed] [Google Scholar]
- [177].Short MA, Gradisar M, Wright H, Lack LC, Dohnt H, Carskadon MA. Time for bed: Parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep. 2011;34:797–800. doi: 10.5665/SLEEP.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- [179].Sisk C, Zehr J. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [180].Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- [181].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [182].Sugishita M, Takashima M, Takeuchi Y, Kato Y, Yamauchi T, Takahashi K. Periodic mother deprivation during the light period reversed the phase of serotonin N-acetyltransferase activity rhythm of the pineal gland in rat pups. Pharmacol Biochem Behav. 1993;46:609–615. doi: 10.1016/0091-3057(93)90551-4. [DOI] [PubMed] [Google Scholar]
- [183].Swaab DF, Zhou JN, Ehlhart T, Hofman MA. Development of vasoactive intestinal polypeptide neurons in the human suprachiasmatic nucleus in relation to birth and sex. Brain Res Dev Brain Res. 1994;79:249–259. doi: 10.1016/0165-3806(94)90129-5. [DOI] [PubMed] [Google Scholar]
- [184].Takahashi K, Hayafuji C, Murakami N. Foster mother rat entrains circadian adrenocortical rhythm in blinded pups. Am J Physiol Endocrinol Metab. 1982;243:E443–E449. doi: 10.1152/ajpendo.1982.243.6.E443. [DOI] [PubMed] [Google Scholar]
- [185].Tate BA, Richardson GS, Carskadon MA. Maturational changes in sleep-wake timing: longitudinal studies of circadian activity rhythm of a diurnal rodent. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social and Psychological Influences. Cambridge University Press; 2002. pp. 40–49. [Google Scholar]
- [186].Thiels E, Alberts JR, Cramer CP. Weaning in rats: II pup behavior patterns. Dev Psychobiol. 1990;23:495–510. doi: 10.1002/dev.420230605. [DOI] [PubMed] [Google Scholar]
- [187].Thomas EMV, Armstrong SM. Effect of ovariectomy and estradiol on unity of female circadian rhythms. Am J Physiol. 1989;257:R1241–R1250. doi: 10.1152/ajpregu.1989.257.5.R1241. [DOI] [PubMed] [Google Scholar]
- [188].Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason TH, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- [189].Tonetti L, Barbato G, Fabbri M, Adan A, Natale V. Mood seasonality: A crosssectional study of subjects aged between 10-25 years. J Affect Dis. 2007;97:155–160. doi: 10.1016/j.jad.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [190].Turek FW, Van Cauter E. Rhythms in Reproduction. In: Knobil E, Neill JD, editors. The Physiolog of Reproduction. Raven; 1994. pp. 487–540. [Google Scholar]
- [191].Van den Bulck J. Television Viewing, Computer Game Playing, and Internet Use and Self-Reported Time to Bed and Time Out of Bed in Secondary School Children. Sleep. 2004;27:101–104. doi: 10.1093/sleep/27.1.101. [DOI] [PubMed] [Google Scholar]
- [192].Vorona RD, Szklo-Coxe M, Wu A, Dubik M, Zhao Y, Ware JC. Dissimilar teen crash rates in two neighboring southeastern Virginia cities with different high school start times. J Clin Sleep Med. 2011;7:145–151D. [PMC free article] [PubMed] [Google Scholar]
- [193].Vosko AM, Hagenauer MH, Hummer DL, Lee TM. Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus) Am J Physiol Regul Integr Comp Physiol. 2009;296:353–361. doi: 10.1152/ajpregu.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wahlstrom KL. Accommodating the Sleep Patterns of Adolescents within Current Educational Structures: An Uncharted Path. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 172–197. [Google Scholar]
- [195].Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding- induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- [196].Weinert D. Ontogenetic development of the mammalian circadian system. Chronobiol Int. 2005;22:179–205. doi: 10.1081/cbi-200053473. [DOI] [PubMed] [Google Scholar]
- [197].Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int. 1994;11:222–231. doi: 10.3109/07420529409067791. [DOI] [PubMed] [Google Scholar]
- [198].Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- [199].Weinert D, Waterhouse J. Daily activity and temperature rhythms do not change spontaneously with age in laboratory mice. Physiol Behav. 1999;66:605–612. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- [200].Wilson ME, Gordon TP. Age differences in the duration of mating periods of female macaques. Dev Psychobiol. 1980;13:637–642. doi: 10.1002/dev.420130610. [DOI] [PubMed] [Google Scholar]
- [201].Wolfson AR, Carskadon MA. Sleep Schedules and Daytime Functioning in Adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- [202].Wolfson AR, Carskadon MA. Understanding Adolescent Sleep Patterns and School Performance: A Critical Appraisal. Sleep Med Rev. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- [203].Wollnik D, Döhler K. Effects of adult or perinatal hormonal environment on ultradian rhythms in locomotor activity of laboratory LEW/Ztm rats. Physiol Behav. 1986;38:229–240. doi: 10.1016/0031-9384(86)90158-7. [DOI] [PubMed] [Google Scholar]
- [204].Wong KY, Graham DM, Berson DM. The retina-attached SCN slice preparation: An in vitro mammalian circadian visual system. J of Biol Rhythms. 2007;22:400–410. doi: 10.1177/0748730407305376. [DOI] [PubMed] [Google Scholar]
- [205].Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1411. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- [206].Yamanaka Y, Honma S, Honma K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes to Cells. 2008;13:497–507. doi: 10.1111/j.1365-2443.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- [207].Yamazaki S, Numano R, Abe M, Akiko H, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei M,H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- [208].Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–260. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- [210].Yang SZ, Wang K, Valladares O, Hannenhalli S, Bucan M. Genome-wide expression profiling and bioinformatics analysis of diurnally regulated genes in the mouse prefrontal cortex. Genome Biol. 2007;8:R247. doi: 10.1186/gb-2007-8-11-r247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- [212].Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of t-e circadian system in the golden hamster. Am J Physiol Regul Integr Comp Physiol. 1980;238:97–101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]