Abstract
BACKGROUND & AIMS
Inflammation may contribute to formation, maintenance, and expansion of cancer stem cells (CSCs), which have the capacity for self-renewal, differentiation, and resistance to cytotoxic agents. We investigated the effects of the inflammatory mediator prostaglandin E2 (PGE2) on colorectal CSC development and metastasis in mice and the correlation between levels of PGE2 and CSC markers in human colorectal cancer (CRC) specimens.
METHODS
Colorectal carcinoma specimens and matched normal tissues were collected from patients at the Mayo Clinic (Scottsdale, AZ) and analyzed by mass spectrometry and quantitative PCR. Human primary CRC cells and mouse tumor cells were isolated using microbeads and flow cytometry and analyzed in sphere-formation and flow cytometry assays. LS-174T cells were sorted by flow cytometry (for CD133+CD44+ and CD133−CD44− cells) and also used in these assays. NSG mice were given cecal or subcutaneous injections of LS-174T or human primary CRC cells. ApcMin/+ mice and NSG mice with orthotopic cecal tumors were given vehicle (controls), PGE2, celecoxib, or/and Ono-AE3-208. PGE2 downstream signaling pathways were knocked down with small hairpin RNAs, expressed from lentiviral vectors in LS-174T cells, or blocked with inhibitors in human primary CRC cells.
RESULTS
Levels of PGE2 correlated with colonic CSC markers (CD133, CD44, LRG5, and SOX2 mRNAs) in human colorectal carcinoma samples. Administration of PGE2 to ApcMin/+ mice increased tumor stem cells and tumor burden, compared with controls. NSG mice given PGE2 had increased numbers of cecal CSCs and liver metastases, compared with controls, after intracecal injection of LS-174T or human primary CRC cells. Alternatively, celecoxib, an inhibitor of prostaglandin-endoperoxide synthase 2 (PTGS2 or COX2), reduced polyp numbers in Apc Min/+ mice, liver metastasis in NSG mice with orthotopic tumors, and numbers of CSCs in ApcMin/+ and NSG mice. Inhibitors or knockdown of PGE receptor 4 (PTGER4 or EP4), PI3K p85α, ERK1, or nuclear factor (NF)-κB reduced PGE2-induced sphere formation by and expansion of LS-174T and/or human primary CRC cells. Knockdown of ERK1 or PI3K p85α also attenuated PGE2-induced activation of NF-κB in LS-174T cells. An EP4 antagonist reduced the ability of PGE2 to induce CSC expansion in orthotopic tumors and to accelerate formation of liver metastases. Knockdown experiments showed that NF-κB was required for PGE2 induction of CSCs and metastasis in mice.
CONCLUSIONS
PGE2 induces CSC expansion by activating NF-κB, via EP4–PI3K and EP4–MAPK signaling, and promotes formation of liver metastases in mice. The PGE2 signaling pathway might therefore be targeted therapeutically to slow colorectal cancer progression.
Keywords: NSAIDs, cyclooxygenase, colon cancer, tumorigenesis
INTRODUCTION
Epidemiologic and experimental evidence strongly implicate chronic inflammation as a risk factor for the development of many cancers, including colorectal cancer (CRC). Nonsteroidal anti-inflammatory drugs (NSAIDs) represent one class of drugs found to have beneficial effects on reducing the risk of CRC. NSAIDs are known to exert certain anti-inflammatory effects by targeting prostaglandin-endoperoxide synthases (PTGS) that include PTGS1 (COX-1) and PTGS2 (COX-2). Previous work confirms that PTGS2 expression is elevated in CRC and is associated with a lower survival rate among CRC patients1, 2. In addition to CRC, PTGS2 levels are also elevated in other premalignant and malignant solid tumors, including stomach, esophagus, liver, pancreas, head and neck, lung, breast, prostate, and bladder cancer and this elevation is associated with decreased survival among these cancer patients as well3. PTGS2 has been shown to play an important role in inflammation and cancer4. However, long-term use of the selective PTGS2 inhibitors and non-selective NSAIDs (except for aspirin) will not be recommended for cancer prevention because of the unacceptable cardiovascular side effects of these drugs, especially in those individuals with a history of atherosclerotic heart disease5. One potential way to avoid these undesired side effects is to only target PTGS-derived prostanoids that mediate the tumor-promoting effects of PTGS2.
PTGS2-derived prostaglandin E2 (PGE2), a pro-inflammatory mediator, is the most abundant prostaglandin found in various types of human malignancies including colorectal, lung, breast, head and neck cancer and is often associated with a poor prognosis6–9. Emerging epidemiologic evidence and a phase II biomarker study showed that urinary PGE2 metabolite (PGE-M) levels were associated with an increased risk of developing colorectal10–12, gastric13, and breast cancer14, 15 and were correlated with disease progression in head and neck squamous cell carcinomas16. PGE2 exerts its effects by binding to EP receptors (EP1, EP2, EP3, and EP4) that belong to the family of seven transmembrane G protein-coupled receptors. Each EP receptor activates its own downstream signaling pathways that transduce a particular biologic affect. PGE2 has been shown to promote tumor epithelial cell proliferation, survival, and migration/invasion via multiple signaling pathways17. However, all of the mechanisms by which PGE2 accelerates CRC formation, progression, and metastasis in complex living systems are not fully understood.
Undifferentiated cancer cells have been referred to as cancer stem cells (CSCs) or tumor-initiating cells because they possess the capacity for self-renewal, differentiation, and innate resistance to cytotoxic agents18, 19. CSCs are thought to be responsible for tumor initiation, growth, metastatic spread, relapse, and recurrence. Several studies have described the presence of CSCs in human CRC and shown that they are capable of initiating tumor development19, 20. However, very little is known about their biology or how they are formed, maintained, and expanded. Emerging evidence supports the hypothesis that chronic inflammation might regulate the development and function of CSCs21 or convert epithelial mesenchymal cells to CSCs22. The observation that NSAIDs can eliminate oncogenic intestinal stem cells via induction of apoptosis in ApcMin/+ mice23 and reduce colonosphere formation in human colorectal carcinoma cell lines in vitro24 prompted us to postulate that PGE2 might accelerate tumor initiation, growth, and liver metastasis by inducing colorectal CSC expansion.
METHODS
Animal experiments
All animal experiments conform to our animal protocols that were approved by the Institutional Animal Care and Use Committee at Arizona State University. NSG and ApcMin/+ mice were obtained from Jackson Laboratory (Bar Harbor, Maine). For the subcutaneous (sub-Q) injection, indicated cell numbers were injected into the flanks of male NSG mice at age of 7 weeks old. For the orthotopic mouse model, 1 × 104 of cells derived from LS-174T, LS-174T/vector, LS-174T/shp65, or 5 × 105 of cells derived from human primary CRC specimen were injected into the cecal wall of male NSG mice at age of 7 weeks old. Additional information on animal treatments is provided in Supplemental Methods.
Isolation of tumor epithelial cells
This study was approved by the Institutional Review Board of the Mayo Clinic. Fresh or frozen tumor specimens were obtained from patients with primary CRC at the Mayo Clinic. Human CRC specimens and mouse cecal tumor tissues were minced and digested in Chang medium with supplement containing 2 mg/ml collagenase III (Worthington Biochemical Corp.) at 37°C, 5% CO2 for 5 hours with occasional shaking. Human epithelial tumor cells were isolated by using human CD326 (EpCAM) MicroBeads (Miltenyi Biotec) according to the manufacturer’s instructions. Mouse colonic adenomas were minced and digested with PBS containing with 0.1%BSA and 12 mg/ml collagenase I (Gibco). Mouse epithelial tumor cells were purified by Flow cytometry sorting using CD326 (EpCAM)-PE antibody (Miltenyi Biotec). Isolated tumor epithelial cells were subjected to in vitro sphere-forming assays, cecal injection, sub-Q injection, or flow cytometry analysis.
Immunohistochemical staining
Paraffin-embedded tissue sections (5 μm thick; n=5 per animal) were stained with anti-CD133 rabbit antibody (1:100, Biorbyt, Cambridge, UK) and anti-CD44v6 mouse monoclonal antibody (1:100, R&D System) in 4°C overnight. The immunohistochemical staining was completed by using a Zymed-Histostain-SP Kit (Zymed, South San Francisco, CA) as described previously25.
Flow cytometry analysis and sorting
Single-cell suspensions in staining buffer (Biolegend) were incubated with anti-hCD44v6-PE (1:20, R&D System), anti-hCD133-FITC (1:10, Miltenyi Biotec), and/or anti-EpCAM (CD326)-APC-Vio770 (1:100, Miltenyi Biotec) antibodies for 30 min on ice and analyzed on a Gallios flow cytometer (Beckman Coulter). CD133+CD44+ and CD133−CD44− LS-174T cells were sorted by flow cytometer and subjected to in vitro sphere-forming and Western blotting assays.
Cell culture
Detailed information about cell culture and treatments is provided in Supplemental Methods.
In vitro sphere-forming assay
For PGE2 treatment, 30,000 cells were cultured in 6-well Ultra-low Attachment surface plate (Costar) with serum-free DMEM/F12 medium containing with indicated dose of PGE2, 10μM ONO-AE-208 (a gift of Ono Pharmaceutical Co., Osaka, Japan), 50μM PD98059 (Calbiochem La Jolla, CA), 10μM LY294002 (Calbiochem), 10μM SC19220 (Cayman Chemical), 10μM AH6809 (Cayman Chemical) and/or vehicle without B27 supplement, EGF, and FGF for 3 weeks. The culture medium was replaced by fresh medium with fresh PGE2, inhibitors, and/or vehicle everyday for three weeks. For regular sphere-forming assays, 30,000 cecal tumor cells isolated from indicated mouse, vehicle-treated cells, or PGE2-treated cells were cultured in 6-well Ultra-low Attachment surface plate with serum-free DMEM/F12 medium containing B27 supplement, 20 ng/ml EGF, and 10 ng/ml FGF without PGE2 for three weeks. The sphere numbers in each well were quantified.
Lentivirus production and stable transfection
ERK1, PI3K p85α, and NF-κB p65 shRNAs were purchased from Open Biosystems. EP1, EP2, EP3, and EP4 shRNAs (lentiviral particles) were obtained from Santa Cruze. Lentivirus production and stable transfection were performed according to the manufacturer’s instructions.
Western blot analysis
Detailed information about Western blotting assay and antibodies is provided in Supplemental Methods.
Quantitative PCR
Total RNA was isolated from human CRC specimens by the TRIzol reagent (Life Technologies) and was reversely transcribed to cDNA using SuperScript III Reverse Transcriptase (Invitrogen). Real-time q-PCR was performed with TaqMan® Gene Expression Assay Mix and TaqMan® Universal PCR Master Mix (Life Technologies) using ViiATM 7 Real-time PCR System (Life Technologies). TaqMan® Gene Expression Assay Mix for CD133, CD44, LGR5, and SOX2 were obtained from Life Technologies. The relative expression of target gene is the averages of triplicates that are normalized against the transcription levels of 18s rRNA.
PGE2 measurement
Tissues were homogenized in PBS with 10% 2,6-di-tert-butyl-p-cresol. PGE2 level were measured using an Agilent 6460 tandem mass spectrometry configured with the Agilent 1200 liquid chromatography system (LC/MS/MS, Agilent Technologies) and normalized with protein concentration.
Statistical analysis
Each in vitro experiment was done at least 3 times and each in vivo experiment was conducted at least twice. Data are presented as mean ± SEM. Comparisons among multiple groups were performed by factorial analysis of variance, followed by Bonferroni test. Comparisons between two groups were performed with Student’s t-test or Mann-Whitney U test where appropriate. Fischer’s exact test was used for categorical variables. p<0.05 was considered significant.
RESULTS
PGE2 levels correlate with the expression of CSC markers in human CRC
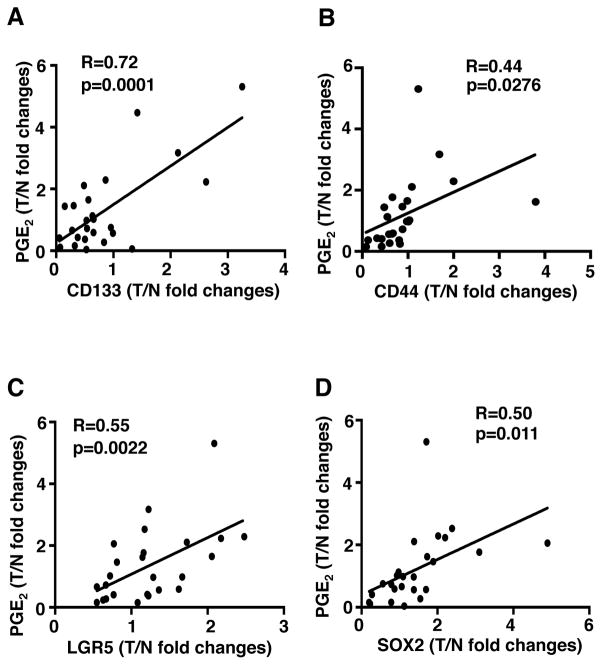
CD133, CD44, CD44v6, LRG5, and SOX2 are informative markers for CSCs in CRC19, 20, 26, 27. However, RT-PCR could not be used to measure CD44v6 mRNA because primers cannot be designed to detect only CD44v6. Therefore, we examined whether PGE2 levels correlate with the expression of CD133, CD44, LRG5, and SOX2 in human colon carcinomas at grade II–III with the matched normal tissues. As shown in Figure 1, the PGE2 levels are positively associated with the expression of CD133 (panel A), CD44 (panel B), LGR5 (panel C), and SOX2 (panel D) at mRNA levels. These results indicate that PGE2 may promote CSC expansion in CRC.
Figure 1. PGE2 levels correlate with the expression of CSC markers in human CRC.
The levels of PGE2 are correlated with the expression of CD133 (panel A), CD44 (panel B) LRG5 (panel C), and SOX2 (panel D) at mRNA levels in 25 pairs of human colorectal carcinomas (T) with matched normal tissues (N). Data were presented as fold changes in cancer specimens as compared to matched normal tissues. Nonparametric Spearman correlation analysis (R value) was performed.
PGE2 increases CSCs and promotes liver metastasis in vivo
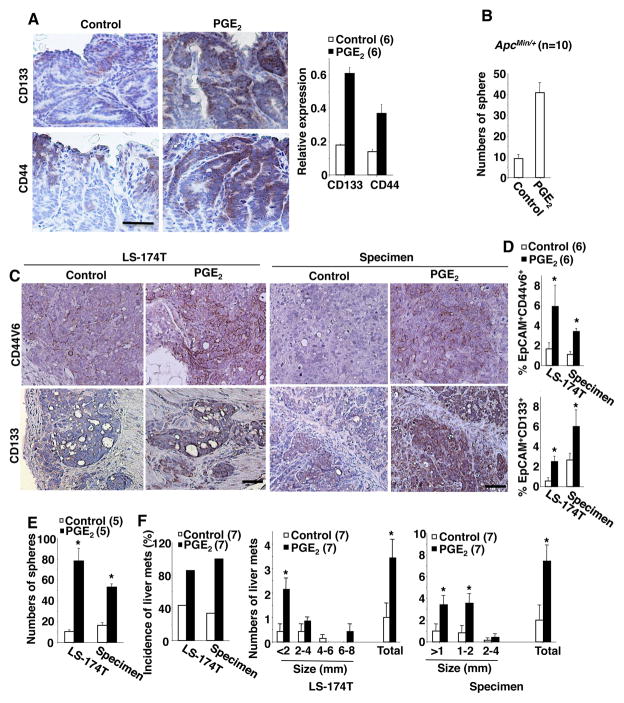
ApcMin/+ mice have been used as a model for human familial adenomatous polyposis and a pre-malignant model for human sporadic CRC. Since NSAIDs reduced oncogenic intestinal stem cells in ApcMin/+ mice23 and the expression of CSC markers has been found in human adenomas28, we first tested whether PGE2 treatment affected oncogenic colonic stem cells in adenomas of ApcMin/+ mice. Treatment of ApcMin/+ mice with PGE2 resulted in an increase in tumor burden (Supplemental Figure S1A) with more CD133− and CD44-positive epithelial cells in colonic adenomas as compared to control mice (Figure 2A). Since no antibody against mouse CD44v6 was available, we utilized an antibody directed against mouse CD44 for IHC staining. Colonic tumor cells isolated from PGE2-treated mice formed more spheres (an index of CSC numbers) than those taken from control mice in in vitro sphere-forming assays (Figure 2B), demonstrating that adenomas from PGE2-treated mice contain more tumor stem cells than those from vehicle-treated mice.
Figure 2. PGE2 increases CSCs and promotes liver metastasis.
(A) Left panel, the representative immunostaining of CD133 and CD44 (brown) in colonic adenomas taken from ApcMin/+ mice treated with PGE2 or vehicle (scale bar, 50 μm). Right panel, relative expression of CD133 and CD44 at mRNA levels in polyps. (B) Formation of spheres from colonic epithelial adenoma cells isolated from ApcMin/+ mice treated with PGE2 or vehicle. (C) The representative immunostaining of CD44v6 and CD133 (brown) in cecal tumors taken from indicated group of mice treated with PGE2 or vehicle (scale bar, 50 μm). (D) Cells isolated from indicated cecal tumors were subjected into flow cytometry analysis. Data represents the percentage of EpCAM+CD44v6+ (top panel) and EpCAM+CD133+ (bottom panel) cells in total cells of cecal tumor taken from above groups. (E) Formation of spheres from cecal tumor cells isolated from mice treated with PGE2 or vehicle in above groups. (F) Incidence (left panel) of liver metastatic tumors and average numbers of liver metastatic tumors (middle and right panels) in mice treated with PGE2 or vehicle in above groups. The error bar indicates ± SEM. *p<0.05.
We also examined whether PGE2 was able to increase CSCs in human colorectal carcinomas. LS-174T cells or primary carcinoma cells isolated from human CRC specimens were injected into the cecal wall of NSG mice. After injection, the mice were treated with vehicle or PGE2. Since both antibodies against human CD44 and CD44v6 are commercially available, we tested both antibodies and found that the CD44v6 antibody is more specific than CD44 antibody in IHC staining and Flow cytometry analysis. Analyses of IHC and flow cytometry revealed that PGE2 increased the number of CD133− and CD44v6-positive epithelial cells found in cecal tumors (Figure 2C–D), although PGE2 treatment did not alter cecal tumor weight (Supplemental Figure S1B). Cecal tumor cells isolated from PGE2-treated mice formed more spheres than those from control mice (Figure 2E). We further evaluated the tumor-initiating ability of these cecal tumor cells. After limiting dilution transplantation, 3 of 6 NSG mice implanted with 2000 cecal tumor cells from PGE2-treated mice developed tumors, whereas none of 6 mice with 2000 cecal tumor cells from vehicle-treated mice formed measurable tumors (Table 1). In addition, tumor cells isolated from PGE2-treated mice exhibited higher growth rates than those from vehicle-treated mice (Supplemental Figure S1C). These results demonstrate that PGE2 treatment results in more tumor-initiating cells compared to vehicle treatment. Moreover, PGE2 treatment promoted metastatic lesions in the liver and increased liver tumor burden overall (Figure 2F). These results demonstrate that exogenous PGE2 treatment promotes CSC expansion and liver metastasis.
Table 1.
Tumorigenicity of cecal tumor cells from mice treated with PGE2 or vehicle
| No. of injected cells | Number of mice with tumors/total injected mice (% of mice with tumors)
|
||||
|---|---|---|---|---|---|
| 27 days | 35 days | 45 days | 56 days | ||
| Vehicle | 10,000 | 0/6 (0) | 3/6 (50) | 4/6 (67) | 4/6 (67) |
| 2,000 | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | |
| 500 | 0/6 (0) | 0/6 (0) | 0/6 (0) | 0/6 (0) | |
| PGE2 | 10,000 | 3/6 (50) | 5/6 (83) | 6/6 (100) | 6/6 (100) |
| 2,000 | 0/6 (0) | 2/6 (33) | 3/6 (50) | 3/6 (50) | |
| 500 | 0/6 (0) | 0/6 (0) | 1/6 (17) | 1/6 (17) | |
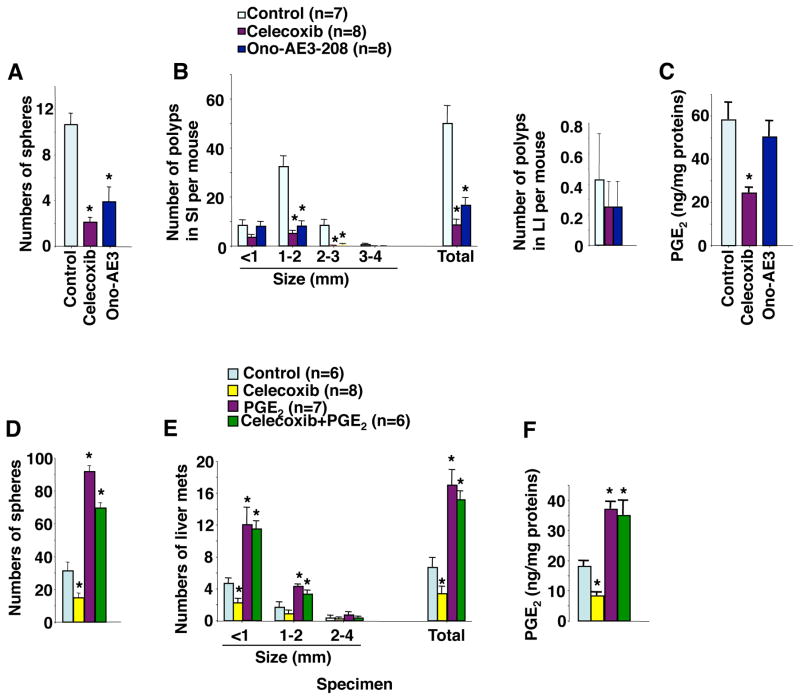
We further determined whether reduction of PGE2 levels by inhibiting PTGS2 (COX-2) activity decreased CSC numbers and inhibited liver metastasis. Treatment of ApcMin/+ mice with a PTGS2 (COX-2) selective inhibitor (celecoxib) resulted in reduction of tumor stem cell numbers (Figure 3A) and tumor burden (Figure 3B, left panel) with decreased PGE2 levels (Figure 3C) in small intestinal adenomas in small intestines. Celecoxib treatment resulted in a trend toward reduction of colonic tumor numbers but did not reach significance (Figure 3B, right panel). After intracecal injection of cells from human CRC specimens into NSG mice, the mice were then treated with vehicle, celecoxib, PGE2, or celecoxib plus PGE2. Celecoxib also reduced cecal CSC numbers (Figure 3D) and the number of liver metastatic tumors (Figure 3E) with decreased cecal PGE2 levels (Figure 3F). Most importantly, exogenous PGE2 treatment reversed the effect of celecoxib on reduction of CSC expansion and liver metastasis (Figure 3D–E). The effect of these agents on cecal tumors was not significant (Supplemental Figure S1D). These results demonstrate that reduction of PGE2 by inhibition of PTGS2 (COX-2) activity suppresses CSC expansion and liver metastasis. Collectively, our results demonstrate that endogenous PGE2 induces CSCs and promotes liver metastasis in vivo.
Figure 3. Reduction of PGE2 suppresses CSCs and inhibits liver metastasis.
(A) Formation of spheres from intestinal adenoma cells isolated from ApcMin/+ mice treated with vehicle, celecoxib, or Ono-AE3-208. (B) Average numbers of polyps at different size and total that includes all sizes in above treatment groups. (C) Intestinal PGE2 levels in above treatment groups. (D) Formation of spheres from cecal tumor cells isolated from NSG mice treated with vehicle, celecoxib, PGE2, or celecoxib plus PGE2 after primary carcinoma cells from human CRC specimen were injected into cecum. (E) Average numbers of liver metastatic tumors at different size and total that includes all sizes in mice as described in panel D. (F) Cecal PGE2 levels in mice as described in panel D. The error bar indicates ± SEM. *p<0.05.
PGE2 directly induces colonic CSCs
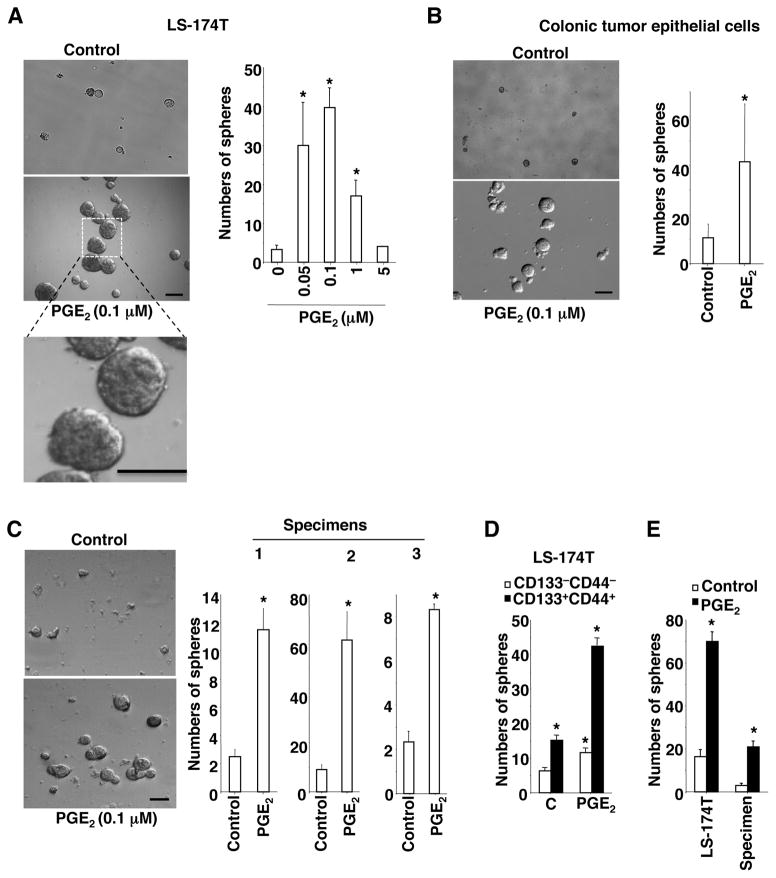
It was not clear whether PGE2 could directly induce CSCs or indirectly regulate CSCs via non-epithelial cells. To address this question, in vitro sphere-forming assays were performed. Treatment of LS-174T cells with PGE2 significantly increased the number of spheres and their size (an index of CSC expansion) (Figure 4A). Since 0.1 μM PGE2 was most effective at inducing sphere formation, this dose was chosen for subsequent studies described below. Similarly, PGE2 also induced sphere formation and expansion in colonic epithelial tumor cells isolated from ApcMin/+ mice and in primary carcinoma cells isolated from three individual CRC patient specimens (Figure 4B–C). To further determine potential mechanisms underlying PGE2 induction of sphere formation and expansion, LS-174T cells were sorted into CD133−CD44− and CD133+CD44+ cells. CD133+CD44+ cells formed more spheres than CD133−CD44− cells without PGE2 treatment (Fig. 4D). Moreover, PGE2 was able to induce sphere formation and expansion (data not shown) in both CD133−CD44− and CD133+CD44+ cells, suggesting that PGE2 may not only induce expansion or survival of existing CSCs but also promote conversion of non-CSC tumor cells to CSCs.
Figure 4. PGE2 directly induces CSCs in tumor epithelial cells.
(A–D) LS-174T cells (A), mouse colonic epithelial tumor cells (B), primary carcinoma cells isolated from three CRC patients (C), and both CD133−/−CD44−/− and CD133+/+CD44+/+ LS-174T cells (D) were treated with vehicle or PGE2 during in vitro sphere-forming assays. Left panels are representative phase contrast photos in panels A–C (scale bar, 50μm). Data in right panels represent the average of sphere numbers from three independent experiments. (E) The LS-174T cells and primary carcinoma cells from human CRC specimens were pretreated with vehicle or PGE2 in regular tissue culture plates for 48 h. After treatment, the cells were subject to in vitro sphere-forming assays without PGE2 treatment. The error bar for panels C indicates ± SD. The error bar for other panels indicates ± SEM. * p<0.05.
PGE2 has been shown to protect intestinal cells from anoikis29. To avoid the effect of PGE2 on anoikis in above in vitro sphere-forming assays, attached LS-174T cells and attached primary carcinoma cells from human CRC specimens were cultured in regular tissue culture plates and then treated with vehicle or PGE2. After treatment, these cells were subjected to in vitro sphere-forming assays without PGE2 treatment. Prior PGE2-treated cells formed more spheres than vehicle-treated cells (Figure 4E), demonstrating that PGE2 induces formation and expansion of cells with intrinsic self-renewal property without involvement of anoikis. Limiting dilution transplantation assays in xenograft models are considered as the gold standard to assess CSCs. After pre-treatment of attached LS-174T cells with PGE2 or vehicle, these cells were injected into the flanks of NSG mice. 8 of 8 mice implanted with as few as 50 PGE2-treated LS-174T cells developed into tumors, whereas 3 of 8 mice with vehicle-treated cells formed any measurable tumor (Supplemental Table 1). Collectively, these results demonstrate that PGE2 can directly induce colonic CSCs by targeting tumor epithelial cells without involvement of other types of cells.
PGE2 induces CSC formation and expansion by activating NF-κB via EP4-PI3K and EP4-MAPK pathways in vitro
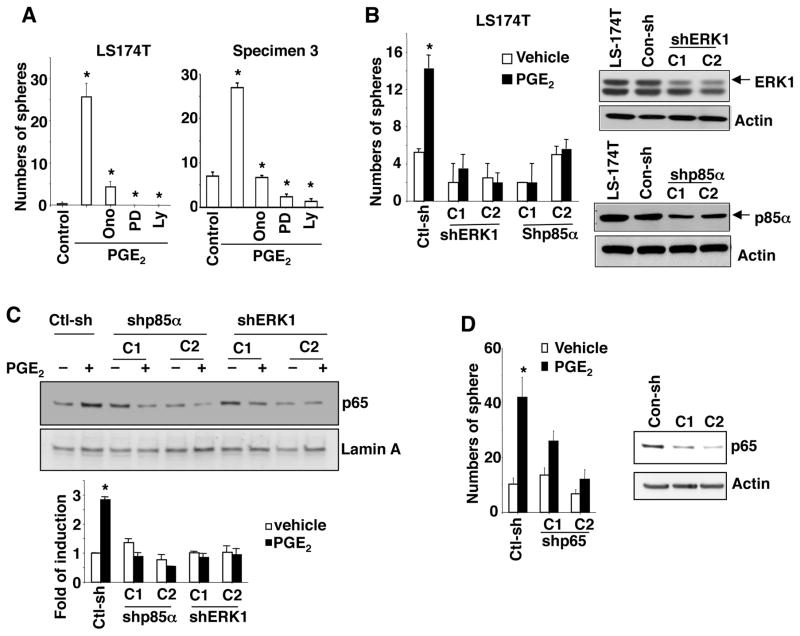
PGE2 exerts its cellular effects by binding to cell surface receptors that activate multiple signaling pathways, including PI3K-AKT and MAPK17, 30. Indeed, treatment of LS-174T or primary carcinoma cells from human CRC specimen with an EP4 antagonist (ONOAE-208), a MEK1 inhibitor (PD98059), or a PI3K inhibitor (Ly294002) reduced PGE2-induced CSCs (Figure 5A). In contrast, treatment with an EP1 antagonist (SC19220) or an EP1-3 antagonist (AH6809) did not block the effect of PGE2 on CSCs (Supplemental Fig. S2A). In addition, both CD133−CD44− and CD133+CD44+ LS-174T cells expressed similar levels of the EP4 receptor (Supplemental Figure S2B). Furthermore, knockdown of ERK1 and PI3K p85α also completely inhibited PGE2 induction of CSCs in LS-174T cells (Figure 5B). These results demonstrate that EP4-PI3K/MAPK signaling pathways are responsible for PGE2 induction of CSCs.
Figure 5. PGE2 induces CSCs by activating NF-κB via EP4-PI3K and EP4-MAPK-pathways.
(A) Formation of spheres in LS-174T cells (left panel) and human primary colon carcinoma cells (right panel) treated with vehicle, PGE2, ONO-AE-208 (Ono), PD98059 (PD), and/or LY294002 (Ly). (B) Formation of spheres in LS-174T/con-sh (non-silencing control shRNA), LS-174T/shERK1, and LS-174T/shp85α cells treated with PGE2 or vehicle (left panel). The protein levels of ERK1 and p85α in indicated cells were measured by Western blotting (right panels). (C) The nuclear NF-κB p65 levels in LS-174T/con-sh, LS-174T/shp85α, and LS-174T/shERK1 cells treated with PGE2 or vehicle for 24 hr. The band density was quantitated by densitometry scanning. The band density of p65 was normalized by the band density of Lamin A. The results were reported as a mean of fold-induction from three independent experiments (low panel). (D) Formation of spheres in LS-174T/con-sh and LS-174T/shp65 cells treated with PGE2 or vehicle (left panel). The protein levels of p65 in these cells were measured as described above (right panel).
Since PI3K and MAPK pathways have been shown to activate NF-κB in many other types of cancer cells31 and another recent study revealed that NF-κB activation promoted intestinal stem cell survival and transformation via effects on Wnt signaling32, we determined whether NF-κB is a downstream target of PI3K and/or MAPK pathways following PGE2 treatment. Indeed, PGE2 enhanced NF-κB p65 nuclear levels in LS-174T/ctl-sh (non-silencing control shRNA), but not in ERK1 and p85α knockdown LS-174T cells (Figure 5C), demonstrating that MAPK and PI3K pathways mediate the effect of PGE2 on NF-κB activation. Moreover, knockdown EP4, but not EP1 and EP2, completely inhibited PGE2-induced nuclear translocation of p65 (Supplemental Figure S2C), demonstrating that EP4 mediates the effect of PGE2 on activation of NF-κB. Moreover, knockdown of p65 attenuated PGE2 induction of CSCs in LS-174T cells (Figure 5D). These results demonstrate that NF-κB is a downstream target of MAPK and PI3K-Akt pathways and required for PGE2 induction of CSCs. Collectively, these results demonstrate that PGE2 induces CSCs by activating NF-κB via both EP4-MAPK and EP4-PI3K-Akt pathways in vitro.
EP4 and NF-κB are required for PGE2 induction of CSCs and liver metastasis
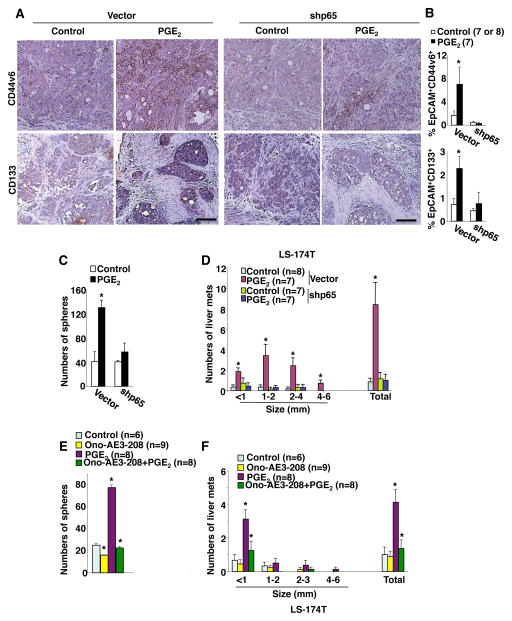
We further examined whether EP4 and NF-κB are required for PGE2 induction of CSCs and liver metastasis in vivo. Analyses of IHC and flow cytometry show that knockdown of NF-κB p65 reduces PGE2 induction of CD133− and CD44v6-positive epithelial cells in cecal tumors as compared to the control (Figure 6A–B). Moreover, p65 knockdown cecal tumor cells isolated from PGE2-treated mice formed similar sphere numbers as p65 knockdown cecal tumor cells isolated from vehicle-treated mice (Figure 6C), demonstrating that NF-κB is required for PGE2 induction of CSCs. Knockdown of p65 also completely inhibited PGE2 promotion of liver metastasis (Figure 6D and Supplemental Figure S3A), but did not affect cecal tumor weight (Supplemental Figure S3B), suggesting that cecal CSCs contribute to liver metastasis. These results demonstrate that NF-κB is required for PGE2 induction of CSCs and liver metastasis in vivo. Moreover, the EP4 antagonist (Ono-AE3-208) attenuated the effect of PGE2 on induction of cecal CSC expansion (Figure 6E) and liver metastasis (Fig. 6F), demonstrating that PGE2 induces CSCs and promotes liver metastasis via EP4. Taken together, our results demonstrate that PGE2 induces CSCs and promotes liver metastasis by activating NF-κB via EP4-MAPK and EP4-PI3K-Akt pathways.
Fig. 6. EP4 and NF-κB are required for PGE2 induction of CSCs and liver metastasis.
(A–B) LS-174T/con-sh or LS-174T/shp65 cells were injected into the cecal walls of NSG mice. After injection, these mice were treated with vehicle or PGE2. (A) The representative immunostaining of CD44v6 and CD133 (brown) in cecal tumors taken from mice treated with PGE2 or vehicle in vector group (left panel) and shp65 group (right panel), scale bar, 50 μm. (B) Data represents the percentage of EpCAM+CD44v6+ (top panel) and EpCAM+CD133+ (bottom panel) cells in total cells of cecal tumor in indicated group of mice treated with PGE2 or vehicle. (C) Formation of spheres from cecal tumor cells isolated from mice treated with PGE2 or vehicle in above groups. (D) Average numbers of liver metastatic tumors in above groups. (E) Formation of spheres from cecal tumor cells isolated from mice treated with vehicle, Ono-AE3-208, PGE2, or Ono-AE3-208 plus PGE2 after LS-174T cells were injected into mouse cecum. (F) Average numbers of liver metastatic tumors at different size and total that includes all sizes in groups as described in panel E. The error bar indicates ± SEM. *p<0.05.
DISCUSSION
Despite recent advances in treatment and effective screening methods, today nearly 50% of patients who present with advanced CRC experience tumor recurrence die within 5 years following treatment. One explanation could be the presence of chemotherapy-resistant CSCs33 that are not being properly targeted. Emerging evidence supports the hypothesis that inflammatory mediators regulate CSC formation and expansion. In vitro studies showed that IL-1β and IL-8, pro-inflammatory cytokines, increased colonosphere and mammosphere formation, respectively34, 35 however, these studies did not demonstrate whether the cells from these spheres possessed properties of CSCs. A recent in vivo study showed that NF-κB p65 is required for crypt stem cell expansion32. It seems likely that other pro-inflammatory mediators could also contribute to CSC regulation. Considering the importance of the PTGS2-derived PGE2 in inflammation and cancer, we postulated that PGE2 could regulate CSCs. Indeed, our in vivo results show that exogenous PGE2 increases mouse oncogenic colonic stem cells, whereas celecoxib decreases these cells with reduction of PGE2 levels. Moreover, our in vivo results also reveal that exogenous PGE2 induces human colonic CSCs and promotes liver metastasis, whereas reduction of endogenous PGE2 levels by inhibiting PTGS2 activity reduces CSC expansion and suppresses liver metastasis, suggesting that PGE2 induces liver metastasis via induction of CSCs. It is well established that both tumor cells and tumor-associated stromal cells, in particular macrophages, highly express PTGS2 and produce high levels of PGE2. A recent report showed that mesenchymal stem cells (MSCs) that were co-cultured with cancer cells also expressed high PTGS2 and produced PGE2. These MSCs have been reported to contribute to a cancer stem cell niche via their production of PGE236. Here we provide direct evidence demonstrating that PGE2 induces CSC expansion by directly targeting on colorectal adenoma and carcinoma cells. Collectively, our findings demonstrate that PGE2 induces CSC expansion and promotes liver metastasis. Our results also suggest that PGE2 promotes CRC initiation, growth, and metastasis via CSCs.
Multiple lines of evidence show that EP1, EP2, and EP4 contribute to intestinal tumorigenesis37. In contrast, EP3 has been shown to inhibit intestinal tumorigenesis37, 38. Previous work showed that treatment of C3L5 breast cancer cells with an EP4 antagonist resulted in reduction of sphere formation in vitro and decreased levels of CSC markers in C3L5 tumor-bearing mice39, indicating that EP4 may mediate PGE2 induction of CSCs in breast cancer. Our in vitro and in vivo results provide direct evidence demonstrating that EP4 mediates the effect of PGE2 on induction of colonic CSC expansion and promotion of liver metastasis. Activation of PI3K-AKT signaling has been shown to enhance intestinal stem cell formation and expansion by enhancing β-catenin nuclear localization and transcriptional activation in mice40. Moreover, a Ras-MAPK pathway is required for maintenance and proliferation of intestinal stem cells in Drosophila41, 42. It is well established that PGE2 activates PI3K and MAPK pathways via EP4 and both pathways can induce NF-κB activation. Here we demonstrate that PGE2 induces CSCs by activating NF-κB via PI3K and MAPK pathways. Although both PI3K and MAPK pathways downstream of EP4 are independent, our results showed that both pathways are required for PGE2 activation of NF-κB. We further demonstrate that EP4, but not EP1 and EP2, mediates the effect of PGE2 on induction of NF-κB activation. More importantly, our in vivo results demonstrate that NF-κB is required for PGE2 induction of CSCs and liver metastasis. These results indicate that an inflammatory pathway, PGE2-NF-κB, mediates the effects of chronic inflammation on CSC expansion. Although PGE2 has been shown to play a role in the regulation of embryonic and adult stem cell homeostasis43–45 as well as in mouse colonic stem cells46, we extended the scope of our research to reveal that PGE2 can also induce CSC expansion.
In conclusion, our findings not only uncover a critical role of PGE2 in regulation of CSCs, but also reveal a novel mechanism for the contribution of chronic inflammation to CSC expansion. Moreover, our in vitro and in vivo results are likely to be clinical relevant because the concentration of PGE2 is positively correlated with the expression of CSS markers in human CRC specimens. Finally, our findings may provide a rationale for development of EP4 antagonists as new therapeutic agents in treatment of CRC patients via eliminating CSCs.
Supplementary Material
Acknowledgments
Grant support: NIH R01 DK047297, NCI R01 CA184820, and NCI P01 CA077839
We thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous support (R.N.D.).
Abbreviations
- CSC
cancer stem cells
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PGE2
Prostaglandin E2
- PTGS (COX)
prostaglandin-endoperoxide synthases (cyclooxygenase)
Footnotes
Disclosures: All authors have nothing to disclose
Conflict of interest statement: All authors have no any conflict interests
Author Contributions: Study concept and design: DW and RND, Acquisition of data: LF, LG, and HS, Analysis and interpretation of data: DW and LF, Writing of manuscript: DW
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1
- Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 2.Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14:8221–7. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot DJ, de Vries EG, Groen HJ, et al. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol. 2007;61:52–69. doi: 10.1016/j.critrevonc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–8. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 6.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–23. [PubMed] [Google Scholar]
- 7.Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.McLemore TL, Hubbard WC, Litterst CL, et al. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988;48:3140–7. [PubMed] [Google Scholar]
- 9.Hambek M, Baghi M, Wagenblast J, et al. Inverse correlation between serum PGE2 and T classification in head and neck cancer. Head Neck. 2007;29:244–8. doi: 10.1002/hed.20503. [DOI] [PubMed] [Google Scholar]
- 10.Cai Q, Gao YT, Chow WH, et al. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol. 2006;24:5010–6. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 11.Shrubsole MJ, Cai Q, Wen W, et al. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prev Res (Phila) 2012;5:336–42. doi: 10.1158/1940-6207.CAPR-11-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JC, Schmidt CR, Shrubsole MJ, et al. Urine PGE-M: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006;4:1358–65. doi: 10.1016/j.cgh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Dong LM, Shu XO, Gao YT, et al. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women’s health study. Cancer Epidemiol Biomarkers Prev. 2009;18:3075–8. doi: 10.1158/1055-9965.EPI-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Taylor JA, Milne G, et al. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013 doi: 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris PG, Zhou XK, Milne GL, et al. Increased Levels of Urinary PGE-M, a Biomarker of Inflammation, Occur in Association with Obesity, Aging, and Lung Metastases in Patients with Breast Cancer. Cancer Prev Res (Phila) 2013;6:428–36. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kekatpure VD, Boyle JO, Zhou XK, et al. Elevated levels of urinary prostaglandin e metabolite indicate a poor prognosis in ever smoker head and neck squamous cell carcinoma patients. Cancer Prev Res (Phila) 2009;2:957–65. doi: 10.1158/1940-6207.CAPR-09-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 19.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 20.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanno T, Matsui W. Development and maintenance of cancer stem cells under chronic inflammation. J Nihon Med Sch. 2011;78:138–45. doi: 10.1272/jnms.78.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C, Liu J, Tang Y, et al. Inflammation linking EMT and cancer stem cells. Oral Oncol. 2012 doi: 10.1016/j.oraloncology.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Qiu W, Wang X, Leibowitz B, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20027–32. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon CM, Kwon JH, Kim JS, et al. Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int J Cancer. 2014;134:519–29. doi: 10.1002/ijc.28381. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Wang H, Shi Q, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–95. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 27.Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–56. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Patel BB, Yu Y, Du J, et al. Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochem Biophys Res Commun. 2009;378:344–7. doi: 10.1016/j.bbrc.2008.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph RR, Yazer E, Hanakawa Y, et al. Prostaglandins and activation of AC/cAMP prevents anoikis in IEC-18. Apoptosis. 2005;10:1221–33. doi: 10.1007/s10495-005-2049-y. [DOI] [PubMed] [Google Scholar]
- 30.Castellone MD, Teramoto H, Williams BO, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi MM, Sung B, Yadav VR, et al. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–30. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwitalla S, Fingerle AA, Cammareri P, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Wang L, Pappan L, et al. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li HJ, Reinhardt F, Herschman HR, et al. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2:840–55. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoji Y, Takahashi M, Kitamura T, et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut. 2004;53:1151–8. doi: 10.1136/gut.2003.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumder M, Xin X, Liu L, et al. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014;105:1142–51. doi: 10.1111/cas.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragab A, Buechling T, Gesellchen V, et al. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30:1123–36. doi: 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang H, Grenley MO, Bravo MJ, et al. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou JY, Ellent DP, Lee S, et al. Cyclooxygenase-2-derived prostaglandin e2 protects mouse embryonic stem cells from apoptosis. Stem Cells. 2007;25:1096–103. doi: 10.1634/stemcells.2006-0505. [DOI] [PubMed] [Google Scholar]
- 44.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan YY, Davidson LA, Callaway ES, et al. Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis. 2014;35:606–12. doi: 10.1093/carcin/bgt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.