Abstract
The hippocampus of patients with schizophrenia displays aberrant excess neuronal activity which affects cognitive function. Animal models of the illness have recapitulated the overactivity in the hippocampus, with a corresponding regionally localized reduction of inhibitory interneurons, consistent with that observed in patients. To better understand whether cognitive function is similarly affected in these models of hippocampal overactivity, we tested a ketamine mouse model of schizophrenia for cognitive performance in hippocampal- and medial prefrontal cortex (mPFC)-dependent tasks. We found that adult mice exposed to ketamine during adolescence were impaired on a trace fear conditioning protocol that relies on the integrity of the hippocampus. Conversely, the performance of the mice was normal on a delayed response task that is sensitive to mPFC damage. We confirmed that ketamine-exposed mice had reduced parvalbumin-positive interneurons in the hippocampus, specifically in the CA1, but not in the mPFC in keeping with the behavioral findings. These results strengthened the utility of the ketamine model for preclinical investigations of hippocampal overactivity in schizophrenia.
Keywords: delayed response task, fear conditioning, hippocampus, overactivity, medial prefrontal cortex
1. Introduction
Neurocognitive deficits are a key clinical feature of schizophrenia that predate the onset of psychosis and predict long-term disability in patients (Corigliano et al., 2014; Eastvold et al., 2007; Green, 1996; Green et al., 2004; Jahshan et al., 2010; Nuechterlein et al., 2014). Existing antipsychotics and nootropics however have limited efficacy in improving cognition and functional outcomes in schizophrenia. Preclinical animal models developed to recapitulate many of the features of the illness have been used to investigate neurocognitive deficits and their treatments (Dudchenko et al., 2013; Lipska and Weinberger, 2000; Moore et al., 2013; Young et al., 2012), and these models commonly pointed to deficits in hippocampal- and prefrontal-mediated functional domains, consistent with their known importance in learning and memory.
Recent evidence from animal models and human neuroimaging studies has indicated altered brain excitability in the medial temporal lobe as a potential driver of pathology in schizophrenia. Specifically, the evidence suggests that hippocampal dysfunction in schizophrenia patients, and also observed in well-established animal models of the schizophrenia, may be due to aberrant hippocampal overexcitability (e.g., Lodge & Grace, 2007; Medoff et al., 2001; Sanderson et al., 2012; Schobel et al., 2009; 2013; Tregellas et al., 2014; Zierhut et al., 2010). In one such study, hippocampal overactivity was observed early during a prodromal stage and predicted clinical progression to overt psychosis within 2 years (Schobel et al., 2009). The level of overactivity has also been found to correlate with worse cognitive performance in schizophrenia patients (Tregellas et al., 2014). Consistent with those findings, aberrant excitability in the hippocampus has been observed in neurodevelopmental animal models of schizophrenia that used the NMDA receptor antagonist ketamine (Schobel et al., 2013) or the antimitotic compound methylazoxymethanol acetate (e.g., Lodge & Grace, 2007). As adults, animals exposed to those agents prenatally or during adolescence have significantly higher hippocampal metabolic basal activity (Schobel et al., 2013) and in vivo hippocampal firing rates (Gill et al., 2011; Lodge & Grace, 2007) compared to vehicle-treated controls. In vitro slice recordings from the treated animals have also provided evidence for neuronal hyperexcitability of principal neurons in the hippocampus that could be partially normalized with diazepam (Sanderson et al., 2012). Thus, both preclinical and clinical data suggest that overactivity is a condition contributing to hippocampal dysfunction in this illness.
One important correlate of underlying circuit perturbation that is relevant to the pathogenesis of overactive hippocampus is the loss of GABAergic inhibitory interneuron populations. Individuals with schizophrenia have normal total number of neurons in the hippocampus (Heckers et al., 1991; Konradi et al., 2011; Schmitt et al., 2009; Walker et al., 2002), but significantly reduced parvalbumin- and somatostatin-expressing interneurons both in patients (Konradi et al., 2011; Torrey et al., 2005; Zhang and Reynolds, 2002) and in animal models (e.g., Gilani et al., 2014, Gill and Grace, 2014; Lodge et al., 2009; Schobel et al., 2013). The loss of inhibitory network integrity in schizophrenia suggests that boosting inhibitory control in the hippocampus may be beneficial. Indeed, treatments with a GABAA α5 positive allosteric modulator in an animal model were shown to be effective in reducing evoked excitatory response in the hippocampus and normalizing dopamine dysfunction (Gill et al., 2011). An alternate strategy involving tightly regulating glutamate transmission was shown to be effective too, at least in reducing hypermetabolism and ameliorating loss of parvalbumin in the mouse hippocampus (Schobel et al., 2013). Whether these treatments, currently still in preclinical testing, are beneficial for enhancing cognition has not been demonstrated yet.
Towards that goal, we set out to determine if the preclinical models used to recapitulate hippocampal hyperactivity in schizophrenia produce cognitive dysfunction. Specifically, we examined a ketamine mouse model that displays hippocampal hyperactivity akin to that seen in schizophrenia patients (Schobel et al., 2013) for functional impairment in hippocampal- and prefrontal-mediated memory tasks. While the ketamine model is well established and produces schizophrenia-like symptoms including impaired cognition in other exposure regimens (e.g., Olney et al., 1999; Neill et al., 2010), it is not known whether the specific induction protocol of ketamine exposure that produced hippocampal overactivity leads to cognitive dysfunction. Differences in induction regimens in this area have been noted to impede consistency in findings and translational integrity (Gilmour et al., 2012). We first established that mice exposed to this ketamine regimen during a month in adolescence show hyper-responsiveness as adults to a dopamine agonist, a commonly used behavioral assay to validate dopaminergic perturbation that is central to the disease. We then showed that these mice displayed impaired hippocampal-dependent trace fear memory but intact medial prefrontal cortex (mPFC)-mediated working memory. The expression of inhibitory interneurons in those brain areas was in line with the behavioral findings; that is, parvalbumin-positive interneurons were decreased in the hippocampus but not in the mPFC.
2. Materials and methods
2.1 Subjects
Male C57/BL6 mice were obtained at 4 weeks old from The Jackson Laboratory (Bar Harbor, Maine). The mice were housed in cohorts of 4 per cage at 25°C and maintained on a 12-hr light/dark cycle. Once ketamine exposure was initiated, the mice were housed individually. All cages were lined with corncob bedding and a nestlet for nest building. Food (Purina autoclave laboratory rodent diet) and water were provided ad libitum. All procedures in the current investigations were approved by the Institutional Animal Care and Committee in accordance with the National Institutes of Health directive.
2.2 Ketamine exposure
Ketamine (VedCo; 100 mg/ml concentration) was diluted to 1.6 mg/ml, and injected at a volume of 10 ml/kg of body weight (Schobel et al., 2013). Mice were injected subcutaneously three times a week (Monday, Wednesday, and Friday) with saline or ketamine (16 mg/kg) for a month starting at 1-mo of age. Following the month treatment, the mice were left undisturbed until adulthood at 4–6 months old for behavioral testing.
2.3 Amphetamine-induced locomotor activity
Each mouse was placed in an open field chamber (42 cm × 42 cm × 30.5 cm) in which locomotion was tracked with the VersaMax animal activity monitoring system (AccuScan Instruments, Columbus, OH). After 30 min of baseline activity, the mouse was taken out of the chamber and injected intraperitoneally with a small dose of amphetamine (0.5 mg/kg in a volume of 10 ml/kg; Sigma). The mouse was then returned to the chamber for another 60 min of activity monitoring. Total distance travelled and movement time were the dependent measures.
2.4 Trace fear conditioning
The trace fear conditioning apparatus and procedures were identical to those described in Smith et al. (2007). Briefly, each conditioning chamber was either square (17.78 cm wide × 17.78 cm deep × 30.48 high; model #H10-35M-04; Coulbourn Instruments, Whitehall, PA) or octagonal (radius 21.59 cm and 30.48 high; model #H10-35M-08; Coulbourn Instruments), with a grid floor through which a footshock could be delivered. Half of the square and half of octagonal chambers were scented with two drops of vanilla extract in the drop pan. During testing, the grid floor was replaced with a solid wooden floor coated with a white colored sealant. A speaker was mounted on the back wall for the delivery of a tone. The tone and shock were created via a peripheral Coulbourn tone generator (model #A69-20) and animal shocker (model #H13-16). A computer that interfaced with Coulbourn Graphic State software controlled all stimuli onset and duration.
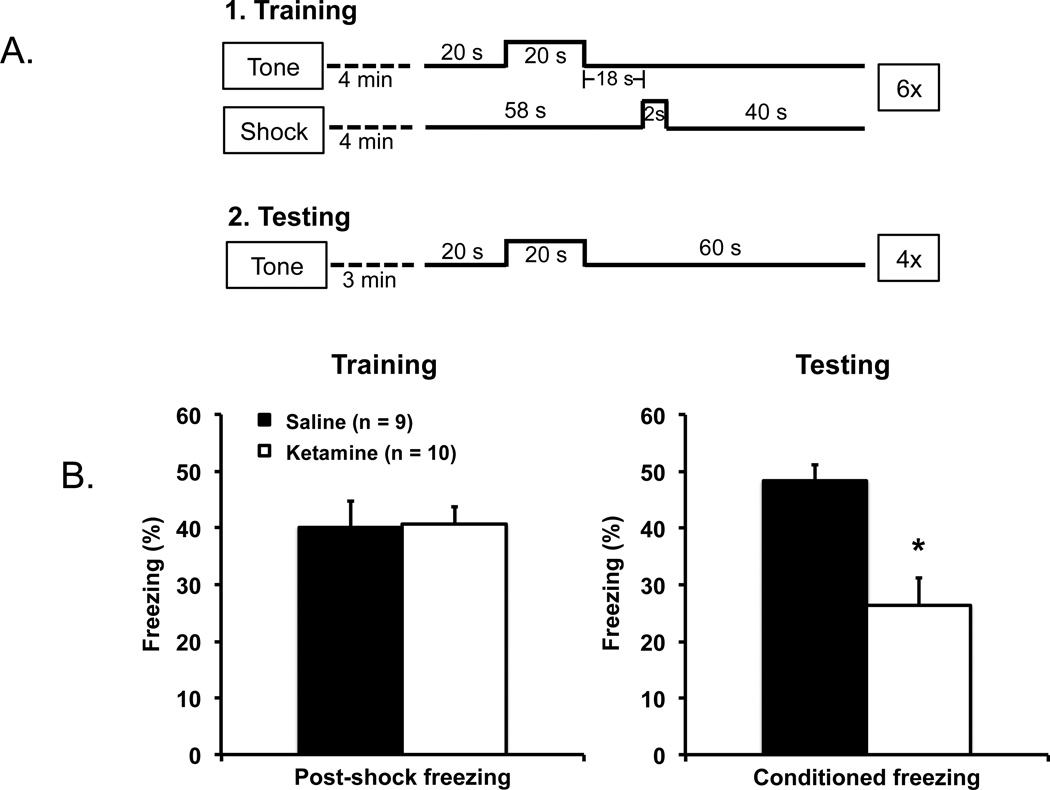
On the first day (acclimatization day), mice were pre-exposed to the training chambers for 12 min; no tone or shock was delivered. On the second day (training day), the mice were placed in the same chambers as on the first day for 4 min to acclimatize. The mice were then given six conditioning trials involving 70-dB tone (conditioned stimulus) and a 2-s, 0.5-mA shock (unconditioned stimulus). Each trial consisted of a 20-s baseline interval, a 20-s tone presentation, an 18-s trace interval, a 2-s shock, and a 40-s post-shock interval. On the third day (testing day), the mice were placed in a novel test chamber for 3 min (i.e., mouse trained in a square chamber was tested in a octagonal chamber, and vice versa), and then given four 100-s testing trials. Each trial consisted of 20-s interval baseline interval, a 20-s tone (70 dB) presentation, and a 60-s post-tone interval (see Figure 2A for schematic representations of the training and testing trials). Scoring was done from a video at 1-s interval increments for freezing during the 40-s post-shock interval on training day and the 60-s post-tone interval on testing day.
Figure 2.
(A) Schematic representations of the training and testing procedures used to assess hippocampal-dependent memory using trace fear conditioning. (B) The ketamine and saline mice displayed the same level of freezing responses during training (post-shock; left panel), but the ketamine-exposed mice showed significantly less freezing 24 hr after training to the conditioned tone than the saline control mice (right panel) suggesting deficit in hippocampal-dependent memory function in the ketamine mice. Error bars indicate mean ± SEM.
2.5 Delayed response task
Mice were tested on a delayed response task in modified operant chambers. The chambers were made of aluminum front and back walls, clear polycarbonate sides and ceiling, and stainless-steel rods floor (Med Associates, St Albans, VT). Each chamber had a programmable food cup for the delivery of liquid reward, and was connected to a vacuum for the removal of liquid. Infrared photocells around the food cup monitored the time spent and number of entries into the cup. Each chamber had two retractable mouse levers to the left and right sides of the food cup. A house light provided ambient light inside the sound-attenuating shell that housed the chambers.
Prior to training, mice were food deprived and pre-exposed to a 10% sucrose solution in their home cages, which served as a reward in the task. The food-deprived mice were water restricted subsequently during training (1 hr water access each day in the home cage) to increase motivation and were maintained at approximately 85% free-feeding body weight throughout the study. At the start of the training phase, the mice were habituated to the chambers and were given food cup magazine training. Each mouse was given 60 trials of sucrose presentation at a pseudo-random interval each day for two days. The sucrose (50 ul of 20% w/v) was presented in the food cup for 5 s before removal by vacuum. Next, the mice were shaped to lever press for sucrose delivery under a fixed ratio schedule (FR-1) for 12 sessions over six days; only one lever was available during each of the two 20-min daily sessions.
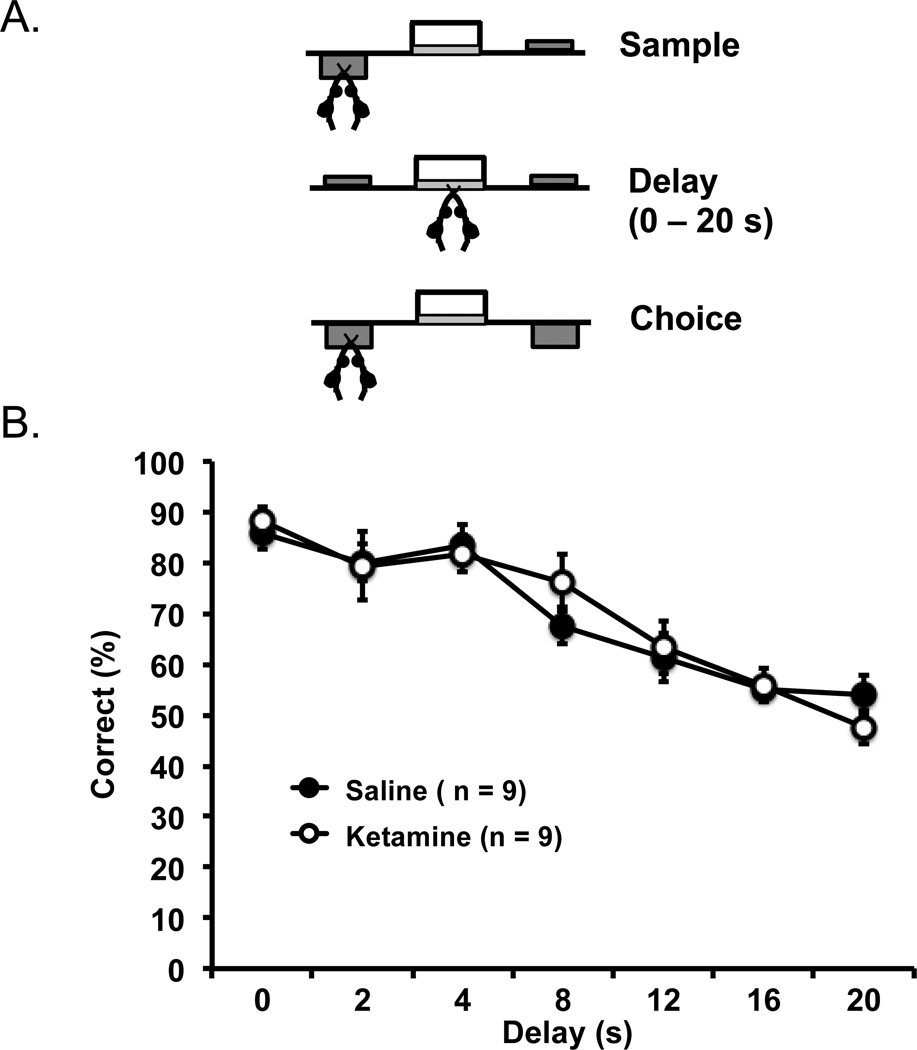
The mice received training of delayed response task procedures that involved sampling, delay, and choice segments (see Figure 3 top panel for schematic representations). On each trial, one pseudo-randomly selected lever was presented for the mice to sample; once pressed, the lever retracted and the mice were required to nosepoke the food magazine. This action initiated an experimentally determined delay interval, after which time both levers were extended for the choice phase. A correct response to the sample lever was rewarded with the delivery of 40–50 µls of sucrose available for 5 s, and a 5-s intertrial interval began (house lights went off for the first 2 s and then turned back on) before the next trial. An incorrect choice resulted in no reward, and the 5-s intertrial interval was activated before the next trial. Each training session was conducted once a day and lasted 40 min. The mice were first trained with no delay between the sample and choice phases. Once the mice achieved a criterion of 80% correct trials on two consecutive days with a minimum of 20 trials, they progressed to a short set of delays (0, 1, 2, 3, 4, 5, and 6 s). Performance of 75% correct trials on two consecutive days further progressed the mice to an intermediate set of delays (1, 2, 4, 8, 10 and 12 s), and then the final test delays (0, 2, 4, 8, 12, 16, and 20 s). Performance over two days of the final test delays was analyzed; each delay was tested with an average of 16 trials.
Figure 3.
(A) Schematic representations of a mPFC-mediated delayed response task in which mice had to press a sample lever (with one lever presented), then undergo varying delay intervals of 0–20 s in the absence of any lever, and finally had to choose between two levers (the previous “sample lever” and an additional lever). A choice of the lever presented prior to the delays resulted in deliverance of a reward. (B) Ketamine- and saline-exposed mice showed progressively decreased delay-dependent accuracy, but were not different from each other in all delay intervals. Error bars indicate mean ± SEM.
2.6 Perfusion and tissue preparation
At the end of behavioral testing, a subset of mice was perfused for parvalbumin immunohistochemistry. The mice were deeply anesthetized with isoflurane and perfused transcardially with sterile phosphate-buffered saline (PBS). The brains were extracted and post-fixed in 4% paraformaldehyde at 4°C for 48 hr, and transferred to 16% sucrose in 4% paraformaldehyde at 4°C for 24 hr. The brains were then frozen with powdered dry ice and stored at −80°C. Just prior to immunohistological processing, the brains were sectioned with a microtome in the coronal plane at 40 µm thickness.
2.7 Immunohistochemistry
Brain sections containing the hippocampus and the mPFC were immunostained for parvalbumin (Swant, Switzerland; catalog number PV 235). Free-floating sections were washed in 0.1 M PBS, and then endogenous peroxidases were quenched in 0.3% hydrogen peroxide in PBS. After additional PBS washes, sections were blocked in 5% normal goat serum in PBS with 0.3% Triton. Sections were then incubated with primary antibody at a dilution of 1:5000 in PBS containing 0.15% Triton and 3% normal goat serum for 48 hr at 4°C with agitation. Following primary antibody incubation, sections were washed in PBS and reacted with biotinylated secondary antibody goat anti-mouse IgG (Vector Laboratories, Burlingame, CA) diluted in PBS with 0.15% Triton and 5% normal goat serum for 45 minutes. The secondary antibody was detected with avidin-biotin complex (ABC Elite; Vector Laboratories) and the avidin-biotin complex was visualized with nickel-enhanced diaminobenzadine (Vector Laboratories). Tissue sections were mounted onto gelatin-coated slides and dried, dehydrated with increasing ethanol concentrations, cleared in xylene, and coverslipped using Permount mounting medium.
Parvalbumin-positive neurons were quantified using a Zeiss Axioplan 2 microscope. All analyses were conducted blind with regards to treatment groups. The lack of immunostaining in one of the brains in the ketamine-exposed mice rendered that mouse brain unsuitable for analysis, and brain sections containing the mPFC of a saline control mouse were not analyzed due to inadequate immunostaining. For the rest of the mice, counts were performed throughout the rostrocaudal extent of the hippocampus (bregma −1.06 to −3.88 mm from a minimum of six histological sections) and mPFC (bregma 1.34 to 2.80 mm from a minimum of three sections); every identifiable parvalbumin-positive interneuron was counted in the sections processed. Region of interest (CA1, CA3, dentate gyrus, and mPFC consisted of prelimbic and infralimbic cortices) were outlined according to mouse brain atlas (Franklin & Paxinos, 1997).
3. Results
3.1 Amphetamine challenge
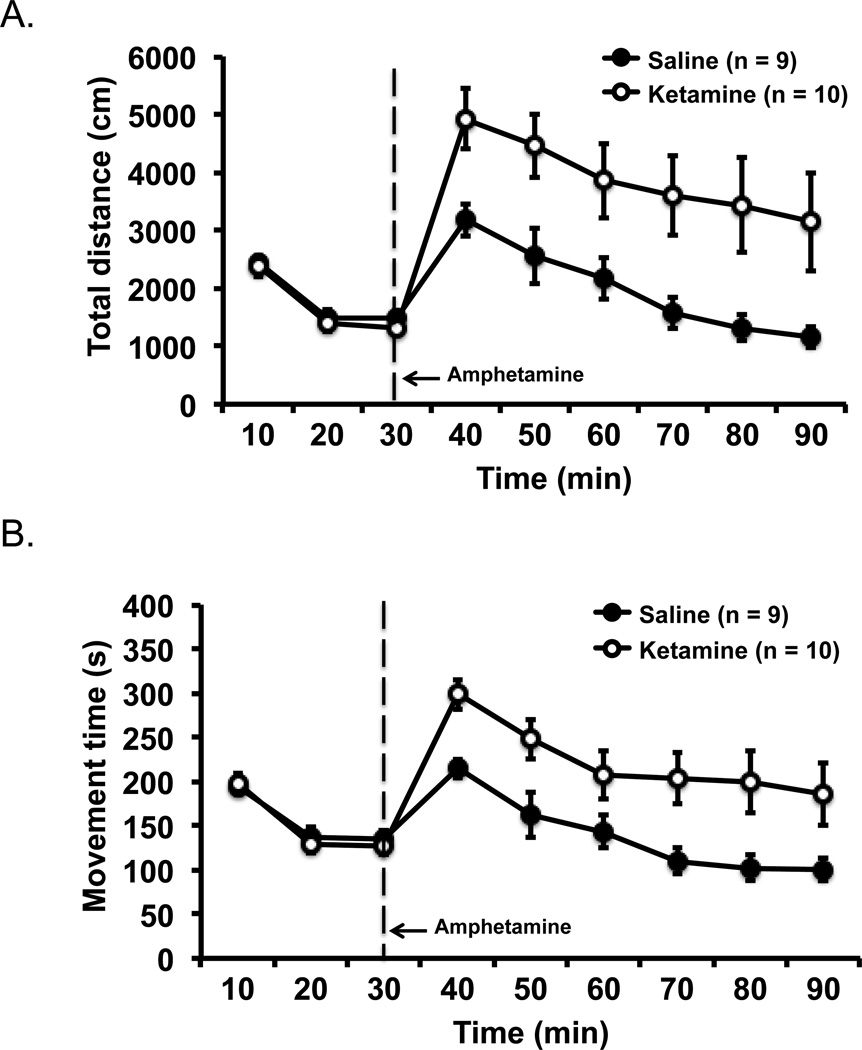
Mice with a history of ketamine (n = 10) or saline (n = 9) exposure during adolescence were challenged with a small dose of amphetamine during adulthood to assess whether the model recapitulated the dysregulation of dopaminergic function that is thought to be a fundamental pathology of schizophrenia. Assessment of baseline activity prior to amphetamine injection showed that ketamine- and saline-exposed mice had identical levels of locomotor activity as measured by distanced travelled [F(2, 34) = 0.33, p = .719 for group by time interaction, and F(1, 17) = 0.48, p = .500 for main effect of group] that decreased over the 30 min period as the mice habituated to the environment [F(2, 34) = 107.51, p = .001 for main effect of time; Figure 1 top pane]. Movement time between the two groups at baseline was also comparable (Figure 1 bottom panel); F(2, 34) = 0.92, p = .408 for interaction, F(1, 17) = 0.09, p = .764 for group effect, and F(2, 34) = 87.69, p = .001 for time effect.
Figure 1.
Hyper-responsiveness to amphetamine challenge in ketamine-exposed mice. Saline- and ketamine-exposed mice showed the same level of activity in the open field at baseline (first 30 min), but the ketamine-exposed mice showed a greater increase in activity after amphetamine injection in total distance travelled (A) and movement time (B) than the saline control mice. Error bars indicate mean ± SEM.
Amphetamine injection noticeably increased locomotor activity in ketamine- and saline-exposed mice from the baseline level, with the ketamine-exposed mice showing a significantly greater increase than the saline control mice (Figure 1). Repeated measures ANOVA of distance travelled post-amphetamine injection showed a significant effect of group, F(1, 17) = 8.11, p = .011, and a significant effect of time as the influence of amphetamine subsided over time in both groups at roughly similar rates, F(5, 85) = 10.44, p = .001; no interaction was thus detected between group and time, F(5, 85) = 0.14, p = .983. The same analysis on movement time as the dependent measure supported the same conclusion. Amphetamine induced higher movement time in ketamine-exposed mice than saline control mice, F(1, 17) = 9.00, p = .008. The amphetamine effect on locomotor activity reduced over time in both groups in a similar fashion, F(5, 85) = 16.73, p = .001 for time effect, and F(5, 85) = 0.31, p = .905 for group by time interaction.
3.2 Hippocampal-dependent trace fear memory
The mice were trained and tested with trace fear conditioning to assess hippocampal function (McEchron et al., 1998; Quinn et al., 2008). Ketamine- (n = 10) and saline-exposed (n = 9) mice were given six tone-foot shock pairings with a 18-s trace interval between them (Chowdhury et al., 2005; Misane et al., 2005; Smith et al., 2007). Freezing after foot shock delivery during training was the same between the two groups, t(17) = 0.09, p = .930 (Figure 2B, left panel), indicating that the mice in both groups were able to display freezing behavior. A day after conditioning, the mice were tested with the tone in the absence of foot shock (Figure 2B, right panel). The ketamine-exposed mice froze significantly less than the saline control mice, t(17) = 3.76, p = .002, a finding that points to impaired hippocampal-dependent memory function.
3.3 mPFC-mediated working memory
Mice were trained in an operant delayed response task that is sensitive to impairment of mPFC and working memory (Sloan et al., 2006). Figure 3B shows the accuracy (percent correct) of the performance of the mice as a function of increasing delay between the sample and choice trials. No differences in performance were detected at any delay between the ketamine- and saline-exposed mice (n = 9 per group), F(6, 96) = 0.72, p = .632 for interaction, and F(1, 16) = 0.04, p = .836 for main effect of group. A significant main effect of delay indicated that the mice were less accurate at longer delays than shorter delays, F(6, 96) = 27.84, p = .001. Taken together, these data suggest that unlike hippocampal-dependent memory function, mPFC-mediated working memory function was intact in mice subjected to this ketamine exposure regimen during adolescence.
3.4 Immunohistochemical assessment
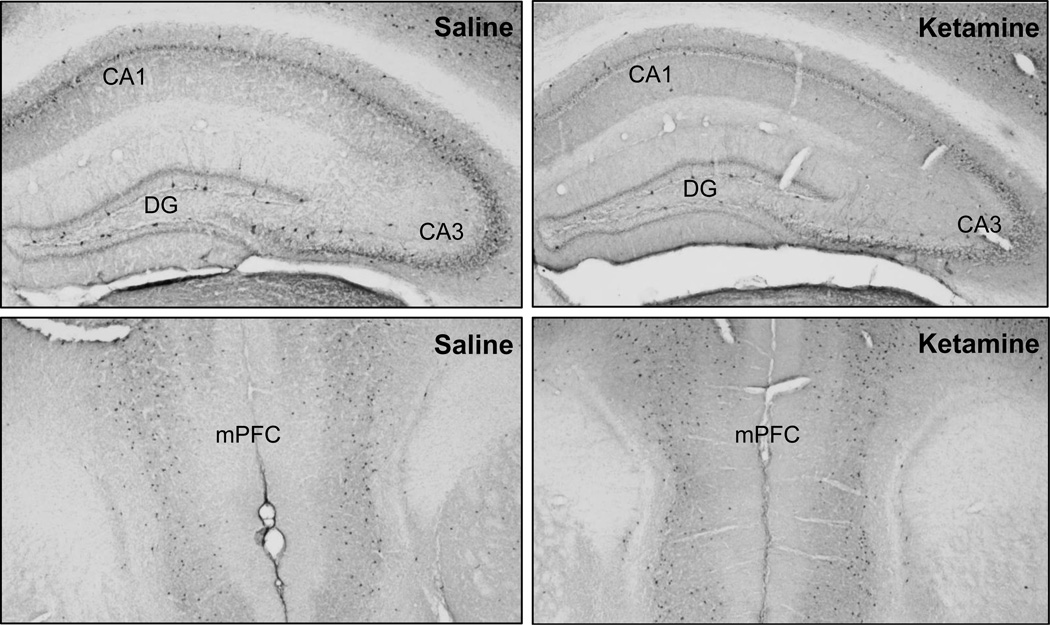
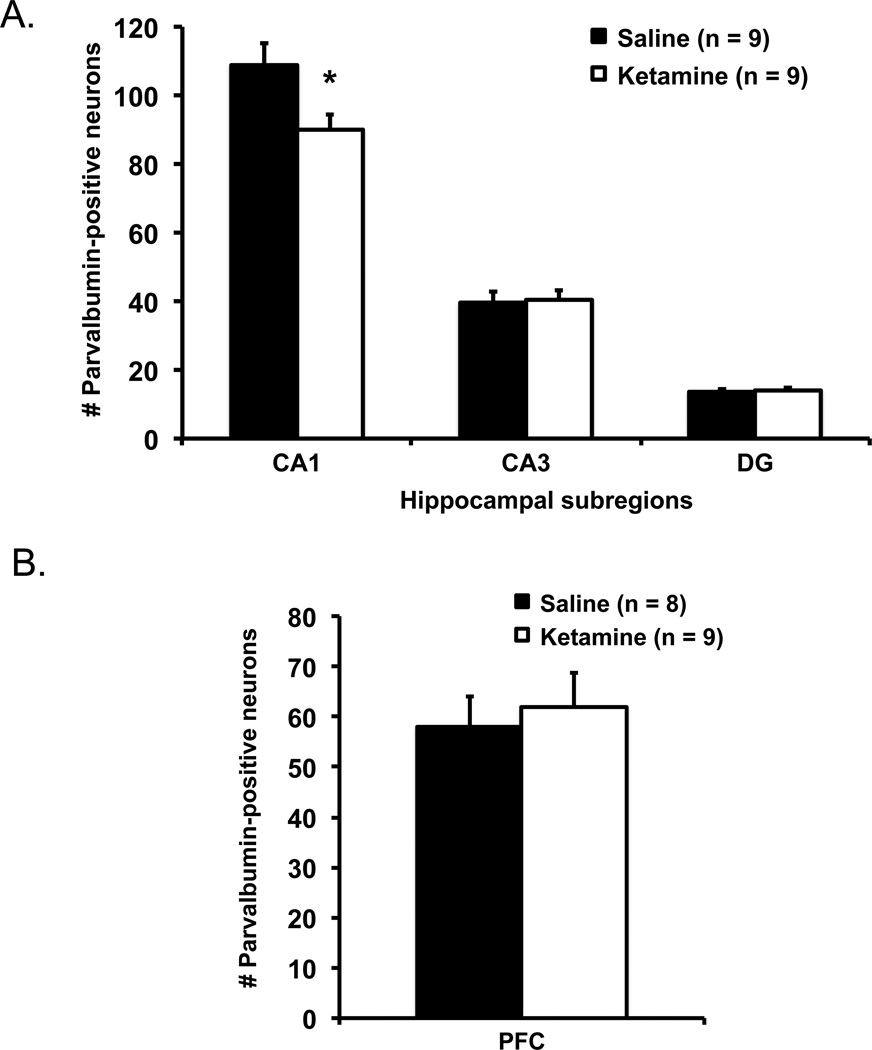
To examine whether the ketamine-exposed mice with impaired hippocampal memory function differed from control mice in the integrity of their hippocampal inhibitory network, the brains of the mice were immunostained for parvalbumin-positive interneurons (Figure 4). Figure 5A shows the number of parvalbumin-positive interneurons in the hippocampal subregions. The ketamine-exposed mice (n = 9) had significantly lower number of parvalbumin-immunoreactive neurons in the CA1 of the hippocampus than saline control mice (n = 9), t(16) = 2.46, p = .026. No differences between the groups were observed in the CA3, t(16) = 0.16, p = .873, or the dentate gyrus, t(16) = 0.33, p = .743. Figure 5B shows the number of parvalbumin-positive interneurons in the mPFC. The ketamine-exposed mice (n = 9) had comparable number of parvalbumin-immunostain neurons in mPFC to those in saline control mice (n = 8), t(15) = 0.42, p = .682.
Figure 4.
Representative photomicrographs of parvalbumin-positive interneurons in the hippocampus (top panel) and mPFC (bottom panel) of ketamine- and saline-treated mice.
Figure 5.
(A) Ketamine-exposed mice showed fewer parvalbumin-positive interneurons in the CA1 but not CA3 or dentate gyrus (DG) of the hippocampus than saline control mice. (B) No differences were detected in the mPFC between the two groups. Error bars indicate mean ± SEM.
4. Discussion
Mice exposed to ketamine intermittently for one month during adolescence were impaired as young adults in hippocampal-dependent long-term memory; no impairment however was seen in mPFC-mediated working memory. Consistent with these behavioral observations, decreased parvalbumin-positive interneurons was found in the CA1 of hippocampus but not the mPFC, a finding that implicates loss of inhibitory control as a contributing factor in disrupting hippocampal-dependent function. Importantly, the ketamine dose, treatment regimen, and age of exposure used here was identical to the induction protocol of an animal neurodevelopmental model of schizophrenia that has been shown to produce hippocampal overactivity similar to that seen in schizophrenia patients (Schobel et al., 2013). Our findings that mice in this model also showed impaired hippocampal-dependent cognition and reduced inhibitory control are consistent with the observation that hippocampal overactivity is associated with cognitive dysfunction in patients (Tregellas et al., 2014). Taken within this context, these results strengthen the utility of this model for preclinical investigations of hippocampal pathology in schizophrenia.
Ketamine administration, both in acute and sub-chronic forms, has been used to recapitulate cognitive as well as positive and negative symptoms of schizophrenia (e.g., Kocsis et al., 2013; Neill et al., 2010). In the exposure protocol that we used (Schobel et al., 2013), mice were administered ketamine during 1–2 mo old, an age that approximates adolescence in humans when the risk for schizophrenia is elevated (Paus et al., 2008), and were behaviorally tested under a drug free condition to avoid motor confounds from the anesthetic effect of ketamine. Against this background, we found that the ketamine mice were impaired in trace fear conditioning, a task that depends on the integrity of the hippocampus (McEchron et al., 1998; Quinn et al., 2008). That the ketamine-exposed mice showed less freezing 24 hr after conditioning compared to the control mice but not during conditioning (post-shock) indicates that the impairment was specific to fear memory and not a general defect in fear response or emotional processing. It is important to note that although the hippocampus is critically involved in trace fear conditioning, the mPFC has also been shown to participate in the process (e.g., Runyan et al., 2004). However, impaired hippocampal function, whether due to damage or aberrant excess neural activity as hypothesized here, appears to be sufficient to interfere with trace fear conditioning (e.g., Burman et al., 2006; McEchron et al., 1998). Importantly, the conditions we set up to test for trace fear learning favor strong hippocampal involvement, namely we used a trace interval (18 s) that approximates those longer intervals (20–40 s) that have been shown to be critically dependent on the hippocampus in addition to engaging the mPFC. Shorter trace intervals (on the range of 5 s) are known to engage the mPFC independent of the hippocampus (Chowdhury et al., 2005; Guimarais et al., 2011). In addition, we tested for recent trace fear memory that is highly sensitive to hippocampal but not mPFC damage; remote fear memory (30-day old and beyond), on the other hand, is sensitive to mPFC but not hippocampal damage (Beeman et al., 2013; Quinn et al., 2008).
In contrast to trace fear conditioning, performance of the ketamine mice on the delayed response task, which critically depends on the mPFC (Sloan et al., 2006), was relatively normal compared to controls. Although deficits in mPFC-mediated working memory tasks have been reported after NMDA antagonist treatment in rodents (e.g., Enomoto and Floresco, 2009; Marquis et al., 2007; Vasconcelos et al., 2015), others, including the present study, have failed to find an impairment (e.g., Li et al., 2003; Stefani and Moghaddam, 2002). Differences in induction protocols and tasks used to assess working memory between studies could account for the disparate findings.
Working memory tasks have been reported to engage the hippocampus in addition to the mPFC (e.g., Lee and Kesner, 2003; Spellman et al., 2015). The degree to which the hippocampus is involved appears to be dependent on the tasks and the conditions of the protocol used. Here, we used a working memory task that has been shown to be dependent on the mPFC but not the hippocampus. In a key finding of a double dissociation, Sloan et al. (2006) found that mPFC lesions interfered with performance on a delayed response task such as that used in our study, but did not affect performance on a spatial reference water maze task; hippocampal lesions, on the other hand, impaired water maze performance but importantly did not affect delayed response task performance. Findings consistent with the lack of hippocampal involvement in delayed response tasks have also been reported by others (Izaki et al., 2007; Winters and Dunnett, 2004; Young et al., 1996). Furthermore, not unlike trace fear conditioning, the hippocampus appears to mediate longer delays in the order of 5 min in delayed nonmatching-to-place tasks; shorter delays (10 s) engage both the mPFC and hippocampus with some redundancy and in a time-limited fashion (Lee and Kesner, 2003). Nonetheless, the lack of a working memory impairment in our study is currently limited to the delayed response task; additional studies using other working memory tasks would be necessary to conclusively show that the sub-chronic ketamine model that we used does not affect mPFC function.
In our study, changes in a GABAergic inhibitory interneuron population seen in the hippocampus but not in the mPFC were in line with the behavioral deficits observed in the former but not latter domains of function. The reduction in parvalbumin-positive interneurons specifically in the CA1 of the hippocampus could impact trace fear memory; molecular signaling in the CA1 has been found to be important for trace fear memory (Brigman et al., 2010; Huang et al., 2010), and parvalbumin interneurons in the CA1 are known to exert synaptic inhibition in the hippocampus for regulating cognitive and psychosis phenotypes relevant to schizophrenia (Gilani et al., 2014). The reduction in parvalbumin-positive interneurons observed in the CA1 was also consistent with the abnormal increase in CA1 basal activity after repeated ketamine administration in mice (Schobel et al., 2013) as well as the elevations of cerebral blood volume in the CA1 subfield of schizophrenia patients (Schobel et al., 2009). In addition, in ketamine-treated mice (Schobel et al., 2013), a reduction in parvalbumin-positive interneurons was also observed in the hippocampus, consistent with our findings. Notably, no reduction of parvalbumin-positive interneurons was observed in the mPFC in the current study, consistent with the behavioral data. Hence, converging evidence points to the CA1 as a key locus of dysfunction in this model for the illness.
The reduction of inhibitory interneuron population such as that seen here with parvalbumin-positive interneurons is known to disrupt oscillatory synchronization of neuron ensembles that has been shown to be important for cognitive processes (e.g., Lodge et al., 2009; Pittman-Polletta et al., 2015; Sohal et al., 2009), and has been suggested as a possible neural basis for pathological symptoms in schizophrenia (Uhlhaas & Singer, 2010; Uhlhaas & Singer, 2015). Relevant to the present model, chronic ketamine administration leads to reduced theta- and gamma-frequency activities in the hippocampus, including those in the CA1, but no significant reduction was found with gamma oscillation in the frontal cortical region (Kittelberger et al., 2012). The disruption of oscillations and neuronal dynamics in the hippocampus may thus serve as another potential functional link in our observations between the effect of ketamine on parvalbumin expression in the CA1 and impaired hippocampal-dependent trace fear memory.
The mice exposed to ketamine responded significantly stronger to amphetamine stimulation than control animals, suggesting the ketamine mice were in a hyperdopaminergic state not unlike that seen in schizophrenia patients (e.g., Laruelle et al., 1996). Interestingly, a potential link between hippocampal overactivity and dopamine system dysfunction may exist and present a novel pathway for treatment of positive symptoms of schizophrenia. Hippocampal output via the subiculum controls dopamine neuron firing in the ventral tegmental area (VTA; Floresco et al., 2001), and excess dopamine activity in the VTA could be reduced by intracranial administration of a GABA agonist into the hippocampus (Gill et al., 2011). Targeting hippocampal overactivity thus has the potential of not only benefiting cognitive performance, but also attenuating dopamine-associated positive symptoms such as psychotic ideation observed in schizophrenia.
We set out to examine whether an animal model of schizophrenia that exhibited hippocampal overactivity also showed impaired cognitive function to equip future development of therapeutics targeting overactivity for pro-cognitive treatment. To this end, our findings of a disrupted hippocampal inhibitory neural circuitry and impaired hippocampal-dependent memory suggest that treatment strategies that boost inhibition in the hippocampus would benefit memory function. Indeed, a recent study that enhanced inhibition via an antiepileptic agent, levetiracetam, was found to be effective in improving sensory processing in a mouse model of gating deficits in schizophrenia (Smucny et al., 2015). Further investigations into the efficacies of such treatments on higher-level cognition are warranted.
Acknowledgments
We thank Austin Sullins, Daniel Burruss, and Robert McMahan for excellent technical support, Dr. Barry Setlow of University of Florida for advice on the delayed response task, and Dr. Michela Gallagher of Johns Hopkins University for financial support, and critical reading and helpful discussion of the manuscript. YS was a Woodrow Wilson Fellow at Johns Hopkins University. This work was supported by the Silvo O. Conte Center, P50MH094268.
Role of funding agencies
None of the funding agencies had a role in collection, management, analysis or interpretation of the data or in preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MTK developed the study design, performed the behavioral experiments, supervised the immunohistochemistry, and drafted the paper. YS performed the immunohistochemistry and analysis. AS and DRS wrote the behavioral protocols and ran the behavioral studies. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors have no actual or potential conflicts of interest that could inappropriately influence this work.
References
- Beeman CL, Bauer PS, Pierson JL, Quinn JJ. Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn. Mem. 2013;20(6):336–343. doi: 10.1101/lm.031161.113. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J. Neurosci. 2010;30(13):4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16(2):103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav. Neurosci. 2005;119(5):1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Corigliano V, De Carolis A, Trovini G, Dehning J, Di Pietro S, Curto M, Donato N, De Pisa E, Girardi P, Comparelli A. Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res. 2014;220(1–2):129–134. doi: 10.1016/j.psychres.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci. Biobehav. Rev. 2013;37(9 Pt B):2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr. Res. 2007;93(1–3):266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Floresco SB. Disruptions in spatial working memory, but not short-term memory, induced by repeated ketamine exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(4):668–675. doi: 10.1016/j.pnpbp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2014;28(4):287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, Chuhma N, Glickstein S, Merker RJ, Xu Q, Small SA, Anderson SA, Ross ME, Moore H. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc. Natl. Acad. Sci. U. S. A. 2014;111(20):7450–7455. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int. J. Neuropsychopharmacol. 2014;17(10):1609–1619. doi: 10.1017/S146114571400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M. NMDA receptors, cognition and schizophrenia--testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62(3):1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Guimarãis M, Gregório A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Front. Behav. Neurosci. 2011;5:89. doi: 10.3389/fnbeh.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch. Gen. Psychiatry. 1991;48(11):1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chiang YW, Liang KC, Thompson RF, Liu IY. Extra-cellular signal-regulated kinase 1/2 (ERK1/2) activated in the hippocampal CA1 neurons is critical for retrieval of auditory trace fear memory. Brain Res. 2010;1326:143–151. doi: 10.1016/j.brainres.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Fujiwara SE, Akema T. Involvement of the rat prefrontal cortex in a delayed reinforcement operant task. Neuroreport. 2007;18(16):1687–1690. doi: 10.1097/WNR.0b013e3282f0b6b6. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24(1):109–120. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct. Funct. 2012;217(2):395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Brown RE, McCarley RW, Hajos M. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci. Ther. 2013;19(6):437–447. doi: 10.1111/cns.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U. S. A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 2003;23(4):1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kim CH, Ichikawa J, Meltzer HY. Effect of repeated administration of phencyclidine on spatial performance in an eight-arm radial maze with delay in rats and mice. Pharmacol. Biochem. Behav. 2003;75(2):335–340. doi: 10.1016/s0091-3057(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23(3):223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis JP, Audet MC, Doré FY, Goulet S. Delayed alternation performance following subchronic phencyclidine administration in rats depends on task parameters. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(5):1108–1112. doi: 10.1016/j.pnpbp.2007.03.017. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8(6):638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15(4):418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Moore H, Geyer MA, Carter CS, Barch DM. Harnessing cognitive neuroscience to develop new treatments for improving cognition in schizophrenia: CNTRICS selected cognitive paradigms for animal models. Neurosci. Biobehav. Rev. 2013;37(9 Pt B):2087–2091. doi: 10.1016/j.neubiorev.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol. Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, Subotnik KL, Bartzokis G. The early longitudinal course of cognitive deficits in schizophrenia. J. Clin. Psychiatry. 2014;75(Suppl 2):25–29. doi: 10.4088/JCP.13065.su1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman-Polletta BR, Kocsis B, Vijayan S, Whittington MA, Kopell NJ. Brain rhythms connect impaired inhibition to altered cognition in schizophrenia. Biol. Psychiatry. 2015;77(12):1020–1030. doi: 10.1016/j.biopsych.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn. Mem. 2008;15(5):368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Moore AM, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004;24(6):1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TM, Cotel MC, O’Neill MJ, Tricklebank MD, Collingridge GL, Sher E. Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62(3):1349–1358. doi: 10.1016/j.neuropharm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta. Neuropathol. 2009;117(4):395–407. doi: 10.1007/s00401-008-0430-y. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain Res. 2006;171(1):116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn. Mem. 2007;14(9):597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Stevens KE, Tregellas JR. The antiepileptic drug levetiracetam improves auditory gating in DBA/2 mice. NPJ Schizophr. 2015;1:e15002. doi: 10.1038/npjschz.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522(7556):309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Effects of repeated treatment with amphetamine or phencyclidine on working memory in the rat. Behav. Brain Res. 2002;134(1–2):267–274. doi: 10.1016/s0166-4328(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am. J. Psychiatry. 2014;171(5):549–556. doi: 10.1176/appi.ajp.2013.13070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol. Psychiatry. 2015;77(12):1001–1009. doi: 10.1016/j.biopsych.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Vasconcelos GS, Ximenes NC, de Sousa CN, Oliveira Tde Q, Lima LL, de Lucena DF, Gama CS, Macêdo D, Vasconcelos SM. Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: Participation of antioxidant, nitrergic and neurotrophic mechanisms. Schizophr. Res. 2015;165(2–3):163–170. doi: 10.1016/j.schres.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, Crow TJ. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am. J. Psychiatry. 2002;159(5):821–888. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]
- Winters BD, Dunnett SB. Selective lesioning of the cholinergic septo-hippocampal pathway does not disrupt spatial short-term memory: a comparison with the effects of fimbria-fornix lesions. Behav. Neurosci. 2004;118(3):546–562. doi: 10.1037/0735-7044.118.3.546. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacolgy. 2012;62(3):1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HL, Stevens AA, Converse E, Mair RG. A comparison of temporal decay in place memory tasks in rats (Rattus norvegicus) with lesions affecting thalamus, frontal cortex, or the hippocampal system. Behav. Neurosci. 1996;110(6):1244–1260. doi: 10.1037//0735-7044.110.6.1244. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr. Res. 2002;55(1–2):1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Zierhut K, Bogerts B, Schott B, Fenker D, Walter M, Albrecht D, Steiner J, Schütze H, Northoff G, Düzel E, Schiltz K. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183(3):187–194. doi: 10.1016/j.pscychresns.2010.03.007. [DOI] [PubMed] [Google Scholar]