Abstract
Study Objectives:
Increasing evidence from laboratory and epidemiologic studies indicates that insufficient sleep may be a risk factor for obesity. Sleep curtailment results in stimulation of hunger and food intake that exceeds the energy cost of extended wakefulness, suggesting the involvement of reward mechanisms. The current study tested the hypothesis that sleep restriction is associated with activation of the endocannabinoid (eCB) system, a key component of hedonic pathways involved in modulating appetite and food intake.
Methods:
In a randomized crossover study comparing 4 nights of normal (8.5 h) versus restricted sleep (4.5 h) in healthy young adults, we examined the 24-h profiles of circulating concentrations of the endocannabinoid 2-arachidonoylglycerol (2-AG) and its structural analog 2-oleoylglycerol (2-OG). We concomitantly assessed hunger, appetite, and food intake under controlled conditions.
Results:
A robust daily variation of 2-AG concentrations with a nadir around the middle of the sleep/overnight fast, followed by a continuous increase culminating in the early afternoon, was evident under both sleep conditions but sleep restriction resulted in an amplification of this rhythm with delayed and extended maximum values. Concentrations of 2-OG followed a similar pattern, but with a lesser amplitude. When sleep deprived, participants reported increases in hunger and appetite concomitant with the afternoon elevation of 2-AG concentrations, and were less able to inhibit intake of palatable snacks.
Conclusions:
Our findings suggest that activation of the eCB system may be involved in excessive food intake in a state of sleep debt and contribute to the increased risk of obesity associated with insufficient sleep.
Commentary:
A commentary on this article appears in this issue on page 495.
Citation:
Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. SLEEP 2016;39(3):653–664.
Keywords: appetite, endocannabinoid, hedonic food intake, hunger, obesity, sleep restriction
Significance.
Insufficient sleep is a putative risk factor for weight gain. Increases in hunger and food intake in sleep-deprived individuals exceed energy demands of extended wakefulness and therefore involve eating for pleasure rather than to fulfill a caloric need. Endocannabinoids are naturally synthesized lipids that bind the same receptors as the active ingredient of marijuana. Activation of these receptors promotes hedonic eating. This study shows that under normal sleep conditions, blood levels of the most abundant endocannabinoid increase from mid-sleep to early afternoon. After sleep restriction, this increase in endocannabinoid level is amplified, coinciding with greater desire for palatable food. The identification of the endocannabinoid system as a mediator of increased hunger following sleep curtailment may help develop novel preventive strategies.
INTRODUCTION
Rates of overweight and obesity have increased rapidly over the past three decades.1 Concurrently, average sleep times have decreased.2 Polls conducted by the Centers for Disease Control and Prevention (CDC) and National Sleep Foundation (NSF), as well as large-scale epidemiological studies, indicate that many Americans experience chronic partial sleep restriction because of voluntary bedtime curtailment.3 A growing body of evidence suggests a link between insufficient sleep duration and increased risk of obesity. A meta-analysis of cross-sectional epidemiologic studies including more than 600,000 adults from around the world found a significant pooled odds ratio for short duration of sleep and obesity of 1.55.4 The majority of prospective studies, whether in children or adults, found that short sleep predicts greater weight gain or the incidence of obesity, after controlling for multiple confounders.5–9 Experimental sleep restriction in the laboratory (4–6 h/night for multiple nights) has adverse effects on insulin sensitivity and glucose tolerance10–12 and increases hunger and food intake.13,14 Further, in a study where caloric intake was strictly controlled, plasma concentrations of the satiety hormone leptin were suppressed and concentrations of the appetite-stimulating hormone ghrelin were elevated, in association with a stimulation of hunger and appetite.15
Recent studies using whole room indirect calorimetry have shown that sleep restriction does increase energy need, but only modestly.16–18 By comparison, the increased hunger and appetite reported by sleep deprived subjects and their increased energy intake in the presence of ad libitum feeding appear to exceed the energy demands of extended wakefulness under sedentary conditions.13–15,18,19 These findings suggest a role for alterations in hedonically driven hunger and appetite as a result of insufficient sleep. The endocannabinoid (eCB) system is involved in the control of feeding, appetite, and energy homeostasis. The eCB system is composed of cannabinoid CB1 and CB2 receptors, the endogenous agonists of these receptors 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (anandamide, AEA), and the enzymes required for the biosynthesis and degradation of the endogenous lipids. The appetite-enhancing effects of cannabinoids appear to be mediated mainly by the CB1 receptor. Production of 2-AG is more than 100-fold larger than that of AEA. The eCBs, measurable in plasma,20 are biosynthesized de novo and bind to CB1 receptors in brain and in peripheral organs involved in energy metabolism, including adipose tissue, endocrine pancreas, muscle, and liver.21–24 It is well established that eCB-dependent CB1 receptor activation is a potent orexigenic signal; agonists of CB1 receptors stimulate feeding, whereas antagonists result in appetite suppression.21 CB1 receptors are found in homeostatic pathways including hypothalamic nuclei known to modulate energy homeostasis via interaction with peripheral peptides (i.e., leptin and ghrelin).25,26 Moreover, activation of the eCB system affects hedonic (motivation and reward) circuits in the mesolimbic system, including the nucleus accumbens and ventral tegmental area. Here eCBs interact with dopamine and opioid pathways, eliciting a preference for highly palatable rewarding food.27–29
There are remarkable parallels between the effects of activation of the eCB system and the effect of experimental sleep restriction. Just as in a state of sleep debt, higher CB1 receptor activity increases feeding behavior in excess of energy need, reduces glucose tolerance, tends to reduce leptin levels and to promote ghrelin release, and stimulates reward and addiction. We therefore hypothesized that sleep restriction activates the eCB system and assessed the 24-h profile of circulating concentrations of the most abundant eCB, 2-AG, and of its structural analog 2-OG, in nonobese healthy individuals who participated in a randomized crossover study comparing 4 days of bedtime restriction to 4.5 h per night to 4 d of 8.5 h bedtimes. We have previously reported the existence of a robust circadian rhythm in circulating eCB concentrations under normal sleep conditions.30
METHODS
Participants
Healthy nonobese men and women between the ages of 18 to 30 y, with a body mass index (BMI; in kg/m2) less than 28 for men and 27 for women and self-reported habitual sleep duration of 7.5–8.5 h between the hours of 23:00 and 09:00, were recruited for participation in this study. Exclusion criteria included: irregular sleep schedule, habitual daytime naps, shift work, travel across time zones in the past 4 weeks, chronic medical condition, acute illness, use of any prescription medications, use of over-the-counter medications or supplements known to affect sleep or glucose metabolism, smoking, marijuana usage, excessive alcohol (more than two drinks per day) or caffeine (> 300 mg per day) consumption, history of psychiatric disorders, or abnormal findings on medical history, physical examination, or routine laboratory testing. All participants underwent overnight laboratory polysomnography to exclude sleep disorders, as well as a standard 75-g oral glucose tolerance test and fasting blood sample collection for routine laboratory analyses. A 12-lead electrocardiogram was also obtained. Healthy individuals who had normal glucose tolerance and no sleep disorders were included. Individuals were administered both the Center for Epidemiologic Studies Depression Scale (CESD; cutoff of > 16 for clinical depression) and the Beck Inventory for Depression (cutoff greater than 10). Only those with both scores below the cutoff were included. Only nonpregnant women were studied, and data collection was scheduled during the follicular phase of the menstrual cycle. All research volunteers gave written informed consent and were paid for their participation.
Study Protocol
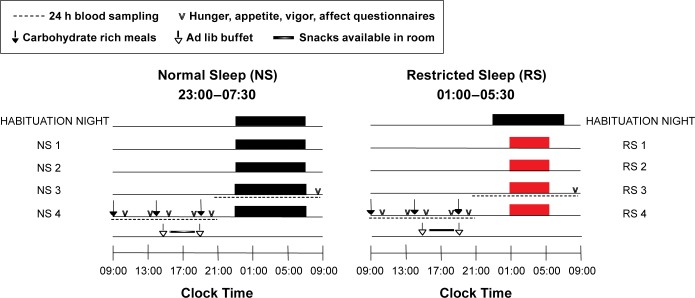
The protocol was approved by the Institutional Review Board of the University of Chicago. Each subject was tested under two sleep conditions, in randomized order, spaced by at least 4 weeks as shown in Figure 1. All study procedures took place in the University of Chicago Clinical Resource Center. During the week preceding each sleep condition, participants were instructed to maintain a standardized schedule of bedtimes (23:00–07:30) and to not deviate from this schedule by more than 30 min. The sleep-wake cycles of the participants were continuously monitored by wrist activity (Actiwatch; Philips Respironics, Bend, OR) to verify adherence. One condition involved 4 consecutive inpatient days with 8.5 h in bed (23:00 to 07:30, normal sleep, NS), whereas the other condition involved 4 days with 4.5 h in bed (01:00 to 05:30, restricted sleep, RS). Each condition was preceded by one night of acclimation to the laboratory environment with 8.5 h in bed. Study participants were limited to sedentary activities during waking hours and were housed in a private room. No naps were allowed. In both conditions, irrespective of sleep/wake time, only dim light (ceiling lights off, shades closed) was allowed in the subject's room from wake until 10:30 and again from 18:00 until bedtime. Dim light was approximately 75– 100 lux, whereas ceiling lights and open shades were approximately 550–600 lux (EXTECH Light Meter, Nashua, NH). Compliance was monitored continuously by research staff. In the afternoon following the second night of each condition, an intravenous sterile heparin-lock catheter was inserted in a forearm vein. The line was kept patent with a slow drip of heparinized saline. Blood sampling was initiated at 21:30 at 15- to 30-min intervals and continued for 24 h. Cortisol, leptin, and ghrelin were assayed on all samples, whereas eCBs were assayed at 60-min intervals. During bedtimes, the catheter was connected to plastic tubing that extended to an adjacent room to sample distally without disturbing the participant. Samples were collected at room temperature in tubes that did not contain inhibitors. Plasma and serum samples were frozen at −80°C until assay.
Figure 1.
The protocol followed a randomized crossover design with two 5-night in-laboratory sessions spaced by at least 1 month. Each session included one habituation night, followed by 4 experimental nights with either 4.5 (RS1-RS4, red bars) or 8.5 (NS1-NS4, black bars) h/night in bed. Over the third experimental night beginning at 21:00, 24-h blood sampling was initiated to examine circulating endocannabinoid, cortisol, leptin, and ghrelin levels (dashed lines). Throughout the following day, identical carbohydrate-rich meals were served at 09:00, 14:00, and 19:00 (denoted by black arrows). Questionnaires to assess hunger, appetite, vigor, and affect were administered at 08:35, 10:35, 13:35, 15:35, 18:35, and 20:35 (denoted by v). In the afternoon following the fourth experimental night, participants were exposed to an ad libitum (ad lib) buffet for lunch at 15:00 and dinner at 19:30 (denoted by open arrows), with a snack period between the meal opportunities (graded line).
Controlled Caloric Intake
Caloric intake was identical under both sleep conditions and was strictly controlled up to the time when the participants were exposed to ad libitum feeding conditions on the day following the fourth night of each condition. Caloric content of the diet was calculated to meet individual participant's caloric requirements for sedentary conditions.31 A registered dietitian from the Clinical Resource Center Metabolic Kitchen supervised the preparation of all meals. Participants were not allowed to consume any foods or beverages that were not provided by the metabolic kitchen. During the 24-h period of blood sampling, participants ate three identical carbohydrate-rich meals (20% fat, 68% carbohydrate, 12% protein), served at 09:00, 14:00, and 19:00. The participants were instructed to consume each meal in its entirety within 20 min.
Ad libitum Feeding
After being fasted since dinner (19:00) the previous night, in the afternoon following the fourth night of each sleep condition participants were presented with an ad libitum buffet of palatable foods, tailored to meet the subject's dietary preferences as determined during an interview with the dietician prior to the initiation of the study. The same individually customized assortment of foods was given during each sleep condition. Participants were instructed to eat as much as they wanted over a 1-h period (from 15:00–16:00) without distraction by staff or investigators. From 16:00 until 19:30, a snack bar was available in the participant's private room. At 19:30, a second ad libitum buffet was offered for dinner, and again participants were instructed to eat as much as they wanted over a 1-h period. Meals and snacks were served in excess to allow for ad libitum intake. Food was weighed before and after each meal and during the snacking period to determine actual consumption. The caloric content and macronutrient composition of all meals and snacks were calculated using Food Processor SQL software (ESHA Research, Salem, OR).
Assessment of Hunger, Appetite, Vigor, and Mood
During the 24-h period of blood sampling when caloric intake was limited to three identical meals, the participants completed four validated computerized visual analog scales (0 to 10 cm32) to assess motivation to eat at six time points: 25 min before and 1 h 35 min after each meal (at 08:35, 10:35, 13:35, 15:35, 18:35, 20:35). At each time point, the participants were asked to score “How hungry do you feel right now?”, “How strong is your desire to eat right now?”, “How much do you think you could eat right now?”, and “How full does your stomach feel right now?”. To assess appetite at each time point, participants were asked to mark on a 10-cm scale how much they would enjoy eating foods from seven different categories (with “not at all” on the left and “very much” on the right) without regard to caloric content, fat content, or a healthy diet.33 A global appetite score was calculated at each time point as the sum of the seven scores (scale: 0–70 cm). Subjective alertness was assessed using the Visual Analog Scale for Global Vigor and Mood,34 which combines the scores on four 10-cm scales (alert, sleepy, weary, and effort) to obtain a Global Vigor score between 0 and 40 cm and the scores on four 10-cm scales (happy, sad, calm, tense) to obtain a Global Mood score between 0 and 40 cm. Data were then divided by 4 to obtain a rating between 0–10.
Sleep Recording
Sleep was recorded by polysomnography (Neurofax EEG-1100A, Nihon Kohden, Foothill Ranch, CA) each night. The recordings were visually scored in 30-sec epochs as wake, rapid eye movement (REM) sleep, or nonrapid eye movement (NREM) sleep stages N1, N2, and N3 according to standardized criteria.35 The following summary variables were calculated: sleep period time (SP, i.e., time interval separating sleep onset from morning awakening), total sleep time (i.e. SP − duration of intrasleep wake periods), sleep efficiency (i.e. total sleep time / time in bed * 100), duration of REM sleep, duration of light NREM sleep (i.e. stages N1+N2), and duration of deep NREM sleep (i.e., stage N3).
Assays
Serum concentrations of 2-AG, the endogenous ligand of the CB1 and CB2 receptors and its structural analog 2-oleoylglycerol (2-OG) were extracted from serum using Bond Elut C18 solid phase extraction columns (Varian Inc, Lake Forest, CA), as previously described.36 The two lipids were quantified in the lipid extracts by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS; Agilent LC-MSD 1100 series, Ramsey, MN) and quantified by isotope dilution as described previously.37
Serum cortisol levels were measured by an immunochemiluminescent assay (Immulite, Los Angeles, CA). Serum leptin and total ghrelin levels were measured by radioimmunoassay (Linco Research, St. Charles, MO).
Analysis of Individual Profiles of Serum Concentrations of 2-AG, 2-OG, Cortisol, Leptin, and Ghrelin
Isolated 2-AG values that represented a relative change of more than 100% in comparison with both the preceding and following values and were not concomitant with a similar change in 2-OG level were assumed to represent assay error and were replaced by linear interpolation. Similar criteria were used to identify aberrant 2-OG values. In the current study, for both 2-AG and 2-OG, a total of 672 values were measured. Fifteen values (2.2%) were interpolated for 2-AG and 14 values (2.1%) were interpolated for 2-OG. A total of 1,932 values were measured for each leptin, ghrelin, and cortisol. No values were interpolated for ghrelin or cortisol, whereas 14 (0.7%) were interpolated for leptin.
To quantify the 24-h profiles of serum levels of 2-AG, 2-OG, cortisol, and leptin, a best-fit curve was calculated for each individual profile using a robust locally weighted nonlinear regression procedure with a window of 2 h for 2-AG and 2-OG and 4 h for cortisol and leptin.38 The peak and the nadir were defined as the maximum and minimum of the regression curve, respectively. The amplitude was defined as half of the difference between the peak and the nadir.
The 24-h variations of ghrelin levels were quantified as previously described.39 To quantify the nocturnal ghrelin elevation, the profiles were smoothed using a four-point moving average and the amplitude of the nocturnal rise was calculated as the difference between the nadir following the evening meal and the maximum level attained between 23:00 and 05:00.
Statistical Analysis
All group values are expressed as mean ± standard error of the mean (SEM). Mean values comparing the normal sleep and restricted sleep conditions were tested with a t-test. Analysis of variance for repeated measures was used to compare variables measured during normal sleep (NS) and restricted sleep (RS). The order of the condition (i.e., NS condition first versus RS condition first) was introduced as covariate in the analysis of hormonal responses, hunger, appetite, vigor, affect, and food intake under ad libitum conditions. Correlations were calculated using the Pearson coefficient.
RESULTS
The protocol is depicted in Figure 1. There are no previous reports on the effect of sleep duration on endocannabinoid levels, and therefore, the sample size of this randomized crossover study could not be estimated using existing data. Instead, we relied on the experience accumulated in eight previous studies of experimental sleep restriction conducted in the controlled environment of the University of Chicago Clinical Resource Center10,11,14,40–44 that all demonstrated a robust effect of insufficient sleep on hormones and metabolism in healthy humans. The median sample size in these eight previous studies was 11 and we conservatively selected a sample size of 15 participants. One participant dropped out after completing one arm of the study, due to personal reasons.
Fourteen individuals, 11 men and 3 women, with a mean age of 23.4 ± 0.8 y and a mean BMI of 23.9 ± 0.7 kg/m2 participated in this study. Six of the 14 participants were tested under the NS condition first, and the remaining eight participants began with the RS condition. When measured prior to the first experimental night of each sleep condition, body weight (NS1: 74.7 ± 2.8 kg versus RS1: 74.6 ± 2.8 kg) and percentage body fat (NS1: 23.8 ± 2.0 % vs. RS1: 23.9 ± 2.2 %) were similar. Body weight did not change within either experimental session, from the evening prior to the first experimental night to the morning following the fourth experimental night (change from NS1 to NS5: - 0.03 kg, P = 0.92; change from RS1 to RS5: −0.26 kg, P = 0.197).
Sleep Duration and Sleep Stages
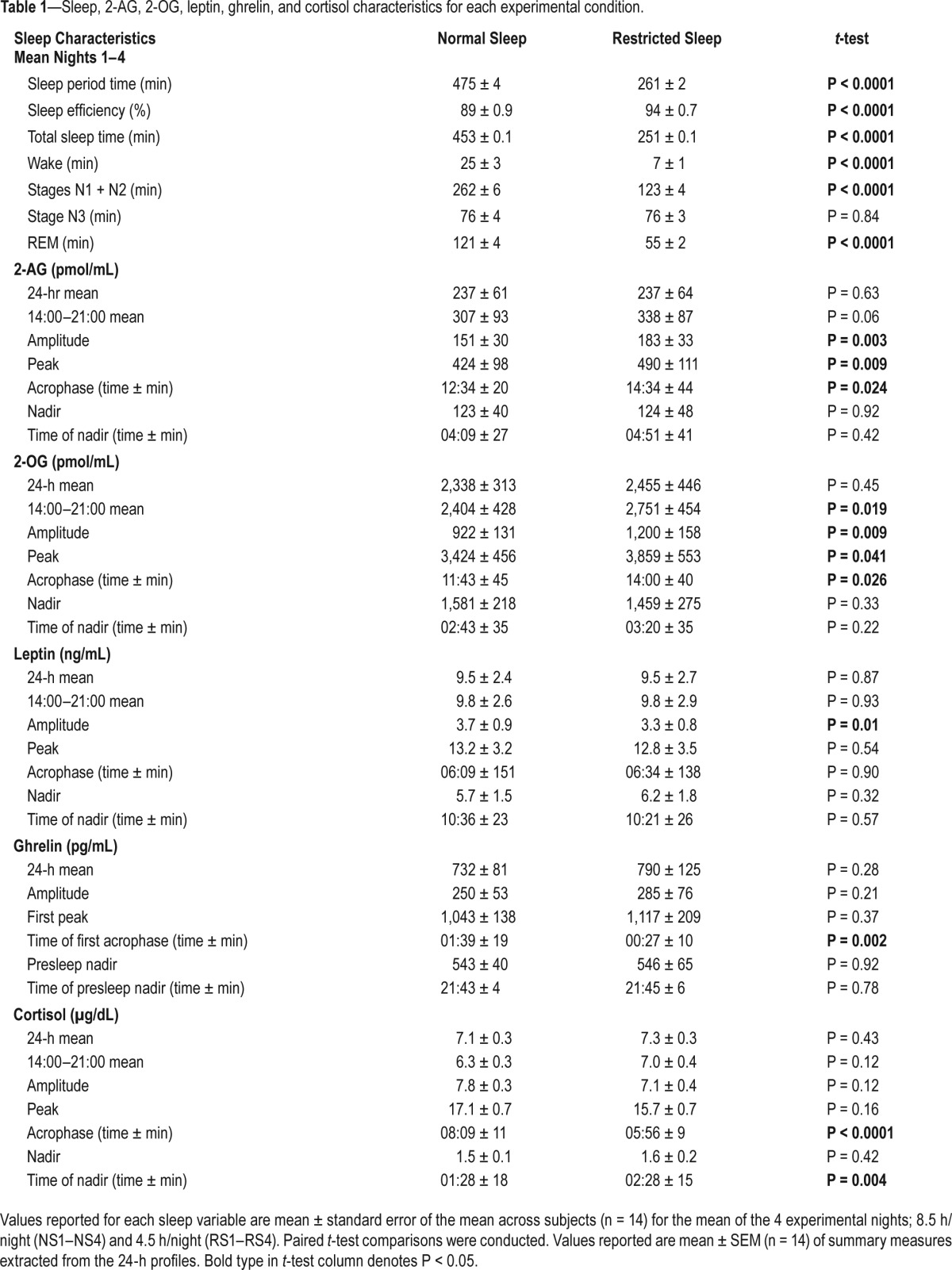
As shown in Table 1, during the NS condition participants slept on average 453 min/night whereas during the RS condition they slept on average 251 min/night (P < 0.0001). As expected, sleep efficiency was higher when time in bed was restricted. The reduction in total sleep time reflected lower amounts of Stage N1 and N2, as well as a reduction of the time spent in REM sleep (Table 1). In contrast, time spent in deep NREM sleep (Stage N3) was nearly identical during RS as compared to NS.
Table 1.
Sleep, 2-AG, 2-OG, leptin, ghrelin, and cortisol characteristics for each experimental condition.
Effect of Sleep Restriction on 24-h Profiles of 2-AG and 2-OG Levels
Mean 24-h serum eCB concentrations were highly stable for a given individual across the two experimental sessions (Supplemental Figure 1A and 1B), even though the sessions were separated by at least 1 month (2-AG: r2 = 0.99, P < 0.0001 and 2-OG: r2 = 0.96, P < 0.0001), indicating strong within-subject reproducibility of the mean circulating concentrations of the two lipids despite wide intersubject variability. Statistics on absolute concentrations of 2-AG and 2-OG are reported in Table 1.
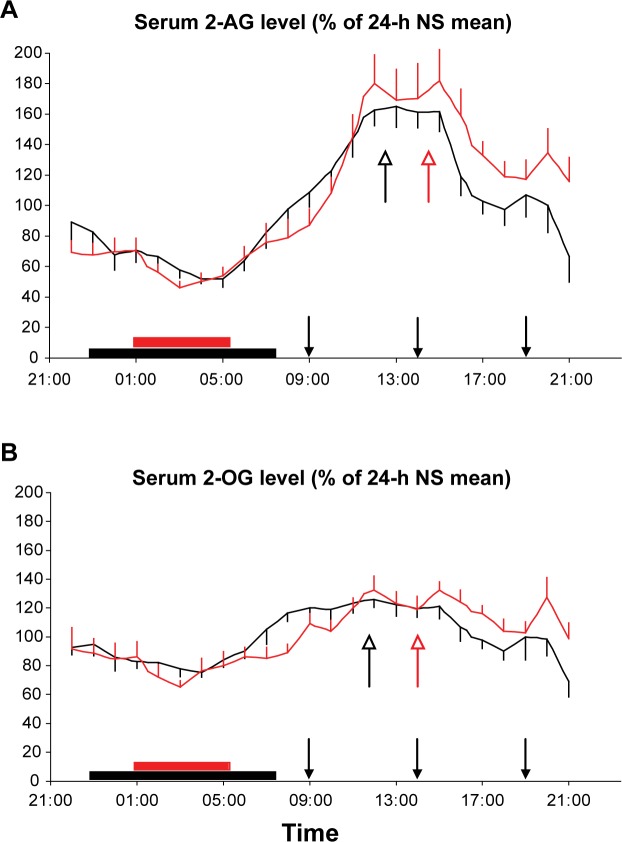
To illustrate the effect of sleep restriction on the wave-shape of the 2-AG and 2-OG profiles, each individual profile was expressed as a percentage of its 24-h mean concentration under NS conditions (Figure 2A and 2B). As we have previously reported,30 under NS conditions, the 24-h profile of 2-AG displayed a large amplitude with peak levels in the early afternoon, around habitual lunchtime. The profile of the structural analog 2-OG, which is synthesized and degraded by processes similar to those controlling blood levels of 2-AG45 but does not bind CB receptors, was roughly parallel to that of 2-AG; but the daytime increase in 2-OG was of shorter duration and of lesser magnitude.28
Figure 2.
Mean 24-h profiles of 2-AG (A) and 2-OG (B), expressed as % of 24-h mean of the normal sleep condition during the restricted 4.5 h (red) or normal 8.5 h (black) sleep condition (n = 14). Vertical bars at each time point represent the standard error of the mean. The sleep period is denoted with red or black bars. Closed arrows represent the identical carbohydrate-rich meals, presented at 09:00, 14:00, and 19:00. Open arrows denote the acrophase of the normal sleep condition (black) and restricted sleep condition (red) profiles. 2-AG, 2-arachidonoylglycerol; 2-OG, 2-oleoylglycerol.
When sleep was restricted, the amplitude of the 24-h profile of 2-AG was increased by an average of 33 ± 11% (P = 0.008), and this increase in amplitude was due to an elevation of the peak by an average of 22 ± 7% (P = 0.006; Figure 2A). Moreover, in the RS condition, the acrophase (time of peak) occurred approximately 2 h later than in the NS condition and elevated concentrations tended to persist until the end of the study (14:00–21:00; P = 0.06; Table 1). In contrast, neither the nadir concentration of 2-AG nor its timing were affected by sleep duration.
Figure 2B shows the mean 24-h profile of 2-OG, also expressed as percent of the 24-h mean of the NS profile. The effect of sleep duration was similar to that observed for 2-AG. The amplitude was increased by an average of 38 ± 15% in RS versus NS condition (P = 0.024). Similarly, the peak increased by 15 ± 7% (P = 0.042), and concentrations remained higher than after NS until the end of the study (14:00–21:00; P = 0.019), whereas there were no significant changes in the nadir 2-OG concentration or timing.
These differences in the 24-h profiles of 2-AG and 2-OG between the two sleep conditions occurred despite identical meals and caloric intake, similar sedentary conditions, and experimental environment, as well as stable BMI.
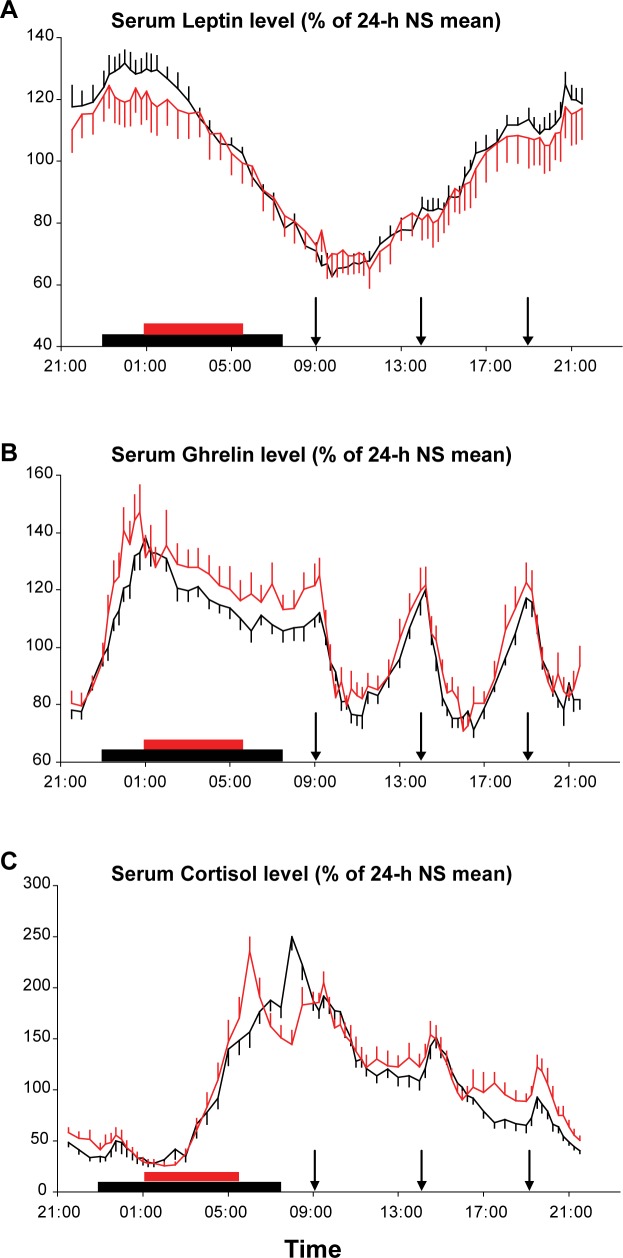
24-h Profiles of Serum Leptin and Ghrelin
Similar to the profiles of 2-AG and 2-OG levels, the 24-h profiles of leptin and ghrelin were expressed as a percentage of the individual mean concentration under NS conditions (Figure 3A and 3B). Overall, mean leptin concentrations were not affected by sleep duration (NS 9.5 ± 2.4 ng/mL versus RS 9.5 ± 2.7 ng/mL; P = 0.97) but the amplitude of the 24-h variation was blunted following RS (NS 3.7 ± 0.9 versus RS 3.3 ± 0.8 ng/mL; P = 0.01, Figure 3A) as has been previously observed in studies of sleep deprivation.40,46 As expected,39,47 the orexigenic factor ghrelin displayed postprandial dips and rebounds and a nocturnal peak (Figure 3B). A trend toward higher nocturnal ghrelin levels during RS was apparent, but not statistically significant (amplitude NS 250 ± 53 pg/mL versus RS 285 ± 76 pg/mL; P = 0.21). The nocturnal peak occurred earlier in the RS than in the NS condition (NS 01:39 ± 19 min versus RS 00:27 ± 10 min; P = 0.002).
Figure 3.
Mean 24-h profiles of leptin (A), total ghrelin (B), and cortisol (C) expressed as % of the 24-h during normal sleep (NS) during the restricted 4.5 h (red) or normal 8.5 h (black) sleep condition (n = 14). Vertical bars at each time point represent the standard error of the mean. The sleep period is denoted with red or black bars. Arrows represent the identical carbohydrate-rich meals, presented at 09:00, 14:00, and 19:00.
The ratio of the ghrelin peak to the leptin peak showed an increase following restricted sleep (P = 0.04), suggesting a shift in neuroendocrine signaling of energy balance consistent with promoting more hunger and less satiety.
24-h Profiles of Serum Cortisol (Figure 3C)
The well-documented circadian rhythm of plasma cortisol levels, with a nocturnal nadir and a morning peak, was present in both sleep conditions. Mean 24-h levels were similar in both sleep conditions. Because of the later bedtime in the RS condition, the inhibitory effect of sleep onset occurred later, resulting in a delay of the nadir by approximately 1 h (NS 01:28 ± 18 min versus RS 02:28 ± 15 min; P = 0.004). Conversely, because wakeup time was advanced in the RS condition, the morning acrophase was advanced (NS 08:09 ± 11 min versus RS 05:56 ± 9 min; P < 0.0001). As in our previous studies of recurrent partial sleep deprivation,11 late afternoon and evening cortisol levels (16:00 to 21:00) were increased in the RS versus NS condition (NS 5.0 ± 0.3 μg/dL versus RS 6.2 ± 0.4 μg/dL; P = 0.011).
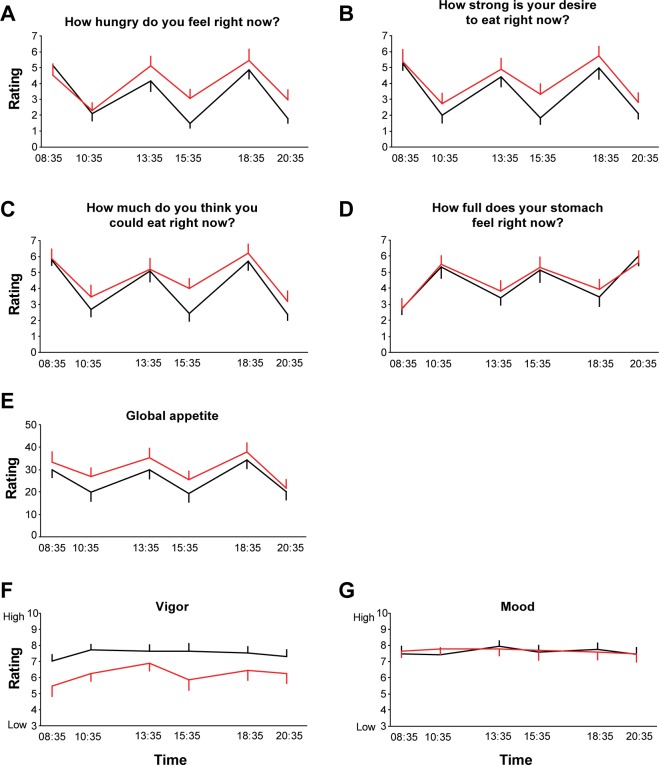
Scores of Hunger, Appetite, Vigor and Mood
Caloric intake was strictly controlled for the entire day of blood sampling and limited to three identical meals. During morning fasting conditions, the ratings of hunger, desire to eat, quantity that could be eaten, fullness, and global appetite did not differ between the NS and RS conditions (Figure 4A–4E). For each scale, the remaining five ratings taken 1 h 35 min after breakfast (10:35), 25 min prior to lunch (13:35), 1 h 35 min after lunch (15:35), 25 min prior to dinner (18:35), and 1 h 35 min after dinner (20:35) were collapsed to derive one mean value for each individual in the NS and RS and conditions. There was no difference between sleep conditions for the mean fullness score (Figure 4D). Despite reporting similar feelings of fullness under both conditions, RS produced a significant increase in ratings of hunger (Figure 4A: NS 2.9 ± 0.3 versus RS 3.8 ± 0.4, P = 0.02), desire to eat (Figure 4B: NS 3.1 ± 0.01 versus RS 3.9 ± 0.4, P = 0.01), quantity of food that could be eaten (Figure 4C: NS 3.7 ± 0.1 versus RS 4.4 ± 0.5, P = 0.05), as well as a trend for an increase in global appetite (Figure 4E: NS 24.7 ± 3.4 versus RS 29.5 ± 3.3, P = 0.07) when compared to the NS condition. When considering all individual time points, in an analysis of variance for repeated measures, the most robust contrast between the RS and NS conditions was for the postlunch (15:35) ratings for all hunger and appetite scales (hunger; P = 0.05, quantity; P = 0.04; strong desire; P = 0.05, global appetite; P = 0.05). As expected, ratings of vigor were significantly lower following RS than NS at all time points (Figure 4F, P < 0.01). Scores of mood were high and nearly identical under both conditions (Figure 4G).
Figure 4.
Ratings of hunger (A), desire to eat (B), quantity of food that could be eaten (C), feeling of fullness (D), global appetite (E), vigor (F), and mood (G) following 3 experimental nights of either 4.5 h (red) or 8.5 h (black) in bed (n = 14). Participants were asked to score visual analog scales 25 min before and 1.5 h after each meal, for a total of six ratings across the day.
Ad libitum Feeding
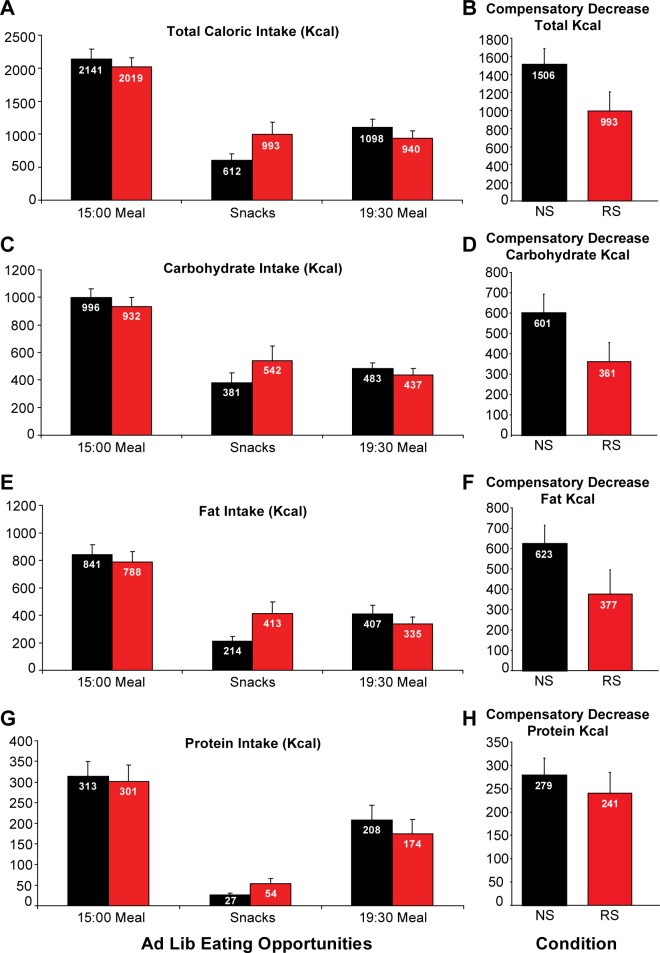
As shown in Figure 5A, when the participants were presented with an ad libitum buffet after either 4 nights of NS or 4 nights of RS, there were no significant differences between the two conditions for total calories eaten during the first meal (NS: 2,141 ± 145 kcal versus RS: 2,019 ± 140 kcal, P = 0.24). It is important to note, however, that although the estimated mean daily caloric need for these participants was 2,313 kcal/day, nearly 90% of this caloric requirement was ingested during this first ad libitum meal in both sleep conditions. Thus, almost all of the daily homeo-static energy need was consumed during the initial meal opportunity. The macronutrient breakdown during this meal was also not affected by sleep duration (Figure 5C, carbohydrate intake: NS 996 ± 65 kcal versus RS 932 ± 65 kcal, P = 0.25; Figure 5E, fat intake: NS 841 ± 76 Kcal versus RS 788 ± 75 kcal, P = 0.31; Figure 5G, protein intake: NS 313 ± 35 kcal versus RS 300 ± 40 kcal, P = 0.52). Thirteen of the 14 participants were considered for analysis of caloric intake during the snack opportunity because one participant was inadvertently not given the same food options in the two sleep conditions. Sleep restriction as compared to NS resulted in a trend for an increase in total caloric intake during the snack period (NS 612 ± 94 kcal versus RS 993 ± 183 kcal; P = 0.085), with consumption of nearly twice as much fat (NS 214 ± 34 kcal versus RS 413 ± 86 kcal; P = 0.062) and protein (NS 27 ± 4 versus RS 54 ± 12; P = 0.072). Carbohydrate consumption was not significantly affected (NS 381 ± 72 kcal versus RS 542 ± 106 kcal; P = 0.218). Most interestingly, the compensatory decrease in food intake after consumption of the first meal (difference between calories ingested during the first ad libitum meal and calories ingested during the subsequent snack period, Figure 5B, 5D, 5F, and 5H) was lower following RS for total caloric intake (NS 1,506 ± 180 kcal versus RS 993 ± 212 kcal; P = 0.02), for carbohydrates (NS 601 ± 93 kcal versus RS 361 ± 94 kcal; P = 0.05), for fat (NS 623 ± 91 kcal versus RS 377 ± 119 kcal; P = 0.05), and to a lesser extent for protein (NS 279 ± 37 kcal versus RS 241 ± 44 kcal; P = 0.07). Thus, although participants ate a meal representing approximately 90% of their daily caloric need in both conditions, they were less able to compensate with a decrease of their subsequent consumption of snacks when they were in a state of sleep debt. We further examined the difference between calories consumed as snacks and kcal intake during the 19:30 meal for both sleep conditions. When sleep restricted, despite ingesting approximately more 50% kcal as snacks, the participants did not reduce significantly their caloric intake during the 19:30 meal (−1 ± 142 kcal, ns). In contrast, under normal sleep conditions, the trend for lower snack intake was compensated with a significant increase in caloric intake at dinner (+511 ± 175 kcal, P = 0.01; difference versus RS: P = 0.073). Thus, the temporal pattern of caloric in-take was modified by sleep loss. Total caloric intake and intake from carbohydrate, fat, and protein during the evening meal were similar for both sleep conditions.
Figure 5.
Caloric intake for each of the ad libitum (ad lib) eating opportunities in the 8.5 h in bed (black) or in the 4.5 h in bed (red) conditions. Eating opportunities included a 15:00 and a 19:30 meal (n = 14), and an intervening period where palatable snacks were presented (n = 13). Data shown are mean (± standard error of the mean). Horizontal panels present Kcal intake for each eating opportunity (left) and compensatory decrease in snack intake after consumption of the 15:00 meal (right) for each sleep condition (back bars: normal sleep; red bars: restricted sleep). From top to bottom: total caloric intake (A,B), carbohydrate intake (C,D), fat intake (E,F), and protein intake (G,H). Please note the difference in eating patterns between the two conditions; in the normal sleep (NS) condition there is a rebound in intake during the dinner meal, however there is no large increase in dinner intake following snack consumption in the RS (restricted sleep) condition. This lack of increase in dinner meal from snack intake is due to high kcal intake during the snack opportunity in the RS group.
DISCUSSION
The current randomized crossover study of sleep restriction versus normal sleep examined the hypothesis that increases in hunger, appetite, and food intake, in a state of sleep debt, are associated with increased activity of the eCB system. This hypothesis was suggested by recent evidence indicating that increases in food intake in conditions of sleep loss clearly exceed the caloric need of extended wakefulness.13–19 The protocol was carefully designed to assess motivation to eat, activity of the peripheral eCB system, and subsequent ad libitum intake of palatable foods. For each sleep condition, caloric intake was rigorously controlled for 4 d, and repeated assessments of subjective hunger and of the simultaneous profile of serum concentrations of the eCB 2-AG were obtained the day following the third experimental night. After the fourth night, the participants were allowed to eat ad libitum from a variety of palatable foods. The novel and important finding of our study is that in a state of sleep debt the amplitude of the robust daily rhythm of circulating concentrations of 2-AG, an endogenous ligand of the CB1 and CB2 receptors, was amplified due to higher and extended peak afternoon concentrations without change in the level or timing of the nocturnal nadir, suggesting that the early afternoon drive for hedonic eating may be stronger and last longer in a state of sleep debt. Simultaneous with the elevation of eCB levels, when the participants were sleep restricted, they reported higher scores of hunger, desire to eat, quantity of food that could be eaten, and appetite, despite reporting feeling as full as when they had normal sleep. These increases in peripheral eCB concentrations could be a mechanism by which recurrent sleep restriction results in excessive food intake, particularly in the form of snacks,13,14 despite minimal increases in energy need. Consistent with this hypothesis, when the participants were faced with an ad libitum buffet in the afternoon following the fourth night of sleep restriction, they were less able to inhibit intake of highly palatable, rewarding snacks despite having ingested 90% of their daily caloric need only 1 to 2 h earlier. This diminished compensatory decrease in snack intake following a large meal in the restricted sleep condition occurred in the late afternoon, coinciding with the time of day when 2-AG levels were most increased relative to the normal sleep condition. The temporal delay of the 2-AG peak, and the persistence of elevated concentrations until the end of day, observed following RS may drive hedonic feeding behavior later in the day in sleep deprived individuals, with adverse consequences on energy homeostasis and weight regulation.48 Indeed, recent data suggest an association among later timing of food intake and weight gain and obesity; animals fed a high fat diet at the “wrong” circadian time gain more weight than controls.48,49 Humans who consume more than 33% of daily energy intake in the evening were twofold more likely to be obese than morning eaters.50 Thus, alterations in the daily rhythm of 2-AG as a result of insufficient sleep may be a contributing factor to increases in hunger, appetite, and food intake associated with a state of sleep debt13–15,18,19 as well as promote evening eating and its adverse metabolic consequences.
Evidence is accumulating to indicate that recurrent sleep restriction disrupts the neuroendocrine control of energy homeostasis.39,40,46,51 Although the findings have not been consistent across all studies, increases in the concentrations of ghrelin, a hunger factor, and decreases in the levels and/or rhythm amplitude of the satiety hormone leptin have been observed in multiple studies.52–54 In the current study, despite the short duration and moderate severity of sleep restriction, the ratio of the ghrelin peak to the leptin peak showed a significant increase following restricted sleep, signaling a shift in neuroendocrine signaling of energy balance consistent with promoting less satiety and more hunger. Daytime levels of ghrelin and leptin were not significantly affected, although a trend for higher postmeal ghrelin levels was apparent. The alterations in the 24-h profiles of 2-AG, leptin, and ghrelin observed after insufficient sleep in the current study are consistent with the hypothesis that stimulation of hedonic eating may precede activation of pathways involved primarily in the control of homeostatic feeding.
Circulating levels of cortisol were higher in the late afternoon following RS, in congruence with previous studies.11 This elevation of cortisol concentrations in the later part of the day was concomitant with the increased concentrations of 2-AG and occurred without any change in mood scores. In exploratory analyses, the increase in late afternoon cortisol concentrations following RS was not correlated with the increase in 2-AG levels.
In the current study, concentrations of 2-OG displayed a profile similar to that observed for 2-AG over the 24-h period, although blunted in comparison, under both sleep conditions. Because 2-OG is a structural analog of 2-AG, and is biosynthesized and degraded by the same enzymes as 2-AG,45 the similarity in findings for 2-AG and 2-OG reinforces the validity of our assessments of the eCB system.
Our study has obvious limitations, including the small sample size, the short duration of sleep restriction, and the relatively low frequency of sampling (i.e., hourly) of eCB concentrations that precluded an estimation of putative pulsatile variations. The protocol does not address the important issue of whether the observed daily variation of eCB levels is partly or largely produced by behavioral time cues, such as the sleep-wake cycle or the feeding schedule. Nonetheless, our findings are relevant to normal life conditions and are clearly signifi-cant and consistent with the epidemiologic evidence.
A growing body of evidence suggests that insufficient sleep may be contributing to the increased risk and prevalence of obesity. Our current observations suggest that alterations in the eCB system may be contributing to the increased drive for food intake observed following sleep restriction, consistent with recent theories suggesting that overweight or obesity are, in part, the consequence of dysregulation in reward pathways. Increased understanding of the mechanisms linking insufficient sleep and the risk of weight gain is important to design preventive strategies.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by Grant Number KL2RR025000 from the National Center for Research Resources to ECH, contract W81XWH-07-2-0071 from the Department of Defense Peer Reviewed Medical Research Program to EVC, RO1 HL-075079 to ET, The Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin to KS, ED, and CJH, and the University of Chicago Institute for Translational Medicine supported by UL1 RR024999. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the Department of Defense, or the National Institutes of Health. The authors have indicated no financial conflicts of interest. Endocannabinoid assays were conducted at the Medical College of Wisconsin. All other aspects of the study were conducted at the University of Chicago.
ABBREVIATIONS
- 2-AG
2-arachidonoylglycerol
- 2-OG
2-oleoylglycerol
- eCB
endocannabinoid
- NS
normal sleep
- RS
restricted sleep
REFERENCES
- 1.Fryar C, Carroll M, Ogden C. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-1962 through 2009-2010. 2012 NCHS Health E-Stat: National Center for Health Statistics. [Google Scholar]
- 2.National Sleep Foundation. Washington, DC: National Sleep Foundation; 2009. Sleep in America Poll: Health and Safety. [Google Scholar]
- 3.National Sleep Foundation. Washington, DC: National Sleep Foundation; 2008. Sleep in America Poll: Sleep, Performance and the Workplace. [Google Scholar]
- 4.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159:S59–66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Bonuck K, Chervin RD, Howe LD. Sleep-disordered breathing, sleep duration, and childhood overweight: a longitudinal cohort study. J Pediatr. 2015;166:632–9. doi: 10.1016/j.jpeds.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 13.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98:1433–9. doi: 10.3945/ajcn.113.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 20.Fanelli F, Di Lallo VD, Belluomo I, et al. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. J Lipid Res. 2012;53:481–93. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 24.Bermudez-Silva FJ, Cardinal P, Cota D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J Psychopharmacol. 2011;26:114–24. doi: 10.1177/0269881111408458. [DOI] [PubMed] [Google Scholar]
- 25.Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 26.Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melis T, Succu S, Sanna F, Boi A, Argiolas A, Melis MR. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett. 2007;419:231–5. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 29.De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology. 2011;63:161–8. doi: 10.1016/j.neuropharm.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanlon E, Tasali E, Leproult R, et al. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab. 2015;100:220–6. doi: 10.1210/jc.2014-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39:5–41. [PubMed] [Google Scholar]
- 32.Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405–15. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 33.Fernstrom MH, Krowinski RL, Kupfer DJ. Appetite and food preference in depression: effects of imipramine treatment. Biol Psychiatry. 1987;22:529–39. doi: 10.1016/0006-3223(87)90180-6. [DOI] [PubMed] [Google Scholar]
- 34.Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 35.Iber C, Ancoli-Israel S, Chesson AJ, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 36.Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel S, Carrier EJ, Ho WS, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–9. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 39.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96:486–93. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 41.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305:2173–4. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broussard JL, Chapotot F, Abraham V, et al. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 2015;58:791–8. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleberg K, Hassing HA, Hansen HS. Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors. 2014;40:363–72. doi: 10.1002/biof.1158. [DOI] [PubMed] [Google Scholar]
- 46.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 47.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 48.Garaulet M, Gomez-Abellan P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2013;27:255–62. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 51.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108:15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killick R, Banks S, Liu PY. Implications of sleep restriction and recovery on metabolic outcomes. J Clin Endocrinol Metab. 2012;97:3876–90. doi: 10.1210/jc.2012-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diabetes Rept. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copinschi G, Leproult R, Spiegel K. The important role of sleep in metabolism. Front Horm Res. 2014;42:59–72. doi: 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.