Abstract
Study Objectives:
Our understanding of the role of neurotransmitters in the control of the electroencephalogram (EEG) has been entirely based on studies of animals with bilateral sleep. The study of animals with unihemispheric sleep presents the opportunity of separating the neurochemical substrates of waking and sleep EEG from the systemic, bilateral correlates of sleep and waking states.
Methods:
The release of histamine (HI), norepinephrine (NE), and serotonin (5HT) in cortical and subcortical areas (hypothalamus, thalamus and caudate nucleus) was measured in unrestrained northern fur seals (Callorhinus ursinus) using in vivo microdialysis, in combination with, polygraphic recording of EEG, electrooculogram, and neck electromyogram.
Results:
The pattern of cortical and subcortical HI, NE, and 5HT release in fur seals is similar during bilaterally symmetrical states: highest in active waking, reduced in quiet waking and bilateral slow wave sleep, and lowest in rapid eye movement (REM) sleep. Cortical and subcortical HI, NE, and 5HT release in seals is highly elevated during certain waking stimuli and behaviors, such as being sprayed with water and feeding. However, in contrast to acetylcholine (ACh), which we have previously studied, the release of HI, NE, and 5HT during unihemispheric sleep is not lateralized in the fur seal.
Conclusions:
Among the studied neurotransmitters most strongly implicated in waking control, only ACh release is asymmetric in unihemispheric sleep and waking, being greatly increased on the activated side of the brain.
Commentary:
A commentary on this article appears in this issue on page 491.
Citation:
Lyamin OI, Lapierre JL, Kosenko PO, Kodama T, Bhagwandin A, Korneva SM, Peever JH, Mukhametov LM, Siegel JM. Monoamine release during unihemispheric sleep and unihemispheric waking in the fur seal. SLEEP 2016;39(3):625–636.
Keywords: unihemispheric sleep, norepinephrine, histamine, serotonin, acetylcholine, fur seal, marine mammals
Significance.
A number of neurotransmitters have been shown to be released at greater levels in waking than in sleep. It has been assumed that these neurotransmitters work together to produce the forebrain and brainstem activation that underlies waking. However, another possibility is that these “waking neurotransmitters” have roles, unrelated to EEG arousal. Examining the fur seal, an animal that can sleep with one side of the brain at a time, we found that the release of norepinephrine, histamine and serotonin are minimal and bilaterally symmetric when one side of the brain is asleep and the other awake. Of the transmitters most strongly implicated in waking control, only acetylcholine was elevated on the waking side. This finding provides a fundamental insight into the neurochemistry of waking and sleep.
INTRODUCTION
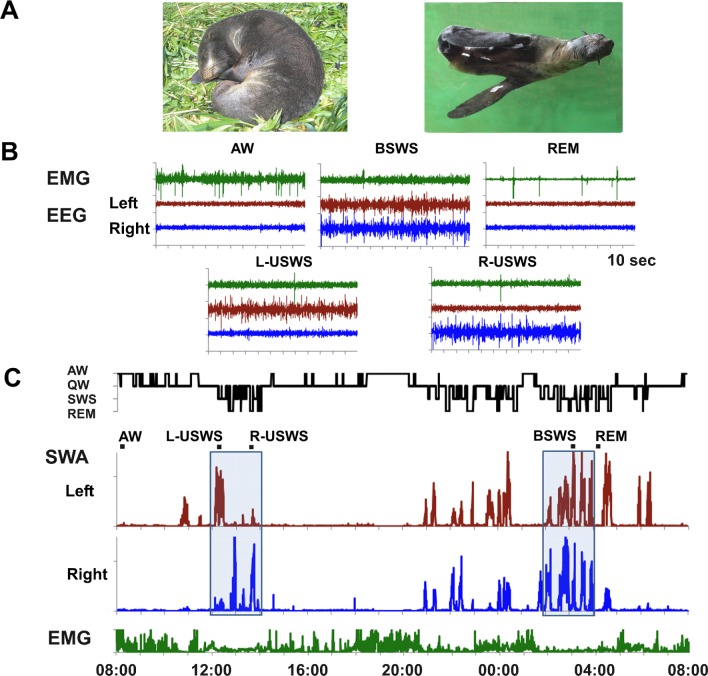
Cetaceans (whales and dolphins) never show bilateral slow waves as seen in most land mammals. Instead they display only unihemispheric sleep (USWS), with the other hemisphere awake (UW). Cetaceans sleep with one eye closed while the other eye is open. Cetaceans also do not appear to exhibit rapid eye movement (REM) sleep.1,2 Fur seals (pinnipeds of the family Otariidae) are semiaquatic mammals. They sleep both on land and in water (Figure 1A). Fur seals have bilateral slow wave sleep (BSWS) and REM sleep as seen in land mammals and USWS/UW as seen in Cetaceans. When sleeping in water, the fur seal sleeps at the surface on the side holding two hind flippers and one fore flipper above the animal in air to reduce heat loss. The fore flipper in water is active and helps to maintain the animal's posture. The fur seal also positions the head and nostrils above the surface to allow regular breathing. During USWS the hemisphere, which is contralateral to the active flipper, is always in a “waking” (or “activated”) state, characterized by the low voltage EEG pattern typical of waking in all mammals. When sleeping on land the fur seal is motionless lying or sitting on the ground. Both on land and in water, during USWS fur seals often open one eye (contralateral to the waking hemisphere) while the other eye contralateral to the “sleeping hemisphere (the hemisphere with a higher voltage EEG slow wave activity) remains tightly closed.3–5 It has been suggested that maintenance of waking in one hemisphere while in USWS enables vision, movement and allows breathing, while minimizing respiratory aspiration of water in fur seals and cetaceans. It also facilitates thermoregulation, protection of neonates, and predator avoidance.1 Some avian species also display short episodes of EEG asymmetry.6,7
Figure 1.
Unihemispheric and bilateral slow wave sleep in the fur seal. (A) Sleep postures in the fur seal. When on land, fur seals usually sleep while lying on their sides. When in water, they sleep at the surface on their sides, paddling with one fore flipper, while holding the other three flippers above the water to maintain their postures. (B) Electroencephalogram (EEG) and electromyogram (EMG) features of active wakefulness (AW), bilateral slow wave sleep (BSWS), rapid eye movement (REM) sleep and left and right unihemispheric sleep (L-USWS, R-USWS). Top and bottom rows are bilateral and asymmetrical states, respectively. (C) Behavioral states, slow wave activity (SWA, EEG power in the range of 1.2–4 Hz) in the two brain hemispheres and integrated neck EMG in a fur seal recorded over a 24-h period while on land. Two boxes highlight a daytime period of USWS (one episode of L-USWS and two episodes of R-USWS) and nighttime period of predominantly BSWS. Two-min polygrams displayed in B are episodes marked in C.
Our knowledge on the neurotransmitters involved in the promotion and maintenance of waking and sleep states has been derived from species with bilaterally symmetrical EEG states, including humans, monkeys, cats, rats, and mice. However, such studies do not indicate whether these transmitters are released in relation to the waking state itself, with its tonic activation of the EEG, or whether the relation is to autonomic control, to motor activity or to emotions occurring within the waking state. Studies of USWS in aquatic mammals allow us to determine which of the many physiological and neurochemical changes seen bilaterally in terrestrial mammals are linked to the EEG-defined state, and which may be related to the behavioral quiescence, cardiorespiratory changes, and sensory input reduction that typically accompany sleep. Our understanding of the mechanisms of USWS in cetaceans is poor due to the obvious difficulty of experimental studies on dolphins and whales. Considering the similarity between the pattern of sleep in cetaceans and otariids, the fur seal appears to be the ideal species to investigate mechanisms of USWS using the tools of experimental physiological research.
In prior studies we found that cortical acetylcholine (ACh) release in fur seals was maximal during active waking (AW), minimal during BSWS, and intermediate both during quiet waking (QW) and REM sleep.8 This pattern is similar to that described in land mammals.9,10 During USWS, cortical ACh release was strongly lateralized in fur seals, with greater levels in the hemisphere displaying “waking” (lower voltage EEG activity).8 In a separate study we showed that that cortical serotonin (5HT) in fur seals release was maximal during AW, progressively decreased during transition to QW and further during BSWS, and that the release was minimal during REM sleep. In contrast to Ach, cortical 5HT release was not lateralized during USWS in the fur seal.11 The current study was undertaken to measure cortical and subcortical release of monoamines that have been implicated in the control of waking—histamine (HI), norepinephrine (NE), and 5HT.
METHODS
Animals
All procedures were approved by the University of California Los Angeles and the Veterans Affairs Greater Los Angeles Healthcare System Committees. All studies were conducted in accordance with the National Institute of Health Guide for the Care and Use of Experimental Animals. Experiments were performed at the Utrish Marine Station of the Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences on the Black Sea, Russia. Data were collected from 2 groups of juvenile northern fur seals (Callorthinus ursinus L.), a total of 8 animals (5 males and 3 females, 18–25 kg, 2–3 years). Fur seals were captured on the Commander Islands (the Western Pacific, Russia) one year before the study and were well adapted to captivity. Five days before surgery, each fur seal to be studied was moved to an empty indoor laboratory pool and placed into the microdialysis recording chamber (1.1 × 0.8 × 0.8 m) for 4–6 h each day for 4 days. Fur seals were fed 1.5 kg fish twice per day (between 8:00 and 09:00 and 18:00 to 19:00) and sprayed with water for 10 min after each feeding. Seals typically approached the water stream and appeared to enjoy the spray, which is an accepted husbandry practice for these marine mammals. During the daytime (8:00 to 20:00), the enclosure was illuminated by artificial light (400–500 lux at floor level); at night the level of illumination was reduced to ≤ 50 lux. Room temperature during recording ranged from 15–25°C, following the adjacent Black Sea environment.
Surgical Procedures
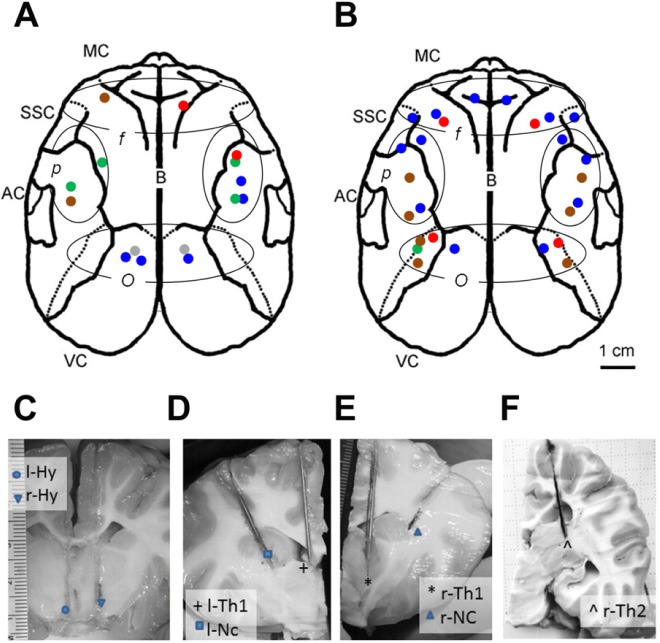
Surgical procedures have been previously described in detail.3–5,8,11 Briefly, in addition to EEG, EMG (electromyogram), and EOG (electrooculogram) electrodes, 1 to 4 pairs of guide cannulas sealed with stylets (CMA Guide Cannulas, CMA Microdialysis AB, Solna, Sweden) were implanted in symmetrical locations in each hemisphere or in subcortical areas (Figure 2). The length of the cortical guide cannulas was 12 mm and the length of subcortical cannulas was 40, 45, or 50 mm. After implantation, the animal was returned to the indoor enclosure and allowed a minimum of 5 days to recover before insertion of the microdialysis probes through the guide cannulas.
Figure 2.
Localization of microdialysis probes in fur seals. (A,B) Reconstructed schematic illustration showing the localization of microdialysis probes used to collect for histamine (HI, n = 15, 5 seals) and norepinephrine (NE, n = 26, 4 seals) in the cerebral cortex of fur seals. Dotted outlines demark the main cortical areas: motor cortex (MC), auditory cortex (AC), somatosensory cortex (SSC), and visual cortex (VC). Ovals demark 3 groups of microdialysis probes based on their localizations in the cortex: f, frontal, p, parietal, o, occipital. Each color represents an individual seal. (C) Localization of left and right probes in the posterior hypothalamus in the area of n. hypothalamus anterior and n. hypothalamus paraventricularis (l-Hy) and in the area of fornix and n. supraopticus (r-Hy) in seal number 3. (D) Localization of left thalamus probe (l-Th1; the dorsal part of medial thalamus) and of left caudate nucleus probe (l-Nc; the anterior part of caudate nucleus) in fur seal number 7. (E) Localization of right thalamus probe (r-Th1; the area of ventral anterior nucleus) and of right caudate nucleus probe (r-Nc; lateral part of caudate nucleus along the external capsule) in seal number 7. (F) Localization of right thalamus probe (r-Th2: the pulvinar, posterior thalamus) in seal number 8.
Microdialysis Procedure
On the morning of the experiment, the fur seal was lightly anesthetized with isoflurane to replace the stylets with micro-dialysis probes. The procedure, lasting on average 1.0 h, was conducted at the 0.5% level of isoflurane following a 5–10 min 1.5–2.0% induction dose. Cortical probes had a 4 mm long semi-permeable polyarylethersulfone membrane (0.5 mm diameter, 20 kDa cut-off; CMA-12 Elite Microdialysis Probe; CMA Microdialysis AB). All probes were 4 mm longer than the guide cannulas so that the membrane was fully projected out of the guide cannula. Shielded Teflon tubing (1.5 m, TJT-10-150HS; Eicom Corporation, Kyoto, Japan) encased within an additional protective sleeve was connected to the inlet and outlet of each probe. While under anesthesia, the fur seal was connected to the polygraph via a low noise cable. After the implantation procedure, the seal was placed in the recording chamber where it remained unrestrained for the duration of the experiment. Fur seals quickly recovered from anesthesia. They did not show any signs of behavioral impairment by 1 h after the cessation of anesthesia. This short anesthetic procedure did not have significant effect on the parameters of sleep during the recording period, including EEG slow wave power in the range of 1.2–4.0 Hz (slow wave activity, SWA) in the right and left hemispheres and the degree of EEG asymmetry (Figure S1, supplemental material).
The tubing from each probe inlet was connected to a syringe pump (ESP-64; Eicom) and the microdialysis probe was per-fused with aCSF (Perfusion Fluid CNS: 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.85 mM MgCl2; CMA Microdialysis AB) at a rate of 1.0 μL/min for NE assays and 1.5 μL/min for HI. The tubing from each probe outlet was connected to a fraction collector (EFC-82; Eicom). After a 4-h stabilization period, samples were collected every 10 min over the course of 42 h, on average. Prior to sample collection, 5.0 μL of anti-oxidant solution (20 mM phosphate buffer, pH 3.5, containing 25 mM EDTA-2Na) was added to each sample vial for NE and 7.5 μL (30 mM phosphate buffer, pH 3.1, containing 100 μM EDTA-2Na) for HI. During collection, samples (15 μL or 22.5 uL for NA and HA, respectively) were kept at 4°C (EFR-82 Cooling Unit; Eicom) and each hour they were transferred to a −80°C freezer and stored until analyzed. Probe placements within cortex and subcortical sites were verified histologically.12,13
Assay of Histamine
Cortical samples were assayed for HI using high-performance liquid chromatography (HPLC) coupled with post-column derivatization and fluorescence detection. Samples maintained at 4°C were injected into the HPLC system using an autosampler (Three pump prototype Autosampling Injector; Eicom). The mobile phase, consisting of 0.1 M phosphate buffer (pH 4.5) containing sodium-1-octanesulfonate (170 mg/L; Nacalai Tesque), EDTA-2Na (50 mg/L; Dojindo Laboratories), and 10% methanol was delivered at a rate of 500 μL/min. HI was separated on a reversed-phased column [Eicompak SC-5ODS column (3.0 ID × 150 mm)] maintained at 40°C (CTC-100 Water Circulator; Eicom). The column eluate was derivatized in a reaction coil maintained at 40°C with OPA (80 mg/L; flow rate 100 μL/min) and 0.5 mol/L K2CO3 (flow rate 100 μl/min) to form a fluorescent derivative. The amount of HI in the reaction mixture was determined using a fluorescence detector with excitation and emission wavelengths of 340 nm and 450 nm, respectively (GL-7453 FL Detector; GL Sciences, Tokyo, Japan). The signal from the detector was recorded using a data acquisition system (EPC-280; Eicom) and analyzed using PowerChrom software (eDAQ). Before each experiment, the HPLC system was tested for linearity and sensitivity using 6 concentrations of HI ranging from 5 fmol (detection limit for HI with a 3:1 signal-to-noise ratio) to 1.5 pmol per injection (16 μL). Every 8 h during sample analysis, the HPLC system was calibrated using an external standard containing a known concentration of HI. Quantification of HI in each dialysate was determined by comparing the sample peak height to the calibration curve generated from the HI standards. Representative chromatograms of HI peaks obtained from samples collected across the sleep-wake cycle are presented in Figure 3A.
Figure 3.
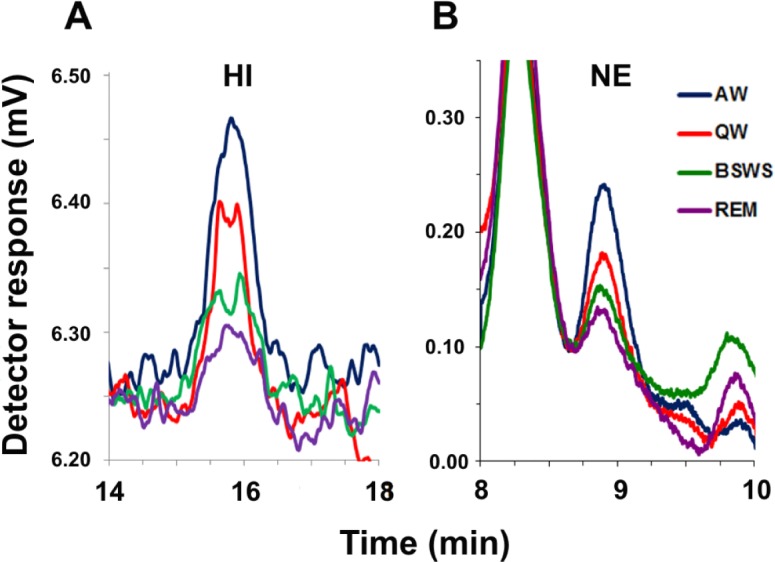
Chromatograms of histamine (HI) and norepinephrine (NE) in fur seals. (A,B) Typical chromatograms displaying the level of HI and NE in dialysates collected during bilaterally symmetrical EEG states in fur seals. AW, active waking; QW, quiet waking; BSWS, bilateral slow wave sleep; REM, rapid eye movement sleep. HI eluted at a retention time of ∼16 min and NA eluted at a retention time of ∼8.9 min.
Assay of Norepinephrine
Cortical and subcortical samples were assayed for NE using high-performance liquid chromatography (HPLC) coupled with electrochemical detection (HTEC-500; Eicom). Samples maintained at 4°C were injected into the HPLC system using an autosampler (832 Temperature Regulator, 402 Syringe Pump, 231 XL Sample Injector; Gilson). The mobile phase, consisting of 0.1 M phosphate buffer (pH 6.0) containing sodium-1-octanesulfonate (400 mg/L; Nacalai Tesque), EDTA-2Na (50 mg/L; Dojindo Laboratories), and 5% methanol, was filtered through a guard (pre) column (PC-03 with CA-ODS packing material; Eicom) and delivered at a rate of 230 μL/min. NE was separated on a reversed phased column (CA-5ODS column ([2.1 ID × 150 mm]) maintained at 25°C. The amount of NE was electrochemically detected by a graphite working electrode (WE-3G; Eicom) set to +450 mV against an Ag/AgCl reference electrode (RE-500). The signal from the detector was recorded and analyzed using PowerChrom software (eDAQ). Before each experiment, the HPLC system was tested for linearity and sensitivity using 10 concentrations of NE ranging from 50 fg (detection limit for NE with a 3:1 signal-to-noise ratio) to 10 pg per injection (12 μL). Every 8 h during sample analysis, the HPLC system was calibrated using an external standard containing a known concentration of NE. Quantification of NE in each dialysate was determined by comparing the sample peak height to the calibration curve generated from the NE standards. Representative chromatograms of NE peaks obtained from samples collected across the sleep-wake cycle are presented in Figure 3B.
Assay of Serotonin
Subcortical samples were assayed for 5HT using high-performance liquid chromatography (HPLC) coupled with electro-chemical detection (HTEC-500; Eicom) as described in our prior study of cortical release.11
Electroencephalogram Recording and Data Analysis
Electrophysiological parameters were recorded using a 16-channel amplifier (Medicor, Hungary or A-M Systems 3500, USA), an ADC-convertor (Power 1401 and Spike 2, CED, Great Britain), and then analyzed as described in detail previously.4,5,8,11 Briefly, for each hemisphere, EEG was visually scored in 20-s epochs as (1) desynchronization (i.e., low amplitude, high frequency waves, stage 1 EEG), (2) low-voltage slow waves (0.5–4.0 Hz) and spindles (stage 2 EEG), or (3) high-voltage slow waves (stage 3 EEG). Waking was characterized by sustained muscle tone and bursts of muscle activity and by stage 1 EEG in both hemispheres. AW was scored when the animal was moving around the recording cage or grooming. Two additional active behaviors were analyzed separately: AW-feeding was scored when the seal was being fed and AW-hosing when the animal was being hosed down with water. QW was scored when none of the above behaviors was occurring in the waking animal, typically when the seal was lying down or sitting either motionless or with occasional shifts in body position during which time its eyes could be open or closed. REM sleep was scored when a desynchronized EEG typical of waking (i.e., stage 1 EEG) in both hemispheres was accompanied by a significant reduction in EMG tone or full atonia, muscle jerks, facial twitches, and rapid eye movements. BSWS was scored when stage 2 and/or stage 3 EEG appeared simultaneously in both hemispheres along with lower or similar amplitude EMG activity as seen during QW. Left or right USWS was scored when stage 3 EEG occurred in one hemisphere and stage 2 or stage 1 EEG occurred in the other hemisphere, or when stage 2 EEG occurred in one hemisphere and stage 1 EEG occurred in the other hemisphere. When in slow wave sleep (SWS) fur seals were usually lying on the chamber floor on their sides or on the bellies. Occasionally slow waves started with the seal in the sitting position. However, both high voltage BSWS and USWS always developed in these experiments while the animals were lying down. REM sleep was always recorded when the animals were lying with their heads resting on the floor.
For each hemisphere, EEG spectral power in the frequency range of 1.2–4 Hz (SWA) was computed in consecutive 5-s epochs by fast Fourier transformation using Spike 2 software. Epochs containing artifacts were excluded from spectral analysis. The average SWA in each hemisphere was then calculated for each sample. To estimate the expression of interhemispheric EEG asymmetry during BSWS, left and right USWS, we used the asymmetry index (AI = (L − R) / (L + R), where L and R were standardized spectral powers in the range of 1.2–4.0 Hz in the left and right hemispheres (SWA), respectively; spectral power in each hemisphere was divided by the average power in the same hemisphere of the same frequency range during QW.5 Thus, the absolute value of AI is a numerical measure of the expression of EEG asymmetry, while the sign is an indication of lateralization of SWA (minus and plus indicates left or right dominance of EEG slow wave power, respectively). The percentage of time spent in each behavioral state was calculated for each sample.
Correlating Concentration of Neurotransmitters and Polygraphic Data
Dialysates were classified as AW-hosing, AW-feeding, AW, QW, BSWS, or REM sleep samples when a single behavioral state occupied ≥ 75% of the sampling interval. When SWS occupied ≥ 75% of the sampling interval but < 25% of it was BSWS, the sample was classified as either left of right USWS (or left and right UW). All remaining samples were classified as mixed state.
Neurotransmitter release was compared during (1) bilaterally symmetrical EEG states: AW, QW, BSWS, and REM sleep, (2) various waking behaviors (AW-hosing, AW-feeding, AW, and QW), and 3) asymmetrical EEG states (left and right USWS).
Mean levels of neurotransmitters (HI or NE) in the cortex during waking and sleep states were calculated for each cortical probe. The analyses revealed that during waking states cortical HI and NE levels were not significantly different between three cortical areas (frontal, parietal and occipital) in fur seals (see Results). For this reason, we have combined data across cortical areas. Statistical tests were then used to evaluate the difference between behavioral states for all cortical probes or separately for probes in the left and right hemispheres using mean values for each probe. When evaluating the effect of behavioral state, we used the probes for which microdialysis samples had been collected for each of the listed states (bilaterally symmetrical EEG states, waking behaviors and asymmetrical EEG states). The pattern of neurotransmitter release in all subcortical areas (the thalamus, hypothalamus, and caudate nucleus) was similar for each transmitter, as it was within each cortical hemisphere. For this reason, we have combined data for the corresponding subcortical sites (the thalamus, hypothalamus, or caudate nucleus). The concentration of neurotransmitter in each sample was normalized to the mean value during QW and BSWS (calculated for all samples of the given probe).
Statistical Analysis
All statistical analyses were performed using Sigma Plot 11. Data were assessed for statistical significance using one-way ANOVA followed by Tukey's post hoc multiple-comparison tests, T-test or Pearson product moment correlation test. Values are given as mean ± SEM.
RESULTS
In agreement with prior studies,3–5 when sleeping on land (in experimental chambers) fur seals displayed both BSWS and SWS with interhemispheric EEG asymmetry. This type of sleep is usually called unihemispheric sleep, unihemispheric slow wave sleep (USWS),1,2,6,7 or asymmetrical SWS.4,5,8,11 USWS is seen in concert with UW in the contralateral hemisphere (Figure 1B, 1C; and Figure S2, supplemental material). In this study more sleep (both SWS and REM sleep) occurred in fur seals during the nighttime. However, both the amplitude of EEG slow waves (SWA) in the sleeping hemisphere and the expression of EEG asymmetry during USWS were comparable during the daytime and nighttime periods (Figure S1).
Localization of Microdialysis Probes
Histamine Probes
The HI probes were located within 3 cortical areas: frontal (n = 2), parietal (n = 8), and occipital (n = 5). Six hundred twenty-five samples collected from 15 cortical probes (7 left and 8 right; Figure 2A) in 5 fur seals (3 males and 2 females, seals numbers 1–5) met the criteria described for non-mixed state. These samples consisted of 29 AW-hosing, 33 AW-feeding, 111 AW, 197 QW, 156 BSWS, 23 left USWS, 47 right USWS, and 29 REM sleep samples.
Norepinephrine Probes
Twenty-six microdialysis probes in a total of 4 seals (3 males and 1 female, seals numbers 3–6) used to collect for cortical NE were located within three cortical areas: frontal (n = 8), parietal (n = 10), and occipital (n = 8). One thousand forty-nine samples collected from a total of 26 cortical probes met the criteria described for non-mixed state (Figure 2B). These samples consisted of 50 episodes of AW-hosing, 43 AW-feeding, 205 AW, 357 QW, 248 BSWS, 49 left USWS, 32 right USWS, and 65 REM sleep. One pair of guide cannulas were also implanted in one seal (male, seal number 3) in the posterior hypothalamus: one (right) probe was located in the area of n. paraventricularis/n. anterior and the second (left) probe was located in the vicinity of fornix/n. supraopticus (Figure 2C). Ninety samples collected from these 2 probes consisted of 2 episodes of AW-hosing, 4 AW-feeding, 9 AW, 16 QW, 33 BSWS, 8 left USWS, 4 right USWS, and 14 REM sleep samples.
Serotonin Probes
A total of 5 subcortical probes were implanted in 2 seals (one male and one female, seals number 7–8) targeting the thalamus and caudate nucleus (Figure 2D–2F). The first probe was located on the left side in the dorsal part of medial thalamus (l-Th1, seal number 7, Figure 2D). The second probe was located on the right side in the ventral anterior nucleus (r-Th1, seal number 7, Figure 2E) and the third probe in the posterior thalamus/pulvinar (r-Th2, seal number 8, Figure 2F). Another 2 probes were located in the ventral and lateral part (adjacent to the external capsule) of the caudate nucleus (adjacent to the external capsule) (l-Nc, r-Nc, respectively; seal number 7, Figure 2D and 2E). Two hundred thirty-five samples were collected from the 3 thalamic probes. They consisted of 97 AW (including 5 AW-hosing and 10 AW-feeding), 68 QW, 28 BSWS, 20 left USWS, and 22 right USWS episodes. One hundred thirty-seven samples were collected from 2 caudate nucleus probes. They consisted of 22 AW, 49 QW, 23 BSWS, 19 left USWS, 22 right USWS, and 2 REM sleep episodes.
As revealed by ANOVA for each of 6 bilaterally symmetrical states (AW-feeding, AW-hosing, AW, QW, BSWS, and REM sleep) the release of both neurotransmitters did not depend on the location of probes within the cortex (in all cases P > 0.05). In all experiments used for the analysis at least a portion of 4 mm long active part of the membrane was located within the gray matter of the cerebral cortex.
Histamine, Norepinephrine, and Serotonin Release Varies with Sleep-Waking State
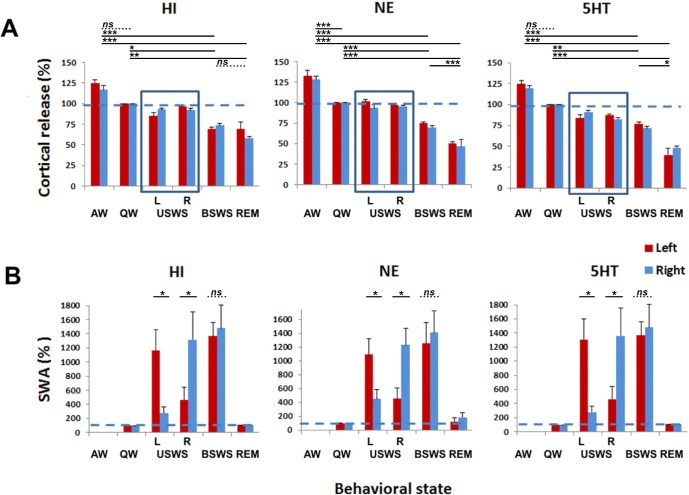
During bilaterally symmetrical EEG states (AW, QW, BSWS, and REM sleep), mean cortical HI release in the fur seal was state-dependent (one-way ANOVA with repeated measures; F 3,11 = 15.951; P < 0.001). Mean HI levels were at 122 ± 3% of QW during AW, 72 ± 2% during BSWS and 63 ± 4% of QW during REM sleep (Figure 4). The Tukey test indicated that the release of HI was significantly different when waking states were compared to both sleep states (AW vs. BSWS and AW vs. REM sleep, both P < 0.001; QW vs. BSWS P = 0.021 and QW vs. REM sleep P = 0.002). The release of HI did not significantly differ between AW and QW or between BSWS and REM sleep (P > 0.05).
Figure 4.
Cortical histamine (HI), norepinephrine (NE) and serotonin (5HT) release during bilaterally symmetrical and asymmetrical states in the fur seal. (A) Cortical HI, NE and 5HT release across the sleep-wake cycle in fur seals. AW, active waking; QW, quiet waking; USWS, unihemispheric slow wave sleep; in the left (L) and right (R) hemispheres; BSWS, bilateral slow wave sleep; REM, rapid eye movement sleep. Each column represents the mean ± SEM of the percent change of HI, NE, and 5HT relative to QW for left and right probes. There was no difference between HI, NE, and 5HT release in the left and right cortical hemispheres for all behavioral states in fur seals including left and right USWS (highlighted by boxes). However, the mean release in the two hemispheres of all 3 neurotransmitters was significantly different between bilateral states (P < 0.05 for all pairs, the post hoc Tukey test after one way ANOVA with repeated measures) except for AW vs. QW for HI and 5HT and BSWS vs. REM sleep for HI and for AW vs. QW for 5HT (P > 0.05 in all cases). The data for cortical 5HT release were collected during a prior study.11 (B) Slow wave activity (SWA; EEG power in the frequency range of 1.2–4.0 Hz) across the sleep-wake cycle in fur seals. Each column represents the mean ± SEM of the percent change in SWA relative to QW. Mean SWA did not differ between two hemispheres during BSWS but it was significantly different between two hemispheres during right and left USWS as determined by power analysis (Table S1, Figure S2, supplemental material). *P < 0.05; **P < 0.01; ***P < 0.001; ns, nonsignificant (P > 0.05).
During bilaterally symmetrical EEG states (AW, QW, BSWS, and REM sleep), mean cortical NE release in the fur seal was also state-dependent (F3,23 = 21.68; P < 0.001). Mean NE levels were at 132 ± 4% of QW levels during AW, at 73 ± 1% during BSWS, and 48 ± 2% during REM sleep. The release of NE was significantly different between all behavioral states (AW vs. QW, BSWS, and REM; QW vs. BSWS and REM sleep; BSWS vs. REM sleep; P < 0.001 for all states).
The pattern of release of cortical HI and NE across AW, QW and BSWS in fur seals was similar (Figure 4). However, during the transition from BSWS to REM there was a more pronounced decrease of NE (on average 34% relative to BSWS values) compared to HI (12% relative to BSWS values). Additional analysis revealed that mean REM to BSWS sleep release ratio of HI was 0.87 ± 0.05 (n = 12 probes, the decrease was significant with P = 0.03, paired t-test), while for NE and 5HT those values were smaller and both identical in magnitude: 0.66 ± 0.02 for NE (n = 24, P < 0.0001) and 0.65 ± 0.05 for 5HT (n = 9, P = 0.0002). ANOVA further demonstrated that the decrease of NE and 5HT during the transition from BSWS to REM was significantly more pronounced than the decrease of HI (F2,42 = 11.370, P < 0.001; P < 0.001 for HI vs. NE and P = 0.002 for HI vs. 5HT; P = 0.998 for 5HT vs. NE).
The pattern of NE and 5HT release during transitions from AW to QW, and from BSWS to REM sleep was also evaluated in 7 subcortical locations (Figure 5A). As with cortical release, mean NE release in the hypothalamus and 5HT release both in the caudate nucleus and in the thalamus were state dependent (ANOVA, F3,61 = 18.061, F3,80 = 19.851, F 2,154 = 25.009, respectively; P < 0.001 in all cases). For both neurotransmitters and at all sites the release was significantly higher during AW when compared to QW, BSWS, and REM (P < 0.05). The difference between other states was not significant (P > 0.05).
Figure 5.
Subcortical norepinephrine (NE) and serotonin (5HT) release in the fur seal. (A) Subcortical NE and 5HT release during main behavioral states in fur seals. (B) Subcortical NE and 5HT release during certain waking activities in fur seals. Each column represents the mean ± SEM of the percent change in NE and 5HT relative to quiet wakefulness (QW). AW, active wakefulness; BSWS, bilateral slow wave sleep; USWS, unihemispheric slow wave sleep; in the hemisphere with a higher voltage EEG slow wave activity (S, “sleeping” hemisphere), or with a low voltage activity desynchronized activity (W, “waking” hemisphere); REM, rapid eye movement sleep; AW-hose, hosing seals down with water; AW-feed, feeding fish; AW, other active waking behaviors. The means were calculated for all combined samples collected from 2 hypothalamus probes (NE-Hy, a total of 90 samples, seal number 3), 3 thalamus probes (5HT-Th, 235 samples, seal numbers 7 and 8) and 2 caudate nucleus probes (5HT-NC, 137 samples, seal number 7). The localizations of the probes are shown in Figure 2. *P < 0.05; ***P < 0.001. Nonsignificant correlations are not marked on these diagrams.
This shows that the pattern of HI, NE (as well as 5HT11) release in the cortex, NE release in the hypothalamus, and 5HT release in the thalamus and in the caudate nucleus during the sleep-wake cycle in the fur seals was in general similar (highest in AW, reduced in QW and BSWS, and lowest in REM sleep). During REM sleep the levels of cortical NE and 5HT drop more substantially compared to BSWS than do levels of cortical HI. In addition, HI levels are less modulated within behavioral states being significantly different between waking (AW and QW) and sleep (SWS and REM sleep) states but not between AW and QW, or SWS and REM sleep.
Histamine, Norepinephrine, and Serotonin Release Is Elevated during Certain Waking States
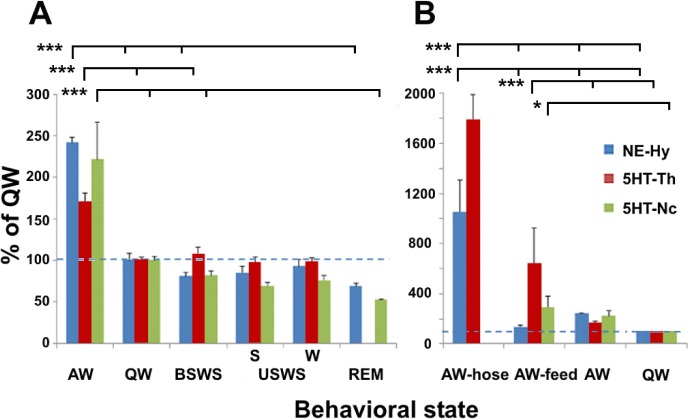
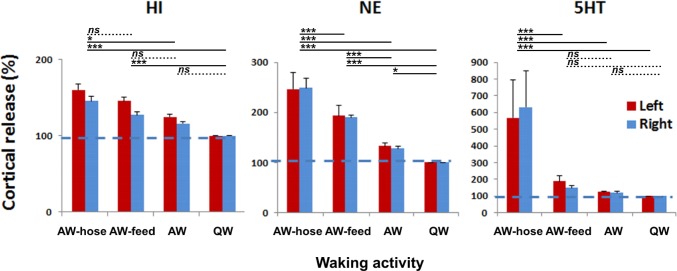
The nature of waking behavior had a significant effect on mean cortical HI release (F3,12 = 11.693, P < 0.001) and mean cortical NE release (F3,18 = 53.639, P < 0.001) (Figure 6). The levels of both neurotransmitters were highest when the seals were being hosed down (AW-hosing), an activity they appeared to enjoy as evidenced by their approach to the water stream.
Figure 6.
Cortical histamine (HI), norepinephrine (NE) and serotonin (5HT) release during different waking behavior in fur seals. Cortical release is expressed as mean ± SEM of the change relative to QW. AW-hose, hosing seals down with water; AW-feed, feeding fish; AW, other active waking behaviors; QW, quiet waking). *P < 0.05; ***P < 0.001; ns, nonsignificant (P > 0.05).
The post hoc Tukey test revealed that the difference in HI release between AW-hosing and AW (P = 0.015) and QW (P < 0.001) was significant as was the difference between AW-feeding and QW (both P < 0.001). The levels of HI did not differ significantly between other waking states (AW-hosing vs. AW-feeding, AW-feeding vs. AW, and AW vs. QW; all P > 0.05).
AW-hosing significantly elevated NE release when compared to AW-feeding, AW (without feeding or hosing) and QW (all P < 0.001). AW-feeding elevated NE release when compared to AW and QW (both P < 0.001). The levels of NE during AW were also significantly higher than during QW (P = 0.021).
As with the cortical release, the type of waking behavior also appears to have a large effect on mean NE release in the hypothalamus, and mean 5HT release both in the caudate nucleus and in the thalamus (ANOVA, F3,31 = 17.352, F2,60 = 22.793, F 3,138 = 59.560, respectively; P < 0.001 in all cases). Hosing the seal down substantially elevated the levels of both neurotransmitters when compared to AW-feeding, AW and QW (P < 0.001 in all cases; Figure 5B). AW-feeding also elevated the levels of 5HT in the thalamus compared to both AW and QW (P < 0.001). However, in the caudate nucleus the increase of 5HT during AW-feeding was significant only compared to QW (P < 0.05) but it was not significant compared to AW (P > 0.05). Feeding did not significantly elevate levels of NE in the hypothalamus compared to both AW and QW.
These findings show that the pattern of HI and NE release from the cortex locations, the level of NE release from the hypothalamus, and the level of 5HT release from thalamus and caudate nucleus in the fur seals were highly elevated during AW-hosing and at a lesser degree during AW-feeding states compared to non-feeding and non-hosing waking states. We can also see differences between the 3 monoamines: both cortical and subcortical 5HT and NE release were maximally elevated during AW-hosing behavior while cortical HI was equally elevated during both AW-hosing and AW-feeding behavior.
Histamine, Norepinephrine, and Serotonin Release Are Not Lateralized during USWS
All of the selected episodes of USWS/UW were characterized by a highly expressed EEG asymmetry as measured by the power spectra amplitude of the two brain hemispheres (Figures 1 and 4; Figure S2). As confirmed by statistical analysis (Table S1, supplemental material), SWA differed significantly between the two hemispheres for all selected episodes of left and right USWS/UW as first scored based on visual criteria. The absolute values of asymmetry index (a measure of the magnitude of EEG asymmetry) for the selected USWS episodes varied between 0.44 and 0.70. At the same time, SWA did not differ between the two hemispheres during sleep episodes scored as BSWS. The absolute asymmetry index for the selected BSWS episodes was smaller than 0.09.
As shown in Figure 7A, mean HI release during USWS in the hemisphere with the higher voltage EEG slow wave activity (“sleeping” hemisphere) was not different from that in the hemisphere with low voltage desynchronized activity (“waking” hemisphere, t10 = 1.105, P = 0.295, the paired t-test). As with HI, mean cortical NE release during USWS did not differ between the hemisphere with slow waves and the hemisphere with low voltage activity (t10 = 0.206, P = 0.841). During USWS, average SWA and cortical HI or NE release were not correlated in 10-min epochs. In addition, there was no correlation between SWA and cortical HI or NE release in the contralateral hemisphere. The same conclusion was reached for cortical 5HT release when analyzing our previously collected data (Figure 8).11 In our prior publication, we showed that inter-hemispheric EEG asymmetry in the fur seal is expressed in the range of 1.2–4, 4–8, 8–12, and 12–16 Hz, and the degree of asymmetry in these 4 frequency ranges as measured by the asymmetry index is positively correlated.5 Based on the prior data we conclude that cortical monoamine release is not related to the pattern of EEG in the range of 1.2–16 Hz. As with cortical NE and HI, the difference between 5HT release during USWS in the left and right hemispheres was not significant in the thalamus (t37 = 0.084, P = 0.933) and in the caudate nucleus (t39 = 0.936, P = 0.339). The same was true for mean NE release during USWS in the hypothalamus (t10 = 0.704, P = 0.437, Figure 7B).
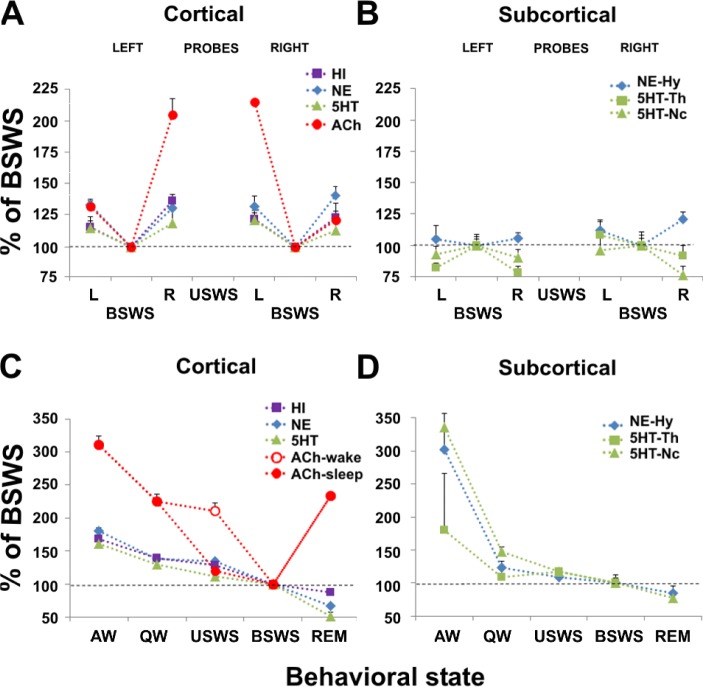
Figure 7.
Release of neurotransmitters during sleep and waking states in the fur seal. (A,B) Cortical and subcortical release of histamine (HI), norepinephrine (NE), serotonin (5HT) and acetylcholine (ACh) during bilateral slow wave sleep (BSWS) and unihemispheric slow wave sleep (USWS) in the left (L) and right (R) hemispheres in fur seals. Each point represents the mean of the percent change in neurotransmitter level relative to BSWS which is calculated separately for the probes located in the left and right cortical hemispheres and subcortical structures (Hy, hypothalamus; Th, thalamus; Nc, caudate nucleus). (C,D) Cortical and subcortical release of HI, NE, 5HT, and ACh during waking and sleep states in fur seals. Each point represents the mean of the percent change in neurotransmitter level relative to BSWS. The release of HI, NE, and 5HT were not lateralized during USWS. It is shown as average for all left and right probes. The release of ACh was lateralized during USWS. For USWS it is shown separately for the waking (open circle) and sleeping (closed circle) hemispheres. During USWS, ACh release is higher on the waking side than on the sleeping side. The release of cortical HI, NE, and 5HT as well as subcortical NE and 5HT during USWS are not lateralized and are comparable to the release of ACh on the sleeping side. The data for cortical ACh and 5HT release were collected during the prior studies.8,11
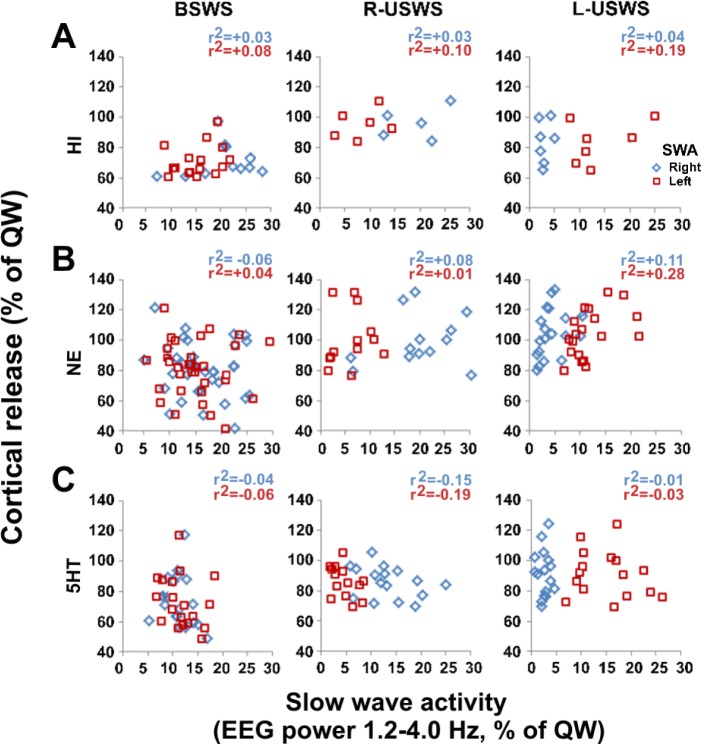
Figure 8.
Slow wave activity (SWA, EEG power in the range of 1.2–4.0 Hz) and cortical monoamine release during bilateral and unihemispheric sleep in the fur seal. Cortical release (Y-axis) is expressed for 10-min epochs as percent of the average release during quiet waking (QW). SWA (X-axis) in 10-min epochs is expressed as percent of the average SWA during QW. BSWS, bilateral slow wave sleep; R-USWS, right unihemispheric slow wave sleep; and L-USWS, left unihemispheric slow wave sleep. HI, histamine (A); NE, norepinephrine (B); and 5HT, serotonin (C). Microdialysis data are shown for left cortical probes. HI data are from 3 left probes (one probe per each of 3 studied seals which displayed both BSWS and USWS; a total of 30 samples). NE data are from 4 left probes (one probe per each of 4 studied seals; a total of 59 samples). 5HT data are from 3 left probes (one probe per each of 3 studied seals which displayed both BSWS and USWS; a total of 59 samples). There was no significant correlation between SWA and cortical monoamine (NE, 5HT and HI) release in the sleeping and waking hemispheres during USWS in fur seals (Pearson product moment correlation, P > 0.05 for all pairs). Similar results were obtained for right cortical samples.
In Figure 7 we compared cortical and subcortical release in the current study with cortical 5HT and cortical ACh levels in the fur seal from our previous studies.8,11 Similar to cortical 5HT, cortical HI, cortical and subcortical NE, and subcortical 5HT release in the fur seal, measured for the first time in the current study, were not lateralized during USWS and UW (Figure 7A, 7B). Cortical ACh release, however, was highly lateralized during USWS (Figure 7A). During USWS, cortical ACh levels in the hemisphere displaying slow waves did not differ from those observed during BSWS, whereas levels in the activated hemisphere were comparable to those observed during QW, a greater than 80% increase (Figure 7C). The pattern of cortical HI, NE, and 5HT release and subcortical 5HT and NE release clearly differs from that of ACh release observed under the same conditions (Figure 7C, 7D).
DISCUSSION
Here we show that over a period of 10 or more minutes USWS in the fur seal, NE and 5HT release are bilaterally symmetrical in the cortex and diencephalon, and HI release is bilaterally symmetrical in the cortex. Of the transmitters examined, only ACh release was asymmetrical in USWS, with significantly greater release in the activated hemisphere. We cannot rule out the possibility that over very short intervals of, for example, 1–3 seconds, symmetric or asymmetric release of the monoamines might occur. But clearly, although we readily detected changes in monoamine release during waking behaviors, the magnitude of any asymmetry during USWS was not signifi-cant for the monoamines, whereas it was for ACh.
Consistent with our finding, systemic blockade of ACh muscarinic receptors has long been known to produce bilateral EEG slowing, even during active behavior.9,14–16 We found that the pattern of NE, HI, and 5HT release across the sleep-wake cycle in each hemisphere of the fur seal is generally similar to that described in several species of terrestrial mammals.17–19 Both in bilaterally sleeping terrestrial mammals and unihemi-spherically sleeping fur seals the release of these monoamines in the brain declined progressively during transition from AW to SWS and then to REM sleep. This universal state dependent pattern of monoamine release correlates with the pattern of neuronal discharge of noradrenergic, histaminergic and serotoninergic neurons firing at highest rate during AW and declining to near complete cessation during REM sleep.20–23
Early concepts of waking being a result of sensory stimulation evolved to the Moruzzi and Magoun concept of a reticular arousal system that received input from sensory systems and was responsible for the generalized activation of the brain in response to sensory input and motivational states. The discovery of the monoaminergic and cholinergic systems within the reticular system, with what appeared to be diffuse, overlapping projections led to idea that they acted redundantly with other systems to produce waking, explaining the recovery of waking with damage to arousal systems.24
The arousal generated by the brainstem midbrain reticular formation can be attributed to the monoaminergic (noradrenergic locus coeruleus [LC], and the serotoninergic dorsal and median raphe nuclei), cholinergic (pedunculopontine and laterodorsal tegmental nuclei [PPT/LTP]), and glutamatergic components of this projection. The arousal pathway from the midbrain reticular formation splits at the level of diencephalon into two branches. The dorsal pathway ascends to the thalamus innervating the intralaminar and reticular nuclei which have been thought to play a critical role in regulating thalamo-cortical transmission and the EEG activity associated with sleep and wakefulness. The ventral pathway includes glutamatergic (parabrachial), NE, 5HT, and dopaminergic (periaqueductal gray) axons. It runs through the lateral hypothalamus and basal forebrain to the cortex.25,26 The activity patterns of these basal forebrain (mostly ACh) neurons correlate with sleep-wake patterns and EEG waveforms and cortical arousal.27,28 The histaminergic arousal system originates from the tuberomammillary area of the posterior hypothalamus projecting to the basal forebrain and cortex.25 The current work suggests that these systems do not contribute equally to EEG control, despite their seemingly similar projections to the cortex. Rather ACh is tonically elevated in the waking state. In contrast, high levels of 5HT, HI, and NE asymmetry are not necessary for the asymmetric EEG of UW and USWS even though we show that they are highly modulated by arousing behaviors in bilateral waking.
We show that both 5HT and NE release are greatly enhanced during motor activation in waking in fur seals, such as AW-hosing (which is always accompanied by extensive grooming of animals) and AW-feeding. At the same time, the increase in NE release during AW-hosing induced activity is smaller than that of 5HT. The highly elevated 5HT release when fur seals were sprayed with water relative to other waking conditions can be interpreted as linked to the increased motor activity.11 This is consistent with prior findings indicating that 5HT neuronal activity is very selectively linked to particular types of movement,29–31 and the activity of the NE containing neurons of the locus coeruleus is correlated with vigilance and presentation of arousing stimuli (e.g., food).32 However, as with other arousal systems one cannot distinguish a possible relation to muscle tone and other variables correlated with alertness from a relation to EEG unless one observes the system under a condition in which these variables can be dissociated from each other. Cataplexy is such a situation, in which muscle tone is lost while EEG activation and alertness is maintained bilaterally. Under this condition, noradrenergic cells completely cease discharge in parallel with the loss of muscle tone, despite a very high degree of EEG activation.20,33 This is also consistent with our findings indicating a pronounced decrease of NE (and 5HT) during the transition from BSWS to REM sleep in fur seals.
Studies of HI neurons have shown that their activity increases during cataplexy relative to that during QW.20,33 Careful behavioral studies of HI and orexin (hypocretin) knockout mice have found distinct roles for these two “waking” neurotransmitters, consistent with the general conclusions of the present investigation. They find that HI, in contrast to orexin, promotes waking through enhanced locomotion. This work also raises the possibility of a differential role of orexin and HI in cognition.34 In prior work, we have reported that orexin release is linked to positive emotions in waking, rather than EEG activation in mice, rats, cats, dogs, and humans.35–38 During aversive states, including pain in humans, orexin cell activity, and release of the orexin peptide is minimal despite intense EEG activation.
As we showed in our prior study, ACh level in fur seals was also highly elevated in REM sleep at levels comparable to that in QW.8 Both stages are characterized by an activated EEG pattern. This suggests that ACh release is linked to cortical activation which is a common feature of waking and REM sleep. At the same time, as shown in the current study monoamine release is, in contrast, associated with level of behavioral arousal and motor activity within the waking state.
The current work extends our knowledge on the role different neurotransmitters in initiation of waking and sleep. Our data show that several transmitters known to be maximally released in waking, and observed to be waking-active in the current study, are not strongly linked to the asymmetric EEG seen during USWS in the fur seal. We cannot rule out the possibility that other transmitters might also be involved in USWS and UW in fur seals and dolphins. Thus, it was shown that that both cholinergic and non-cholinergic neurons in the rat basal forebrain (presumably mainly GABA and glutamate neurons) need to be lesioned to cause loss of wakefulness.39 Thus, the role of the glutamatergic and GABAergic system in maintenance of aspects of waking as well as bilateral and asymmetrical sleep states could be productively investigated.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by grants from National Science Foundation (0919929), National Institute of Health MH064109, DA034748, Russian Fund for Basic Research (13-04-01704, 14-04-32075), Medical Research Service of the Dept. of Veterans Affairs, Utrish Dolphinarium Ltd., National Science and Engineering Council of Canada, and Canadian Institutes of Health Research. The authors have indicated no financial conflicts of interest. This study was performed at the University of California Los Angeles, Los Angeles, USA and Severtsov Institute of Ecology and Evolution, Moscow, Russia.
ACKNOWLEDGMENTS
The authors thank Dr. Paul Manger for help with localization of microdialysis probes in the fur seal brains and personnel of the Utrish Marine Station (Russia) for help with animal care. Author contributions: OIL, LMM, JLL and JMS designed research; JLL, POK, OIL, TK, AB, SMK, and JMS performed research; LMM and JHP contributed unpublished reagents/analytic tools; JLL, POK, TK, OIL, and JMS analyzed data; OIL, JLL and JMS wrote the paper.
ABBREVIATIONS
- 5HT
serotonin
- ACh
acetylcholine
- AI
asymmetry index
- AW
active wakefulness
- BSWS
bilateral slow wave sleep
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- HI
histamine
- Hy
hypothalamus
- LC
locus coeruleus
- Nc
caudate nucleus
- NE
norepinephrine
- PPT/LTP
pedunculopontine and laterodorsal tegmental nuclei
- QW
quiet wakefulness
- REM sleep
rapid eye movement sleep
- SWA
slow wave activity
- SWS
slow wave sleep
- Th
thalamus
- USWS
unihemispheric slow wave sleep
- UW
unihemispheric wakefulness
REFERENCES
- 1.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–84. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–4. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]
- 3.Lyamin OI, Mukhametov LM. Organization of sleep in the northern fur seal. In: Sokolov VE, Aristov AA, Lisitzina TU, editors. The northern fur seal. Systematic, morphology, ecology, behavior. Moscow: Nauka; 1998. p. 280.p. 302. (in Russian) [Google Scholar]
- 4.Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, Siegel JM. Fur seals display a strong drive for bilateral slow-wave sleep while on land. J Neurosci. 2008;28:12614–21. doi: 10.1523/JNEUROSCI.2306-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM, Siegel JM. Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J Sleep Res. 2008;17:154–65. doi: 10.1111/j.1365-2869.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 6.Lesku JA, Vyssotski AL, Martinez-Gonzalez D, Wilzeck C, Rattenborg NC. Local sleep homeostasis in the avian brain: convergence of sleep function in mammals and birds? Proc Biol Sci. 2011;278:2419–28. doi: 10.1098/rspb.2010.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rattenborg NC, Lima SL, Amlaner CJ. Facultative control of avian unihemispheric sleep under the risk of predation. Behav Brain Res. 1999;105:163–72. doi: 10.1016/s0166-4328(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM. Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J Neurosci. 2007;27:11999–2006. doi: 10.1523/JNEUROSCI.2968-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–2. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- 10.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 11.Lapierre JL, Kosenko PO, Kodama T, et al. Symmetrical serotonin release during asymmetrical slow-wave sleep: implications for the neurochemistry of sleep-waking states. J Neurosci. 2013;33:2555–61. doi: 10.1523/JNEUROSCI.2603-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supin AY, Popov VV, Mass AM. Boston, MA: Kluwer Academic; 2001. The sensory physiology of aquatic mammals. [Google Scholar]
- 13.Lim RKS, Liu CN, Moffitt RL. Springfield, IL: Charles C. Thomas; 1960. A stereotaxic atlas of the dog's brain. [Google Scholar]
- 14.Lhalainen J, Sarajarvi T, Rasmusson D, et al. Effects of memantine and donepezil on cortical and hippocampal acetylcholine levels and object recognition memory in rats. Neuropharmacology. 2011;61:891–99. doi: 10.1016/j.neuropharm.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–11. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lena I, Parrot S, Deschaux O, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 19.Chu M, Huang ZL, Qu WM, Eguchi N, Yao MH, Urade Y. Extracellular histamine level in the frontal cortex is positively correlated with the amount of wakefulness in rats. Neurosci Res. 2004;49:417–20. doi: 10.1016/j.neures.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Wu MF, Gulyani S, Yao E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–99. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–26. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–8. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MF, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep- waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554:202–15. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–8. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones BM. Basic mechanisms of sleep-wake states. In: Kryger MK, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. New York, NY: Saunders; 2005. pp. 136–53. [Google Scholar]
- 26.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–40. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maan Gee Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–9. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornal CA, Martin-Cora FJ, Jacobs BL. “Fatigue” of medullary but not mesencephalic raphe serotonergic neurons during locomotion in cats. Brain Res. 2006;1072:55–61. doi: 10.1016/j.brainres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 31.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:57–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 32.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–80. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the posterior hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–34. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anaclet C, Parmentier R, Ouk K, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–38. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blouin AM, Fried I, Wilson CL, et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Com. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci. 2011;31:15455–67. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;l;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch Ital Biol. 2011;149:492–8. doi: 10.4449/aib.v149i4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.