Abstract
Purpose
Obstructive sleep apnea (OSA) has been implicated in complications of cardiovascular disease, including arrhythmias and sudden cardiac death (SCD). Prolonged QT interval is associated with arrhythmias and SCD in patients with cardiovascular disease and apparently healthy humans. Apneic episodes during sleep in OSA patients are associated with QT prolongation due to increased vagal activity, but it is not understood whether chronic QT prolongation persists during normoxic daytime wakefulness.
Methods
To determine whether daytime QT intervals in OSA patients are prolonged compared to control subjects, we recruited 97 (76 male, 21 female) newly diagnosed patients with OSA [apnea-hypopnea index (AHI) ≥5 events/h] and 168 (100 male, 68 female) healthy volunteers (AHI <5 events/h) and measured daytime resting QT and RR intervals from the electrocardiograms to determine QT prolongation corrected for heart rate (QTc).
Results
All subjects with OSA were older and heavier, with increased heart rate and significantly increased AHI, arousal index, and reduced oxygen saturation (SpO2) during sleep, and spent less time in sleep with >90% SpO2 compared to respective controls. QTc in patients with OSA (410±3.3 for male and 433±5.6 for female) was significantly increased compared to respective control groups (399±2.9 for male and 417±2.9 for female), after adjustment for age and body mass index.
Conclusions
Our data show that OSA in either men or women is associated with a significant increase in resting daytime QTc. The propensity for ventricular arrhythmias in patients with OSA may be a result of abnormalities in resting cardiac repolarization.
Keywords: Sleep, cardiovascular disease, risk factors, QT interval
Introduction
Obstructive sleep apnea (OSA) has been increasingly implicated in the pathogenesis and complications of cardiovascular disease [1], including arrhythmias [2], and sudden cardiac death (SCD) [3]. Prolonged QT interval on a surface electrocardiogram (ECG) is associated with arrhythmias and SCD in patients with cardiovascular disease [4] and even in apparently healthy humans [5-8]. The QT interval reflects ventricular activity, and encompasses myocardial depolarization and repolarization. QT prolongation results from delayed repolarization and is associated with dispersion of refractoriness that, in turn, creates a substrate for ventricular arrhythmias with after-depolarization acting as triggers [9]. QT intervals change with heart rate, and QT prolongation corrected for heart rate (QTc) is considered to be a marker of risk for arrhythmias and SCD [10,4,11,12]. One important advantage of the QTc interval as a marker of risk of SCD is that the duration of QTc in healthy volunteers is stable and its short- and long-term reproducibility is high [13-15].
Normal sleep is associated with QT prolongation due to sleep-related changes in autonomic function [16]. OSA results in repetitive and severe nocturnal hypoxemia and sleep disturbances due to frequent breathing-related arousals [17]. Apneic episodes in OSA are associated with both significant QT prolongation due to increased vagal activity and abrupt QT shortening during post-apnea due to increased sympathetic tone and/or vagal withdrawal [18]. QT intervals that are prolonged due to hypoxemia return to normal after withdrawal of the hypoxic stimulus [19-21]. Limited data are available in patients with OSA addressing abnormalities of ventricular repolarization, which may play an important role in the genesis of ventricular arrhythmia in OSA. Specifically, it is not clear whether the QT interval is chronically prolonged in patients with OSA even during normoxic daytime wakefulness. Additionally, men have shorter QTc [22] and increased prevalence of OSA compared to age-matched females [23]. Therefore, we measured QTc in newly diagnosed and untreated patients with OSA to determine whether both men and women with OSA have increased QTc compared to respective control subjects.
Methods
Subjects
Subjects that presented for sleep studies at Mayo Clinic Sleep Laboratory who were free of cardiovascular (familial cardiac diseases, cardiac structural defects, coronary arterial diseases, heart failure and cardiac arrhythmias), cerebrovascular (stroke), and any chronic disease, had never been treated for OSA, and were on no medications, were recruited for the study. The control subjects were recruited from the community who also were free of any acute or chronic disease and on no medications. 97 (76 male, 21 female) newly diagnosed adult patients with OSA [apnea-hypopnea index (AHI) ≥5 events per h] and 168 (100 male, 68 female) healthy volunteers (AHI <5 events per h) in whom OSA was excluded by overnight sleep study were recruited for recording of daytime resting ECG, respiration and blood pressure (BP). The study was approved by the Institutional Human Subjects Review Committee at Mayo Clinic, Rochester, MN.
Polysomnography
The presence and severity of OSA in all subjects were determined by standard overnight polysomnography (E-Series Comprehensive Networked-Linked Amplifier, Compumedics USA Inc., Charlotte, USA), including electroencephalography, electrooculography, electromyography, finger-pulse oximeter readings, thermistor measurements of oro-nasal airflow, and measurements of rib-cage and abdominal movements of breathing. An apnea was defined as complete cessation of airflow for at least 10 seconds. Hypopnea was defined as 30% or greater reduction of respiratory signals for at least 10 seconds associated with oxygen desaturation of ≥4%. The AHI was calculated as the total number of respiratory events per h of sleep.
Recording of ECG, respiration, and blood pressure
Subjects were studied between 7:00 AM and 10:00 AM. Subjects rested supine in a quiet room for approximately 30 min before acquisition of ECG and blood pressure measurements. Standard lead-II surface ECG (ECG Bioamplifier; Gould Electronics, Cleveland, Ohio, USA) and respiration with a thoracic belt (Pneumotrace: Gould Electronics, Cleveland, Ohio, USA) were recorded continuously for 10 min during supine rest. Blood pressure was measured from the brachial artery with an automatic sphygmomanometer (Dinamap; Critikon Inc., Tampa, FL).
Data storage and analysis
The data were digitized on a computer and stored for subsequent analysis. All signals were sampled at a frequency of 400Hz. RR and QT intervals were calculated from lead II of the surface ECG using an ECG analysis module (AD Instruments, Colorado Springs, CO, USA). The ECG module provides a graphical interface of recorded ECG with automatic identification of beginning and end of each wave form of the ECG waves and manual control on the automatically selected markers of each wave form. RR and QT intervals were measured from continuous 5-min recordings. The QT intervals were measured from the onset of the Q- or R-wave to the termination of the T-wave, which was determined by the intersection of a tangent drawn to the steepest slope of the down-sloping portion of the T-wave and the isoelectric baseline. The beginning of the Q- and R-waves and the end of T-wave were identified by an automated computer program and manually edited after careful observation of the ECG. QTc was calculated by Bazett's formula [24], where QTc=QT/RR1/2. The investigators involved in ECG analyses were blinded to the diagnosis of OSA.
Statistical Analysis
Statistical analyses were conducted with JMP 7.0.1 (SAS Institute, Cary, NC, USA). Categorical variables were described as proportion, and continuous variables were expressed as means and standard deviations (SD). Differences between OSA and control groups for both male and female subjects were calculated using the Chi-square test for categorical variables and Student t-test for continuous variables. Bivariate associations of QTc and severity of OSA (AHI and SpO2) were analyzed using Spearman correlation coefficients. An analysis of covariance (ANCOVA) model was used with covariates including age and body mass index (BMI). A P-value <0.05 was considered statistically significant for all analyses.
Results
Demographics, hemodynamics, and sleep profile in OSA and control subjects
Baseline characteristics of men and women with or without OSA are described in Table 1. Both men and women with OSA were older and heavier compared to respective control subjects. Diastolic and mean blood pressures were increased in men with OSA compared to men without OSA. Heart rate was significantly elevated in patients with OSA compared to control subjects, for both male and female. Sleep duration, efficiency, and latency and REM sleep latency were not significantly different between men or women with OSA compared to respective control subjects. However, men with OSA did have significantly increased Stage 1 and Stage 2 sleep and significantly reduced slow-wave and REM sleep compared to men without OSA. AHI and arousal index (AI) events were significantly increased in both men and women with OSA compared to respective control subjects. Although, awake SpO2 was not different between men and women with OSA and controls, mean SpO2 and lowest SpO2 during sleep and percent time spent with SpO2 >90% were significantly reduced in both men and women with OSA compared to respective control subjects.
Table 1. Demographics, hemodynamics, and sleep profile in male and female subjects with or without obstructive sleep apnea (OSA).
| Male subjects | Female subjects | |||
|---|---|---|---|---|
| Control (n=100) |
OSA (n=76) |
Control (n=68) |
OSA (n=21) |
|
| Age, yrs | 39±14 | 47±12* | 37±13 | 49±12# |
| BMI, kg/m2 | 28±6 | 32±5* | 28±6 | 37±9# |
| Systolic BP, mmHg | 129±15 | 133±12 | 126±20 | 130±12 |
| Diastolic BP, mmHg | 72±7 | 79±7* | 72±9 | 68±5 |
| Mean BP, mmHg | 91±9 | 97±8* | 90±12 | 89±4 |
| Heart Rate, beats per min | 62±11 | 66±11* | 66±12 | 74±11# |
| Sleep Time, min | 409±53 | 353±97 | 387±61 | 384±24 |
| Sleep Efficiency, % | 77±19 | 80±12 | 80±17 | 78±12 |
| Sleep Latency, min | 19±48 | 9±10 | 12±15 | 16±25 |
| REM Latency, min | 127±106 | 120±72 | 121±86 | 138±83 |
| Stage 1 Sleep, % | 11±9 | 17±10* | 12±8 | 16±8 |
| Stage 2 Sleep, % | 50±10 | 57±11* | 50±11 | 53±9 |
| Slow-Wave Sleep, % | 24±10 | 13±9* | 20±9 | 17±6 |
| REM Sleep, % | 16±7 | 14±7* | 18±7 | 14±6 |
| Awake SpO2, % | 97±1 | 97±1 | 97±2 | 97±2 |
| Mean SpO2, % | 96±2 | 94±2* | 97±2 | 94±2# |
| Lowest SpO2, % | 90±5 | 79±12* | 92±4 | 82±8# |
| >90% SpO2, min | 100±1 | 95±8* | 100±1 | 98±2# |
| AI, events per h | 21±15 | 44±21* | 21±9 | 36±26# |
| AHI, events per h | 1±1 | 32±30* | 1±1 | 19±18# |
AHI, apnea-hypopnea index; AI, arousal index; REM, rapid eye movement; SpO2, oxygen saturation by finger-pulse oximeter.
Values are means±SD.
P<0.05, OSA vs control for male subjects.
P<0.05, OSA vs control for female subjects.
RR, QT, and QTc in OSA and Control Subjects
RR intervals were significantly reduced in men with OSA compared to control subjects after adjustment of age and BMI (Figure 1A). QT intervals between OSA and control groups were not significantly different for both men and women. However, QTc was significantly increased in OSA compared to control groups for both men (Figure 1A) and women (Figure 1B) after adjustment for age and BMI (P=0.01). Correlation analysis indicated that the severity of OSA, whether defined by AHI, AI, or mean or lowest SpO2 during sleep, was not significantly associated with daytime QTc in either men or women.
Figure 1.
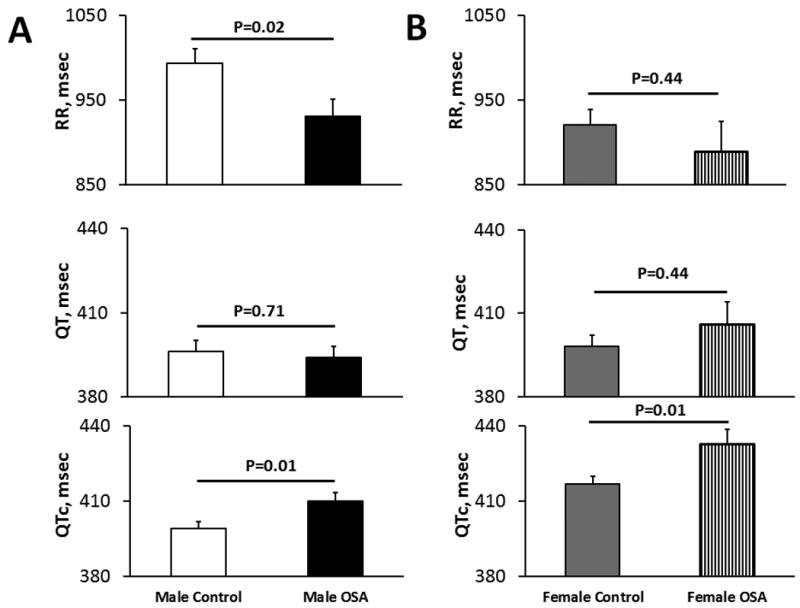
RR, QT, QTc in men with OSA and control subjects (A) and in women with OSA and control subjects (B) after adjustment for age and BMI. QTc is increased in both men and women with OSA compared to respective control. Data are expressed as least square means±SEM.
Discussion
Our results indicate that QTc was significantly increased in both men and women with OSA compared to respective control subjects without OSA after adjustment of age and BMI.
Cardiovascular diseases including cardiac arrhythmias [17] and SCD [3] are common in patients with OSA. QT-interval prolongation in OSA patients has been considered as a lethal combination, particularly in infants, causing sudden unexpected death [25]. Ventricular arrhythmias have been observed frequently in patients with severe OSA [26]. These arrhythmias correlate with the degree of oxygen desaturation, and effective therapy is associated with suppression of arrhythmias [27,28]. Marked sympathetic activation due to severe nocturnal hypoxemia in sleep apneics, in combination with hypoxia-mediated lowering of arrhythmic thresholds [29], may be an important factor in the genesis of arrhythmias.
The autonomic nervous system contributes importantly to day-to-day variation in the QT interval [30] by direct effects on the ventricular myocardium [31,32] and by sinus-node influences on heart rate and thus, rate-related changes in QT interval. Repeated nocturnal apneic events in OSA patients result in carry-over sustained daytime activation of the sympathetic nervous system [33]. Indeed, resting daytime heart rate in sleep apnea patients is higher than in matched controls [34], suggesting increase in daytime sympathetic drive. Recently, prolonged QT interval in OSA patients has been reported [35,21,36]. Therefore, the increased QTc in both men and women with OSA found in our study may be associated with a chronically elevated sympathetic drive, even during daytime normoxic conditions.
Roche et al. studied the effects of acute hypoxia on QT interval in 11 healthy volunteers and found a significant prolongation of QT and increased QTc [21]. The QT intervals returned to normal following withdrawal of the hypoxic stimuli. A recent report suggested a significant QT prolongation after withdrawal of CPAP treatment in patients with OSA [37]. Sleep is associated with changes in QT interval variability in OSA patients, and the QT variability also correlates with the severity of OSA [38]. QT-interval prolongation has been reported during nocturnal hypoxemia in patients with chronic obstructive-airway disease [39] and in patients with coronary artery disease who manifested intermittent decreases in oxygen saturation of blood [40]. Increased QTc in patients with OSA may be associated with repeated nighttime hypoxia and consistently elevated sympathetic drive during daytime normoxic conditions. Our results suggest that severity of OSA as defined by AHI, AI or SpO2 is not associated with increased QT prolongation in either men or women. However, the mechanisms of hypoxia-related prolongation of QT intervals are not yet clear and require further study.
Several factors may determine the inter-individual variation in QT interval, including age [41], gender [41], BMI [42], electrolyte imbalance [43], congenital [44] or acquired [45] diseases, and certain medications [46]. Healthy women have significantly elevated QTc compared to men matched for age and BMI [22]. Longer baseline resting QT intervals in women may predispose them to drug-induced prolongation of ventricular repolarization and consequent cardiac arrhythmias [47,48]. Women also have a higher incidence of torsades de pointes, and women with congenital long QT syndrome are more susceptible to arrhythmias than men [49]. Therefore, we compared QTc between OSA and control groups separately for men and women. Our study showed that both men and women with OSA have significantly elevated QTc compared to respective control groups.
Strengths of our study include the analysis of a relatively large number of OSA patients who were free from cardiovascular disease and not on any medications, including those affecting QT interval. The larger sample size allowed multivariate adjustment of age and BMI, both of which may have significant effects on inter-individual variations in QT intervals. Additionally, the signal-averaged resting electrocardiograms of 3 to 5 min used for QT-interval analysis limits the respiration-related beat-to-beat RR and thus, QT changes. Considering the possible effects of gender on QT intervals for females, we compared OSA and control subjects separately for men and women. One of the limitations of the current study was the absence of 12-lead ECG recordings. In addition to measurements of QT intervals in individual leads, the 12 lead-ECG would allow us to quantify QTd in addition to QTc. QTd is another marker of cardiac repolarization abnormality and risk of SCD. Several recent studies have reported increased QTd in adults and children with OSA [35,50]. Therefore, measurements of QTd in OSA patients might identify the risk of arrhythmias in these patients.
In conclusion, we found that OSA in either men or women is associated with a significant increase in resting daytime QTc. However, mechanisms of increased QTc in patients with OSA are not clear. The propensity for ventricular arrhythmias in patients with OSA may be a result of abnormalities in resting cardiac repolarization.
Acknowledgments
The authors are grateful to J. Denise Wetzel, CCHMC Medical Writer, for critical review of the manuscript.
Sources of Support: These studies were supported by a Perkins Memorial Award, an American Heart Association Scientist Development Grant (0730129N, AS) and AHA Fellowship Grant (09-20069G, FSK), and by the National Institutes of Health (NIH) grants HL-70302, HL-65176, TW05463, TW05469, and 1 UL1 RR024150. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflict of Interest: Dr. Somers has served as a consultant for Respicardia, NeuPro, and ResMed and is an investigator on studies funded with grants from the Philips Respironics Foundation.
Dr. Pressman has received funding from the Philips Respironics Foundation.
The other authors declare that they have no conflict of interest.
References
- 1.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52(5):490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 3.Pearce S, Saunders P. Obstructive sleep apnoea can directly cause death. Thorax. 2003;58(4):369. doi: 10.1136/thorax.58.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 5.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84(4):1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 6.de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150(12):1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- 7.de Bruyne MC, Hoes AW, Kors JA, Hofman A, van Bemmel JH, Grobbee DE. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study. Eur Heart J. 1999;20(4):278–284. doi: 10.1053/euhj.1998.1276. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Nelson JC, Kronmal RA, Zhang ZM, Robbins J, Gottdiener JS, Furberg CD, Manolio T, Fried L. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study) Am J Cardiol. 2001;88(2):118–123. doi: 10.1016/s0002-9149(01)01604-6. [DOI] [PubMed] [Google Scholar]
- 9.Davey P. QT interval and mortality from coronary artery disease. Prog Cardiovasc Dis. 2000;42(5):359–384. doi: 10.1053/pcad.2000.0420359. [DOI] [PubMed] [Google Scholar]
- 10.Munger RG, Prineas RJ, Crow RS, Changbumrung S, Keane V, Wangsuphachart V, Jones MP. Prolonged QT interval and risk of sudden death in South-East Asian men. Lancet. 1991;338(8762):280–281. doi: 10.1016/0140-6736(91)90419-p. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57(6):1074–1077. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 12.Wheelan K, Mukharji J, Rude RE, Poole WK, Gustafson N, Thomas LJ, Jr, Strauss HW, Jaffe AS, Muller JE, Roberts R, et al. Sudden death and its relation to QT-interval prolongation after acute myocardial infarction: two-year follow-up. Am J Cardiol. 1986;57(10):745–750. doi: 10.1016/0002-9149(86)90606-5. [DOI] [PubMed] [Google Scholar]
- 13.Krasemann T, Strompen C, Blumenberg J, Gehrmann J, Burkhardtsmaier G, Vogt J. Changes of the corrected QT interval in healthy boys and girls over day and night. Eur Heart J. 2009;30(2):202–208. doi: 10.1093/eurheartj/ehn452. [DOI] [PubMed] [Google Scholar]
- 14.Kautzner J, Yi G, Camm AJ, Malik M. Short- and long-term reproducibility of QT, QTc, and QT dispersion measurement in healthy subjects. Pacing Clin Electrophysiol. 1994;17(5 Pt 1):928–937. doi: 10.1111/j.1540-8159.1994.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 15.Veglio M, Maule S, Matteoda C, Quadri R, Valentini M, Pecchio O, Piancino G, Chiandussi L. Use of corrected QT interval in autonomic function testing: assessment of reproducibility. Clin Auton Res. 1996;6(6):309–312. doi: 10.1007/BF02556300. [DOI] [PubMed] [Google Scholar]
- 16.Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol. 1983;52(1):55–59. doi: 10.1016/0002-9149(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 17.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 18.Gillis AM, Stoohs R, Guilleminault C. Changes in the QT interval during obstructive sleep apnea. Sleep. 1991;14(4):346–350. doi: 10.1093/sleep/14.4.346. [DOI] [PubMed] [Google Scholar]
- 19.Horii M, Takasaki I, Ohtsuka K, Tsukiyama H, Takahashi A, Hatori Y, Hakuta T. Changes of heart rate and QT interval at high altitude in alpinists: analysis by Holter ambulatory electrocardiogram. Clin Cardiol. 1987;10(4):238–242. doi: 10.1002/clc.4960100406. [DOI] [PubMed] [Google Scholar]
- 20.Fuenmayor AJ, Stock FU, Fuenmayor AC, Fuenmayor PA. QT interval and final portion of T wave: measurements and dispersion in infants born at high altitude. Int J Cardiol. 2002;82(2):123–126. doi: 10.1016/s0167-5273(01)00616-7. [DOI] [PubMed] [Google Scholar]
- 21.Roche F, Reynaud C, Pichot V, Duverney D, Costes F, Garet M, Gaspoz JM, Barthelemy JC. Effect of acute hypoxia on QT rate dependence and corrected QT interval in healthy subjects. Am J Cardiol. 2003;91(7):916–919. doi: 10.1016/s0002-9149(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann MH, Timothy KW, Frankovich D, Fromm BS, Keating M, Locati EH, Taggart RT, Towbin JA, Moss AJ, Schwartz PJ, Vincent GM. Age-gender influence on the rate-corrected QT interval and the QT-heart rate relation in families with genotypically characterized long QT syndrome. J Am Coll Cardiol. 1997;29(1):93–99. doi: 10.1016/s0735-1097(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 23.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 24.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1918;7:353–370. [Google Scholar]
- 25.Smith TA, Mason JM, Bell JS, Francisco JT. Sleep apnea and Q-T interval prolongation--a particularly lethal combination. Am Heart J. 1979;97(4):505–508. doi: 10.1016/0002-8703(79)90399-5. [DOI] [PubMed] [Google Scholar]
- 26.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101(4):392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 28.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60(9):781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich H, Borchard U, Hafner D, Hirth C. Antiarrhythmic and electrophysiological actions of flecainide, bepridil and amiodarone on isolated heart preparations during controlled hypoxia. Archives internationales de pharmacodynamie et de therapie. 1985;274(2):267–282. [PubMed] [Google Scholar]
- 30.Murakawa Y, Inoue H, Nozaki A, Sugimoto T. Role of sympathovagal interaction in diurnal variation of QT interval. Am J Cardiol. 1992;69(4):339–343. doi: 10.1016/0002-9149(92)90230-v. [DOI] [PubMed] [Google Scholar]
- 31.Magnano AR, Holleran S, Ramakrishnan R, Reiffel JA, Bloomfield DM. Autonomic nervous system influences on QT interval in normal subjects. J Am Coll Cardiol. 2002;39(11):1820–1826. doi: 10.1016/s0735-1097(02)01852-1. [DOI] [PubMed] [Google Scholar]
- 32.Ahnve S, Vallin H. Influence of heart rate and inhibition of autonomic tone on the QT interval. Circulation. 1982;65(3):435–439. doi: 10.1161/01.cir.65.3.435. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177(3):385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 34.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 35.Cicek D, Lakadamyali H, Gokay S, Sapmaz I, Muderrisoglu H. Effect of obstructive sleep apnea on heart rate, heart rate recovery and QTc and P-wave dispersion in newly diagnosed untreated patients. Am J Med Sci. 2012;344(3):180–185. doi: 10.1097/MAJ.0b013e318239a67f. [DOI] [PubMed] [Google Scholar]
- 36.Barta K, Szabo Z, Kun C, Munkacsy C, Bene O, Magyar MT, Csiba L, Lorincz I. The effect of sleep apnea on QT interval, QT dispersion, and arrhythmias. Clin Cardiol. 2010;33(6):E35–39. doi: 10.1002/clc.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi VA, Stoewhas AC, Camen G, Steffel J, Bloch KE, Stradling JR, Kohler M. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: data from a randomized controlled trial. Eur Heart J. 2012;33(17):2206–2212. doi: 10.1093/eurheartj/ehs073ehs073. [pii] [DOI] [PubMed] [Google Scholar]
- 38.Baumert M, Smith J, Catcheside P, McEvoy RD, Abbott D, Sanders P, Nalivaiko E. Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. Sleep. 2008;31(7):959–966. [PMC free article] [PubMed] [Google Scholar]
- 39.Tirlapur VG, Mir MA. Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N Engl J Med. 1982;306(3):125–130. doi: 10.1056/NEJM198201213060301. [DOI] [PubMed] [Google Scholar]
- 40.De Olazabal JR, Miller MJ, Cook WR, Mithoefer JC. Disordered breathing and hypoxia during sleep in coronary artery disease. Chest. 1982;82(5):548–552. doi: 10.1378/chest.82.5.548. [DOI] [PubMed] [Google Scholar]
- 41.Kassotis J, Costeas C, Bedi AK, Tolat A, Reiffel J. Effects of aging and gender on QT dispersion in an overtly healthy population. Pacing Clin Electrophysiol. 2000;23(7):1121–1126. doi: 10.1111/j.1540-8159.2000.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 42.el-Gamal A, Gallagher D, Nawras A, Gandhi P, Gomez J, Allison DB, Steinberg JS, Shumacher D, Blank R, Heymsfield SB. Effects of obesity on QT, RR, and QTc intervals. Am J Cardiol. 1995;75(14):956–959. doi: 10.1016/s0002-9149(99)80700-0. [DOI] [PubMed] [Google Scholar]
- 43.Vervaet P, Amery W. QTc-measurements: a case-control study on serum electrolytes. Acta Cardiol. 1993;48(6):565–578. [PubMed] [Google Scholar]
- 44.Johansson BW, Jorming B. Hereditary prolongation of QT interval. Br Heart J. 1972;34(7):744–751. doi: 10.1136/hrt.34.7.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu EY, Clemo HF, Ellenbogen KA. Acquired QT Prolongation: Mechanisms and Implications. Cardiol Rev. 1998;6(6):319–324. doi: 10.1097/00045415-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Henderson W, Chu J, Hoffman R. QTc prolongation and drugs. Clin Pharmacol Ther. 2001;70(6):567–568. [PubMed] [Google Scholar]
- 47.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. Jama. 1993;270(21):2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 48.Lu HR, Remeysen P, Somers K, Saels A, De Clerck F. Female gender is a risk factor for drug-induced long QT and cardiac arrhythmias in an in vivo rabbit model. J Cardiovasc Electrophysiol. 2001;12(5):538–545. doi: 10.1046/j.1540-8167.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- 49.Kimbrough J, Moss AJ, Zareba W, Robinson JL, Hall WJ, Benhorin J, Locati EH, Medina A, Napolitano C, Priori S, Schwartz PJ, Timothy K, Towbin JA, Vincent GM, Zhang L. Clinical implications for affected parents and siblings of probands with long-QT syndrome. Circulation. 2001;104(5):557–562. doi: 10.1161/hc3001.093501. [DOI] [PubMed] [Google Scholar]
- 50.Khositseth A, Nantarakchaikul P, Kuptanon T, Preutthipan A. QT dispersion in childhood obstructive sleep apnoea syndrome. Cardiol Young. 2011;21(2):130–135. doi: 10.1017/S1047951110001514. doi:S1047951110001514. [pii] 10.1017/S1047951110001514. [DOI] [PubMed] [Google Scholar]


