Abstract
Background and purpose
Because of a low prevalence of severe carotid stenosis in the general population screening for presence of asymptomatic carotid artery stenosis (ACAS) is not warranted. Possibly, for certain subgroups screening is worthwhile. The present study aims to develop prediction rules for the presence of ACAS (>50% and >70%).
Methods
Individual participant data from four population-based cohort studies (Malmö Diet and Cancer Study, Tromsø Study, Carotid Atherosclerosis Progression Study, and Cardiovascular Health Study; totaling 23,706 participants) were pooled. Multivariable logistic regression was performed to determine which variables predict presence of ACAS (>50% and >70%). Calibration and discrimination of the models was assessed and bootstrapping was used to correct for overfitting.
Results
Age, sex, history of vascular disease, systolic and diastolic blood pressure, TC/HDL ratio, diabetes mellitus and current smoking were predictors of stenosis (>50% and >70%). The calibration of the model was good confirmed by a non-significant Hosmer and Lemeshow test for moderate (p=0.59) and severe stenosis (p=0.07). The models discriminated well between participants with and without stenosis, with an AUC corrected for over optimism of 0.82 (95%CI 0.80–0.84) for moderate stenosis and of 0.87 (95% CI 0.85–0.90) for severe stenosis. The regression coefficients of the predictors were converted into a score chart to facilitate practical application.
Conclusions
A clinical prediction rule was developed that allows identification of subgroups with high prevalence of moderate (>50%) ACAS and severe (>70%). When confirmed in comparable cohorts, application of the prediction rule may lead to a reduction in the number needed to screen for ACAS.
Keywords: Asymptomatic carotid artery stenosis, Prediction, Screening
Introduction
Stroke is among the leading causes of morbidity, long-term disability and mortality in both men and women in nearly all high, middle and low income countries.1;2 As such stroke poses a substantial economic burden in terms of health care and societal costs worldwide.3
Stenosis of the internal carotid artery is a major risk factor for stroke. In individuals with symptoms of cerebral ischemia (i.e., transient ischemic attack or a minor disabling stroke) and with a carotid stenosis of 50% or over, high risks of a recurrent event have been reported: the risk of stroke was 21% at 2 weeks after the first TIA or stroke, and 32% at 12 weeks.4 Treatment of symptomatic carotid stenosis has been well established. In general, symptomatic patients suitable for surgery with more than 70% carotid artery stenosis are recommend to have an carotid endarterectomy.5
Asymptomatic carotid artery stenosis (ACAS) is also related to a higher risk of stroke. Older studies among individuals not receiving optimal cardiovascular preventive treatment showed estimates for an annual risk for stroke of approximately 2–4% for patients with severe (>70%) carotid stenosis.6–9 An observational study reported 10- and 15-year stroke risks being 10% and 17%, respectively.10 These untreated risk estimates put individuals with an ACAS in the very high risk group based on the ESC/AHA guidelines on cardiovascular disease prevention.11;12 As such ACAS individuals should receive best medical treatment involving antihypertensive and lipid-lowering drugs in addition to lifestyle advice (dietary measures, weight loss, quitting smoking, restriction alcohol consumption, increase exercise). Indeed, several studies have shown lower incidence rates among those with optimal cardiovascular preventive medications. A more recent study showed stroke risks of around 0.5% per year for 70% to 99% ACAS patients.13
Population screening for ACAS has been suggested as a way to reduce the burden of stroke. In earlier days this was based on the notion that revascularisation in combination with preventive therapy would be the most optimal treatment to reduce stroke risk. However, nowadays the lower risk estimates seems to favour a more conservative treatment choice as opposed to revascularisation.14 And thus screening for ACAS is meant to identify those at high risk of stroke.
This study aims at developing prediction rules for identification of individuals with a high probability of having a moderate (>50%) or severe (>70%) asymptomatic carotid artery stenosis in the general population.
Methods
Study population
We used individual participant data from four observational studies on cardiovascular diseases (Tromsø Study, Malmö Diet and Cancer Study [MDCS], Carotid Atherosclerosis Progression Study [CAPS] and Cardiovascular Health Study [CHS]).15–19 In brief, the Tromsø Study is a population-based prospective study in Tromsø, Norway. All inhabitants aged 55 to 74 years and 5% to 10% samples of other 5-year-age groups aged ≥ 25 years were invited. In total, 6,727 participants (attendance rate 77%) were screened with ultrasound examination of the right carotid artery and valid written informed consent was available in 6,659 participants.15 In the population-based Malmö Diet and Cancer Study (MDCS), a total of 28 449 participants attended between 1991 and 1996 (attendance rate 41%). A random sample of 6,103 (20%) participants had an ultrasound examination.16;17 In the Carotid Atherosclerosis Progression Study (CAPS), members of a German primary healthcare scheme were invited of whom 6,962 (attendance rate 21%) agreed to take part to be screened with ultrasound examination.18 The Cardiovascular Health Study is a community-based, prospective study of people aged ≥ 65 years including 5,888 participants (attendance rate 57%) who were screened with ultrasound examination.19 All studies excluded symptomatic patients and obtained information on degree of stenosis and potential determinants thereof.
Baseline characteristics
The following baseline characteristics were recorded in each study: age, sex, presence of diabetes mellitus, history of coronary and/or cerebrovascular disease, and information on medication use. In addition, data on blood pressure, lipid levels, current smoking, waist circumference and body mass index (BMI) were recorded.
Outcomes
Moderate ACAS was defined as ≥50% stenosis and severe ACAS as ≥70% stenosis, measured by Doppler ultrasonography supported by B-mode ultrasound imaging. When both carotid arteries were measured, we used the most severe stenosis grade observed.
Model development
Missing values were imputed with single regression techniques using information from all individuals without missing values on that variable, since deleting subjects with missing values often leads to biased findings and to a loss of statistical power.20 The grade of stenosis was missing in 0.2% of the participants, predictors were missing for 0.1% to 5.2% of the participants. Restricted cubic spline functions and graphs were used to determine whether continuous variables could be analyzed as linear terms or required a transformation.21
For the continuous predictors age, systolic and diastolic blood pressure, and TC/HDL ratio, a linear relationship with outcome was found to be a good approximation after assessment of nonlinearity using restricted cubic splines. All candidate predictors for moderate stenosis were included in a multivariable logistic model and were step by step excluded using the likelihood ratio test with a p-value above 0.20. All analyses were stratified by study.
As most of the predictors to identify individuals with a high probability of ACAS being present, were similar to those used in the Framingham Risk Score (FRS), we additionally compared Number Needed to Screen (NNS) with the FRS.
Model performance
To study the performance of the final prediction model, we assessed its discrimination and calibration. Discrimination is the ability of the model to distinguish between participants with or without moderate (>50%) or severe (>70%) stenosis, and is quantified as the area under the receiver operating characteristic curve (AUC). An AUC ranges from 0.5 (no discrimination) to 1 (perfect discrimination). Calibration refers to the agreement between the predicted probabilities and observed frequencies of stenosis degree, which was tested with the Hosmer-Lemeshow statistic.22 To create a good overview we selected the same predictors for moderate and severe stenosis.
Model validation
Prediction models derived with multivariable regression analysis are known for overestimated regression coefficients. These results in overestimated predictions when applied in new participants.22;23 Therefore, we validated our model internally with bootstrapping techniques where in each bootstrap sample the entire modeling process was repeated.23 This resulted in a shrinkage factor for the regression coefficients.22 The bootstrap procedure was also used to estimate the AUC corrected for over-optimism. The corrected AUC may be considered as an estimate of discriminative ability expected in future similar participants.
Clinical application
To facilitate practical application of the model, the regression coefficients of the predictors in the model for severe stenosis were converted into points on a score chart. The total points (sum scores) were linked to the risk of the presence of moderate or severe stenosis.
The total points (sum scores) were linked to the risk of the presence of moderate or severe stenosis. Various cut off values were introduced, categorizing patients as having a very low risk, low risk, intermediate risk or high risk (see Table 4). The numbers needed to screen (NNS), sensitivities, specificities and the positive and negative predicted values of these thresholds were calculated. Additionally, we used the European Systematic COronary Risk Evaluation (SCORE) risk chart to estimate the number of individuals at high risk for developing vascular disease (10-year risk of ≥ 20%) and who are recommended antihypertensive and lipid-lowering drugs.24
Table 4.
Model performance for severe and moderate stenosis
| Moderate stenosis | |||||||||
| Prediction score |
Total no. of participants |
No. of participants with moderate stenosis |
prevalence | SE (%) | SP (%) | PPV (%) | NPV (%) | NNS | |
| Very low risk | ≤ 9 points | 8417 | 26 | 0.3% | |||||
| Low risk | 10–11 points | 4044 | 36 | 0.9% | 94.4% | 36.1% | 2.9% | 99.7% | 35 |
| Intermediate risk | 12–13 points | 3998 | 57 | 1.4% | 86.7% | 53.3% | 3.6% | 99.5% | 28 |
| High risk | ≥ 14 points | 7247 | 346 | 4.8% | 74.4% | 70.3% | 4.8% | 99.3% | 21 |
| Total | 23706 | 465 | 2.0% | ||||||
| Severe stenosis | |||||||||
| Prediction score |
Total no. of participants |
No. of participants with severe stenosis |
prevalence | SE (%) | SP (%) | PPV (%) | NPV (%) | NNS | |
| Very low risk | ≤ 9 points | 8417 | 2 | 0.0% | |||||
| Low risk | 10–11 points | 4044 | 5 | 0.1% | 98.4% | 35.7% | 0.8% | 100.0% | 122 |
| Intermediate risk | 12–13 points | 3998 | 14 | 0.4% | 94.5% | 52.8% | 1.1% | 99.9% | 94 |
| High risk | ≥ 14 points | 7247 | 106 | 1.5% | 83.5% | 69.7% | 1.5% | 99.9% | 68 |
| Total | 23706 | 127 | 0.5% | ||||||
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; NNS, number needed to screen.
Results
Study population
General characteristics of the study population are presented in Table 1. The mean age was 61 ± 12 years and 46% of the participants were men. Crude differences between studies are a consequence of different inclusion criteria across studies, in particular difference in age range. Overall, fifteen percent of the participants had a history of vascular disease (coronary heart disease and/or stroke). This prevalence of a history of vascular disease varied from 3% to 40% between the cohorts. Overall, 465 (2%) of the 23,706 participants had moderate stenosis and 127 (0.5%) had severe stenosis. The prevalence of severe stenosis among participants without a history of vascular disease 0.3% (95% CI 0.2–0.4%), among participants with a history of coronary heart disease 1.9% (95% CI 1.4–2.4%) and among participants with a history of stroke was 3.5% (95% CI 2.1–4.9%).
Table 1.
General characteristics of the study population, by cohort.
| Tromsø | MDCS | CAPS | CHS | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants | 6659 | 6103 | 5056 | 5888 | 23706 | |||||
| Age (years) | 60.2 | ± 10.2 | 57.5 | ± 5.9 | 50.1 | ± 13.1 | 72.8§ | ± 5.6 | 60.5 | ± 12.1 |
| Male sex | 3298 | (49.5%) | 2572 | (42.1%) | 2471 | (48.9%) | 2495 | (42.4%) | 10836 | (45.7%) |
| History of vascular disease | 954 | (14.3%) | 167 | (2.7%) | 155 | (3.1%) | 2213 | (37.6%) | 3489 | (14.7%) |
| Coronary heart disease | 825 | (12.4%) | 102 | (1.7%) | 108 | (2.1%) | 1997 | (33.9%) | 3032 | (12.8%) |
| Stroke | 182 | (2.7%) | 69 | (1.1%) | 52 | (1.0%) | 349 | (5.9%) | 652 | (2.8%) |
| Body mass index (kg/m2) | 26.1 | ± 4.0 | 25.9 | ± 4.0 | 26.6 | ± 4.1 | 26.7 | ± 4.7 | 26.3 | ± 4.2 |
| Waist-hip ratio | 0.87 | ± 0.08 | 0.85 | ± 0.09 | 0.95 | ± 0.11 | 0.93 | ± 0.09 | 0.90 | ± 0.10 |
| Hypertension* | 4006 | (60.2%) | 3891 | (63.8%) | 1320 | (26.1%) | 3681 | (62.5%) | 12898 | (54.4%) |
| Systolic blood pressure (mmHg) | 145 | ± 23 | 141 | ± 19 | 128 | ± 17 | 137 | ± 22 | 138 | ± 21 |
| Diastolic blood pressure (mmHg) | 83 | ± 13 | 87 | ± 9 | 77 | ± 10 | 71 | ± 11 | 80 | ± 13 |
| Diabetes mellitus† | 217 | (3.3%) | 277 | (4.5%) | 134 | (2.7%) | 722 | (12.3%) | 1350 | (5.7%) |
| Current smoking | 2118 | (31.8%) | 1724 | (28.2%) | 1057 | (20.9%) | 700 | (11.9%) | 5599 | (23.6%) |
| Hyperlipidemia‡ | 2518 | (37.8%) | 2447 | (40.1%) | 720‖ | (14.2%) | 1563 | (26.5%) | 7248 | (30.6%) |
| TC/HDL ratio | 4.7 | ± 1.6 | 4.8 | ± 1.5 | 3.8 | ± 1.1 | 4.2 | ± 1.3 | 4.4 | ± 1.4 |
| Moderate stenosis | ||||||||||
| Observed prevalence | 1.8% | 0.6% | 0.8% | 4.4% | 2.0% | |||||
| Expected prevalence# | 1.9% | 1.3% | 2.6% | 2.1% | 2.0% | |||||
| Severe stenosis | ||||||||||
| Observed prevalence | 0.7% | 0.1% | 0.2% | 1.1% | 0.5% | |||||
| Expected prevalence# | 0.8% | 0.3% | 0.8% | 0.5% | 0.5% | |||||
Hypertension is defined as a systolic blood pressure above 140mmHg or an diastolic blood pressure above 90mmHg.
Diabetes mellitus is defined as a glucose level above 6.9mmol/l or anti diabetic medicine use.
Hyperlipidemia defined as an TC/HDL ratio above 5mmol/l or statin use.
inclusion criteria: age 65 and older.
classification method: peak systolic velocity ratio method (Tromso), lumen diameter reduction (MDCS), cross sectional area reduction method (CAPS), peak systolic velocity (CHS).
given the risk factor distribution of the total study population.
Model development
Table 2 presents the results from the multivariable analysis for severe stenosis. Age, sex, history of vascular disease, systolic and diastolic blood pressure, TC/HDL ratio, diabetes mellitus and current smoking were independent predictors of moderate and severe stenosis. The positive relation with systolic pressure in combination with an inverse relation with diastolic pressure in one regression model indicates the relevance of pulse pressure in the relation with risk of stenosis. As the fit of the model was better with systolic and diastolic pressure in the model as compared with pulse pressure alone, we decided to present the current model. The calibration of the model was good confirmed by a non-significant Hosmer and Lemeshow test for moderate stenosis (p=0.585), and for severe stenosis (p=0.071). The models discriminated well between participants with and without stenosis, with an AUC corrected for over optimism of 0.82 (95%CI 0.80–0.84) for moderate stenosis and of 0.87 (95% CI 0.85–0.90) for severe stenosis.
Table 2.
Multivariable predictors for presence of moderate and severe and stenosis
| Odds ratio (95% confidence interval)* | ||
|---|---|---|
| Predictors | Moderate stenosis (>50%) |
Severe stenosis (>70%) |
| Age (per 10 years) | 1.8 (1.6–2.1) | 2.2 (1.7–2.8) |
| Male sex | 1.5 (1.2–1.8) | 2.5 (1.7–3.6) |
| History of vascular disease | 1.9 (1.6–2.3) | 2.5 (1.7–3.5) |
| Systolic blood pressure (per 10 mmHg) | 1.3 (1.2–1.4) | 1.3 (1.2–1.5) |
| Diastolic blood pressure (per 10 mmHg) | 0.7 (0.6–0.7) | 0.7 (0.6–0.8) |
| TC/HDL ratio (per point) | 1.2 (1.1–1.3) | 1.2 (1.1–1.4) |
| Diabetes mellitus | 1.3 (1.0–1.8) | 1.6 (1.0–2.5) |
| Current smoking | 2.3 (1.8–2.8) | 3.0 (2.1–4.4) |
| Area under the ROC curve† | 0.82 (0.80–0.84) | 0.87 (0.85–0.90) |
TC, TC, total cholesterol; HDL, high-density lipoprotein
Adjusted for overoptimism (regression coefficients were shrunk by 7% in model for severe stenosis and 2% in model for moderate stenosis).
Adjusted for optimism with bootstrapping techniques.
Clinical application
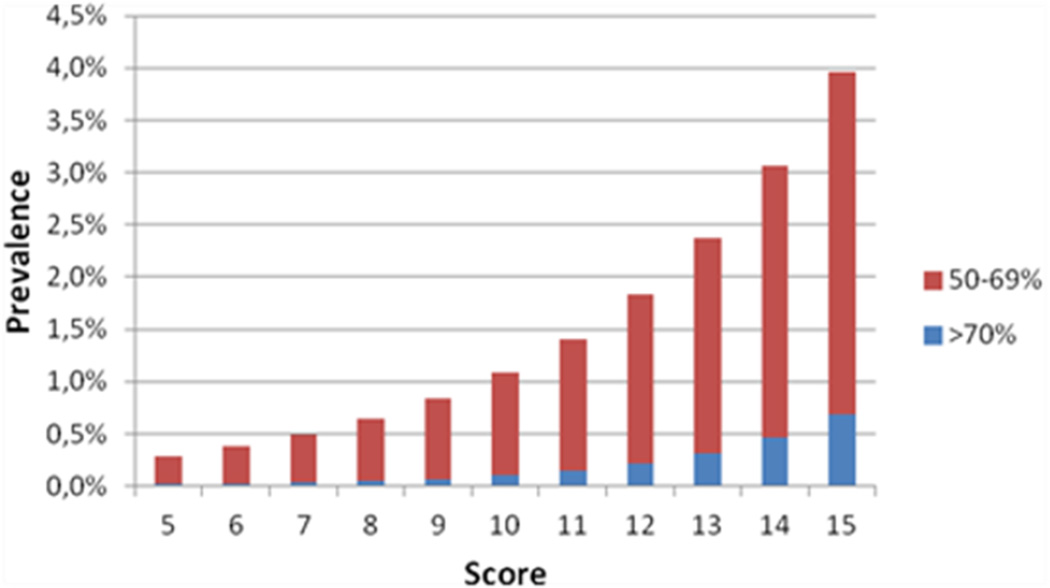
The regression coefficients of the predictors of the final model were converted into a score chart to facilitate practical application (Table 3). As an example how to use this chart, a 65-year-old men, non-smoker, presenting with a SBP of 160, a DBP of 80, with normal lipid levels, no diabetes and no history of vascular disease, will have a sum score of 14 (7+2+0+4+1+0+0+0). This corresponds to a risk of moderate stenosis of 3.1% and a risk of severe stenosis of 0.5% (Figure 1).
Table 3.
Stenosis score chart
| Characteristic | Value | Stenosis score |
|---|---|---|
| Age (years) | < 50 | 0 |
| 50–59 | 4 | |
| 60–69 | 7 | |
| 70+ | 9 | |
| Sex | male | 2 |
| female | 0 | |
| History of vascular disease | no | 0 |
| yes | 3 | |
| Systolic blood pressure (mmHg) | < 125 | 0 |
| 125–139 | 2 | |
| 140+ | 4 | |
| Diastolic blood pressure (mmHg) | < 75 | 2 |
| 75–84 | 1 | |
| 85+ | 0 | |
| TCHDL ratio | < 5 | 0 |
| 5+ | 1 | |
| Diabetes mellitus | no | 0 |
| yes | 1 | |
| Current smoking | no | 0 |
| yes | 3 | |
- LPsevere stenosis = − 10.74 + 0.384 × sum score;
- LPmoderate stenosis = − 7.16 + 0.265 × sum score.
Using these models, the mean predicted probability of severe stenosis was 0.5% and the mean predicted probability of moderate stenosis was 2.0%. We estimated the intercepts for other prevalence figures, so when the model is applied in a population with other prevalence rates of stenosis, the intercept of the model can be adjusted (Webtable 1). When the prevalence of stenosis is lower, the intercept needs to be more negative, leading to lower predicted prevalences.
Figure 1.
Predicted probability of stenosis according to score categories.
Table 4 shows the distribution of participants with and without moderate or severe stenosis across different risk categories. These results are of relevance to indicate the consequences of screening in particular groups. For participants at high stenosis risk (n=7247; 31% of the population), the prevalence of moderate stenosis is estimated to be 4.8% and the number needed to screen is 21. About 68% of the participants in this high-risk category who are initially free of vascular disease have a high cardiovascular risk (regardless of the degree of stenosis) and should receive lifestyle and drug interventions. When using a lower risk threshold for screening means that somewhat more participants with stenosis will be detected but against the expense of screening many more individuals. For example, in participants at intermediate to high stenosis risk (n=11245; 47% of the population), the prevalence of moderate stenosis is estimated to be 3.6% and the number needed to screen is 28.
When we used the Framingham Risk Score estimate of 10 year risk of 20% or above, the number needed to screen for detection of one ACAS was 64. When a lower Framingham Risk Score cut point was used, e.g. 10 year risk of 7.5% as recently proposed, the number needed to screen was much higher.
Discussion
We developed a prediction model that allows identification of participants that might benefit from screening for asymptomatic carotid artery stenosis. We found that age, sex, TC/HDL ratio, systolic and diastolic blood pressure level, history of vascular disease, diabetes and smoking are strong predictors for the probability of having a moderate and severe ACAS. With the use of our prediction rule groups good be identified in whom the number needed to screen to detect one ACAS is between 21 and 35. When the Framingham risk score was used, with a cut point of a 10 year risk of 20% or above, 64 individuals would be needed to screen to detect one ACAS.
Comparison with existing literature
We did not come across studies that specifically aimed at developing a prediction rule for the presence of ACAS. Etiologic focused studies reported determinants of carotid artery stenosis. These studies suggested that elevated blood pressure, smoking, cholesterol levels, increasing age and male sex were associated with presence of carotid artery stenosis.15;25;26 These observations are compatible with our findings. Presence of a bruit over the carotid artery has been evaluated as a means to identify individuals at high risk of a carotid stenosis, but was found to be unreliable.27
Strengths and limitations
The major strength of this study is the large number of individuals that were included in our population-based cohorts. This gave us the opportunity to present a precise and accurate prediction rule. Using bootstrapping techniques, we demonstrated that the prediction rule was robust. The shrinkage factor was close to 1, suggesting a stable model and the calibration after correction for over optimism also was very good (AUC 0.87 for severe (>70%) stenosis). In addition, not all data were available for each participant. With imputation techniques, we were able to use all participants instead of only complete cases. This results in a prediction rule with increased precision. Although there are differences in the methods of measurement of degree of stenosis between studies we are not concerned about the validity of our prediction model. The Tromsø study measured only the right carotid artery. We believe that this had little effect on the prediction rule. Those with a stenosis are the cases, and in Tromsø some of the cases will be in the ‘reference’ population. As the prevalence of stenosis is rather low, the effect of having some few cases in the much larger reference population will not affect the magnitudes and direction of the risk factor relations. Having only one side does however affect the prevalence of stenosis in the population. This means that the prevalence of stenosis is underestimated to some extent. Furthermore, the discrimination between presence and absence of ACAS may be affected by the variation in diagnostic criteria. Unfortunately, there is no easy way to reclassify study participants using uniform stenosis criteria.
Clinical implication
Due to the improvement in drug therapy the annual rate of ipsilateral stroke associated with asymptomatic carotid stenosis has fallen from 2–4% to <1% in the last 20 years.28 Therefore, the balance between benefit and risk for surgery in patients with ACAS has changed. So carotid revascularization as mainly underlying reason for screening for ACAS seems not applicable anymore. Yet, individuals with an ACAS are at very high risk of any future cardiovascular events, and should be considered for best medical treatment following the cardiovascular risk management guidelines and should obtain lifestyle guidance. Our model may help in that respect.
Conclusions
In conclusion, our clinical prediction rule that allows identification of subgroups with relatively high prevalence of moderate (>50%) or severe (>70%) ACAS. When population based screening for ACAS is considered, use of the prediction rule is recommended to identify subgroups in order to reduce the number needed to screen substantially.
Supplementary Material
Acknowledgments
None
Sources of funding
This study is supported by an unconditional grant from The Netherlands Organization for Health Research and Development (ZonMW, project No. 6230.0046).
Footnotes
Conflicts of interest/Disclosures
None
References
- 1.Primatesta P, Allender S, Ciccarelli P, Doring A, Graff-Iversen S, Holub J, et al. Cardiovascular surveys: manual of operations. Eur J Cardiovasc Prev Rehabil. 2007;14:S43–S61. doi: 10.1097/01.hjr.0000277988.18096.3b. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, et al. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 4.Fairhead JF, Mehta Z, Rothwell PM. Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology. 2005;65:371–375. doi: 10.1212/01.wnl.0000170368.82460.b4. [DOI] [PubMed] [Google Scholar]
- 5.Kakisis JD, Avgerinos ED, Antonopoulos CN, Giannakopoulos TG, Moulakakis K, Liapis CD. The European Society for Vascular Surgery guidelines for carotid intervention: an updated independent assessment and literature review. Eur J Vasc Endovasc Surg. 2012;44:238–243. doi: 10.1016/j.ejvs.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Hennerici M, Hulsbomer HB, Hefter H, Lammerts D, Rautenberg W. Natural history of asymptomatic extracranial arterial disease. Results of a long-term prospective study. Brain. 1987;110:777–791. doi: 10.1093/brain/110.3.777. [DOI] [PubMed] [Google Scholar]
- 7.Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000;342:1693–1700. doi: 10.1056/NEJM200006083422302. [DOI] [PubMed] [Google Scholar]
- 8.Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485–1490. doi: 10.1161/01.str.22.12.1485. [DOI] [PubMed] [Google Scholar]
- 9.O'Holleran LW, Kennelly MM, McClurken M, Johnson JM. Natural history of asymptomatic carotid plaque. Five year follow-up study. Am J Surg. 1987;154:659–662. doi: 10.1016/0002-9610(87)90238-8. [DOI] [PubMed] [Google Scholar]
- 10.Nadareishvili ZG, Rothwell PM, Beletsky V, Pagniello A, Norris JW. Long-term risk of stroke and other vascular events in patients with asymptomatic carotid artery stenosis. Arch Neurol. 2002;59:1162–1166. doi: 10.1001/archneur.59.7.1162. [DOI] [PubMed] [Google Scholar]
- 11.Perk J, De BG, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 13.den Hartog AG, Achterberg S, Moll FL, Kappelle LJ, Visseren FL, van der Graaf Y, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke. 2013;44:1002–1007. doi: 10.1161/STROKEAHA.111.669267. [DOI] [PubMed] [Google Scholar]
- 14.Abbott AL, Bladin CF, Levi CR, Chambers BR. What should we do with asymptomatic carotid stenosis? Int J Stroke. 2007;2:27–39. doi: 10.1111/j.1747-4949.2007.00096.x. [DOI] [PubMed] [Google Scholar]
- 15.Mathiesen EB, Joakimsen O, Bonaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromso Study. Cerebrovasc Dis. 2001;12:44–51. doi: 10.1159/000047680. [DOI] [PubMed] [Google Scholar]
- 16.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Life-course perspective on socioeconomic differences in carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1704–1711. doi: 10.1161/01.atv.0000032006.75577.24. [DOI] [PubMed] [Google Scholar]
- 17.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17:299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz MW, von KS, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 20.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 24.The Dutch College of General Practitioners. Multidisciplinary guideline on cardiovascular risk management (revision 2011) Houten: Bohn Stafleu van Loghum; 2011. [Google Scholar]
- 25.Bots ML, Breslau PJ, Briet E, de Bruyn AM, van Vliet HH, van den Ouweland FA, et al. Cardiovascular determinants of carotid artery disease. The Rotterdam Elderly Study. Hypertension. 1992;19:717–720. doi: 10.1161/01.hyp.19.6.717. [DOI] [PubMed] [Google Scholar]
- 26.Beks PH, Mackaay AJ, de VH, de Neeling JN, Bouter LM, Heine RJ. Carotid artery stenosis is related to blood glucose level in an elderly Caucasian population: the Hoorn Study. Diabetologia. 1997;40:290–298. doi: 10.1007/s001250050676. [DOI] [PubMed] [Google Scholar]
- 27.Ratchford EV, Jin Z, Di Tullio MR, Salameh MJ, Homma S, Gan R, et al. Carotid bruit for detection of hemodynamically significant carotid stenosis: the Northern Manhattan Study. Neurol Res. 2009;31:748–752. doi: 10.1179/174313209X382458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies KJ, Thapar A, Kasivisvanathan V, Shalhoub J, Davies AH. Review of transatlantic cardiovascular best medical therapy guidelines - recommendations for asymptomatic carotid atherosclerosis. Curr Vasc Pharmacol. 2013;11:514–523. doi: 10.2174/1570161111311040015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.