Review of how the availability and transport of chloride influence host defense.
Keywords: hypochlorous acid, myeloperoxidase, neutrophil oxidants, phagocytes
Abstract
Salt provides 2 life-essential elements: sodium and chlorine. Chloride, the ionic form of chlorine, derived exclusively from dietary absorption and constituting the most abundant anion in the human body, plays critical roles in many vital physiologic functions, from fluid retention and secretion to osmotic maintenance and pH balance. However, an often overlooked role of chloride is its function in innate host defense against infection. Chloride serves as a substrate for the generation of the potent microbicide chlorine bleach by stimulated neutrophils and also contributes to regulation of ionic homeostasis for optimal antimicrobial activity within phagosomes. An inadequate supply of chloride to phagocytes and their phagosomes, such as in CF disease and other chloride channel disorders, severely compromises host defense against infection. We provide an overview of the roles that chloride plays in normal innate immunity, highlighting specific links between defective chloride channel function and failures in host defense.
Introduction
Humans acquire chloride only through intestinal absorption from food and its seasoning, with adult daily consumption of 5–11 g of sodium chloride. The serious health consequences of inadequate as well as excess salt intake underscore the importance of moderated salt ingestion [1–3]. Because of links between salt intake and optimal health, salt availability and possession have greatly influenced social, economic, and cultural aspects of human history (Box 1).
Salt in history
Salt comes from multiple sources, including sea water, mineral deposits (rock salt), surface encrustations, saline lakes, and brine springs. Because of its importance to survival, salt has become a valuable commodity and a source of wealth. Archaeological evidence as far back as 6050 B.C. indicates that the neolithic people of the Precucuteni culture in Romania extracted salt by boiling salt-laden spring water through the process of briquetage [4]. The oldest saltworks can be dated to at least 6000 B.C. at Xiechi Lake near Yuncheng in Shanxi, China, where salt was harvested from the lakeshore [5]. As a result of uneven geographical distribution and limited transportation means in the ancient times, salt availability, exploitation, and possession influenced the politics, economics, and culture throughout human history [2, 6]. For example, various ancient religious rituals included salt offerings [7], and governments of antiquity imposed salt taxes and monopolized salt supplies. Salt-tax aversion sparked the French Revolution, and the British monopoly of salt caused the Indian War of Independence.
For centuries, medical practitioners have recognized the therapeutic benefits of salt [2, 6]. The Edwin Smith papyrus of ancient Egypt, written in the third pre-Christian millennium, recommends salt for the treatment of chest wounds. The papyrus Ebers (1600 B.C.) describes the use of salt in formulations for laxatives and anti-infectives, and Hippocrates (460 B.C.), the father of Western medicine, observed the healing effects of seawater on the injured hands of fishermen. Greek medicine applied salt topically to skin lesions and prescribed drinking salty or mineralized waters for digestive troubles and inhaled salt for respiratory diseases. Galen (129–200 A.D.), physician in ordinary to the Roman emperor, included salt in remedies for infected wounds, skin diseases, callosities, and indigestion. Pharmacists of the 19th century commonly recommended the internal use of salt for digestive upsets, goiter, glandular diseases, intestinal worms, dysentery, dropsy, epilepsy, and syphilis. External application of salt was advised in cases of rash, swelling, inflammation, and burns. Many salt therapies persist in modern medical practices, ranging from supportive care to therapeutic interventions (Box 2). Even though salt frequently has been used clinically, the molecular mechanisms underlying many of the observed therapeutic effects, especially the antiseptic action, remain largely undefined.
Current practice of salt therapy
Even today, salt constitutes an important component of medicinal therapy and can be injected, inhaled, or ingested [6]. Routinely infused into patients as fluid-replacement therapy [8, 9], isotonic sodium chloride solution (saline) can be used as an irrigating solution to drain, cleanse, or moisten organs or tissues [10]. Dental salt is used to treat plaques of gingivitis and caries [11]. Salt baths are frequently recommended to treat psoriasis, atopic dermatitis, chronic eczema, and arthritis [12]. In the past, steam vapor from salt water was inhaled as treatment of chronic diseases of the upper and lower respiratory tract [13]. Today, patients with respiratory diseases directly inhale aerosols of salt vapor, nebulized by use of oxygen, compressed air, or ultrasonic power, to promote pulmonary toilet. Inhalation of hypertonic saline has been used routinely in treating CF and other chronic lung diseases [14–18]. Saline ingestion can replace electrolytes lost during diarrhea and can increase gastric and pancreatic secretion to modulate digestion [19].
CHLORIDE AND NEUTROPHIL ANTIMICROBIAL ACTION
Adult humans are home to ∼100 trillion microrganisms, outnumbering host cells by ∼10:1 [20] and representing a sizable reservoir of potential infectious agents. To combat any organisms that breach mechanical barriers, the host has developed a powerful immune-defense system, which includes soluble and cellular effectors that act in a coordinated fashion to eliminate invading microbes and restore homeostasis. In normal individuals, the cells critical for first-line protection are phagocytes [21, 22], first observed and described by Elie Metchnikoff [23] more than 1 century ago (and see ref. [24]). Internalization and destruction of encountered microbes by phagocytes, largely neutrophils and macrophages in humans, constitute a functionally critical host defense mechanism that is evolutionary conserved in multicellular organisms [25, 26]. Confined in a membrane-bound, specialized compartment—the phagosome-ingested microbes undergo assault by phagocyte-derived toxins, including antimicrobial proteins stored in granules and reactive oxygen species generated de novo during cell activation [27]. Optimal microbicidal action in phagosomes depends on synergistic interactions among the antimicrobial agents whose generation and activity rely on regulated ion flux.
Stimulated phagocytes exhibit a burst in oxygen consumption that reflects the activity of a multicomponent NOX, whose components are segregated in resting cells but assemble into a functional complex after agonist exposure [28]. Activated neutrophils, monocytes, and macrophages assemble NOX on their phagosomal and plasma membranes and generate oxidants in the lumen of phagosomes or into the extracellular space. During the process, cytoplasmic components (p47phox, p67phox, p40phox, and GTP-bound Ras-related C3 botulinum toxin substrate 1 or 2) associate with flavocytochrome b, a heterodimeric membrane protein composed of gp91phox (aka NOX2) and p22phox. Similar to the other members of the NOX protein family, the phagocyte oxidase operates fundamentally as an electron transferase, shuttling electrons from NADPH across phagosomal or plasma membranes to molecular oxygen, thereby generating the superoxide anion [29]. As evidence of the robust nature of the transfer, it is estimated that formyl peptide-stimulated neutrophils produce ∼5 × 106 superoxide anions/cell [30], a process that requires the translocation of an identical number of electrons. Such electron flux depolarizes the membrane at a rate of ∼1.1 V/s and unchecked, will reach a membrane potential at which electron transfer and NADPH activity will cease [31]. The nominal driving force for electron transport into the phagosome is ∼+160 mV, derived from the difference between standard redox potentials of NADPH2+ in cytoplasm and superoxide anion across the phagosomal membrane [31]. DeCoursey et al. [32] experimentally demonstrated the voltage dependence of NOX, whereby depolarization of the phagocyte membrane inhibits electron transfer through NOX. As a consequence, continuous activity of the oxidase requires compensation of the negative charge, accumulating on the side of the membrane to which electrons flow. Compelling evidence indicates that the Hv1 mediates the bulk of the charge compensation required [32–35]. Zn2+ inhibition of proton channels [36] or that genetic inactivation of Hv1 [37, 38] substantially reduces NOX activity, convincingly proving the requirement of proton-dependent charge compensation for oxidant production by phagocytes.
Superoxide anion is the immediate product of oxygen reduction by the phagocyte oxidase but exerts little, if any, direct antimicrobial action. Instead, superoxide anion rapidly undergoes conversion to H2O2, an oxidant with modest antimicrobial action, which in turn, reacts with the granule protein MPO to catalyze the 2-electron reduction of chloride to yield the potent microbicide HOCl [39, 40]. With MPO representing ∼5% of the total dry weight of human neutrophils, these phagocytes are well suited to support robust HOCl generation. Winterbourn et al. [41] have credibly modeled the reactions of H2O2 and MPO in neutrophils and found that during the respiratory burst, a phagosome with 1.2 fl vol generates HOCl at a rate of ∼0.16 fmol/min or ∼1.6 × 106 HOCl molecules/s. If abundant phagosomal chloride is available, 28–72% of the oxygen consumed is converted to HOCl [42–45]. Consequently, HOCl generation in phagosomes requires a continuous supply of chloride.
Antimicrobial action of HOCl
The longstanding use of hypochlorite, the salt form of HOCl or bleach, as an antiseptic agent in medicinal, industrial, and domestic settings [46], provides testimony to recognition of its potent and broad-spectrum antimicrobial action. Although the specific molecular mechanisms for HOCl-mediated antimicrobial action with human neutrophils are incompletely understood [47, 48], HOCl has potent biochemical reactivity with a wide variety of biologic substrates [49–51]. Sulfur-containing compounds, such as cysteine, methionine, or glutathione, are especially susceptible to oxidation by HOCl. The sulfur group in cysteine is typically oxidized into sulfenic acid, sulfonic acid, or disulfide if intramolecular or intermolecular thiol groups are available, whereas oxidation of the sulfur group in methionine yields methionine sulfoxide or dehydromethionine or sulfilimine [52–54]. In addition to oxidizing vulnerable residues, HOCl chlorinates macromolecules. Primary and secondary amines are converted into the respective chloramines, which are also capable of chlorinating and oxidizing other molecules [55, 56].
Because of its broad chemical reactivity, HOCl attacks multiple targets within microbes, including enzymes essential for maintaining structural integrity and energy production. As neutrophils encounter and ingest microorganisms with a wide variety of structural components, including cell walls, polysaccharide capsules, and LPS, the broad spectrum of activity of HOCl provides a defense system that matches the diversity of its targets [57]. Compared with H2O2, the product of NOX, HOCl is ∼10 million-fold more reactive against thiols; reaction rate, 2.9 M−1s−1 for H2O2 [58] versus 3 × 107 M−1s−1 for HOCl [51]. Consequently, any cryptic, vulnerable targets that become exposed are only transiently more likely to react with HOCl than with H2O2. In fact, HOCl-mediated killing of susceptible bacteria occurs within milliseconds [59] and in the case of Escherichia coli, with remarkable efficiency, requiring ∼108 molecules of HOCl versus ∼1011 molecules of H2O2 [60]. The relative speed of bactericidal action against Klebsiella pneumoniae, another important human pathogen, favors HOCl as well, with viability reduced in half after 60 min exposure to 1 μM HOCl versus 1 mM H2O2 [61]. Taken together, its high levels of production, broad spectrum of susceptible targets, and robust chemical reactivity make HOCl ideally suited to arm human neutrophils to meet a wide range of microbial challenges.
Chloride sources available to neutrophil phagosomes
For neutrophils to sustain HOCl production, there needs to be an accessible reservoir of chloride anion and a mechanism to deliver chloride into phagosomes. Potential sources for phagosomes to obtain chloride include the extracellular fluid, neutrophil granules, and cytoplasm. As phagosomes enclose their internalized objects tightly, the chloride internalized from the extracellular space incidentally during phagocytosis is limited and consumed rapidly [41] and insufficient to meet the chloride demand. Granules may contain prestored chloride, but their total content is believed to be limited, given the minimal increase in phagosomal volume after granule fusion. However, the relatively high-chloride concentration in the cytoplasm of resting neutrophils and monocytes, measured at 80–100 mM [62–65], may meet the immediate needs of phagosomes. Over the initial 5–10 min of stimulation by PMA or phagocytosis of Candida albicans or Staphylococcus aureus, neutrophils release 70–80% of their cytosolic chloride reservoir to extracellular space and presumably to phagosomes as well [65, 66]. We have used a novel, chloride-sensitive fluorochrome conjugated to zymosan particle to probe the chloride concentration within phagosomes of human neutrophils [67, 68]. The assay conditions include the addition of sodium azide to inhibit MPO-mediated oxidation of the probe. Under these experimental conditions, the concentration of chloride in the lumen of zymosan-containing phagosomes is ∼70 mM under steady-state conditions [67], although this value likely overestimates the ambient chloride concentration in phagosomes, as the consumption of chloride anion by MPO-mediated HOCl production is blocked by sodium azide. The observed accumulation of chloride in phagosomes, while the cytosol is drastically losing chloride, creates a paradox for anion flow, as the NOX depolarization of the phagosomal membrane would drive anions out of the phagosome. Chloride redistribution relies on specialized protein carriers, as the anion cannot permeate lipid membranes freely. Multiple classes of anion channels preferentially transporting Cl– have been characterized in mammalian cells [69–71]. However, the mechanisms that support the seeming counterflow of chloride anion into phagosomes have not been fully explicated (vide infra).
CFTR-mediated chloride flux into phagosomes
Dedicated chloride channels or transporters in mammalian cells include cAMP/PKA-activated Cl– channels, such as CFTR [72, 73], voltage-gated Cl– channels, or H+/Cl– exchangers, such as ClC family members [74], CaCCs, including TMEM16A and TMEM16B [75–80], ligand-gated Cl– channels (GABA and glycine gated) [81, 82], and volume-regulated Cl– channels [83]. To date, only CFTR [84–86] and ClC3 [87, 88] have been identified in phagocytes and thus implicated in phagocytic host defense. CFTR is a phosphorylation-dependent, voltage-independent chloride channel. Based on its primary protein sequence, CFTR belongs to the adenine nucleotide-binding cassette (ATP-binding cassette transporter) transporter family, possessing 2 MSDs, 2 NBDs, and an R domain [73, 89, 90]. The MSDs form the Cl‒-selective pore, the NBDs regulate channel gating, and the R domain controls channel activity.
Expressed in mature human neutrophils [85, 91–93], CFTR resides in the membranes of secretory vesicles [85]. During phagocytosis, secretory vesicles fuse with nascent phagosomes, thereby incorporating CFTR into the phagosomal membrane and providing a means by which to transport chloride from cytoplasm into the phagosomal lumen. Neutrophils from patients with a variety of genotypes of CF achieve subnormal levels of chloride in phagosomes [68], and pharmacologic inhibition of CFTR in normal neutrophils reduces the influx of chloride into phagosomes to levels seen in CF neutrophils [68, 85]. Taken together, these data demonstrate that maintaining optimal chloride concentration in neutrophil phagosomes requires normal CFTR activity. Supporting this interpretation are studies that report decreased chlorination and killing of ingested Pseudomonas aeruginosa by neutrophils from patients with CF [85, 94]. Observations from animal-infection models reinforce the role for CFTR in phagocytic host defense. Neutrophils from zebrafish embryos with reduced expression of the cftr gene (Cftr morphants) exhibit a reduced respiratory burst response and directional migration, and the embryos fail to resist infection with Pseudomonas [95]. Mice with only myeloid CFTR inactivation have phagocytic host-defense defects and impaired pulmonary clearance of bacteria [96].
Cation-dependent chloride flux into phagosomes
In addition to CFTR, other chloride carriers in neutrophils must contribute to phagosomal chloride accumulation. Neutrophils express ClC3, a member of the intracellular ClC protein family of chloride-protein exchangers (ClC3 to -7) [97, 98] that are broadly expressed and predominantly on compartments of the endosomal/lysosomal pathways [99]. As a result of its strong voltage dependence and strong outward rectification, ClC3 should be inactive at luminal-positive voltages [99]. However, ClC3 is also pH dependent, whereby low pH uncouples the Cl–/H+ interchange and turns ClC3 into exclusively a chloride channel [100]. This uncoupling process is nearly completed at pH 6.2, which is physiologically achievable in macrophage phagosomes, as macrophages acidify phagosomes rapidly to pH 4–5 after phagocytosis [101]. Neutrophil phagosomes, however, remain nearly neutral for prolonged periods of time after phagocytosis, followed by slow acidification [102]. In that setting, the pH-induced conversion of ClC3 from an antiporter to a conducting channel may serve as a control mechanism to allow chloride entry into the phagosomal lumen at the permissive phagosomal membrane potential and pH gradient. Although the precise role of ClC3 in neutrophil biology remains incompletely defined, murine neutrophils lacking ClC3 exhibit blunted NOX activity. In addition, studies in smooth muscle cells demonstrate that ClC3 provides the countercurrent in endosomes after TNF-α or IL-1β stimulation of NOX1 [103], a homolog of the phagocyte NOX.
Cation influx is a prerequisite for chloride to enter phagosomal lumen. Hv1, which compensates for electron flux through NOX [104, 105], could generate a net-positive charge in the phagosomal lumen that would facilitate chloride anion influx only to the extent that there is a driving force for passive proton influx. In other words, cytoplasmic pH must be lower than phagosomal pH. This situation seems improbable for macrophages, whose phagosomes are acidic, but could occur in neutrophils, as their phagosomes remain neutral, and cytoplasmic pH drops sharply, immediately upon phagocytosis [106]. On the other hand, phagosomal acidification in murine macrophages is attenuated drastically in hydrogen voltage-gated channel 1 knockout mice [107], indicating that proton channels do contribute to this process, although the V-ATPase is thought to drive acidification. The V-ATPase operates as a rotary proton-pumping nanomotor [108–110], catalyzing the hydrolysis of ATP to create an electrochemical proton gradient across membranes and thereby, driving proton accumulation in intracellular compartments [111]. In macrophages, the electrical potential across the phagosomal membrane measures ∼27 mV, with the inside lumen positive. Inactivation of the V-ATPase by concanamycin A reduces the phagosomal membrane potential in macrophages from ∼+27 mV (lumen positive) to +17 mV [112]. Thus, the V-ATPase could support an electrical potential that would facilitate chloride entry into the macrophage phagosome. Neutrophils also express the proton pump, but its contribution to phagosomal membrane electrical potential has not been studied.
In addition to chloride channels or exchangers that permit phagosomal chloride transport, CCCs can also facilitate phagosomal accumulation of chloride. Expressed in phagocytes and phagosomes [113], the KCC mediates coupled electroneutral movement of K+ and Cl‒ across cytoplasmic and phagosomal membranes [113, 114] without affecting the electrical potential. As a result of the high tonicity of K+, robust KCC activity will alter cell or organelle volume [115]. However, although activated neutrophils increase their cell volumes by ∼20% [116], phagosomal volume tends not to change significantly. Thus, the contribution of KCC to phagosomal chloride movements requires further study.
CaCCs are calcium- and voltage-gated chloride channels, whose broad expression, including epithelia, smooth muscle, and neurons, predicts their potential expression in phagocytes [117, 118]. Stimulation of GPCRs mobilizes calcium in the cytosol and triggers CaCC-mediated cell responses. Ligands to GPCRs in smooth muscle cells include ATP, acetylcholine, endothelin-1, angiotensin II, lysophosphatidic acid, and histamine [119]. Conspicuously in phagocytes, all of the chemoattractants (N-formylated peptides and chemokines) stimulate GPCRs and calcium signaling to activate and mobilize phagocytes [120, 121], which would surely evoke calcium-dependent chloride transport in phagocytes.
Proposed model for phagosomal chloride acquisition
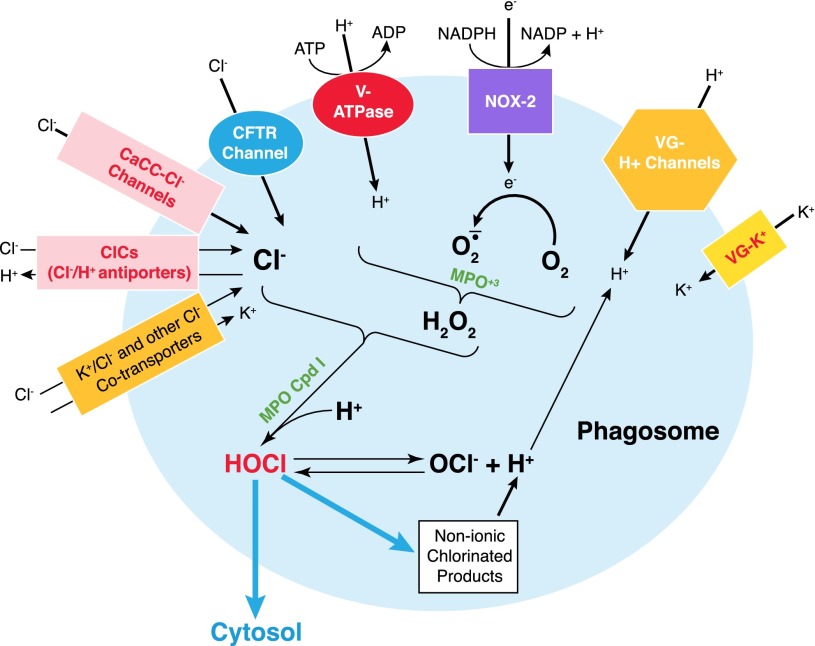
Multiple mechanisms sustain intraphagosomal chloride concentration that can support HOCl production (Fig. 1). NOX and V-ATPase provide the bulk of electrogenic activity in phagosomes. The voltage-dependent H+ channels (e.g., Hv1) and ClCs (e.g., ClC3) are conditionally electrogenic at permissive pH. Hv1-mediated proton influx compensates for the electron flow through NOX, whereas net-positive charges created by the V-ATPase pump could be balanced by an influx of an anion, such as Cl– via Cl– channels or exchangers. Furthermore, protons accumulated within the phagosome by the combined action of Hv1 and V-ATPase regulate luminal pH and, thus, sustain generation of H2O2 and HOCl. The phagosomal membrane potential governs the activity of not only Hv1 but also ClCs and CaCls, thereby indirectly contributing to the regulation of chloride flux.
Figure 1. Ionic trafficking and charge balance in neutrophil phagosomes.
NOX (Nox2) transports electrons from the cytoplasm to the phagosomal lumen to reduce molecular O2 to O2‒. The consequent membrane potential build-up is neutralized by cation influx through multiple cation channels or pumps, including Hv1 (VG-H+), voltage-gated potassium (VG-K+) channels, and the V-ATPase proton pump. Ferric MPO (MPO3+) dismutates superoxide anion to H2O2, whereas MPO compound I (MPO Cpd I) oxidizes Cl‒ to produce chlorine bleach (HOCl). Phagosomal chloride acquisition is achieved through the following: 1) the PKA-activated chloride channel (e.g., CFTR channel); 2) ClCs (e.g., ClC3 2Cl‒/H+ antiporter); 3) CaCCs; and 4) CCCs (e.g., KCC).
In addition to the membrane potential, a chemical gradient drives chloride into the phagosome. Although Cl– is released as HOCl reacts with thiols and thioesters, most Cl– becomes unavailable, consumed in nonionic-chlorinated products or lost as HOCl escapes from phagosomes. Therefore, both the electronic gradient, generated by V-ATPase, Hv1, and Cl‒/H+ exchanger, and the chemical gradient, derived from chloride consumption, together drive chloride redistribution into phagosomes and sustain HOCl production.
CFTR CHLORIDE CHANNEL AND HOST DEFENSE
The clinical consequences of defective chloride transport on host defense highlight the importance of chloride for the maintenance of a normal immune state. Patients with CF, a genetic disease, as a result of inherited defects in CFTR [122, 123], frequently succumb to bacterial pulmonary infection. Although pulmonary function is normal at birth in CF humans and animals, frequent and recurrent infections fuel the chronic inflammation that induces airway injury and structural changes, leading over time to bronchiectasis and deterioration in pulmonary function [124, 125]. The predominant pulmonary findings in the lungs of patients with CF include neutrophil infiltration, purulent small airway obstruction, and chronic bacterial infection [126]. Lung infection in CF has a distinct pathogen profile that contrasts with that seen in infections occurring in the general population. Pathogens include P. aeruginosa, Burkholderia cepacia, S. aureus, and Haemophilus influenzae. It is noteworthy that the proclivity of patients with CF for infections with B. cepacia and with S. aureus overlaps with the spectrum of pathogens seen in patients with CGD [127, 128], an inherited impairment of NOX [129–131]. Given the evidence indicating defective neutrophil function in patients with CF, the parallel between CGD and CF is intriguing, although patients with CGD experience frequent and severe infections in organ systems other than the lung, whereas infectious complications in CF are largely restricted to the airways. The clinically relevant question becomes how the CFTR chloride channel defect renders CF patients more susceptible to recurrent and relapsing infections in the respiratory tract with these particular opportunistic pathogens.
Pulmonary host defense reflects the combined activities of resident cells, such as pulmonary epithelial cells and tissue macrophages, and recruited immune cells, most notably, neutrophils and monocytes. Epithelial and mucosal dysfunction contributes to the pathogenesis of the associated lung disease in CF [132]. Effective clearance of inhaled particulates relies on ASL, a shallow layer of liquid atop airway epithelium that possesses a gel-like mucus layer and a sol-like liquid phase. Under normal circumstances, the mucus layer serves to trap inhaled microorganisms and particles to restrict spreading in the lung, whereas the liquid phase bathes and lubricates epithelial cilia to facilitate mechanical movement [133, 134]. Synchronized ciliary movement sweeps trapped microbes and particulate matter toward the mouth to be expectorated or swallowed. Epithelial chloride secretion, sodium absorption, and secondarily, water retention jointly regulate the volume and composition of the ASL. In CF airway epithelia, dysfunctional CFTR reduces chloride and bicarbonate secretion, thereby decreasing the osmotic force for water retention in ASL. The changes in the quality and quantity of ASL impede mucociliary clearance [135] and diminish the potency of airway antimicrobials, such as β-defensins [136], a fundamental component of innate immunity in airways [133], thereby creating a static milieu to support microbial colonization. After local defenses are overwhelmed, pulmonary infection ensues with sequential recruitment of neutrophils and monocytes to the lung and into the ASL.
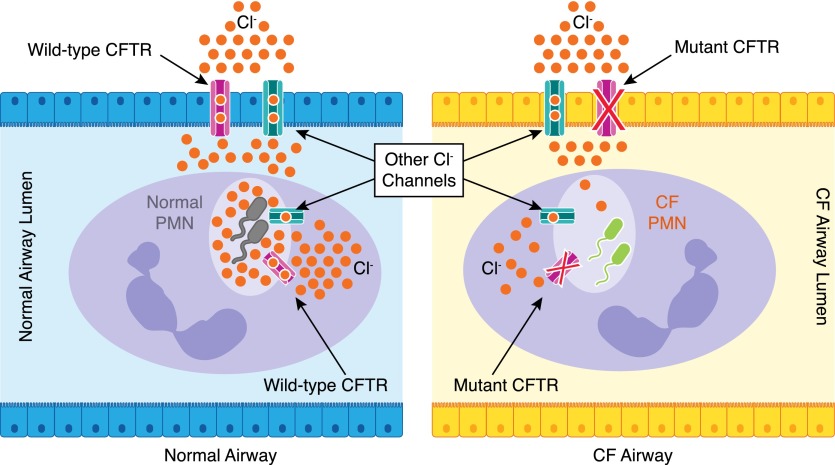
With contributions from other chloride channels, CFTR in airway epithelia sustains ASL chloride levels, which in turn, provide a source for stimulated neutrophils to replenish cytoplasmic stores within minutes. Within neutrophils, CFTR transports chloride from the cytoplasm to phagosomes to support HOCl production. Hence, defective CFTR expression in airway epithelial cells compounds the inherent phagocytic host defect (Fig. 2).
Figure 2. Comparison of neutrophil chloride flux in normal and CF airways.
(Left) Normal airway epithelial cells express a functional CFTR channel and other chloride channels that transport chloride to the airway lumen. In addition, normal neutrophils express the normal CFTR and other chloride channels. Thus, the normal chloride supply chain ensures sufficient phagocyte chloride supplies to support sustained generation of chlorine bleach (HOCl), optimally to kill the phagocytosed pathogens. (Right) CF airway epithelial cells and neutrophils both lack functional CFTR expression, which together, limits chloride transport to neutrophil phagosomes and thereby compromises microbial killing. PMN, polymorphonuclear leukocytes.
Recent studies provide some insight into the relative contributions of defective CFTR activity in neutrophils versus airway epithelia with respect to host defense. Mice with conditional inactivation of Cftr in myeloid tissues have defective phagosomal HOCl production and microbial killing, which lead to incompetent lung bacterial clearance and reduced survival in response to pulmonary challenge with Pseudomonas [96]. However, the reduction in survival was less than that of mice with ubiquitous CFTR deletion in myeloid cells and airway epithelia [137]. These data clearly indicate that defective CFTR activities in neutrophils and in airway epithelia synergize to undermine normal host defense in the lung.
OTHER CHLORIDE CHANNELS OR CARRIERS AND HOST DEFENSE
In addition to CF, other chloride carrier defects have been causally linked to clinical disorders, such as neurodegeneration and lysosomal storage disease, Batten disease, and Dent’s disease [70, 99, 138]. However, with the exception of CF, the contribution of specific chloride transport defects to impaired host defense remains understudied.
Voltage-gated chloride channels and host defense
As discussed earlier, the organelle-associated ClCs mediate Cl– flux across intracellular membranes and participate in organelle membrane potential neutralization, chloride acquisition, and acidification of compartments. Because of the critical role of endocytosis and phagocytosis in antigen presentation and microbial clearance, dysfunction of these intracellular ClCs undermines optimal host defense. Mice that lack functional ClC3 have a complex phenotype, including growth retardation, blindness, kyphoscoliosis, seizures, severe degeneration of the hippocampus and retina, and premature death [139–141]. It is noteworthy that ClC3−/−mice develop sepsis after surgical injury and are more susceptible to infection, suggesting the presence of defective host defense [87]. ClC3−/− neutrophils have reduced NOX activity, diminished phagocytosis, abnormal shape change and chemotaxis, and impaired transendothelial migration [142]. Although dysfunction of ClC3 or CFTR blocks normal phagocytic chloride acquisition, mutants in each gene exhibit phenotypic differences. For example, CF neutrophils appear to have normal chemotaxis, as CF patients display exuberant neutrophil infiltration in the lungs [126] and neutrophils from myeloid, CFTR-inactivated mice migrate normally to infected lungs [96].
Early endosomes in epithelial cells in the kidney and intestine and in macrophages [88] express ClC5, and defective ClC5, the molecular basis for Dent’s disease, causes urinary losses of low molecular-weight proteins, phosphate, and calcium, culminating in recurrent nephrolithiasis in affected patients [143, 144]. Abnormal endocytosis likely compromises normal processing and presentation of antigens by macrophages. ClC5−/− mice exhibit defects in IL-6-dependent immune processes and increased susceptibility to ulcerative colitis [88] However, links between chloride transport and endocytosis remain incompletely elucidated. Recent data show that it is a chloride-proton exchange rather than the pure chloride conductance of ClC5 that plays a crucial role in endocytosis [145]. Interestingly, a CFTR-mediated chloride-channel defect also decreases renal endocytosis and accumulation of aminoglycoside drugs [146], although to a lesser extent than in the ClC5 antiporter defect, suggesting the importance of endosomal chloride in endocytosis.
Unlike ClC5, which localizes to early endosomes, ClC6 and -7 are expressed in lysosomes and late endosomes [147–149]. Disruption of ClC6 or ClC7 in mice leads to neuronal ceroid lipofuscinoses (Batten disease), a lysosomal storage disorder [147, 149], facilitating vesicular acidification and chloride accumulation [150, 151]. Although defects of ClC6 and -7 cause neurodegeneration, distinct phenotypes can be distinguished [152]. ClC6−/− mice display mild behavioral changes and a normal lifespan but exhibit up-regulation of inflammatory genes, implicating immune responses in some phase of the pathogenesis of the disease [149]. The ClC7 defect displays severe retinal and CNS degeneration and early death by 7 wk old [148]. ClC7 contributes to bone resorption, as ClC7−/− causes osteopetrosis or marble bone disease [153] and lysosomal degradation of internalized proteins [154], potentially affecting resolution of inflammation and correct antigen presentation. If expressed in phagocytes, a possibility given their broad tissue-expression pattern, ClC6 and -7 may contribute to host defense.
CaCCs and host defense
CaCCs are outwardly rectifying and activated by cytosolic Ca2+ at a positive membrane potential [70, 117]. These chloride channels play important roles in epithelial fluid secretion [78, 155], sensory transduction and adaptation [80, 156], regulation of smooth muscle tone [119, 157], control of neuronal [158], and cardiac [159] excitability and nociception [160]. Encoded by the ANO/TMEM16 family genes, TMEM16A (ANO1) and TMEM16B (ANO2) are the first 2 family members identified to be associated with Ca2+ signaling via GPCRs [78, 161]. Mice lacking TMEM16A (Tmem16a−/−) fail to thrive during postnatal life, with ∼90% dying within the first 9 d of life, and display a congenital defect in tracheal cartilage [155, 162]. Interestingly, similar congenital cartilaginous defects occur in CFTR−/− mice [155]. Parallels between Tmem16a−/− mice and CFTR−/− mice extend to the gastrointestinal tract as well; the former fail to generate slow waves in intestines, thus lacking gastrointestinal peristalsis, whereas CFTR−/− mice have severe intestinal obstruction, which is believed to lead to early death before or during weaning [163]. These phenotypic similarities between the 2 chloride-channel knockout mice may reflect a shared defect in an activity requiring normal supply of chloride by both transporters. Recognition that CF mice develop a host defense defect provides the basis for speculation that the Tmem16a−/− mice might likewise be immunologically compromised. Direct evidence linking CaCCs to host defense comes from a recent study of Drosophila, demonstrating that flies lacking the TMEM16 gene Subdued succumb to gut bacterial infection [164]. The Subdued defect may block the production of antimicrobial oxidants and result in increased bacterial proliferation and higher lethality [164].
KCCs and host defense
Ubiquitously expressed [114], KCC responds to cell swelling [165], thiol reagents [166], or internal magnesium depletion [167]. It plays important roles in blood-pressure control and cell-volume regulation [168]. Sun and colleagues [113] reported that mice lacking KCC activity have an impaired oxidant production and have increased susceptibility to infection after intraperitoneal challenge with bacteria. Evidence shows that KCC regulates NOX assembly by altering membrane recruitment and phosphorylation of essential oxidase components [113]. As KCC is volume activated, and phagocyte volume swells after stimulation, defective KCC may affect chloride transport from extracellular space to cytoplasm, therefore impairing phagocytic host defense by reducing the cytoplasmic reservoir of the chloride ion.
CONCLUDING REMARKS
Sodium and chloride in the human body are analogized as “the king and queen of electrolytes” [138]. Despite the prominence of chloride, representing 70% of the total negative ion content, this anion was considered only a companion ion to sodium and potassium, receiving little scientific attention in its own right for a long time. The genuine interest in chloride arose when chloride-carrier defects were causally linked to clinical diseases. In addition to its many well-established physiologic functions, chloride operates as a critical element in host immunity. Inadequate supplies of chloride undermine optimal host-defense capacity by compromising the production of HOCl in human neutrophil phagosomes. This and other causative links between chloride channelopathies and host-defense failure provide new insights into the long-observed antiseptic properties of salt.
Acknowledgments
For this research, the Wang laboratory is currently funded by a Louisiana State University Health Sciences Center research grant, and the Nauseef laboratory is supported by Grants AI070958 and AI044642 from the U.S. National Institutes of Health and by a merit review award and given use of facilities at the Iowa City Department of Veterans Affairs Medical Center (Iowa City, IA, USA). The authors thank the reviewers of our manuscript for helpful suggestions that improved clarity and accuracy of the text. The authors extend special thanks to Dr. Fred Lamb (Vanderbilt University) for discussions on ClCs and to Dr. Thomas DeCoursey (Rush University Medical Center) for informative conversations on charge compensation and for assistance in editing sections of the text for clarity.
Glossary
- ANO1/2
anoctamin 1/2
- ASL
airway surface liquid
- CaCC
calcium-activated Cl− channel
- CCC
cation-chloride cotransporter
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- CGD
chronic granulomatous disease
- ClC
chloride-conducting ion channel
- GPCR
G protein-coupled receptor
- HOCl
hypochlorous acid
- Hv1
voltage-gated proton channel
- KCC
K+/Cl− cotransporter
- MPO
myeloperoxidase
- MSD
membrane-spanning domain
- NBD
nucleotide-binding domain
- NOX
NADPH oxidase
- PKA
protein kinase A
- R
regulatory domain
- TMEM
transmembrane protein
- V-ATPase
vacuolar-type ATPase
REFERENCES
- 1.Frisoli T. M., Schmieder R. E., Grodzicki T., Messerli F. H. (2012) Salt and hypertension: is salt dietary reduction worth the effort? Am. J. Med. 125, 433–439. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo M., Capasso G., Di Leo V. A., De Santo N. G. (1994) A history of salt. Am. J. Nephrol. 14, 426–431. [DOI] [PubMed] [Google Scholar]
- 3.Kotchen T. A., Cowley A. W. Jr., Frohlich E. D. (2013) Salt in health and disease—a delicate balance. N. Engl. J. Med. 368, 1229–1237. [DOI] [PubMed] [Google Scholar]
- 4.Weller O., Dumitroaia G. (2005) The earliest salt production in the world: an early Neolithic exploitation in Poiana Slatinei-Lunca, Romania. Antiquity 79, 1–4. [Google Scholar]
- 5.Kurlansky M. (2002) Salt: A World History. Walker and Co., New York. [Google Scholar]
- 6.Denton D. (1982) The Hunger for Salt. An Anthropological, Physiological and Medical Analysis. Springer-Verlag, Berlin, Heidelberg, New York. [Google Scholar]
- 7.Ritz E. (1996) The history of salt—aspects of interest to the nephrologist. Nephrol. Dial. Transplant. 11, 969–975 (http://www.academia.edu/936358). [DOI] [PubMed] [Google Scholar]
- 8.Greig E. D. (1946) The treatment of cholera by intravenous saline injections; with particular reference to the contributions of Dr. Thomas Aitchison Latta of Leith. Edinburgh Med. J. 53, 256–263. [PMC free article] [PubMed] [Google Scholar]
- 9.Baskett T. F. (2002) William O’Shaughnessy, Thomas Latta and the origins of intravenous saline. Resuscitation 55, 231–234. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe P. J., Fawcett T. N. (2002) Wound cleansing: the evidence for the techniques and solutions used. Prof. Nurse 18, 95–99. [PubMed] [Google Scholar]
- 11.Marthaler T. M. (2013) Salt fluoridation and oral health. Acta Med. Acad. 42, 140–155. [DOI] [PubMed] [Google Scholar]
- 12.Adler-Cohen C., Czarnowicki T., Dreiher J., Ruzicka T., Ingber A., Harari M. (2012) Climatotherapy at the Dead Sea: an effective treatment modality for atopic dermatitis with significant positive impact on quality of life. Dermatitis 23, 75–80. [DOI] [PubMed] [Google Scholar]
- 13.Chervinskaya A. V., Zilber N. A. (1995) Halotherapy for treatment of respiratory diseases. J. Aerosol Med. 8, 221–232. [DOI] [PubMed] [Google Scholar]
- 14.Da Dalt L., Bressan S., Martinolli F., Perilongo G., Baraldi E. (2013) Treatment of bronchiolitis: state of the art. Early Hum. Dev. 89 (Suppl 1), S31–S36. [DOI] [PubMed] [Google Scholar]
- 15.Nagakumar P., Doull I. (2012) Current therapy for bronchiolitis. Arch. Dis. Child. 97, 827–830. [DOI] [PubMed] [Google Scholar]
- 16.Kellett F., Robert N. M. (2011) Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir. Med. 105, 1831–1835. [DOI] [PubMed] [Google Scholar]
- 17.Reeves E. P., McElvaney N. G. (2012) The facilitating effect of hypertonic saline on resolution of airway inflammation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 185, 226–227. [DOI] [PubMed] [Google Scholar]
- 18.Reeves E. P., Molloy K., Pohl K., McElvaney N. G. (2012) Hypertonic saline in treatment of pulmonary disease in cystic fibrosis. ScientificWorldJournal 2012, 465230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nightingale J., Woodward J. M.; Small Bowel and Nutrition Committee of the British Society of Gastroenterology (2006) Guidelines for management of patients with a short bowel. Gut 55 (Suppl 4), iv1–iv12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underhill D. M., Ozinsky A. (2002) Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20, 825–852. [DOI] [PubMed] [Google Scholar]
- 22.Flannagan R. S., Jaumouillé V., Grinstein S. (2012) The cell biology of phagocytosis. Annu. Rev. Pathol. 7, 61–98. [DOI] [PubMed] [Google Scholar]
- 23.Metchnikoff E. (1905) Immunity in Infective Diseases. Cambridge University Press, London, UK. [Google Scholar]
- 24.Cavaillon J. M. (2011) The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J. Leukoc. Biol. 90, 413–424. [DOI] [PubMed] [Google Scholar]
- 25.Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- 27.Nauseef W. M. (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219, 88–102. [DOI] [PubMed] [Google Scholar]
- 28.Nauseef W. M. (2008) Biological roles for the NOX family NADPH oxidases. J. Biol. Chem. 283, 16961–16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babior B. M. (2004) NADPH oxidase. Curr. Opin. Immunol. 16, 42–47. [DOI] [PubMed] [Google Scholar]
- 30.Yagisawa M., Yuo A., Yonemaru M., Imajoh-Ohmi S., Kanegasaki S., Yazaki Y., Takaku F. (1996) Superoxide release and NADPH oxidase components in mature human phagocytes: correlation between functional capacity and amount of functional proteins. Biochem. Biophys. Res. Commun. 228, 510–516. [DOI] [PubMed] [Google Scholar]
- 31.Decoursey T. E. (2003) Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579. [DOI] [PubMed] [Google Scholar]
- 32.DeCoursey T. E., Morgan D., Cherny V. V. (2003) The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534. [DOI] [PubMed] [Google Scholar]
- 33.Demaurex N., El Chemaly A. (2010) Physiological roles of voltage-gated proton channels in leukocytes. J. Physiol. 588, 4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki M., Takagi M., Okamura Y. (2006) A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592. [DOI] [PubMed] [Google Scholar]
- 36.Chvapil M., Stankova L., Zukoski C. IV, Zukoski C. III (1977) Inhibition of some functions of polymorphonuclear leukocytes by in vitro zinc. J. Lab. Clin. Med. 89, 135–146. [PubMed] [Google Scholar]
- 37.Ramsey I. S., Ruchti E., Kaczmarek J. S., Clapham D. E. (2009) Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA 106, 7642–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Chemaly A., Okochi Y., Sasaki M., Arnaudeau S., Okamura Y., Demaurex N. (2010) VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J. Exp. Med. 207, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klebanoff S. J. (1968) Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 95, 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klebanoff S. J., Kettle A. J., Rosen H., Winterbourn C. C., Nauseef W. M. (2013) Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 93, 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winterbourn C. C., Hampton M. B., Livesey J. H., Kettle A. J. (2006) Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 281, 39860–39869. [DOI] [PubMed] [Google Scholar]
- 42.Foote C. S., Goyne T. E., Lehrer R. I. (1983) Assessment of chlorination by human neutrophils. Nature 301, 715–716. [DOI] [PubMed] [Google Scholar]
- 43.Weiss S. J., Klein R., Slivka A., Wei M. (1982) Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J. Clin. Invest. 70, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas E. L., Grisham M. B., Jefferson M. M. (1983) Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J. Clin. Invest. 72, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman A. L., Hampton M. B., Senthilmohan R., Winterbourn C. C., Kettle A. J. (2002) Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 277, 9757–9762. [DOI] [PubMed] [Google Scholar]
- 46.Rutala W. A., Weber D. J. (1997) Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 10, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winterbourn C. C., Kettle A. J. (2013) Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 18, 642–660. [DOI] [PubMed] [Google Scholar]
- 48.Hurst J. K. (2012) What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 53, 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies M. J. (2011) Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 48, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pattison D. I., Hawkins C. L., Davies M. J. (2009) What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem. Res. Toxicol. 22, 807–817. [DOI] [PubMed] [Google Scholar]
- 51.Pattison D. I., Davies M. J. (2001) Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 14, 1453–1464. [DOI] [PubMed] [Google Scholar]
- 52.Deborde M., von Gunten U. (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-kinetics and mechanisms: a critical review. Water Res. 42, 13–51. [DOI] [PubMed] [Google Scholar]
- 53.Peskin A. V., Turner R., Maghzal G. J., Winterbourn C. C., Kettle A. J. (2009) Oxidation of methionine to dehydromethionine by reactive halogen species generated by neutrophils. Biochemistry 48, 10175–10182. [DOI] [PubMed] [Google Scholar]
- 54.Ronsein G. E., Winterbourn C. C., Di Mascio P., Kettle A. J. (2014) Cross-linking methionine and amine residues with reactive halogen species. Free Radic. Biol. Med. 70, 278–287. [DOI] [PubMed] [Google Scholar]
- 55.Rosen H., Klebanoff S. J., Wang Y., Brot N., Heinecke J. W., Fu X. (2009) Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci. USA 106, 18686–18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurst J. K., Albrich J. M., Green T. R., Rosen H., Klebanoff S. (1984) Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J. Biol. Chem. 259, 4812–4821. [PubMed] [Google Scholar]
- 57.Nauseef W. M. (2014) Myeloperoxidase in human neutrophil host defence. Cell. Microbiol. 16, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imlay J. A. (2003) Pathways of oxidative damage. Annu. Rev. Microbiol. 57, 395–418. [DOI] [PubMed] [Google Scholar]
- 59.Albrich J. M., Hurst J. K. (1982) Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 144, 157–161. [DOI] [PubMed] [Google Scholar]
- 60.Lymar S. V., Hurst J. K. (1995) Role of compartmentation in promoting toxicity of leukocyte-generated strong oxidants. Chem. Res. Toxicol. 8, 833–840. [DOI] [PubMed] [Google Scholar]
- 61.Hirche T. O., Gaut J. P., Heinecke J. W., Belaaouaj A. (2005) Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J. Immunol. 174, 1557–1565. [DOI] [PubMed] [Google Scholar]
- 62.Simchowitz L., De Weer P. (1986) Chloride movements in human neutrophils. Diffusion, exchange, and active transport. J. Gen. Physiol. 88, 167–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ince C., Thio B., van Duijn B., van Dissel J. T., Ypey D. L., Leijh P. C. (1987) Intracellular K+, Na+ and Cl− concentrations and membrane potential in human monocytes. Biochim. Biophys. Acta 905, 195–204. [DOI] [PubMed] [Google Scholar]
- 64.Baron D. N., Ahmed S. A. (1969) Intracellular concentrations of water and of the principal electrolytes determined by analysis of isolated human leucocytes. Clin. Sci. 37, 205–219. [PubMed] [Google Scholar]
- 65.Busetto S., Trevisan E., Decleva E., Dri P., Menegazzi R. (2007) Chloride movements in human neutrophils during phagocytosis: characterization and relationship to granule release. J. Immunol. 179, 4110–4124. [DOI] [PubMed] [Google Scholar]
- 66.Green J. N., Kettle A. J., Winterbourn C. C. (2014) Protein chlorination in neutrophil phagosomes and correlation with bacterial killing. Free Radic. Biol. Med. 77, 49–56. [DOI] [PubMed] [Google Scholar]
- 67.Painter R. G., Wang G. (2006) Direct measurement of free chloride concentrations in the phagolysosomes of human neutrophils. Anal. Chem. 78, 3133–3137. [DOI] [PubMed] [Google Scholar]
- 68.Painter R. G., Marrero L., Lombard G. A., Valentine V. G., Nauseef W. M., Wang G. (2010) CFTR-mediated halide transport in phagosomes of human neutrophils. J. Leukoc. Biol. 87, 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Planells-Cases R., Jentsch T. J. (2009) Chloride channelopathies. Biochim. Biophys. Acta 1792, 173–189. [DOI] [PubMed] [Google Scholar]
- 70.Duran C., Thompson C. H., Xiao Q., Hartzell H. C. (2010) Chloride channels: often enigmatic, rarely predictable. Annu. Rev. Physiol. 72, 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verkman A. S., Galietta L. J. (2009) Chloride channels as drug targets. Nat. Rev. Drug Discov. 8, 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheppard D. N., Welsh M. J. (1999) Structure and function of the CFTR chloride channel. Physiol. Rev. 79 (1 Suppl) S23–S45. [DOI] [PubMed] [Google Scholar]
- 73.Riordan J. R. (2005) Assembly of functional CFTR chloride channels. Annu. Rev. Physiol. 67, 701–718. [DOI] [PubMed] [Google Scholar]
- 74.Zifarelli G., Pusch M. (2007) CLC chloride channels and transporters: a biophysical and physiological perspective. Rev. Physiol. Biochem. Pharmacol. 158, 23–76. [DOI] [PubMed] [Google Scholar]
- 75.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594. [DOI] [PubMed] [Google Scholar]
- 76.Hartzell C., Putzier I., Arreola J. (2005) Calcium-activated chloride channels. Annu. Rev. Physiol. 67, 719–758. [DOI] [PubMed] [Google Scholar]
- 77.Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215. [DOI] [PubMed] [Google Scholar]
- 79.Pifferi S., Dibattista M., Menini A. (2009) TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch. 458, 1023–1038. [DOI] [PubMed] [Google Scholar]
- 80.Stöhr H., Heisig J. B., Benz P. M., Schöberl S., Milenkovic V. M., Strauss O., Aartsen W. M., Wijnholds J., Weber B. H., Schulz H. L. (2009) TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J. Neurosci. 29, 6809–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sigel E., Steinmann M. E. (2012) Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 287, 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avila A., Nguyen L., Rigo J. M. (2013) Glycine receptors and brain development. Front. Cell. Neurosci. 7, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009) Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimura K., Nakamura H., Trapnell B. C., Chu C. S., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. (1991) Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 19, 5417–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Painter R. G., Valentine V. G., Lanson N. A. Jr., Leidal K., Zhang Q., Lombard G., Thompson C., Viswanathan A., Nauseef W. M., Wang G., Wang G. (2006) CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 45, 10260–10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di A., Brown M. E., Deriy L. V., Li C., Szeto F. L., Chen Y., Huang P., Tong J., Naren A. P., Bindokas V., Palfrey H. C., Nelson D. J. (2006) CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 8, 933–944. [DOI] [PubMed] [Google Scholar]
- 87.Moreland J. G., Davis A. P., Bailey G., Nauseef W. M., Lamb F. S. (2006) Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J. Biol. Chem. 281, 12277–12288. [DOI] [PubMed] [Google Scholar]
- 88.Alex P., Ye M., Zachos N. C., Sipes J., Nguyen T., Suhodrev M., Gonzales L., Arora Z., Zhang T., Centola M., Guggino S. E., Li X. (2010) Clcn5 knockout mice exhibit novel immunomodulatory effects and are more susceptible to dextran sulfate sodium-induced colitis. J. Immunol. 184, 3988–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riordan J. R., et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073. [DOI] [PubMed] [Google Scholar]
- 90.Lukacs G. L., Verkman A. S. (2012) CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 18, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Y., Song K., Painter R. G., Aiken M., Reiser J., Stanton B. A., Nauseef W. M., Wang G. (2013) Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J. Innate Immun. 5, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su X., Looney M. R., Su H. E., Lee J. W., Song Y., Matthay M. A. (2011) Role of CFTR expressed by neutrophils in modulating acute lung inflammation and injury in mice. Inflamm. Res. 60, 619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoshimura K., Rosenfeld M. A., Nakamura H., Scherer E. M., Pavirani A., Lecocq J. P., Crystal R. G. (1992) Expression of the human cystic fibrosis transmembrane conductance regulator gene in the mouse lung after in vivo intratracheal plasmid-mediated gene transfer. Nucleic Acids Res. 20, 3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Painter R. G., Bonvillain R. W., Valentine V. G., Lombard G. A., LaPlace S. G., Nauseef W. M., Wang G. (2008) The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 83, 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phennicie R. T., Sullivan M. J., Singer J. T., Yoder J. A., Kim C. H. (2010) Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 78, 4542–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng H. P., Zhou Y., Song K., Hodges C. A., Drumm M. L., Wang G. (2014) Neutrophil-mediated phagocytic host defense defect in myeloid Cftr-inactivated mice. PLoS ONE 9, e106813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picollo A., Pusch M. (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436, 420–423. [DOI] [PubMed] [Google Scholar]
- 98.Scheel O., Zdebik A. A., Lourdel S., Jentsch T. J. (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436, 424–427. [DOI] [PubMed] [Google Scholar]
- 99.Stauber T., Weinert S., Jentsch T. J. (2012) Cell biology and physiology of CLC chloride channels and transporters. Compr. Physiol. 2, 1701–1744. [DOI] [PubMed] [Google Scholar]
- 100.Matsuda J. J., Filali M. S., Collins M. M., Volk K. A., Lamb F. S. (2010) The ClC-3 Cl−/H+ antiporter becomes uncoupled at low extracellular pH. J. Biol. Chem. 285, 2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huynh K. K., Grinstein S. (2007) Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 71, 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jankowski A., Scott C. C., Grinstein S. (2002) Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 277, 6059–6066. [DOI] [PubMed] [Google Scholar]
- 103.Miller F. J. Jr., Filali M., Huss G. J., Stanic B., Chamseddine A., Barna T. J., Lamb F. S. (2007) Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ. Res. 101, 663–671. [DOI] [PubMed] [Google Scholar]
- 104.Murphy R., DeCoursey T. E. (2006) Charge compensation during the phagocyte respiratory burst. Biochim. Biophys. Acta 1757, 996–1011. [DOI] [PubMed] [Google Scholar]
- 105.DeCoursey T. E. (2010) Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 25, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morgan D., Capasso M., Musset B., Cherny V. V., Ríos E., Dyer M. J., DeCoursey T. E. (2009) Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl. Acad. Sci. USA 106, 18022–18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El Chemaly A., Nunes P., Jimaja W., Castelbou C., Demaurex N. (2014) Hv1 proton channels differentially regulate the pH of neutrophil and macrophage phagosomes by sustaining the production of phagosomal ROS that inhibit the delivery of vacuolar ATPases. J. Leukoc. Biol. 95, 827–839. [DOI] [PubMed] [Google Scholar]
- 108.Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929. [DOI] [PubMed] [Google Scholar]
- 109.Nishi T., Forgac M. (2002) The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3, 94–103. [DOI] [PubMed] [Google Scholar]
- 110.Marshansky V., Rubinstein J. L., Grüber G. (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim. Biophys. Acta 1837, 857–879. [DOI] [PubMed] [Google Scholar]
- 111.Breton S., Brown D. (2013) Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 28, 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinberg B. E., Touret N., Vargas-Caballero M., Grinstein S. (2007) In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc. Natl. Acad. Sci. USA 104, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y. T., Shieh C. C., Delpire E., Shen M. R. (2012) K⁺-Cl⁻ cotransport mediates the bactericidal activity of neutrophils by regulating NADPH oxidase activation. J. Physiol. 590, 3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adragna N. C., Di Fulvio M., Lauf P. K. (2004) Regulation of K-Cl cotransport: from function to genes. J. Membr. Biol. 201, 109–137. [DOI] [PubMed] [Google Scholar]
- 115.Gamba G. (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 85, 423–493. [DOI] [PubMed] [Google Scholar]
- 116.Grinstein S., Furuya W., Cragoe E. J. Jr. (1986) Volume changes in activated human neutrophils: the role of Na+/H+ exchange. J. Cell. Physiol. 128, 33–40. [DOI] [PubMed] [Google Scholar]
- 117.Pedemonte N., Galietta L. J. (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459. [DOI] [PubMed] [Google Scholar]
- 118.Huang F., Rock J. R., Harfe B. D., Cheng T., Huang X., Jan Y. N., Jan L. Y. (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. USA 106, 21413–21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Large W. A., Wang Q. (1996) Characteristics and physiological role of the Ca(2+)-activated Cl− conductance in smooth muscle. Am. J. Physiol. 271, C435–C454. [DOI] [PubMed] [Google Scholar]
- 120.Murphy P. M. (1997) Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 34, 311–318. [PubMed] [Google Scholar]
- 121.Murphy P. M. (1994) The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633. [DOI] [PubMed] [Google Scholar]
- 122.Collins F. S. (1992) Cystic fibrosis: molecular biology and therapeutic implications. Science 256, 774–779. [DOI] [PubMed] [Google Scholar]
- 123.Welsh M. J., Ramsey B. W., Accurso F., Cutting G. (2001) Cystic fibrosis. In Metabolic and Molecular Basis of Interited Disease. (Scriver C. R., ed.), McGraw-Hill, New York, 5121–5188. [Google Scholar]
- 124.Stoltz D. A., Meyerholz D. K., Pezzulo A. A., Ramachandran S., Rogan M. P., Davis G. J., Hanfland R. A., Wohlford-Lenane C., Dohrn C. L., Bartlett J. A., Nelson G. A. IV, Chang E. H., Taft P. J., Ludwig P. S., Estin M., Hornick E. E., Launspach J. L., Samuel M., Rokhlina T., Karp P. H., Ostedgaard L. S., Uc A., Starner T. D., Horswill A. R., Brogden K. A., Prather R. S., Richter S. S., Shilyansky J., McCray P. B. Jr., Zabner J., Welsh M. J. (2010) Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2, 29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hampton T. H., Ballok A. E., Bomberger J. M., Rutkowski M. R., Barnaby R., Coutermarsh B., Conejo-Garcia J. R., O’Toole G. A., Stanton B. A. (2012) Does the F508-CFTR mutation induce a proinflammatory response in human airway epithelial cells? Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L509–L518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davis P. B., Drumm M., Konstan M. W. (1996) Cystic fibrosis. Am. J. Respir. Crit. Care Med. 154, 1229–1256. [DOI] [PubMed] [Google Scholar]
- 127.Segal B. H., Grimm M. J., Khan A. N., Han W., Blackwell T. S. (2012) Regulation of innate immunity by NADPH oxidase. Free Radic. Biol. Med. 53, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Porter L. A., Goldberg J. B. (2011) Influence of neutrophil defects on Burkholderia cepacia complex pathogenesis. Front. Cell. Infect. Microbiol. 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berendes H., Bridges R. A., Good R. A. (1957) A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn. Med. 40, 309–312. [PubMed] [Google Scholar]
- 130.Holmes B., Page A. R., Good R. A. (1967) Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J. Clin. Invest. 46, 1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matute J. D., Arias A. A., Wright N. A., Wrobel I., Waterhouse C. C., Li X. J., Marchal C. C., Stull N. D., Lewis D. B., Steele M., Kellner J. D., Yu W., Meroueh S. O., Nauseef W. M., Dinauer M. C. (2009) A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 114, 3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen T. S., Prince A. (2012) Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med. 18, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knowles M. R., Boucher R. C. (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 109, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boucher R. C. (2003) Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 445, 495–498. [DOI] [PubMed] [Google Scholar]
- 135.Pezzulo A. A., Tang X. X., Hoegger M. J., Alaiwa M. H., Ramachandran S., Moninger T. O., Karp P. H., Wohlford-Lenane C. L., Haagsman H. P., van Eijk M., Bánfi B., Horswill A. R., Stoltz D. A., McCray P. B. Jr., Welsh M. J., Zabner J. (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schutte B. C., McCray P. B. Jr. (2002) [beta]-Defensins in lung host defense. Annu. Rev. Physiol. 64, 709–748. [DOI] [PubMed] [Google Scholar]
- 137.Bonfield T. L., Hodges C. A., Cotton C. U., Drumm M. L. (2012) Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J. Leukoc. Biol. 92, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berend K., van Hulsteijn L. H., Gans R. O. (2012) Chloride: the queen of electrolytes? Eur. J. Intern. Med. 23, 203–211. [DOI] [PubMed] [Google Scholar]
- 139.Dickerson L. W., Bonthius D. J., Schutte B. C., Yang B., Barna T. J., Bailey M. C., Nehrke K., Williamson R. A., Lamb F. S. (2002) Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res. 958, 227–250. [DOI] [PubMed] [Google Scholar]
- 140.Stobrawa S. M., Breiderhoff T., Takamori S., Engel D., Schweizer M., Zdebik A. A., Bösl M. R., Ruether K., Jahn H., Draguhn A., Jahn R., Jentsch T. J. (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29, 185–196. [DOI] [PubMed] [Google Scholar]
- 141.Yoshikawa M., Uchida S., Ezaki J., Rai T., Hayama A., Kobayashi K., Kida Y., Noda M., Koike M., Uchiyama Y., Marumo F., Kominami E., Sasaki S. (2002) CLC-3 deficiency leads to phenotypes similar to human neuronal ceroid lipofuscinosis. Genes Cells 7, 597–605. [DOI] [PubMed] [Google Scholar]
- 142.Volk A. P., Heise C. K., Hougen J. L., Artman C. M., Volk K. A., Wessels D., Soll D. R., Nauseef W. M., Lamb F. S., Moreland J. G. (2008) ClC-3 and IClswell are required for normal neutrophil chemotaxis and shape change. J. Biol. Chem. 283, 34315–34326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Piwon N., Günther W., Schwake M., Bösl M. R., Jentsch T. J. (2000) ClC-5 Cl− -channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408, 369–373. [DOI] [PubMed] [Google Scholar]
- 144.De Stefano S., Pusch M., Zifarelli G. (2013) A single point mutation reveals gating of the human ClC-5 Cl−/H+ antiporter. J. Physiol. 591, 5879–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novarino G., Weinert S., Rickheit G., Jentsch T. J. (2010) Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science 328, 1398–1401. [DOI] [PubMed] [Google Scholar]
- 146.Raggi C., Fujiwara K., Leal T., Jouret F., Devuyst O., Terryn S. (2011) Decreased renal accumulation of aminoglycoside reflects defective receptor-mediated endocytosis in cystic fibrosis and Dent’s disease. Pflugers Arch. 462, 851–860. [DOI] [PubMed] [Google Scholar]
- 147.Kasper D., Planells-Cases R., Fuhrmann J. C., Scheel O., Zeitz O., Ruether K., Schmitt A., Poët M., Steinfeld R., Schweizer M., Kornak U., Jentsch T. J. (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kornak U., Kasper D., Bösl M. R., Kaiser E., Schweizer M., Schulz A., Friedrich W., Delling G., Jentsch T. J. (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215. [DOI] [PubMed] [Google Scholar]
- 149.Poët M., Kornak U., Schweizer M., Zdebik A. A., Scheel O., Hoelter S., Wurst W., Schmitt A., Fuhrmann J. C., Planells-Cases R., Mole S. E., Hübner C. A., Jentsch T. J. (2006) Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. USA 103, 13854–13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Graves A. R., Curran P. K., Smith C. L., Mindell J. A. (2008) The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788–792. [DOI] [PubMed] [Google Scholar]
- 151.Neagoe I., Stauber T., Fidzinski P., Bergsdorf E. Y., Jentsch T. J. (2010) The late endosomal ClC-6 mediates proton/chloride countertransport in heterologous plasma membrane expression. J. Biol. Chem. 285, 21689–21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pressey S. N., O’Donnell K. J., Stauber T., Fuhrmann J. C., Tyynelä J., Jentsch T. J., Cooper J. D. (2010) Distinct neuropathologic phenotypes after disrupting the chloride transport proteins ClC-6 or ClC-7/Ostm1. J. Neuropathol. Exp. Neurol. 69, 1228–1246. [DOI] [PubMed] [Google Scholar]
- 153.Weinert S., Jabs S., Supanchart C., Schweizer M., Gimber N., Richter M., Rademann J., Stauber T., Kornak U., Jentsch T. J. (2010) Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl− accumulation. Science 328, 1401–1403. [DOI] [PubMed] [Google Scholar]
- 154.Wartosch L., Fuhrmann J. C., Schweizer M., Stauber T., Jentsch T. J. (2009) Lysosomal degradation of endocytosed proteins depends on the chloride transport protein ClC-7. FASEB J. 23, 4056–4068. [DOI] [PubMed] [Google Scholar]
- 155.Rock J. R., O’Neal W. K., Gabriel S. E., Randell S. H., Harfe B. D., Boucher R. C., Grubb B. R. (2009) Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J. Biol. Chem. 284, 14875–14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Billig G. M., Pál B., Fidzinski P., Jentsch T. J. (2011) Ca2+-activated Cl− currents are dispensable for olfaction. Nat. Neurosci. 14, 763–769. [DOI] [PubMed] [Google Scholar]
- 157.Hwang S. J., Blair P. J., Britton F. C., O’Driscoll K. E., Hennig G., Bayguinov Y. R., Rock J. R., Harfe B. D., Sanders K. M., Ward S. M. (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 587, 4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frings S., Reuter D., Kleene S. J. (2000) Neuronal Ca2+ -activated Cl− channels—homing in on an elusive channel species. Prog. Neurobiol. 60, 247–289. [DOI] [PubMed] [Google Scholar]
- 159.Duan D. (2009) Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J. Physiol. 587, 2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cho H., Yang Y. D., Lee J., Lee B., Kim T., Jang Y., Back S. K., Na H. S., Harfe B. D., Wang F., Raouf R., Wood J. N., Oh U. (2012) The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021. [DOI] [PubMed] [Google Scholar]
- 161.Duran C., Hartzell H. C. (2011) Physiological roles and diseases of Tmem16/anoctamin proteins: are they all chloride channels? Acta Pharmacol. Sin. 32, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rock J. R., Futtner C. R., Harfe B. D. (2008) The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev. Biol. 321, 141–149. [DOI] [PubMed] [Google Scholar]
- 163.Guilbault C., Saeed Z., Downey G. P., Radzioch D. (2007) Cystic fibrosis mouse models. Am. J. Respir. Cell Mol. Biol. 36, 1–7. [DOI] [PubMed] [Google Scholar]
- 164.Wong X. M., Younger S., Peters C. J., Jan Y. N., Jan L. Y. (2013) Subdued, a TMEM16 family Ca²⁺-activated Cl⁻channel in Drosophila melanogaster with an unexpected role in host defense. eLife 2, e00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saitta M., Cavalier S., Garay R., Cragoe E. Jr., Hannaert P. (1990) Evidence for a DIOA-sensitive [K+,Cl−-cotransport system in cultured vascular smooth muscle cells. Am. J. Hypertens. 3, 939–942. [DOI] [PubMed] [Google Scholar]
- 166.Lauf P. K., Bauer J., Adragna N. C., Fujise H., Zade-Oppen A. M., Ryu K. H., Delpire E. (1992) Erythrocyte K-Cl cotransport: properties and regulation. Am. J. Physiol. 263, C917–C932. [DOI] [PubMed] [Google Scholar]
- 167.Lauf P. K., Adragna N. C. (2000) K-Cl cotransport: properties and molecular mechanism. Cell. Physiol. Biochem. 10, 341–354. [DOI] [PubMed] [Google Scholar]
- 168.Delpire E., Mount D. B. (2002) Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 64, 803–843. [DOI] [PubMed] [Google Scholar]