Abstract
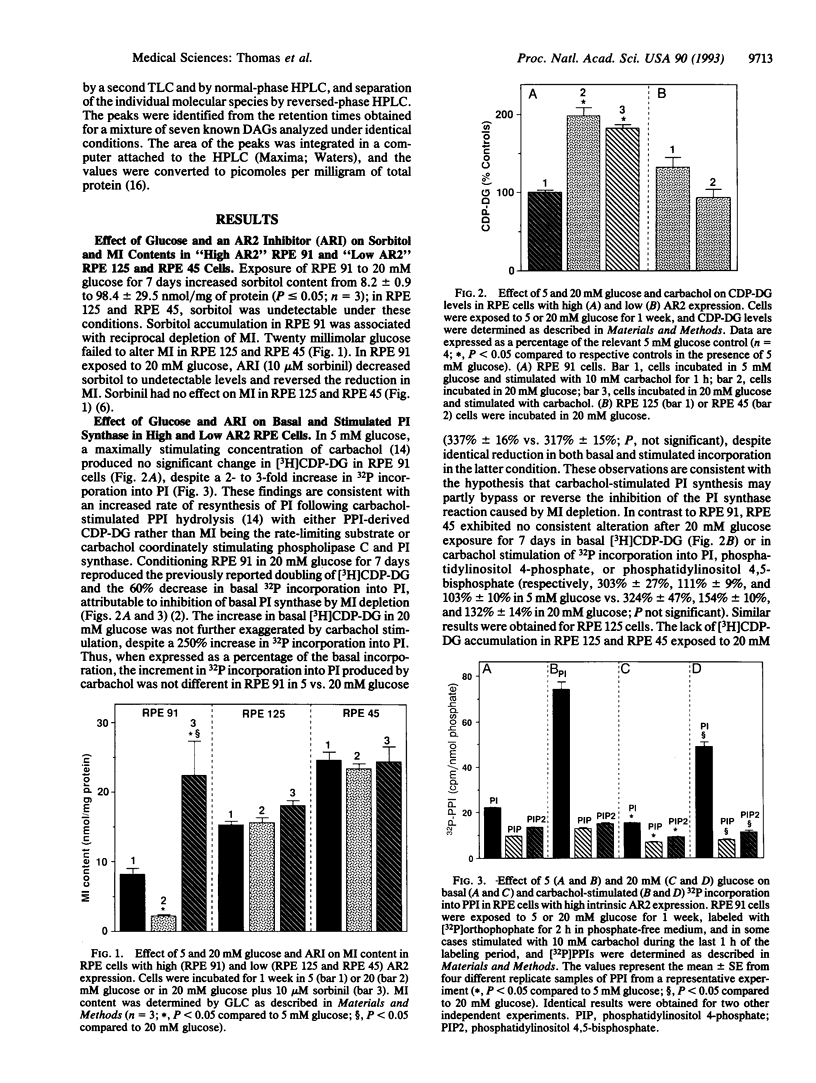
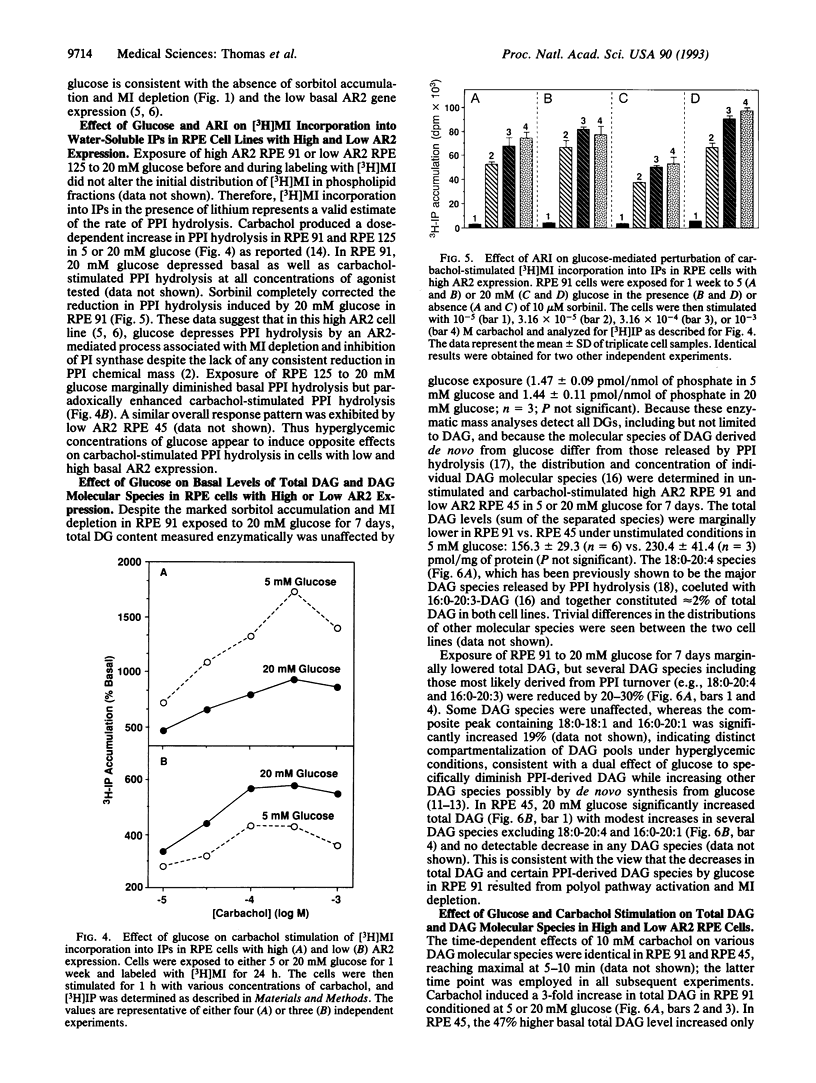
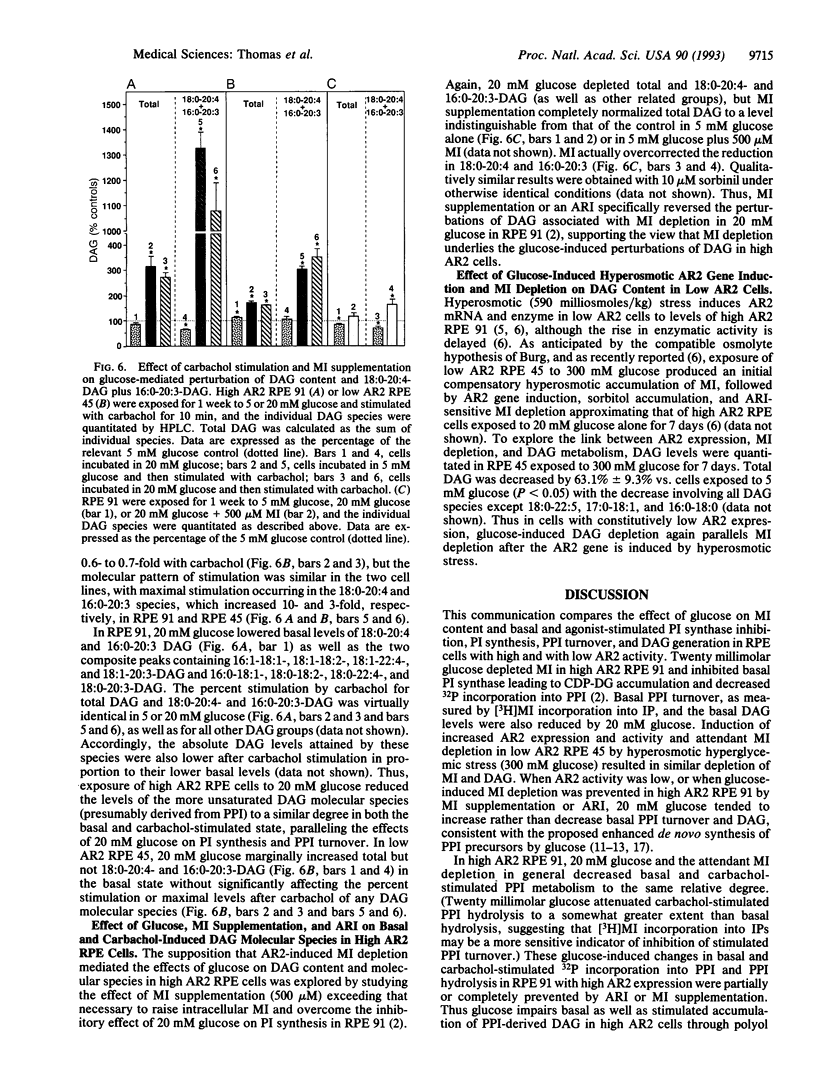
Physiological hyperglycemia has been speculated to alter phosphoinositide (PPI; inositol phospholipid) signal transduction in cells prone to diabetic complications by two separate mass-action mechanisms with antiparallel putative effects on diacylglycerol (DAG): (i) sorbitol-induced depletion of myo-inositol leads to diminished PPI synthesis and turnover and DAG release, and (ii) elevated glucose-derived DAG precursors enhance de novo DAG synthesis. Because the first mechanism is mediated by aldose reductase (AR2), which converts glucose to sorbitol, the effects of glucose on basal and stimulated PPI signaling were explored in lines of cultured human retinal pigment epithelial cells differing widely in their basal AR2 gene expression and enzymatic activity. The results suggest that the effects of glucose on PPI signaling vary inversely with the level of AR2 activity and parallel the extent of AR2-induced myo-inositol depletion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Yajima Y., Kador P. F., Kuwabara T., Kinoshita J. H. Localization of aldose reductase in the human eye. Diabetes. 1984 Jun;33(6):562–566. doi: 10.2337/diab.33.6.562. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Robertson S. Essential fatty acid diet supplementation. Effects on peripheral nerve and skeletal muscle function and capillarization in streptozocin-induced diabetic rats. Diabetes. 1991 May;40(5):532–539. doi: 10.2337/diab.40.5.532. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Del Monte M. A., Rabbani R., Diaz T. C., Lattimer S. A., Nakamura J., Brennan M. C., Greene D. A. Sorbitol, myo-inositol, and rod outer segment phagocytosis in cultured hRPE cells exposed to glucose. In vitro model of myo-inositol depletion hypothesis of diabetic complications. Diabetes. 1991 Oct;40(10):1335–1345. [PubMed] [Google Scholar]
- Feldman E. L., Randolph A. E., Johnston G. C., DelMonte M. A., Greene D. A. Receptor-coupled phosphoinositide hydrolysis in human retinal pigment epithelium. J Neurochem. 1991 Jun;56(6):2094–2100. doi: 10.1111/j.1471-4159.1991.tb03471.x. [DOI] [PubMed] [Google Scholar]
- Galvao C., Shayman J. A. The phosphatidylinositol synthase of proximal tubule cells. Biochim Biophys Acta. 1990 May 1;1044(1):34–42. doi: 10.1016/0005-2760(90)90215-j. [DOI] [PubMed] [Google Scholar]
- Go M., Sekiguchi K., Nomura H., Kikkawa U., Nishizuka Y. Further studies on the specificity of diacylglycerol for protein kinase C activation. Biochem Biophys Res Commun. 1987 Apr 29;144(2):598–605. doi: 10.1016/s0006-291x(87)80008-6. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer-Greene S., Sima A. A. Pathogenesis of diabetic neuropathy: role of altered phosphoinositide metabolism. Crit Rev Neurobiol. 1989;5(2):143–219. [PubMed] [Google Scholar]
- Henry D. N., Del Monte M., Greene D. A., Killen P. D. Altered aldose reductase gene regulation in cultured human retinal pigment epithelial cells. J Clin Invest. 1993 Aug;92(2):617–623. doi: 10.1172/JCI116629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- Lee C., Fisher S. K., Agranoff B. W., Hajra A. K. Quantitative analysis of molecular species of diacylglycerol and phosphatidate formed upon muscarinic receptor activation of human SK-N-SH neuroblastoma cells. J Biol Chem. 1991 Dec 5;266(34):22837–22846. [PubMed] [Google Scholar]
- Lee T. S., Saltsman K. A., Ohashi H., King G. L. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Lockett M. J., Tomlinson D. R. The effects of dietary treatment with essential fatty acids on sciatic nerve conduction and activity of the Na+/K+ pump in streptozotocin-diabetic rats. Br J Pharmacol. 1992 Feb;105(2):355–360. doi: 10.1111/j.1476-5381.1992.tb14258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy A., Lurie K. G., Ghosh A., Wilson J. M., MacGregor L. C., Matschinsky F. M. Diabetes and the myo-inositol paradox. Diabetes. 1990 Oct;39(10):1305–1312. doi: 10.2337/diab.39.10.1305. [DOI] [PubMed] [Google Scholar]
- Ludvigson M. A., Sorenson R. L. Immunohistochemical localization of aldose reductase. I. Enzyme purification and antibody preparation--localization in peripheral nerve, artery, and testis. Diabetes. 1980 Jun;29(6):438–449. doi: 10.2337/diab.29.6.438. [DOI] [PubMed] [Google Scholar]
- Nakamura J., Del Monte M. A., Shewach D., Lattimer S. A., Greene D. A. Inhibition of phosphatidylinositol synthase by glucose in human retinal pigment epithelial cells. Am J Physiol. 1992 Apr;262(4 Pt 1):E417–E426. doi: 10.1152/ajpendo.1992.262.4.E417. [DOI] [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Sonobe M., Yasuda H., Hisanaga T., Maeda K., Yamashita M., Kawabata T., Kikkawa R., Taniguchi Y., Shigeta Y. Amelioration of nerve Na(+)-K(+)-ATPase activity independently of myo-inositol level by PGE1 analogue OP-1206.alpha-CD in streptozocin-induced diabetic rats. Diabetes. 1991 Jun;40(6):726–730. doi: 10.2337/diab.40.6.726. [DOI] [PubMed] [Google Scholar]
- Stevens M. J., Henry D. N., Thomas T. P., Killen P. D., Greene D. A. Aldose reductase gene expression and osmotic dysregulation in cultured human retinal pigment epithelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):E428–E438. doi: 10.1152/ajpendo.1993.265.3.E428. [DOI] [PubMed] [Google Scholar]
- Stinson A. M., Wiegand R. D., Anderson R. E. Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes. Exp Eye Res. 1991 Feb;52(2):213–218. doi: 10.1016/0014-4835(91)90261-c. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Baier L. D., Harlow J. E., Smith S. R., Ostrow E., Williamson J. R. Diabetes-induced glomerular dysfunction: links to a more reduced cytosolic ratio of NADH/NAD+. Kidney Int. 1992 Apr;41(4):778–788. doi: 10.1038/ki.1992.121. [DOI] [PubMed] [Google Scholar]
- de la Rubia G., Oliver F. J., Inoguchi T., King G. L. Induction of resistance to endothelin-1's biochemical actions by elevated glucose levels in retinal pericytes. Diabetes. 1992 Dec;41(12):1533–1539. doi: 10.2337/diabetes.41.12.1533. [DOI] [PubMed] [Google Scholar]


