Abstract
Cytomegalovirus (CMV) infection negatively influences both short- and long-term outcomes after cardiothoracic transplantation. In heart transplantation, registry analyses have shown that CMV immunoglobulin (CMVIG) with or without virostatic prophylaxis is associated with a significant reduction in mortality and graft loss versus no prophylaxis, particularly in high-risk donor (D)+/recipient (R)− transplants. Randomized comparative trials are lacking but retrospective data suggest that addition of CMVIG to antiviral prophylaxis may reduce rates of CMV-related events after heart transplantation, including the incidence of acute rejection or chronic allograft vasculopathy. However, available data consistently indicate that when CMVIG is used, it should be administered with concomitant antiviral therapy, and that evidence concerning preemptive management with CMVIG is limited, but promising. In lung transplantation, CMVIG should again only be used with concomitant antiviral therapy. Retrospective studies have shown convincing evidence that addition of CMVIG to antiviral prophylaxis lowers CMV endpoints and mortality. The current balance of evidence suggests that CMVIG prophylaxis reduces the risk of bronchiolitis obliterans syndrome, but a controlled trial is awaited. Overall, the relatively limited current data set suggests that prophylaxis with CMVIG in combination with antiviral therapy appears effective in D+/R− heart transplant patients, whereas in lung transplantation, addition of CMVIG in recipients of a CMV-positive graft may offer an advantage in terms of CMV infection and disease.
In this article, we will review clinical reports investigating the use of cytomegalovirus (CMV) immunoglobulin (CMVIG) in heart and lung transplantation. In particular, we will focus on the evidence reached by several studies conducted over a wide time span, thus reflecting diverse eras of clinical practice, aiming to dissect the role CMVIG should play in the current setting of antiviral and immunosuppressive strategies. In addition, we will highlight unmet needs and unanswered questions needing further investigation.
Use of CMVIG Prophylaxis
There is wide variability in the modalities of CMVIG use for CMV prophylaxis among thoracic transplant centers. Although a small number of centers use CMVIG universally, it is more commonly selectively administered in high-risk or very high-risk cases. Across all organ types, approximately 20% of centers use CMVIG in donor (D)+/recipient (R)− transplants or other specific situations,1 whereas an international survey of lung transplant centers in 2010 indicated that approximately every third center uses CMVIG in D+/R− transplants.2 Many centers do not administer CMVIG prophylactically, relying entirely on antiviral agents. The updated CMV Consensus Conference of the Transplantation Society included the option of treatment with CMVIG in addition to antiviral prophylaxis therapy.3 The report pointed out that the combination is most widely used in high-risk procedures, such as thoracic or intestinal transplants, in conjunction with antiviral prophylaxis, consistent with the International Society of Heart and Lung Transplantation Guidelines for the Care of Heart Transplant Recipients.4
In this article, we consider studies which have supported the role of CMVIG in thoracic transplantation. It should be noted, however, that some studies were performed before effective oral antiviral medications were available, and thus their relevance needs to be assessed in the context of current therapeutic strategies. Nevertheless, we have sought to dissect the available evidence to shed light on the potential role of CMVIG amidst modern anti-CMV prophylaxis strategies, pointing out where data are lacking and proposing possible future directions for study.
As is often the case, thoracic transplantation may gain insights from experience in abdominal organs.5-9 In the preganciclovir era, a double-blind, placebo-controlled study in 141 liver transplants found that CMVIG prophylaxis without antiviral prophylaxis reduced the risk of severe CMV disease from 26% to 12% overall (relative risk, 0.39; 95% confidence interval, 0.17-0.89), although surprisingly not for the D+/R− subpopulation.6 More recently, an analysis of data from the Scientific Registry of Transplant Recipients supported a benefit for CMVIG administration versus patients who received no CMVIG or antiviral therapy in terms of improved graft survival and a trend toward better patient survival in high-risk transplants.7 An analysis of registry data from 2805 liver transplant patients given CMVIG with or without antiviral therapy found the risk of graft loss or death to be reduced after CMVIG treatment versus no prophylaxis, but only when given in combination with antiviral therapy.8 Lastly, a meta-analysis of prospective randomized trials in solid organ transplants of different types (predominantly kidney) has examined the effect of CMVIG prophylaxis.9 Compared with patients who received no CMVIG, there was a marked reduction in all-cause mortality which took into account CMV-related deaths (Table 1). However, the study population included few thoracic transplant recipients and only 2 of the 11 studies assessed included concomitant antiviral therapy.9
TABLE 1.
Registry analyses and meta-analyses of CMVIG prophylaxis
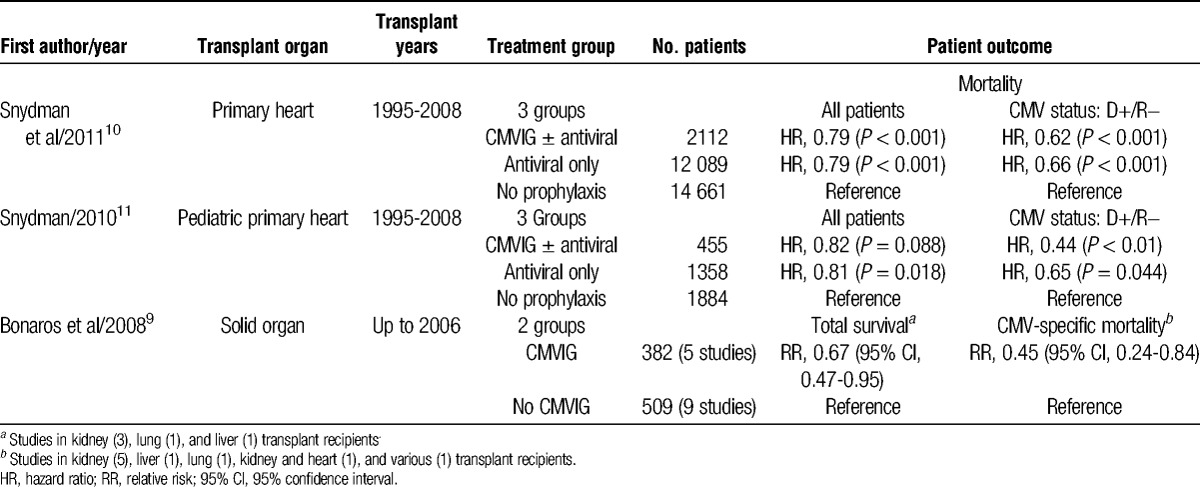
Translation of these results to the thoracic transplantation setting is limited by the scarcity of studies and lack of randomized trials, despite the fact that heart or lung transplant recipients are at increased risk for CMV disease compared with other types of solid organ transplantation12,13 and could particularly benefit from additional protection. As alluded to above, 2 important caveats need to be considered when interpreting results:
(1) The era of analyses should be taken into consideration. The CMV infection was poorly diagnosed in the 1980s and early 1990s compared with recent years, when antigenemia and, latterly, polymerase chain reaction–based monitoring became more widely available. Additionally, early studies of CMVIG prophylaxis in thoracic transplant recipients frequently did not include antiviral therapy, which is now routine in high-risk or even all patients.
(2) There are several CMVIG products, which are not identical. Modifications of the original Cytotect 10% CMVIG product, including exclusion of β-propiolactone treatment, resulted in a higher concentration of anti-CMV antibodies and significantly greater inhibition of T-cell proliferation and cytokine production versus the original Cytotect CP preparation in vitro.14 A comparison of the 2 most widely used commercial preparations, Cytotect and Cytogam, shows that both contain comparable amounts of high-avidity CMV-specific IgG and similar neutralization activity directed against CMV glycoproteins.15 Data relating to other products cannot necessarily be regarded as relevant to these 2 preparations.
Registry Analyses
Data analysis from transplant registries offers large populations and long follow-up, permitting detection of differences in mortality and graft survival rates between treatment strategies. However, few or no data are available on the types of agents used (either CMVIG or antiviral agents), the route of antiviral administration, the duration of therapy, or the dosing regimens, making it difficult to draw firm conclusions about differences between treatment groups.
Snydman and colleagues10 undertook an analysis of the effect of CMV prophylaxis in 28 862 recipients of a primary heart transplant registered with the Scientific Registry of Transplant Recipients. At hospital discharge, patients were receiving CMVIG, usually with an antiviral agent as well (1514 of 2112 patients), antiviral therapy only (n = 12 089), or no CMV prophylaxis (n = 14 661). The frequency of D+/R− transplants was highest in the cohort receiving CMVIG (30%). The CMVIG, with or without antiviral therapy, was an independent predictor for higher patient and graft survival (Table 1). The adjusted risk for death or graft loss was also significantly lower for recipients treated with CMVIG monotherapy versus no-CMV prophylaxis (Table 1). However, there were no significant differences in patient/graft survival rates between CMVIG-only therapy versus antiviral therapy, or between CMVIG with antivirals or antivirals alone.10 The same group performed a similar analysis in pediatric heart transplant recipients.11 After 7 years of follow-up, the benefit for CMVIG was again most pronounced in the high-risk (D+/R−) population. As in the adult analysis, treatment with CMVIG alone, CMVIG with antiviral therapy, or antivirals alone showed no apparent differences in long-term patient or graft survival rates. Interestingly, however, there was a lower risk for acute rejection in children discharged on CMVIG alone versus only antiviral therapy (hazard ratio, 0.61; P = 0.04)15; a similar analysis was not performed in the adult cohort.10
Heart Transplantation
Early nonrandomized studies suggested that CMVIG prophylaxis reduced the incidence of CMV infection and disease in D+/R− heart transplants compared with that seen in seropositive patients,16,17 indicating that passive immunization offers the same protection against CMV as naturally acquired anti-CMV resistance. Few randomized studies have assessed the use of CMVIG for prophylaxis or preemptive management in heart transplantation, and the available data are largely confirmed to high-risk populations (Table 2).18-23 In 1995, Aguado et al21 published the results of a prospective, randomized trial in 31 CMV-seropositive patients receiving heart transplants from seropositive or seronegative donors who were treated with CMVIG or intravenous (IV) ganciclovir prophylaxis. Those randomized to CMVIG (Cytotect) received 100 mg/kg within 24 hours of transplant, and at 2, 4, 6, 8, and 10 weeks posttransplant with no antiviral therapy. In the ganciclovir group, ganciclovir was given at 5 mg/kg twice a day for 14 days within 48 hours of transplant, with the dose adjusted based on renal function. All patients received OKT3 with cyclosporine, steroids and azathioprine. Six months after CMV, infection rates were similar but CMV disease was significantly more frequent in the CMVIG monotherapy group.21 No patient died of CMV-related causes. However, a large 5-year single-arm retrospective analysis in 377 heart transplant patients24 observed a 3.6% incidence of CMV-related deaths in CMV-seronegative recipients receiving CMVIG only, concluding that additional prophylaxis with ganciclovir is advisable in this high-risk subgroup.24 No randomized trial has compared CMVIG with or without antiviral therapy in heart transplantation, but a retrospective study in 207 D+/R− transplants reported significantly lower rates of CMV disease and CMV-related disease with the addition of IV ganciclovir.19 These findings support recommendations that when CMVIG is used, it should be administered in conjunction with antiviral prophylaxis therapy.3
TABLE 2.
Comparative studies of CMVIG in heart transplantation
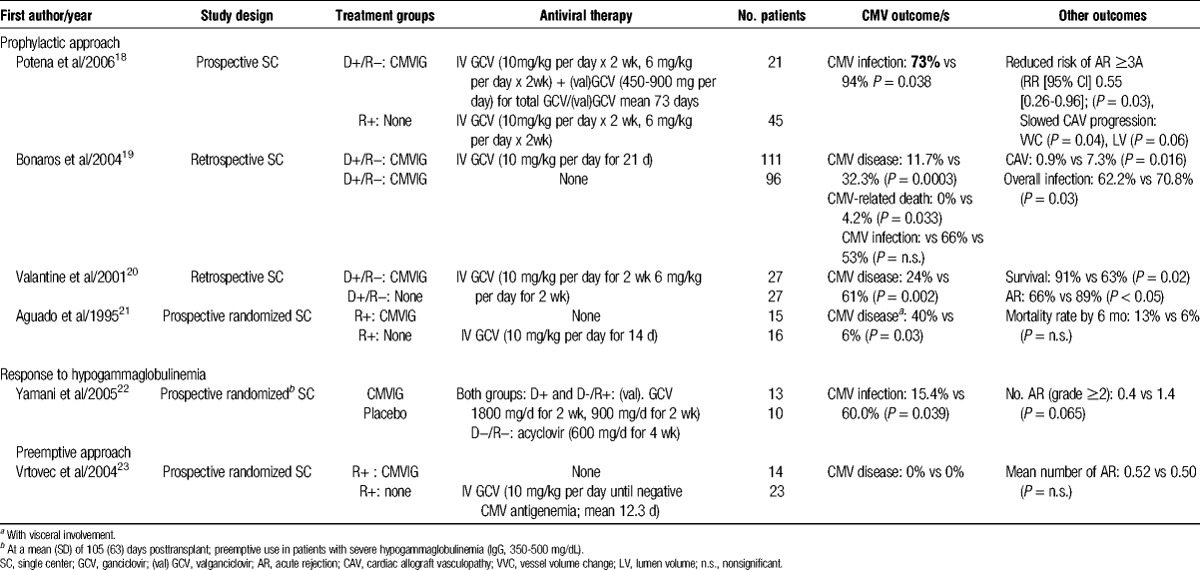
Randomized comparisons of CMVIG plus IV ganciclovir versus IV ganciclovir alone are lacking. A retrospective analysis of 27 D+/R− transplant patients compared with 27 matched historical controls by Valantine et al20 found significant benefits for the addition of CMVIG. Over 3 years of follow-up, the rate of CMV disease was significantly lower in the cohort given CMVIG with IV ganciclovir therapy, showing a significant increase in survival. Potena et al18 described outcomes in a series of 66 heart transplant patients in whom CMV prophylaxis was tailored according to risk status. All CMV-seropositive recipients received a standard course of IV ganciclovir for 4 weeks. All D+/R− recipients received the same course of IV ganciclovir, but this was supplemented with CMVIG for the first 4 months posttransplant. In addition, oral valganciclovir was given for a mean of 6 weeks after ganciclovir therapy in the D+/R− group, but not in lower-risk patients. The high-risk D+/R− patients, under the intensive prophylactic regimen, had a lower rate of CMV infection than R+ patients without CMVIG or valganciclovir (Table 2). However, the specific contribution of CMVIG is difficult to ascertain because CMVIG-treated patients also received prolonged prophylaxis with antiviral therapy.18 In a subsequent analysis, Potena and colleagues25 showed that the benefit of prolonged prophylaxis with the addition of CMVIG influenced CMV kinetics and replication burden, with D+/R− patients unexpectedly experiencing lower CMV DNA titers in mononuclear and polymorphonuclear cells, suggesting a more effective recovery of CMV immunity than in R+ patients.
One study, by Yamani et al,22 investigated initiation of CMVIG in response to low IgG levels. Twenty-three patients who developed moderate hypogammaglobulinemia (350-500 mg/dL) were randomized to CMVIG (Cytogam, 150 mg/kg until IgG >500 mg/dL; mean, 1.4 doses per patient) or placebo, at a mean of 105 days posttransplant.22 All patients received oral acyclovir or valganciclovir, depending on risk status. The CMVIG treatment group had a much lower rate of CMV infection at month 12 posttransplant (Table 2). Another randomized trial compared preemptive therapy with CMVIG (Cytogam) versus IV ganciclovir, in seropositive heart transplant recipients in whom CMV antigenemia was detected by week 12 based on the presence of pp65 antigen.23 A single dose of CMVIG (200 mg/kg) was given in the CMVIG group (n = 14). The IV ganciclovir 5 mg/kg was given every 12 hours until the CMV antigenemia test was negative (mean, 12.3 days). No patient in either group developed CMV disease, and there was no difference in the rates of superinfections.23 These promising findings for use of CMVIG as preemptive therapy await confirmation in a larger trial.
Regarding the impact on acute rejection, there is suggestive evidence that CMVIG may significantly influence the incidence of this complication.18,20,22 Two nonrandomized trials found that D+/R− patients given CMVIG prophylaxis had a significantly lower incidence of acute rejection than untreated R+ patients18 or D+/R− patients.20 Preemptive CMVIG treatment in patients with moderate hypogammaglobulinemia showed a trend to fewer rejection episodes in a randomized trial.22 However, 1 small randomized trial found no difference in the rate of rejection between patients given CMVIG versus IV ganciclovir, both as preemptive therapy.23 Anti-CMV strategies may also indirectly lower the risk for rejection by reducing CMV event rates. A meta-analysis of anti-CMV prophylactic or preemptive antiviral regimens found a significant reduction in risk of allograft rejection,26 as did a registry analysis of CMVIG prophylaxis in children undergoing heart transplantation.11 It is unclear, however, whether an effect on rejection is partly mediated by the well-known immunomodulatory effects of immunoglobulins27 rather than an entirely CMVIG-specific effect.
The CMV is believed to contribute to the development of native atherosclerosis28 as well as graft coronary remodeling after heart transplantation,29,30 with higher rates of cardiac allograft vasculopathy reported in CMV-seropositive heart transplant recipients.24 Two studies have performed intravascular ultrasound (IVUS) in patients receiving CMVIG with antiviral therapy.18,20 In a retrospective analysis by Valantine et al,20 IVUS was performed at 3 years posttransplant in 19 D+/R− patients given CMVIG with IV ganciclovir and 12 matched controls given IV ganciclovir alone. Mean intimal thickness was significantly lower in the CMVIG group (0.16 versus 0.45 mm, P < 0.001), as was the proportion of patients with intimal thickness 0.3 mm (15% vs 56%; P = 0.01). Potena and colleagues performed IVUS in a subset of 42 patients from their study in which D+/R− patients received CMVIG prophylaxis with IV ganciclovir and subsequent oral valganciclovir, and CMV-seropositive patients were given only IV ganciclovir.18 There was a significantly smaller decrease in vessel volume and lumen volume in the patients given CMVIG, indicating less graft vascular disease, supporting the concept that aggressive anti-CMV strategies may improve graft vascular protection.
It remains an open question as to what contribution is made by addition of CMVIG to prolonged antiviral prophylaxis and to what extent the IgG-related properties of CMVIG may play a CMV-independent role in graft protection. It seems reasonable to speculate that the immunomodulatory properties of CMVIG could be a factor in the reduced rate of rejection and allograft vasculopathy observed in CMV D+/R− patients. Indeed, an extended duration of antiviral prophylaxis alone does not seem to achieve a graft-protective effect, because studies comparing different durations of antiviral prophylaxis have failed to demonstrate differences in rejection outcomes.31,32 In addition, in recent years, the concept of endothelial injury mediated by donor-specific antibodies (DSA) has emerged, and immunoglobulin therapies may disrupt DSA-mediated damage. Unfortunately, published studies of CMVIG in thoracic transplantation have so far not included DSA monitoring, and this possibility requires testing in an appropriately designed prospective study.
Lung Transplantation
Few comparative studies have used CMVIG in lung or heart-lung transplantation (Table 3).20,33-38 One randomized trial by Kruger et al33 compared CMVIG prophylaxis versus no prophylaxis in 44 CMV-seropositive lung transplant patients. The CMVIG alone did not influence the rate of CMV infection, mortality, or the incidence or severity of CMV pneumonitis. However, the treatment regimens for diagnosed CMV infections were not homogeneous. The literature contains a small number of case reports of successful CMV prophylaxis in high-risk lung transplant patients using CMVIG alone,39,40 but also cases in which patients given only CMVIG prophylaxis developed positive antigenemia.40 As in heart transplantation, it appears advisable to use CMVIG only in combination with antiviral therapy.
TABLE 3.
Comparative studies of CMVIG in lung and heart-lung transplantation
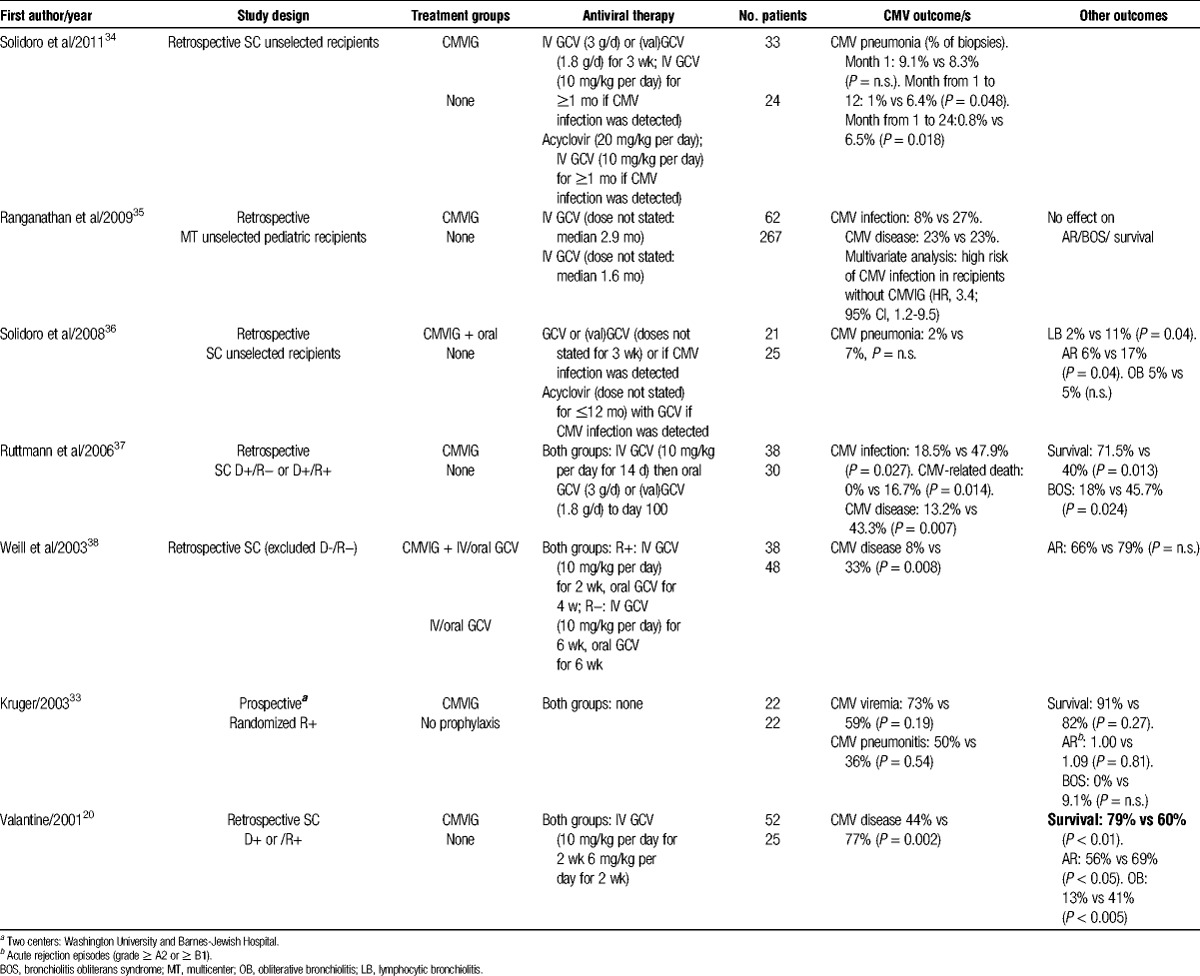
Retrospective analyses have compared outcomes with CMVIG prophylaxis in combination with ganciclovir versus ganciclovir alone in lung transplant patients.20,37,38 Two of these were performed in at-risk serostatus patients20,35; the third excluded D-/R− transplants.37 Each of these analyses showed a convincing benefit for addition of CMVIG in terms of CMV endpoints, including CMV disease. This translated to substantial survival benefits at 3 years after lung transplantation: in a study of 38 patients by Ruttmann et al37 as well as in an analysis of 77 lung or heart-lung transplants by Valantine et al.20 However, in the study by Ruttmann and colleagues,37 which analyzed patients transplanted during 1994 to 2004, all patients received IV ganciclovir but oral ganciclovir was switched to the better-absorbed valganciclovir after 2003. Since CMVIG therapy was initiated in 2000, valganciclovir was more widely used in the CMVIG cohort. In the population analyzed by Valantine et al,20 IV ganciclovir was given for only 4 weeks compared with the 6 to 12 months which is now standard practice. The relevance of antiviral therapy in CMVIG-treated patients was highlighted by a small single-center retrospective analysis, in which CMVIG prophylaxis with IV ganciclovir was reported to be more efficacious in D+/R− lung transplant patients than CMVIG with oral ganciclovir.40
A recent single-center retrospective study analyzed rates of CMV pneumonia in 57 lung transplants of all CMV serotype matches. In total, 148 biopsies were collected from 33 recipients given CMVIG with a short course of ganciclovir or valganciclovir (days 21 to 42 posttransplant) and 155 biopsies from 24 control patients given oral acyclovir for 24 months, with IV ganciclovir added in the event of CMV antigenemia or confirmation of clinically suspected CMV infection. The CMVIG did not influence the rate of pneumonia during the first month, compatible with its mode of action, but subsequently resulted in a marked and significant reduction in tissue pneumonia up to the final follow-up at 24 months.34
The available data do not show a consistent effect for CMVIG, with or without concomitant antiviral therapy, on rates of acute rejection after lung transplantation,33,35,38 except for 2 retrospective studies.20,36
Regarding chronic rejection (bronchiolitis obliterans syndrome), the analyses by Ruttmann et al37 and Valantine et al20 reported a significant reduction in the rate of bronchiolitis obliterans syndrome using CMVIG prophylaxis, whereas other studies did not report consistent data.33,35,36 Relating to this, the immunomodulatory properties of CMVIG have been discussed above in the context of heart transplantation.
Data on use of CMVIG as preemptive therapy in lung transplantation is restricted to a small number of cases in which CMVIG with oral ganciclovir achieved antigenemia neutralization within the first month of treatment.40
Ranganathan et al35 undertook a multicenter retrospective analysis of 329 children given ganciclovir prophylaxis, 62 of whom were also given CMVIG using various regimens. Multivariate modeling showed that patients without CMVIG were 3-fold more likely to develop CMV infection. The authors found no effect of CMVIG administration on risk for developing CMV disease, possibly because the study population included patients at all levels of risk.
One large (n = 1157) single-center retrospective analysis of lung transplant recipients from 1989 to 2011, who received CMVIG for 4 weeks with ganciclovir and/or valganciclovir, found a low rate of posttransplant lymphoproliferative disease (1.5%) compared with the literature,41 but comparative studies are lacking.
CMVIG Dosing in Thoracic Transplantation
The manufacturer's recommendations for Cytotect in solid organ transplantation advise that administration should start on the day of transplantation, with at least 6 doses (50 mg/kg) given at intervals of 2 to 3 weeks. For Cytogam, 5 doses of 150 mg/kg are recommended by the manufacturer, starting within 72 hours of transplant then at 2 weekly intervals, followed by 2 doses of 10 mg/kg to week 16. A variety of regimens have been used in thoracic transplant studies, including occasional administration of a single high dose but more typically at the recommended doses given up to 7 times. One retrospective, single-center study compared different dosing regimens for CMVIG in D+/R− heart transplant patients.42 Patients were given 1 dose of IV nonspecific immunoglobulin therapy (500 mg/kg) followed by either 1 or 5 doses of CMVIG (Cytogam, 125 mg/kg). The antiviral regimens comprised IV and oral ganciclovir or acyclovir, and differed between groups, negating a robust comparison between groups. However, the patients who received 5 doses of CMVIG showed markedly lower rates of CMV disease at 2 years posttransplant than those given only 1 dose (17% or 33% with multiple-dose CMVIG, depending on antiviral therapy, versus 75% with single-dose CMVIG, P < 0.01).42 In terms of duration, 1 trial in recipients of a CMV-seropositive lung transplant given CMVIG for only 1 month showed good efficacy when given with ganciclovir,37 but treatment for a longer period, broadly compatible with the manufacturer's recommendations, is more standard.
CONCLUSIONS
In light of the need to provide effective and safe anti-CMV strategies in thoracic organ transplantation, without exposing patients to an increased risk of drug toxicity or viral resistance, we reviewed the available literature to identify evidence for a benefit of CMVIG in this specific high-risk patient population.
Although scattered across several small, single-center, and often retrospective studies, and frequently limited by the absence of current antiviral therapy, the available data suggest that CMVIG, when associated with antiviral therapy, may provide an additional benefit in preventing CMV disease and manifestations of chronic rejection in thoracic transplant recipients. This benefit appears particularly apparent in D+/R− heart and lung transplant patients, achieving a low rate of CMV disease and effective prevention of CMV-related death.18-20,37,38 It must be noted, however, that prophylaxis with CMVIG alone is not advisable.21 The favorable safety profile of CMVIG as compared with ganciclovir derivatives and other antiviral agents permits its administration as rescue or adjunctive therapy: in patients with leucopenia (a common side effect of ganciclovir), especially those receiving mycophenolic acid immunosuppression; in patients who are intolerant to antiviral agents; or in those who are under intense immunosuppression (eg, antirejection treatment with high-dose steroids or lymphocyte-depleting therapy).
On the other hand, the studies reviewed herein also highlighted the issues relating to CMVIG strategy that require further investigation. These include the lack of adequately sized prospective randomized studies including seropositive recipients; specific investigation of the mechanism of action of CMVIG, potentially involving the recovery of CMV cell-mediated immunity; data on the possible positive interaction with mammalian target of rapamycin inhibitors, immunosuppressants known to be associated with a low risk of CMV infection; and the immunomodulatory effect of CMVIG in preempting the onset of donor-specific antibodies, a CMV-independent effect that could partially explain some of the benefits observed with CMVIG,18,20,37 and that may be related to a class effect of immunoglobulins.
Footnotes
This supplement was funded by Biotest AG, Dreieich, Germany.
F.R. has received advisory board fees from Biotest. L.P. has received advisory board fees from Biotest, Novartis, and Diaxonhit. N.Y. has received a speaker's honorarium from Biotest AG. F.W. received advisory board fees from Biotest. F.C. has no relevant conflicts of interest in relation to Biotest or other pharmaceutical companies.
All authors attended a meeting to discuss the content of this supplement and review the available evidence, after which the article was developed by a freelance medical writer. All authors undertook a detailed critique of draft texts and approved the final article for submission.
REFERENCES
- 1. Le Page AK, Jager MM, Kotton CN, et al. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation. 2013; 95: 1455– 1460. [DOI] [PubMed] [Google Scholar]
- 2. Zuk DM, Humar A, Weinkauf JG, et al. An international survey of cytomegalovirus management practices in lung transplantation. Transplantation. 2010; 90: 672– 676. [DOI] [PubMed] [Google Scholar]
- 3. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96: 333– 360. [DOI] [PubMed] [Google Scholar]
- 4. Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010; 29: 914– 956. [DOI] [PubMed] [Google Scholar]
- 5. Snydman DR, Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med. 1987; 317: 1049– 1054. [DOI] [PubMed] [Google Scholar]
- 6. Syndman DR, Werner BG, Dougherty NN, et al. Cytomegalovirus immune globulin prophylaxis in liver transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1993; 119: 984– 991. [DOI] [PubMed] [Google Scholar]
- 7. Snydman DR, Ulsh P. The association between cytomegalovirus immune globulin (CMVIG) and recipient and graft survival in CMV donor positive/ CMV recipient negative renal transplant recipients. Am J Transplant. 2010; 10(Suppl 4): 326. [Google Scholar]
- 8. Fisher RA, Kistler KD, Ulsh P, et al. The association between cytomegalovirus immune globulin and long-term recipient and graft survival following liver transplantation. Transpl Infect Dis. 2012; 14: 121– 131. [DOI] [PubMed] [Google Scholar]
- 9. Bonaros N, Mayer B, Schachner T, et al. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008; 22: 89– 97. [DOI] [PubMed] [Google Scholar]
- 10. Snydman DR, Kistler KD, Ulsh P, et al. The impact of CMV prevention on long-term recipient and graft survival in heart transplant recipients: analysis of the Scientific Registry of Transplant Recipients (SRTR) database. Clin Transplant. 2011; 25: E455– E462. [DOI] [PubMed] [Google Scholar]
- 11. Snydman DR, Kistler KD, Ulsh P, et al. Cytomegalovirus prevention and long-term recipient and graft survival in pediatric heart transplant recipients. Transplantation. 2010; 90: 1432– 1438. [DOI] [PubMed] [Google Scholar]
- 12. Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012; 14: 633– 641. [DOI] [PubMed] [Google Scholar]
- 13. da Cunha-Bang C, Sørensen SS, Iversen M, et al. Factors associated with the development of cytomegalovirus infection following solid organ transplantation. Scand J Infect Dis. 2011; 43: 360– 365. [DOI] [PubMed] [Google Scholar]
- 14. van Gent R, Jaadar H, Tjon AS, et al. T-cell inhibitory capacity of hyperimmunoglobulins is influenced by the production process. Int Immunopharmacol. 2014; 19: 142– 144. [DOI] [PubMed] [Google Scholar]
- 15. Germer M, Schϋttrumpf J. Functional characterisation of human cytomegalovirus IgG preparations. Transplant Int. 2014; 27: 21 [Abstract 060]. [DOI] [PubMed] [Google Scholar]
- 16. Metselaar HJ, Balk AH, Mochtar B, et al. Cytomegalovirus seronegative heart transplant recipients. Prophylactic use of anti-CMV immunoglobulin. Chest. 1990; 97: 396– 399. [DOI] [PubMed] [Google Scholar]
- 17. Balk AH, Wiemar W, Rothbarth PH, et al. Passive immunization against cytomegalovirus in allograft recipients. The Rotterdam Heart Transplant Program experience. Infection. 1993; 21: 195– 200. [DOI] [PubMed] [Google Scholar]
- 18. Potena L, Holweg CT, Chin C, et al. Acute rejection and cardiac allograft vascular disease is reduced by suppression of subclinical cytomegalovirus infection. Transplantation. 2006; 82: 398– 405. [DOI] [PubMed] [Google Scholar]
- 19. Bonaros NE, Kocher A, Dunkler D, et al. Comparison of combined prophylaxis of cytomegalovirus hyperimmune globulin plus ganciclovir versus cytomegalovirus hyperimmune globulin alone in high-risk heart transplant recipients. Transplantation. 2004; 77: 890– 897. [DOI] [PubMed] [Google Scholar]
- 20. Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation. 2001; 72: 1647– 1652. [DOI] [PubMed] [Google Scholar]
- 21. Aguado JM, Gomez-Sanchez MA, Lumbreras C, et al. Prospective randomized trial of efficacy of ganciclovir versus that of anti-cytomegalovirus (CMV) immunoglobulin to prevent CMV disease in CMV-seropositive heart transplant recipients treated with OKT3. Antimicrob Agents Chemother. 1995; 39: 1643– 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamani MH, Avery R, Mawhorter SD, et al. The impact of Cytogam on cardiac transplant recipients with moderate hypogammaglobulinemia: a randomized single-center study. J Heart Lung Transplant. 2005; 24: 1766– 1769. [DOI] [PubMed] [Google Scholar]
- 23. Vrtovec B, Thomas CD, Radovancevic R, et al. Comparison of intravenous ganciclovir and cytomegalovirus hyperimmune globulin pre-emptive treatment in cytomegalovirus-positive heart transplant recipients. J Heart Lung Transplant. 2004; 23: 461– 465. [DOI] [PubMed] [Google Scholar]
- 24. Kocher AA, Bonaros N, Dunkler D, et al. Long-term results of CMV hyperimmune globulin prophylaxis in 377 heart transplant recipients. J Heart Lung Transplant. 2003; 22: 250– 257. [DOI] [PubMed] [Google Scholar]
- 25. Potena L, Holweg CT, Vana ML, et al. Frequent occult infection with cytomegalovirus in cardiac transplant recipients despite antiviral prophylaxis. J Clin Microbiol. 2007; 45: 1804– 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalil AC, Levitsky J, Lyden E, et al. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005; 143: 870– 880. [DOI] [PubMed] [Google Scholar]
- 27. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013; 13: 176– 189. [DOI] [PubMed] [Google Scholar]
- 28. Nieto FJ, Adam E, Sorlie P, et al. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996; 94: 922– 927. [DOI] [PubMed] [Google Scholar]
- 29. Fearon WF, Potena L, Hirohata A, et al. Changes in coronary arterial dimensions early after cardiac transplantation. Transplantation. 2007; 83: 700– 705. [DOI] [PubMed] [Google Scholar]
- 30. Potena L, Grigioni F, Ortolani P, et al. Relevance of cytomegalovirus infection and coronary-artery remodeling in the first year after heart transplantation: a prospective three-dimensional intravascular ultrasound study. Transplantation. 2003; 75: 839– 843. [DOI] [PubMed] [Google Scholar]
- 31. Humar A, Limaye AP, Blumberg EA, et al. Extended valganciclovir prophylaxis in D+/R− kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation. 2010; 90: 1427– 1431. [DOI] [PubMed] [Google Scholar]
- 32. Finlen Copeland CA, Davis WA, Snyder LD, et al. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant. 2011; 30: 990– 996. [DOI] [PubMed] [Google Scholar]
- 33. Kruger RM, Paranjothi S, Storch GA, et al. Impact of prophylaxis with Cytogam alone on the incidence of CMV viremia in CMV-seropositive lung transplant recipients. J Heart Lung Transplant. 2003; 22: 754– 763. [DOI] [PubMed] [Google Scholar]
- 34. Solidoro P, Delsedime L, Costa C, et al. Effect of CMV-immunoglobulins (Cytotect Biotest) prophylaxis on CMV pneumonia after lung transplantation. New Microbiol. 2011; 34: 33– 36. [PubMed] [Google Scholar]
- 35. Ranganathan K, Worley S, Michaels MG, et al. Cytomegalovirus immunoglobulin decreases the risk of cytomegalovirus infection but not disease after pediatric lung transplantation. J Heart Lung Transplant. 2009; 28: 1050– 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solidoro P, Libertucci D, Delsedime L, et al. Combined cytomegalovirus prophylaxis in lung transplantation: effects on acute rejection, lymphocytic bronchitis/bronchiolitis, and herpesvirus infections. Transplant Proc. 2008; 40: 2013– 2014. [DOI] [PubMed] [Google Scholar]
- 37. Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006; 81: 1415– 1420. [DOI] [PubMed] [Google Scholar]
- 38. Weill D, Lock BJ, Wewers DL, et al. Combination prophylaxis with ganciclovir and cytomegalovirus (CMV) immune globulin after lung transplantation: effective CMV prevention following daclizumab induction. Am J Transplant. 2003; 3: 492– 496. [DOI] [PubMed] [Google Scholar]
- 39. Novick RJ, Menkis AH, McKenzie FN, et al. Should heart-lung transplant donors and recipients be matched according to cytomegalovirus serologic status? J Heart Transplant. 1990; 6: 699– 706. [PubMed] [Google Scholar]
- 40. García-Gallo CL, Gil PU, Laporta R, et al. Is gammaglobulin anti-CMV warranted in lung transplantation? Transplant Proc. 2005; 37: 4043– 4045. [DOI] [PubMed] [Google Scholar]
- 41. Jaksch P, Wiedemann D, Kocher A, et al. Effect of cytomegalovirus immunoglobulin on the incidence of lymphoproliferative disease after lung transplantation: single-center experience with 1157 patients. Transplantation. 2013; 95: 766– 772. [DOI] [PubMed] [Google Scholar]
- 42. Czer LS, Ruzza A, Vespignani R, et al. Prophylaxis of cytomegalovirus disease in mismatched patients after heart transplantation using combined antiviral and immunoglobulin therapy. Transplant Proc. 2011; 43: 1887– 1892. [DOI] [PubMed] [Google Scholar]


