Abstract
OBJECTIVE
We aimed to elucidate whether potato consumption is associated with a higher risk of type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
We analyzed data in three cohorts consisting of U.S. male and female health professionals without diabetes, cardiovascular disease, and cancer at baseline: 70,773 women from the Nurses’ Health Study (1984–2010), 87,739 women from Nurses’ Health Study II (1991–2011), and 40,669 men from the Health Professionals Follow-up Study (1986–2010). Potato consumption was assessed quadrennially using validated food frequency questionnaires (FFQs), and we calculated 4-year change in potato consumption from consecutive FFQs. Self-reported T2D diagnosis was confirmed using a validated supplementary questionnaire.
RESULTS
During 3,988,007 person-years of follow-up, 15,362 new cases of T2D were identified. Higher consumption of total potatoes (including baked, boiled, or mashed potatoes and french fries) was significantly associated with an elevated risk for T2D: the pooled hazard ratio (HR) of T2D compared with <1 serving/week was 1.07 (95% CI 0.97–1.18) for 2–4 servings/week and 1.33 (95% CI 1.17–1.52) for ≥7 servings/week after adjustment for demographic, lifestyle, and dietary factors. In addition, the pooled HRs of T2D for every 3 servings/week were 1.04 (95% CI 1.01–1.08) for baked, boiled, or mashed potatoes, and 1.19 (95% CI 1.13–1.25) for french fries. We further estimated that the HR of T2D was 0.88 (95% CI 0.84–0.91) for replacing 3 servings/week of total potatoes with the same amount of whole grains. Last, in comparison with stable potato consumption, every 3-servings/week increment of potato consumption in 4 years was associated with a 4% (95% CI 0–8%) higher T2D risk.
CONCLUSIONS
Greater consumption of potatoes, especially french fries, was associated with a higher T2D risk, independent of BMI and other risk factors. Replacement of potatoes with whole grains was associated with a lower T2D risk.
Introduction
Potatoes are widely consumed in the U.S. and European countries as a staple food (1). Potatoes primarily contain starches and generally have a high glycemic index (GI) and glycemic load (GL) (2,3). Because a higher GI and GL of an overall diet is associated with a higher risk of type 2 diabetes (T2D) (4,5), greater potato consumption has the potential to increase risk for T2D. However, a controversy has been raised in the U.S. and U.K. regarding whether potatoes should be regarded as vegetables in dietary recommendations (6–8). Currently, in the U.S. national food guide, called “MyPlate,” potatoes are considered a vegetable (9), whereas potatoes are grouped with cereals in the U.K. national food guide, the “eatwell plate” (10). The U.S. Institute of Medicine recently recommended that white potatoes be allowed as an eligible vegetable of the Special Supplemental Nutrition Program for Women, Infants, and Children (11). Such inconsistencies may originate from different considerations, including the historical nature of potatoes and policies of trade, agriculture, and food, as well as the limited and mixed evidence of the association of potato consumption with health outcomes such as T2D (12–17).
In this analysis, therefore, we aimed to examine whether greater potato consumption was associated with an increased risk of T2D using data from the Nurses’ Health Study (NHS), NHSII, and the Health Professionals Follow-up Study (HPFS). In addition, we examined whether consumption of individual potato foods may be differentially associated with T2D risk. Moreover, to strengthen causal inference, we further examined whether increased potato consumption over time was associated with subsequent incidence of T2D. The change analysis was intended to mimic intervention studies in which dietary changes or food substitutions might affect subsequent disease risk.
Research Design and Methods
Study Population
The NHS enrolled 121,701 female nurses in 1976 (18); the NHSII was launched in 1989, enrolling 116,430 female nurses (19); and the HPFS was established in 1986 with the enrollment of 51,529 male health professionals (20). Every 2 years since baseline, follow-up questionnaires have been mailed to the participants to collect and update information on lifestyle practices and the occurrence of chronic diseases. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard School of Public Health, Boston, MA. The completion of the self-administered questionnaire was considered to imply informed consent.
Exclusion Criteria
We excluded participants who reported having diabetes, cardiovascular disease, or cancer at baseline (n = 9,806 in 1984 for NHS, 6,234 in 1991 for NHSII, and 7,182 in 1986 for HPFS). We also excluded those who had missing data for individual potato foods or an unusual level of total energy intake (<500 or >3,500 kcal/day for NHS and NHSII, and <800 or >4,200 kcal/day for HPFS; n = 750 for NHS, 3,047 for NHSII, and 1,162 for HPFS); those whose diagnosis date of T2D was undetermined (n = 259 for HPFS); and those who did not respond to any follow-up questionnaire after baseline survey (n = 385 for NHS, 578 for NHSII, and 658 for HPFS). After excluding these participants, 70,773 women in NHS, 87,739 women in NHSII, and 40,669 men in HPFS were available for the analysis.
Assessment of Potato Consumption
A semiquantitative food frequency questionnaire (FFQ) was used to assess and update participants’ habitual diet in the past year; this was sent to participants in 1984, 1986, and every 4 years thereafter in NHS, and quadrennially since 1991 in NHSII and since 1986 for HPFS. In all FFQs we asked the participants how often, on average, they consumed each food with a standard portion size (1 medium or 1 cup for baked, boiled, or mashed potatoes; 4 oz or 1 serving for french fries). Participants could choose from nine possible responses, ranging from “never, or less than once per month” to “6 or more times per day.” Total potato consumption was calculated by summing the frequency of eating baked, boiled, or mashed potatoes and french fries. We included baked, boiled, or mashed potatoes as a single food item; although their glycemic properties were quite similar, they had a large variance that resulted from differences in preparation methods and the temperature of the food when served: GI was 86 ± 6 for baked potatoes, 82 ± 7 for boiled potatoes, and 87 ± 3 for instant mashed potatoes (3). Consumption of chips was not included in total potato consumption because we combined potato and corn chips in a question. These semiquantitative FFQs were previously validated against diet records (21,22) (Supplementary Methods). In addition, primarily based on the U.S. Department of Agriculture Nutrition Database (2), we also estimated individuals’ intake of nutrients, including total starch (i.e., amylopectin and amylose) (23,24).
Assessment of Diabetes
In all three cohorts T2D incidence was queried in the biennial follow-up questionnaires, and a supplementary questionnaire was used to confirm the self-reported diagnosis and actual diagnosis date. A T2D diagnosis was confirmed if participants met the National Diabetes Data Group criteria (25,26) (detailed in the Supplementary Methods). In prior validation studies, among 62 self-reported cases of T2D confirmed by the supplementary questionnaire, 61 cases (98%) in the NHS and 57 of 59 cases (97%) in the HPFS were reconfirmed after an endocrinologist reviewed the medical records (27,28).
Statistical Analysis
Evaluation of Potato Consumption in Relation to T2D Risk
We calculated the person-years from the return date of the baseline FFQ to the date of T2D diagnosis, date of death, return date of the most recent valid follow-up questionnaire, or the end of follow-up (2010 for NHS and HPFS, 2011 for NHSII), whichever came first. To represent long-term diet and minimize within-person variation, we calculated and used the cumulative average of dietary intake based on valid FFQ assessments through the follow-up (29). In a sensitivity analysis we also examined the primary hypothesis using simply updated consumption levels instead of cumulative average. We used the following potato consumption categories: for total potato foods, less than one, one, two to four, five to six, and seven or more servings/week; for individual potato foods, almost never, one to three servings/month, and one, two to four, and five or more servings/week. Of note, the lowest two consumption categories of baked, boiled, or mashed potatoes were combined into a reference group to maintain an adequate sample size. To minimize missing covariates, we carried forward the last valid value to replace missing values of physical activity (9.4% for NHS, 9.3% for NHSII, and 15.1% for HPFS). For missing values of covariates, including BMI, physical activity, smoking status, oral contraceptive use, menopausal status, and postmenopausal hormone use, we created a dummy variable when making categories for each covariate.
All analyses were conducted separately in each cohort first, and then we pooled the results from three cohorts. The hazard ratios (HR) and 95% CIs of T2D were estimated for consumption levels of total and individual potato foods using time-dependent Cox regression. The regression analysis was stratified jointly by age and calendar year and was adjusted for BMI at baseline, ethnicity, family history of diabetes, and updated values of physical activity, cigarette smoking, alcohol intake, multivitamin use, menopausal status and postmenopausal hormone use (women only), oral contraceptive use (NHSII only), total energy intake, and the modified alternate Healthy Eating Index (aHEI) score (30) (assessment of covariates is detailed in the Supplementary Methods). We mutually adjusted for individual potato foods when examining their associations with T2D risk. To evaluate the impact of potential confounding or intermediate factors, we conducted two sensitivity analyses: 1) further adjusting for the GL values of overall diet, excluding potatoes, and 2) adjusting for updated BMI instead of baseline BMI.
Moreover, we examined associations using energy-adjusted residuals of potato intake as exposure or using simply updated intake levels instead of cumulative average of intake levels to evaluate the robustness of the results of the main analyses. Furthermore, to examine the influence of adjustment for major dietary variables, we conducted primary analyses by including intakes of individual food groups, including red meat, fish, whole grains, sugar-sweetened beverages, coffee, fruits, fruit juices, vegetables, and nuts (all fifths), instead of the modified aHEI score. Finally, to assess the potential impact of reverse causation resulting from health issues such as a prediabetic condition, we excluded incident T2D cases in the first 2 years of follow-up.
The proportional hazards assumption was tested by including interaction terms between consumption of total and individual potato foods and follow-up duration, and the assumption was not violated (P > 0.05 for all tests). Linear trend was examined by modeling the median values of potato consumption categories as a continuous variable. According to the assumption that the biological effects of potato intake were similar among study populations with different demographics, we pooled the multivariable-adjusted HRs from three cohorts using a fixed-effects model and examined the heterogeneity of associations among the cohorts using the Cochrane Q statistic and the I2 statistic. We examined interactions with BMI, smoking status, physical activity, and the modified aHEI score in relation to T2D risk by examining product terms between a dichotomous variable and potato intake in the full model using the Wald test.
We further estimated potential effects of substituting consumption of whole grains for consumption of total and individual potato foods (31). To estimate substitution effects of healthy foods for potato in a realistic setting, we used a serving-based replacement instead of an energy-based replacement. By including continuous variables of whole-grain intake and total or individual potato food intake in the same model, point estimates and variances for each exposure and the covariance between them were obtained. Point estimate for the replacement of total or individual potato foods with whole grains was calculated as the differences in point estimates of whole grains and potatoes, and SE was calculated as the square root of the sum of their variances − (2 × covariance).
Evaluation of Changes in Potato Consumption With Subsequent Risk of T2D
We calculated changes in the consumption of total and individual potato foods in every 4-year interval and evaluated associations with subsequent T2D risk. In this analysis we further excluded participants with missing changes in potato consumption. A total of 48,183 women from NHS, 73,328 women from NHSII, and 29,090 men from HPFS were included in the analysis. Based on the considerations of statistical power, we used the following categories: decreased ≥4 servings/week, decreased 1.0–3.9 servings/week, no change (±0.9 serving/week), increased 1.0–3.9 servings/week, and increased ≥4 servings/week for total potato foods; decreased ≥1 serving/week, decreased 0.5–0.9 serving/week, no change (±0.5 serving/week), increased 0.5–0.9 serving/week, and increased ≥1 serving/week for individual potato foods. Similar to the main analysis, we stratified jointly by age and calendar year, and adjusted for ethnicity, family history of diabetes, initial BMI, baseline values of multivitamin use, menopausal status and postmenopausal hormone use (women only), oral contraceptive use (NHSII only), and both initial and change values of physical activity, cigarette smoking, total energy intake, and the modified aHEI score (30).
To examine whether the association between potato intake and T2D risk is mediated by the GL of the overall diet or total carbohydrate intake, we further adjusted for initial and change variables of GL of the overall diet or total carbohydrate intake in sensitivity analyses.
Statistical analyses were performed with SAS version 9.3 (SAS Institute, Inc.). All P values were two-sided, and statistical significance was defined as P < 0.05.
Results
Risk Profiles at Baseline in Three Cohorts
During 3,988,007 person-years of follow-up, 15,362 T2D cases were identified (NHS: 7,436 cases/1,584,572 person-years; NHSII: 4,621 cases/1,610,311 person-years; HPFS: 3,305 cases/793,124 person-years). Average consumption of baked, boiled, or mashed potatoes was much larger than that of french fries in each category of total potato consumption (Supplementary Table 1). Total potato consumption showed a left-skewed distribution (Table 1) and was correlated with total energy intake; consumption of red meat, sugar-sweetened beverages, and trans fat; and prevalence of current smoking. It was inversely correlated with age, physical activity, modified aHEI score, and the likelihood of multivitamin use.
Table 1.
Baseline characteristics of participants according to total potato consumption in the NHS, NHSII, and HPFS
| Total potato consumption, servings/week | |||||
|---|---|---|---|---|---|
| <1 | 1 | 2–4 | 5–6 | ≥7 | |
| NHS (1984) | |||||
| Participants, n | 8,498 | 12,840 | 22,871 | 23,508 | 3,056 |
| Age, years | 51.8 (6.8) | 50.8 (7.0) | 50.1 (7.2) | 48.9 (7.1) | 51.2 (7.2) |
| BMI, kg/m2 | 24.5 (4.3) | 24.9 (4.5) | 24.8 (4.5) | 25.0 (4.8) | 24.9 (4.7) |
| Physical activity, MET-hours/week | 17.1 (24.2) | 15.9 (23.8) | 14.0 (19.6) | 12.5 (18.1) | 13.2 (27.0) |
| Alcohol intake, g/day | 7.0 (11.2) | 7.0 (11.3) | 7.1 (11.2) | 6.9 (11.3) | 6.5 (11.8) |
| Current smoker, % | 22.0 | 22.8 | 23.7 | 25.7 | 26.1 |
| Caucasian, % | 95.2 | 96.7 | 98.1 | 98.8 | 99.3 |
| Family history of diabetes, % | 25.8 | 25.1 | 25.4 | 25.6 | 25.1 |
| Multivitamin use, % | 42.9 | 39.7 | 37.7 | 33.5 | 28.9 |
| Ever menopausal hormone use, % | 26.1 | 23.6 | 22.0 | 18.6 | 21.9 |
| History of hypertension, % | 20.5 | 21.6 | 19.5 | 19.2 | 20.7 |
| History of hypercholesterolemia, % | 8.3 | 8.1 | 7.6 | 6.6 | 8.9 |
| Total energy intake, kcal/day | 1,393 (457) | 1,525 (457) | 1,719 (477) | 1,966 (505) | 2,165 (544) |
| Total starch intake, g/day | 50.5 (20.0) | 55.0 (16.5) | 59.0 (14.9) | 62.1 (13.7) | 66.2 (14.1) |
| GL | 94.3 (24.1) | 96.9 (20.8) | 99.3 (18.9) | 101.1 (17.1) | 107.7 (19.2) |
| Red meat intake, servings/day | 0.77 (0.59) | 0.92 (0.59) | 1.11 (0.62) | 1.41 (0.68) | 1.52 (0.86) |
| Fish intake, servings/day | 0.29 (0.28) | 0.28 (0.26) | 0.27 (0.23) | 0.25 (0.21) | 0.25 (0.22) |
| Whole-grain intake, servings/day | 0.4 (0.4) | 0.4 (0.4) | 0.4 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| Total fruit intake, servings/day | 1.44 (1.13) | 1.38 (1.04) | 1.35 (0.99) | 1.27 (0.92) | 1.29 (1.02) |
| Total vegetable intake, servings/day | 2.96 (1.76) | 2.97 (1.64) | 3.06 (1.56) | 3.10 (1.52) | 3.24 (1.66) |
| Coffee intake, servings/day | 2.42 (1.85) | 2.40 (1.83) | 2.42 (1.83) | 2.46 (1.86) | 2.45 (1.89) |
| Sugar-sweetened beverage intake, servings/day | 0.16 (0.46) | 0.22 (0.51) | 0.28 (0.54) | 0.40 (0.65) | 0.46 (0.78) |
| Polyunsaturated fat–to–saturated fat ratio | 0.74 (0.35) | 0.72 (0.33) | 0.70 (0.30) | 0.68 (0.27) | 0.67 (0.30) |
| Trans fat intake, g/day | 1.64 (0.63) | 1.78 (0.60) | 1.90 (0.59) | 2.07 (0.56) | 2.00 (0.61) |
| Modified aHEI score | 41.9 (10.1) | 39.5 (9.6) | 36.6 (9.3) | 32.3 (8.8) | 31.3 (9.8) |
| NHSII (1991) | |||||
| Participants, n | 8,366 | 16,046 | 31,193 | 29,965 | 2,169 |
| Age, years | 36.6 (4.6) | 36.3 (4.7) | 36.0 (4.7) | 36.0 (4.7) | 35.7 (4.8) |
| BMI, kg/m2 | 23.7 (4.6) | 24.1 (4.9) | 24.4 (5.1) | 25.0 (5.6) | 25.6 (6.5) |
| Physical activity, MET-hours/week | 25.5 (32.7) | 22.6 (28.7) | 20.7 (27.3) | 19.0 (24.7) | 21.2 (29.8) |
| Alcohol intake, g/day | 3.1 (6.1) | 3.2 (6.0) | 3.2 (6.2) | 3.1 (6.0) | 3.0 (6.8) |
| Current smoker, % | 10.7 | 10.8 | 12.1 | 13.2 | 15.2 |
| Caucasian, % | 91.6 | 93.9 | 96.0 | 97.4 | 97.6 |
| Family history of diabetes, % | 16.3 | 16.1 | 15.5 | 16.2 | 17.2 |
| Multivitamin use, % | 47.8 | 46.1 | 43.8 | 41.5 | 40.0 |
| Ever use menopausal hormones, % | 3.1 | 2.9 | 3.0 | 3.2 | 4.1 |
| Current oral contraceptive use, % | 10.7 | 10.7 | 11.1 | 10.7 | 11.3 |
| History of hypertension, % | 5.7 | 5.6 | 5.7 | 6.5 | 7.0 |
| History of hypercholesterolemia, % | 13.7 | 13.6 | 14.1 | 14.7 | 16.0 |
| Total energy intake, kcal/day | 1,442 (472) | 1,560 (479) | 1,734 (490) | 2,019 (524) | 2,367 (565) |
| Total starch intake, g/day | 77.0 (25.6) | 77.3 (21.8) | 78.0 (19.1) | 81.2 (17.5) | 85.1 (18.1) |
| GL | 123.4 (26.4) | 121.7 (23.1) | 120.6 (21.0) | 121.6 (19.5) | 125.2 (21.8) |
| Red meat intake, servings/day | 0.42 (0.41) | 0.56 (0.43) | 0.75 (0.47) | 1.00 (0.57) | 1.24 (0.81) |
| Fish intake, servings/day | 0.27 (0.30) | 0.26 (0.25) | 0.26 (0.24) | 0.27 (0.24) | 0.30 (0.33) |
| Whole-grain intake, servings/day | 0.7 (0.5) | 0.6 (0.4) | 0.5 (0.4) | 0.4 (0.3) | 0.4 (0.4) |
| Total fruit intake, servings/day | 1.23 (1.04) | 1.19 (0.97) | 1.17 (0.92) | 1.18 (0.92) | 1.12 (0.96) |
| Total vegetable intake, servings/day | 3.14 (2.42) | 3.02 (2.12) | 3.09 (2.03) | 3.29 (2.03) | 3.41 (2.28) |
| Coffee intake, servings/day | 0.36 (0.82) | 0.33 (0.78) | 0.32 (0.77) | 0.30 (0.75) | 0.29 (0.79) |
| Sugar-sweetened beverage intake, servings/day | 0.28 (0.66) | 0.34 (0.70) | 0.45 (0.82) | 0.60 (0.94) | 0.73 (1.11) |
| Polyunsaturated fat–to–saturated fat ratio | 0.56 (0.20) | 0.53 (0.17) | 0.52 (0.15) | 0.51 (0.14) | 0.52 (0.17) |
| Trans fat intake, g/day | 1.30 (0.55) | 1.46 (0.56) | 1.64 (0.58) | 1.80 (0.60) | 1.92 (0.77) |
| Modified aHEI score | 43.4 (10.1) | 40.7 (9.8) | 37.6 (9.5) | 34.0 (9.3) | 32.3 (10.4) |
| HPFS (1986) | |||||
| Participants, n | 3,722 | 6,819 | 13,192 | 15,179 | 1,757 |
| Age, years | 54.8 (9.4) | 53.5 (9.4) | 52.8 (9.5) | 52.1 (9.4) | 53.6 (9.7) |
| BMI, kg/m2 | 24.8 (5.0) | 24.8 (5.0) | 24.9 (4.7) | 25.0 (4.8) | 24.8 (5.1) |
| Physical activity, MET-hours/week | 22.9 (34.0) | 22.3 (30.4) | 22.0 (30.2) | 20.1 (27.7) | 22.2 (32.0) |
| Alcohol intake, g/day | 10.8 (15.1) | 11.0 (15.4) | 11.2 (14.9) | 12.0 (15.7) | 11.4 (16.3) |
| Current smoker, % | 8.2 | 9.5 | 8.6 | 10.5 | 10.9 |
| Caucasian, % | 89.1 | 93.1 | 95.5 | 96.9 | 96.9 |
| Family history of diabetes, % | 18.7 | 18.9 | 18.4 | 19.1 | 19.4 |
| Multivitamin use, % | 48.2 | 43.2 | 42.6 | 39.1 | 36.7 |
| History of hypertension, % | 21.2 | 20.6 | 18.9 | 18.0 | 20.1 |
| History of hypercholesterolemia, % | 10.6 | 10.8 | 10.8 | 9.3 | 10.4 |
| Total energy intake, kcal/day | 1,614 (514) | 1,720 (532) | 1,907 (545) | 2,229 (601) | 2,597 (658) |
| Total starch intake, g/day | 59.4 (26.5) | 63.2 (22.0) | 67.3 (19.9) | 70.8 (17.9) | 76.8 (19.2) |
| GL | 120.5 (31.6) | 122.6 (27.8) | 124.3 (25.6) | 125.0 (23.0) | 132.9 (26.3) |
| Red meat intake, servings/day | 0.76 (0.70) | 0.87 (0.67) | 1.03 (0.72) | 1.42 (0.82) | 1.68 (1.10) |
| Fish intake, servings/day | 0.34 (0.33) | 0.32 (0.29) | 0.33 (0.28) | 0.32 (0.28) | 0.34 (0.33) |
| Whole-grain intake, servings/day | 0.6 (0.6) | 0.6 (0.5) | 0.6 (0.5) | 0.5 (0.4) | 0.5 (0.5) |
| Total fruit intake, servings/day | 1.56 (1.55) | 1.50 (1.27) | 1.50 (1.25) | 1.43 (1.14) | 1.44 (1.22) |
| Total vegetable intake, servings/day | 2.80 (1.68) | 2.83 (1.67) | 3.02 (1.67) | 3.13 (1.61) | 3.38 (1.82) |
| Coffee intake, servings/day | 1.81 (1.75) | 1.84 (1.74) | 1.90 (1.77) | 2.02 (1.83) | 2.04 (1.92) |
| Sugar-sweetened beverage intake, servings/day | 0.18 (0.44) | 0.20 (0.43) | 0.25 (0.47) | 0.36 (0.58) | 0.45 (0.76) |
| Polyunsaturated fat–to–saturated fat ratio | 0.62 (0.27) | 0.59 (0.22) | 0.58 (0.21) | 0.54 (0.17) | 0.55 (0.21) |
| Trans fat intake, g/day | 2.41 (1.18) | 2.62 (1.14) | 2.75 (1.09) | 3.11 (1.07) | 3.10 (1.27) |
| Modified aHEI score | 43.1 (10.4) | 42.3 (10.2) | 41.0 (10.3) | 37.5 (10.1) | 36.6 (11.1) |
Data are mean (SD) unless otherwise indicated. MET, metabolic equivalent.
Association Between Habitual Potato Consumption and Risk of T2D
Total potato consumption was significantly associated with increased risk for T2D in age-adjusted analysis (Table 2). Adjusting for demographic, lifestyle, and modified aHEI score, as well as baseline BMI, largely weakened these associations, but the statistical significance remained. These associations were consistent among all three cohorts (for linear trend: P for heterogeneity = 0.32; I2 = 2.3%). Comparing seven or more servings/week with less than one serving/week of total potato consumption, the pooled, multivariable-adjusted HR of T2D was 1.33 (95% CI 1.17–1.52). In addition, each additional three servings/week of total potato consumption was associated with a multivariable-adjusted HR of T2D of 1.13 (1.09–1.17). For individual potato foods, greater consumption of baked, boiled, or mashed potatoes, and french fries, was associated with an increased risk of T2D after multivariable adjustment (Table 3). The pooled HRs of T2D for five or more servings/week were 1.08 (1.00–1.16) for baked, boiled, or mashed potatoes compared with less than one serving/week, and 1.32 (1.13–1.55) for french fries compared with less than one serving/month. For every three servings/week, the pooled HRs of T2D were 1.04 (1.01–1.08) for baked, boiled, or mashed potatoes and 1.19 (1.13–1.25) for french fries. By contrast, multivariable adjustment of covariates, especially baseline BMI, largely attenuated the association for consumption of potato/corn chips to null: comparing five or more servings/week with less than one serving/month, the HR was 0.90 (0.82–0.99) for the full model including demographic, lifestyle, and the modified aHEI score, and 0.93 (0.85–1.02) for the model that was further adjusted for baseline BMI.
Table 2.
HRs (95% CI) of T2D according to total potato consumption
| Total potato consumption, servings/week | Every 3 servings/week | P value for trend | |||||
|---|---|---|---|---|---|---|---|
| <1 | 1 | 2–4 | 5–6 | ≥7 | |||
| NHS | |||||||
| Cases/person-years | 222/76,255 | 678/188,652 | 3,625/765,323 | 2,742/520,932 | 169/33,410 | ||
| Model 1* | 1.00 | 1.14 (0.98–1.32) | 1.33 (1.16–1.53) | 1.56 (1.36–1.79) | 1.73 (1.42–2.11) | 1.23 (1.18–1.28) | <0.0001 |
| Model 2† | 1.00 | 1.18 (1.01–1.37) | 1.34 (1.17–1.54) | 1.49 (1.29–1.72) | 1.56 (1.27–1.92) | 1.16 (1.11–1.21) | <0.0001 |
| Model 3‡ | 1.00 | 1.16 (0.99–1.35) | 1.25 (1.09–1.44) | 1.31 (1.14–1.51) | 1.34 (1.09–1.64) | 1.08 (1.04–1.13) | 0.0003 |
| Model 4§ | 1.00 | 1.08 (0.93–1.26) | 1.15 (1.00–1.32) | 1.22 (1.05–1.40) | 1.27 (1.04–1.56) | 1.08 (1.04–1.13) | 0.0003 |
| NHSII | |||||||
| Cases/person-years | 124/78,479 | 400/215,170 | 2,156/788,609 | 1,782/498,008 | 159/30,045 | ||
| Model 1* | 1.00 | 1.05 (0.86–1.29) | 1.34 (1.12–1.60) | 1.96 (1.64–2.36) | 3.35 (2.64–4.23) | 1.57 (1.49–1.65) | <0.0001 |
| Model 2† | 1.00 | 1.04 (0.85–1.28) | 1.27 (1.05–1.52) | 1.70 (1.41–2.05) | 2.49 (1.95–3.17) | 1.41 (1.34–1.49) | <0.0001 |
| Model 3‡ | 1.00 | 1.02 (0.83–1.24) | 1.15 (0.96–1.39) | 1.44 (1.19–1.74) | 2.04 (1.60–2.61) | 1.30 (1.23–1.37) | <0.0001 |
| Model 4§ | 1.00 | 0.95 (0.78–1.16) | 0.99 (0.82–1.19) | 1.09 (0.90–1.31) | 1.38 (1.08–1.76) | 1.12 (1.05–1.18) | 0.0002 |
| HPFS | |||||||
| Cases/person-years | 120/34,791 | 311/89,791 | 1,466/350,216 | 1,266/291,602 | 142/26,723 | ||
| Model 1* | 1.00 | 0.95 (0.77–1.18) | 1.04 (0.86–1.25) | 1.13 (0.93–1.36) | 1.44 (1.13–1.84) | 1.12 (1.06–1.19) | <0.0001 |
| Model 2† | 1.00 | 1.00 (0.81–1.24) | 1.10 (0.91–1.33) | 1.18 (0.97–1.44) | 1.47 (1.14–1.89) | 1.12 (1.05–1.19) | 0.0003 |
| Model 3‡ | 1.00 | 0.99 (0.80–1.23) | 1.06 (0.88–1.29) | 1.10 (0.90–1.34) | 1.33 (1.03–1.72) | 1.07 (1.01–1.14) | 0.03 |
| Model 4§ | 1.00 | 0.94 (0.76–1.17) | 1.03 (0.85–1.24) | 1.09 (0.89–1.32) | 1.38 (1.07–1.78) | 1.10 (1.03–1.17) | 0.004 |
| Pooled¶ | |||||||
| Model 3‡ | 1.00 | 1.08 (0.97–1.20) | 1.17 (1.07–1.29) | 1.29 (1.17–1.42) | 1.52 (1.33–1.73) | 1.17 (1.13–1.22) | <0.0001 |
| Model 4§ | 1.00 | 1.01 (0.91–1.12) | 1.07 (0.97–1.18) | 1.15 (1.04–1.26) | 1.33 (1.17–1.52) | 1.13 (1.09–1.17) | <0.0001 |
*Model 1 was adjusted for age (years).
†Model 2 was adjusted for age; ethnicity (Caucasian, African American, Hispanic, or Asian); smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day], or missing); alcohol intake [0, 0.1–4.9, 5.0–14.9, or ≥15.0 g/day for women and 0, 0.1–4.9, 5.0–29.9, or ≥30.0 g/day for men); multivitamin use (yes or no); physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 metabolic equivalents of task-hours/week, or missing); a family history of diabetes (yes or no); menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, past, or current hormone use], or missing, for women); oral contraceptive use (never, past, current, or missing, for NHS II); and total energy intake (kcal/day).
‡Model 3 was adjusted for variables in model 2 plus modified aHEI score (fifths).
§Model 4 was adjusted for variables in model 3 plus baseline BMI (<23, 23.0–24.9, 25.0–26.9, 27.0–28.9, 29.0–30.9, 31.0–32.9, 33.0–34.9, 35.0–36.9, 37.0–38.9, 39.0–40.9, 41.0–42.9, 43.0–44.9, and ≥45.0 kg/m2, or missing).
¶Results from all cohorts were pooled using a fixed-effects meta-analysis.
Table 3.
Pooled, adjusted HRs (95% CI) of T2D for consumption of different potato foods*
| Consumption | Every 3 servings/week | P values for trend | |||||
|---|---|---|---|---|---|---|---|
| Almost never | 1–3 servings/month | 1 serving/week | 2–4 servings/week | ≥5 servings/week | |||
| Baked, boiled, or mashed potatoes | |||||||
| Cases/person-years | 1,108/416,491 | 2,928/958,417 | 8,568/2,050,019 | 2,758/563,081 | |||
| Multivariable-adjusted HR† | 1.00 | 1.04 (0.97–1.11) | 1.06 (1.00–1.14) | 1.09 (1.01–1.18) | 1.05 (1.01–1.08) | 0.01 | |
| Further adjustment for baseline BMI | 1.00 | 1.02 (0.95–1.09) | 1.03 (0.96–1.10) | 1.08 (1.00–1.16) | 1.04 (1.01–1.08) | 0.01 | |
| French fries | |||||||
| Cases/person-years | 2,270/831,523 | 6,493/1,704,668 | 4,082/987,853 | 2,315/432,871 | 202/31,091 | ||
| Multivariable-adjusted HR† | 1.00 | 1.21 (1.15–1.27) | 1.33 (1.25–1.41) | 1.54 (1.44–1.65) | 1.84 (1.57–2.15) | 1.41 (1.34–1.48) | <0.0001 |
| Further adjustment for baseline BMI | 1.00 | 1.11 (1.06–1.17) | 1.17 (1.11–1.24) | 1.26 (1.18–1.35) | 1.32 (1.13–1.55) | 1.19 (1.13–1.25) | <0.0001 |
*HRs from all cohorts were pooled using a fixed-effects meta-analysis.
†HRs were adjusted for age (years); ethnicity (Caucasian, African American, Hispanic, or Asian); smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day], or missing); alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥15.0 g/day for women, and 0, 0.1–4.9, 5.0–29.9, or ≥30.0 g/day for men); multivitamin use (yes or no); physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 metabolic equivalents of task-hours/week, or missing); a family history of diabetes (yes or no); menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, past, or current hormone use], or missing, for women); oral contraceptive use (never, past, current, or missing, for NHS II); total energy intake (kcal/day); modified aHEI score (fifths), baked, boiled or mashed potatoes (for french fries), and french fries (for baked, boiled, or mashed potatoes).
There was a significant interaction between total potato consumption with BMI (P < 0.001) and physical activity (P = 0.01) in relation to T2D risk (Supplementary Table 2). The positive associations between total potato consumption and T2D risk were somewhat stronger among individuals with low BMI than those with high BMI, and among those who had high physical activity than those engaging in little physical activity. No significant interaction was observed for aHEI score (P = 0.09) or smoking status (P = 0.69).
Sensitivity Analyses
To evaluate the potential mediational factors for the association between total potato consumption and T2D risk, as well as the robustness of the association, we conducted four additional analyses (Supplementary Table 3). To examine whether the association was confounded by the quality and quantity of carbohydrates, we adjusted for the GL values from all foods except potatoes; such an adjustment did not substantially change the positive association. To assess the impact of BMI change on the association between total potato consumption and T2D risk, we adjusted for time-varying BMI instead of baseline BMI and found similar associations. When we used energy-adjusted residuals of potato intake, we observed largely similar associations. When we used simply updated consumption levels instead of the cumulative average of consumption, the positive associations were largely unchanged. Furthermore, when we adjusted for individual dietary factors instead of the aHEI score, the associations were modestly attenuated, but the statistical significance persisted. Last, when we excluded T2D cases identified during the first 2 years of follow-up, the positive association did not drastically change.
Potential Impact of Substituting Whole Grains for Total or Individual Potato Foods
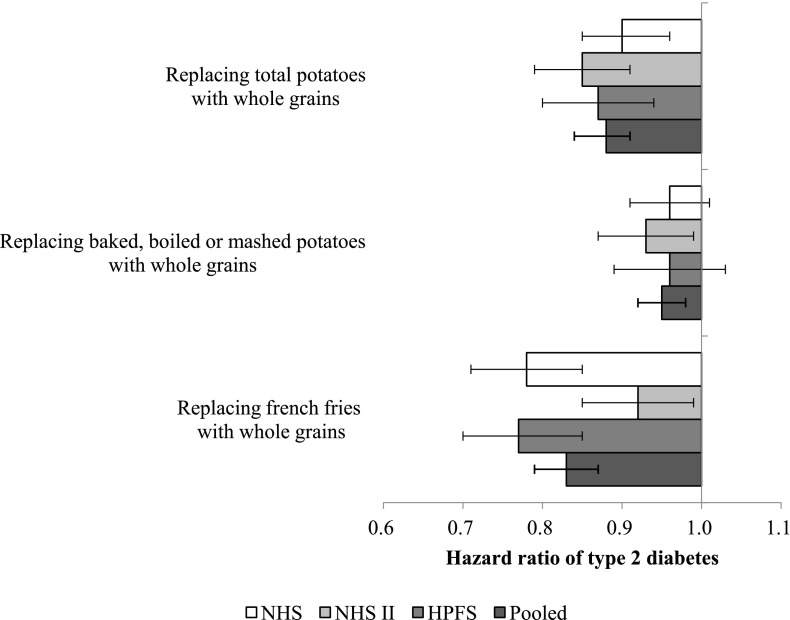
We estimated the potential benefits of substituting whole grains for total or individual potato foods as the main source of carbohydrates (Fig. 1). The pooled HR of T2D was 0.88 (95% CI 0.84–0.91) for replacing three servings/week of total potato foods with three servings/week of whole grains. The corresponding HRs were 0.95 (0.92–0.98) for baked, boiled, or mashed potatoes and 0.83 (0.79–0.87) for french fries.
Figure 1.
The impact of replacing potato foods with whole grains on risk of type 2 diabetes. All HRs were adjusted for age (years), ethnicity (Caucasian, African American, Hispanic, or Asian); baseline BMI (<23, 23.0–24.9, 25.0–26.9, 27.0–28.9, 29.0–30.9, 31.0–32.9, 33.0–34.9, 35.0–36.9, 37.0–38.9, 39.0–40.9, 41.0–42.9, 43.0–44.9, or ≥45.0 kg/m2, or missing); smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day], or missing); alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥15.0 g/day for women and 0, 0.1–4.9, 5.0–29.9, or ≥30.0 g/day for men); multivitamin use (yes or no); physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent of task-hours/week, or missing); a family history of diabetes (yes or no); menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, past, or current hormone use], or missing for women); oral contraceptive use (never, past, current, or missing for those in NHSII); total energy intake (kilocalories/day); modified aHEI score (fifths), baked, boiled, or mashed potatoes (for french fries), and french fries (for baked, boiled, or mashed potatoes). Error bars represent the 95% CIs.
Association of 4-Year Change in Potato Consumption With Subsequent Risk for T2D
In addition to the association between habitual potato consumption and T2D risk, we further examined the associations between 4-year changes in the consumption of total or individual potato foods and subsequent T2D risk (Table 4). Compared with stable intake over time, increased consumption of total potatoes, especially french fries, was associated with a higher T2D risk after multivariate adjustment for baseline and changes in demographic, lifestyle, and dietary factors. The pooled, multivariable-adjusted HR of T2D was 1.04 (95% CI 1.00–1.08; P value for heterogeneity = 0.61; I2 = 1.0%) for every three servings/week increase of total potato foods over a 4-year period. The corresponding HRs were 1.03 (1.00–1.07) for baked, boiled, or mashed potatoes and 1.10 (1.05–1.15) for french fries. Although baseline consumption of chips was not associated with T2D risk, increased consumption of chips during the follow-up period was associated with a higher risk: the HR was 1.04 (1.00–1.09) for every one serving/week increased intake of potato/corn chips over a 4-year period. In sensitivity analyses, after adjusting for the initial and changed GL values of the overall diet or total carbohydrate intake, the positive association remained statistically significant (Supplementary Table 4).
Table 4.
HRs (95% CI) of T2D for change in the consumption of potato foods in NHS, NHSII, and HPFS
| Change in potato consumption, servings/week | Every 3 servings/week | P value for trend | ||||||
|---|---|---|---|---|---|---|---|---|
| Decreased ≥4.0 | Decreased 1.0–3.9 | No change (±0.9) | Increased 1.0–3.9 | Increased ≥4.0 | ||||
| Total potato foods | ||||||||
| NHS (cases/person-years) | 191/28,461 | 971/187,321 | 2,703/541,523 | 851/156,280 | 124/21,717 | |||
| Model 1* | 1.12 (0.94–1.33) | 0.90 (0.83–0.97) | 1.00 | 1.14 (1.05–1.23) | 1.21 (1.01–1.45) | 1.11 (1.05–1.17) | 0.0002 | |
| Model 2† | 1.12 (0.94–1.33) | 0.92 (0.85–1.00) | 1.00 | 1.03 (0.96–1.12) | 1.00 (0.84–1.20) | 1.03 (0.97–1.09) | 0.37 | |
| Model 3‡ | 1.09 (0.92–1.30) | 0.91 (0.84–0.99) | 1.00 | 1.02 (0.95–1.11) | 0.98 (0.82–1.18) | 1.03 (0.97–1.09) | 0.36 | |
| NHSII (cases/person-years) | 163/33,584 | 670/205,114 | 1,601/566,999 | 629/175,691 | 140/28,291 | |||
| Model 1* | 1.06 (0.87–1.29) | 0.89 (0.80–0.98) | 1.00 | 1.37 (1.24–1.50) | 1.94 (1.63–2.31) | 1.30 (1.23–1.39) | <0.0001 | |
| Model 2† | 1.08 (0.89–1.31) | 0.93 (0.84–1.02) | 1.00 | 1.15 (1.05–1.26) | 1.25 (1.04–1.49) | 1.10 (1.04–1.18) | 0.002 | |
| Model 3‡ | 1.02 (0.84–1.24) | 0.92 (0.83–1.02) | 1.00 | 1.07 (0.97–1.17) | 1.12 (0.93–1.34) | 1.07 (1.00–1.13) | 0.05 | |
| HPFS (cases/person-years) | 90/19,274 | 458/97,614 | 1,079/253,156 | 419/88,724 | 101/16,963 | |||
| Model 1* | 1.11 (0.87–1.42) | 1.06 (0.94–1.19) | 1.00 | 1.10 (0.98–1.23) | 1.41 (1.15–1.72) | 1.07 (0.99–1.15) | 0.07 | |
| Model 2† | 1.07 (0.84–1.37) | 1.08 (0.96–1.22) | 1.00 | 1.04 (0.92–1.16) | 1.19 (0.97–1.47) | 1.01 (0.94–1.09) | 0.71 | |
| Model 3‡ | 1.00 (0.78–1.28) | 1.06 (0.94–1.19) | 1.00 | 1.03 (0.92–1.15) | 1.15 (0.93–1.42) | 1.02 (0.95–1.10) | 0.59 | |
| Pooled‡§ | 1.05 (0.93–1.17) | 0.94 (0.89–1.00) | 1.00 | 1.04 (0.98–1.10) | 1.08 (0.96–1.20) | 1.04 (1.00–1.08) | 0.04 | |
| Decreased ≥1.0 | Decreased 0.5–0.9 | No change (±0.4) | Increased 0.5–0.9 | Increased ≥1.0 | Every 1 serving/week | |||
| Baked, boiled, or mashed potatoes | ||||||||
| NHS (cases/person-years) | 1,120/210,194 | 390/72,267 | 2,198/426,228 | 227/54,448 | 894/168,856 | |||
| Model 3‡ | 0.92 (0.85–1.00) | 1.06 (0.95–1.20) | 1.00 | 0.89 (0.76–1.04) | 1.00 (0.92–1.08) | 1.02 (0.97–1.07) | 0.43 | |
| NHSII (cases/person-years) | 729/210,285 | 271/104,153 | 1,363/442,467 | 252/83,115 | 579/166,915 | |||
| Model 3‡ | 0.92 (0.83–1.02) | 0.93 (0.81–1.07) | 1.00 | 1.14 (0.97–1.34) | 1.04 (0.94–1.15) | 1.07 (1.01–1.14) | 0.03 | |
| HPFS (cases/person-years) | 465/104,335 | 166/34,727 | 919/212,317 | 145/29,960 | 443/92,537 | |||
| Model 3‡ | 1.01 (0.89–1.15) | 1.06 (0.89–1.27) | 1.00 | 1.09 (0.88–1.35) | 1.04 (0.92–1.18) | 1.01 (0.93–1.09) | 0.84 | |
| Pooled‡§ | 0.94 (0.89–0.99) | 1.02 (0.94–1.10) | 1.00 | 1.02 (0.93–1.13) | 1.02 (0.96–1.08) | 1.03 (1.00–1.07) | 0.06 | |
| French fries | ||||||||
| NHS (cases/person-years) | 196/31,887 | 820/154,752 | 2,886/596,764 | 706/118,407 | 199/27,958 | |||
| Model 3‡ | 0.90 (0.75–1.08) | 0.92 (0.84–1.00) | 1.00 | 1.09 (1.00–1.19) | 1.03 (0.88–1.19) | 1.12 (1.03–1.21) | 0.007 | |
| NHSII (cases/person-years) | 330/77,569 | 486/179,887 | 1,468/511,349 | 540/160,311 | 363/77,017 | |||
| Model 3‡ | 0.89 (0.76–1.04) | 0.93 (0.84–1.04) | 1.00 | 1.08 (0.98–1.20) | 1.09 (0.96–1.23) | 1.12 (1.04–1.20) | 0.003 | |
| HPFS (cases/person-years) | 206/36,105 | 359/78,189 | 1,022/252,608 | 343/70,415 | 190/33,501 | |||
| Model 3‡ | 1.06 (0.87–1.30) | 1.05 (0.92–1.19) | 1.00 | 1.13 (0.99–1.28) | 1.11 (0.95–1.31) | 1.05 (0.95–1.16) | 0.34 | |
| Pooled‡§ | 0.93 (0.84–1.03) | 0.95 (0.89–1.01) | 1.00 | 1.09 (1.03–1.16) | 1.07 (0.99–1.17) | 1.10 (1.05–1.15) | <0.0001 | |
*Model 1 was adjusted for age (years).
†Model 2 was adjusted for age (years); ethnicity (Caucasian, African American, Hispanic, or Asian); smoking status (never-never, current-past, never/past-current, past-past, or current-current smoker); a family history of diabetes (yes or no); baseline values of multivitamin use (yes or no); postmenopausal status and hormone use (premenopause, postmenopause [never, past, or current postmenopausal hormone use], or missing); oral contraceptive use (never, past, current, or missing, for NHS II); potato food intake; and initial values and changes of alcohol intake (for initial value: 0, 0.1–4.9, 5.0–14.9, ≥15.0 g/day, or missing [for women] and 0, 0.1–4.9, 5.0–29.9, ≥30.0 g/day, or missing [for men]; for change value: fifths or missing); physical activity (for initial value: <3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥27.0 metabolic equivalents of task-hours/week, or missing; for change value: fifths or missing); total energy intake (kilocalories/day for initial value, fifths for change value); and modified aHEI score (fifths for both initial and change values).
‡Model 3 was adjusted for the variables in model 2 plus initial BMI (<23, 23.0–24.9, 25.0–26.9, 27.0–28.9, 29.0–30.9, 31.0–32.9, 33.0–34.9, and ≥35.0 kg/m2, or missing).
§Results from all cohorts were pooled using a fixed-effects meta-analysis.
Conclusions
In these three cohorts of U.S. men and women, higher consumption of total and individual potato foods was positively associated with T2D risk. Moreover, increased consumption of total potato foods, especially french fries over a 4-year period, was associated with a subsequently increased risk of developing T2D. These associations were independent of demographic, anthropometric, lifestyle, and dietary factors related to T2D, were robust in various sensitivity analyses, and largely persisted within populations with various baseline risk profiles of diabetes. Furthermore, we estimated that substitution of whole grains for total potato foods; baked, boiled, or mashed potatoes; or french fries was associated with a lower risk for T2D.
Evidence of potato consumption in relation to T2D risk was sparse. In three cross-sectional and case-control studies, intake of potatoes or french fries was positively associated with insulin resistance and prevalent T2D (12–14). In a Finnish cohort comprising 4,303 men and women, participants who consumed >283 g/day of total potatoes had 42% higher incident diabetes medication use than those consuming <132 g/day of potatoes (15). In the Women’s Health Study, which comprised 39,876 female health professionals, total potato consumption was not associated with diabetes risk after multivariable adjustment (16).
To our knowledge, this analysis is among the first investigations to examine changes in potato food intake in relation to subsequent risk of developing T2D. The positive associations observed in this analysis further corroborate the hypothesis that increased potato food intake may lead to an increased risk for T2D. Since this is an observational study, we cannot establish the causality between potato consumption and the development of T2D. In clinical trials, maintaining high adherence to a dietary intervention for a long time is typically difficult, in part because of dietary changes contradicting participants’ long-term dietary preferences. Poor adherence dilutes the true effect of an intervention. By contrast, although our study design cannot completely eliminate potential confounding, the findings from a natural experiment with realistic change in potato consumption may be more externally generalizable than those from a well-controlled experiment.
The strength of this analysis includes the comprehensive evaluation of the relationship between potato consumption and T2D risk, with a unique study design as well as a large sample size, long duration of follow-up, repeated measurements of exposure during follow-up, and use of data from multiple cohort studies. In addition, the use of a prospective study design helped to minimize recall bias of diet after the occurrence of disease, which is of particular concern in retrospective studies (32). Such a bias may potentially explain why true associations as demonstrated in clinical trials are more likely to be distorted in cross-sectional and case-control studies than in prospective studies, as in the case of Mediterranean diet in relation to risk for T2D (33). This study has several limitations that are worth discussing as well. Measurement errors were inevitable in the estimates of potato consumption using FFQs (21,22). Adjusting for energy intake and the use of a cumulative average of consumption can reduce the impact of measurement errors to some extent (29). In general, random errors in exposure assessments tend to attenuate true associations toward the null. Although we included major possible confounders of lifestyle and dietary factors in the multivariable analysis, residual or unmeasured confounding may still exist. Last, the generalizability of our findings may be limited to health professionals with European ancestry.
In conclusion, greater concurrent and increased potato consumption was associated with a higher risk for T2D. Moreover, increased consumption of french fries was positively associated with subsequent T2D risk. The findings from this study suggest that replacing baked, boiled, or mashed potatoes and french fries with whole grains may result in reduced risk for diabetes. Potatoes are considered to be a healthful vegetable in the National Guideline of Healthy Eating (MyPlate) established by the U.S. Department of Agriculture and the Review of Special Supplemental Nutrition Program for Women, Infants, and Children Food Packages by the Institute of Medicine. However, the current findings cast serious doubts on this classification.
Supplementary Material
Article Information
Funding. This study was funded by research grants from the National Cancer Institute (CA87969, CA176726, CA55075, CA50385, and CA167552) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK58845 and DK082486). Q.S. was supported by a career development award (R00HL098459) from the National Heart, Lung, and Blood Institute.
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors are not affiliated with the funding institutions.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. I.M. conceived of the study concept, designed the study, performed statistical analysis, and prepared the manuscript. E.B.R. and J.E.M. collected data. W.C.W. collected data and obtained funding. F.B.H. designed the study, collected data, and obtained funding. Q.S. conceived of the study, designed the study, and collected data. I.M. and Q.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0547/-/DC1.
A slide set summarizing this article is available online.
References
- 1.The Statistics Division of the FAO. FAOSTAT. Available from http://faostat.fao.org/. Accessed 24 February 2015
- 2.U.S. Department of Agriculture, Agriculture Research Service. USDA National Nutrient Database for Standard Reference, Release 27. 2014. Available from http://www.ars.usda.gov/ba/bhnrc/ndl. Accessed 24 February 2015
- 3.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr 2011;106:1649–1654 [DOI] [PubMed] [Google Scholar]
- 5.Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr 2013;97:584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster J. The great potato debate: nutritionists insist spuds ARE vegetables [article online]. Daily Mail, 2 April 2011. Available from http://www.dailymail.co.uk/health/article-1372728/The-great-potato-debate-Nutritionists-insist-spuds-ARE-vegetables.html. Accessed 16 November 2015
- 7.Rimm EB, Appel LJ, Williams CL, Van Horn L. Don’t subsidize potatoes for the poor: column [article online]. USA Today, 22 May 2014. Available from http://www.usatoday.com/story/opinion/2014/05/22/potato-wic-hunger-nutrition-lobby-agriculture-column/9433799/. Accessed 24 February 2015
- 8.Collins S, Udall M. Excluding potatoes makes no sense: opposing view [article online]. USA Today, 11 May 2014. Available from http://www.usatoday.com/story/opinion/2014/05/11/wic-white-potatoes-nutrition-editorials-debates/8979877/. Accessed 24 February 2015
- 9.U.S. Department of Agriculture. ChooseMyPlate.gov [Internet]. Available from http://www.choosemyplate.gov/. Accessed 10 August 2014
- 10.National Health Service. NHS choices. The eatwell plate. Last reviewed 12 June 2015. Available from http://www.nhs.uk/Livewell/Goodfood/Pages/eatwell-plate.aspx. Accessed 16 November 2015
- 11.Institute of Medicine Review of WIC Food Packages: An Evaluation of White Potatoes in the Cash Value Voucher: Letter Report. Washington, DC, National Academies Press, 2015 [PubMed] [Google Scholar]
- 12.Ylönen SK, Virtanen SM, Groop L; Botnia Research Group . The intake of potatoes and glucose metabolism in subjects at high risk for Type 2 diabetes. Diabet Med 2007;24:1049–1050 [DOI] [PubMed] [Google Scholar]
- 13.Midhet FM, Al-Mohaimeed AA, Sharaf FK. Lifestyle related risk factors of type 2 diabetes mellitus in Saudi Arabia. Saudi Med J 2010;31:768–774 [PubMed] [Google Scholar]
- 14.Khosravi-Boroujeni H, Mohammadifard N, Sarrafzadegan N, et al. Potato consumption and cardiovascular disease risk factors among Iranian population. Int J Food Sci Nutr 2012;63:913–920 [DOI] [PubMed] [Google Scholar]
- 15.Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–448 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Serdula M, Janket SJ, et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004;27:2993–2996 [DOI] [PubMed] [Google Scholar]
- 17.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 2006;83:284–290 [DOI] [PubMed] [Google Scholar]
- 18.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 2004;164:2235–2240 [DOI] [PubMed] [Google Scholar]
- 19.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–1473 [DOI] [PubMed] [Google Scholar]
- 20.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–199 [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 25.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 26.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540 [DOI] [PubMed] [Google Scholar]
- 30.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett WC. Recall of remote diet. In Nutritional Epidemiology. 3rd ed. New York, Oxford University Press, 2013, p. 142–149 [Google Scholar]
- 33.Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014;63:903–911 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.