Abstract
Background
Essential tremor (ET) is a very prevalent neurological disease. Although familial and sporadic ET are assumed to have different age at onset distributions, no detailed study of this question has been carried out.
Methods
Using a carefully-characterized sample of 376 ET cases (232 [61.7%] familial) enrolled in a clinical-epidemiological study, we contrasted the age of onset distributions in familial vs. sporadic ET.
Results
Familial ET had a lower age at onset distribution, regardless of current age. The majority (71 [86.6%] of 82) childhood onset ET cases were familial rather than sporadic. Additionally, onset of ET occurred after age 40 in a majority of cases (125 [53.9%] of 232 with familial ET and 118 [81.9%] of 144 with sporadic ET), and in approximately one-quarter to one-half of cases, after age 60 years.
Conclusions
The age of onset of ET differs between familial and sporadic ET and furthermore, is variable within each of these groups. Childhood onset ET is usually familial, and the small number of identified exceptions could be due to de novo mutations. Understanding the heterogeneity in onset age will provide insights into the nature of underlying etiological and patho-biological processes, about which little is presently known.
Keywords: essential tremor, epidemiology, genetics, familial, sporadic, age of onset
Introduction
Although essential tremor (ET) is highly heritable [1-4], numerous ET cases do not have an identified family history [1, 2]. This observation, as well as others, indicates that environmental factors are also likely to play a role in the etiology of this disease [5-9]. Surprisingly, few if any clinical differences between familial and sporadic ET have been identified. One possible exception is age of onset: the two forms of ET are commonly assumed to differ with respect to age of onset, with earlier onset in the familial than in the sporadic form [10-12]. However, no detailed study has been carried out of the age of onset distributions in the familial vs. sporadic ET. Using a carefully-characterized sample of nearly 400 ET cases enrolled in a clinical epidemiological study, we contrasted the distributions of age of onset in familial vs. sporadic ET, to identify distinct patterns of age of onset. Understanding the heterogeneity in age of onset will provide insights into the nature of the underlying etiological and patho-biological processes, about which so little is presently known.
Methods
Participants
ET cases were enrolled in a study of environmental risk factors for ET at Columbia University Medical Center (CUMC) [13]. Hence, they were not enrolled based on the presence vs. absence of family history of ET. Upon enrollment, a trained tester obtained written informed consent, approved by the CUMC Institutional Review Board, from all participants. ET cases were identified from two primary sources: a computerized billing database of all ET patients who were seen at least once at the Center for Parkinson’s Disease and Other Movement Disorders at CUMC over the past 5 years, and the International Essential Tremor Foundation (IETF) [13]. IETF members who lived in the New York metropolitan area were mailed advertisements and volunteered as participants [13, 14]. All enrollees had received diagnoses of ET from their treating neurologists and lived within a 2-hour driving distance of CUMC [13, 14]. After enrollment, all diagnoses were confirmed using published diagnostic criteria, as outlined below [13, 14].
Clinical Evaluation
Each case underwent an in-person evaluation that included a series of demographic and clinical questionnaires. Age of onset was defined as the self-reported age at which the individual first noted tremor. A prior study indicated that this age is reliably reported by ET cases [15]. Each case was asked whether he or she had one or more relatives with ET or tremor and, if so, to provide additional demographic and clinical information on each affected relative.
Each case also underwent a 20-minute videotaped neurological examination, which included an assessment of postural tremor, five tests of kinetic tremor, and assessments of head (neck), voice and jaw tremors [13]. Each videotaped examination was reviewed by E.D.L., who rated the severity of postural and kinetic arm tremors (range = 0 – 3) using a reliable and valid clinical rating scale, assigning a total tremor score (range 0 - 36) [13]. Diagnoses of ET were re-confirmed by E.D.L. based on the available data using Washington Heights Inwood Genetic Study of Essential Tremor (WHIGET) criteria (moderate or greater amplitude kinetic tremor [tremor rating ≥ 2] during three or more tests or a head tremor, in the absence of Parkinson’s disease, dystonia or another cause) [13]. These diagnostic criteria for ET were developed for a population-based genetic study and, based on data from approximately 2,000 normal (non-diseased controls), the criteria carefully specify the specific examination maneuvers during which tremor should be present and the severity of tremor that should be evident during these maneuvers to distinguish normal from ET. The WHIGET criteria have been shown to be both reliable [16] and valid [17], have been used by tremor investigators in the United States and internationally [18-27].
Definitions
Familial ET (ETF) was defined using both liberal and conservative criteria. Using liberal criteria, ETF was the presence, by the proband’s report, of at least one first- or second-degree relative with “ET” or “tremor”; sporadic ET (ETS) was defined as the absence of at least one such relative. Using conservative criteria, ETF was the presence, by the proband’s report, of at least one first- or second-degree relative with “ET”; sporadic ET (ETS) was defined as the absence of at least one such relative.
Analyses
Analyses were initially performed using the liberal definition for ETF and then repeated using the conservative definition of ET. As in prior studies, childhood onset ET was defined as age 18 or younger [28]. As age of onset was not normally distributed (Kolmogorov-Smirnov test, p-value <0.001), a non-parametric test (Mann-Whitney test) was used when comparing groups by age of onset. Several of our analyses/tables also took current age into consideration, as current age and age of onset are highly correlated.
Results
There were 388 ET cases, of whom 376 (97%) provided information on age of onset and were included in the analysis (Table 1). ETF was identified in 232 (61.7%) cases using the liberal definition and 117 (31.1%) cases using the conservative definition. The current age of ETF was similar to that of ETS: median = 71.0 for ETF using liberal definition vs. 71.0 for ETS (Mann-Whitney test = 1.24, p = 0.22), and median = 71.0 for ETF using conservative definition vs. 71.0 for ETS (Mann-Whitney test = 0.09, p = 0.93).
Table 1.
Demographic and clinical features of 376 ET cases
| Demographic and clinical features | Familial ET (n = 232) |
Sporadic ET (n = 144) |
All Cases (n = 376) |
|---|---|---|---|
| Current age in years | 66.6 ± 15.8 Median = 71 |
68.9 ± 14.0 Median = 71 |
67.5 ± 15.1 Median = 71 |
| Female gender | 124 (53.4) | 72 (50.0) | 196 (52.1) |
| Education in years | 15.4 ± 3.6 | 14.7 ± 4.1 | 15.1 ± 3.8 |
| Total tremor score * | 19.5 ± 7.2 | 17.6 ± 7.2 | 18.8 ± 7.2 |
| Tremor duration in years * | 27.5 ± 19.2 | 15.0 ± 14.9 | 22.8 ± 18.7 |
| Age of tremor onset in years * | 39.1 ± 22.2 | 53.9 ± 19.8 | 44.7 ± 22.5 |
| Currently taking medication for tremor * | 139 (59.9) | 65 (45.1) | 204 (54.3) |
All values are means ± standard deviations or proportions (percentages).
p <0.05 (familial ET vs. sporadic ET).
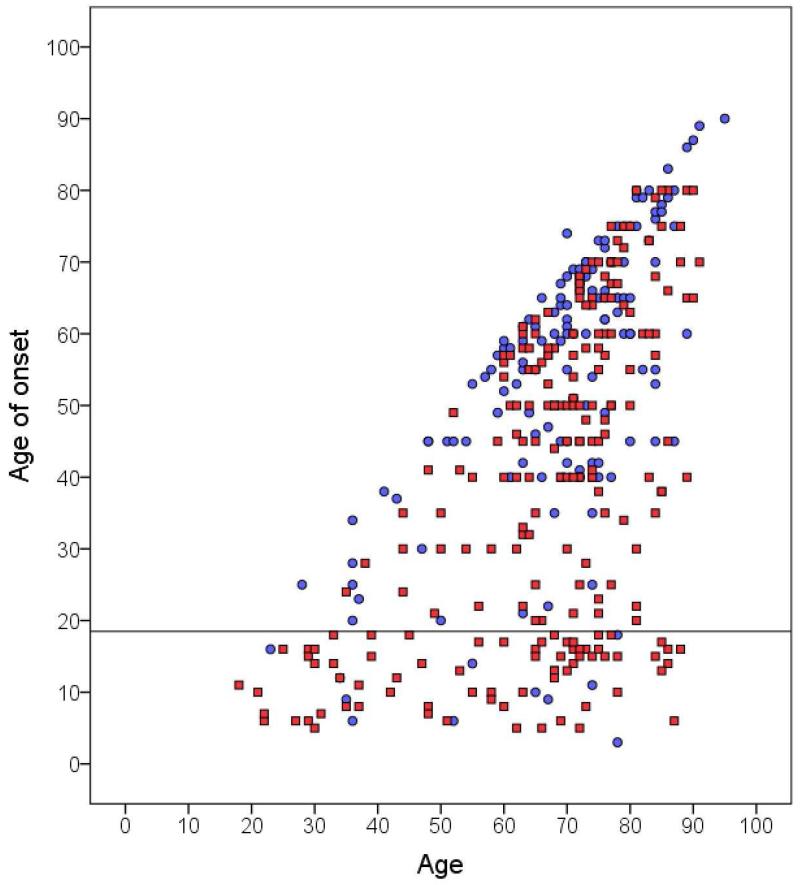
We plotted age by age of onset, comparing ETF (liberal definition) to ETS (Figure 1). Several patterns were evident:
Overall, the age of onset of ETF cases was younger than that of ETS (i.e., note the downward shift in the distribution of red squares relative to blue circles in Figure 1). The mean ± standard deviation age of onset was 39.1 ± 22.2 [median = 40.0 years] for ETF vs. 53.9 ± 19.8 [59.0 years] for ETS (Mann-Whitney z = 6.13, p < 0.001).
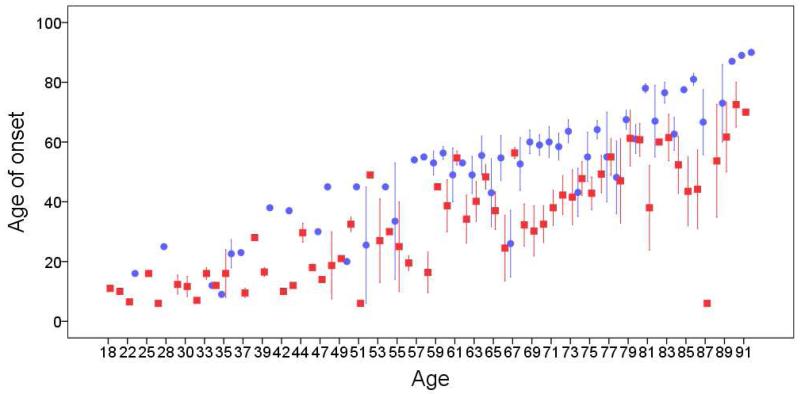
In general, at each current age, the mean age of onset of ETF cases was lower than that of ETS cases (Table 2 and Figure 2).
Among all childhood onset (age of onset ≤18 years, N = 82) cases, 71 (86.6%) had familial as opposed to sporadic ET (Figure 1, Table 3).
Childhood onset ET was not always familial – 11 (13.4%) of 82 cases with onset ≤18 years had ETS (Figure 1, Table 3). Conversely, 11 (7.6%) of 144 ETS cases had an age of onset that was ≤18 years.
Age at onset occurred ≥40 years in majority of both familial (54%, N = 125) and sporadic (82%, N = 118) cases (Figure 1, Table 3).
ETF seemed to have two peaks in age of onset (see Table 3 and two clusters of red squares in Figure 1): a childhood peak (≤18 years) that comprised 71 (30.6%) cases and an older peak (≥ 40 years) that comprised 125 (53.9%) cases (Figure 1). The remaining 36 (15.5%) cases were between ages 19 and 39 years.
Age at onset occurred at age 60 or older in a significant proportion of both familial (22.8%, N = 53) and sporadic (48.6%, N = 70) cases (Figure 1, Table 3).
Figure 1.
Current age in years (X axis) by age of onset in years (Y axis) in ETF (red squares) and ETS (blue circles). Childhood onset cases (age at onset ≤18 years) appear below the horizontal line.
Table 2.
Age of onset by current age stratum in ETF vs. ETS
| Current age stratum (years) |
n | Age of onset (years, ETF) 1 |
n | Age of onset (years, ETS) 1 |
Significance 2 |
|---|---|---|---|---|---|
| <25 | 4 | 8.5 ± 2.4 [8.5] | 1 | 16 (n = 1) | NA |
| 25 – 29 | 5 | 11.8 ± 5.3 [15.0] | 1 | 25 (n = 1) | NA |
| 30 – 34 | 7 | 12.3 ± 4.7 [14.0] | 1 | 12 (n = 1) | NA |
| 35 – 39 | 7 | 16.0 ± 7.8 [15.0] | 7 | 20.7 ± 10.1 [23.0] | p = 0.38 |
| 40 – 44 | 5 | 22.2 ± 11.1 [24.0] | 2 | 37.5 ± 0.7 [37.5] | p = 0.095 |
| 45 – 49 | 6 | 18.2 ± 12.5 [16.0] | 3 | 40.0 ± 8.7 [45.0] | p = 0.048 |
| 50 – 54 | 7 | 29.1 ± 15.1 [30.0] | 5 | 32.2 ± 18.2 [45.0] | p = 0.64 |
| 55 – 59 | 8 | 22.9 ± 14.1 [19.5] | 6 | 47.0 ± 16.4 [53.5] | p = 0.01 |
| 60 – 64 | 28 | 42.1 ± 17.0 [48.0] | 14 | 51.8 ± 11.1 [55.5] | p = 0.04 |
| 65 – 69 | 32 | 36.8 ± 19.6 [42.0] | 18 | 48.4 ± 18.6 [57.0] | p = 0.025 |
| 70 – 74 | 44 | 41.0 ± 19.7 [45.0] | 33 | 56.3 ± 15.0 [61.0] | P < 0.001 |
| 75 – 79 | 39 | 49.7 ± 20.5 [55.0] | 23 | 58.2 ± 18.4 [65.0] | p = 0.081 |
| 80 – 84 | 19 | 53.4 ± 20.7 [60.0] | 18 | 66.8 ± 12.2 [71.5] | p = 0.049 |
| ≥85 | 21 | 50.0 ± 21.2 [65.0] | 12 | 77.4 ± 13.0 [79.5] | p = 0.001 |
All values are means ± standard deviations [medians].
Mann-Whitney test comparing age of onset in ETF vs. ETS.
NA = not applicable
Figure 2.
Current age in years (x axis) by age of onset in years (y axis) of ETF cases (red squares) vs. ETS cases (blue circles). Circles and squares represent mean age of onset at each current age and bars represent 1 standard error.
Table 3.
Number (proportion) of ETF vs. ETS cases in each age of onset stratum
| Age of onset stratum (years) | ETF (n = 232) | ETS (n = 144) |
|---|---|---|
| By 5-year age of onset stratum | ||
| <5 | 0 (0.0) | 1 (0.7) |
| 5 - 9 | 19 (8.2) | 4 (2.8) |
| 10 – 14 | 21 (9.1) | 4 (2.8) |
| 15 – 18 1 | 31 (13.4) | 2 (1.4) |
| 19 – 24 1 | 12 (5.2) | 5 (3.5) |
| 25 – 29 | 5 (2.2) | 4 (2.8) |
| 30 – 34 | 11 (4.7) | 2 (1.4) |
| 35 – 39 | 8 (3.4) | 4 (2.8) |
| 40 – 44 | 17 (7.3) | 11 (7.6) |
| 45 – 49 | 14 (6.0) | 15 (10.4) |
| 50 – 54 | 18 (7.8) | 8 (5.6) |
| 55 – 59 | 23 (9.9) | 14 (9.7) |
| 60 – 64 | 16 (6.9) | 19 (13.2) |
| 65 – 69 | 15 (6.5) | 21 (14.6) |
| 70 – 74 | 11 (4.7) | 10 (6.9) |
| 75 – 79 | 6 (2.6) | 12 (8.3) |
| 80 – 84 | 5 (2.2) | 4 (2.8) |
| ≥85 | 0 (0.0) | 4 (2.8) |
| By larger age of onset stratum | ||
| ≤18 | 71 (30.6) | 11 (7.6) |
| 19 - 39 | 36 (15.5) | 15 (10.4) |
| 40 - 59 | 72 (31.0) | 48 (33.3) |
| ≥ 60 | 53 (22.8) | 70 (48.6) |
Percentages are column percentages.
These two age strata were modified slightly in order to better present data for childhood onset ET (i.e., onset ≤ 18 years).
The findings using the conservative definition of ETF were similar to those presented above, with similar patterns as noted above in 1 – 7 (data not shown).
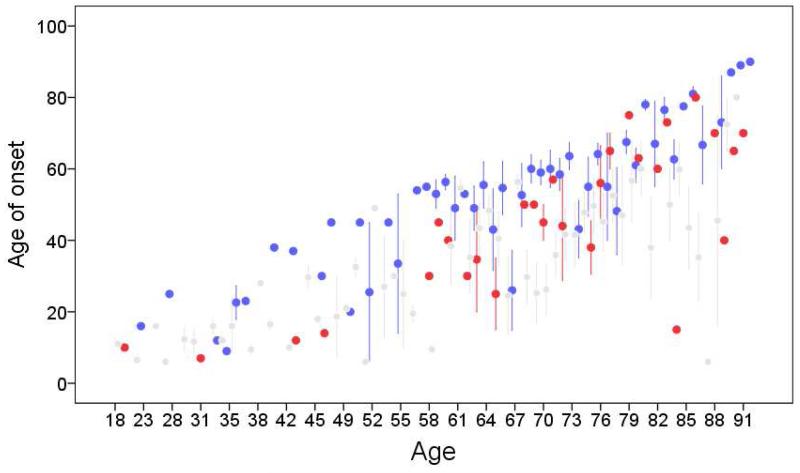
One possible explanation for apparently younger age at onset in familial ET is that ETF cases are more aware of their own tremor than are sporadic cases, because they have relatives with tremor. That is, even if the actual age at onset is the same in both groups, familial ET cases may report a younger age of onset because of greater awareness of their tremor. To explore this possibility, we performed a secondary analysis in which we stratified ETF cases into those who reported having affected relatives in preceding generations (e.g., grandparents, parents, aunts, or uncles) vs. those who reported affected relatives only in the same or younger generations (e.g., siblings, children) (Figure 3). In general, at each current age, the age of onset of ETF cases in the latter group (with affected relatives only in the same or younger generations) remained younger than that of ETS cases (Figure 3). This suggests that the observed difference in age of onset between ETF and ETS is not solely the result of reporting bias.
Figure 3.
Current age in years (x axis) by age of onset in years (y axis). Red circles are ETF cases whose affected relatives are in the same generation or in a younger generation than the proband. Gray circles are ETF cases whose affected relatives are in preceding generations. Blue circles are ETS cases (blue circles). Circles represent means and each bar represents 1 standard error.
Discussion
Familial ET is widely believed to have an earlier age onset than sporadic ET, but no detailed analysis of the distributions of age of onset has been done. In this study, we found a number of patterns.
Age of onset varied widely within both forms of ET; however, in general, the age of onset of ETF cases was statistically significantly younger than that of ETS cases. Curiously, childhood onset was rare in ETS but was common in ETF. Yet this dearth of childhood onset ETS cases did not account entirely for the observed younger age of onset of ETF cases than ETS cases. On closer inspection (Figure 2, Table 2), one can see that at each current age, the age of onset of ETF cases was lower than that of ETS cases. Hence, having a genetic predisposition for ET not only increases disease risk [1], but it also seems to lower the age of disease onset.
In ETF but not ETS, there seemed to be two peaks in age of onset, a young peak and an older peak. In ETS, by contrast, while there were some young-onset cases, there was no young onset peak. This suggests that the genetic predisposition results in an early onset form of disease during childhood. Thus, the genetic predisposition not only increases disease risk, and lowers age of onset at each age across the age spectrum, as discussed above, but it also results in a childhood onset form of the disease. Why some cases of ETF begin in childhood whereas others do not begin until elderly life, is not clear, but could be related to the nature of the underlying susceptibility gene or genes as well as their combination with environmental factors.
A small number, 7.6%, of ETS cases had childhood onset ET. These cases had no apparent family history. One explanation is that they did not correctly recall their age of onset, misattributing it to a younger age. Another explanation is they may have had affected relatives about which they were unaware. Alternatively, they may have had a genetic form of ET, but had no affected relatives due to reduced penetrance or variable expressivity. A final possibility is that their disease was triggered by an early, unidentified environmental exposure or that the basis for the tremor, if genetic, was due to a de novo mutation.
Despite a younger age at onset distribution of ETF, cases continued to arise even after age 60 years; indeed, these cases accounted for nearly 1 in 4 ETF cases. These data suggest that the presence of variable expressivity.
Overall, the data paint a picture of a disease for which, even in its familial form, there is a broad range of disease onset, with cases arising from the first decade of life all the way to the ninth decade. Hence, there seem to be an array of forces ranging from those that push onset at a very early age (i.e., even during early childhood) (e.g. rare highly penetrant mutations) to those whose influence on disease expression seems muted until advanced age (“uncommon” risk factors).
The current study had a number of limitations. First, our study utilized patients from a single cohort and it would be of value to extend these studies to additional cohorts. Second, in some cases, age of onset can be mis-remembered, so it is possible that some of our data on age of onset lack precision. Third, we asked our cases to self-report the presence of a family history but did not examine their immediate or extended families. This, too, could have resulted in some misclassification of ETF as ETS and vice versa.
The study also had several strengths. First, the large sample of nearly 400 ET cases provided sufficient clinical observations to be able to detect a range of clinical patterns. Second, all cases were diagnosed with ET using stringent research criteria. Third, we also considered in our analyses alternative definitions of ETF.
In summary, the age of onset of ET differs between ETF and ETS and furthermore, is variable within each of these groups. Understanding the sources of this heterogeneity will provide some insight into the nature of underlying etiological and patho-biological processes, about which so little is presently known.
Funding/Acknowledgements
Dr. Louis has received research support from the National Institutes of Health (NIH): NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R21 NS077094 (co-Investigator), and NINDS #R01 NS36630 (co-Investigator). This funding body played no role in the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Footnotes
Competing Interests
Dr. Louis has no competing interests.
Dr. Clark has no competing interests.
Dr. Ottman has no competing interests.
Statistical Analyses: The statistical analyses were conducted by Elan D. Louis.
References
- 1.Louis ED, Ford B, Frucht S, Barnes LF, M XT, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49:761–769. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ottman R. How familial is familial tremor? The genetic epidemiology of essential tremor. Neurology. 1996;46:1200–1205. doi: 10.1212/wnl.46.5.1200. [DOI] [PubMed] [Google Scholar]
- 3.Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain. 2007;130:1456–1464. doi: 10.1093/brain/awm018. [DOI] [PubMed] [Google Scholar]
- 4.Tan EK, Schapira AH. Hunting for genes in essential tremor. Eur J Neurol. 2008;15:889–890. doi: 10.1111/j.1468-1331.2008.02226.x. [DOI] [PubMed] [Google Scholar]
- 5.Scarmeas N, Louis ED. Mediterranean diet and essential tremor. A case-control study. Neuroepidemiology. 2007;29:170–177. doi: 10.1159/000111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED. Environmental epidemiology of essential tremor. Neuroepidemiology. 2008;31:139–149. doi: 10.1159/000151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez-Jiménez FJ, de Toledo-Heras M, Alonso-Navarro H, Ayuso-Peralta L, Arévalo-Serrano J, Ballesteros-Barranco A, Puertas I, Jabbour-Wadih T, Barcenilla B. Environmental risk factors for essential tremor. Eur Neurol. 2007;58:106–113. doi: 10.1159/000103646. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Factor-Litvak P, Liu X, Vonsattel JP, Galecki M, Jiang W, Zheng W. Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology. 2013;38:131–135. doi: 10.1016/j.neuro.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, Langston JW, Koller WC. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001;57:1389–1391. doi: 10.1212/wnl.57.8.1389. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. 2007;29:208–12. doi: 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Ottman R. Study of possible factors associated with age of onset in essential tremor. Mov Disord. 2006;21:1980–1986. doi: 10.1002/mds.21102. [DOI] [PubMed] [Google Scholar]
- 12.Brin MF, Koller W. Epidemiology and genetics of essential tremor. Mov Disord. 1998;13(Suppl 3):55–63. doi: 10.1002/mds.870131310. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Gerbin M, Galecki M. Essential tremor 10, 20, 30, 40: clinical snapshots of the disease by decade of duration. Eur J Neurol. 2013;20:949–954. doi: 10.1111/ene.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED. When do essential tremor patients develop head tremor? Influences of age and duration and evidence of a biological clock. Neuroepidemiology. 2013;41:110–115. doi: 10.1159/000351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED, Schonberger RB, Parides M, Ford B, Barnes LF. Test-retest reliability of patient information on age of onset in essential tremor. Mov Disord. 2000;15:738–741. doi: 10.1002/1531-8257(200007)15:4<738::aid-mds1024>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998;13:287–293. doi: 10.1002/mds.870130215. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord. 2001;16:668–673. doi: 10.1002/mds.1144. [DOI] [PubMed] [Google Scholar]
- 18.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 19.Inzelberg R, Mazarib A, Masarwa M, Abuful A, Strugatsky R, Friedland RF. Essential tremor prevalence is low in Arabic villages in Israel: door-to-door neurological examinations. J Neurol. 2006;253:1557–1560. doi: 10.1007/s00415-006-0253-5. [DOI] [PubMed] [Google Scholar]
- 20.Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, Meco G. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 21.Farrer M, Gwinn-Hardy K, Muenter M, DeVrieze FW, Crook R, Perez-Tur J, Lincoln S, Maraganore D, Adler C, Newman S, MacElwee K, McCarthy P, Miller C, Waters C, Hardy J. A chromosome 4p haplotype segregating with Parkinson’s disease and postural tremor. Hum Mol Genet. 1999;8:81–85. doi: 10.1093/hmg/8.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Dogu O, Sevim S, Louis ED, Kaleagasi H, Aral M. Reduced body mass index in patients with essential tremor: a population-based study in the province of Mersin, Turkey. Arch Neurol. 2004;61:386–389. doi: 10.1001/archneur.61.3.386. [DOI] [PubMed] [Google Scholar]
- 23.Gatto EM, Roca MC, Raina G, Micheli F. Low doses of topiramate are effective in essential tremor: a report of three cases. Clin Neuropharmacol. 2003;26:294–296. doi: 10.1097/00002826-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED. Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J Neurol Sci. 2009;287:138–142. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE. Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry. 2005;76:684–690. doi: 10.1136/jnnp.2004.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, Wharen RE. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology. 2000;54:2342–2344. doi: 10.1212/wnl.54.12.2342. [DOI] [PubMed] [Google Scholar]
- 27.Seijo-Martinez M, Del Rio MC, Alvarez JR, Prado RS, Salgado ET, Esquete JP, Sobrido-Gómez MJ. Prevalence of Essential Tremor on Arosa Island, Spain: a Community-based, Door-to-Door Survey. Tremor Other Hyperkinet Mov (N Y) 2013 Sep 3;:3. doi: 10.7916/D89P30BB. pii: tre-03-192-4299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis ED, Fernandez-Alvarez E, Dure L, Frucht S, Ford B. Association between male gender and pediatric essential tremor. Mov Disord. 2005;20:904–906. doi: 10.1002/mds.20483. [DOI] [PubMed] [Google Scholar]