Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative therapy for patients with myelodysplastic syndrome (MDS). Donor T cells are critical for graft-versus-tumor effect (GVT) but carry the risk of graft-versus-host disease (GVHD). CD34 selection with immunomagnetic beads has been an effective method of depleting alloreactive donor T cells from the peripheral blood graft and has been shown to result in significant reduction in acute and chronic GVHD. We analyzed the outcomes of 102 adults (median age 57.6 years) with advanced MDS who received a CD34 selected allo-HSCT between January 1997 and April 2012 at Memorial Sloan Kettering Cancer Center. The cumulative incidence (CI) of grade II-IV acute GVHD at day 100 was 9.8% (95% CI: 5.0-16.5%) and at day 180, 15.7% (95% CI: 9.4-23.4%). The CI of chronic GVHD at 1 year was 3.9% (95% CI: 1.3-9.0%). The CI of relapse at 1 year was 11.8% (95% CI: 6.4-18.9%) and at 2 years 15.7% (95% CI: 9.4-23.4%). Forty-eight patients were alive with a median follow-up of 71.7 months. The overall survival (OS) at 2 years was 56.9% (95% CI: 48-67.3%) and at 5 years, 49.3% (95% CI: 40.4-60.2%). Relapse-free survival (RFS) at 2 years was 52.0% (95% CI: 41.9-61.1%) and at 5 years, 47.6% (95% CI: 37.5-56.9%). The CI of non-relapse mortality was 7.8% (95% CI: 3.7-14.1%) at day 100, 22.5% (95% CI 15.0-31.1%) at 1 year and 33.4% (95% CI:24.2-42.6%) at 5 years post-transplant. Chronic GVHD/relapse-free survival (CRFS) overlapped with RFS. These findings demonstrate that ex-vivo T- cell depleted (TCD) allo-HSCT by CD34 selection offers long term OS and RFS with low incidences of acute and chronic GVHD and without an increased risk of relapse.
Keywords: CD34 selection, Advanced myelodysplastic syndromes, Acute and chronic GVHD, Relapse, Relapse/chronic GvHD-free survival, myelodysplastic syndrome, allogeneic transplantation, T cell depletion
Introduction
Over the past few years, there have been major advances in understanding the biology of MDS, particularly regarding the role of molecular mutations in predicting outcomes1-6. Despite these exciting findings there have been few new treatment options for patients with MDS. Hypomethylating agents, 5-azacytidine and 2'-deoxy-5-azacytidine, are FDA approved drugs for the treatment of MDS with an overall response rate of 30% to 60% 7,8. However, these are not curative treatments whereas allo-HSCT remains the only curative treatment available for patients with MDS 9,10.
Acute and chronic graft-versus-host diseases (GVHD) remain significant post-transplant complications with cumulative incidence ranging from 30 to 60% 11,12. The most effective method to prevent GVHD is depletion of the alloreactive T lymphocytes from the allograft 13. The efficacy of ex-vivo CD34 selection strategies in reducing the risk of acute and chronic GVHD without higher relapse rates has been reported in previous publications 14-17. These findings were confirmed in a prospective multicenter trial sponsored by the Bone Marrow Transplant Clinical Trial Network in patients with acute myeloid leukemia in complete hematologic remission 13.
We previously reported the outcomes of 49 patients who underwent TCD allo-HSCT for advanced MDS (RAEB-1, RAEB-2 and AML evolved from MDS) from matched related donors15 and showed that TCD allo-HSCT results in favorable outcomes with low incidence of GVHD and relapse rates comparable to unmodified allo-HSCT provided that the blast count is reduced prior to transplant. We report herein the outcomes of 102 patients who received a TCD allo-HSCT from matched and mismatched related and unrelated donors.
Patients and methods
Patients
Between January 1997 and April 2012, 102 adults with advanced MDS underwent TCD allo-HSCT at Memorial Sloan Kettering Cancer Center. Eligibility criteria for transplantation included a diagnosis of advanced MDS (RAEB-1, RAEB-2) and AML post MDS (evolved from a well established diagnosis of MDS or clearly documented cytopenia for at least 6 months prior to diagnosis of AML), age <75, absence of active infection, and lack of co-existing cardiac, pulmonary, hepatic, or renal dysfunction that preclude administration of the cytoreductive regimen. HLA typing was performed using high resolution DNA sequence-specific oligonucleotide typing for HLA-A,-B,-C, -DRB1, and -DQB1 loci. Patients typed using intermediate resolution methods were retyped later with high-resolution methods and this is the HLA typing that was used to define the level of mismatch. Written informed consent for treatment was obtained from all patients and donors. Approval for this retrospective review was obtained from the center's Institutional Review and Privacy Board.
Patients were followed according to standard clinical practice; engraftment, acute and chronic GvHD, relapse, causes of death and other relevant transplant outcomes were captured in real time and these data were stored in institutional data base. This study was carried out using these collected data and review of patient's medical records.
Patients and disease characteristic are summarized in table 1. The MDS subtypes and prognostic classification at diagnosis and before transplantation were determined according to the World Health Organization (WHO) 18 and the Revised International Prognostic Scoring (IPSS-R) criteria 19. Patients who responded to pre-cytoreduction treatment were classified into 2 groups: those who achieved hematologic remission and those who had a second refractory cytopenia phase. Complete hematologic remission was defined as cellular marrow with <5% blasts, no overt dysplasia and full recovery of blood counts with neutrophils >1000/uL, platelets of >100,000/uL, red cell transfusion independent and no circulating blasts. Second refractory cytopenia was defined as<5% blasts in the bone marrow and no circulating blasts but persistent cytopenias 20,21.
Table 1.
Patients characteristics
| Variable | No. (%) of patients |
|---|---|
| No. of patients | 102 |
| Male sex- no. | 53 (52.0%) |
| Patient age (years)-median (range) | 57.6(21.9-73.0) |
| 20-49 | 24 (23.5%) |
| 50-65 | 61 (59.8%) |
| >65 | 17 (16.7%) |
| Etiology | |
| De-novo | 82 (80.4%) |
| Therapy related | 15 (14.7%) |
| Post aplastic anemia/MPD | 3/2 (4.9%) |
| Disease status at diagnosis by WHO criteria | |
| RA/RCMD | 27(26.5%) |
| RAEB I | 31 (30.4%) |
| RAEB II | 44 (43.1%) |
| IPSS-R at Diagnosis | |
| Very low | 5 (4.9%) |
| Low | 13 (12.7%) |
| Intermediate | 21 (20.6%) |
| High | 31(30.4%) |
| Very high | 26 (25.5%) |
| Missing data | 6 (5.9%) |
| Disease status at transplant by WHO criteria | |
| CR | 36 (35.3%) |
| RA/RCMD | 46 (45.1%) |
| RAEB I | 16 (15.7%) |
| RAEB II | 4 (3.9%) |
| IPSS-R at time of transplant | |
| Very low | 9 (8.8%) |
| Low | 37 (36.3%) |
| Intermediate | 28 (27.5%) |
| High | 18 (17.6%) |
| Very high | 8(7.8%) |
| Missing data | 2 (2.0%) |
| Time from diagnosis to transplant (months) | |
| <6 months | 25 (24.5%) |
| 6-12 months | 34 (33.3%) |
| 12-24 months | 20 (19.6%) |
| >24 months | 23 (22.5%) |
| HCT-CI | |
| 0 | 18 (17.6%) |
| 1-2 | 26 (25.5%) |
| ≥3 | 57 (55.0%) |
| Missing data | 1 (1.0%) |
Co-morbidities
Evaluation of co-morbidities and assignment of scores were done using consistent definitions for coding the 17 components of the HCT-CI 22.
Pre-transplant therapy
Ninety nine patients were treated prior to admission for transplant in order to induce remission or to decrease MDS tumor burden; 32 patients received low dose chemotherapy or hypomethylating agents, and 67 patients received high dose induction chemotherapy; three patients did not receive any induction therapy prior to starting preparative regimen.
Preparative regimen
All patients were prepared for transplant with a myeloablative regimen, 15 with a hyperfractionated total body irradiation (HFTBI)-based regimen and 87 with a chemotherapy only regimen (table 2) 15,16. The use of chemotherapy-based preparative regimen started in 2000 and it included busulfan, melphalan 140 mg/m2 and fludarabine 125 mg/m2. Busulfan at the dose of 8.0 mg/kg intravenous was given until October 2008 when the dose was increased to 9.6 mg/kg. First dose pharmacokinetics was performed for busulfan to target a steady-state level of 600-900 ng/ml, with the desired level closer to 900 ng/ml. All but 6 patients (5.9%) received ATG to prevent graft rejection. Eighty -seven patients (90.6%) received rabbit ATG (2.5 to 5 mg/kg), and 9 patients (9.4%) received horse ATG (30 mg to 60 mg/kg).
Table 2.
Transplant characteristics
| Variable | No. (%) of patients |
|---|---|
| Conditioning regimen | |
| High dose hyperfractionated TBI (1375Cgy-1500cGy) containing regimens | 15 (14.7%) |
| HFTBI, Thiotepa (10 mg/kg),Cytoxan (120mg/kg) | 9 |
| HFTBI, Thiotepa (10mg/kg),Fludarabine (125mg/m2) | 6 |
| Chemotherapy based regimens | 87 (85.3%) |
| Busulphan (8-9.6mg/kg),Melphalan (140mg/m2), Fludarabine (125mg/m2) | 86 |
| Busulphan (12.8 mg/kg), Fludarabine (150mg/m2) | |
| Donor type | |
| Related | |
| HLA match | 37 (36.3%) |
| HLA mismatch | 2 (2.0%) |
| Unrelated | |
| HLA match | 38 (37.3%) |
| HLA mismatch | 25 (24.5%) |
| CD 34 selection method | |
| Clinimacs | 36 (35%) |
| Isolex | 66 (65%) |
Source of hematopoietic stem cells
All patients received peripheral blood stem cells (PBSC). Seventy-five donors were HLA matched; 37 related and 38 unrelated. Twenty-seven donors were mismatched, 25 unrelated and 2 related. With high resolution typing, the level of mismatch was: 5/10 (n=2), 7/10 (n=1), 8/10 (n=5) and 9/10 (n=19).
CD34 selection
CD34 selection of granulocyte colony-stimulated factor (G-CSF) -mobilized PBSCs was accomplished by positive selection of CD34+ stem cells using initially the ISOLEX 300i Magnetic Cell Separator (Baxter, Deerfield, IL) and subsequent sheep RBC rosette depletion (n=66) 16, and since October 2010 using the CliniMACS CD34 Reagent System23 (Miltenyi Biotech, Gladbach, Germany) (n=36). The TCD-PBSC allograft was infused within 24-48 hours after completion of cytoreduction. No pharmacologic post-transplant GVHD prophylaxis was given.
Donor Leukocyte Infusions (DLI) and Cytotoxic T Lymphocytes (CTL)
Nine patients received DLI and 3 CTL. Four received DLI for relapse and 3 for increasing mixed chimerism with no evidence of hematologic relapse. Two patients received DLI as treatment for an opportunistic infection. CMV CTL were given to 1 patient, EBV CTL to 1 patient and dual specific CMV and EBV cells were given to another patient.
Supportive care
All patients received supportive care and prophylaxis against opportunistic infections according to standard guidelines. Patients conditioned with busulfan received seizure prophylaxis with phenytoin or Keppra (since 2008). Granulocyte colony-stimulating factor was given beginning on day + 7 post-transplant.
Outcome definitions
Engraftment
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥500/ul. Engraftment was confirmed by documentation of chimerism in the bone marrow cells using karyotype or fluorescent in situ hybridization (FISH) of the X and Y chromosomes in sex mismatched donor-recipient pairs and by measurement of DNA restriction fragments length polymorphism or short tandem repeats in sex matched pairs.
Graft failure
Primary graft failure was defined as the absence of neutrophil recovery (≥500/ul) by day 28 and bone marrow biopsy ≤5% cellularity. Secondary graft failure was defined as loss of ANC to <500/ul after primary engraftment, with bone marrow biopsy showing less than 5% cellularity.
Acute and chronic graft-versus-host disease
Acute and chronic GVHD were evaluated according to the IBMTR 11 and the NIH 12 criteria in patients who survived ≥ 21 and ≥100 days with engraftment, respectively. Since these criteria are relatively recent, particularly the NIH criteria, acute and chronic GvHD in patients transplanted prior to these criteria, were graded according to the Keystone24 criteria for acute GvHD and Sullivan25 criteria for chronic GvHD. The staging and characteristics on GvHD were collected in real time and the final grading was established by a GvHD Consensus Committee. Conversion of GvHD grading according to current criteria was made by the main investigators of this manuscript and validated by the GvHD Consensus Committee and was based on review of previously captured GvHD data and chart review.
Relapse
Hematologic relapse was defined as recurrence of cytopenias associated with marrow morphologic changes diagnostic of MDS. Cytogenetic relapse was defined as recurrence of pretransplantation chromosome abnormalities.
Relapse free survival
Was defined as being alive and without evidence of relapse (as above).
Chronic GVHD/relapse-free survival (CRFS)
This is a composite outcome and we have used the BMT CTN definition that is used for the GVHD prophylaxis trial (BMT CTN 1301). The time to event outcomes are defined as moderate to severe chronic GVHD by the NIH consensus criteria, disease relapse or death by any cause.
Causes of death
Causes of death were defined according to the NMDP's algorithm 26.
Data collection
This was a restrospective analysis
Statistical methods
Estimates of overall survival, relapse-free survival, and chronic GVHD/relapse-free survival were calculated using the Kaplan-Meier method 27 with survival distributions compared across patient or treatment characteristics using the log-rank test statistic. Relapse or death were considered events for relapse-free survival, while chronic GVHD, relapse, or death were considered events for chronic GVHD/relapse-free survival. Cumulative incidence estimates of acute and chronic GVHD, relapse and non-relapse related mortality were based on the cumulative incidence method for competing risks. Relapse, death in the absence of relapse, and relapse or death in the absence of GVHD were considered competing risks for non-relapse related mortality (NRM), relapse, and GVHD, respectively. Evaluation of differences in these outcomes based on day 100 CD4 counts was done via a landmark analysis. Gray's test was used to compare the incidence of relapse, NRM and GVHD across groups28. The Cox proportional hazards model was employed to investigate the cause-specific hazard of relapse in adjusted multivariate models. All tests were two-sided and considered significant at the 0.05 level. Statistical analyses were completed using R version 3.1.2 (http://www.r-project.org/).
Results
Disease status before conditioning
Of the 102 patients, 99 were treated prior to start of cytoreduction to induce remission or to reduce the blast count. Disease status at the time of diagnosis and at transplant is summarized in table 1. Disease had progressed prior to transplant in 66 patients. In 27 patients with RA/RCMD, their disease progressed to RAEB-1 in 8, to RAEB-2 in 6, and to AML in 13. In 20 patients with RAEB-1, their disease progressed to RAEB-2 in 5 patients and to AML in 15. In 19 patients with RAEB-2, their disease progressed to AML. The majority of patients (83%) were in CR or a second cytopenic phase before conditioning. The median time from diagnosis to transplant in patients who were diagnosed with RA/RCMD was 34.3 months (range 4.7-161.1 months), in patients with RAEB-1 6.5 months (1.8-43.6), and in patients with RAEB-2 8.2months (range: 2.3- 28.6).
Engraftment
Median cell dose was 7.2×106 CD34/kg (range: 0.6 to 28.8×106/kg). Three patients died before day 28 and were not evaluable for engraftment. The median time to neutrophil engraftment was 11 days (range 8-21 days). Graft rejection occurred in one patient who underwent a second unmodified transplant from the original donor and died on day 107 from first transplant.
Acute and chronic graft-versus-host disease
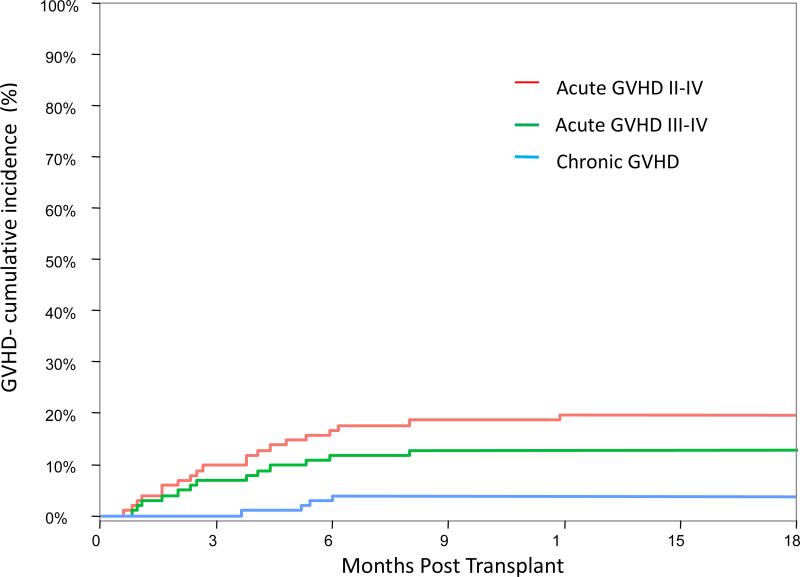
Ninety-nine patients were evaluable for GVHD. The cumulative incidence of grade II-IV aGVHD at day 100 was 9.8% (95% CI: 5.0-16.5%) and at 6 months was 15.7% (95% CI: 9.4-23.4%) (Figure 1). Six patients (6.8%) developed grade I skin only that resolved with topical steroids and 13 patients (13.1%) required systemic immunosuppressive treatment. The only factors that were found to be associated with aGVHD were: recipient male gender (p=0.021) and donor type (p=0.023). The CI of grade II-IV aGVHD at 6 months in recipients of a matched related donor grafts was 5.4% (95% CI: 0.9-16.1%), in recipients of a matched unrelated donor grafts was 21.1% (95% CI: 9.7-35.2%) and in recipients of a mismatched donor grafts was 22.2% (95% CI: 8.8-39.4%). Chronic GVHD developed only in 4 patients with CI at 1 year of 3.9% (95% CI: 1.3-9.0%). Three patients had mild and one patient had moderate chronic GVHD and required systemic immunosuppression .
Figure 1.
Cumulative incidence of acute and chronic graft versus host disease.
Relapse
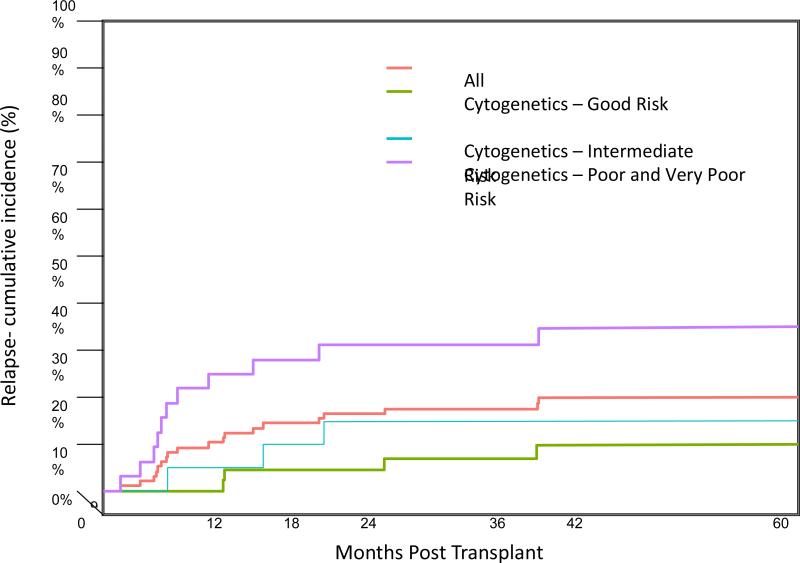
Disease relapsed in 19 patients with a cumulative incidence at 1 year of 11.8% (95% CI: 6.4-18.9%) and at 2 years of 15.7% (95% CI: 9.4-23.4%)(Figure 2). The majority of relapses (63%) occurred within the first year post-transplant, 2 patients relapsed before day 100 with the earliest relapse on day 46 post-transplant. Of 19 relapsed patients only 3 are alive.
Figure 2.
The cumulative incidence of relapse for the whole group of patients is shown in the red line. Relapse was significantly higher for patients with poor and very poor cytogenetic abnormalities according to IPSS-R categories, blue line (p=0.01)
High-risk cytogenetic abnormalities according to IPSS-R at time of diagnosis and pre-transplant therapy with hypomethylating agents were associated with higher relapse incidence (Table 5). In patients with poor and very poor risk cytogenetic abnormalities at diagnosis the CI of relapse at 1 year post-transplant was 25.0% (95% CI: 11.5-41.1%) and at 2 years was 31.2% (95% CI: 16.0-47.8%) (Figure 2). The CI of relapse at 1 year was significantly lower in patients with good (4.3%, 95% CI: 0.8-13.0%) and intermediate (5.3%, 95% CI: 0.3-22.0%) risk cytogenetic abnormalities (p=0.011). To determine the effect of cytogenetic remission on relapse, 28 patients in the poor and very poor risk cytogenetic categories were divided into two groups according to the level of cytogenetic remission before conditioning, 16 achieved hematologic and cytogenetic remission and 12 were in hematologic remission but had persistent cytogenetic abnormalities. The cumulative incidence of relapse at 1 year was 25.0% in both groups. The 1-year CI of relapse among patients treated with hypomethylating agents/low dose chemotherapy was 18.8% (95% CI: 7.5-34.0%) compared to 9.0% (95% CI: 3.6-17.3%) in patients treated with induction chemotherapy (p=0.031). In a multivariate model adjusted for both cytogenetic risk at diagnosis and pre-transplant therapy, cytogenetic risk at diagnosis remained a significant predictor of relapse (p=0.003). Intermediate (HR=1.45, 95% CI 0.32-6.49) or poor/very poor (HR=6.09, 95% CI 1.91-19.43) cytogenetic risk at diagnosis was associated with greater risk of relapse, as compared to good cytogenetic risk at diagnosis.
Table 5.
Correlation of relapse and NRM with patient, disease, and transplant characteristics
| Relapse % (95% CI) | NRM % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| 1 year | 2 year | p-value | day 100 | 1 year | 5 years | p-value | |
| Overall | 11.8 (6.4-18.9) | 15.7 (9.4-23.4) | - | 7.8 (3.7-14.1) | 22.5 (15.0-31.1) | 33.4 (24.2- | - |
| Age | 0.389 | 0.230 | |||||
| ≤ 50 | 11.5 (2.8-27.1) | 23.1 (9.1-40.8) | 0.00 (NA) | 15.4 (4.7-31.9) | 26.9(11.6- | ||
| 50-65 | 12.7 (5.9-22.2) | 14.3 (7.0-24.1) | 7.9 (2.9-16.3) | 20.6 (11.6-31.4) | 31.8 (20.7- | ||
| > 65 | 7.7 (0.4-30.6) | 7.7 (0.4-30.6) | 23.1 (5.1-48.5) | 46.2 (17.7-70.8) | 53.8 (22.8- | ||
| Etiology | 0.843 | 0.222 | |||||
| 10.3 (5.1-17.8) | 14.9 (8.4-23.3) | 6.9 (2.8-13.5) | 21.8 (13.8-31.1) | 32.3 (22.7- | |||
| Therapy-related | 20.0 (4.4-43.5) | 20.0 (4.4-43.5) | 13.3 (2.0-35.4) | 26.7 (7.7-50.6) | 40.0 (15.4-63.9) | ||
| Cytogenetic risk at diagnosis | 0.011 | 0.338 | |||||
| Very good/good | 4.3 (0.8-13.0) | 4.3 (0.8-13.0) | 12.8 (5.1-24.0) | 23.4 (12.5-36.3) | 34.0 (20.9- | ||
| Intermediate | 5.3 (0.3-22.0) | 15.8 (3.7-35.6) | 0.00 (NA) | 5.3 (0.3-22.1) | 21.1 (6.2- | ||
| Poor/very poor | 25.0 (11.5-41.1) | 31.2 (16.0-47.8) | 6.3 (11-18.4) | 28.1 (13.8-44.4) | 37.5 (20.8- | ||
| Pre-transplant therapy | 0.031 | 0.071 | |||||
| Chemotherapy | 9.0 (3.6-17.3) | 10.5 (4.5-19.2) | 9.0 (3.6-17.3) | 28.4 (18.1-39.5) | 38.9 (27.2- | ||
| Hypomethylating agents | 18.8 (7.5-34.0) | 28.1 (13.8-44.4) | 6.3 (11-18.4) | 9.4 (2.3-22.5) | 21.9 (9.4-37.6) | ||
| Conditioning regimen | 0.352 | 0.841 | |||||
| Chemotherapy | 10.3 (5.1-17.8) | 13.8 (7.5-22.0) | 9.2 (4.3-16.4) | 21.8 (13.8-31.1) | 33.3 (23.7- | ||
| Total Body Irradiation | 20.0 (4.5-43.5) | 26.7 (7.6-50.8) | 0.00 (NA) | 26.7 (7.7-50.6) | 33.3(11.3-57.5) | ||
| Match Type | 0.607 | 0.807 | |||||
| Match-related | 10.8 (03.4-23.3) | 16.2 (6.4-29.9) | 5.4 (0.9-16.1) | 21.6 (10.0-36.1) | 32.6 (18.0- | ||
| Match-unrelated | 13.2 (4.7-26.0) | 13.2 (4.7-26.0) | 13.2 (4.7-26.0) | 23.7 (11.6-38.2) | 31.6 (17.5- | ||
| Mismatch | 11.1 (2.7-26.3) | 18.5 (6.5-35.3) | 3.7 (0.03-16.2) | 22.2 (8.8-39.4) | 37.0 (19.2- | ||
| HCT-CI | 0.845 | 0.382 | |||||
| 0 | 5.6 (0.3-23.1) | 11.1 (1.7-30.5) | 0.00 (NA) | 16.7 (3.9-37.2) | 22.2(6.6- | ||
| 1-2 | 11.5 (2.8-27.2) | 15.4 (4.6-31.9) | 7.7 (13-22.1) | 23.1 (9.1-40.7) | 30.8 (14.3- | ||
| ≥ 3 | 14.0 (6.5-24.4) | 17.5 (8.9-28.5) | 10.5 (4.2-20.1) | 22.8 (12.9-344) | 36.8 (24.4- | ||
| CD 34 selection | 0.797 | 0.664 | |||||
| Clinimacs | 8.3(2.1-20.3) | 13.9 (5.0-27.3) | 8.3(2.1-20.3) | 22.2(10.3-37.0) | 30.6(16.4- | ||
| Isolex | 13.6 (6.7-23.1) | 16.7 (8.8-26.7) | 7.6(2.8-15.6) | 22.7(13.5-33.5) | 34.8 (23.5- | ||
Survival
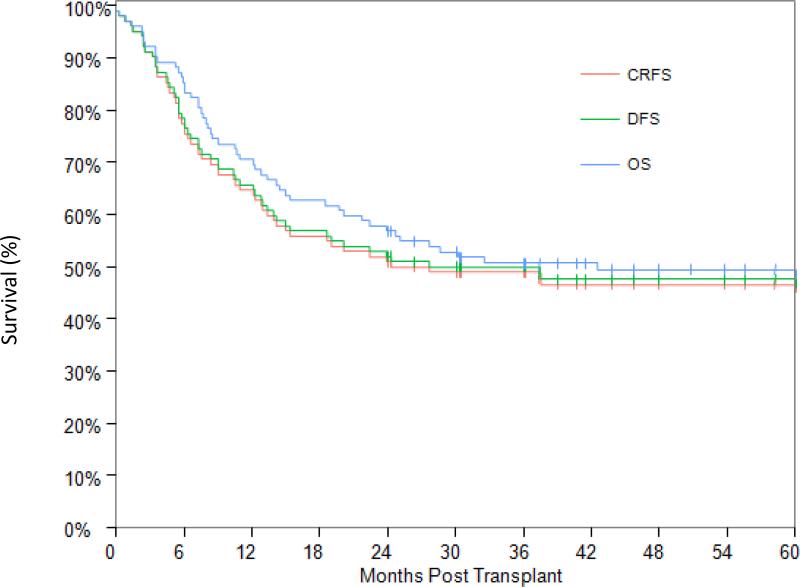
At time of analysis, 48 patients were alive with a median of 6.0 years post-transplant (range: 2.0 months to 12.6 years). The OS and RFS at 2 years post-transplant were 56.9% (95% CI: 48.0-67.3%) and 52.0% (95% CI: 41.9-61.1%), respectively, and at 5 years 49.3% (95% CI: 40.4-60.2%) and 47.6% (95% CI: 37.5-56.9%), respectively (Figure 3). The only factor that was found to be associated with worse OS and RFS was high-risk cytogenetic abnormalities at time of diagnosis (Table 4). Older age (>65) was also associated with worse OS; 38.5% (95% CI: 14.1-62.8%) at 2 years and 15.4% (95% CI: 1.2-45.3%) at 5 years (p=0.074), though age didn't affect RFS (p=0.127). There was a trend for a lower OS and RFS in patients with a high co-morbidity score, but these differences did not reach statistical significance (p=0.140 and 0.253, respectively). The CRFS in this cohort of patients overlapped with that of RFS and it was 51.0% (95% CI: 40.9-60.2) at 2 years and 46.6% (95% CI: 36.6-56.0) at 5 years post-transplant, reflecting the very low incidence of chronic GVHD.
Figure 3.
Overall survival, relapse free survival and chronic GVHD/relapse-free survival.
Table 4.
Correlation of OS and RFS with patient, disease, and transplant characteristics
| OS % (95% CI) | RFS % (95% CI) | |||||
|---|---|---|---|---|---|---|
| 2 year | 5 year | p-value | 2 year | 5 year | p-value | |
| Overall | 56.9 (48.0-67.3) | 49.3 (40.4 -60.2) | - | 52.0 (41.9-61.1) | 47.6 (37.5-56.9) | |
| Age | 0.07 | 0.127 | ||||
| <= 50 | 57.7 (36.8- 73.9) | 49.1 (28.8- 66.6) | 50.0 (29.9-67.2) | 45.0 (25.2-63.0) | ||
| 50-65 | 60.3 (47.2-71.2) | 55.4 (42.3-66.7) | 55.6 (42.5-66.8) | 53.9 (40.9-65.3) | ||
| > 65 | 38.5 (14.1-62.8) | 15.4 (1.2-45.3) | 38.5 (14.1-62.8) | 20.5 (3.9-46.3) | ||
| Etiology | 0.109 | 0.140 | ||||
| Primary/secondary | 59.8 (48.7-69.2) | 50.8 (39.7-60.9) | 54.0 (43.0-63.8) | 48.8 (37.8-58.9) | ||
| Therapy related | 40.0 (16.5-62.8) | 40.0 (16.5-62.8) | 40.0 (16.5-62.8) | 40.0 (16.5-62.8) | ||
| Cytogenetic risk at diagnosis | 0.002 | <0.001 | ||||
| Very good/good | 63.8 (51.5-79.2) | 55.9 (43.0-72.8) | 61.7 (49.3-77.3) | 56.6 (43.8-73.1) | ||
| Intermediate | 73.7 (56.3- 96.4) | 63.2 (44.8-89.0) | 68.4 (50.4-92.9) | 63.2 (44.8-89.0) | ||
| Poor/very poor | 40.6 (26.7-61.8) | 34.4 (21.3-55.5) | 31.2 (18.7-52.2) | 27.8 (15.8-48.8) | ||
| Cytogenetic risk at transplant | 0.220 | 0.162 | ||||
| Very good/good | 59.7 (49.7-71.8) | 52.6 (42.4-65.2) | 55.8 (45.8-68.1) | 50.1 (40.0-62.8) | ||
| Intermediate | 41.7 (21.3-81.4) | 41.7 (21.3-81.4) | 41.7 (21.3-81.4) | 41.7 (21.3-81.4) | ||
| Poor/very poor | 53.8 (32.6-89.1) | 38.5 (19.3-76.5) | 38.5 (19.3-76.5) | 38.5 (19.3-76.5) | ||
| Pre-Transplant Therapy | 0.619 | 0.856 | ||||
| Chemotherapy | 53.7 (43.0-67.1) | 48.7 (38.0-62.5) | 52.2 (41.5-65.7) | 47.4 (36.8-61.1) | ||
| Hypomethylating agents | 62.5 (47.8-81.7) | 49.2 (34.3-70.4) | 50.5 (35.4-70.7) | 45.0 (30.1-67.4) | ||
| Conditioning regimen | 0.847 | 0.516 | ||||
| Chemotherapy | 58.6 (49.1-69.9) | 49.8 (40.2-61.7) | 54.0 (44.5-65.6) | 48.9 (39.3-60.7) | ||
| Total Body irradiation | 46.7 (27.2-80.2) | 46.7 (27.2-80.2) | 40.0 (21.5-74.3) | 40.0 (21.5-74.3) | ||
| Match type | 0.602 | 0.506 | ||||
| Match-related | 59.5 (45.6-77.6) | 53.9 (40.0-72.7) | 54.1 (40.2-72.8) | 51.2 (37.4-70.2) | ||
| Match-unrelated | 57.9 (44.1-75.9) | 52.1 (38.3-70.9) | 55.3 (41.5-73.6) | 52.4 (386-71.0) | ||
| Mismatch | 51.9 (36.1-74.6) | 39.5 (24.4-63.9) | 44.4 (29.2-67.8) | 35.9 (21.4-60.2) | ||
| HCT-CI | 0.140 | 0.253 | ||||
| 0 | 66.7 (48.1-92.4) | 66.7 (48.1-92.4) | 66.7 (48.1-92.4) | 60.0 (40.8-88.3) | ||
| 1-2 | 61.5 (45.4-83.4) | 57.4 (41.2-80.1) | 53.8 (37.7-76.9) | 53.8 (37.7-76.9) | ||
| > 3 | 52.6 (41.1-67.3) | 41.7 (30.6-56.9) | 47.4 (36.0-62.3) | 42.0 (31.0-57.0) | ||
| CD 34 selection method | ||||||
| Clinimacs | 58.3(44.3-76.9) | 55.3(41.1-74.3) | 0.538 | 55.6(41.5-74.4) | 50.5 (35.7-71.4) | 0.520 |
| Isolex | 56.1 (45.3-69.4) | 47.0 (36.3-60.7) | 50.0 (39.3-63.6) | 45.5 (34.9-59.2) | ||
Non-relapse mortality and causes of death
Fifty-four patients (52.9%) died by the time of this analysis; 17 (31.5%) due to relapse and 37(68.5%) due to non-relapse causes (Table 3). The main causes of non-relapse deaths in this series were infections (33.3%), followed by GVHD (9.2%) and organ toxicity (7.4%). Among the 5 patients in whom the cause of death was attributed to GVHD, 3 had active infection at time of death. Among the infectious causes, the most common were bacterial and viral infections.
Table 3.
Causes of death
| Cause of death | No. of patients (%) |
|---|---|
| Relapse | 17 (31.5%) |
| Infections | 18 (33.3%) |
| Bacterial | 8 |
| Viral | 8 |
| Fungal | 1 |
| Other (PCP) | 1 |
| GVHD | 5 (9.3%) |
| Other | 7 (13.0%) |
| Recurrence of primary malignancy | 2 (MCL, neuroblastoma) |
| Secondary malignancy | 1 (Lung Ca) |
| Portal HTN and GI bleed | 1 |
| Acute hemolytic anemia | 1 |
| Post surgical death | 1 |
| Pericarditis | 1 |
| Toxicity | 4 (7.4%) |
| VOD | 1 |
| Lung | 3 |
| Graft failure | 2 (3.7%) |
| Graft rejection | 1 |
| Late graft failure | 1 |
| Organ failure | 1 (1.9%) |
| Respiratory failure | 1 |
| Total | 54 |
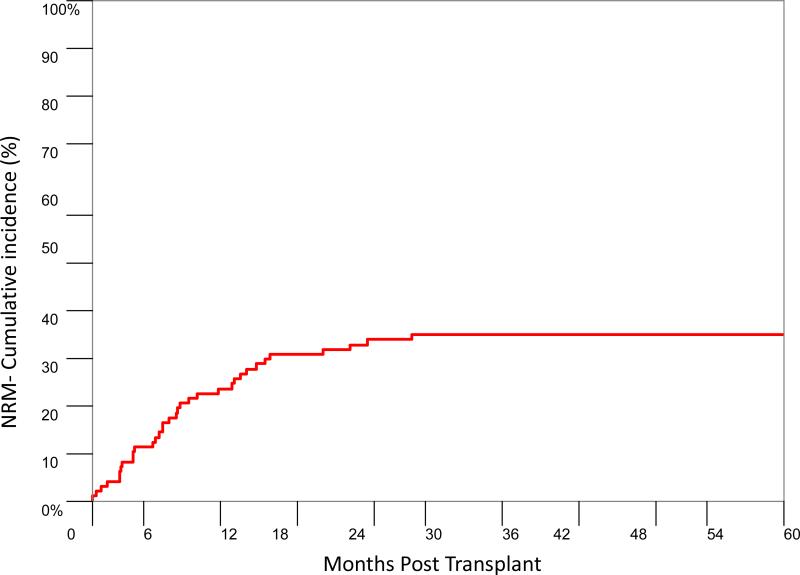
The CI of NRM at day 100 post transplant was 7.8% (95% CI: 3.7-14.1%) and at 1 year was 22.5% (95% CI: 15.0-31.1%) (Figure 4). The NRM at 1 year for patients whose CD4 count was less than 100/μL at day 100 post-transplant was higher compared to patients whose CD4 count was higher (20.6% vs 11.4% p=0.070). Patients who received induction chemotherapy prior to transplant had higher NRM (9.0% by day 100 and 28.4% by 1 year) in comparison to patients who received hypomethylating agents or low dose chemotherapy pre-transplant (6.3% by day 100 and 9.4% by 1 year, p=0.071).
Figure 4.
Cumulative incidence of non-relapse mortality.
Discussion
We demonstrate in this large cohort of patients with advanced MDS that ex vivo TCD-HSCT by positive CD34 selection produce an OS and a RFS that are comparable to that reported after unmodified transplants 29-32 with a much lower incidence of acute GVHD and negligible incidence of cGVHD and without a high relapse rate.
The method of TCD used in this series of advanced MDS patients has been extensively used by us and others and the efficacy of this method in preventing acute and chronic GVHD has been well documented in AML 14,33, ALL 34, CML 14 and NHL 16, as well as a small series of patients with advanced MDS 15. More recently, a multicenter prospective phase 2 trial in patients with AML in remission sponsored by the BMT CTN confirmed our findings 13. Moreover, comparison of our TCD experience with unmodified transplants in MDS following either myeloablative conditioning (MAC) or reduced intensity conditioning (RIC) shows that CD34 selected allo-HSCT significantly lowers acute and chronic GVHD. The early experience with unmodified transplants for MDS has been mostly with MAC and this was associated with a high incidence of acute GVHD and a high incidence of NRM related to the toxicities of the preparative regimens31. The use of RIC in MDS patients has decreased the toxicities and the NRM as well as the incidence of acute GVHD without changing the incidence of chronic GVHD; however RIC is associated with a higher incidence of relapse. The overall effect of these different complications has resulted in similar OS and RFS in MAC and RIC transplants for MDS patients with a significant proportion of them suffering from GVHD 31. Although the composite outcome CRFS - a parameter that measures freedom from ongoing morbidity and represents ideal HCT recovery - has not been reported in previous studies of allo-HSCT in MDS, a recent study 35 including MDS patients and other hematological malignancies reported a 20% difference in RFS and CRFS at 1 year post-transplant. This difference was due to moderate to severe acute and chronic GVHD. We show in this series that the CRFS is almost identical to RFS given that the incidence of moderate to severe chronic GvHD is quite low (<1%).
The advantages and disadvantages of TCD transplant in comparison to unmodified transplants have not been properly assessed in a randomized trial including exclusively high-risk MDS patients. The NMDP trial comparing unmodified to TCD transplants in a variety of hematological diseases showed no differences in OS and RFS, although the incidence of GVHD was significantly lower in recipients of TCD transplants36. The main limitation of this trial was that T cell depletion was performed by more than one method and resulted in different levels of T cells depletion. A recent CIBMTR study compared retrospectively the outcomes of 44 patients who participated in the CTN sponsored phase 2 trial of TCD by CD34 selection with the Miltenyi device 13 with those of 84 recipients of unmodified transplants requiring post-transplant immunosuppression transplanted during the same time period 37. There were no differences in rates of graft rejection, leukemia relapse, treatment-related mortality, RFS and OS. However at 1 year, 54% and 12% of patients were still on immunosuppression in the immune suppression therapy (IST) and TCD cohorts, respectively. TCD was associated with a higher GVHD-free survival at 2 years compared with IST (41% v 19%, respectively; P=0 .006). A large randomized trial is now undergoing to determine the optimal GVHD prevention strategy in the context of MAC. This trial performed under the auspices of the National Heart, Lung and Blood Institute (NHLBI) as well as the National Cancer Institute (NCI) will compare CD34 selection to post transplant cyclophosphamide to a CNI/MTX and its primary endpoint is CRFS.
Our method of ex-vivo TCD also produces equivalent or better outcomes when compared to invivo TCD with alemtuzumab. Potter et al 38 recently published their experience with an alemtuzumab-based RIC regimen in patients with MDS and AML evolved from MDS. The OS, RFS, NRM, relapse and chronic GVHD at 5 years post-transplant were 44%, 33%, 26%, 51% and 19%, respectively. The incidence of chronic GVHD was higher in the alemtuzumab treated patients, 19% vs 4% in our series, likely due to the depth of TCD. The ex vivo method used at our institution produce a 3-4 log depletion compared to 2 log depletion caused by alemtuzumab. Also, despite the use of RIC, the NRM at 1-year post transplant in the alemtuzumab series was 20% similar to 24% in our cohort of patients who received MAC regimen. One significant difference comparing these two series is the higher relapse rate in the alemtuzumab group, 40% at 2 years post transplant despite the fact that majority of patients were transplanted without excess blasts, versus 19.8% in our cohort. The lower relapse rate in our cohort of patients is likely attributed to the higher intensity preparative regimen.
Another emerging method of in vivo TCD is with post-transplant high dose cyclophosphamide. The outcomes of this approach in patients with hematologic malignancies were assessed in a multi-institutional prospective study reported by Kanakry et al 39, where all patients received MAC followed by high dose post transplant cyclophosphamide as the sole GVHD prophylaxis. Donors were matched related or unrelated and graft source was bone marrow. While grade II-IV acute GVHD was as high as 51% by day 100 post-transplant, grade III-IV was only 15% and chronic GVHD was as low as 14%. NRM was 9% at day 100 post-transplant and 16% at 1-year post transplant, with relapse rate of 22% at 2 years post -transplant. One of the potential advantages of this approach is that cyclophosphamide does not deplete memory T cells and patients are not severely immunodeficient and may have a lower rate of infectious complications. The CTN trial described before will hopefully determine whether this method can produce the same outcomes as CD34 selection and pharmacologic GVHD prophylaxis.
An intensive conditioning is required prior to TCD transplant to provide a more intense anti-leukemic effect as the graft-versus-MDS effect 32,40,41 might be reduced and also to induce a more profound immunosuppression and thereby to decrease the risk of graft rejection. High dose TBI based conditioning regimen was used more frequently in the early days, however the majority of patients (86%) received a chemotherapy only preparative regimen. We didn't observe difference in outcomes comparing full dose TBI based regimen and chemotherapy only based regimen, however the TBI group included only 2 patients older then 60 and no patients older than 65 compared to 35 patients older than 60 and 17 older than 65 in the chemotherapy based conditioning regimen. The chemotherapy only myeloablative condoning regimen combining busulfan, melphalan, fludarabine and rabbit ATG was well tolerated with an overall NRM at day 100 of 7.8% (CI: 3.7-14.1%). Higher NRM rates, 23.1% (95% CI: 5.1-48.5%) were seen in patients older than 65 who also had a higher HCT-CI (85% with HCT CI of >3). Although there was no statistically significant differences in NRM between the different age groups (p=0.230), intuitively older patients are expected to tolerate less well the myeloablative preparative regimen necessary for T cell depleted transplant. With the growth of the elderly population, patients in their 7th and 8th decades of live are now candidates for allo-HSCT and that underscores the importance of developing less toxic preparative regimens to benefit particularly older patients.
The relapse rate in this series disproves the belief that recipients of TCD transplants have higher relapse rates as compared to unmodified transplants. The Fred Hutchinson Cancer Center reported a CI of relapse at 2 years ranging between 16% for patients with low risk cytogenetic risk group by IPSS to 35% for patients with high-risk disease29,42 which is similar to our outcomes. Similarly, a CIBMTR study showed relapse rates in the order of 25-28% and higher in patients with poor risk cytogenetic abnormalities or patient who underwent NMA conditioning regimen 43. Relapse strongly correlated in our series with cytogenetic risk at diagnosis, similarly to what has been published for patients undergoing unmodified transplant in a large retrospective study reported by EBMT 44. Patients with poor risk cytogenetic abnormalities had a relapse rate of 31% at 1 year compared to only 17% in patients with intermediate risk and 8% in patients with low risk. The poor outcomes of patients who relapse after allo- HSCT 45-47 irrespective of the type of transplant emphasizes the need for better strategies such as molecular mutations to identify patients who are at higher risk for relapse for whom post-transplant prophylaxis against relapse should be offered. One of these approaches is adoptive immune therapy with un-manipulated or leukemia antigen specific cytotoxic lymphocytes. A great advantage TCD transplants is that the probability of GVHD is low and therefore adoptive immune therapy is more feasible.
Administration of chemotherapy to patients with advanced MDS prior to conditioning has been a matter of debate over the years 10,48-50. Based on our previous published experience 15, the majority of patients underwent treatment prior to initiation of cytoreduction therapy and had less than 10% blasts at time of admission for transplant. The results of this study confirm our earlier observations that achieving hematologic remission or a minimal residual disease state prior to TCD transplant significantly reduces the risk of post-transplant relapse. In contrast to the importance of reduction of blasts and achieving morphologic remission prior to transplant, we could not demonstrate in this series that achieving cytogenetic remission prior to transplant reduce relapse risk. Induction chemotherapy and hypomethylating agents are the two main options to reduce disease burden prior to transplant and there are pros and cons to each option 51. Damaj et al 52 studied the outcome of 163 patients with MDS who were treated pre-transplant by either azacitidine, induction chemotherapy or azacitidine preceded or followed by induction chemotherapy and reported no difference in OS, RFS, relapse and NRM when comparing azacitidine to induction chemotherapy. However, patients who received both azacitidine and induction chemotherapy had worse outcomes, particularly high risk of NRM, and lower OS and RFS. In our cohort there was no difference in OS and RFS among patients who were treated with either induction chemotherapy or azacitidine, however there was higher relapse incidence in the azacitidine group and a trend toward lower NRM. Prospective studies are needed to define the best treatment to achieve remission before transplant.
NRM was the main cause of failure in this series of patients and this abrogated the benefits of reduced GVHD. The main cause of NRM in our patients was infection, accounting for 33% of causes of death. The basis for this has been established; the TCD methods used in these patients causes profound depletion of immunocompetent T cells and as a result patients are severely immunodeficient 53. Moreover, the process of immune reconstitution is extremely slow 54 particularly in older patients who have poor thymic function 55. This was confirmed in this cohort, the median CD4 count at 3 and 6 months post-transplant were 116 cells/μL (range 0-1233cell/μL) and 136 cells/μL (range 0-722 cells/μL) respectively.
In summary, the outcomes of patients with advanced MDS transplanted with TCD allografts are favorable and are similar to those reported for patients who underwent unmodified transplant with the advantage of much lower incidence of acute and chronic GVHD. Delayed immune recovery and infections remains the main obstacle to the success of TCD allo-HSCT. However, freedom from GVHD and no need for the use of immunosuppressive medications can be used as a platform for early post transplant intervention in the form of specific cytotoxic CTLS to treat mostly viral infections and to enhance GVL without paying the price of GVHD.
Highlights.
CD34 selected stem cell transplantation offers long term survival for patient with advanced MDS.
CD34 selected stem cell transplantation has low incidence of acute and chronic GVHD.
Relapse rates after CD34 selected stem cell transplantation are not higher compared to unmodified transplants.
Infections are the main cause of mortality after CD34 selected stem cell transplantation.
Acknowledgements
This study was supported in part by NIH P01 CA23766 grant, NIH (U10-HL069315), the MDS Research Fund, the Bone Marrow Transplant Research Fund, The Satlin BMT Research Fund, The Byrne Fund and the Bergstein Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: RT, SC, and HCM contributed to the acquisition and analyzed the data, interpret the data and wrote the manuscript. PH and SMD analyzed, interpret the data and contributed to the writing of the manuscript. MM and DYH contributed to the acquisition of the data. EBP, AAJ, JDG, MAP, DMP, CS, VK, JWY, ROR, and SG wrote the manuscript
Conflict-of-interest disclosure: The other authors have no competing financial interest to declare.
References
- 1.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–27. doi: 10.1182/blood-2013-08-518886. quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejar R, Stevenson KE, Caughey BA, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30:3376–82. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy DM, Bejar R, Stevenson K, et al. NRAS mutations with low allele burden have independent prognostic significance for patients with lower risk myelodysplastic syndromes. Leukemia. 2013;27:2077–81. doi: 10.1038/leu.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter MJ, Shen D, Ding L, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366:1090–8. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter MJ, Shen D, Shao J, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013;27:1275–82. doi: 10.1038/leu.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gore SD. New ways to use DNA methyltransferase inhibitors for the treatment of myelodysplastic syndrome. Hematology Am Soc Hematol Educ Program. 2011;2011:550–5. doi: 10.1182/asheducation-2011.1.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation for myelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia. 1998;12(Suppl 1):S25–9. [PubMed] [Google Scholar]
- 10.Warlick ED, Cioc A, Defor T, et al. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–8. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–51. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbano-Ispizua A, Brunet S, Solano C, et al. Allogeneic transplantation of CD34+-selected cells from peripheral blood in patients with myeloid malignancies in early phase: a case control comparison with unmodified peripheral blood transplantation. Bone Marrow Transplant. 2001;28:349–54. doi: 10.1038/sj.bmt.1703154. [DOI] [PubMed] [Google Scholar]
- 15.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–9. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 22.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–63. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(R) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18:690–7. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 25.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9. [PubMed] [Google Scholar]
- 26.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 28.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 29.Chang C, Storer BE, Scott BL, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–87. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–11. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 31.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 32.Hiramoto N, Kurosawa S, Tajima K, et al. Positive impact of chronic graft-versus-host disease on the outcome of patients with de novo myelodysplastic syndrome after allogeneic hematopoietic cell transplantation: a single-center analysis of 115 patients. Eur J Haematol. 2014;92:137–46. doi: 10.1111/ejh.12214. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–90. [PubMed] [Google Scholar]
- 34.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:208–13. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner JE, Thompson JS, Carter SL, et al. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–41. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 37.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter VT, Krishnamurthy P, Barber LD, et al. Long-term outcomes of alemtuzumab-based reduced-intensity conditioned hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myelogenous leukemia secondary to myelodysplastic syndrome. Biol Blood Marrow Transplant. 2014;20:111–7. doi: 10.1016/j.bbmt.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campregher PV, Gooley T, Scott BL, et al. Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:965–71. doi: 10.1038/sj.bmt.1705840. [DOI] [PubMed] [Google Scholar]
- 41.Weisdorf D, Zhang MJ, Arora M, et al. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18:1727–33. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120:1398–408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenecke C, Gohring G, de Wreede LC, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic syndromes: a retrospective multicenter study of the European Society of Blood and Marrow Transplantation. Haematologica. 2015;100:400–8. doi: 10.3324/haematol.2014.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:1027–32. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 46.McIver ZA, Yin F, Hughes T, et al. Second hematopoietic SCT for leukemia relapsing after myeloablative T cell-depleted transplants does not prolong survival. Bone Marrow Transplant. 2013;48:1192–7. doi: 10.1038/bmt.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakai K, Kanda Y, Fukuhara S, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19:396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 49.Damaj G, Mohty M, Robin M, et al. Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2014;20:1349–55. doi: 10.1016/j.bbmt.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Field T, Perkins J, Huang Y, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45:255–60. doi: 10.1038/bmt.2009.134. [DOI] [PubMed] [Google Scholar]
- 51.Yakoub-Agha I, Deeg J. Are hypomethylating agents replacing induction-type chemotherapy before allogeneic stem cell transplantation in patients with myelodysplastic syndrome? Biol Blood Marrow Transplant. 2014;20:1885–90. doi: 10.1016/j.bbmt.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 52.Damaj G, Duhamel A, Robin M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol. 2012;30:4533–40. doi: 10.1200/JCO.2012.44.3499. [DOI] [PubMed] [Google Scholar]
- 53.Keever CA, Small TN, Flomenberg N, et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989;73:1340–50. [PubMed] [Google Scholar]
- 54.Lewin SR, Heller G, Zhang L, et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood. 2002;100:2235–42. [PubMed] [Google Scholar]
- 55.Castermans E, Hannon M, Dutrieux J, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96:298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]