Abstract
Transcription factor expression fluctuates during β-cell ontogeny, and disruptions in this pattern can affect the development or function of those cells. Here we uncovered that murine endocrine pancreatic progenitors express high levels of the homeodomain transcription factor Prox1, whereas both immature and mature β-cells scarcely express this protein. We also investigated if sustained Prox1 expression is incompatible with β-cell development or maintenance using transgenic mouse approaches. We discovered that Prox1 upregulation in mature β-cells has no functional consequences; in contrast, Prox1 overexpression in immature β-cells promotes acute fasting hyperglycemia. Using a combination of immunostaining and quantitative and comparative gene expression analyses, we determined that Prox1 upregulation reduces proliferation, impairs maturation, and enables apoptosis in postnatal β-cells. Also, we uncovered substantial deficiency in β-cells that overexpress Prox1 of the key regulator of β-cell maturation MafA, several MafA downstream targets required for glucose-stimulated insulin secretion, and genes encoding important components of FGF signaling. Moreover, knocking down PROX1 in human EndoC-βH1 β-cells caused increased expression of many of these same gene products. These and other results in our study indicate that reducing the expression of Prox1 is beneficial for the expansion and maturation of postnatal β-cells.
Introduction
Islet β-cells, the most abundant endocrine cell type in the adult mammalian pancreas, are key for glucose homeostasis because they supply insulin to the entire body. Genetic or metabolic conditions that disrupt the complex physiology of β-cells can lead to diabetes, a prevalent life-threatening disease. Understanding the molecular mechanisms that specify the fate of β-cells in the embryonic pancreas and guide their final maturation in the postnatal pancreas is fundamental to engineer cells suitable for replacement therapy and develop better treatments for patients with diabetes (1,2).
All pancreatic endocrine cell types (i.e., insulin+ β-cells, glucagon+ α-cells, somatostatin+ δ-cells, pancreatic polypeptide+ (PP) cells, and ghrelin+ ε-cells) originate from progenitors that commonly express the transcription factor (TF) neurogenin 3 (Neurog3) (3,4). The majority of these progenitors form during a developmental period called the secondary transition, which in mice occurs between embryonic day (E) 12.5 and 15.5 (4). Once the distinct proendocrine cell lineages are specified, these cells proceed to differentiate and form clusters that gradually delaminate from the pancreatic epithelium. In mice, islet formation begins shortly before birth, with β-cells being allocated toward the central region that constitutes the islet core and the α-cells, δ-cells, ε-cells, and PP cells being positioned toward the periphery to form the islet mantle (4).
Studies in mice reveal that TF expression changes dramatically during the secondary transition, with some factors being upregulated and others being downregulated in the newly specified endocrine cell lineages (4). In β-cells, TF expression continues to change well into postnatal stages until the final maturation state is reached and the complex regulatory networks that maintain the functional status are established (1,2,4). Loss-of-function and gain-of-function studies have shown that altering TF expression can be detrimental to endocrine development, β-cell maturation, and β-cell maintenance (1,2,4–6).
The family of homeodomain TFs comprises several critical regulators of β-cell development and maintenance (1,4). We previously reported expression of a divergent member of this family named Prox1 in endocrine progenitors and islet cells of mice (7). We also identified that Prox1 activity in the pancreas is necessary for endocrine progenitor formation and α-cell differentiation (7) but is dispensable for β-cell formation (8). Prox1 expression in endocrine pancreatic cells is uniformly expressed at high levels in all endocrine progenitors (i.e., Neurog3+ cells), but mature islet cells have variable levels. In particular, we found that in the adult pancreas, only those cells located in the islet mantle retain high Prox1 expression (i.e., α-cells, δ-cells, PP cells, and ε-cells [7]). The notable lack of Prox1 expression in β-cells suggests that this step might be necessary for their specification and/or maturation. Here, we used a transgenic mouse approach to investigate whether sustained Prox1 expression is incompatible with β-cell development or maintenance. We report that β-cell maturation and expansion are drastically impaired in the presence of high levels of Prox1.
Research Design and Methods
Mice
Jojo-Prox1 (9), Neurog3-Cre (10), RIP-Cre (11), and Pax4+/− (12) mice were maintained and genotyped as previously reported. JoJo-Prox1;RIP-Cre mice (hereafter named Prox1betaOE) were generated from crosses of RIP-Cre mice (expressing Cre recombinase using the rat insulin 1 (Ins1) promoter [11]) with JoJo-Prox1 mice (carrying a CAG-loxP-eGFP-Stop-loxP-Prox1-Ires-β-gal transgene [9]). JoJo-Prox1;Neurog3-Cre mice (hereafter named Prox1endOE) were produced from crosses of Neurog3-Cre mice (expressing Cre in endocrine pancreatic precursors [10]) with JoJo-Prox1 mice. Mice were treated according to criteria outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal experiments were reviewed and approved by the St. Jude Animal Care and Use Committee.
Fasting and Nonfasting Blood Glucose
Blood glucose levels from the tail vein in mice that were fasted overnight or fasted and fed for 1 h were measured with the CONTOUR Blood Glucose Monitoring System (Bayer HealthCare LLC).
Intraperitoneal Glucose Tolerance Test
Mice were fasted overnight and blood glucose (t = 0) was measured from the tail vein as above. Glucose (2 mg dextrose/g body weight) in sterile PBS was injected intraperitoneally, and blood glucose levels were measured at 20, 40, 60, and 120 min postinjection.
ELISA for Insulin
Blood was collected (cardiac puncture) from mice that were fasted overnight and fed 1 h with regular chow. The Rat/Mouse Insulin ELISA Kit (Millipore) was used for serum insulin quantification as per the manufacturer’s directions.
Tissue Processing
Mouse embryos or pancreata of newborn mice were prepared for cryosectioning as described by Wang et al. (7). Cardiac perfusion (4% paraformaldehyde/PBS) was used for mice after postnatal day (P) 15 that were previously anesthetized with Avertin.
Immunostaining
Immunohistostaining of frozen sections was performed as previously described (13). MafA detection required antigen retrieval (1% SDS, 5 min). Images were obtained with a Zeiss Axioskop 2 microscope or a confocal/multiphoton laser-scanning Zeiss LSM 510 META microscope and processed with Adobe Photoshop 7.0 (Adobe Systems). Supplementary Table 1 lists the antibodies.
Morphometric Analysis and Quantification of Fluorescence Intensity
Whole pancreata of control and Prox1endOE mice (n = 3–5 per group) were sectioned (10 μm), and three representative sections were used for cell counting and/or morphometric analyses. Only Prox1endOE breeders constantly producing >50% Prox1endOE hyperglycemic offspring were used here. Islets were stained with anti-synaptophysin antibodies, and anti-Ki67 antibodies to visualize proliferating cells. AxioVision software version 4.7 (Zeiss) was used for morphometric analyses. Three representative sections from control, normoglycemic, and hyperglycemic Prox1endOE pancreata were also stained with anti-Prox1 and anti-insulin antibodies and imaged at equal exposure levels, and the intensity of fluorescent signals was quantified using SlideBook version 5.5.
Quantitative Real-Time PCR
RNA isolation and quantitative PCR (qPCR) were performed as described by Seth et al. (14). Mice older than 2 weeks were perfused with RNAlater (Invitrogen), and the dissected pancreata were incubated overnight at 4°C in RNAlater before RNA extraction. 18s rRNA expression was used to normalize gene expression levels. Supplementary Table 2 lists the qPCR primers.
Microarray Analysis
Total RNA from pancreata of control and Prox1endOE mice (n = 3) was isolated, and RNA quality was assessed using the Agilent 2100 Bioanalyzer system. Gene expression was analyzed using the Mouse Genome 430 2.0 GeneChip Array (Affymetrix) at the Hartwell Center for Bioinformatics & Biotechnology, St. Jude Children’s Research Hospital. The GeneChip Operating Software was used for fluorescence detection, and data calculations were performed using the MAS5 statistical algorithm and the Affymetrix GeneChip Operating Software version 1.4. ANOVA, gene set enrichment analysis (GSEA), and local pooled error t test were used for data analyses. Microarray data were deposited in Gene Expression Omnibus under accession number GSE68133 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=sbgpmkkqdpitdgt&acc=GSE68133).
Retroviral Transduction of β-TC6 Cells
The human PROX1 cDNA was cloned into an MSCV-IRES-GFP plasmid (15), and 293T cells were transfected with MSCV-PROX1-IRES-GFP or MSCV-IRES-GFP vectors and two plasmids carrying the viral packaging proteins. The viral particle supernatant was harvested 24 h later, filtered (0.45-μm gauze), and added to β-TC6 cells (grown to 70% confluence) in the presence of 0.8 µg/mL Polybrene. The next day, the viral supernatant was replaced with complete growth medium and the cells were collected 48 h postinfection for RNA isolation (Qiagen RNeasy Mini Kit).
In Silico Analysis of Mouse MafA Sequences
The mouse genome (assembly GRCm38.p3, January 2012) was scanned for evolutionarily conserved regions (ECR Browser; http://ecrbrowser.dcode.org/) and partially analyzed for Prox1-binding sites (–10 kb and +10 kb of the transcription start site) (14,16) in mouse MafA using the TRANSFAC (https://portal.biobase-international.com/cgi-bin/build_t/idb/1.0/searchengine/start.cgi) and JASPAR (http://jaspar.genereg.net/cgi-bin/jaspar_db.pl?rm=browse&db=core&tax_group=vertebrates) bioinformatics tools.
Chromatin Immunoprecipitation
α-TC1 (clone 9; American Type Culture Collection [ATCC]) and β-TC6 cells (ATCC) were fixed with 1% paraformaldehyde/PBS (10 min, room temperature) and nuclear chromatin was collected. The harvested chromatin was fragmented using micrococcal nuclease (SimpleChIP Enzymatic Chromatin Immunoprecipitation Kit; Cell Signaling Technology), as per the manufacturer’s instructions. The chromatin was precleared with rabbit IgG and immunoprecipitated with anti-Prox1 antibody or isotype-specific IgG. Immunoprecipitates were washed and eluted, protein-DNA complexes were de-cross-linked, and DNA was purified using QIAquick columns (Qiagen). qPCR results were normalized to input DNA and expressed as fold change over IgG control. Supplementary Table 2 lists the qPCR primers.
Knockdown Experiments
EndoC-βΗ1 cells (4 × 106) were treated with ON-TARGETplus small interfering RNA of human PROX1 (J-016913–08, 0.5 nmol) or a nontargeting control (D001810) (GE 1003) using Buffer V (VVCA-1003; Lonza, Walkersville, MD) and an Amaxa Nucleofector 2 (Program G-016; Lonza). RNA was collected either 72 or 96 h postnucleofection using TRIzol reagent (Life Technologies), and the iScript cDNA Synthesis Kit (Bio-Rad) was used for cDNA synthesis. The qPCR reactions were performed with the gene primers listed in Supplementary Table 2 on a LightCycler 480 II (Roche) and analyzed by the ∆∆CT method. GAPDH was used for normalization.
Statistical Analyses
Microsoft Office Excel and the two-tailed Student t test were used for statistical analyses. P < 0.05 was considered statistically significant.
Results
Prox1 Overexpression Does Not Affect the Function or Integrity of Mature Islet β-Cells
Immunostaining analysis of mouse pancreatic tissues revealed that embryonic (Fig. 1A), postnatal (Fig. 1B), and adult (Fig. 1C) α-cells expressed high levels of Prox1, and embryonic and adult δ-cells, ε-cells, and PP cells expressed moderate to high levels (Supplementary Fig. 1A–C and data not shown). In contrast, this TF was barely detectible in embryonic (Fig. 1A), postnatal (Fig. 1B), and adult (Fig. 1C) β-cells, whereas Pdx1 (a distant homeodomain relative) was highly expressed in this cell population throughout life (Fig. 1C′) (1,2,4). These results indicate that after specification of pancreatic endocrine progenitors, Prox1 expression is uniquely downregulated in the β-cell lineage. This notion was supported by the observation that in Pax4-null mice that lack β-cells, Prox1 was highly expressed in almost every islet cell (Supplementary Fig. 1D and D′) (12).
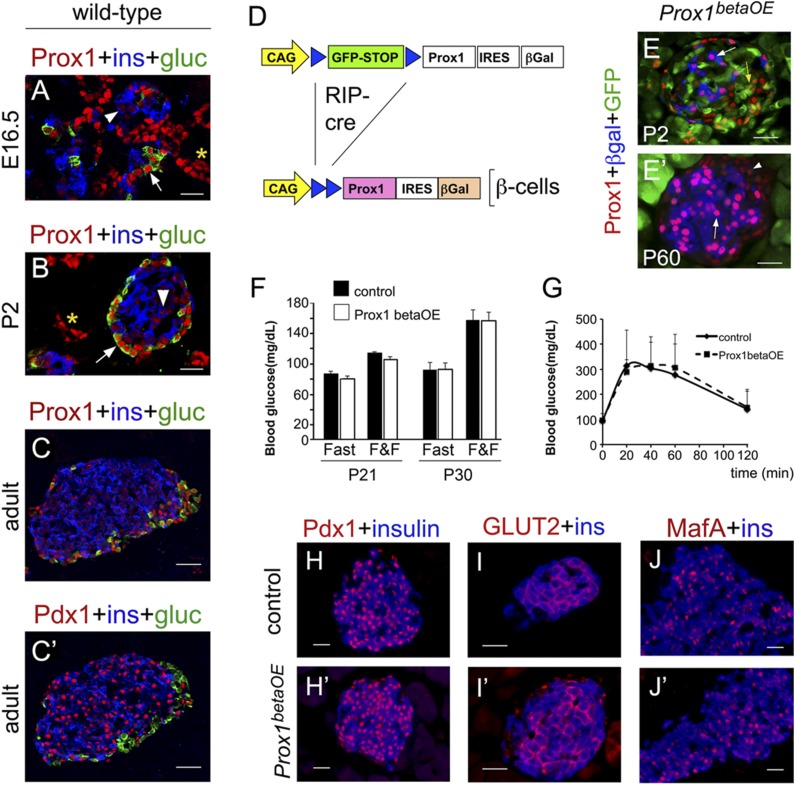
Figure 1.
Prox1 upregulation does not affect mature β-cell function. Prox1 (red) was expressed at very low levels in insulin+ cells (blue, arrowheads) and at high levels in glucagon+ cells (green, arrows) in the pancreata of wild-type mouse embryos (A), newborns (B), and adults (C). Asterisks indicate Prox1 expression in the pancreatic ducts. C′: Compared with Prox1, Pdx1 (red) levels were noticeably higher in insulin+ pancreatic cells (blue). C and C′ are consecutive sections. D: RIP-Cre–mediated excision of a GFP-STOP cassette in the Jojo-Prox1 transgene activated the expression of exogenous Prox1 and the β-gal reporter in β-cells of Prox1betaOE mice. E: Very few cells expressed the β-gal reporter (blue, arrow) and high Prox1 (red) in Prox1betaOE pancreata at P2. GFP expression (green) denoting lack of Cre activity was very extensive in the islet core (yellow arrow) at this stage. E′: Numerous cells expressed high Prox1 (red, arrow) and β-gal (blue) throughout the islet core in Prox1betaOE adult pancreata. The arrowhead indicates Prox1 normal expression in peripheral islet cells. Both the blood glucose levels under fed (fast and fed [F&F]) and fasting (Fast) conditions (F) and the glucose clearance response (G) were comparable between Prox1betaOE and control mice (RIP-Cre). The expression of Pdx1 (red [H and H′]), GLUT2 (red [I and I′]), and MafA (red [J and J′]) in insulin+ cells (blue) was indistinguishable between control (RIP-Cre [H–J]) and Prox1betaOE (H′–J′) adult pancreata. A–C and C′ are confocal images. G: Error bars represent +SEM values; n = 5–6 mice per genotype. Scale bars: 25 μm.
Our immunostaining results suggested that high Prox1 expression could be detrimental to β-cells. Therefore, we overexpressed Prox1 in mature β-cells using a novel transgenic mouse strain that we named JoJo-Prox1;RIP-Cre or Prox1betaOE. In Prox1betaOE mice, Cre deletes a floxed-eGFP-STOP sequence and activates Prox1 and β-gal in only insulin-expressing cells (11), with the CAG promoter sustaining Prox1 and β-gal expression in these cells (Fig. 1D). We observed β-gal/Prox1HIGH expression in only a few insulin+ cells of Prox1betaOE mice at P2–P7 (Fig. 1E and Supplementary Fig. 2A and B) and extensive β-gal/Prox1HIGH expression in insulin+ cells of Prox1betaOE adult mice (Fig. 1E′ and Supplementary Fig. 2C). Overall, Prox1 upregulation was largely restricted to mature β-cells in our Prox1betaOE mouse model.
Prox1betaOE mice had normal blood glucose levels under fasting and nonfasting conditions (Fig. 1F), and their response to a glucose tolerance test was no different from that of control (RIP-Cre) mice (Fig. 1G). Also, both the islet architecture and the expression of various β-cell–specific markers (e.g., Pdx1, insulin, GLUT2, and MafA) were normal in Prox1betaOE pancreata (compare Fig. 1H–J with H′–J′). These results reveal that Prox1 overexpression in mature β-cells does not alter the function or integrity of these cells.
Prox1 Overexpression in Immature β-Cells Affects Glucose Homeostasis
We produced a second transgenic mouse strain (Prox1endOE) by crossing JoJo-Prox1 mice with Neurog3-Cre mice to investigate the effects of Prox1 overexpression in immature β-cells (Fig. 2A). Similar to a genetic fate mapping study using Neurog3-Cre mice (17), we detected β-gal expression in the brain (including the ventral thalamus), spinal cord, heart, and pancreas in Prox1endOE embryos harvested at E14.5 (Supplementary Fig. 3). Also, as predicted from the Neurog3-Cre–mediated deletion of the floxed-eGFP-STOP sequence in endocrine precursors (10), most islets were β-gal+/Prox1HIGH+/GFP− and most acini were β-gal−/Prox1−/GFP+ in Prox1endOE newborn and adult pancreata (Supplementary Fig. 3H and I). Furthermore, we uncovered that the majority of β-cells expressed high levels of Prox1 in pancreata of Prox1endOE mice dissected at perinatal (Fig. 2B and C) and adult (Fig. 2D) stages. Therefore, this model is different from Prox1betaOE mice by expressing this TF in early, immature embryonic β-cells.
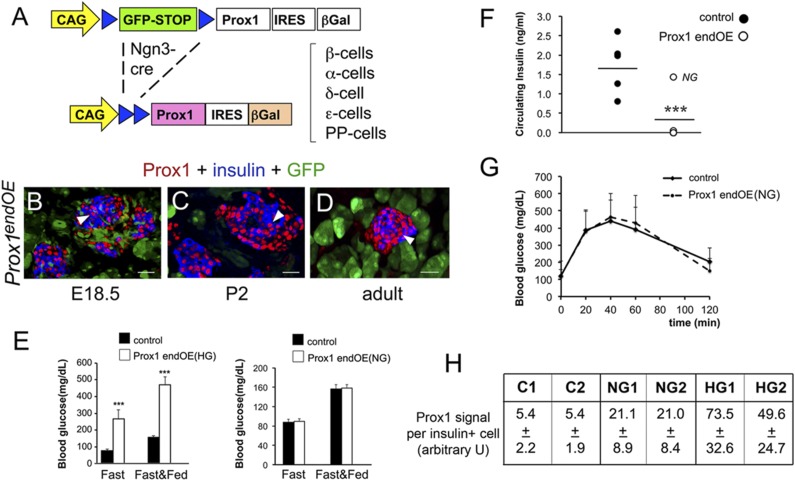
Figure 2.
Prox1 overexpression in immature β-cells causes hyperglycemia and hypoinsulinemia. A: Neurog3-Cre deleted a GFP-STOP cassette and activated exogenous expression of both Prox1 and β-gal in endocrine precursors and islet cells of Prox1endOE mice. Insulin+ cells (blue) of Prox1endOE mice expressed high levels of Prox1 (red and arrowheads) at embryonic (B), newborn (C), and adult (D) stages. Note that the islet cells are GFP− and the surrounding nonislet cells are GFP+. E: Prox1endOE(HG) mice showed hyperglycemia under fed (Fast&Fed) and fasting (Fast) conditions (control, Neurog3-Cre mice; n = 15–20 mice per genotype). In contrast, Prox1endOE(NG) mice and control mice (Neurog3-Cre) had similar glucose levels under fed and fasting conditions (n = 4–5 mice per genotype). F: Blood insulin levels were significantly lower in Prox1endOE(HG) mice than in control mice (Neurog3-Cre). A single mouse in the Prox1endOE group classified as normoglycemic (NG) showed blood insulin levels comparable to those of control mice (n = 5 mice per genotype). G: Prox1endOE(NG) mice and control mice (Neurog3-Cre) had similar glucose clearance after intraperitoneal glucose tolerance test (n = 9–12 mice per genotype). H: Prox1 immunofluorescence in single insulin+ cells was about fourfold higher in normoglycemic pancreata (NG, n = 2) and 9- to 13-fold higher in hyperglycemic pancreata (HG, n = 2), compared with controls (C, n = 2). Error bars represent +SEM values. ***P < 0.001. B–D are confocal images. Scale bars: 25 μm.
All Prox1endOE mice looked normal at birth and survived past the weaning stage. However, 40–50% of transgenic animals began to look ill at roughly 1 month and were killed (Supplementary Fig. 4A). Blood analysis uncovered severe hyperglycemia (Fig. 2E) and hypoinsulinemia (Fig. 2F) in these mice (designated Prox1endOE[HG] or hyperglycemic). In contrast, the circulating levels of glucose (fasting and nonfasting) (Fig. 2E) and insulin (Fig. 2F) and the response to intraperitoneal glucose tolerance test (Fig. 2G) were normal in a second cohort of healthy-looking Prox1endOE mice (designated Prox1endOE[NG] or normoglycemic). Results of quantifying the Prox1-immunofluorescent signals in individual insulin+ cells showed that Prox1 expression was much higher in Prox1endOE(HG) β-cells (9- to 13-fold) than either Prox1endOE(NG) (threefold) or Ngn3-Cre (control) β-cells (Fig. 2H). Immunostaining results revealed widespread β-gal and insulin coexpression in Prox1endOE(HG) pancreata (Supplementary Fig. 4B) and more restricted colocalization of these proteins in Prox1endOE(NG) pancreata (Supplementary Fig. 4B′). Similarly, Prox1HIGH/β-gal+ expression was very extensive in Prox1endOE(HG) islets (Supplementary Fig. 4C) and sparse in Prox1endOE(NG) islets (Supplementary Fig. 4C′). These results suggest that Prox1HIGH causes β-cell dysfunction in Prox1endOE(HG) mice.
Prox1 Overexpression Decreases Proliferation in Immature β-Cells
Immunostaining analysis showed the normal distribution of core insulin+ cells and peripheral glucagon+ cells in Prox1endOE islets (compare Fig. 3A–C with A′–C′). However, many islets looked abnormally small at P15 in some Prox1endOE pancreata (Fig. 3B and B′) and uniformly hypoplastic in adult Prox1endOE(HG) pancreata (Fig. 3E and C′). Conversely, only normal-sized islets were found in adult Prox1endOE(NG) pancreata (compare Fig. 3D with F).
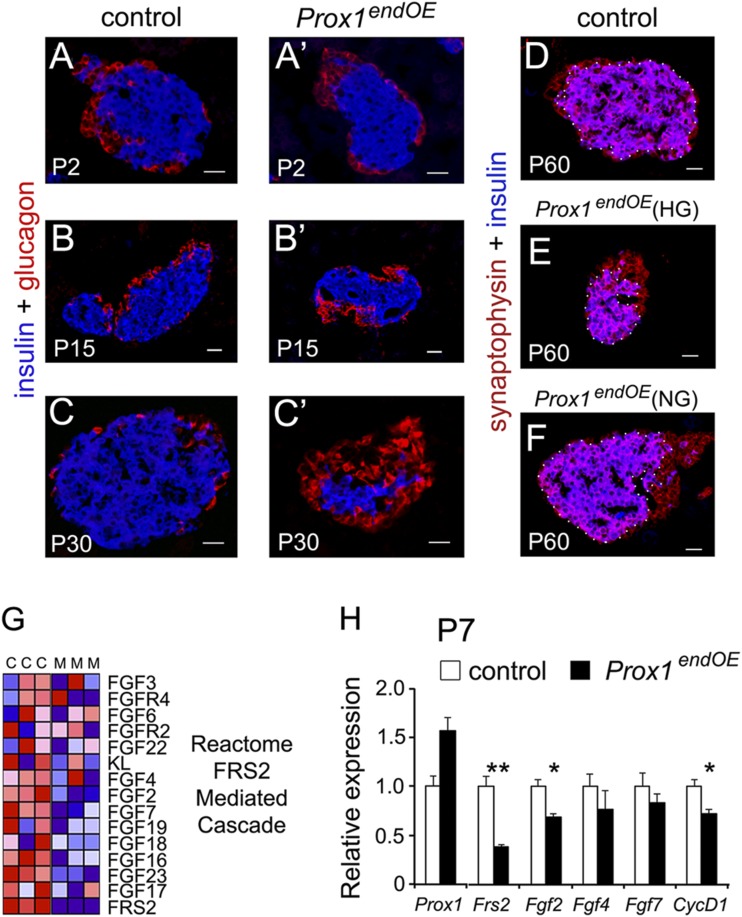
Figure 3.
Prox1endOE(HG) adult mice exhibit islet hypoplasia. A–C and A′–C′: Immunostaining for insulin (blue) and glucagon (red) showing that the mass of β-cells decreases after P2 in pancreatic specimens from Prox1endOE mice. C′ is an islet of a Prox1endOE(HG) mouse. Immunostaining for the pan-islet marker synaptophysin (red) and insulin (blue) showing that at P60, the sizes of islets from control (D) and Prox1endOE(NG) (F) mice are comparable and the size of islets of Prox1endOE(HG) mice is abnormally small (E). The white dots demarcate the insulin-positive area. G: Heat map representation of transcripts involved in FGF signaling that were consistently decreased in Prox1endOE pancreata at P15. “C” and “M” are control (Neurog3-Cre) and Prox1endOE triplicates. H: qPCR analysis showed that Frs2, Fgf2, and CycD1 transcripts were significantly decreased in Prox1endOE pancreata at P7 (tissues from litters of parents that consistently produced only hyperglycemic transgenic offspring were used for these experiments). Data represent the mean (±SEM) of three independent experiments. *P < 0.05; **P < 0.01. Scale bars: 25 μm.
Quantitative results demonstrated that Prox1 overexpression decreases postnatal β-cell proliferation by P2 and P7 in Prox1endOE pancreata (Table 1), and both the total β-cell area and the average islet size were reduced at P7 (Table 1). TUNEL staining also revealed that apoptotic β-cells were more abundant in adult Prox1endOE(HG) than Prox1endOE(NG) pancreata (average TUNEL+ cells/insulin+ area were 1.6 ± 0.14 [control], 1.5 ± 0.3 [NG], and 6.6 ± 3.1 [HG]; n = 2). However, TUNEL+/insulin+ cells were largely absent in both pancreata of control and Prox1endOE mice at P2–P15 (data not shown). Therefore, although the survival of pancreatic β-cells was markedly affected in Prox1endOE(HG) adult mice, this alteration was probably not a direct consequence of Prox1 overexpression.
Table 1.
Results of morphometric and cell proliferation analyses
| Islet size | Total β-cell area | Total α-cell area | Relative β-cell area | Relative α-cell area | β-Cell proliferation index | |
|---|---|---|---|---|---|---|
| P2 | ||||||
| Control (n = 3) | 8.2 ± 0.4 | 6.0 ± 0.2 | 2.0 ± 0.2 | 74.0 ± 2.0 | 25.0 ± 3.5 | 32.0 ± 0.2 |
| Prox1endOE (n = 5) | 7.2 ± 0.7 | 4.9 ± 0.5 | 2.7 ± 0.4 | 69.0 ± 1.6 | 38.2 ± 2.5 | 14.1 ± 2.8 |
| P value | 0.33 | 0.12 | 0.09 | 0.09 | 0.02 | 0 |
| P7 | ||||||
| Control (n = 3) | 9.6 ± 1.1 | 8.0 ± 0.8 | 1.7 ± 0.3 | 82.6 ± 2.2 | 18.6 ± 3.0 | 9.2 ± 0.6 |
| Prox1endOE (n = 6) | 6.3 ± 0.7 | 4.8 ± 0.6 | 1.9 ± 0.1 | 73.5 ± 2.0 | 32.9 ± 3.4 | 3.4 ± 0.5 |
| P value | 0.03 | 0.02 | 0.5 | 0.03 | 0.03 | 0.001 |
Islet size = synaptophysin+ area × 103 U area. Total (β/α) cell area = (insulin/glucagon)+ area × 103 U area. Relative (β/α) cell area = (insulin/glucagon)+ area/total islet area × 103 U area. β-Cell proliferation index = number of Ki67+ cells/insulin+ area. U are arbitrary units as determined by the AxioVision software. Numbers in bold indicate significant P values. Results are shown as mean ± SEM.
To further understand how Prox1 overexpression affects β-cells, the gene expression profiles of control and Prox1endOE pancreata at P15 were compared by microarray analysis. GSEA uncovered upregulation of immune response pathways in Prox1endOE pancreata (Supplementary Fig. 5A). However, these changes were not uniform across all samples and their potential relevance was not investigated.
Significantly, GSEA results showed downregulation of pathways involved with cell proliferation and β-cell development (Supplementary Fig. 5A–C). These results were consistent with our previous finding that both the proliferation index and the mass of β-cells were decreased in Prox1endOE mice after P7 (Table 1). GSEA data also revealed that pathways related to FGF signaling were downregulated in P15 Prox1endOE pancreata (Supplementary Fig. 5B and C and Fig. 3G). qPCR results corroborated significant decreases in Frs2 (encoding an adaptor protein that links FGFR-1 activation to the MAPK cascade) (18), Fgf2, and CycD1 transcripts (Fig. 3H) in Prox1endOE pancreata at P7. These findings are intriguing because a published study (19) showed that blocking FGFR-1 decreases the number of β-cells and causes diabetes in mice. Therefore, reduced FGF signaling could be a factor reducing β-cell mass in pancreata of Prox1endOE mice.
Sustained High Prox1 Expression Impairs β-Cell Maturation
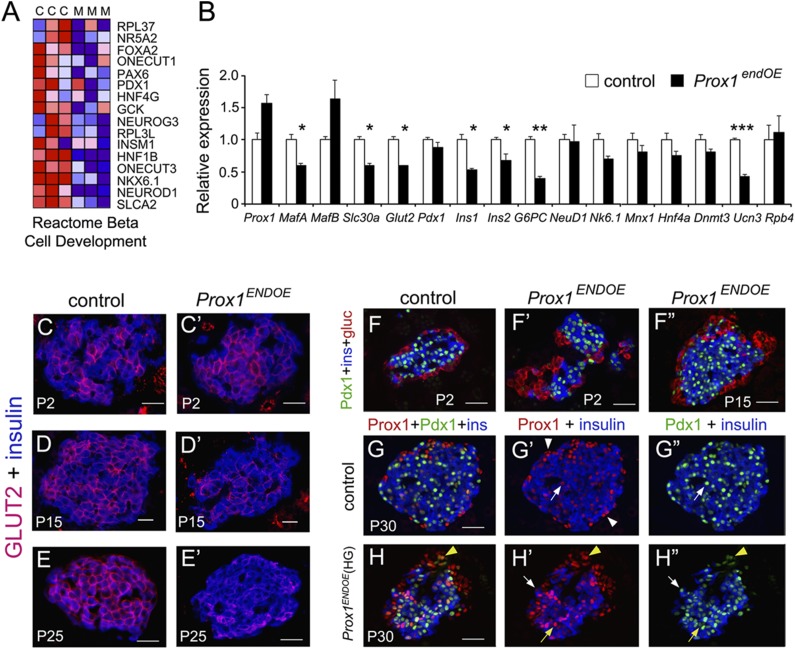
Similar to the GSEA results showing downregulation of β-cell development pathways in Prox1endOE pancreata at P15 (Fig. 4A and Supplementary Fig. 5B and C), qPCR analysis uncovered reduced expression of various transcripts encoding TFs that control β-cell development in Prox1endOE pancreata at P7 (Fig. 4B). Whereas some of those changes were not significant and probably paralleled the decrease in β-cell numbers caused by Prox1 overexpression (Table 1), some were significantly and specifically reduced at P7 in Prox1endOE pancreata (Fig. 4B). These included the following: MafA (encoding a critical regulator of β-cell development [20]), Ucn3 (encoding a distinctive marker of β-cell maturation [21]), Slc30a8 (encoding a zinc transporter required for insulin maturation and storage [22]), G6pc2 (encoding a regulator of fasting glucose levels [23]), Slc2a2/Glut2 (encoding the main glucose transporter in mouse β-cells [24]), and both the Ins1 and Ins2 transcripts (Fig. 4B).
Figure 4.
High levels of Prox1 affect β-cell gene expression. A: Heat map representation of transcripts involved in β-cell development that were consistently decreased in Prox1endOE pancreata at P15. “C” and “M” are control (Neurog3-Cre) and Prox1endOE triplicates. B: qPCR analysis of control (Neurog3-Cre) and Prox1endOE pancreata dissected at P7 to compare the expression of transcripts associated with β-cell maturation or transcripts encoding β-cell TFs (Slc30a = Slc30a8, G6PC = G6PC2, NeuD1 = NeuroD1, and Nk6.1 = Nkx6.1). Data represent the mean (±SEM) of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. GLUT2 expression in insulin+ cells of Prox1endOE mice was normal at P2 (C and C′) and considerably reduced at P15 (D and D′) and P25 (E and E′). F–F′′: Pdx1 expression in insulin+ cells of Prox1endOE mice was normal at P2–P15. G–G′′: Prox1 (red) and Pdx1 (green) colocalize in insulin+ cells (G′ and G′′, arrows) but not in peripheral islet cells (G′, arrowheads) in pancreata of adult control mice. H–H′′: Prox1 colocalizes with Pdx1 in both insulin+ cells (arrows) and insulin− peripheral cells (yellow arrowhead) in pancreata of Prox1endOE(HG) mice. Yellow arrows indicate a Prox1HIGH/Pdx1LOW β-cell, and white arrows indicate a Pdx1HIGH/Prox1LOW cell. Notice that peripheral cells expressing Pdx1LOW and no insulin are restricted to the smallest islets of Prox1endOE(HG) adult mice. Scale bars: 25 μm.
Immunostaining results showed that GLUT2 protein expression was very deficient in β-cells of Prox1endOE mice at P15 (Fig. 4D and D′) and nearly absent in many islets of Prox1endOE(HG) mice at P25 (Fig. 4E and E′). In contrast, the distribution of insulin proteins in the islet core (compare Fig. 4F with F′ and F′′ and Fig. 4G with H) or Pdx1 expression in β-cells (compare Fig. 4F with F′ and F′′ and Fig. 4G′′ with H′′) was unaffected. We conclude that insufficient expression of insulin and some key components of the glucose-stimulated insulin secretion machinery contributed to fostering hyperglycemia and hypoinsulinemia in Prox1endOE(HG) mice.
Defective Expression of MafA and MafB in β-Cells Overexpressing Prox1
MafA expression levels were decreased whereas MafB was abnormally increased in pancreata of Prox1endOE mice at P7 (Fig. 4B). These results were intriguing because the MafA and MafB TFs have distinct expression patterns and roles during mouse β-cell production and function (25–27). Mouse MafA is solely expressed in both immature and mature β-cells (Fig. 5A–D), with activity required postnatally for β-cell maturation (27). Deficiency of MafA in murine β-cells can lead to changes in gene expression similar to those seen in pancreata of Prox1endOE mice, including reduced expression of Slc30a8, G6pc2, Slc2a2/Glut2, and Ins1/Ins2 (26,28). Thus, some aspects of the Prox1endOE phenotype can be attributed to the deficiency of MafA.
Figure 5.
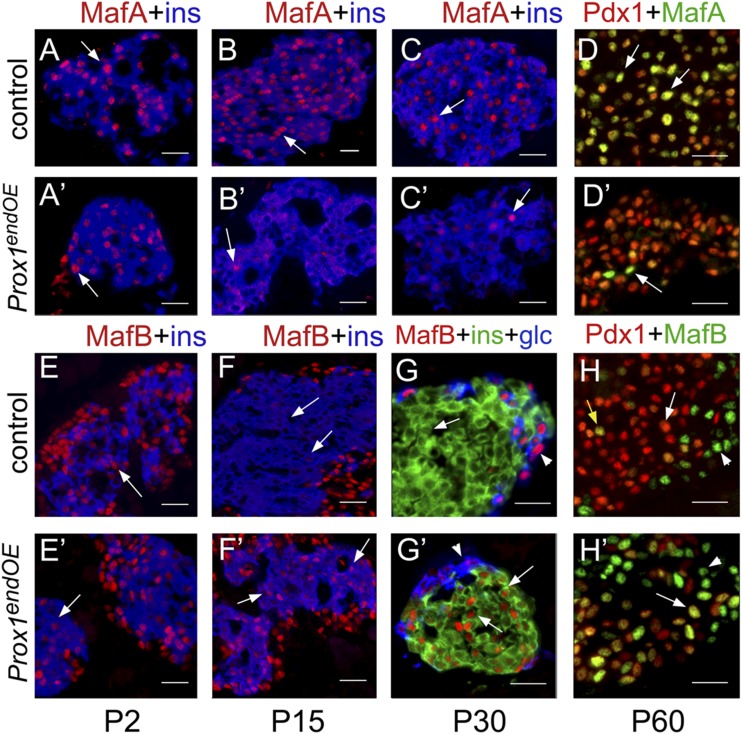
β-Cells that overexpress Prox1 have abnormal MafA and MafB expression. MafA (red in A–C; green in D) colocalized extensively with insulin (blue; A–C, arrows) and Pdx1 (red; D, arrows) in pancreata of control mice (Neurog3-Cre). Most insulin+ cells expressed MafA at P2 (A′, arrow), and only very few insulin+ cells expressed MafA at P15 (B′, arrow) and P30 (C′, arrow) in pancreata of Prox1endOE mice. The P30 Prox1endOE mouse showed hyperglycemia. D and D′: Most Pdx1+ (red) islet cells did not express MafA (green) in pancreata of adult Prox1endOE(HG) mice. The arrow in D′ indicates a rare MafA+/Pdx1+ cell. E and E′: Insulin+ cells (blue, arrows) expressed MafB (red) extensively in islets from control and Prox1endOE mice at P2. MafB (red) was no longer expressed in β-cells (insulin+ [F and G] and Pdx1+ [H], arrows) from control (Neurog3-Cre) mice after P15. Arrowheads indicate MafB+ α-cells; yellow arrow (H) indicates a rare MafB+/Pdx1+ islet cell. MafB expression (red, arrows) persisted in insulin+ cells (F′ and G′) and Pdx1+ cells (H′) of P15–P60 Prox1endOE mice. P30 and P60 transgenic pancreata were from Prox1endOE(HG) mice. The arrowheads in G′ and H′ indicate the normal peripheral MafB+ cells that lack insulin or Pdx1 expression. Scale bars: 20 μm (D, D′, H, and H′) or 25 μm (A–C, A′–C′, E–G, and E′–G′).
Immunostaining results showed comparable distribution of MafA proteins in insulin+ cells of control and Prox1endOE mice at embryonic (data not shown) and newborn (P2) stages (Fig. 5A and A′). However, MafA was visibly reduced and often restricted to the cytoplasm in insulin+ cells of Prox1endOE pancreata by P15 (Fig. 5B′). MafA was nearly undetectable in insulin+ cells (Fig. 5C′) and Pdx1+ cells (Fig. 5D′) in Prox1endOE(HG) pancreata, whereas all colocalized in control pancreata (Fig. 5D). These results corroborate that Prox1 overexpression in postnatal β-cells deeply impairs MafA expression.
In silico analysis of MafA sequences identified three potential Prox1-binding sites (14,16) in upstream regions of the mouse, rat, and guinea pig genes (Prox1 BS.I-III) (Supplementary Fig. 6A). Also, chromatin immunoprecipitation experiments revealed enrichment of endogenous Prox1 at a putative binding site located ∼2.6 kb upstream of the transcription start site in the mouse glucagonoma α-TC1 cells (Supplementary Fig. 6B). In contrast, chromatin immunoprecipitation approaches did not show enrichment of Prox1 at any of the potential binding sites in the chromatin of mouse insulinoma β-TC6 cells (Supplementary Fig. 6B and data not shown), not unexpected because of low expression levels. Moreover, Prox1 overexpression in β-TC6 cells significantly reduced Ins2 and G6pc2 transcript levels without affecting MafA transcript expression (Supplementary Fig. 6C). These results suggest that loss of MafA in insulin+ cells of Prox1endOE mice may not directly result from Prox1 upregulation.
MafB is exclusively expressed in embryonic and immature β-cells of rodents and its function is critical for early β-cell development (25,26). In contrast to MafA, Prox1 upregulation increased MafB transcripts in pancreata of P7 Prox1endOE mice (Fig. 4B). MafB expression is normally shut off soon after birth in insulin+ cells (26) (i.e., compare P2 [Fig. 5E] to P15 [Fig. 5F], P30 [Fig. 5G], and P60 [Fig. 5H] in control pancreata). In contrast, MafB was extensively coexpressed with insulin and Pdx1 at P2 (Fig. 5E′), P15 (Fig. 5F′), and P30 (Fig. 5G′) in Prox1endOE and Prox1endOE(HG) mice. This persistent MafB expression in insulin+ cells of Prox1endOE adult mice provides further evidence for Prox1HIGH levels impairing β-cell maturation. Notably, islet MafA and MafB expression was unaffected in the pancreata of Prox1endOE(NG) adult mice (Supplementary Fig. 7). These data demonstrate that severe MafA deficiency and abnormal retention of MafB in β-cells were specific to the Prox1endOE(HG) phenotype.
PROX1 Knockdown Increases Transcripts Associated With β-Cell Maturation in Human EndoC-βH1 Cells
EndoC-βH1 cells were derived from human fetal pancreas (29) and produce many specific β-cell markers, secrete insulin in response to glucose and secretagogues, and proliferate extensively. EndoC-βH1 cells express both MAFA and MAFB, a unique feature of the human islet β-cell (30). The majority of EndoC-βH1 cells also expressed moderate to high PROX1 (Supplementary Fig. 8). A small interfering (si) RNA approach was used to investigate if PROX1 downregulation affects the expression of genes associated with β-cell maturation in EndoC-βH1 human cells.
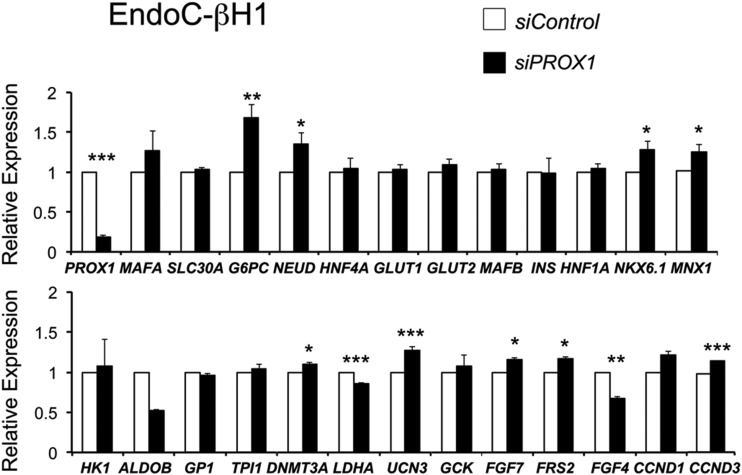
PROX1 transcript and protein levels were reduced by 80% in EndoC-βH1 cells expressing siPROX1 when compared with control siGFP treatment (Fig. 6). MAFA transcript levels appear upregulated (albeit not significantly) by siPROX1, whereas MAFB levels were unaffected by siPROX1 (Fig. 6). Most notable, PROX1 knockdown in EndoC-βH1 cells led to significant increases in transcripts encoding key β-cell TFs (NKX6.1, NEUROD1, and MNX1 [1,2,4]), markers of β-cell maturation (UCN3 and G6PC2), FGF signaling components (FRS2 and FGF7), and cell cycle regulators (CCND3) (Fig. 6). Also, of note, siPROX1 decreased LDHA transcripts in EndoC-βH1 cells (Fig. 6), a “disallowed gene” of the adult β-cell due to effects on mitochondrial metabolism. Glucose-induced insulin secretion in EndoC-βH1 cells is improved after knockdown of HK1 and LDHA (31). Because expression of disallowed genes in EndoC-βH1 cells suggests these represent a model of the immature human β-cells (30,31), repression of PROX1 may be important to human β-cell maturation.
Figure 6.
PROX1 knockdown increases transcripts associated with β-cell maturation in human EndoC-βH1 cells. qPCR results showing significant upregulation of transcripts associated with β-cell maturation, FGF signaling, and proliferation, after PROX1 knockdown (siPROX1) in human EndoC-βH1 cells. RNA used here was harvested 72 h posttransfection and data represent the mean (±SEM) of three independent experiments. SLC30A = SLC30A8, G6PC = G6PC2, and NEUD = NEUROD. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Some TFs that are normally expressed in multipotent pancreatic progenitors are downregulated or suppressed in β-cells at later stages, and gain-of-function studies show that reintroducing their expression harms glucose homeostasis. For example, ectopic expression of Hnf6 in murine β-cells leads to defective islet morphogenesis and diabetes (5,32,33), and Sox9 misexpression in mature murine β-cells decreases the production of insulin and leads to hyperglycemia (34). In a reciprocal manner, premature expression of the β-cell–specific TF MafA in multipotent pancreatic progenitors reduces their proliferation capacity and decreases endocrine cell formation (6). Thus, the expression of individual TFs must be carefully controlled throughout β-cell ontogeny to correctly assemble the regulatory networks that specify cell fate, promote differentiation, and maintain proper physiology. In agreement with this notion, we discovered that Prox1 overexpression obstructs the expansion and maturation of postnatal β-cells and causes severe hyperglycemia in mice.
Murine pancreatic β-cells become functionally mature in the first 2 weeks after birth, and during this critical period, the potential to secrete insulin in response to circulating glucose levels is acquired (35,36). The activity of MafA is essential to β-cell maturation because it controls the glucose-responsive transcription of insulin and key components of the glucose-stimulated insulin secretion machinery (20,25). Our study found that β-cells overexpressing Prox1 rapidly lose MafA expression after birth and develop a pathology sharing similarities with mice with conditional MafA inactivation in β-cells, including persistent expression of MafB in insulin+ cells, reduction in the β-cell mass, and deficient expression of Slc2a2/Glut2, Slc30a8, and G6pc2 (20,26). These results are consistent with the current view that postnatal β-cells of rodents have to switch from a MafB+/MafA+ to a MafA+/MafB− status for full maturation (20,27).
In spite of the similarities between Prox1 overexpression and MafA deficiency in mice, there are significant and important differences in their phenotypes. In particular, only Prox1 overexpression decreases β-cell proliferation, promotes β-cell apoptosis (although likely not directly), and produces hyperglycemia (20,26). Also, of note, the Prox1endOE phenotype recapitulated some defects that were previously reported in mice misexpressing Hnf6 in β-cells, such as GLUT2 deficiency, reduced pancreatic insulin content, decreased MafA expression, and fasting hyperglycemia (5,30). However, it is unlikely that Hnf6 played any role here because the β-cells of Prox1endOE mice did not express this TF (data not shown). Thus, Prox1endOE mice represent a unique model of defective β-cell maturation, expansion, and survival.
Our study did not conclusively identify other alterations that in conjunction with the loss of MafA fostered hyperglycemia in Prox1endOE mice. However, we hypothesize that defective FGF signaling could be one of those cooperating factors since this pathway was downregulated in Prox1endOE pancreatic tissues, and attenuation of FGF signaling promotes diabetes in mice (19). The specific contributions of FGF signaling to the Prox1endOE(HG) phenotype are outstanding issues warranting further investigation. Another interesting finding is that Prox1 upregulation reduced Frs2 transcripts in murine pancreatic tissues, whereas downregulation increased expression of the FRS2 human homolog in EndoC-βH1 cells. Moreover, various transcripts associated with driving β-cell maturation were increased in EndoC-βH1 cells upon knockdown of PROX1. Our results support the conclusion that high Prox1 expression is unfavorable for rodent and human β-cell maturation.
The segregation of a “normoglycemic” versus “hyperglycemic” Prox1 phenotype was another intriguing observation. Our experimental evidence suggests that these distinct outcomes were influenced by several factors: 1) the timing of Prox1 upregulation in β-cells (i.e., dysfunction only associated with earlier immature/newborn expression), 2) the extent of Prox1 overproduction in β-cells, and 3) the penetrance of Prox1 overexpression in the islet β-cell population. On the other hand, we discard the possibility that those distinct phenotypes were influenced by the extent of Prox1 overexpression in extrapancreatic tissues (particularly the ventral thalamic region that produces neurons that control appetite) because Prox1endOE mice were not obese or displayed abnormally excessive appetite. Moreover, Prox1endOE(HG) animals had specific and intrinsic β-cell alterations (e.g., decreased proliferation and loss of GLUT2 and MafA expression).
In conclusion, this study uncovered that Prox1 downregulation is a prerequisite to expand the β-cell mass after birth and for proper maturation of this lineage. Our findings warrant investigation of PROX1 expression in current protocols of directed differentiation of insulin+ cells from human-induced pluripotent stem cells or embryonic stem cells and suggest that manipulating the levels of this TF could increase the production of glucose-responsive β-cells for therapy. Finally, results of genome-wide association studies identifying single nucleotide polymorphisms in regulatory regions of human PROX1 that correlate with diabetes predisposition (37–43) open the possibility that some of those single nucleotide polymorphisms lead to Prox1 upregulation in immature β-cells.
Supplementary Material
Article Information
Acknowledgments. The authors thank G. Oliver (Department of Genetics, St. Jude Children's Research Hospital) for providing the Jojo-Prox1 mouse strain, J. Ye (Department of Genetics, St. Jude Children's Research Hospital) for help with the cell culture experiments, the Hartwell Center for Bioinformatics & Biotechnology and the Cell and Tissue Imaging Center of St. Jude Children’s Research Hospital, and Vani Shanker (St. Jude Children’s Research Hospital Scientific Editing Department) for editing the manuscript.
Funding. This study was supported by the National Institutes of Health (T32 DK007061 to H.A.C. and DK090570 to R.S.) and funds from the American Lebanese Syrian Associated Charities (B.S.-P.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.P. performed most of the experiments with the assistance of J.S. E.M.W. and H.A.C. performed the experiments in EndoC-βH1 cells. Y.D. performed the in silico analysis of MafA and prepared the retroviral constructs. G.N. assisted in the microarray data analysis. R.S. supervised the study. J.S. assisted L.P. with performing the experiments. G.G. provided the backbone retroviral vectors. P.L.H. provided the RIP-Cre mice. B.S.-P. conceived, designed, and directed the study and wrote the manuscript. B.S.-P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0713/-/DC1.
References
- 1.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev 2008;22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seymour PA, Sander M. Historical perspective: beginnings of the beta-cell: current perspectives in beta-cell development. Diabetes 2011;60:364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 4.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011;240:530–565 [DOI] [PubMed] [Google Scholar]
- 5.Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development 2000;127:2883–2895 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura W, Bonner-Weir S, Sharma A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev Biol 2009;333:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol 2005;286:182–194 [DOI] [PubMed] [Google Scholar]
- 8.Westmoreland JJ, Kilic G, Sartain C, et al. Pancreas-specific deletion of Prox1 affects development and disrupts homeostasis of the exocrine pancreas. Gastroenterology 2012;142:999–1009.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol 2010;8:e1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 2004;270:443–454 [DOI] [PubMed] [Google Scholar]
- 11.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000;127:2317–2322 [DOI] [PubMed] [Google Scholar]
- 12.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997;386:399–402 [DOI] [PubMed] [Google Scholar]
- 13.Westmoreland JJ, Wang Q, Bouzaffour M, Baker SJ, Sosa-Pineda B. Pdk1 activity controls proliferation, survival, and growth of developing pancreatic cells. Dev Biol 2009;334:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seth A, Ye J, Yu N, et al. Prox1 ablation in hepatic progenitors causes defective hepatocyte specification and increases biliary cell commitment. Development 2014;141:538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall AD, Picchione F, Geltink RI, Grosveld GC. PAX3-FOXO1 induces up-regulation of Noxa sensitizing alveolar rhabdomyosarcoma cells to apoptosis. Neoplasia 2013;15:738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charest-Marcotte A, Dufour CR, Wilson BJ, et al. The homeobox protein Prox1 is a negative modulator of ERRalpha/PGC-1alpha bioenergetic functions. Genes Dev 2010;24:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelling M, Anthwal N, McNay D, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol 2011;349:406–416 [DOI] [PubMed] [Google Scholar]
- 18.Kouhara H, Hadari YR, Spivak-Kroizman T, et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997;89:693–702 [DOI] [PubMed] [Google Scholar]
- 19.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse β-cells leads to diabetes. Nature 2000;408:864–868 [DOI] [PubMed] [Google Scholar]
- 20.Hang Y, Stein R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metab 2011;22:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 2012;30:261–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimienti F, Devergnas S, Pattou F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 2006;119:4199–4206 [DOI] [PubMed] [Google Scholar]
- 23.Bouatia-Naji N, Rocheleau G, Van Lommel L, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 2008;320:1085–1088 [DOI] [PubMed] [Google Scholar]
- 24.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell 1988;55:281–290 [DOI] [PubMed] [Google Scholar]
- 25.Artner I, Blanchi B, Raum JC, et al. MafB is required for islet beta cell maturation. Proc Natl Acad Sci USA 2007;104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artner I, Hang Y, Mazur M, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 2010;59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hang Y, Yamamoto T, Benninger RK, et al. The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes 2014;63:1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Moriguchi T, Kajihara M, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 2005;25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravassard P, Hazhouz Y, Pechberty S, et al. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest 2011;121:3589–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharfmann R, Pechberty S, Hazhouz Y, et al. Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest 2014;124:2087–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhawan S, Tschen S-I, Zeng C, et al. DNA methylation directs functional maturation of pancreatic β cells. J Clin Invest 2015;125:2851–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tweedie E, Artner I, Crawford L, et al. Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of beta-cells. Diabetes 2006;55:3264–3270 [DOI] [PubMed] [Google Scholar]
- 33.Wilding Crawford L, Tweedie Ables E, Oh YA, Boone B, Levy S, Gannon M. Gene expression profiling of a mouse model of pancreatic islet dysmorphogenesis. PLoS One 2008;3:e1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev 2013;27:2563–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jermendy A, Toschi E, Aye T, et al. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia 2011;54:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 2005;360:2211–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker A, Sharp SJ, Timpson NJ, et al. Association of genetic loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes 2011;60:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dupuis J, Langenberg C, Prokopenko I, et al.; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Zhang R, Wang C, et al. Variants from GIPR, TCF7L2, DGKB, MADD, CRY2, GLIS3, PROX1, SLC30A8 and IGF1 are associated with glucose metabolism in the Chinese. PLoS One 2010;5:e15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingelsson E, Langenberg C, Hivert MF, et al.; MAGIC investigators . Detailed physiologic characterization reveals diverse mechanisms for novel genetic loci regulating glucose and insulin metabolism in humans. Diabetes 2010;59:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kretowski A, Adamska E, Maliszewska K, et al. The rs340874 PROX1 type 2 diabetes mellitus risk variant is associated with visceral fat accumulation and alterations in postprandial glucose and lipid metabolism. Genes Nutr 2015;10:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecompte S, Pasquetti G, Hermant X, et al. Genetic and molecular insights into the role of PROX1 in glucose metabolism. Diabetes 2013;62:1738–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris AP, Voight BF, Teslovich TM, et al.; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.