Abstract
In vitro drug release kinetics studies are routinely performed to examine the ability of new drug formulations to modulate drug release. The underlying assumption is that the studies are performed in a sufficiently dilute solution, where the drug release is not limited by the solubility and the difference in release kinetics profile reflects the performance of a drug carrier in vivo. This condition is, however, difficult to meet with poorly water-soluble drug formulations, as it requires a very large volume of release medium relative to the formulation mass, which makes it challenging to measure the drug concentration accurately. These difficulties are aggravated with nanoparticle (NP) formulations, which are hard to separate from the release medium and thus require a dialysis bag or repeated high-speed centrifugation for sampling. Perhaps for these reasons, drug release kinetics studies of NPs of poorly water-soluble drugs are often performed in suboptimal conditions in which the NPs are not sufficiently diluted. However, such a practice can potentially underestimate drug release from NPs, leading to an inaccurate prediction that the NPs will attenuate the drug activity in vivo. Here we perform release kinetics studies of two different NP formulations of paclitaxel, a representative poorly water-soluble drug, according to common practices in the literature. We find that the drug release from NPs can be substantially underestimated depending on the choice of the release medium, NP/medium ratio, and handling of release samples. We discuss potential consequences of underestimating drug release, ending with suggestions for future studies with NP formulations of poorly water-soluble drugs.
Keywords: poorly water-soluble drugs, paclitaxel, solubility, nanoparticles, in vitro release kinetics, sustained release
Graphical abstract
1. INTRODUCTION
Nanoparticles (NPs) are used in various drug delivery applications. NPs can be designed to attenuate drug release so that they have minimal side effects on nontarget tissues during circulation.1 NPs are also used to solubilize poorly water-soluble drugs.2 For example, polymeric micelles help disperse poorly water-soluble drugs in water by encapsulating the drugs in hydrophobic cores while facing water via hydrophilic shells.3,4 Alternatively, poorly water-soluble drugs can be processed into nanocrystals, pure drug particles of nanometric dimensions stabilized with surface active agents, to increase their dissolution rate in water.5–7 Irrespective of the purposes of NPs, in vitro drug release kinetics (cumulative drug release vs time profiles, also called dissolution kinetics) are almost always examined to demonstrate their ability to attenuate or enhance drug release.
Release kinetics studies or dissolution tests of NPs are performed by various methods.8 In the dialysis method, NP suspension is placed in a dialysis bag with a specified molecular weight cutoff (MWCO), and drug molecules diffusing out of the bag are frequently sampled for quantitative analysis. Alternatively, NPs are suspended in a finite volume of release medium and incubated with agitation. The suspension is spun down at certain time points to separate a supernatant, which is sampled and analyzed to determine the amount of drug released during the interval. A standardized United States Pharmacopoeia (USP) method is also available. In the USP apparatus 4 (flow-through cell apparatus) method, NPs are put in a small dialysis unit and placed in a small volume cell, through which the release medium is passed at a constant flow rate and analyzed at regular time points.9
Irrespective of the test method, the assumption underlying the release kinetics studies is that the dose range of the NP products and the volume of release medium satisfy sink conditions (defined as the volume of medium at least three times that required to form a saturated solution of a drug10): i.e., the drug release is not limited by the solubility, and the difference in release kinetics profile reflects the performance of NPs as a drug carrier in vivo. To meet this requirement, it is important that one use a sufficient volume of release medium for the NPs. However, in the case of poorly water-soluble drugs, satisfying the sink condition can be quite challenging as it means a very low ratio of NP mass to the volume of release medium. A disadvantage of using a large volume of release medium is that drug analysis gets difficult due to the low concentration. In order to alleviate this difficulty, the sampled solution is concentrated prior to analysis or a dissolution aid such as surfactants or cosolvents is included in the release medium to increase the drug solubility (hence the ratio of NP mass to medium volume).10,11
Given these requirement and constraints in fulfilling a sink condition, it is very important to know an accurate solubility value of a drug and set up appropriate experimental conditions in studying drug release kinetics from NPs. Nevertheless, we observe that solubility values of paclitaxel (PTX), a representative poorly water-soluble drug, reported in the literature vary over a range of orders of magnitude, and the release kinetics studies of PTX-loaded NPs are performed under different understandings of a sink condition. Here, we revisit the current practice of drug release kinetics studies on NP formulations of poorly water-soluble drugs and discuss potential pitfalls and consequences. We first determine the solubility and stability of PTX in potential release media (PBS, PBS with 0.2% Tween 80, and PBS with 50% fetal bovine serum (FBS)) and perform release kinetics studies of different PTX NP formulations in those media according to common practices in the literature. We discuss our results and other studies published in 2005–2014 based on our stability/solubility data, ending with suggestions for future studies with NP formulations of poorly water-soluble drugs.
2. EXPERIMENTAL SECTION
2.1. Determination of PTX Solubility in PBS, 0.2% Tween 80/PBS, and 50% FBS/PBS
PTX solubility in PBS (pH 7.4), PBS containing 0.2 v/v% Tween 80 (Tween/PBS), and PBS containing 50 v/v% FBS (FBS/PBS) were determined by incubating excess PTX (0.6–2.4 mg) in 1 mL of each medium at 37 °C for 7 or 24 h with agitation. Samples were centrifuged at 10 000 rpm for 20 min to separate a supernatant. PTX dissolved in PBS and Tween/PBS was directly analyzed with high performance liquid chromatography (HPLC) as described in Section 2.7. PTX dissolved in FBS/PBS was filtered with 0.45 μm PVDF syringe filters, extracted with ethyl acetate as described in Section 2.7, and analyzed with HPLC.
PTX solubility was alternatively determined by diluting 10 mg/mL PTX stock solution dimethyl sulfoxide (DMSO) in each medium. To determine PTX solubility in PBS and Tween/PBS, PTX/DMSO solution (10 mg/mL) was first diluted with Tween/PBS to make 1 mg/mL of PTX solution, which was further diluted with PBS to final concentrations of 0.1–20 μg/mL (n = 3) or with Tween/PBS to 1–70 μg/mL (n = 3). Samples were incubated at 37 °C for 24 h with shaking. Finally, PTX solutions were separated by filtration with 0.45 μm PVDF syringe filters and analyzed with HPLC. To determine PTX solubility in FBS/PBS, PTX/DMSO solution (10 mg/mL) was sequentially diluted with FBS/PBS to yield final concentrations of 25–300 μg/mL (n = 3). Samples were incubated at 37 °C for 7 or 24 h with shaking. At the end of the incubation, the samples were centrifuged at 10 000 rpm for 20 min, and the supernatant was filtered with 0.45 μm PVDF syringe filters, extracted with ethyl acetate as described in Section 2.7, and analyzed with HPLC.
To determine how quickly PTX precipitated in PBS at 37 °C, PTX solution in PBS at a concentration of 20 μg/mL was prepared by diluting 10 mg/mL PTX/DMSO solution with PBS (final DMSO concentration: 0.2%), aliquoted by 1 mL, and incubated at 37 °C with shaking. At predetermined time points, three aliquots were taken and centrifuged at 3000 rpm for 5 min. The supernatants were additionally centrifuged at 10 000 rpm for 20 min to remove precipitates and analyzed with HPLC.
2.2. Stability of PTX in 0.2% Tween 80/PBS
PTX solution (1.5 μg/mL) in Tween/PBS was prepared by diluting 10 mg/mL PTX/DMSO solution with Tween/PBS (final DMSO concentration: 0.015%). The solution was divided into several 1 mL aliquots, and the initial PTX concentration was determined with seven of them. The remaining aliquots were incubated at 37 °C, and three aliquots were taken at predetermined time points and kept at −80 °C until HPLC analysis. The frozen samples were thawed and analyzed with HPLC. The concentration of intact PTX at each time point was divided by the original PTX concentration (1.5 μg/mL) and expressed as the percentage of original PTX.
2.3. Preparation of PTX Nanoparticles (PTX/NPs)
PLGA NPs loaded with PTX (PTX/NPs) were prepared by the single emulsion solvent evaporation method. Briefly, 20 mg of PLGA and 2.5 mg of PTX were dissolved in 1 mL of dichloromethane (DCM) and emulsified in 4 mL of 4% poly(vinyl alcohol) (PVA) solution by probe sonication. The o/w emulsion was dispersed in deionized (DI) water and stirred for 1 h, followed by rotary evaporation for another hour to ensure DCM evaporation. Finally, NPs were collected by centrifugation and washed three times with water. The NPs were lyophilized with trehalose as a lyoprotectant.
2.4. Preparation of PTX Nanocrystals (PNC) and Human Serum Albumin-Stabilized PNC (aPNC)
PTX nanocrystals (PNC) were prepared according to the published method.12 Briefly, 4 mg/mL PTX/ethanol solution was added to 20 mL of DI water and stirred for 10 min in a round-bottom flask immerged in a sonication bath filled with ice water. The formed PNC was filtered through a 100 nm polycarbonate membrane and resuspended in DI water. To further stabilize PNC, 1 mg/mL PNC suspension was mixed with 2 mg/mL of human serum albumin solution and incubated for 1.5 h at room temperature. The human serum albumin-stabilized PNC (aPNC) was collected by centrifugation (10 000 rpm, 15 min) and washed with DI water twice.
2.5. Release Kinetics of PTX/NPs in PBS, Tween/PBS, or FBS/PBS via Centrifugation Method
To determine the PTX content in NPs, the freeze-dried PTX/NPs was dissolved in a mixture of acetonitrile/water (50:50) for 2 h, and the supernatant was analyzed with HPLC. For release kinetics studies of PTX/NPs, the freeze-dried PTX/NPs equivalent to 4.4 or 27 μg of PTX were suspended in 1 mL of release medium (PBS, Tween/PBS, or FBS/PBS) and incubated at 37 °C with constant agitation. At predetermined time points, the suspension was centrifuged at 10 000 rpm for 10 min at room temperature to separate NP pellets and supernatants. Then 0.8 mL of supernatant was sampled and replaced with the same volume of fresh medium in which the NP pellet was resuspended and returned for further incubation. The sampled supernatant was analyzed immediately (PBS and Tween/PBS) or stored frozen (FBS/PBS) until HPLC analysis. At the end of the release experiment, the remaining NPs were dissolved in 1 mL of acetonitrile/water (50:50) for 2 h (PBS and Tween/PBS) or processed with the same extraction method as release samples (FBS/PBS) to determine the unreleased PTX.
2.6. Release Kinetics of PNC and aPNC in PBS via Dialysis Method
PNC or aPNC equivalent to 200 μg of PTX were suspended in 3 mL of PBS, put in a dialysis cassette (MWCO 3500), placed in 200 mL of PBS, and incubated at 37 °C under constant agitation. At timed intervals, 5 mL of release medium was sampled and replaced with 5 mL of fresh PBS.
2.7. HPLC Analysis of PTX
PTX in PBS or PTX in Tween/PBS solution was analyzed with HPLC after filtration with a 0.45 μm PVDF syringe filter with no other treatment. Optionally, PTX in PBS sample was mixed with acetonitrile in 1:1 volume ratio and then filtered for HPLC analysis. PTX in FBS/PBS was extracted with ethyl acetate prior to HPLC analysis. Briefly, 1 mL of PTX solution in FBS medium with 10 μg of carbamazepine as an internal standard was mixed with 3 mL of ethyl acetate and shaken on a rotating shaker for 40 min. The mixture was then centrifuged at 4000 rpm for 15 min to separate an organic layer, which was transferred to a new glass vial and dried under vacuum. The dried sample was resuspended in the HPLC mobile phase, filtered through a 0.45 μm syringe filter, and analyzed by HPLC. A calibration curve was drawn with PTX solutions in FBS medium in known concentrations, treated in the same manner as the sample solutions. PTX was analyzed with HPLC equipped with UV detector (1100 series, Agilent Technologies, Palo Alto, CA) and an Ascentis C18 column (25 cm × 4.6 mm, particle size 5 μm) (Supelco, St. Louis, MO, USA). The mobile phase was a mixture of acetonitrile and water (50:50) run in the isocratic mode at a flow rate of 1 mL/min. PTX was detected at 227 nm.
3. RESULTS
3.1. PTX Solubility in PBS, Tween/PBS, and FBS/PBS
The reported values of PTX solubility in deionized water or phosphate-buffered saline (PBS, pH 7.4) range from 0.3 to 30 μg/mL (Supporting Table 1). PTX solubility in a medium containing a surfactant such as Tween 80 is reported to be much higher: up to >100 μg/mL (in 3% Tween 80).13 PTX solubility in calf serum is defined as 171 μg/mL.14 We evaluated PTX solubility in PBS, PBS containing 0.2% Tween 80 (Tween/PBS), and PBS containing 50% FBS (FBS/PBS) by suspending excess amounts of PTX in each medium and measuring the concentration of dissolved PTX. The results showed a similar trend as those in the literature, although our values fell in lower ends of the reported ranges. PTX solubility in PBS at 37 °C was measured at ∼0.2 μg/mL with no specific trend according to the incubation time, although the values were variable due to the limited sensitivity of HPLC (Figure 1a). PTX solubility in Tween/PBS was measured to be 3.3 μg/mL irrespective of the incubation time (Figure 1b). PTX solubility in FBS/PBS was measured at 35 μg/mL after 7 h incubation at 37 °C, much higher than those in PBS or Tween/PBS, which confirmed the solubilizing effect of serum proteins (Figure 1c). Notably, PTX concentration measured after 24 h incubation was 25 μg/mL, 28.6% lower than that after 7 h. This difference is attributable to the instability of PTX in serum, reported in our previous study15 as well as in others.16,17 This indicates that, if PTX release kinetics studies are performed in serum-containing medium and the medium is not sampled and analyzed frequently, one may not recover 100% of PTX from the formulation due to the degradation of released PTX. In contrast, PTX was relatively more stable in Tween/PBS, maintaining 94% of the initial concentration for 1 day (Figure 1d). This means that, as long as the medium is sampled at least once a day, PTX stability in Tween/PBS is less likely to be a problem.
Figure 1.
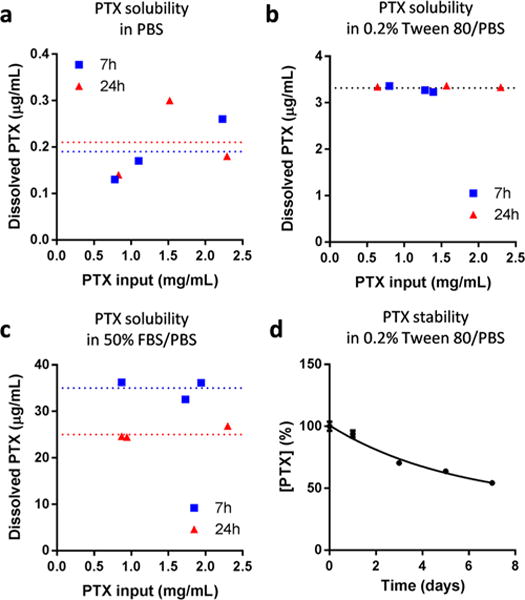
Paclitaxel (PTX) solubility in (a) PBS, (b) 0.2% Tween 80/PBS, and (c) 50% FBS/PBS. PTX solubility in each medium was determined by incubating excess PTX (0.6–2.4 mg) in 1 mL of medium at 37 °C for 7 or 24 h with agitation. Samples were centrifuged to remove precipitated PTX and analyzed with HPLC. (d) Stability of 1.5 μg/mL PTX in 0.2% Tween 80/PBS at 37 °C.
PTX solubility was alternatively measured with solutions prepared by diluting a concentrated PTX/DMSO stock solution in each medium. This method helped handle small quantities of PTX with greater accuracy than the previous method. However, a small trace of DMSO in the solution (maximum 0.2% in PBS, 0.7% in Tween/PBS, and 3% in FBS/PBS) seemed to have affected PTX dissolution, resulting in slightly higher solubility values after 24 h incubation in 37 °C (∼0.4 μg/mL in PBS and 3.9 μg/mL in Tween/PBS, Supporting Figure 1a,b). In FBS/PBS, PTX concentration increased linearly with the PTX input and never reached a limit at least by 300 μg/mL (Supporting Figure 1c). PTX concentrations measured after 24 h incubation at 37 °C were about 50% of those incubated for 7 h, confirming the instability of PTX in FBS/PBS. Of note, PTX solubility in Tween/PBS showed interesting variability across the measurements repeated four times. While the saturation solubility was measured to be 3.9 μg/mL in the presence of far excess PTX (Supporting Figure 1b), PTX solutions prepared in the range of 4–18 μg/mL in Tween/PBS showed concentrations greater than the saturation solubility to varying degrees in each experiment, indicating the formation of supersaturated solutions. This result indicates that one may observe variable solubility values in Tween/PBS, depending on the degree of supersaturation.
3.2. Release Kinetics of PTX NPs in PBS, Tween/PBS, or FBS/PBS via Centrifugation Method
We then prepared two types of PTX NPs (polymeric NPs and nanocrystals) and tested their release kinetics with conventional methods (centrifugation or dialysis methods). First of all, PTX was encapsulated in polymeric NPs (PTX/NPs) using the single emulsion method. PTX/NPs were spherical and had an average diameter of 161 nm (Supporting Figure 2). PTX release from PTX/NPs was tested using PBS, Tween/PBS, or FBS/PBS as release media. NPs equivalent to 4.4 μg of PTX was suspended in 1 mL of each release medium, creating a condition exceeding the solubility limit (in PBS), close to the solubility (in Tween/PBS), or satisfying the sink condition (in FBS/PBS). At regular intervals, 80% of the release medium (0.8 mL) was sampled after centrifugation and replaced with fresh buffers, and the sampled medium was analyzed by HPLC. In FBS/PBS, which satisfied a sink condition from the initial time point, NPs released 50.7 ± 9.1% of the loaded PTX upon the addition of the release medium and 98.7 ± 11.0% in 72 h (Figure 2a). Similarly, in Tween/PBS, NPs released 56.6 ± 1.2% of the loaded PTX immediately and 83.9 ± 1.3% in 72 h (Figure 2a). It is worth mentioning that NPs in Tween/PBS did not satisfy the sink condition at the initial time point but resulted in a similar trend as in FBS/PBS. This may be attributable to the fact that Tween/PBS was capable of forming supersaturated PTX solution in the range of 4–18 μg/mL (Supporting Figure 1b). On the other hand, PTX release in PBS was relatively small, reaching a cumulative release of 34.2 ± 6.4% in 72 h (Figure 2a). Since the total amount of PTX dispersed as NPs in PBS (4.4 μg/mL) was above the PTX solubility (0.2 μg/mL), we initially thought that PTX release was inhibited due to the low PTX solubility. However, the sum of total release (34.2%) and unreleased PTX (3.8%) fell far short of 100%, unlike those in Tween/PBS or FBS/PBS (Supporting Figure 3), suggesting a potential sample loss during the sampling or sample treatment. We thus added acetonitrile to the sampled PBS medium in 1:1 volume ratio and reanalyzed the samples. We found that a much greater amount of PTX was present in the sampled medium (46.1 ± 1.4% as immediate release and 78.7 ± 3.2% as cumulative release by 72 h) than initially measured. This indicates that PTX was released in PBS to a similar level as in Tween/PBS and FBS/PBS but quickly precipitated out in the sampled medium due to the low solubility in PBS. When analyzed as sampled (without additional acetonitrile), the precipitated PTX was removed during the HPLC sample preparation (i.e., filtration) and excluded from the analysis, which was avoided in the second analysis by the addition of acetonitrile. This result underscores the importance of keeping the ratio of total drug in NPs to medium volume below the drug solubility limit in the release kinetics studies. If this condition is not met (as in PBS in our case), one may observe low drug levels in the medium and incorrectly interpret them as sustained drug release, when in reality the drug has already been released and precipitated out in the sampled medium.
Figure 2.
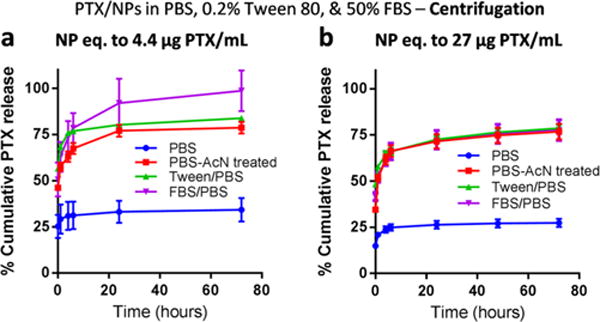
Release kinetics of PTX/NPs in media containing PBS, FBS, or Tween 80. PTX/NPs equivalent to (a) 4.4 μg or (b) 27 μg of PTX were suspended in 1 mL of release medium (PBS, Tween/PBS, or FBS/PBS) and incubated at 37 °C with constant agitation. At predetermined time points, the suspension was centrifuged to separate NP pellets and supernatants. Then 0.8 mL of supernatant was sampled and replaced with the same volume of fresh medium. The NP pellet was resuspended and returned for further incubation. The sampled supernatant was analyzed as sampled (PBS and Tween/PBS), with the addition of an equal volume of acetonitrile (PBS-AcN treated), or after extraction with ethyl acetate (FBS/PBS).
The release kinetics of PTX/NPs was also studied using a greater amount of NPs per release medium (NPs equivalent to 27 μg of PTX in 1 mL of release medium), which was comparable to typical conditions described in the literature (Supporting Tables 2 and 3) (hence ending up violating sink conditions for all samples at the initial time point). A similar trend was observed (Figure 2b), with the cumulative release in PBS being the least when directly measured but similar to those in Tween/PBS and FBS/PBS when analyzed with additional acetonitrile. Interestingly, PTX concentrations in sampled media (4.0 μg/mL in PBS and 13.1 μg/mL in Tween/PBS at the first sampling time point) were much greater than its solubility limit in each medium (0.2 μg/mL in PBS and 3.3 μg/mL in Tween/PBS). This may be explained by the increase in dissolution rate due to the small particle size of PTX NPs, followed by temporary supersaturation of PTX in the release medium. However, since the extent of supersaturation can vary (Supporting Figure 1b), one may not be sure that the result will be reproducible.
3.3. Release Kinetics of PNC and aPNC in PBS via Dialysis Method
PTX was also formulated as nanocrystals (PNC) by nonsolvent and temperature-induced crystallization.7 PNC was optionally coated with human serum albumin to produce albumin-stabilized PNC (aPNC). Both PNC (Supporting Figure 2) and aPNC were rod-shape particles with a length of ∼400 nm and a width of ∼100 nm. PTX release kinetics from PNC or aPNC was evaluated using the dialysis method and PBS as a release medium. Here, the NCs equivalent to 200 μg PTX was suspended in 3 mL of PBS, put in a dialysis cassette, and incubated in 200 mL of PBS with regular sampling and analysis, to make it comparable to typical conditions described in the literature (Supporting Tables 2). Figure 3a shows that PTX release (dissolution) from the NCs was very slow, reaching less than 10% cumulative release in 10 days. Given that the PTX concentration in the dialysis cassette was 66.7 μg/mL, far exceeding the solubility (0.2 μg/mL), and that the dialysis membrane could delay PTX diffusion into the release medium, we suspected that PTX might have precipitated in the dialysis cassette. Indeed a significant fraction of PTX remained in the dialysis cassette after 10 days. To estimate how quickly PTX precipitated in a dialysis cassette, we prepared a 20 μg/mL PTX solution in PBS (<1/3 of the initial concentration in a dialysis cassette) and sampled the solution at different time points to quantify the dissolved PTX. PTX rapidly precipitated out in less than 30 min, leaving PTX in solution only to the solubility level (Supporting Figure 4). This result suggests that even though the small size of NCs had increased the dissolution rate of PTX, the dissolved PTX might have undergone reprecipitation in the dialysis cassette. In other words, PTX detected in the release medium did not necessarily reflect the dissolution of NCs but that of PTX precipitates entrapped in the cassette. Since only the dissolved PTX could pass the membrane and became diluted in the release medium, the PTX concentration in the release medium was <0.1 μg/mL, below the solubility limit, at any time point (Figure 3b).
Figure 3.
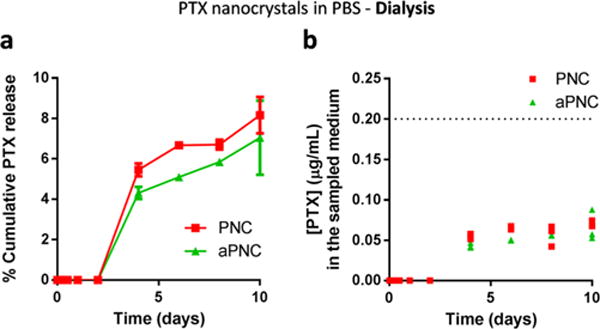
(a) Release kinetics of PNC and aPNC in PBS: PNC or aPNC equivalent to 200 μg of PTX were suspended in 3 mL of PBS, put in a dialysis cassette (MWCO 3500), placed in 200 mL of PBS, and incubated at 37 °C under constant agitation. At timed intervals, 5 mL of release medium was sampled and replaced with 5 mL of fresh PBS. (b) PTX concentration in the sampled medium at each time point. Symbols indicate each replicate. The dotted line indicates the saturation solubility of PTX in PBS.
4. DISCUSSION
Our study demonstrates that release kinetics of a poorly water-soluble drug may be much underestimated when the ratio of the NP mass to release medium is not sufficiently low because the released drug reprecipitated in the sampled medium (Figure 2) or in the dialysis cassette (Figure 3). In light of this observation, we reviewed articles reporting in vitro release kinetics of PTX from NPs or other sustained delivery formulations, published in 2005–2014. In studies using the centrifugation method, PTX concentration in a test tube ranged from 25 to 1000 μg/mL (Supporting Tables 2 and 3). In studies using the dialysis method, the concentration of PTX provided as NPs in total release medium (sum of the medium in dialysis bag and the bulk medium in which the bag was placed) was kept less than the saturation solubility, or the bulk medium was frequently replaced in most cases. However, the PTX concentration in a dialysis bag ranged from 40 to 1500 μg/mL (Supporting Tables 2 and 3), exceeding the water solubility of PTX. These studies conclude that their NP formulations achieve sustained PTX release over various time periods. However, given that these concentration ranges are far above the solubility (0.2 μg/mL), we suspect that even if the drug release had been much faster in reality they would not have been able to detect it. As our release kinetics studies show, when PTX is present in excess of the solubility limit in the medium, the drug can precipitate out in the system shortly after it is released out of the formulation. This translates to a low drug level in the release medium, which can be incorrectly interpreted as sustained drug release. From this perspective, we revisit some of the previous studies with conflicting bioactivity results. For example, with slow in vitro drug release kinetics, one may expect that a NP formulation will be less effective than a free drug control. Some studies do report the attenuated bioactivity of NPs relative to free PTX.13,18–22 However, in many cases, PTX NP formulations are not any less toxic than a free drug control.23–30 This may be interpreted as a consequence of enhanced cellular drug uptake or retention of NPs,23,26,29,30 but it could also be premature drug release, which has been ignored in the release kinetics study. A potential hazard of underestimating in vitro drug release is that it can mislead to a prediction that a NP formulation will attenuate the drug activity during circulation and thus help reduce its side effects on nontarget tissues. However, unlike in vitro, NPs face an ultimate sink condition in the body, where the released drug is continuously diluted and undergoes protein binding, and can thus show very different drug release behaviors and biological performances than expected from the in vitro release studies. This may partly explain why many NPs expected to be effective in vitro do not readily translate to clinically effective products.
In order for in vitro release kinetics to provide some predictive potential, it is necessary that the release studies be performed with release media that simulate critical features of in vivo systems and sampling methods that maintain simplicity and convenience of in vitro tests. PBS is the most simple and common medium for the release kinetics studies, but it requires a very low ratio of NPs to medium volume especially for poorly water-soluble drugs, which is met at the price of accuracy of the analysis. To avoid the analytical limitation, some have measured drug remaining as NPs in the system at regular time points as an indirect estimate of drug release, where the difference between the initial and remaining dose is considered the released drug.31 This is a good alternative to measuring the released drug, as long as the released drug remains stable in the medium. Serum-containing buffers may be a reasonable choice of release medium for mimicking a physiological fluid with a complex composition that affects drug release. Because of the solubilizing effect of serum proteins, these media are also good for achieving a sink condition at a reasonably high concentration. However, PTX in serum-containing medium requires an additional extraction step to separate PTX from the proteins prior to analysis. Moreover, PTX is unstable in serum-containing solution; thus, the drug release may be underestimated unless the medium is exchanged frequency. We find that Tween/PBS is most recommendable among those tested in this study, as PTX in Tween/PBS is more stable than in FBS/PBS, does not require extra sample treatment for HPLC analysis, and generates a similar release profile as that in FBS/PBS, which satisfied a sink condition. However, Tween/PBS may not be compatible with the dialysis method. If Tween/PBS is used to disperse NPs in a dialysis bag, the released drug will be entrapped in the surfactant micelles and not freely pass the membrane. Conversely, if Tween/PBS is used as the bulk medium, NPs isolated in the bag cannot make a direct contact with the surfactants that aid in its dissolution in the medium, and the released drug can reprecipitate in the dialysis bag.
Centrifugation and dialysis are most widely used for sampling the release medium, but both have critical limitations. Centrifugation method requires centrifugation at a high speed for separating NPs from the free drug at each sampling. The pressure generated during the centrifugation can disturb the equilibrium between released drug and NPs and make it difficult to resuspend the NPs for further incubation. In addition, the separation is often incomplete, leading to cumulative errors in measurement of the released drug. Dialysis method eliminates the need for a separation step, but the fact that the dialysis membrane itself functions as a diffusion barrier creates a different problem, especially for the poorly water-soluble drugs. As observed in this study, a poorly water-soluble drug, accumulating in the bag due to the delay in diffusion across the membrane, can reprecipitate into larger aggregates, which then drive the apparent release kinetics. A similar concern has been raised by Anderson et al., who studied release kinetics of lipophilic drug-loaded liposomes with the dialysis method and observed reversible binding of the released drug to the liposomes within the dialysis bag.31 Given these disadvantages, it is worthwhile to consider various alternative methods proposed over the years. For example, Szoka et al. used agarose hydrogel to accommodate liposomes for noninvasive separation of the released drug from the carrier.32 Alternatively, a biphasic dissolution model is a conceivable option for studying the release kinetics of poorly water-soluble drugs.11 Here, a water-immiscible organic solvent with a low density (e.g., octanol) is laid over an aqueous release medium that contains the formulation. A drug released into the medium partitions into the organic layer due to the lipophilicity, keeping the aqueous medium from saturation.11 This method can, at least in theory, maintain the sink condition without excessive dilution and/or invasive sampling, although it is necessary to find a way to keep NPs from direct contact with the organic solvent.
In summary, our study illustrates how in vitro release kinetics studies of poorly water-soluble drugs designed without considering the solubility limitation can result in underestimation of drug release and a misleading conclusion of sustained drug release. To reasonably simulate in vivo conditions in which NPs are administered, the ratio of a drug in NP form to the initial volume of the release medium should be sufficiently lower than the saturation solubility of the drug. Inclusion of a dissolution aid in the release medium can help meet this requirement without compromising sample detection as long as it is in direct contact with NPs. In any combinations of release media and sampling methods, it is desirable to analyze the remaining NPs at the end of the study and check the mass balance, in order to exclude potential underestimation of drug release. Our discussion is limited to PTX NPs, but the same consideration can be extended to other formulations of drugs with similar stability and solubility limitations.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 EB017791 and a Grant from the Lilly Endowment, Inc. to College of Pharmacy, Purdue University. We acknowledge the Fellowship support from the Egyptian Government Ministry of Higher Education Missions Sector to S.A.A. and the Ronald W. Dollens Scholarship support for B.S. We also thank Samyang Genex Corp (Seoul, Korea) for the kind donation of paclitaxel and the NAL Pharmaceuticals Ltd. (Monmouth Junction, NJ) for the gift support.
Footnotes
Supporting Information
Supporting Figures and Tables. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
References
- 1.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Merisko-Liversidge EM, Liversidge GG. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds. Toxicol Pathol. 2008;36(1):43–48. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 3.Batrakova EV, Bronich TK, Vetro JA, Kabanov AV. Polymer Micelles as Drug Carriers. In: Torchilin VP, editor. Nanoparticulates as Drug Carriers. Imperial College Press; London: 2006. pp. 57–93. [Google Scholar]
- 4.Yu BG, Okano T, Kataoka K, Kwon G. Polymeric Micelles for Drug Delivery: Solubilization and Haemolytic Activity of mphotericin B. J Controlled Release. 1998;53(1–3):131–136. doi: 10.1016/s0168-3659(97)00245-9. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Liu G, Ma J, Wang X, Zhou L, Li X, Wang F. Application of Drug Nanocrystal Technologies on Oral Drug Delivery of Poorly Soluble Drugs. Pharm Res. 2013;30(2):307–324. doi: 10.1007/s11095-012-0889-z. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Yeo Y. Nanocrystals for the Parenteral Delivery of Poorly Water-Soluble Drugs. Curr Opin Solid State Mater Sci. 2012;16(6):295–301. doi: 10.1016/j.cossms.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao R, Hollis CP, Zhang H, Sun L, Gemeinhart RA, Li T. Hybrid Nanocrystals: Achieving Concurrent Therapeutic and Bioimaging Functionalities toward Solid Tumors. Mol Pharmaceutics. 2011;8(5):1985–1991. doi: 10.1021/mp200154k. [DOI] [PubMed] [Google Scholar]
- 8.Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol Pharmaceutics. 2013;10(6):2093–2110. doi: 10.1021/mp300697h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Z. In Vitro Dissolution Testing with Flow-through Method: A Technical Note. AAPS PharmSciTech. 2009;10(4):1401–1405. doi: 10.1208/s12249-009-9339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The United States Pharmacopeia: The National Formulary (USP37/NF32) The United States Pharmacopeial Convention, Inc; Rockville, MD: 2014. [Google Scholar]
- 11.Phillips DJ, Pygall SR, Cooper VB, Mann JC. Overcoming Sink Limitations in Dissolution Testing: A Review of Traditional Methods and the Potential Utility of Biphasic Systems. J Pharm Pharmacol. 2012;64(11):1549–1559. doi: 10.1111/j.2042-7158.2012.01523.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R, Hollis CP, Zhang H, Sun L, Gemeinhart RA, Li T. Hybrid Nanocrystals: Achieving Concurrent Therapeutic and Bioimaging Functionalities toward Solid Tumors. Mol Pharmaceutics. 2011;8(5):1985–1991. doi: 10.1021/mp200154k. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. Enhanced Solubility and Stability of Pegylated Liposomal Paclitaxel: In Vitro and in Vivo Evaluation. Int J Pharm. 2007;338(1–2):317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Lovich MA, Creel C, Hong K, Hwang CW, Edelman ER. Carrier Proteins Determine Local Pharmacokinetics and Arterial Distribution of Paclitaxel. J Pharm Sci. 2001;90(9):1324–1335. doi: 10.1002/jps.1085. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj G, Kim MR, Mohammed SI, Yeo Y. Hyaluronic Acid-Based Hydrogel for Regional Delivery of Paclitaxel to Intraperitoneal Tumors. J Controlled Release. 2012;158(3):386–392. doi: 10.1016/j.jconrel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willey TA, Bekos EJ, Gaver RC, Duncan GF, Tay LK, Beijnen JH, Farmen RH. High-Performance Liquid Chromatographic Procedure for the Quantitative Determination of Paclitaxel (Taxol) in Human Plasma. J Chromatogr. 1993;621(2):231–238. doi: 10.1016/0378-4347(93)80100-i. [DOI] [PubMed] [Google Scholar]
- 17.Ringel I, Horwitz SB. Taxol Is Converted to 7-Epitaxol, a Biologically Active Isomer, in Cell Culture Medium. J Pharmacol Exp Ther. 1987;242(2):692–698. [PubMed] [Google Scholar]
- 18.Kim JH, Kim YS, Kim S, Park JH, Kim K, Choi K, Chung H, Jeong SY, Park RW, Kim IS, Kwon IC. Hydrophobically Modified Glycol Chitosan Nanoparticles as Carriers for Paclitaxel. J Controlled Release. 2006;111(1–2):228–234. doi: 10.1016/j.jconrel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Liang HF, Chen CT, Chen SC, Kulkarni AR, Chiu YL, Chen MC, Sung HW. Paclitaxel-Loaded Poly(Gamma-Glutamic Acid)-Poly(Lactide) Nanoparticles as a Targeted Drug Delivery System for the Treatment of Liver Cancer. Biomaterials. 2006;27(9):2051–2059. doi: 10.1016/j.biomaterials.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Saravanakumar G, Min KH, Min DS, Kim AY, Lee CM, Cho YW, Lee SC, Kim K, Jeong SY, Park K, Park JH, Kwon IC. Hydrotropic Oligomer-Conjugated Glycol Chitosan as a Carrier of Paclitaxel: Synthesis, Characterization, and in Vivo Biodistribution. J Controlled Release. 2009;140(3):210–217. doi: 10.1016/j.jconrel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZP, Feng SS. In Vitro Investigation on Poly(Lactide)-Tween 80 Copolymer Nanoparticles Fabricated by Dialysis Method for Chemotherapy. Biomacromolecules. 2006;7(4):1139–1146. doi: 10.1021/bm050953v. [DOI] [PubMed] [Google Scholar]
- 22.Malavaud BA, LeVisage C, Rioux-Leclercq N, Haller M, Breton P, Leong K. Efficacy of Paclitaxel Released from Bio-Adhesive Polymer Microspheres on Model Superficial Bladder Cancer. J Urol. 2004;171(4):188–188. doi: 10.1097/01.ju.0000103922.12319.59. [DOI] [PubMed] [Google Scholar]
- 23.Liu SQ, Tong YW, Yang YY. Thermally Sensitive Micelles Self-Assembled from Poly(N-Isopropylacrylamide-Co-N,N-Dimethylacrylamide)-B-Poly(D,L-Lactide-Co-Glyco Lide) for Controlled Delivery of Paclitaxel. Mol Biosyst. 2005;1(2):158–165. doi: 10.1039/b501756b. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Feng SS. Self-Assembled Nanoparticles of Poly(Lactide)–Vitamin E Tpgs Copolymers for Oral Chemotherapy. Int J Pharm. 2006;324(2):191–198. doi: 10.1016/j.ijpharm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Tan JP, Fukushima K, Nederberg F, Yang YY, Waymouth RM, Hedrick JL. Thermoresponsive Nanostructured Polycarbonate Block Copolymers as Biodegradable Therapeutic Delivery Carriers. Biomaterials. 2011;32(23):5505–5514. doi: 10.1016/j.biomaterials.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Li L, Liu J, Hennink WE, Zhuo R. Facile Fabrication of Thermo-Responsive and Reduction-Sensitive Polymeric Micelles for Anticancer Drug Delivery. Macromol Biosci. 2012;12(5):703–711. doi: 10.1002/mabi.201100459. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J. Amphiphilic Carboxymethyl Chitosan-Quercetin Conjugate with P-Gp Inhibitory Properties for Oral Delivery of Paclitaxel. Biomaterials. 2014;35(26):7654–7665. doi: 10.1016/j.biomaterials.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 28.Gu Q, Xing JZ, Huang M, Zhang X, Chen J. Nanoformulation of Paclitaxel to Enhance Cancer Therapy. J Biomater Appl. 2013;28(2):298–307. doi: 10.1177/0885328212446822. [DOI] [PubMed] [Google Scholar]
- 29.Song N, Liu W, Tu Q, Liu R, Zhang Y, Wang J. Preparation and in Vitro Properties of Redox-Responsive Polymeric Nanoparticles for Paclitaxel Delivery. Colloids Surf B. 2011;87(2):454–463. doi: 10.1016/j.colsurfb.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Tang XL, Cai SY, Zhang RB, Liu P, Chen HB, Zheng Y, Sun LL. Paclitaxel-Loaded Nanoparticles of Star-Shaped Cholic Acid-Core Pla-Tpgs Copolymer for Breast Cancer Treatment. Nanoscale Res Lett. 2013;8(1):420–432. doi: 10.1186/1556-276X-8-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modi S, Anderson BD. Determination of Drug Release Kinetics from Nanoparticles: Overcoming Pitfalls of the Dynamic Dialysis Method. Mol Pharmaceutics. 2013;10(8):3076–3089. doi: 10.1021/mp400154a. [DOI] [PubMed] [Google Scholar]
- 32.Peschka R, Dennehy C, Szoka FC., Jr A Simple in Vitro Model to Study the Release Kinetics of Liposome Encapsulated Material. J Controlled Release. 1998;56(1–3):41–51. doi: 10.1016/s0168-3659(98)00067-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


