Abstract
Background
The CAV1 gene encodes caveolin-1 expressed in cell types relevant to atherosclerosis. Cav-1-null mice showed a protective effect on atherosclerosis under the ApoE−/− background. However, it is unknown whether CAV1 is linked to CAD and MI in humans. In this study we analyzed a tagSNP for CAV1 in intron 2, rs3807989, for potential association with CAD.
Methods and Results
We performed case-control association studies in three independent Chinese Han populations from GeneID, including 1,249 CAD cases and 841 controls in Population I, 1,260 cases and 833 controls in Population II and 790 cases and 1,212 controls in Population III (a total of 3,299 cases and 2,886 controls). We identified significant association between rs3807989 and CAD in three independent populations and in the combined population (Padj=2.18×10−5, OR=1.19 for minor allele A). We also detected significant association between rs3807989 and MI (Padj=5.43×10−5, OR=1.23 for allele A). Allele A of SNP rs3807989 was also associated with a decreased level of LDL cholesterol. Although rs3807989 is a tagSNP for both CAV1 and nearby CAV2, allele A of SNP rs3807989 was associated with an increased expression level of CAV1 (both mRNA and protein), but not CAV2.
Conclusions
The data in this study demonstrated that rs3807989 at the CAV1/CAV2 locus was associated with significant risk of CAD and MI by increasing expression CAV1 (but not CAV2). Thus, CAV1 becomes a strong candidate susceptibility gene for CAD/MI in humans.
Keywords: Coronary artery disease (CAD) and myocardial infarction (MI), Atherosclerosis, Single nucleotide polymorphism (SNP), rs3807989, CAV1 and CAV2, Genome-Wide Association Study (GWAS)
1. Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide [1, 2]. CAD is caused by stenosis of one of coronary arteries due to plaque formation. When the stenosis is severe or a plaque ruptures, blood flow through a coronary artery is blocked, which causes thrombosis, myocardial infarction (MI) and sudden death. Multiple factors can influence the development of CAD and MI, for example, the age, gender, smoking, alcohol intake, hypertension, obesity, diabetes mellitus (DM), and genetic factors as well as interactions between genetic factors and environmental factors [3]. Since 2007, large scale genome-wide association studies (GWAS) have identified more than 50 genomic variants or single nucleotide polymorphisms (SNPs) that either increase or decrease risk of CAD/MI. These SNPs include rs599839 on 1p13, rs17465637 on 1q41, rs2943634 on 2q36, rs1420101 on 2q12, rs12619285 on 2q13, rs4857855 on 3q21, rs6903956 on 6p24, rs4143832 on 5q31, rs6922269 on 6q25, rs1333049 on 9p21, rs501120 on 10q11, rs3184504 on 12q24, rs17228212 on 15q22 and the other loci such as 1p32, 2q33, 3q22, 7p22, 10p11, 10q23, 11q22, 15q25, 21q22, and 19p23 [4-17]. Most GWAS for CAD/MI were performed in European ancestry populations, but recently GWAS in the Chinese population have been reported, too. Our group reported the first GWAS for CAD in the Chinese population and identified the C6orf105 gene (now referred to as ADTRP) as a susceptibility gene for CAD in the Chinese population only [15]. Later, another GWAS reported 4 SNPs associated with CAD in the Chinese population [16]. Recently, we developed a candidate pathway GWAS that combines eQTL analysis and mining of GWAS data and identified two SNPs in the complement system associated with CAD [18]. Some CAD variants identified by GWAS showed susceptibility of CAD across multiple ethnic populations, for example, SNPs on 9p21 [19-22]. However, some CAD risk variants showed ethnic specificity, for example, rs3184504 on 12q24 and rs17228212 on 15q22 with a 0 or very low frequency of the minor allele (MAF) in the Chinese population, rs12619285 on 2q13, rs4857855 on 3q21 and rs4143832 on 5q31 not replicated in the Chinese population [23, 24], and rs6903956 on 6p24 increasing risk of CAD only in the Chinese population to date [15, 25, 26]. Nevertheless, all genomic variants identified to date in aggregate accounted for <20% of heritability of CAD [17]. Considering the estimated heritability of 40% to 60% of CAD, a majority of CAD heritability remains missing, an observation referred to as missing heritability [27, 28]. Therefore, a major challenge for the field of genetics of CAD is to identify the rest of genomic variants that account for missing heritability.
The candidate gene approach is potentially one of the effective strategies to identify missing heritability of CAD. Almost all candidate gene studies failed to identify true CAD/MI risk variants before GWAS mostly due to the small sample sizes used in those studies and lack of rigorous independent replication. The small sample size generated false positive signals that are rarely replicated. With large sample cohorts available now for CAD/MI, we speculate that the candidate gene approach may become one of the prevailing strategies to identify missing heritability in the post-GWAS era. In this study, we employed the candidate gene approach to identify significant association between CAV1 and CAD.
The CAV1 gene is a small gene with 3 exons that encodes caveolin-1, one of the three members of the caveolin family, that assembles caveolae as a coat and scaffolding protein [29]. The caveolae plays an important role in many signaling pathways, ionic conductance and lipid regulation [29, 30]. Interesting studies on Cav1-null mice indicated that Cav1 was involved in insulin resistance, hypertension, atherosclerosis and lipoprotein metabolism [31-35]. Because insulin resistance, hypertension, and lipoprotein metabolism are all associated with risk of atherosclerosis, we hypothesized that genomic variant in CAV1 was associated with susceptibility to CAD and/or MI in humans. Thus, we selected a tagSNP in CAV1, rs3807989, and tested its association with CAD and MI using a case-control study design. Previous GWAS in populations of European ancestry found that SNP rs3807989 was associated with the electrocardiographic PR interval and QRS duration [36-38] as well as AF [39, 40], but not with CAD and/or MI. We studied three independent Chinese populations with a total of 6,185 subjects (3,299 CAD cases and 2,886 controls) from GeneID [15, 18, 41-48]. Significant association was found between SNP rs3807989 and CAD/MI in all three populations and the large combined population. Moreover, eQTL analysis and ELISA protein expression analysis found that allele A of SNP rs3807989 was associated with an increased expression level of CAV1 mRNA or protein.
2. Materials and Methods
2.1. Study subjects
The subjects in this study were all from the Chinese GeneID database, which is one of the largest GeneBank databases for cardiovascular diseases and contains more than 80,000 study subjects with several different types of diseases, including CAD/MI, atrial fibrillation, ventricular arrhythmias, hypotension, stroke, congenital heart disease and controls in China. All study subjects were all of Han ethnic origin by self-description. This study was approved by appropriate local institutional review boards on human subject research and conformed to the guidelines set forth by the Declaration of Helsinki. All participants have provided written informed consent.
A total of 6,185 subjects were characterized, including 3,299 CAD patients/cases and 2,886 non-CAD controls. The subjects were from three independent populations: Population I as the discovery population and Population II and Population III as independent replication populations (Table 1). There were 1,249 CAD cases and 841 controls in Population I, 1,260 cases and 833 controls in Population II, and 790 cases and 1,212 controls in Population III (Table 1). The numbers of MI patients in Population I, II and III were 568, 609 and 304, respectively (Table 1).
Table 1.
Clinical and Demographic Characteristics of Study Subjects.
| Characteristics | Population I | Population II | Population III | Population I+II+III (Combined) | ||||
|---|---|---|---|---|---|---|---|---|
| CAD (n=1,249) | Control (n=841) | CAD (n=1,260) | Control (n=833) | CAD (n=790) | Control (n=1,212) | CAD (n=3,299) | Control (n=2,886) | |
| Male, n (%) | 816 (65.33) | 509 (60.52) | 819 (65.00) | 495 (59.42) | 510 (64.56) | 818 (67.49) | 2,145 (65.02) | 1,822 (65.13) |
| Age, years (mean±sd)* | 65.08±12.57 | 58.80±10.68 | 66.18±12.26 | 59.52±10.85 | 58.30±10.26 | 57.97±11.80 | 63.87±12.34 | 58.66±11.23 |
| Hypertension, n (%) | 922 (73.82) | 178 (21.17) | 798 (63.33) | 149 (17.89) | 528 (66.84) | N/A | 2248 (68.14) | N/A |
| DM, n (%) | 265 (21.22) | 39 (4.64) | 261 (20.71) | 41 (4.92) | 136 (17.22) | N/A | 662 (20.06) | N/A |
| Smoker, n (%) | 330 (26.42) | 56 (6.66) | 303 (24.04) | 63 (7.56) | 294 (37.22) | N/A | 927 (28.10) | N/A |
| Drinker, n (%) | 195 (15.61) | 27 (3.21) | 203 (16.11) | 46 (5.52) | 14 (1.77) | N/A | 412 (12.49) | N/A |
| Lipid lowering medication, n (%) | 125 (10.00) | 7 (0.83) | 111 (8.80) | 7 (0.84) | N/A | N/A | N/A | N/A |
| AF, n (%) | 89 (7.12) | 10 (1.19) | 94 (7.46) | 15 (1.80) | 35 (4.43) | N/A | 243 (7.37) | N/A |
| MI, n (%) | 568 (45.48) | N/A | 609 (48.33) | N/A | 304 (38.48) | N/A | 1,481(44.89) | N/A |
For quantitative traits, data were presented as means±SD.
The diagnosis of CAD was made by at least two independent cardiologists according to the standard guidelines established by the ACC/AHA. A patient with >70% of luminal stenosis in one or more main vessels detected by coronary angiography, coronary artery bypass graft (CABG), percutaneous coronary intervention (PCA), and/or MI was diagnosed as a CAD case. The diagnosis of MI was based on typical chest pain sustained for at least 30 min, characteristic electrocardiographic patterns of acute MI, and elevation of troponin I or T and cardiac enzymes such as creatine kinase-MB and lactate dehydrogenase. Patients with congenital heart disease, childhood hypertension, type I diabetes mellitus, myocardial spasm, and myocardial bridge identified by angiography were excluded [15, 18, 46]. Subjects without history of MI or detectable stenosis evaluated by coronary angiography were defined as controls. The demographic and other relevant clinical information, if present, were all obtained from the medical records.
2.2. Isolation of genomic DNA and genotyping of SNP rs3807989
Human genomic DNA was extracted from peripheral blood samples using the Wizard Genomic DNA Purification Kit (Promega Corporation).
SNP rs3807989 was genotyped using a Rotor-Gene™ 6000 High Resolution Melt system (Corbett Life Science, Concorde, NSW, Australia). The procedures of PCR and the high-resolution melting analysis (HRM) were described by us previously [15, 41, 43, 45-48]. The sequences for primers for HRM genotyping and sequencing are: HRM forward primer, 5′-CGC GAC CCT AAA CAC CTC AA-3′ and reverse primer, 5’-TGA TTC TTT TTT GTC CTC TGG TGT C-3’; Sequencing forward primer, 5’- ATC CCT CCT CTC TGT TCA AGT TC-3’ and sequencing reverse primer, 5’- TGG CCT CAC GTG TTC ATT ATC-3’.
2.3. Real -Time quantitative RT-PCR
Total RNA was isolated from human peripheral blood leukocytes using the Trizol reagent (Life Technologies, Gaithersburg, MD). The RNA samples were quantified, reverse-transcribed and used for real-time qRT-PCR analysis with the Faststart Universal SYBR Green Master Kit (Roche Applied Science, Indianapolis, IN) as described by us previously [15, 18, 47]. The primers used in the study were 5′-CGC GAC CCT AAA CAC CTC AA-3′ (forward) and 5′-TGC CGT CAA AAC TGT GTG TCC-3′ (reverse) for CAV1, 5′-GCC ATG CCC TCT TTG AAA TCA-3′ (forward) and 5′-AAG GCA GAA CCA TTA GGC AGG-3′ (reverse) for CAV2, and 5′-AAG GTG AAG GTC GGA GTC AAC-3′ (forward) and 5′-GGG GTC ATT GAT GGC AAC AAT A-3′ (reverse) for the GAPDH reference gene (internal standard). The ΔΔCq method (RQ=2−ΔΔCq ,ΔΔCq is for the individual, ΔCq is the calibrator) was used to determine the differences of the mean expression levels of CAV1 and CAV2 among different genotypes for SNP rs3807989 as described [15, 18, 47].
2.4. Measurement of expression levels of CAV1 protein in human serum samples
Serum samples were collected from whole blood samples from human study subjects. We measured the serum concentration of the CAV1 protein using an enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp. , USA) according to the instructions from the manufacturer. Absorbance was read on a spectrophotometer (VERSA max microplate reader, USA) at 450 nm.
2.5. Statistical analyses
Power analysis of each replication population was conducted using the Power and Sample Size Calculation program (PS version 3.0.43) (http://biostat.mc.vanderbilt.edu). Linkage disequilibrium (LD) analysis was performed using the physical map information of CAV1 and CAV2 and the MAF of the CHB population from HapMap (http://www.hapmap.org, phase1, 2 & 3) and Haploview 4.2. (http://www.broadinstitute.org). The assumptions for LD analysis were r2 of 0.80 and MAF of 0.20. Hardy–Weinberg linkage disequilibrium analysis was performed with PLINK version 1.07 (http://pngu.mgh.harvard.edu/~purcell/plink/archive.shtml) in each control population. The 2×2 Pearson χ2 contingence tables were used for allelic association analysis. The 2×3 Pearson χ2 contingence tables were used for genotypic association analysis. Odds ratios (ORs) and corresponding 95% confidential intervals were calculated using PLINK version 1.07 or SPSS version.17.0. For association analyses, we also performed multiple logistic regression analysis to adjust significant covariates for CAD using SPSS version.17.0.
To detect the association between SNP rs3807989 with serum lipid levels, we used SPSS version.17.0 to assess genotypic association by linear regression under an additive, dominant and recessive genetic model.
For real-time qRT-PCR data, unpaired student's t tests and ANOVA were used for statistical analysis with SPSS version.17.0.
For ELISA data, unpaired student's t tests and ANOVA were used for statistical analysis with SPSS version.17.0.
3. Results
3.1. Significant allelic association between CAV1 SNP rs3807989 and CAD
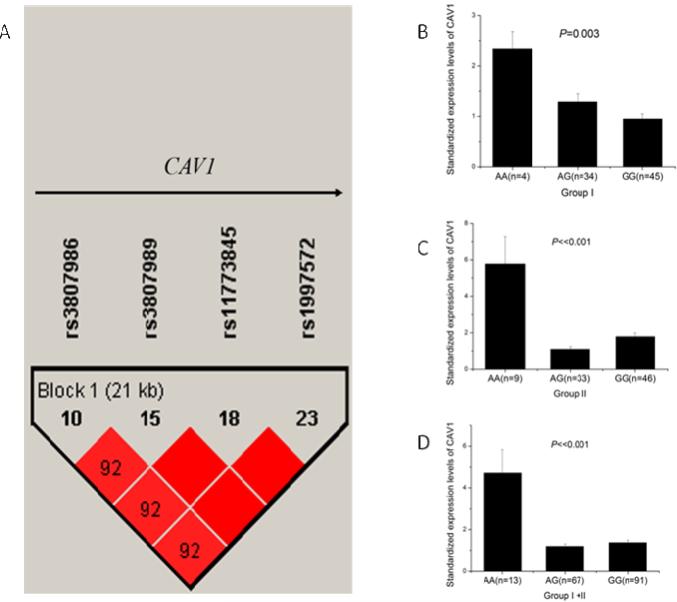
CAV1 is a small gene that spans a genomic region of 36,391 bp. Haploview 4.2 analysis showed that CAV1 contained only one LD with four SNPs (rs3807986, rs3807989, rs11773845, and rs1997572) when r2 was 0.80 and MAF was 0.20 (Fig. 1A). Any one of the four SNPs can sufficiently capture all genomic information of CAV1. Therefore, we selected rs3807989 in intron 2 as the tagSNP for CAV1. To determine whether CAV1 tagSNP rs3807989 was associated with CAD, we performed genetic association analysis in three independent Chinese Han populations. Populations I, II, and III consisted of 1,249 CAD cases and 841 controls, 1,260 cases and 833 controls, and 790 cases and 1,212 controls, respectively (Table 1). The demographic and clinical characteristics of the study populations are shown in Table 1.
Fig. 1.
Identification of a tagSNP for CAV1 by linkage disequilibrium (LD) analysis and significant association of SNP rs3807989 with expression of CAV1 mRNA. (A) Overview of LD of the 36.4 kb genomic region spanning CAV1. The LD structure was derived from MAF of SNPs for the Chinese population from the HapMap database (http://www.hapmap.org, phase1, 2&3) using Haploview 4.2. CAV1 has only one LD and rs3807989 captures all genomic information of the LD. (B-D) eQTL analysis of rs3807989 for CAV1 in two independent groups of study subjects. Major allele G of rs3807989 was associated with decreased expression of CAV1 mRNA , whereas minor allele A was associated with increased expression of CAV1 mRNA under an additive model in two independent groups of samples (B, C) and in the combined group (D).
SNP rs3807989 was genotyped in Population I. Genotypic frequencies of rs3807989 were in Hardy-Weinberg equilibrium in the control population (Phwe=0.85). Significant allelic association was identified between SNP rs3807989 and CAD with an odds ratio or OR of 1.24 for minor allele A (Pobs=3.23×10−3) (Table 2). After adjusting for significant covariates of hypertension, DM, smoking, alcohol consumption, gender and age, the association remained significant (Padj=8.04×10−3, OR=1.27 for minor allele A) (Table 2). Because rs3807989 was shown to be associated with AF, we further adjusted the association for AF and found it to remain significant (Padj=1.40×10−2, OR=1.25 for minor allele A). Therefore, the A allele of SNP rs3807989 is the risk allele in development of CAD.
Table 2.
Allelic association of SNP rs3807989 with CAD and MI in Chinese Han populations and the combined population.
| Study population | Sample size | Major/Risk Allele | MAF (case/control) | Before adjustment | After adjustment | |||
|---|---|---|---|---|---|---|---|---|
| Case/Control | P obs | OR (95%CI) | P adj | OR (95%CI) | ||||
| CAD | Population I | 1,249/841 | G/A | 0.277/0.237 | 3.23E-03 | 1.24 (1.07-1.43) | 8.04E-03 | 1.27 (1.06-1.51) |
| Population II | 1,260/833 | G/A | 0.300/0.261 | 6.87E-03 | 1.21 (1.05-1.39) | 1.98E-03 | 1.29 (1.10-1.52) | |
| Population III | 790/1,212 | G/A | 0.344/0.302 | 5.89E-03 | 1.21 (1.06-1.38) | 5.67E-03 | 1.21 (1.06-1.39) | |
| Combined | 3,299/2,886 | G/A | 0.302/0.269 | 1.84E-04 | 1.16 (1.07-1.26) | 2.18E-05 | 1.19 (1.10-1.29) | |
| Subgroups | Male | 2,145/1,822 | G/A | 0.306/0.273 | 1.43E-03 | 1.17 (1.06-1.29) | 4.71E-04 | 1.19 (1.08-1.32) |
| Female | 1,154/1,064 | G/A | 0.295/0.268 | 0.052 | 1.14 (0.99-1.30) | 1.76E-02 | 1.19 (1.03-1.36) | |
| MI | Population I | 568/841 | G/A | 0.276/0.237 | 1.71E-02 | 1.23 (1.04-1.46) | 2.87E-02 | 1.27 (1.03-1.58) |
| Population II | 609/833 | G/A | 0.305/0.261 | 1.51E-02 | 1.23 (1.04-1.44) | 9.60E-03 | 1.30 (1.07-1.58) | |
| Population III | 304/1,212 | G/A | 0.370/0.302 | 4.35E-04 | 1.40 (1.16-1.68) | 4.62E-04 | 1.40 (1.16-1.69) | |
| Combined | 1,481/2,886 | G/A | 0.306/0.269 | 2.25E-04 | 1.20 (1.09-1.32) | 5.43E-05 | 1.23 (1.11-1.36) | |
| Subgroups | Male | 1,036/1,822 | G/A | 0.295/0.273 | 8.12E-03 | 1.17 (1.04-1.32) | 4.60E-03 | 1.19 (1.06-1.34) |
| Female | 445/1,064 | G/A | 0.313/0.268 | 8.04E-03 | 1.26 (1.06-1.50) | 3.25E-03 | 1.33 (1.10-1.62) | |
Pobs: P value for association before adjusting for covariates by 2×2 contingence tables using PLINK version 1.07; Padj: P value for association after adjusting for covariates of hypertension, DM, smoking, alcohol intake, gender and age by multiple logistic regression analysis using SPSS version17.0; OR: odds ratio; 95% CI: 95% confidential interval.
For case control association studies, initial novel, significant association needs to be replicated in at least one other independent population. Therefore, we studied SNP rs3807989 in Population II and Population III. Statistical power analysis showed that Population II and Population III had a power of >80% and >90%, respectively, to replicate the association between SNP rs3807989 and CAD based on the OR and MAF from Population I.
SNP rs3807989 was genotyped in Populations II and III. Genotypic frequencies of rs3807989 were in Hardy-Weinberg equilibrium in the two control populations (Phwe=0.11 in Population II and Phwe=0.34 in Population III). Significant allelic association was identified between SNP rs3807989 and CAD in both Population II and Population III (Pobs=6.87×10−3, OR=1.21 for minor allele A in Population II; Pobs=5.89×10−3, OR=1.21 for minor allele A in Population III) (Table 2). After adjusting for significant covariates of hypertension, DM, smoking, alcohol drinking, gender and age, the association remained significant in both replication populations (Padj=1.98×10−3, OR=1.29 for minor allele A in Population II; Padj=5.67×10−3, OR=1.21 for minor allele A in Population III) (Table 2). These data validate the finding from the discovery population (I) that the minor allele A of SNP rs3807989 in CAV1 conferred a risk of CAD. The association remained significant after further adjustment for AF in Population II (Padj=1.24×10−3, OR=1.31 for minor allele A). No adjustment for AF was performed in Population III due to lack of clinical data on AF.
3.2. Significant allelic association between CAV1 SNP rs3807989 and MI
We also analyzed the association between rs3807989 and MI. There are 568, 609 and 304 MI patients in Population I, II and III, respectively. Significant allelic association was identified between SNP rs3807989 and MI in all three independent populations (Pobs=1.71×10−2, OR=1.23 for minor allele A in Population I; Pobs=1.51×10−2, OR=1.23 for minor allele A in Population II; Pobs=4.35×10−4, OR=1.40 for minor allele A in Population III) (Table 2). After adjusting for significant covariates of hypertension, DM, smoking, alcohol drinking, gender and age, the association remained significant (Padj=2.87×10−2, OR=1.27 for minor allele A in Population I; Padj=9.60×10−3, OR=1.30 for minor allele A in Population II; Padj=4.62×10−4, OR=1.40 for minor allele A in Population III) (Table 2). These data suggest that the minor allele A of SNP rs3807989 in CAV1 conferred a risk of MI. The association remained significant after further adjustment for AF in Population I (Padj=3.72×10−2, OR=1.27 for minor allele A) and II (Padj=8.13×10−3, OR=1.32 for minor allele A). No adjustment for AF was performed in Population III due to lack of clinical data on AF.
3.3. Significant allelic association of SNP rs3807989 with CAD and MI in the combined population
To further assess the association between SNP rs3807989 and CAD or MI, we combined the three populations together. This generated a large case control association study population for CAD (3,299 cases and 2,886 controls) and for MI (1,481 cases and 2,886 controls). The association between SNP rs3807989 and CAD or MI became much more significant before and after adjustment of covariates in the combined CAD population (Pobs=1.84×10−4, OR=1.16; Padj=2.18×10−5, OR=1.19) and in the combined MI population (Pobs=2.25×10−4, OR=1.20; Padj=5.43×10−5, OR=1.23) (Tables 2). When the study populations were divided into male groups and female groups, the association between rs3807989 and CAD and MI remained significant in both male populations and female populations (Table 2). The data from three independent populations and the combined population provided strong genetic evidence that the minor allele A of SNP rs3807989 conferred a risk of both CAD and MI.
The control study subjects are mostly individuals undergoing physical examinations offered free for the active working groups by the government agencies or individual's working units/institutions. The free physical examinations are not readily available for retired individuals (men, 60 years old or above; women, 50-55 years old or above). Therefore, the average age of the controls was younger than that of CAD/MI patients/cases (Table 1). To further minimize the confounding of age and sex, we generated case control populations for CAD and MI by matching each individual case to a control (Table 3). There are 2,187 cases and 2,187 controls matched for age and sex in the CAD population. Among 2,187 CAD cases, 958 study subjects were affected with MI, too (Table 3). The association between rs3807989 and CAD was highly significant (Padj=6.48×10−6, OR=1.24) (Table 3). When divided into a male group and a female group, the association remained significant in both male populations and female populations (Table 3). Similar findings were made for MI (Table 3).
Table 3.
Allelic association of SNP rs3807989 with CAD and MI in age- and sex-matched case-control populations.
| Study population | Sample size | Major/Risk Allele | MAF (case/control) | Before adjustment | After adjustment | |||
|---|---|---|---|---|---|---|---|---|
| Case/Control | P obs | OR (95%CI) | P adj | OR (95%CI) | ||||
| CAD | 2,187/2,187 | G/A | 0.309/0.265 | 6.39E-06 | 1.24 (1.13-1.36) | 6.48E-06 | 1.24 (1.13-1.36) | |
| Subgroups | Male | 1,505/1,505 | G/A | 0.311/0.268 | 2.21E-04 | 1.23 (1.10-1.38) | 2.23E-04 | 1.23 (1.10-1.38) |
| Female | 682/682 | G/A | 0.304/0.260 | 9.43E-03 | 1.25 (1.06-1.48) | 9.49E-03 | 1.25 (1.06-1.48) | |
| MI | 958/2,187 | G/A | 0.313/0.265 | 1.24E-04 | 1.26 (1.12-1.42) | 1.20E-04 | 1.26 (1.12-1.42) | |
| Subgroups | Male | 718/1,505 | G/A | 0.306/0.268 | 9.04E-03 | 1.20 (1.05-1.38) | 9.16E-03 | 1.20 (1.05-1.38) |
| Female | 240/682 | G/A | 0.333/0.260 | 1.93E-03 | 1.43 (1.14-1.79) | 1.67E-03 | 1.44 (1.15-1.80) | |
Pobs: P value for association before adjusting for covariates by 2×2 contingence tables using PLINK version 1.07; Padj: P value for association after adjusting for covariates of gender and age by multiple logistic regression analysis using SPSS version17.0; OR: odds ratio; 95% CI: 95% confidential interval.
3.4. Genotypic association between rs3807989 and serum lipids levels
Atherosclerosis is usually associated with lipid levels, thus, we analyzed potential association between SNP rs3807989 and lipid levels, including the plasma concentrations of total cholesterol (TC), LDL-C, HDL-C and triglyceride (TG). For association analysis with lipid levels, we excluded any study subject with potential use of satins and other lipid lowering medications both in cases and controls to rule out the interference of pharmacological interventions. The means ± SE serum LDL-C levels were 2.63 ± 0.06 mmol/L for the AA genotype, 2.68 ± 0.05 mmol/L with for the AG genotype, and 2.68 ± 0.08 mmol/L for the GG genotype (Table 3). We found that SNP rs3807989 was significantly associated with LDL-C levels under an additive model and a dominant model (Padj =0.03 and 0.02, respectively) (Table 4). Thus, the minor allele A of SNP rs1122608 was associated with a decreased level of LDL-C. No significant association was identified between SNP rs3807989 and TG, TC, or HDL-C.
Table 4.
Clinical and demographic characteristics of serum lipids levels
| Lipid | Case (2,592) | Control (707) | P value |
|---|---|---|---|
| Total cholesterol (TC, mmol/L) * | 4.32±0.02 | 4.56±0.07 | <0.001 |
| LDL-C (mmol/L) * | 2.65±0.04 | 3.25±0.14 | <0.001 |
| HDL (mmol/L) * | 1.16±0.03 | 1.49±0.23 | 0.02 |
| Triglyceride (TG, mmol/L) * | 1.69±0.03 | 1.56±0.05 | 0.02 |
For quantitative traits, data were shown as means±SE
3.5. Real-Time RT-PCR and ELISA analyses identified significant association between SNP rs3807989 and the expression level of CAV1, but not that of CAV2
SNP rs3807989 is located in the 2nd intron of the CAV1 gene, therefore it may be associated with the expression level of CAV1. We performed real-time qRT-PCR analysis with two sets of total RNA samples isolated from leukocytes: one with 83 study subjects and the other with 88 subjects. Real-time qRT-PCR analysis showed that the expression levels of the CAV1 mRNA were significantly different among carriers with the AA, AG, and GG genotypes in the first 83 subjects (P=0.003) (Fig. 1B). The expression levels of the CAV1 mRNA were significantly higher in carriers with AA genotype than those with AG or GG genotypes (Fig. 1B). In order to validate the finding, we performed real-time qRT-PCR analysis in another 88 subjects (Fig. 1C). The expression levels of the CAV1 mRNA were still significantly higher in carriers with the AA genotype than those with AG or GG genotypes (P=1.76×10−9) (Fig. 1C). When the two groups of study subjects were combined, significant association between SNP rs3807989 and the expression levels of CAV1 remained highly significant (P=8.00×10−13) (Fig. 1D).
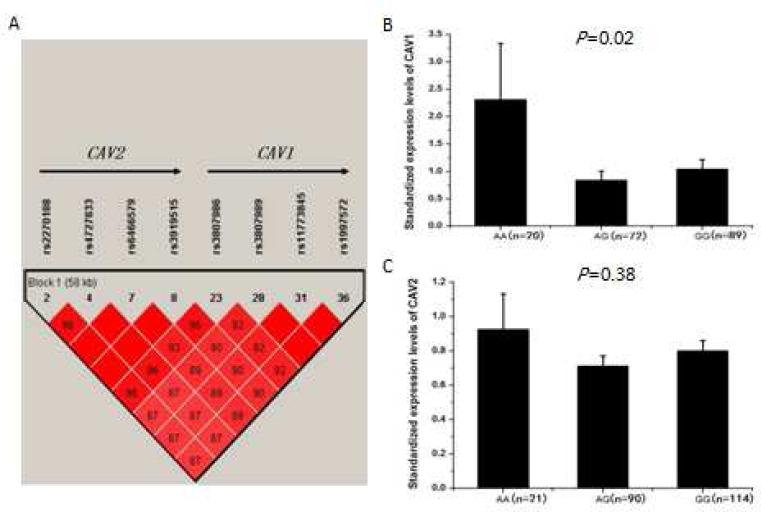
Further LD analysis revealed that CAV1 and CAV2 are located in the same LD block and rs3807989 can capture genomic information for both CAV1 and CAV2 (Fig. 2A). Therefore, it is important to determine whether tagSNP rs3807989 is also associated with the expression level of CAV2. We studied another independent group of study subjects with real-time qRT-PCR analysis. As shown in Fig. 2B, the expression levels of the CAV1 mRNA were, as expected, significantly different among carriers with the AA, AG, and GG genotypes (P=0.02). On the other hand, rs3807989 was not associated with the expression level of the CAV2 mRNA (P=0.38) (Fig. 2C).
Fig. 2.
Significant association of SNP rs3807989 with expression of CAV1 mRNA, but not with expression of CAV2. (A) Overview of LD of the 61.8 kb genomic region spanning both CAV1 and CAV2. The LD structure was computed as in Fig. 1A. CAV1 and CAV2 are in the same LD and rs3807989 captures all genomic information of the LD. (B, C) eQTL analysis of rs3807989 for both CAV1 and CAV2 located at the same LD block. Major allele G of rs3807989 was associated with decreased expression of CAV1 mRNA (B), but not with expression of CAV2 (C) under an additive model in a group of study subjects.
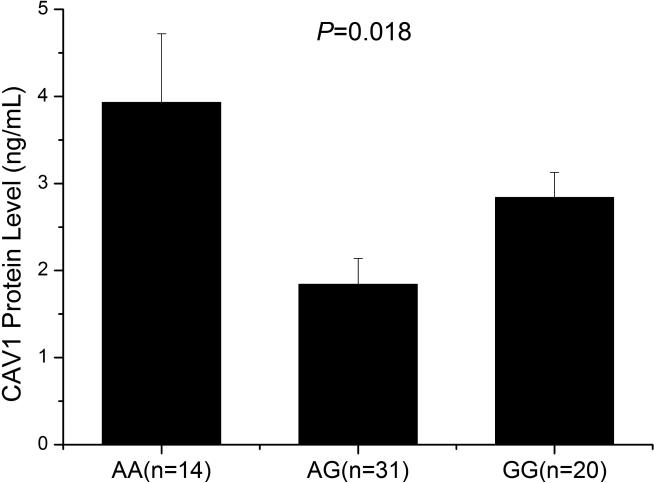
To further confirm the association between SNP rs3807989 and the expression level of CAV1, we also performed ELISA analysis to measure the expression level of the CAV1 protein with 65 human serum samples, including 14 carriers with the AA genotype, 31 carriers with the AG genotype and 20 carriers with the GG genotype. As shown in Fig. 3, the expression levels of the CAV1 protein were significantly different among carriers with the AA, AG, and GG genotypes (P=0.018).
Fig. 3.
Significant association of SNP rs3807989 with expression of the CAV1 protein in serum samples. ELISA analysis showed that major allele G of rs3807989 was associated with decreased expression of the CAV1 protein.
Together, these data suggests that SNP rs3807989 is significantly associated with the expression level of CAV1 but not that of CAV2.
4. Discussion
In this study, we identified significant association between tagSNP rs3807989 in intron 2 of the CAV1 gene and CAD as well as MI. The genetic evidence was particularly strong. The association between SNP rs3807989 and CAD was significant in all three independent populations and in the large combined population (3,299 cases and 2,886 controls). The association was significant before and after adjustment of significant covariates of CAD (Pobs=1.84×10−4, OR=1.16 for minor allele A; Padj=2.18×10−5, OR=1.19) (Table 2). Similar findings were made for MI (1,481 cases and 2,886 controls; Pobs=2.25×10−4, OR=1.20; Padj=5.43×10−5, OR=1.23) (Table 2). Minor allele A conferred a risk in development of CAD and MI, whereas the major allele G played a protective role. This is the first time that rs3807989 in the CAV1 gene is linked to CAD and MI in humans. To the best of our knowledge, no GWAS to date reported significant association between rs3807989 or other SNPs in CAV1 and CAD/MI. We also mined two GWAS databases for CAD and found that one SNP, rs193567, showed a nominal P value of 0.04 for association with CAD in a Wuhan population, but the association was not significant in a Beijing population (P=0.59).
We then tried to identify the underlying mechanism by which SNP rs3807989 increases risk of CAD. Because SNP rs3807989 is located in the 2nd intron of the CAV1 gene, we hypothesized that it affected the expression level of CAV1. We performed real-time qRT-PCR analysis in two groups of study subjects and found that the expression levels of the CAV1 mRNA were significantly higher in carriers with the AA genotype than those with AG or GG genotypes in two independent populations as well as in the combined population (Fig. 1B-1D). Further eQTL analysis showed that although CAV1 and CAV2 are located in the same LD block, rs3807989 was associated with the expression level of CAV1, but not CAV2 (Fig. 2B-2C). ELISA analysis also revealed significant association between rs3807989 and the expression level of the CAV1 protein (Fig. 3). These data indicate that the minor allele A of SNP rs3807989 was associated significantly with an increased expression level of CAV1. Therefore, it is likely that SNP rs3807989 increased expression of CAV1, which then leads to development of CAD and MI. The human findings were consistent with the observations made in Cav1-null mice. Frank et al [49] reported that Cav1−/− knockout mice showed a 70% decrease in atherosclerotic lesion area under ApoE−/− background. Engel et al [50] reported that Cav1−/−ApoE−/− mice showed a 15-fold reduction in plaque size and infiltration of fewer inflammation cells such as macrophages, T cells and neutrophils. Fernandez-Hernando et al [32] reported that Cav1−/− ApoE−/− mice showed inhibition of atherosclerosis, but re-expression of Cav1 in endothelial cells expanded atherosclerotic lesions. The same group later created transgenic mice that overexpress Cav1 in endothelial cells under ApoE−/− background and these mice showed increased atherosclerosis [34]. Together, all these human and mouse studies suggest that increased CAV1 expression increases risk of CAD, whereas decreased CAV1 expression or Cav1 deficiency inhibits development of CAD.
We also found that minor allele A of SNP rs3807989 was associated with a decrease of the LDL-C levels although the effect was very small. This result is consistent with the studies from Cav1−/− knockout mice with increased large, apoB-containing and remnant lipoproteins (VLDL-sized and IDL/LDL-sized fractions) when fed with Western diet [49]. This may be related to the role of caveolin-1 in transcytosis of LDL [50].
GWAS in populations of European ancestry found that SNP rs3807989 was significantly associated with atrial fibrillation (AF), however, controversial results were reported in Chinese replication studies [51, 52]. We have recently carried out additional replication studies and follow-up meta-analysis with 4 Asian populations and found a significant association between rs3807989 and AF (P=3.40×10−4, OR=0.81 for minor allele A or 1.24 for major allele G) [48]. However, in contrast to CAD and MI, the major allele G of rs3807989 is the risk allele for AF, whereas the minor allele A is a protective allele for AF. We speculate that the opposite effects of SNP rs3807989 on CAD/MI and AF may be related to the distinct roles of CAV1 in cardiomyocytes (AF) and endothelial cells (CAD and MI). In cardiomyocytes, caveolin-1 plays an important role in regulation of ionic currents by interacting with ion channels such as Kir2.1, KCNH2, HCN4, Nav1.8 and Nav1.5 [53-57]. Major allele G of rs3807989, which decreases expression of CAV1, may alters cardiac potassium or sodium currents, leading to development of AF. In contrast, Fernandez-Hernando et al [32, 34] showed that the role of Cav1 in atherosclerosis involves endothelial cells. Minor allele A of rs3807989, which increases expression of CAV1, may decrease endothelial cell proliferation and migration and nitric oxide production, leading to increased risk of CAD.
In conclusion, for the first time we identified the significant association between SNP rs3807989 in intron 2 of CAV1 and CAD and MI. We also demonstrated that minor allele A of rs3807989 was associated with an increased expression level of CAV1 (but not CAV2), thereby leading to development of CAD and MI. These data identifies a new candidate susceptibility gene for CAD and MI and further our understanding of genetic bases of CAD.
Table 5.
Genotypic association between rs3807989 and serum lipids levels under three different genetic models.
| Lipid | Subgroups | P adj | Effect | ||||
|---|---|---|---|---|---|---|---|
| Additive* | Recessive* | Dominant* | Additive* | Recessive* | Dominant* | ||
| Total cholesterol (TC, mmol/L) | Combined | 0.158 | 0.747 | 0.100 | −0.024 (−0.119 to 0.019) | −0.006 (−0.184 to 0.132) | −0.028 (−0.164 to 0.014) |
| Male | 0.597 | 0.625 | 0.338 | −0.012 (−0.119 to 0.068) | 0.011 (−0.161 to 0.267) | −0.021 (−0.179 to 0.062) | |
| Female | 0.067 | 0.146 | 0.118 | −0.053 (−0.188 to 0.006) | −0.042 (−0.388 to 0.058) | −0.045 (−0.228 to 0.026) | |
| LDL-C (mmol/L) | Combined | 0.030 | 0.289 | 0.027 | −0.038 (−0.280 to −0.014) | −0.018 (−0.469 to 0.140) | −0.039 (−0.366 to −0.022) |
| Male | 0.252 | 0.476 | 0.283 | −0.025 (−0.231 to 0.061) | −0.016 (−0.455 to 0.212) | −0.024 (−0.291 to 0.085) | |
| Female | 0.058 | 0.431 | 0.043 | −0.055 (−0.514 to 0.009) | −0.023 (− 0.841 to 0.359) | −0.059 (−0.693 to −0.011) | |
| HDL (mmol/L) | Combined | 0.804 | 0.318 | 0.376 | −0.004 (−0.192 to 0.149) | 0.017 (−0.192 to 0.590) | −0.016 (−0.321 to 0.121) |
| Male | 0.490 | 0.082 | 0.930 | 0.015 (−0.126 to 0.264) | 0.038 (−0.051 to 0.842) | −0.002 (−0.263 to 0.241) | |
| Female | 0.270 | 0.700 | 0.222 | −0.032 (−0.503 to 0.141) | −0.011 (−0.883 to 0.594) | −0.035 (−0.682 to 0.158) | |
| Triglyceride (TG, mmol/L) | Combined | 0.696 | 0.505 | 0.898 | −0.007 (−0.082 to 0.055) | −0.011 (−0.210 to 0.103) | −0.002 (−0.094 to 0.083) |
| Male | 0.672 | 0.653 | 0.770 | 0.009 (−0.072 to 0.112) | 0.010 (−0.162 to 0.259) | 0.006 (−0.101 to 0.136) | |
| Female | 0.138 | 0.041 | 0.439 | −0.042 (−0.171 to 0.024) | −0.058 (−0.455 to −0.010) | −0.022 (−0.177 to 0.077) | |
Padj, P value obtained from multiple linear modeling after adjustment for age, gender, hypertension, DM, smoking, and alcohol intake using SPSS v17.0; β, effect size related to the minor allele, using SPSS v17.0
Additive model = AA/AG/GG; recessive model = AA/AG+GG; dominant model = AA+AG/GG.
Highlight.
● Identification of a new susceptibility gene (CAV1) for CAD and MI;
● First demonstration of association between a genomic variant in CAV1 and CAD/MI;
● The minor allele A of CAV1 tagSNP rs3807989 increases risk of CAD and MI;
● Allele A of rs3807989 is associated with increased CAV1 expression, but not CAV2 expression.
Acknowledgments
We thank the study subjects for their participation and support of this study and all members of the GeneID team for help and assistance.
Sources of Funding
This study was supported by the China National Natural Science Foundation Key Program (31430047), Chinese National Basic Research Programs (973 Programs 2013CB531101 and 2012CB517801), Hubei Province's Outstanding Medical Academic Leader Program, Hubei Province Natural Science Key Program (2014CFA074), the China National Natural Science Foundation grant (91439129, NSFC-J1103514), NIH/NHLBI grants R01 HL121358 and R01 HL126729, Specialized Research Fund for the Doctoral Program of Higher Education from the Ministry of Education, and the “Innovative Development of New Drugs” Key Scientific Project (2011ZX09307-001-09).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests/disclosures
The authors have declared no conflict of interest specific to this article, however, a related project on functional characterization of the CAD gene ADTRP is funded by Bayer HealthCare.
Reference
- 1.Zhang XH, Lu ZL, Liu L. Coronary heart disease in China. Heart. 2008;94:1126–1131. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torpy JM, Burke AE, Glass RM. Coronary heart disease risk factors. JAMA. 2009;302:2388. doi: 10.1001/jama.302.21.2388. [DOI] [PubMed] [Google Scholar]
- 4.The Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. The New England journal of medicine. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helgadottir A, Thorleifsson G, Magnusson KP, Gretarsdottir S, Steinthorsdottir V, Manolescu A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 8.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 11.Myocardial Infarction Genetics Consortium. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 13.Coronary Artery Disease (C4D) Genetics Consortium A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 14.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Xu CQ, He Q, Cai JP, Li XC, Wang D, et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet. 2011;43:345–349. doi: 10.1038/ng.783. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Wang L, Chen S, He L, Yang X, Shi Y, et al. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet. 2012;44:890–894. doi: 10.1038/ng.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Yang Q, Xiong H, Wang L, Cai J, Wang F, et al. Candidate pathway-based genome-wide association studies identify novel associations of genomic variants in the complement system associated with coronary artery disease. Circ Cardiovasc Genet. 2014;7:887–894. doi: 10.1161/CIRCGENETICS.114.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assimes TL, Knowles JW, Basu A, Iribarren C, Southwick A, Tang H, et al. Susceptibility locus for clinical and subclinical coronary artery disease at chromosome 9p21 in the multi-ethnic ADVANCE study. Hum Mol Genet. 2008;17:2320–2328. doi: 10.1093/hmg/ddn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117:1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen GQ, Li L, Rao S, Abdullah KG, Ban JM, Lee BS, et al. Four SNPs on chromosome 9p21 in a South Korean population implicate a genetic locus that confers high cross-race risk for development of coronary artery disease. Arterioscler Thrombosis Vascular Biol. 2008;28:360–365. doi: 10.1161/ATVBAHA.107.157248. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Zhang X, He M, Cheng L, Chen Y, Hu FB, et al. Associations between single nucleotide polymorphisms on chromosome 9p21 and risk of coronary heart disease in Chinese Han population. Arterioscler Thrombosis Vascular Biol. 2008;28:2085–2089. doi: 10.1161/ATVBAHA.108.176065. [DOI] [PubMed] [Google Scholar]
- 23.Ye H, Hong Q, Li Y, Xu X, Huang YI, Xu L, et al. A lack of association between the rs12619285 polymorphism and coronary heart disease. Exp Ther Med. 2015;9:1309–1313. doi: 10.3892/etm.2015.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian J, Huang Y, Huang RS, Xu L, Le Y, Yang X, et al. Meta-analyses of four eosinophil related gene variants in coronary heart disease. J Thromb Thrombolysis. 2013;36:394–401. doi: 10.1007/s11239-012-0862-z. [DOI] [PubMed] [Google Scholar]
- 25.Guo CY, Gu Y, Li L, Jia EZ, Li CJ, Wang LS, et al. Association of SNP rs6903956 on chromosome 6p24.1 with angiographical characteristics of coronary atherosclerosis in a Chinese population. PLoS One. 2012;7:e43732. doi: 10.1371/journal.pone.0043732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang EW, Peng LY, Zheng JX, Wang D, Xu QY, Huang L, et al. Common Variants in Promoter of ADTRP Associate with Early-Onset Coronary Artery Disease in a Southern Han Chinese Population. PLoS One. 2015;10:e0137547. doi: 10.1371/journal.pone.0137547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peden JF, Farrall M. Thirty-five common variants for coronary artery disease: the fruits of much collaborative labour. Hum Mol Genet. 2011;20:R198–205. doi: 10.1093/hmg/ddr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watkins H, Farrall M. Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet. 2006;7:163–173. doi: 10.1038/nrg1805. [DOI] [PubMed] [Google Scholar]
- 29.Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: Implications for human disease. Cardiovasc Res. 2006;70:42–49. doi: 10.1016/j.cardiores.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 31.Pojoga LH, Underwood PC, Goodarzi MO, Williams JS, Adler GK, Jeunemaitre X, et al. Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab. 2011;96:E1288–1292. doi: 10.1210/jc.2010-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. American journal of physiology Cell physiology. 2008;295:C242–248. doi: 10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, Stefansdottir H, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–122. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 37.Pfeufer A, van Noord C, Marciante KD, Arking DE, Larson MG, Smith AV, et al. Genome-wide association study of PR interval. Nat Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42:1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubitz SA, Lunetta KL, Lin H, Arking DE, Trompet S, Li G, et al. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol. 2014;63:1200–1210. doi: 10.1016/j.jacc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi L, Li C, Wang C, Xia Y, Wu G, Wang F, et al. Assessment of association of rs2200733 on chromosome 4q25 with atrial fibrillation and ischemic stroke in a Chinese Han population. Hum Genet. 2009;126:843–849. doi: 10.1007/s00439-009-0737-3. [DOI] [PubMed] [Google Scholar]
- 42.Ren X, Xu C, Zhan C, Yang Y, Shi L, Wang F, et al. Identification of NPPA variants associated with atrial fibrillation in a Chinese GeneID population. Clin Chim Acta. 2010;411:481–485. doi: 10.1016/j.cca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Wang F, Wang B, Li X, Li C, Wang D, et al. Minor allele C of chromosome 1p32 single nucleotide polymorphism rs11206510 confers risk of ischemic stroke in the Chinese Han population. Stroke. 2010;41:1587–1592. doi: 10.1161/STROKEAHA.110.583096. [DOI] [PubMed] [Google Scholar]
- 44.Cheng X, Shi L, Nie S, Wang F, Li X, Xu C, et al. The same chromosome 9p21.3 locus is associated with type 2 diabetes and coronary artery disease in a Chinese Han population. Diabetes. 2011;60:680–684. doi: 10.2337/db10-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Wang F, Yang Y, Fu F, Xu C, Shi L, et al. Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum Genet. 2011;129:239–246. doi: 10.1007/s00439-010-0912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Huang Y, Yin D, Wang D, Xu C, Wang F, et al. Meta-analysis identifies robust association between SNP rs17465637 in MIA3 on chromosome 1q41 and coronary artery disease. Atherosclerosis. 2013;231:136–140. doi: 10.1016/j.atherosclerosis.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Xiong X, Xu C, Zhang Y, Li X, Wang B, Wang F, et al. BRG1 variant rs1122608 on chromosome 19p13.2 confers protection against stroke and regulates expression of pre-mRNA-splicing factor SFRS3. Hum Genet. 2014;133:499–508. doi: 10.1007/s00439-013-1389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Wang C, Wang X, Xu C, Wu M, Wang P, Tu X, Wang QK. Significant association between CAV1 Variant rs3807989 on 7p31 and atrial fibrillation in a Chinese Han population. J Am Heart Assoc. 4(2015) doi: 10.1161/JAHA.115.001980. doi: 10.1161/JAHA.115.001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 50.Engel D, Beckers L, Wijnands E, Seijkens T, Lievens D, Drechsler M, et al. Caveolin-1 deficiency decreases atherosclerosis by hampering leukocyte influx into the arterial wall and generating a regulatory T-cell response. FASEB J. 2011;25:3838–3848. doi: 10.1096/fj.11-183350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li G, Zhang R, Gao L, Zhang S, Dong Y, Yin X, et al. Lack of association between rs3807989 in cav1 and atrial fibrillation. Int J Clin Exp Pathol. 2014;(7):4339–4344. [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Ni B, Lin Y, Chen XG, Chen M, Hu Z, et al. The rs3807989 G/A polymorphism in CAV1 is associated with the risk of atrial fibrillation in Chinese Han populations. Pacing Clin Electrophysiol. 2015;(38):164–170. doi: 10.1111/pace.12494. [DOI] [PubMed] [Google Scholar]
- 53.Ambrosini E, Sicca F, Brignone MS, D'Adamo MC, Napolitano C, Servettini I, et al. Genetically-induced dysfunctions of Kir2. 1 channels: implications for short QT3 syndrome and autism-epilepsy phenotype. Hum Mol Genet. 2014;23:4875–4876. doi: 10.1093/hmg/ddu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J, Lin S, Choy PC, Shen X, Deng C, Kuang S, et al. The regulation of the cardiac potassium channel (HERG) by caveolin-1. Biochem Cell Biol. 2008;86:405–415. doi: 10.1139/o08-118. [DOI] [PubMed] [Google Scholar]
- 55.Barbuti A, Scavone A, Mazzocchi N, Terragni B, Baruscotti M, DiFrancesco D. A caveolin-binding domain in the HCN4 channels mediates functional interaction with caveolin proteins. J Mol Cell Cardiol. 2012;53:187–195. doi: 10.1016/j.yjmcc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Ohman E, Nilsson A, Madeira A, Sjögren B, Andrén PE, Svenningsson P. Use of surface plasmon resonance coupled with mass spectrometry reveals an interaction between the voltage-gated sodium channel type X α-subunit and caveolin-1. J Proteome Res. 2008;7:5333–5338. doi: 10.1021/pr800498t. [DOI] [PubMed] [Google Scholar]
- 57.Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E, et al. NaV1. 5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835–4842. doi: 10.1242/jcs.123901. [DOI] [PubMed] [Google Scholar]