Abstract
Background
Corneal allograft survival dramatically decreases in hosts with inflamed or vascularized recipient beds. We have previously shown that in rejected corneal allografts regulatory T cells (Tregs) demonstrate diminished Foxp3 expression and immunoregulatory function. Treatment with low doses of IL-2 selectively expands Tregs and has been proposed for the treatment of autoimmune diseases. In this study we investigated the effect of low-dose IL-2 administration on Treg function and corneal allograft survival.
Methods
Allogeneic corneal transplantation was performed on inflamed host beds. Low-dose systemic IL-2 was administered starting three days before grafting until six weeks after transplantation. Frequencies of Tregs as well as their immunosuppressive function and antigen specificity were assessed using flow cytometry, in vitro proliferation assays and adoptive transfer experiments. Frequencies of effector T cells (Teff) and graft infiltrating immune cells were measured at 2 weeks post-transplantation. Long-term allograft survival was evaluated for up to 9 weeks using Kaplan-Meier survival analysis.
Results
Treatment with low-dose IL-2 significantly increased frequencies of CD4+CD25+Foxp3+ Tregs and their immunosuppressive function. It also suppressed alloimmune response as shown by the decreased CD4+IFNγ+T cell frequencies and graft infiltration of CD45+ and CD4+ cells. Clinical evaluation of the grafts showed significant improvement in long-term corneal allograft survival in the IL-2 treated group compared to controls.
Conclusions
Our study is the first to report that treatment with low-dose IL-2 increases survival of corneal allografts. We propose that IL-2-mediated Treg expansion can be an effective tool to prevent alloimmunity and to improve long-term allograft survival in transplantation.
Introduction
Corneal transplantation is the most commonly performed tissue transplantation worldwide.1 Despite very high (above 90%) survival rates in recipients with non-vascularized and non-inflamed graft beds, survival rates significantly decline (under 50%) when transplants are placed onto vascularized or inflamed host beds associated with conditions such as prior graft rejection, infections or trauma.2 These results are seen despite treatment with high doses of non-specific immunosuppressive medications, which often do not promote long-term survival and may have severe side effects such as glaucoma, cataracts and opportunistic infections.1,2 Therefore, new strategies are required to modulate the immune system without conventional immunosuppressive agents and improve transplant survival in ‘high-risk’ patients with inflamed host beds.
Regulatory T cells (Tregs) are key modulators of the immune response. Unlike broad-acting immunosuppressive regimens, Tregs display antigen-specific and long-lasting effects,3 therefore, several Treg-centered therapies have been introduced, such as in vivo expansion of Tregs by administration of low-dose interleukin (IL)-2.4 Tregs (but not effector T cells) constantly express CD25, the α subunit of the IL-2 receptor (IL-2Rα), which dramatically increases the affinity of IL-2 to bind its receptor.5 Thus, low concentrations of IL-2 selectively activate Tregs, whereas high doses will stimulate Tregs as well as effector T cells (Teffs) and natural killer cells.6
Interleukin-2 regulates the development and survival of Tregs,7-9 and maintains Treg suppressive function by promoting Foxp3 expression and subsequent production of immunoregulatory molecules.10 Administration of low-dose IL-2 has been shown to be a promising approach to treat autoimmune conditions such as chronic refractory graft-versus-host-disease (GVHD),11 hepatitis C virus-induced vasculitis12 and type 1 diabetes13 by restoring Treg homeostasis and promoting the induction of tolerance. Furthermore, in experimental models of transplantation, low-doses of IL-2 have been combined with rapamycin or IL-2 mAb (JES6-1) to increase survival of skin and islet cell allografts.14,15 In this study, we demonstrate that systemic administration of low-dose IL-2 alone promotes Treg frequencies and function, reduces activation of T cells and their infiltration into grafts, and thus increases long-term allograft survival in corneal transplantation, the most common form of transplantation performed worldwide.
Materials and Methods
Animals
Eight- to 10-week-old male BALB/c (H-2d), C57BL/6 (H-2b) and C3H (H-2k) mice were obtained from Charles River Laboratories, Wilmington, MA. Animals were kept in a pathogen-free environment at the Schepens Eye Research Institute Animal Care Facility. Animals were treated in accordance with the Schepens Eye Research Institute Animal Care and Use Committee and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research. Mice were anesthetized for surgical procedures using intraperitoneal (i.p.) injections of 120 mg/kg Ketamine and 20 mg/kg Xylazine.
Orthotopic corneal transplantation onto inflamed graft beds
Corneal transplantation was performed using C57BL/6 (B6) corneal grafts and BALB/c hosts with inflamed graft beds as described previously.16 Briefly, three interrupted 8-shaped sutures (11–0 nylon, 50μm diameter needle; Sharpoint; Angiotech, Vancouver, BC, Canada) were placed in the corneas of BALB/c hosts to induce inflamed and neovascularized graft beds 14 days before transplantation. The central cornea of donor B6 mice was marked with a 2 mm diameter trephine and excised with Vannas scissors (Storz Instruments Company, San Damis, CA) and transplanted into a similarly prepared host bed of 1.5 mm diameter on the right eye of recipient (BALB/c) mice with 8 interrupted 11-0 nylon sutures (Sharpoint; Vanguard, Houston, TX).17 Eyelids were closed immediately after the surgery using 8-0 sutures and kept closed for two days. Corneal sutures were removed 7 days post-transplantation.
Evaluation of graft survival
Transplanted corneas were examined weekly in a blinded fashion for 9 weeks (or until sacrificed for analyses) using a slit-lamp microscope. A standardized opacity grading system was used.17 Grafts with opacity scores of >2 (i.e. a level of opacity that obscures recognition of iris details) for at least two consecutive weeks at week 2 post-transplantation and onwards were considered as immune-rejected (SDC Figure 1). Eyes that underwent complications during or after surgery including intraoperative hemorrhage, cataract, infection, or synechia as well as grafts that became opaque in the first two weeks after transplantation and never became clear were excluded from the analysis.
Interleukin-2 administration
Interleukin-2 (1μg/25g mouse body weight; Biolegend, San Diego, CA) diluted in 100 μl sterile normal saline was administered by intraperitoneal (i.p.) injection in transplant recipients once/day starting three days before transplantation until one week after transplantation. Further injections were continued twice/week up to six weeks post-surgery. The control group received 100 μl i.p. injection of sterile saline.
Cornea digestion and lymph node cell preparation
Single cell suspensions were prepared from corneal tissues and draining submandibular and cervical lymph nodes two weeks after transplantation as previously described.18 In brief, corneas were digested in RPMI media (Lonza, Walkersville, MD) containing 2 mg/ml collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 2 mg/ml DNase I (Roche, Basel, Switzerland) for 60 min at 37°C, and then filtered through a 70-μm cell strainer. Draining lymph nodes (DLNs) in the cervical and submandibular regions were collected and a single cell suspension was prepared.
Flow cytometry
All cell suspensions were incubated with an Fc receptor blocking antibody (R&D Systems, Minneapolis, MN) before they were stained with the following antibodies: anti-CD45 (30-F11), anti-CD25 (PC61.5), anti-Foxp3 (FJK-16s), and anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) (UC10-4B9) from eBioscience (San Diego, CA); and anti-CD4 (RM4-5) and anti-IFN-γ (XMG1.2) from Biolegend (San Diego, CA). In order to stain intracellular IFN-γ expression, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma-Aldrich) and Ionomycin (500 ng/ml; Sigma-Aldrich) for 6 hours in the presence of Golgistop (0.7 μl/100 μl media; BD Biosciences, San Jose, CA). For intracellular staining of IFN-γ and intranuclear staining of Foxp3, cells were fixed and permeabilized with appropriate buffers (eBioscience). Isotype controls were used for all antibodies. Cells were analyzed using the LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ) and Summit v4.3 Software (DAKO Colorado Inc., Fort Collins, CO).
Cell sorting
CD4+CD25+ suppressor cells and CD4+CD25− responder cells were isolated either by magnetic separation using the Treg isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany), or by flow sorting (MoFlo™ XDP, Beckman Coulter, United States) from lymph nodes of naïve and transplanted BALB/c mice. Purity of isolated Tregs was > 90% as measured by flow cytometry. Antigen presenting cells (APCs) were obtained by depleting T cells (CD90.2+ cells) from splenocytes of C56BL/6 mice using a CD90.2 isolation kit (Miltenyi Biotec).
Treg suppression assay
CD4+CD25+ Tregs (5 × 104) from IL-2 treated and saline injected BALB/c hosts and CD4+CD25− responder cells (1 × 105) from naïve BALB/c mice were isolated from the DLNs using magnetic separation, then co-cultured for 5 days in the presence of 1 × 105 APCs (isolated by magnetic sorting) from B6 or C3H splenocytes, the latter to provide unrelated (third party) antigen. It was determined through our preliminary studies that Teff to Treg ratio of 2 to 1 would result in 50% reduction in T cell proliferation; therefore, we used same numbers of Teffs and Tregs in our experiments (SDC Figure 2). Proliferation of responder T cells was measured using the BrdU incorporation assay (Millipore, Billerica, MA).
Real Time-PCR
Using Trizol (Ambion, Life Technologies, Grand Island, NY) tissues were homogenized on ice and RNA was precipitated in the aqueous phase using 70% ethanol, followed by subsequent extraction and purification using RNeasy Micro kit (Qiagen, Valencia, CA, USA). Reverse transcription of total RNA was conducted using Superscript III kit (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using TaqMan Universal PCR Mastermix (Applied Biosystems, Foster City, CA, USA) and preformulated primers for IL-10 (Mm00439614_m1), Foxp3 (Mm00475162_m1), IFNγ (Mm01168134_m1) and GAPDH (Mm99999915_gl). The PCR conditions were 40 cycles at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, followed by final extension at 72 °C for 10 min. The results were analyzed by the comparative threshold cycle method and normalized by GAPDH as an internal control.
Adoptive transfer of Tregs
Tregs were isolated using flow sorting from the DLNs of IL-2 and saline injected graft recipients at three weeks after transplantation and were transfused i.v. (80,000 cells per mouse) to four different groups of allograft recipients at 18 hours post-surgery. The time point for Treg adoptive transfer was decided according to our previous observation that considerable numbers of graft-derived APCs were detected in the draining LNs of recipients at 24h post-transplantation.19 Allograft survival rate in each group (n= 6 per group) was monitored up to 5 weeks post-transplantation.
Statistical analyses
Student's t test was used to compare two groups. P values <0.05 were considered statistically significant. The Kaplan-Meier survival curve was used to determine graft survival and Wilcoxon Rank test was used to compare survival rates between the groups.
Results
IL-2 treatment increases the frequencies and function of regulatory T cells
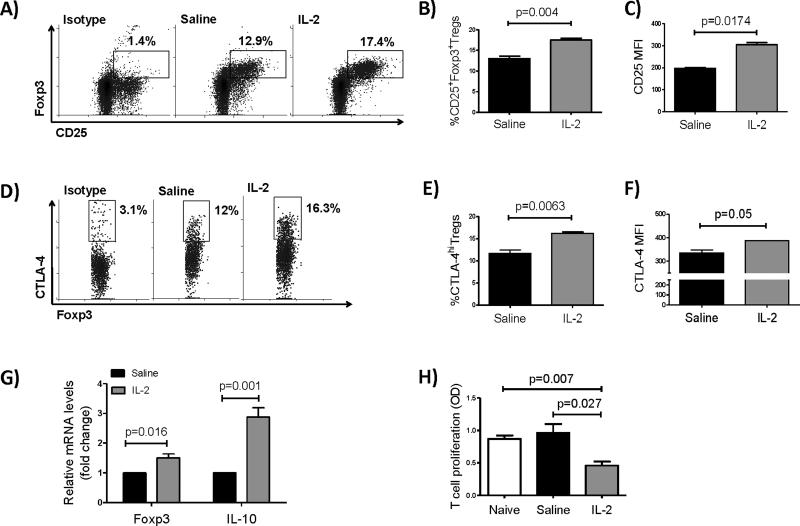
Previous studies in our lab have demonstrated reduced immunosuppressive function of Tregs in hosts rejecting their corneal allografts, reflected by decreased expression of Foxp3, IL-10 and TGF-β, as well as a diminished anti-proliferative potential of Tregs.20 We observed that systemic treatment with 1μg/day of IL-2 significantly increased frequencies of CD25+Foxp3+ Tregs among CD4+ cells, as well as Treg expression of CD25 and CTLA-4 in the DLNs of graft recipients at day 7 post-transplantation (Figure 1A-F). Levels of mRNA for IL-10 and Foxp3 also showed significant increases in the IL-2 treated group compared to controls (Figure 1G). To assess the suppressive capacity of expanded Tregs in vitro, we performed a T cell proliferation assay at day 7 after transplantation. CD4+CD25− T cells from lymph nodes of naive BALB/c mice were sorted and co-cultured with CD4+CD25+Tregs (from IL-2 treated recipients, saline injected mice, or naïve controls) and APCs from the spleen of a B6 mouse. Five days after culturing, T cell proliferation was significantly decreased when CD4+CD25− T cells were co-cultured with Tregs from IL-2 treated recipients compared to either saline injected or naïve controls, indicative of an increase in Treg suppressive function after IL-2 treatment (Figure 1H).
Figure 1. Low-dose IL-2 treatment expands CD4+CD25+Foxp3+ regulatory T cells and increases their immunosuppressive function.
Corneal transplantation onto inflamed graft beds was performed and low doses of IL-2 (1μg/25g mouse body weight) or saline (control) were administered. Draining lymph nodes were analyzed at day 7 post-transplantation using flow cytometry. (A) Representative dot plots of saline treated and IL-2 treated recipients showing frequencies of CD25+Foxp3+Tregs among CD4+ cells. (B, C) Bar graphs showing mean ± standard deviation (SD) of the frequencies of CD4+CD25+Foxp3+ Tregs and CD25 MFI in CD4+CD25+ Tregs, respectively (frequencies are measured among total CD4+ lymph node cells). (D) Representative dot plots of saline treated and IL-2 treated recipients showing frequencies of CTLA-4hi Tregs among CD4+CD25+Foxp3+T cells. (E, F) Bar graphs showing mean ± SD of CTLA-4+ Tregs and CTLA-4 MFI among CD25+CTLA-4hi Tregs, respectively (frequencies are measured among total CD4+CD25+ lymph node cells). (G) Relative changes in mRNA levels of IL-10 and Foxp3 in DLNs. (H) Treg suppression assay showing proliferation of naïve CD4+CD25−T cells in the presence of CD4+CD25+Tregs isolated from IL-2 treated vs. saline injected mice or naïve controls (N=5 mice/group).
IL-2 treatment decreases IFN-γ+ effector T cell frequencies and leukocyte infiltration of grafts
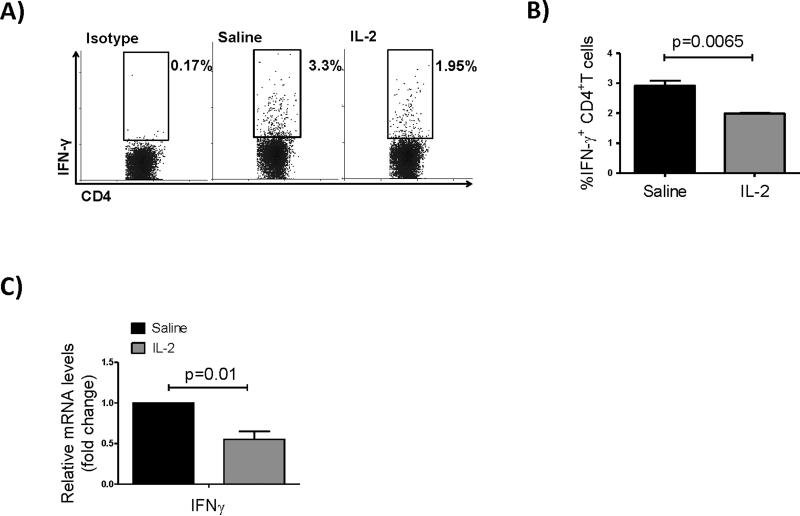
IFNγ-producing activated T cells are known as the principal mediators of alloimmunity and corneal allograft rejection.21,22 Therefore, we measured frequencies of CD4+IFN-γ+ Teffs in DLNs of graft recipients 2 weeks post-transplantation. Flow cytometric analysis showed a marked decrease in Teffs in the IL-2 treated group compared to controls (Figure 2A, B). Quantification of mRNA expression levels for IFNγ in the DLNs also showed significant reduction in IL-2 treated hosts compared to controls (Figure 2C).
Figure 2. Low-dose IL-2 treatment decreases IFN-γ+ effector T cell frequencies.
(A) Representative flow dot plots showing IFN-γ expressing CD4+ T cells in the draining lymph nodes of saline treated (control) and IL-2 treated recipients at 2 weeks post-transplantation. (B) Bar graph showing mean ± SD frequencies of IFN-γ+ CD4 T cells (frequencies are measured among total CD4+ lymph node cells). (C) Bar graphs showing relative changes in mRNA levels of IFNγ in DLNs (N=4 mice/group).
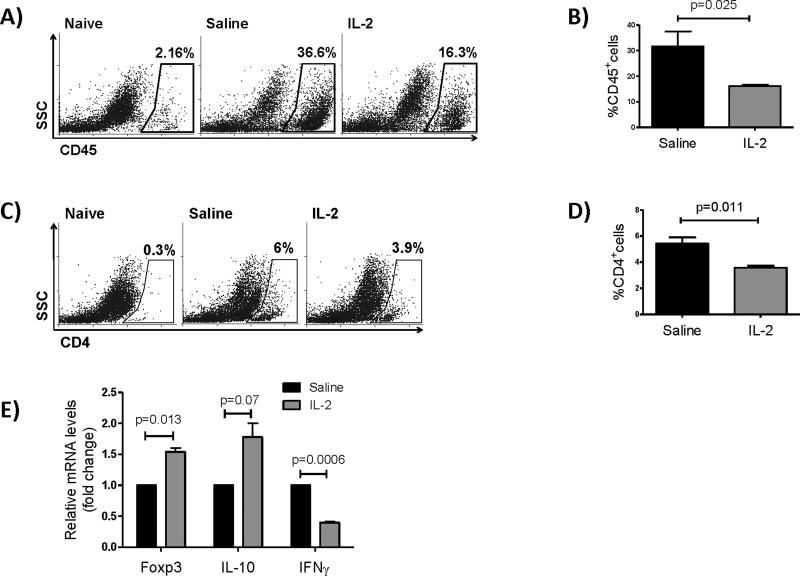
Inflammatory cells home to the graft site via the efferent arm through blood vessels where they can exert their damage. In order to determine the effect of IL-2 on leukocytic infiltration of allografts, we examined CD45+ leukocyte and CD4+ T cell infiltration in grafted corneas using flow cytometry. The frequencies of CD45+ cells were significantly diminished in corneas of IL-2 treated mice (48% reduction from 31.64% to 16.14%) 2 weeks after transplantation indicating suppressed corneal inflammation after treatment with IL-2 (Figure 3A, B). Frequencies of CD4+ T cells infiltrating the corneas were also significantly reduced in grafts of IL-2 treated mice (34% reduction from 5.4% to 3.5%) (Figure 3C, D). In addition, RT-PCR analysis of the corneal tissue revealed significant increases in IL-10 and Foxp3 and a significant decrease in IFNγ mRNA levels, suggesting the predominance of immunoregulatory vs. inflammatory cytokine production by the cells infiltrating the graft in IL-2 treated group compared to controls (Figure 3E).
Figure 3. Low-dose IL-2 treatment decreases corneal infiltration of leukocytes.
Two weeks post-transplantation corneas of IL-2–treated and control mice (naïve BALB/c mice without transplantation and saline-treated mice) were collected and analyzed for their expression of CD45 and CD4. (A, B) Representative flow dot plots and bar graphs showing frequencies of CD45+ leukocytes. (C, D) Representative flow dot plots and bar graphs showing frequencies of CD4+ T cells. (E) Relative changes in mRNA levels of IL-10, Foxp3 and IFNγ in corneal tissues at two weeks after transplantation (Bar graphs show mean ± SD, N=4 mice/group, data represents results of three independent experiments).
IL-2 treatment increases long-term corneal allograft survival in recipients with inflamed host beds
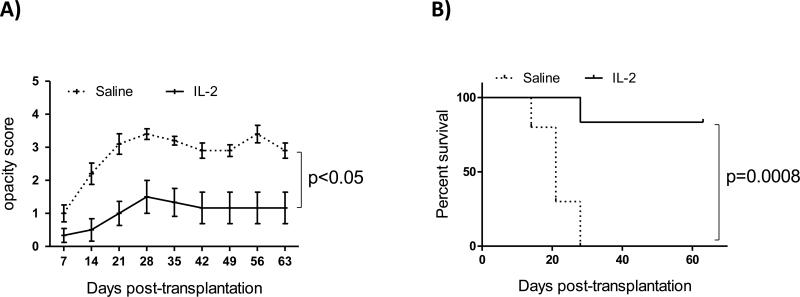
To evaluate the effect of IL-2 on long-term allograft survival, recipient mice were treated with IL-2 for 6 weeks and grafts were examined weekly up to 9 weeks. Graft opacity scores were significantly decreased in the IL-2 treated group compared to controls (Figure 4A, p<0.05 at all the time points). Similar to previous reports from our lab on the survival of high risk corneal transplantation,23 all the grafts in the control group were rejected by week 4 post-transplantation, whereas in the IL-2 treated group 83% of the grafts survived. To determine the long-term effect of IL-2 on graft survival, we stopped the treatment at week 6 and assessed graft survival for 3 additional weeks. Accepted corneas remained clear until the end of the follow-up at day 63 post-transplantation despite discontinuation of IL-2 treatment more than three weeks earlier (Fig 4B, p=0.0008).
Figure 4. Low-dose IL-2 treatment decreases graft opacity and increases corneal allograft survival.
Corneal transplantation (N=6-10/group) was performed onto inflamed graft beds and low doses of IL-2 (1μg/25g mouse body weight) or saline (control) were administered once daily starting three days before transplantation up to one week after transplantation; IL-2 (or saline) treatment was continued twice/week until 8 weeks post-transplantation. (A) Graft opacity scores were significantly decreased at all the time points (p<0.05); error bars show standard error. (B) Weekly examination of grafts for nine weeks post-transplantation revealed a significant increase in graft survival in IL-2 treated mice compared to controls (83% vs. 0%, p=0.0008), data represents result of an individual experiment.
IL-2 expanded Tregs show antigen-specific immunoregulatory function in vitro and in vivo
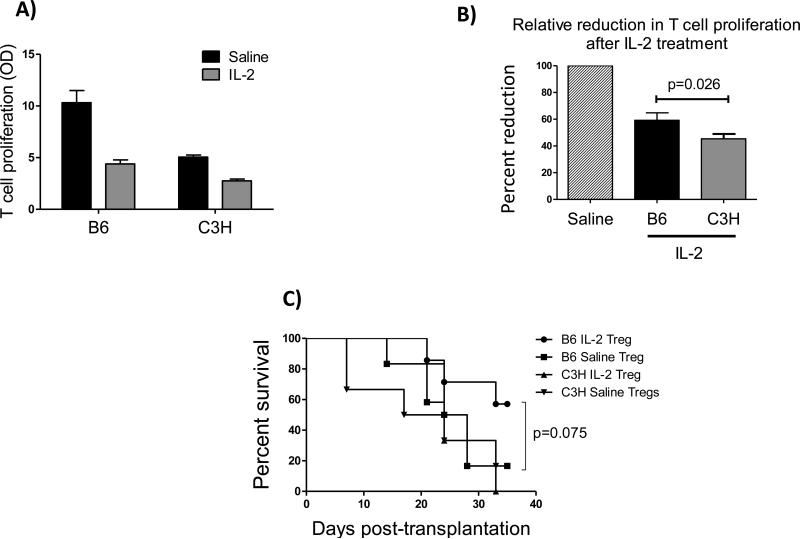
To determine antigen specificity of expanded Tregs in vitro, we performed Treg suppression assays in the presence of either B6 or C3H APCs at two weeks post-transplantation. Using flow sorting, CD4+CD25+ Tregs from DLNs of IL-2 and saline injected mice were isolated and co-cultured with CD4+CD25− responder T cells from naïve BALB/c mice (50,000 Tregs and 100,000 Teff per well). APCs were isolated from spleens of B6 and C3H mice through magnetic sorting and added to the culture (100,000 cells per well). T cell proliferation assay five days after the co-culture showed a 15% increase in Treg suppressive activity in the IL-2 treated group compared to saline controls, when responder T cells were stimulated by B6 APCs compared to C3H APCs (63% vs. 48%, p=0.026 ), demonstrating antigen specific activity of expanded Tregs using low-dose IL-2 (Figure 5A, B).
Figure 5. IL-2 expanded Tregs show allo-antigen specificity in vitro and in vivo.
(A, B) Treg suppression assay showing proliferation of naïve CD4+CD25−T cells in the presence of CD4+CD25+Tregs isolated from IL-2 treated vs. saline injected mice in the presence of B6 (donor type) or C3H (third party) APCs (N=5 mice/group), data represents results of two independent experiments. (C) Adoptive transfer of Tregs from IL-2 or saline injected BALB/c recipients of B6 donors to a new set of hosts (N=7/group). Tregs were isolated using flow sorting at 3 weeks post-transplantation; 80,000 Tregs per recipient were i.v. transferred to two different groups of hosts (BALB/c mice receiving transplants from either B6 or C3H donors) 18 hour post-surgery. Graft survival was monitored up to 5 weeks post-transplantation, which revealed an increase survival when hosts were injected with Tregs from IL-2 treated group with prior encounter to the same donor antigen (n = 6-7/group, Kaplan-Meier survival analysis).
To study whether Tregs from IL-2 treated mice are capable of inducing immune tolerance, we adoptively transferred these Tregs to a new set of BALB/c hosts that received transplants from either B6 or C3H donors. Our results showed a marked increase in survival of grafts from B6 donors when transfused with Tregs from IL-2 treated mice compared to saline injected controls (57% vs. 16.6%, p=0.075), whereas in recipients of C3H corneas, adoptive transfer of Tregs from either saline or IL-2 treated mice did not increase allograft survival (16% and 0%, respectively) (Figure 5C).
Discussion
Allogeneic corneal transplants performed on inflamed and vascularized host beds are rejected at high rates due to swift allosensitization of the recipients by graft APCs.24 Primed Teffs are then recruited to the graft site where they exert their damage.25 Tregs play a modulatory role in the alloimmune response through a wide array of regulatory mechanisms, such as induction of apoptosis in Teffs by IL-2 deprivation, or inactivating them through production of granzymes, perforins, and immunoregulatory cytokines. Tregs can also indirectly inhibit T cell activation by aggregating around APCs and preventing them to bind to Teffs. In addition, Tregs down-regulate expression of costimulatory molecules in APCs (CD80/CD86) as well as their expression of indoleamine 2,3-dioxygenase (IDO) via CTLA-4 ligation, all of which render APCs tolerogenic and less effective in sensitizing T cells.26-28 We have previously shown that in rejected corneal allografts host Tregs show decreased immunomodulatory function reflected in their decreased levels of Foxp3 expression, immunoregulatory cytokine production, and in vitro suppressive capacity.20 Therfore, expanding the Treg pool and increasing their tolerogenic potential is an attractive approach for increasing transplant survival in graft recipients that are at high risk for rejection. In this study, we demonstrate that low-dose IL-2 increases long-term corneal allograft survival in recipients with inflamed host beds by expanding Tregs and inhibiting Teff activation.
Interleukin-2 was first identified as a potent growth factor for T cells,29 and administration of IL-2 has been used to stimulate Teffs to treat metastatic melanoma, renal cell carcinoma, and patients with HIV;30,31 however, often with suboptimal results in part because of simultaneous expansion of Tregs.30 Further studies have shown that IL-2 or IL-2Rα deficient animals die of lymphoproliferative disease that is preventable by adoptive transfer of Tregs, clarifying the critical role of IL-2 in the generation and function of Tregs.32 Although all recently activated T cells transiently express IL-2Rα (CD25), regulatory T cells are the major responders to low concentrations of IL-2 due to their constant and high expression of CD25.6 Therefore, it has been suggested that lower doses of this cytokine can selectively expand Tregs without concurrently stimulating Teffs.36
Previous studies on Treg therapy to promote transplant survival have suggested that administration of Tregs prior to transplantation is more efficient than their introduction after transplantation, as there is rapid invasion of the grafted tissue by Teffs followed by a delayed arrival of Tregs, which would be ineffective to control tissue damage.4 In addition, based on the available literature on the use of low dose IL-2 for in vivo expansion of Tregs, 15,36 and our preliminary observations that three systemic injections of low-dose IL-2 (once/day) induced significant Treg expansion (SDC Figure 3), we decided to start IL-2 injections three days before transplantation. It is also reported that Treg numbers go down to baseline levels at around two weeks after discontinuation of the treatment.15 Therefore, we decided to continue IL-2 injections to maintain effective prevention of T cell priming. Also, IL-2 injections were performed more frequently during the first week after transplantation, the period of time that host sensitization mainly occurs in high-risk corneal allografts.31 We decreased frequencies to twice a week from week two until six weeks post-transplantation, a time point by which all high-risk corneal allografts are rejected. 23,34
Reports of low-dose IL-2 therapy in other animal models of transplantation have been promising. In a study by Webster et al. in diabetic mice, 80% of islet cell allografts survived when mice were treated with three i.p. injections of IL-2 combined with murine anti-IL-2 antibody (JES6-1).15 The JES6-1 mAb binds to IL-2 and increases its half-life, while facilitating its binding to IL-2Rα on Tregs.35 In the same study the authors reported that administration of similar amounts of IL-2 alone did not improve graft survival,15 suggesting that higher doses of IL-2, when used without the antibody, are required to induce immune tolerance in islet cell allografts. In mouse models of skin transplantation, IL-2 treatment increased graft survival in single MHC class II disparate models,36 however, when both CD4+ and CD8+ T cell alloreactivity were present (with both MHC class I and class II disparities), IL-2 mediated Treg expansion was not sufficient to prolong allograft survival. These results are in accord with our previous studies, in which we showed CD4+ T cells (and not CD8+ T cells) are principal mediators of allograft rejection in corneal grafts, as opposed to skin transplants where both CD4+ and CD8+ simultaneously contribute to allograft rejection.37 In corneal transplantation, combination of IL-2 and rapamycin was shown to be effective in reduction of graft inflammation very early after grafting; however, no long-term survival data on the allografts were reported, and the immunomodulatory mechanisms of IL-2 therapy alone in transplantation were not studied.38 In a more recent study in skin transplantation, combination of IL-2 and rapamycin showed a significant increase in allograft survival.14 In humans, two recent clinical trials have reported significant therapeutic benefits of low-dose IL-2 in bone marrow transplanted patients with GVHD that received immunosuppressive drugs such as glucocorticoids and rapamycin.11,39 The efficacy of IL-2 treatment was also demonstrated in mouse models of GVHD when rapamycin was added to destroy alloreactive Teffs, particularly in the acute phase of the disease.40,41 In our study, however, we demonstrate significant improvement of corneal allograft survival through administration of IL-2 alone, which could be explained by the relatively low immunogenicity of corneal allografts compared to pancreatic, skin and bone marrow transplants that contain large numbers of MHC-IIhi antigen presenting cells, making these grafts more prone to rejection.
In conclusion, this is the first report to show that low-dose IL-2 treatment alone effectively enhances corneal allograft survival through expansion of Tregs. We also show for the first time that Tregs from IL-2 treated hosts demonstrate alloantigen specific activity and are capable of inducing long-term allograft tolerance. Our previous work has shown that the inflammatory microenvironment in vascularized graft host beds, which constitutes close to a quarter of clinical cases of corneal transplantation, promotes accelerated T cell sensitization with blunted immune regulatory responses.23 Our current data suggest that enhancing Treg frequencies and their immunosuppressive function using low-dose IL-2 can largely counterbalance this inflammatory microenvironment, and thus be a potentially effective strategy for improving long-term alloimmune tolerance in hosts at high-risk of rejecting their corneal transplants.
Supplementary Material
Acknowledgments
This study was funded by NIH Grant EY12963. The authors would like to thank Dr. Susanne Eiglmeier (Schepens Eye Research Institute) for her editorial assistance and helpful discussions regarding the project, and Dr. Jing Hua (Schepens Eye Research Institute) for her valuable input.
Abbreviations
- APC
antigen presenting cell
- DLN
draining lymph node
- GVHD
graft-versus-host disease
- IL-2Rα
interleukin-2 receptor α (CD25)
- i.p.
intraperitoneal
- i.v.
intravenous
- mAb
monoclonal antibody
- PMA
phorbol 12-myristate 13-acetate
- Teff
effector T cell
- Treg
regulatory T cell
Footnotes
Disclosure: The authors have no financial conflicts of interest.
- Maryam Tahvildari: Research design, performance of the research, data analysis and writing the paper.
- Masahiro Omoto: Performance of the research, data analysis.
- Yihe Chen: Performance of the research.
- Parisa Emami-Naeini: Performance of the research.
- Takenori Inomata: Performance of the research.
- Thomas H. Dohlman: Performance of the research.
- Abigail E. Kaye: Performance of the research.
- Sunil K. Chauhan: Research design, data analysis and writing the paper.
- Reza Dana: Research design, data analysis and writing the paper.
References
- 1.Streilein JW, Yamada J, Dana MR, Ksander BR. Anterior chamber-associated immune deviation, ocular immune privilege, and orthotopic corneal allografts. Transplantation proceedings. 1999 May;31(3):1472–1475. doi: 10.1016/s0041-1345(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 2.Qazi Y, Hamrah P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. Journal of clinical & cellular immunology. 2013 Nov 20;2013(Suppl 9) doi: 10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May 30;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012 Feb;4(1):11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005 Nov 18;310(5751):1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 6.Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 7.Barron L, Dooms H, Hoyer KK, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. Journal of immunology. 2010 Dec 1;185(11):6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005 Nov;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 9.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nature reviews. Immunology. 2004 Sep;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 10.Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Annals of the New York Academy of Sciences. 2013 Apr;1283:8–12. doi: 10.1111/nyas.12011. [DOI] [PubMed] [Google Scholar]
- 11.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. The New England journal of medicine. 2011 Dec 1;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. The New England journal of medicine. 2011 Dec 1;365(22):2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 13.Hartemann A, Bensimon G, Payan CA, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. The lancet. Diabetes & endocrinology. 2013 Dec;1(4):295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 14.Pilon CB, Petillon S, Naserian S, et al. Administration of Low Doses of IL-2 Combined to Rapamycin Promotes Allogeneic Skin Graft Survival in Mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Dec;14(12):2874–2882. doi: 10.1111/ajt.12944. [DOI] [PubMed] [Google Scholar]
- 15.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009 Apr 13;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Investigative ophthalmology & visual science. 1996 Nov;37(12):2485–2494. [PubMed] [Google Scholar]
- 17.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992 Oct;54(4):694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. Journal of immunology. 2007 Sep 15;179(6):3672–3679. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. The Journal of experimental medicine. 2002 Jan 21;195(2):259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. Journal of immunology. 2009 Jan 1;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000 Sep;19(5):625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Cunnusamy K, Niederkorn JY. IFN-gamma blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Dec;13(12):3076–3084. doi: 10.1111/ajt.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohlman TH, Omoto M, Hua J, et al. VEGF-trap Aflibercept Significantly Improves Long-term Graft Survival in High-risk Corneal Transplantation. Transplantation. 2015 Apr;99(4):678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 24.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. Journal of immunology. 2004 Oct 1;173(7):4464–4469. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 25.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. International ophthalmology. 2008 Jun;28(3):209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May 30;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunology. 2007 Dec;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 28.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007 Nov 22;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 29.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA : the journal of the American Medical Association. 1994 Mar 23-30;271(12):907–913. [PubMed] [Google Scholar]
- 31.Abrams D, Levy Y, Losso MH, et al. Interleukin-2 therapy in patients with HIV infection. The New England journal of medicine. 2009 Oct 15;361(16):1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson BH. IL-2, regulatory T cells, and tolerance. Journal of immunology. 2004 Apr 1;172(7):3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 33.Bayer AL, Pugliese A, Malek TR. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunologic research. 2013 Dec;57(1-3):197–209. doi: 10.1007/s12026-013-8452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boisgerault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009 Jan 15;87(1):16–23. doi: 10.1097/TP.0b013e318191b38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006 Mar 31;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 36.Vokaer B, Charbonnier LM, Lemaitre PH, Le Moine A. Impact of interleukin-2-expanded regulatory T cells in various allogeneic combinations on mouse skin graft survival. Transplantation proceedings. 2012 Nov;44(9):2840–2844. doi: 10.1016/j.transproceed.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001 Aug 15;167(4):1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wang W, Xu J, Le Q. Effect of rapamycin and interleukin-2 on regulatory CD4+CD25+Foxp3+ T cells in mice after allogenic corneal transplantation. Transplant Proc. 2013 Mar;45(2):528–537. doi: 10.1016/j.transproceed.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014 Apr 15;20(8):2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perol L, Martin GH, Maury S, Cohen JL, Piaggio E. Potential limitations of IL-2 administration for the treatment of experimental acute graft-versus-host disease. Immunol Lett. Dec. 2014;162(2 Pt B):173–184. doi: 10.1016/j.imlet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011 Aug 25;118(8):2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.