Abstract
Accurate identification of breast cancer patients most likely to benefit from adjuvant systemic therapies is crucial. Better understanding of differences between methods can lead to an improved ER, PgR, and HER-2 assessment. The purpose of this preplanned translational research is to investigate the correlation of central IHC/FISH assessments with microarray mRNA readouts of ER, PgR, and HER-2 status in the MINDACT trial and to determine if any discordance could be attributed to intratumoral heterogeneity or the DCIS and normal tissue components in the specimens. MINDACT is an international, prospective, randomized, phase III trial investigating the clinical utility of MammaPrint in selecting patients with early breast cancer for adjuvant chemotherapy (n = 6694 patients). Gene-expression data were obtained by TargetPrint; IHC and/or FISH were assessed centrally (n = 5788; 86 %). Macroscopic and microscopic evaluation of centrally submitted FFPE blocks identified 1427 cases for which the very same sample was submitted for gene-expression analysis. TargetPrint ER had a positive agreement of 98 %, and a negative agreement of 95 % with central pathology. Corresponding figures for PgR were 85 and 94 % and for HER-2 72 and 99 %. Agreement of mRNA versus central protein was not different when the same or a different portion of the tumor tissue was analyzed or when DCIS and/or normal tissue was included in the sample subjected to mRNA assays. This is the first large analysis to assess the discordance rate between protein and mRNA analysis of breast cancer markers, and to look into intratumoral heterogeneity, DCIS, or normal tissue components as a potential cause of discordance. The observed difference between mRNA and protein assessment for PgR and HER-2 needs further research; the present analysis does not support intratumoral heterogeneity or the DCIS and normal tissue components being likely causes of the discordance.
Keywords: Breast cancer, ER, PgR, HER2, Concordance, Tumor heterogeneity, Hormone receptor, IHC/FISH, Gene expression, TargetPrint
Introduction
Accurate identification of breast cancer patients most likely to benefit from adjuvant systemic therapies is crucial. Estrogen receptor (ER) and progesterone receptor (PgR) content in the primary tumor of patients with early-stage invasive breast cancer are powerful predictors of response to adjuvant endocrine therapies. It is recommended that endocrine receptors be measured in all primary breast cancer specimens, and endocrine expression is the primary basis for selection of adjuvant systemic therapy [1, 2]. HER-2 positivity in breast cancer is a prognostic factor of tumor aggressiveness and a predictive factor for response to anti HER-2 treatment. Early and accurate HER-2 testing of all breast cancer patients at primary diagnosis is essential for optimal disease management [3].
ASCO/CAP recommendations for optimal ER, PgR, and HER-2 assessments describe the laboratory testing requirements for testing, and serve as the golden standard for current early breast cancer patient diagnostics [3, 4].
Other methods for assessing ER, PgR, and HER-2 such as mRNA microarray and RT-PCR expression have been commercially available for quite some time and are being acknowledged as potential reliable future diagnostic adjuncts depending on clinical utility studies [3–5].
Several large studies are available comparing mRNA versus pathology and generally reporting overall good concordance; these studies, however, do not address the likely causes of the discordances encountered [6, 7].
Better understanding of differences between methods can lead to an improved ER, PgR, and HER-2 assessment. mRNA-based assays could potentially serve as an adjunct tool for ER, PgR, and HER-2 assessment.
A previous analysis of a subset of the MINDACT population (n = 619) indicated that local pathology results for ER, PgR, and HER-2 were in overall good to high concordance with central pathology, confirming the high quality of stratification in MINDACT [8]. The current pre-planned analysis of the complete MINDACT dataset allows for the unique opportunity of looking into the concordance rate and the possible causes of any discordance between mRNA-based and IHC/FISH assessments of hormone receptors and HER2.
MINDACT is an international, prospective, randomized, phase III trial investigating the clinical utility of MammaPrint® versus standard clinicopathological criteria (Adjuvant!Online) to select patients with early breast cancer for adjuvant chemotherapy. Central assessment of the main standard histopathological features of the tumors were performed as well as mRNA expression analysis of ER, PgR, and HER-2, providing a unique opportunity to look at concordance and discordance between these methodologies and look into potential causes of any discordances found.
The aim of this pre-planned translational research was to evaluate the agreement of central IHC/FISH assessments versus microarray mRNA readouts by TargetPrint (commercially available microarray-based test) of ER, PgR, and HER-2, and to assess whether any differences between mRNA and central pathology could be due to intratumoral heterogeneity or by the DCIS and/or normal tissue components in the samples undergone mRNA assays.
Methods
Patients
Female patients (N = 6694) with histologically proven operable invasive breast cancer and 0–3 positive lymph nodes were enrolled in MINDACT between February 2007 and July 2011 [9, 10]. Clinical Trials number: NCT00433589. The protocol was approved by independent ethics committees and medical authorities. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
Tumor samples
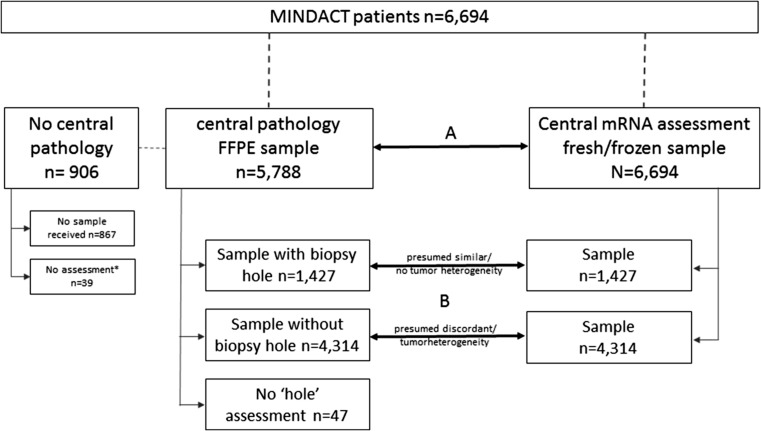
Prior to enrollment for randomization and stratification, local pathology assessment of hormone receptor and HER-2 status was determined, and a frozen core biopsy (3–6 mm) of the surgical tumor sample was sent to Agendia NV (Amsterdam, The Netherlands) for MammaPrint and TargetPrint analyses. For translational research, a representative diagnostic paraffin tissue block of each tumor was sent from each participating center to the European Institute of Oncology (EIO), (Milan, Italy) for central pathology re-assessment (Fig. 1). TargetPrint readout was available for all 6694 patients. Central pathology results were unavailable for 867 patients because the sample had not been submitted for central assessment. Among the 5788 patients with central pathology results, 39 had incomplete data (1 for ER, 7 for PgR, 32 for HER-2) and 12 equivocal HER-2 IHC and FISH (Fig. 2).
Fig. 1.
Sample schedule for translational preplanned analysis, with the 2 comparative analyses indicated: A The agreement of central IHC/FISH assessments versus microarray mRNA readouts by TargetPrint® of ER, PgR, and HER-2 and B Sub-study to assess whether the differences between mRNA and central pathology could be caused by tumor heterogeneity
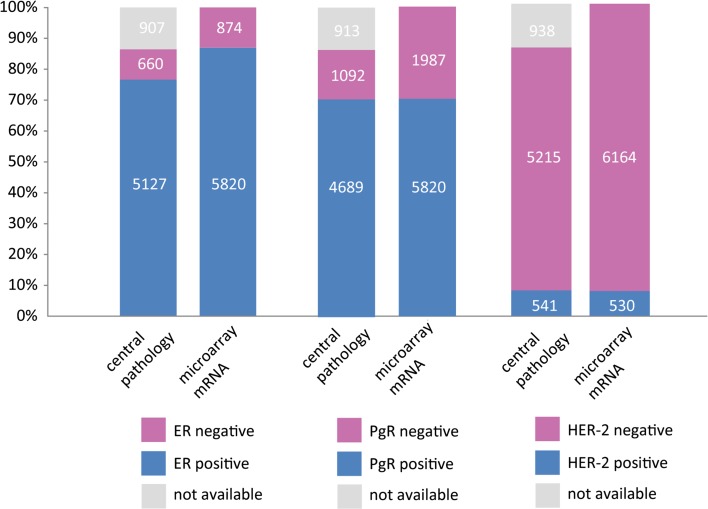
Fig. 2.
Data overview and sample availability
ER, PgR, and HER-2 assessment
Gene-expression data and central laboratory assessments were assessed as described previously [8].
mRNA and IHC/FISH discordancy analysis
To investigate tumor heterogeneity, we assessed whether the same tumor sample submitted to the the central pathology laboratory was also used for the gene-expression analysis. When a core biopsy punch hole was found in the blocks submitted for central pathology, it was assumed that the very same samples underwent central assessment and TargetPrint assay. In absence of such a hole, the assessments were assumed not to have been performed on the same block, but on a different part of the tumor. The 6694 FFPE (H&E) slides were assessed for presence or absence of a core biopsy punch hole in the tumor sample (Fig. 3).
Fig. 3.
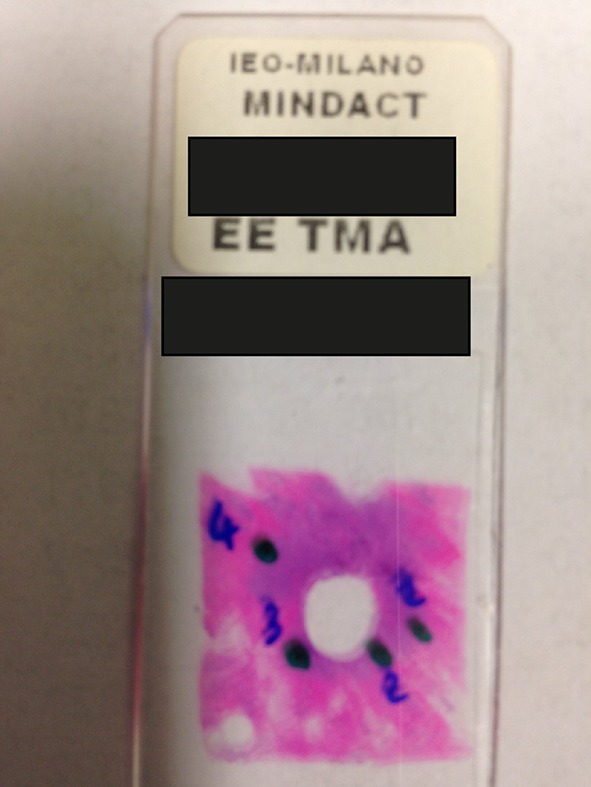
H&E-stained section with a core biopsy punch hole caused by the MammaPrint biopsy device provided in the sample kit
Additional retrospective investigations were carried out to investigate whether any differences between mRNA assays and central pathology for ER and HER-2 could be explained by the DCIS and/or normal tissue components in the biopsy sample submitted for TargetPrint analysis. To this purpose, 141 blinded scanned H&E sections of the frozen samples submitted for TargetPrint analyses were reviewed by the central pathology laboratory, and the extent of DCIS and normal tissue was semi-quantitatively assessed as a percent of the whole section area. These cases were selected as follows: 31 ER-negative cases by central pathology assessment and ER-positive by TargetPrint analysis; 30 randomly selected control samples ER-negative both by central IHC and TargetPrint analysis; 50 HER-2-negative cases by central IHC/FISH and HER-2-positive by TargetPrint analysis; and30 randomly selected control samples HER-2-negative both by central IHC/FISH and TargetPrint. The selection was performed by the trial statistician with no interference from the pathologist and molecular biologists.
Statistical analysis
Only the independent statistician of the EORTC had simultaneous access to both clinical and genomic data and performed the correlation analysis. Statistical calculations were conducted using SAS® 9.2 (SAS Institute Inc., Cary, USA). This pre-planned translational research investigated 2 comparisons A and B, depicted in Fig. 1. Analysis (A) investigated the agreement of central IHC/FISH assessments versus microarray mRNA readouts by TargetPrint. The statistics included positive and negative agreement [11]; positive (PPV) and negative (NPV) predictive value; percentage of concordance; and Cohen’s κ coefficient [12]. And secondly (B), using Fisher’s exact test for association, we investigated whether the differences between mRNA and central pathology could be caused by tumor heterogeneity or by the occurrence of DCIS and/or normal tissue.
Results
Correlation central pathology and TargetPrint for ER, PgR, and HER-2 (A)
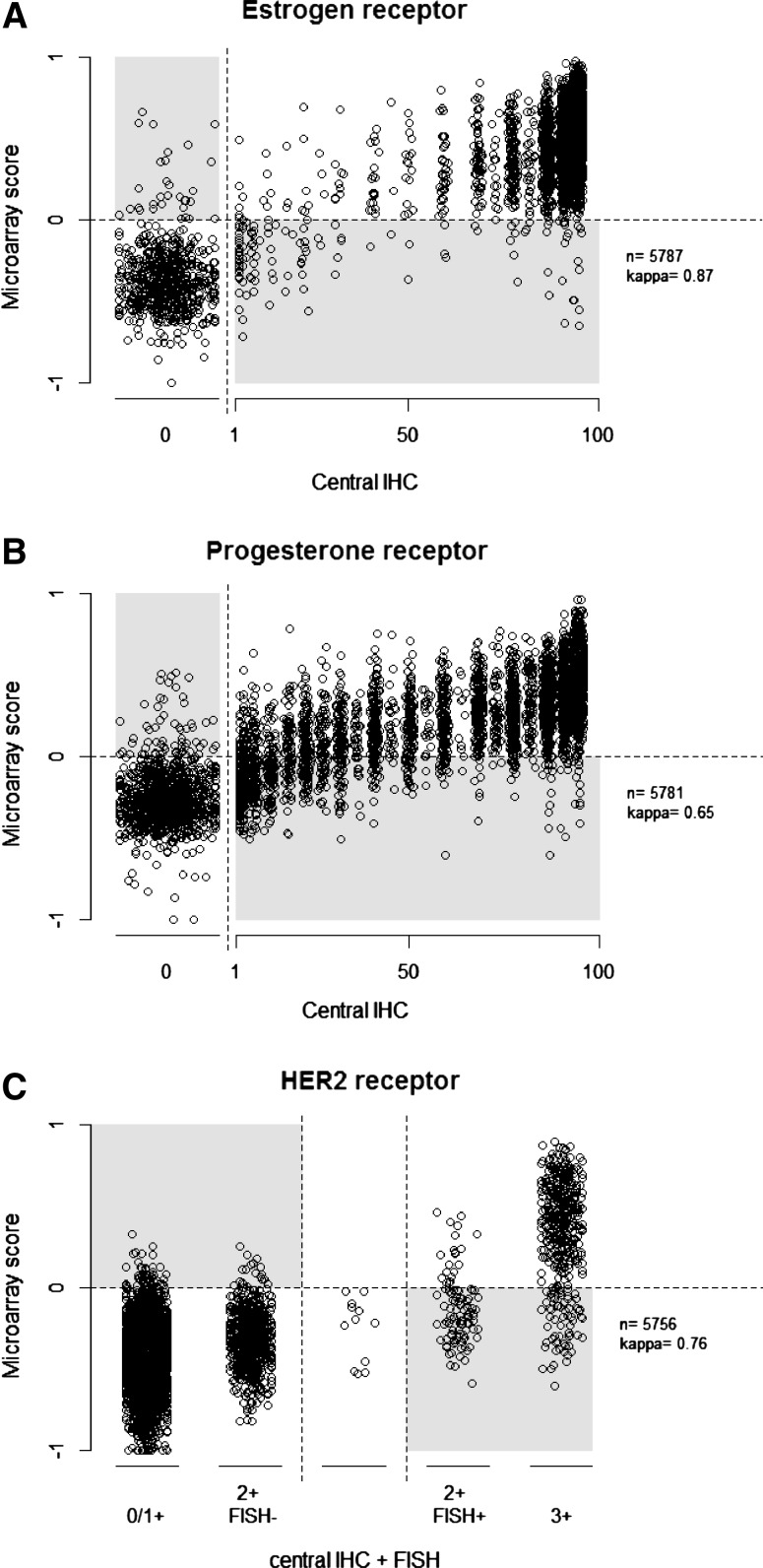
Figures 4A through 4C show the central pathology assessments for ER, PgR, and HER-2 as integer percentages and for HER-2 as five categories. The TargetPrint results are mRNA expression scores on a continuous scale. For ER and PgR, most discordances were seen in the lower ranges of expression.
Fig. 4.
A–C Comparative depiction of mRNA score on a continuous scale for TargetPrint versus central pathology assessment as integer percentage for ER (A), PgR (B), and as five categories for HER-2 (C). Random trimmed noise was added to the data points to increase visibility
Comparison of central assessment with TargetPrint (Table 1) indicated a highly similar overall performance, with a concordance of 97 % (κ = 0.87) for ER, 96 % for HER-2 (κ = 0.76), and a slightly lower concordance for PgR (87 %; κ = 0.65).
Table 1.
Agreement of central pathology versus mRNA Agreement statistics of central pathology assessment versus TargetPrint, including 2 IHC cut-offs for ER and PgR (1 and 10 %)
| Concordance (95 % CI) | Kappa (95 % CI) | Positive agreement | Negative agreement | PPV | NPV | Sample size | |
|---|---|---|---|---|---|---|---|
| ER (1 %) | 97.3 % (96.9–97.7) | 0.874 (0.855–0.894) | 97.6 (5002/5127) | 95.3 (629/660) | 99.4 (5002/5033) | 83.4 (629/754) | 5787 |
| ER (10 %) | 98.1 % (97.7–98.4) | 0.913 (0.897–0.929) | 98.6 (4993/5065) | 94.5 (682/722) | 99.2 (4993/5033) | 90.5 (682/754) | 5787 |
| PgR (1 %) | 86.9 % (86.0–87.7) | 0.649 (0.626–0.671) | 85.3 (3998/4689) | 93.8 (1024/1092) | 98.3 (3998/4066) | 59.7 (1024/1715) | 5781 |
| PgR (10 %) | 90.4 % (89.7–91.2) | 0.761 (0.743–0.780) | 90.8 (3908/4303) | 89.3 (1320/1478) | 96.1 (3908/4066) | 77.0 (1320/1715) | 5781 |
| HER-2 | 96.1 % (95.6–96.6) | 0.757 (0.726–0.788) | 72.3 (391/541) | 98.6 (5142/5215) | 84.3 (391/464) | 97.2 (5142/5292) | 5756 |
For ER, the positive agreement was 98 % and the negative agreement was 95 % with a PPV of 99.4 and an NPV of 83.4. The positive agreement for PgR was 85 % and the negative agreement was 94 %. PPV was 98.3 and the NPV 59.7. For HER2, the positive agreement for TargetPrint was 72 %, with a PPV of 84.3, and an NPVof 97.2.
Also provided in Table 2 are the agreement statistics for ER and PgR by central pathology using 10 % invasive tumor cells as cut-off instead of 1 % compared with TargetPrint. For both receptors, the agreement increases significantly when using the 10 % cut-off for the IHC results, reaching a concordance of 98 % (κ = 0.91) for ER and of 90 % for PgR (κ = 0.76).
Table 2.
Tumor heterogeneity analysis of central pathology versus mRNA
| Concordance (95 % CI) | Kappa (95 % CI) | Positive agreement | Negative agreement | PPV | NPV | Sample size | |
|---|---|---|---|---|---|---|---|
| ER Hole | 97.0 % (96.1–97.9) | 0.864 (0.825–0.904) | 97.4 (1217/1250) | 94.1 (160/170) | 99.2 (1217/1227) | 82.9 (160/193) | 1420 |
| ER No hole | 97.5 % (97.0–97.9) | 0.880 (0.858–0.903) | 97.7 (3673/3761) | 95.8 (459/479) | 99.5 (3673/3693) | 83.9 (459/547) | 4240 |
| PgR Hole | 87.8 % (86.0–89.4) | 0.666 (0.621–0.710) | 86.5 (996/1151) | 92.9 (250/269) | 98.1 (996/1015) | 61.7 (250/405) | 1420 |
| PgR No hole | 86.8 % (85.7–87.8) | 0.647 (0.621–0.673) | 85.1 (2919/3432) | 94.0 (754/802) | 98.4 (2919/2967) | 59.5 (754/1267) | 4234 |
| HER-2 Hole | 95.8 % (94.8–96.9) | 0.764 (0.706–0.822) | 73.6 (109/148) | 98.4 (1245/1265) | 84.5 (109/129) | 97.0 (1245/1284) | 1413 |
| HER-2 No hole | 96.2 % (95.6–96.8) | 0.756 (0.720–0.792) | 71.8 (280/390) | 98.7 (3775/3826) | 84.6 (280/331) | 97.2 (3775/3885) | 4216 |
Agreement statistics for central pathology assessments versus TargetPrint, for central samples with a biopsy hole and thus assumed to have no tumor heterogeneity in the sample sent for mRNA analysis, and samples without such a hole and thus assumed to be heterogeneous from the mRNA sample
Analyses of discordant mRNA and IHC/FISH results (B)
Table 2 shows how the agreement statistics for samples with a hole versus samples without such a hole are very similar for all measures of agreement.
Fisher’s exact test shows that the extent of DCIS and normal tissue in the sample analyzed for TargetPrint analysis is not associated with the discordant result for ER (Table 3). For HER-2 however, the test shows a greater extent of DCIS and/or normal cells for TargetPrint HER-2-negative and IHC/FISH HER-2-negative compared to TargetPrint HER-2-positive and IHC/FISH HER-2-negative (Table 4). However, given that the sample size is rather small this could be a chance finding.
Table 3.
DCIS and/or normal tissue component analysis for ER
| ER | TargetPrint negative n = 30 | TargetPrint positive n = 31 | Total n = 61 | Fisher exact test p value |
|---|---|---|---|---|
| % DCIS or normal cells | ||||
| 0 | 19 (63 %) | 15 (48 %) | 34 (56 %) | 0.30 |
| 1–30 | 7 (23 %) | 13 (42 %) | 20 (33 %) | |
| 31–60 | 2 (7 %) | 3 (10 %) | 5 (8 %) | |
| >60 | 1 (3 %) | 0 (0 %) | 1 (2 %) | |
| No slide found | 1 (3 %) | 0 (0 %) | 1 (2 %) |
Fisher’s exact test for the association of DCIS and/or normal tissue components and ER discordant rates, comparing 30 cases both negative by TargetPrint and central assessment with 31 cases positive by TargetPrint and negative at central assessment
Table 4.
DCIS and/or normal tissue component analysis for HER-2
| HER-2 | TargetPrint negative n = 30 | TargetPrint positive n = 50 | Total n = 80 | Fisher exact test p value |
|---|---|---|---|---|
| % DCIS or normal cells | ||||
| 0 | 17 (57 %) | 40 (80 %) | 57 (71 %) | 0.0067 |
| 1–30 | 11 (37 %) | 10 (20 %) | 21 (26 %) | |
| 31–60 | 1 (3 %) | 0 (0 %) | 1 (1 %) | |
| >60 | 0 (0 %) | 0 (0 %) | 0 (0 %) |
Fisher’s exact test for the association of DCIS and/or normal tissue components and HER-2 discordant rate, comparing 30 cases both negative by TargetPrint and central assessment with 50 cases positive by TargetPrint and negative at central assessment
Discussion
The current study confirms the previously reported high level of concordance between microarray TargetPrint readout and central pathology analyses for ER, with lower negative agreement for PgR and lower positive agreement for HER-2. These results confirm that mRNA assessment of ER by TargetPrint may be a reliable adjunct to IHC assessment [8].The lower agreement for PgR has been reported by several others [7, 13, 14]. Whether mRNA PgR assessment is indeed more strongly associated with clinical outcome compared with IHC PgR assessment as suggested is a hypothesis to be tested when MINDACT outcome data will become available [15]. The positive agreement for HER-2 mRNA analysis in the current series is only 72 %, and in line with previously reported figures for mRNA analysis [16, 17].
The agreement for ER and PgR between central pathology and TargetPrint results increases significantly using 10 % invasive tumor cells as an IHC cut-off instead of 1 %, reaching a concordance rate of 98 % (κ = 0.91) for ER and of 90 % for PgR (κ = 0.76). This may be explained by the TargetPrint originally having been trained on the 10 % cut-off, although none of the training samples had a score between 1 and 10 % [5]. Whether this improvement in concordance will also lead to improved endocrine responsiveness will need to be tested, since there are also indications for these samples having basal-like features implying reduced endocrine responsiveness and increased sensitivity to chemotherapy [18].
The likely causes of the discordant results between mRNA readout and pathology assessment of ER, PgR, and HER-2 have not been elucidated thus far. Suggested possible causes such as intratumoral heterogeneity, DCIS, and/or normal tissue components have not yet been analyzed in a randomized patient cohort. The MINDACT trial is a prospectively designed study to determine the clinical utility of MammaPrint in selecting patients with early breast cancer for adjuvant chemotherapy and enrolled 6694 patients. The extensive sample availability per patient in the central pathology laboratory, together with whole genome mRNA expression analysis provides for a unique comparative dataset able to address these issues: Could tumor heterogeneity or the DCIS and normal tissue components be responsible for the discordant results when different areas of the tumor are analyzed by IHC and mRNA assays? Because the same level of agreement for mRNA versus central pathology protein assessment was obtained when the very same tissue samples or different blocks were analyzed by the 2 methods, it is concluded that tumor heterogeneity cannot justify the reported discordant results. Similarly, the observed differences between mRNA and protein assessment for hormone receptors and HER-2 cannot be justified by the extent of DCIS and normal tissue.
In conclusion, in this large randomized multicenter pan-European trial setting with central pathology and mRNA assessments available for 86 % of patients, TargetPrint mRNA assessment shows high concordance with central assessment of ER, but lower concordance rates for PgR and HER-2. This means that mRNA assessment of ER by TargetPrint can be a reliable adjunct to IHC assessment, but for PgR and HER-2 the stand of care remains IHC assessment.
The current analysis indicates that tumor heterogeneity and extent of DCIS and normal tissue components are not the likely causes of any differences between mRNA and protein assessment. This insight may ultimately lead to further research to determine what biological differences are being detected by the two methods.
Since MINDACT outcome data are expected in 2016, the clinical implications for differences between the two assessments for PgR and HER-2 can be tested.
Acknowledgments
We are grateful to all women participating in this study, all the investigators, surgeons, pathologists, and research nurses, the National Coordinating Centers/BIG Groups (BOOG, CaCTUS, CEEOG, FNCLCC, GOIRC, IBCSG, SAKK, SOLTI, WSG), and World Courier. This trial has funding grants from the European Commission Framework Programme VI (FP6-LSHC-CT-2004-503426), the Breast Cancer Research Foundation, Novartis, F. Hoffman La Roche, Sanofi-Aventis, the National Cancer Institute (NCI), the EBCC-Breast Cancer Working Group (BCWG grant for the MINDACT biobank), the Jacqueline Seroussi Memorial Foundation (2006 JSMF award), Prix Mois du Cancer du Sein (2004 award), Susan G. Komen for the Cure (SG05-0922-02), Fondation Belge Contre le Cancer (SCIE 2005-27), Dutch Cancer Society (KWF), Association Le Cancer du Sein, Parlons-en!, Deutsche Krebshilfe, and the Grant Simpson Trust and Cancer Research UK. This trial was supported by the EORTC Charitable Trust. Whole genome analysis was provided in kind by Agendia.
Compliance with ethical standards
Conflict of Interest
FS and LSS were employees for Agendia at the time of analysis. AG is an employee of Agendia. LV is a founder of Agendia and has stock ownership. All remaining authors have declared no competing interests.
Footnotes
On behalf of the TRANSBIG Consortium & the MINDACT investigators.
References
- 1.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3.2010
- 2.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;2007(25):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roepman P, Horlings HM, Krijgsman O, Kok M, Bueno-de-Mesquita JM, Bender R, et al. Microarray-based determination of estrogen receptor, progesterone receptor, and HER2 receptor status in breast cancer. Clin Cancer Res. 2009;15(22):7003–7011. doi: 10.1158/1078-0432.CCR-09-0449. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Baehner FL, Butler SM, Thompson EA, Dueck AC, Jamshidian F, et al. The relationship between quantitative human epidermal growth factor receptor 2 gene expression by the 21-gene reverse transcriptase polymerase chain reaction assay and adjuvant trastuzumab benefit in Alliance N9831. Breast Cancer Res. 2015;17(1):133. doi: 10.1186/s13058-015-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badve SS, Baehner FL, Gray RP, Childs BH, Maddala T, Liu M-L, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26(15):2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 8.Viale G, Bogaerts J, Slaets L, Rutgers E, van’t Veer L, Piccart-Gebhart MJ, et al. High concordance of protein (by IHC), gene (by FISH; HER2 only) and microarray readout (by TargetPrint) of ER/PR/HER2: results from the MINDACT trial. Ann Oncol. 2014;25(4):816–823. doi: 10.1093/annonc/mdu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, Delaloge S, Van’t Veer L, Rubio IT, et al. The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;00:2742–2749. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, Delaloge S, Van ‘t Veer LJ, Rubio IT et al. Baseline results of the EORTC 10041/MINDACT TRIAL (Microarray In Node 0-3 positive Disease may Avoid ChemoTherapy). ECCO 2013
- 11.US Food and Drug Administration. Guidance for industry and FDA staff: Statistical guidance on reporting results from studies evaluating diagnostic tests. http://medical.cms.itri.org.tw/pdf/u14.pdf Accessed 13 May 2015
- 12.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 13.Ma XJ, Hilsenbeck SG, Wang W, Ding L, Sgroi DC, Bender RA, et al. The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol. 2006;24:4611–4619. doi: 10.1200/JCO.2006.06.6944. [DOI] [PubMed] [Google Scholar]
- 14.Kraus JA, Dabbs DJ, Beriwal S, Bhargava R. Semi-quantitative immunohistochemical assay versus oncotype DX(®) qRT-PCR assay for estrogen and progesterone receptors: an independent quality assurance study. Mod Pathol. 2012;25(6):869–876. doi: 10.1038/modpathol.2011.219. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabbs DJ, Klein ME, Mohsin SK, Tubbs RR, Shuai Y, Bhargava R. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol. 2011;29:4279–4285. doi: 10.1200/JCO.2011.34.7963. [DOI] [PubMed] [Google Scholar]
- 17.Baehner FL, Achacoso N, Maddala T, Shak S, Quesenberry CP, Jr, Goldstein LC, et al. Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J Clin Oncol. 2010;28:4300–4306. doi: 10.1200/JCO.2009.24.8211. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30(7):729–734. doi: 10.1200/JCO.2011.36.2574. [DOI] [PubMed] [Google Scholar]