Abstract
Myeloid leukemias, although a heterogeneous group of hematopoietic stem cell neoplasms, are arguably among the most suited for active specific immunotherapy. Nevertheless, clinical development of myeloid leukemia vaccine lagged behind similar approaches in other solid and hematological malignancies. The recent identification of apparently specific leukemia antigens and advances in understanding the fundamentals of tumor immunology have helped initiate a number of early phase clinical studies evaluating the safety and clinical efficacy of this approach. Here we review the recently identified and characterized putative leukemia antigens, the main vaccination strategies employed by most investigators and the results of clinical studies of immunotherapy of myeloid leukemias. Although these studies are early and often difficult to interpret, they offer evidence that effective immunity to leukemia could be induced following vaccination, and that clinical benefit can sometimes be observed, thus setting the stage for future development of this strategy and in the combinatorial approaches to treatment of myeloid leukemias that incorporate immunotherapy.
Keywords: immunotherapy, vaccine, antigen, myeloid leukemia, T lymphocyte
Background
Ongoing efforts to improve long-term outcomes and cure patients with myeloid malignancies remain challenged by the relatively marginal benefits seen with newer agents, despite the development of novel therapeutics that appear to alter the natural history of some of these diseases. Unfortunately, most patients with myeloid diseases will eventually succumb to their disease. The development of non-cross-reactive modalities, like immunotherapy, may offer alternative strategies that focus on creating a lasting antitumor effect in hopes of curing many of our patients. Myeloid leukemias, both acute and chronic, constitute a heterogeneous group of clonal stem cell malignancies that are particularly suited for immunotherapeutic intervention. There are a variety of reasons why this is indeed the case. First, myeloid leukemias express both human lymphocyte antigen (HLA)-I and -II molecules, and their downregulation is infrequently observed in leukemic blasts.1 They also express a number of adhesion/costimulatory membrane molecules including CD54, CD58, CD80 and CD86,2–4 and release a number of immunomodulatory soluble mediators, such as IL1b, IL6 and TNF-α.5 Second, the leukemic blasts typically exist in physical niches within the marrow microenvironment and/or peripheral blood compartments that are relatively accessible to antigen-specific T cells as well as other nonantigen-specific immunocytes. For example, the stromal microenvironment as a biophysical barrier is less well developed in myeloid tumors compared to their solid tumor deposits. Third, myeloid disorders are often characterized by chromosomal translocations that result in chimeric proteins, which are unique leukemia antigens that may offer target specificity. Another important factor that suggests myeloid tumors are well suited to immunotherapeutic strategies is our ability to achieve major cytoreduction, attain a state of measurable, minimal residual disease that not only provide a clinical scenario amenable to immunotherapeutic intervention, but also allows for quantification of its impact. And finally, the window of immune recovery or reconstitution following cytotoxic-based induction therapy or stem cell transplantation offers a unique opportunity to prime an immune response and circumvent potential leukemia-induced peripheral tolerance. This article will review the current knowledge of the putative leukemia-associated antigens, their mechanisms of antigenicity, and the generation of antigen-specific immune responses (as well as the potential development of tumor immunity) through immunotherapeutic approaches in myeloid leukemias.
Potential myeloid leukemia-associated antigens
Several categories of potential tumor antigens recognized by T cells that have been identified through various approaches, although mainly through the generation of T-cell clones from cancer patients through in vitro antigen stimulation or by combining serological analysis with antigen cloning techniques (that is, serological expression cloning).6 It is noteworthy that most known leukemia-associated antigens are often critically involved in the initiation or maintenance of leukemogenesis. This feature suggests the generation of tumor escape by downregulation of antigen expression and/or processing would negatively impact the active tumor thereby making this an unlikely mechanism of tumor resistance and allowing for such antigens to remain as viable immune targets. Leukemia-associated antigens identified thus far fall into one of the following categories as being either unique to the leukemia, overexpressed by the leukemia or shared between the malignant clone and normal tissues albeit restricted to gonadal tissues.
Unique leukemia antigens are essentially altered proteins that are involved in the regulation, initiation or maintenance of leukemogenesis (Table 1). In many of these cases, the antigens arise as a result of chromosomal translocations which provide an exclusively tumor-specific chimeric protein, with peptide epitopes derived from the joining regions of these chimeras and serve as potential targets for T-cell recognition and effector function. Less commonly, mutated oncogenes, such as ras oncogenes, may also serve as a unique tumor antigen. The chimeric BCR-ABL fusion protein resulting from the t(9;22) translocation7 in Philadelphia-chromosome-positive chronic myeloid leukemia (CML) patients is the prototypical example of this category of antigens. Not only is this chimeric molecule tumor specific, but it is also essential for the transformed phenotype in CML and it displays limited variability with only two chromosomal breakpoints.7 This breakpoint results in the ABL coding sequences upstream (5′) of exon II on chromosome 9 being translocated to chromosome 22 and fused in-frame with the BCR gene downstream (3′) of exon III, resulting in the most common chimeric messenger RNA transcript (b3a2), which is translated into a chimeric protein (p210BCR-ABL).7 Translation of b3a2 messenger RNA results in the coding of a unique amino acid, lysine, within the fusion region. This finding suggests selectivity even within the unique target of BCR-ABL prompting several investigators to use BCR-ABL peptides in an attempt to elicit CML-specific T-cell responses. Using several HLA-A2-, -A3-, -A22- and -B8-restricted overlapping peptides (that include this unique lysine) to bind to their respective HLA alleles, in vitro T-cell proliferation responses were successfully induced when the peptide was either loaded onto HLA-matched antigen-presenting cells or onto HLA-B8-positive CML cells.8–10 However, when these b3a2 peptides were used to elicit b3a2-specific T-lymphocyte lines in vitro, the resulting T cells could not specifically lyse fresh CML cells that had not previously been pulsed with the peptide10 suggesting either low avidity of the generated peptide-specific cytotoxic T lymphocyte (CTL) or lack of processing and/or presentation of the peptide on CML cells. Interest in this phenomenon has been further fueled by recent identification of b3a2-specific CTLs in the peripheral blood of chronic phase CML patients by using soluble b3a2 peptide/MHC tetramers.11 Although the tetramer-positive CTLs from the patients were not examined for their ability to kill autologous CML target cells, b3a2-specific CTL elicited in vitro from healthy donors were able to kill CML cells. It is possible that bcr-abl fusion peptides may also be targets of CTL immunity and that CML cells are indeed capable of processing and presenting HLA-restricted immunogenic peptides derived from the bcr-abl fusion protein. Furthermore, Gannage et al.,12 made the interesting observation that spontaneously induced bcr-abl-specific CTLs can be identified in the peripheral blood of 61% of CML patients. These findings, buoyed by recent clinical results showing that vaccination of CML patients with bcr-abl breakpoint fusion peptides resulted in a peptide-specific immune response has stoked the interest in immunization strategies targeting this unique leukemia antigen.13 Less proverbial, and certainly less well-studied, putative unique leukemia antigens include the DEK-CAN fusion peptide derived from the t(6;9) translocation in patients with acute myeloid leukemia (AML)14 and the expression products of the AML/ETO or PML-RARα rearranged genes.15,16 Interestingly, CD4+ lymphocytes from acute promyelocytic leukemia patients were shown to recognize an HLA-II-restricted peptide derived from the fusion region of the PML-RARα.17 Clinical development of immunotherapies based on this observation is yet to be pursued.
Table 1.
Potential myeloid leukemia-associated antigens
| Antigen | Function |
|---|---|
| BCR-ABL | Chimeric protein |
| PML/RAR-α | Chimeric protein |
| Neutrophil elastase (ELA2) | Neutral serine protease |
| Proteinase 3 (PRTN3) | Neutral serine protease |
| Wilm's tumor antigen 1 (WT1) | Zinc-finger transcription factor |
| Human telomerase reverse transcription (hTERT) | |
| Survivin | Inhibitor of apoptosis |
| p53 | Tumor-suppressor gene |
| Mutated ras | Oncogene |
Overexpressed leukemia antigens are a second class of leukemia-associated antigens and are comprised of myeloid-restricted proteins that are aberrantly overexpressed in transformed myeloid cells relative to their normal hematopoietic progenitor counterparts. The two notable examples of such differentiation antigens are both associated with granule formation: proteinase 3 (PRTN3) and neutrophil elastase (ELA2). These serine proteases are stored in the primary azurophilic granules and are normally overexpressed at the promyelocyte stage of myeloid differentiation.18–20 Interestingly, PRTN3 appears to be important in maintaining the leukemogenic phenotype as its inhibition by the use of antisense oligonucleotides halts cell division and induces terminal differentiation of the HL-60 promyelocytic leukemia cell line.21 In addition, both PRTN3 and ELA2 are believed to be the antigens recognized by autoreactive pathogenic T- and B-lymphocyte clones associated with Wegener's granulomatosis and possibly other small-vessel vasculitides syndromes.22–24 Molldrem et al.25–27 used algorithms based on the known anchor motifs of HLA class I-restricted peptides to identify PR1, an HLA-A2.1-restricted nonamer derived from both PRTN3 and ELA2, as a leukemia-associated antigen. Peptides derived from the amino acid sequence of the protein and predicted to have high-affinity binding to HLA-A2.1 were synthesized, confirmed to bind and then used to elicit peptide-specific CTLs in vitro from healthy donor lymphocytes. PR1 peptide could elicit CTLs from HLA-A2.1+ healthy donors in vitro and T-cell immunity to PR1 was evident not only in healthy donors but also in many patients with CML who are in remission. These PR1-specific CTLs show preferential cytotoxicity toward allogeneic HLAA2.1+ myeloid leukemia cells over HLA-identical normal donor marrow.26 In addition, PR1-specific CTLs inhibit colony-forming unit granulocyte-macrophage from the marrow of CML patients, but not from normal HLA-matched donors,27 which suggested that leukemia progenitors could potentially be targeted by the antigen-specific T cells.
Another important leukemia-associated antigen is the Wilms’ tumor gene, WT1. Its antigenicity results from its marked overexpression by most human leukemias including MDS, CML and acute lymphoblastic and nonlymphoblastic leukemias.28 WT1 encodes a zinc finger transcription factor involved in the regulation of cell proliferation, differentiation and apoptosis. In leukemias, however, WT1 is linked to an oncogenic function as constitutive expression of WT1 results in growth promotion and differentiation arrest whereas its inhibition by antisense oligonucteotide results in growth inhibition.29,30 Using the same peptide binding motifs, several WT1 protein-derived CTL epitopes restricted by both HLA-A*0201 and HLA-A*2402 were identified.28 Moreover, CTLs specific for such epitopes were generated and shown to preferentially recognize and lyse leukemia cells and inhibit colony formation by CD34+ progenitor cells from CML patients.31,32 The fact that antibodies against WT1 were detected in patients with AML,32 further underscores the immunogenicity of this leukemia antigen as it is capable of engendering both cellular and humoral antileukemic immune responses.
The human telomerase reverse transcription (hTERT) antigen is expressed, or overexpressed, by the majority of human tumors, including leukemias and hTERT-specific CTLs capable of killing hTERT+ tumor cells have successfully generated in vitro from both healthy donors and cancer patients.33 Survivin, a member of the inhibitors of apoptosis gene family, has also been shown to be abundantly expressed in tumor cells, including myeloid leukemias. CTLs specific for survivin were again demonstrated to specifically recognize and lyse survivin-positive primary malignant cells from patients with chronic lymphatic leukemia and AML.34,35 Finally, surface molecules preferentially expressed in leukemia cells, such as CD45 and CD33, have been explored as potential immunogenic target for leukemia;36,37 however, it remains to be seen whether these antigens are immunologically ‘relevant’ for endogenous or vaccine-induced antileukemia immune responses.
Shared tumor antigens are those present on various tumor types of different histological derivations and whose expression in normal tissues is restricted to the gonadal tissues (for example, cancer-testis antigens such as MAGE, BAGE and GAGE).38,39 Although their ubiquity on many cancers makes them an attractive target for clinical vaccine development, their analysis in hematological malignancies has lagged behind their identification and utilization in solid tumors. Only recently has PRAME (preferentially expressed antigen of melanoma) been shown to be expressed in 47% of AML patients.40 To date, further analysis of the expression of this class of tumor antigens and their relevance to immune responses in leukemia patients is yet to be done.
Immunotherapeutic strategies against myeloid leukemias
The development of vaccines against cancers has been challenging and no more so than in the area of myeloid malignancies, in part due to a somewhat unique set of challenges. Far and away, the most important factor is the need to break tolerance to antigens against which the host has been actively tolerized.41,42 The fact that myeloid neoplasms likely share a common cell of origin with those of the immune system highlights that hosts must be partially, if not completely, tolerant of such neoplasms. The difficulty of trying to create vaccine formulations that can induce robust and sustained responses capable of producing meaningful immunity although remaining safe so as to not generate autoimmunity against normal tissues is likewise evident. The vaccination strategies employed in antileukemia immunotherapy to date have fallen largely into two categories; those that utilize molecularly defined antigens or epitopes and those that use primarily whole tumor cells as the source of antigen. Both strategies employ immunomodulatory strategies to enhance the immunogenicity of the antigen(s), facilitate their processing and presentation, augment T-cell priming, and/or downregulate innate or antigen-specific suppressive cells or molecules of the immune system.
Vaccines using leukemia-associated antigens have been driven by the discovery and molecular characterization of many potential tumor rejection antigens in myeloid leukemias and have led to a flurry of studies evaluating their safety and efficacy in early phase trials. One such developmental study involved CML patients that were given escalating doses of BCR-ABL fusion region peptides (including four HLA class I-restricted peptides from b3a2 and a 25-mer HLA class II epitope43) given in incomplete Freund's adjuvant.13 The vaccines were well tolerated and importantly, peptide-specific T-cell proliferation was demonstrated in half the patients who received the highest dose of peptides (0.5–1.5 mg). This dose was then selected for a phase II efficacy trial that enrolled 14 patients with chronic phase CML.44 Investigators were encouraged as all patients had the detection of delayed-type hypersensitivity (DTH) and antigen-induced interferon-γ production by T cells by enzyme-linked immunosorbent spot (ELISPOT) was seen in 6 out of 12 patients tested. Unfortunately, no leukemia-specific cytotoxic activity was noted and no definite vaccine-induced clinical responses were observed. Undeterred by the initially disappointing, and largely unexplained lack of correlation between immune and clinical surrogate end points, an Italian group conducted a single-arm phase II study where 16 CML patients with stable residual disease after achieving maximal response to either imatinib mesylate or interferon-α therapy were vaccinated with six b3a2 peptides and the QS-21 adjuvant.45 Again, all patients tested demonstrated specific BCR-ABL immune responses as measured by DTH, in vitro proliferation, or interferon-γ production by ELISPOT, but importantly, five patients achieved complete cytogenetic responses as the first potential link to clinical responses. This trial was a single-arm study with patients continuing on their primary therapy of imatinib mesylate or interferon-α therapy making the determination of the relative contribution of the vaccine versus the primary therapies difficult.
One attempt to minimize the impact of ongoing therapies selected 37 patients with refractory or relapsed disease spanning a spectrum of refractory myeloid tumors, including AML, CML and myelodysplastic syndromes. These patients received PR1 peptide in incomplete Freund's adjuvant plus granulocyte-macrophage colony-stimulating factor (GM-CSF) every 3 weeks for a total of three vaccinations.44 A significant increase of PR1-specific T cells after vaccination was observed in 22 out of 37 (60%) patients and clinical remission was observed in 11 (30%) patients. Moreover, durable molecular remissions were noted in three patients with refractory AML, which lasted for up to 3 years follow up. Furthermore, the observed clinical responses were shown to positively correlate with the induction of PR1-specific T cells that had higher TCR avidity compared to nonresponders (P = 0.02). This observation is important in that it provides a clue to a qualitative aspect of the immune response that is often omitted from immune monitoring of vaccine studies. Such feature, that is, the induction of higher avidity T cells might be more relevant to vaccine efficacy than the quantitative estimation of immune responses. Final results of this study are eagerly awaited.
The widespread overexpression of WT1 by myeloid leukemias and the identification of a number of HLA-restricted peptide epitopes derived from its amino acid sequence led to its testing as a potential immunogen in early phase clinical trials. In a phase I dose escalation study, 14 patients with either AML or MDS in remission were vaccinated in a phase I dose-ranging study. The vaccine consisted of natural, or modified 9-mer WT1 peptide emulsified with montanide ISA51 adjuvant and was given in 2-week intervals. An increased frequency of WT1-specific T cells was demonstrated as early as after two immunizations.28 Importantly, clinical end points (reduction in leukemia blast cells and/or WT1 transcripts) appeared to correlate with the noted the immune response (measured quantitatively by tetramer staining). A phase I/II study building upon this work in Germany has recently completed accrual.46 Of 23 evaluable patients with AML and 2 with RAEB given a median of 11 (range 3–25 days) vaccinations, one durable CR (lasting 514 days) and 13 s.d. (99–339 days) were observed. No significant toxicity was noted. There was a statistically significant association between T-cell responses by both the WT1 tetramer staining and the WT1 peptide-specific cytokine response to the observed surrogate clinical end points.47
Heat-shock protein 70 (HSP70) peptide complexes were also studied as immunotherapy for patients with CML who remained with measurable disease despite ongoing therapy with imatinib mesylate.48 HSP70 is a molecular chaperone of peptides to the MHC class-I and class-II antigen-processing pathway often upregulated, in part due to cellular damage or stress. Hsp70PCs were purified from the leukopheresed peripheral blood mono-nuclear cells and were administered in eight weekly intradermal injections without adjuvant. The investigators reported clinical responses as measured by lowering of bcr/abl transcript levels in 13 of 20 patients, immunologic responses as measured by an increase in the frequency of CML-specific interferon (IFN)-γ-producing cells and IFN-γ-secreting natural killer cells in the blood, and a positive relationship between the two.48 One interesting aspect of this approach is its reliance on the ability of HSP to provide naturally processed and presented peptides that should potentially reflect with some fidelity the density and composition of the antigenic peptide repertoire in leukemia cells.
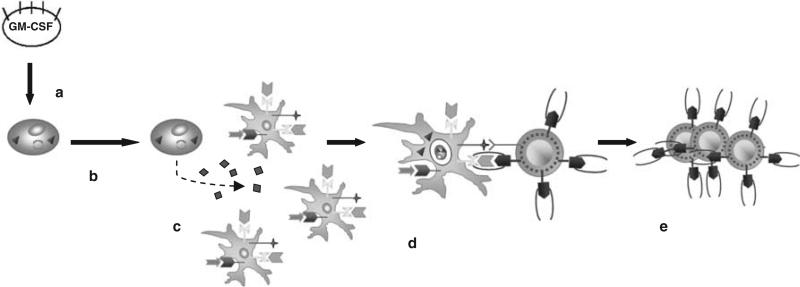
A second strategy focuses on the utilization of whole cell-based vaccines. As such, use of a whole cell-based vaccine affords the ability to provide a panel of molecules whose antigenic hierarchy is more representative of the antigenicity of leukemia cells in vivo. The disadvantages are the cost of generating cell-based vaccines and the difficulty of monitoring induced immune responses given the lack of certainty as to which antigen(s) prime T cells. The potential efficacy of this approach relies on the premise that tolerance can be preferentially broken against tumor-selective antigens versus self-antigens. In fact, such selectivity seems possible within the realm of immunostimulatory and/or costimulatory signals that direct the processing and presentation of tumor antigens by host-derived antigen presenting cells. This concept is highlighted by seminal studies in the 1990s that showed that the main mechanism of priming tumor antigen-specific T cells in vivo involves the cross-presentation of such antigens by bone marrow-derived antigen presenting cells.49,50 This observation fueled interest in developing tumor cell-based vaccines that were genetically altered to enhance the recruitment of patient-derived dendritic cells for their in vivo antigen processing and cross-presentation to potentially tumor antigen-specific lymphocytes (Figure 1). One study Dranoff et al.51 compared the impact of 10 different cytokines and costimulatory molecules that were transduced into poorly immunogenic autologous tumor cells. This work revealed that immunization with cells engineered to express GM-CSF produced a robust, potent and long-lasting systemic tumor immunity that was mediated by both CD8+ and CD4+ T lymphocytes. Given the relative accessibility of leukemia cells, the generation of autologous tumor cell-based vaccines is relatively easy and in fact, additional preclinical studies supported the utility of this vaccination approach for hematologic neoplasms.52 However, the realization that the majority of the antigens identified for myeloid malignancies thus far belong to the ‘shared’ category combined with the phenomenon of cross-presentation of tumor antigens by host-derived dendritic cells being the main mechanism of T-cell priming, suggested that allogeneic vaccines might be equally effective across the HLA barrier among many patients. The prototypical example of this approach is an allogeneic cell-based vaccine made of an erythroleukemia cells established from a CML patient in a blast crisis (K562) that were engineered to express GM-CSF and dubbed K562/GM-CSF.53 In addition to local production of the stimulatory cytokine GM-CSF, these cells lacked the expression of HLA class I and II molecules that minimize the risk of allogeneic rejection with repeated vaccination. Thus, K562/GM-CSF held potential as a ‘bystander’ cell to provide adjuvant paracrine excretion of GM-CSF when admixed with unmodified autologous tumor cells serving as the source of antigen as well as potential as a ‘whole cell’ vaccine providing both the potentially relevant CML-related antigens and the stimulatory GM-CSF for patients with CML.
Figure 1.
Cross-presentation of tumor-associated antigen by patient-derived antigen presenting dendritic cells. (a) Autologous or allogeneic tumor cells are transfected with the gene for granulocyte-macrophage colony-stimulating factor (GM-CSF); (b) irradiated GM-CSF producing cells are injected into patients; (c) local production of GM-CSF helps to induce the recruitment and maturation of dendritic cells; (d) dendritic cells process and cross-present shed antigen from tumor cells to antigen-specific T cells; (e) primed T cells proliferate and migrate to sites of tumor.
An early stage clinical trial designed to evaluate the safety and efficacy of a tumor vaccine administered to patients with AML in the periautologous stem cell transplantation period. Following induction and consolidation, a pretransplant vaccination of 1 × 108 autologous leukemia cells admixed with 4 × 107 K562/GM-CSF bystander cells was given to patients. This immunization was then followed 2 weeks later with leukopheresis with the goal of harvesting primed leukemia-specific lymphocytes that would then be reinfused along with the autologous stem cells following myeloablative preparative regimen of cyclophosphamide and busulphan. The patients then went on to receive eight booster immunizations following immune reconstitution. Of 20 evaluable patients, 11 had measurable decreases in WT1 transcripts by reverse transcriptase (RT)–PCR that were associated with a longer relapse-free survival (RFS). In addition, at a mean follow-up of 2 years, patients who developed a DTH reaction to irradiated autologous AML blasts had a 100% RFS, compared to 60% RFS in patients who did not mount a DTH. As commonly observed in many vaccine trials, the relative contribution of the immunization versus that of concurrently delivered therapeutic intervention is difficult to ascertain in this nonrandomized trial. Nonetheless, this study provides more tantalizing evidence of the potential ability to engender leukemia-specific immune responses using the whole cell-based vaccination strategy. Another study used the same cell line as an allogeneic vaccine in 19 patients with CML. The patients were given four immunizations with 1 × 108 irradiated K562/GM-CSF alone at 3-week intervals following the attainment of maximum cytogenetic response to imatinib. The group's median age was 52 years and their median disease duration was 57 months. Of the 19 patients, 4 were considered to have significant disease burden with fluorescence in situ hybridization (FISH)-positive disease as their best previous response to imatinib, 2 of whom became FISH negative following completion of four planned vaccinations. Interestingly, one patient achieved PCR negativity and one achieved a > 1 log reduction in disease burden measured by PCR. Of the 15 patients whose best previous response to imatinib mesylate was FISH negative but PCR positive, 4 became PCR negative postvaccine and 4 experienced > 1 log reduction by PCR. Moreover, mean PCR levels for the 19 patients declined between pre- and postvaccine measures (P = 0.01). Only one patient progressed having entered the study with a heavy disease burden (30% FISH positive). Intriguingly, these interesting clinical responses did not appear to correlate with any immune responses as tested against a panel of potential myeloid antigen targets. In addition, although the clinical responses are exciting, interpretation of the impact of the vaccines must be made cautiously due to the fact that patients were continued on imatinib mesylate therapy during and beyond the immunotherapy period.54
Conclusions and future directions
Canvassing the field of immunology and immunotherapy of myeloid malignancies, it is difficult to draw any solid conclusions at this stage of development. Thus far, the only clinically meaningful immunotherapeutic modality in leukemia remains allogeneic stem cell transplants and the infusion of donor lymphocytes and even this approach remains unclear whether or not this observation is indeed a tumor antigen-specific immune response or whether it is an inseparable part of an alloreactive response to the host. What is clear, however, is the fact that several questions will need to be addressed in order for immunotherapy of myeloid leukemias to move beyond the experimental realm. The first question relates to which antigens are immunologically and clinically relevant. It is well known that antigen-specific immune responses, both cellular and humoral, can be detected in many patients with cancers; however, which, if any, among these putative antigens will be relevant to the induction of protective and/or therapeutic immunity, remains to be elucidated. In addition, which cells or molecules of the immune system are relevant in the context of antileukemia vaccination? Research in the past has focused almost exclusively on T and B lymphocytes. More recently, the role of natural killer cells in mediating antileukemia immunity in the context of allogeneic stem cell transplantation has become a hot topic for investigations.55–57 It will be interesting to see whether the role of these cells, long relegated to a second-class status among immunologist, can be better understood and eventually utilized in the context of antileukemia vaccination and immunotherapy. The role of the immunological checkpoints, that is, barriers that operate at a number of levels, and involve the tumor, its tumor microenvironment and various components of the innate and adaptive immune systems is also being actively dissected.58 Interventions that aim at negotiating these checkpoints in the context of vaccination are being tested in early phase studies. The potential of such interventions to enhance the antitumor immunity without sacrificing specificity will be a great boost to the clinical development of leukemia and other tumor vaccines. Another challenge will be to rationally combine the vaccines with other active therapeutics for a maximum clinical benefit. Preclinical studies point to synergy between vaccine-induced immune responses and blockade of vascular endothelial growth factor pathway or hypomethylating agents, two classes of drugs with proven activity against myeloid tumors.59,60
Although single-arm studies evaluating vaccine efficacy in myeloid leukemia patients can provide tantalizing evidence of efficacy, only better designed studies can help glean the impact of vaccine approaches on patient outcome. Specifically, randomized trials, although costly, time consuming and labor intensive, are probably the only way to circumvent potential confounding variables that may erroneously account for any observed clinical benefit such as inadvertent patient selection bias. Finally, the identification of a reliable biomarker that can predict with an acceptable degree of sensitivity and specificity, clinical benefit from therapeutic vaccinations will be a great boost to the clinical development of such vaccines and the immune monitoring thereof.
Footnotes
Conflict of interest
The authors do not have financial or any other conflicts of interest to declare.
References
- 1.Elghetany MT. Surface marker abnormalities in myelodysplastic syndromes. Hematologica. 1998;83:1104–1115. [PubMed] [Google Scholar]
- 2.Bene MC. Immunophenotyping of acute leukemias. Immunol Lett. 2005;98:9–21. doi: 10.1016/j.imlet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Notter M, Willinger T, Erben U, Thiel E. Targeting of B7-1 (CD80) immunoglobulin G fusion protein to acute myeloid leukemia blasts increases their costimulatory activity for autologous remission T cells. Blood. 2001;97:3138–3145. doi: 10.1182/blood.v97.10.3138. [DOI] [PubMed] [Google Scholar]
- 4.Bruserud O. Acute myelogenous leukemia blasts as accessory cells during T lymphocyte activation: possible implications for future therapeutic strategies. Leukemia. 1999;13:1175–1178. doi: 10.1038/sj.leu.2401452. [DOI] [PubMed] [Google Scholar]
- 5.Lotem J, Sachs L. Hematopoietic cytokines inhibit apoptosis induced by transforming growth factor beta 1 and cancer chemotherapy compounds in myeloid leukemia cells. Blood. 1992;80:1750–1757. [PubMed] [Google Scholar]
- 6.Jager D. Potential target antigens for immunotherapy identified by serological expression cloning (SEREX). Methods Mol Biol. 2007;360:319–326. doi: 10.1385/1-59745-165-7:319. [DOI] [PubMed] [Google Scholar]
- 7.Kurzrock R, Talpaz M. The molecular pathology of chronic myelogenous leukemia. Br J Hematol. 1991;79:34–37. doi: 10.1111/j.1365-2141.1991.tb08116.x. [DOI] [PubMed] [Google Scholar]
- 8.Kessler JH, Bres-Vlooemans SA, van Veelen PA, de Ru A, Huijbers IJ, Camps M, et al. BCR-ABL fusion regions as a source of multiple leukemia-specific CD8+ T-cell epitopes. Leukemia. 2006;20:1738–1750. doi: 10.1038/sj.leu.2404354. [DOI] [PubMed] [Google Scholar]
- 9.Bocchia M, Wentworth PA, Southwood S, Sidney J, McGraw K, Scheinberg DA, et al. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood. 1995;85:2680–2684. [PubMed] [Google Scholar]
- 10.Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, et al. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood. 1996;87:3387–3392. [PubMed] [Google Scholar]
- 11.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, et al. Direct evidence that leukemia cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98:2887–2893. doi: 10.1182/blood.v98.10.2887. [DOI] [PubMed] [Google Scholar]
- 12.Gannage M, Abel M, Michaellet AS, Delluc S, lambert M, Giraudier S, et al. Ex vivo characterization of multiepitopic tumor specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and cellular immunotherapy. J Immunol. 2005;174:8210–8218. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- 13.Pinilla-Ibarz J, Cathcart K, Krontsvit T, Soignet S, Bocchia M, Caggiano J, et al. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint peptides generates specific immune responses. Blood. 2000;95:1781–1787. [PubMed] [Google Scholar]
- 14.von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, et al. The translocation (6;9) associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia specific dek-can mRNA. Mol Cell Biol. 1992;12:1687–1697. doi: 10.1128/mcb.12.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de The’ H, Lavau C, Marchino A, Chomienne C, Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 16.Downing JR, Head Dr, Curcio-Brint AM, Hulshof MG, Motroni TA, Raimondi SC, et al. An AML1/ETO fusion transcript is consistently detected by RNA based polymerase chain reaction in acute myelogenous leukemia containing the (8;21)(q22;q22) translocation. Blood. 1993;81:2860–2865. [PubMed] [Google Scholar]
- 17.Gambacorti-Passerini C, Grignani F, Arienti F, Pandolfi PP, Pelicci PG, Parmiani G. Human CD4 lymphocytes specifically recognize a peptide representing a fusion region of the hybrid protein pml/RARalpha present in acute promyelocytic leukemia cells. Blood. 1993;81:1369–1375. [PubMed] [Google Scholar]
- 18.Sturrock AB, Franklin KF, Rao G, Marshall BC, Rebentisch MB, Lemons RS, et al. Structure, chromosomal assignment, and expression of the gene for proteinase-3. The Wegener's granulomatosis autoantigen. J Biol Chem. 1992;267:1193–1199. [PubMed] [Google Scholar]
- 19.Chen T, Meier R, Ziemiecki A, Fey MF, Tobler A. Myeloblastin/proteinase 3 belongs to the set of negatively regulated primary response genes expressed during in vitro myeloid differentiation. Biochem Biophys Res Commun. 1994;200:1130–1135. doi: 10.1006/bbrc.1994.1568. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Berat N, Minowada J, Tsuji-Takayama K, Drexler H, Lanotte M, Wieslander J, et al. The phylogeny of proteinase 3/myeloblastin, the autoantigen in Wegener's granulomatosis, a myeloperoxidase as shown by immunohistochemical studies on human leukemia cell lines. Clin Immunol Immunopathol. 1994;70:51–59. doi: 10.1006/clin.1994.1010. [DOI] [PubMed] [Google Scholar]
- 21.Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989;59:959–968. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- 22.Borregaard N, Cowland JB. Granules of human neutrophilic polymorphonuclear leukocytes. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 23.Brouwer E, Stageman CA, Huitema MG, Limburg PC, Kallenberg CG. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener's granulomatosis. Clin Exp Immunol. 1994;98:448–453. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franssen CF, Stageman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, et al. Antiproteinase 3- and myeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–2206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 25.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 26.Molldrem J, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony forming units. Blood. 1997;90:2529–2534. [PubMed] [Google Scholar]
- 27.Molldrem JJ, Lee PP, Wang C, Champlin RE, Davis MM. A PR1-human leukocyte antigen A2 tertramer can be used to isolate low frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chromic myelogenous leukemia. Cancer Res. 1999;59:2675–2681. [PubMed] [Google Scholar]
- 28.Oka Y, Tsuboi A, Kawakami M, Elisseeva OA, Nakajima H, Udaka K, et al. Development of WT1 peptide cancer vaccine against hematopoietic malignancies and solid cancer. Curr Med Chem. 2006;13:2345–2352. doi: 10.2174/092986706777935104. [DOI] [PubMed] [Google Scholar]
- 29.Inoue K, Tamaki H, Ogawa H, Oka Y, Soma T, Tatekawa T, et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood. 1998;91:2969–2976. [PubMed] [Google Scholar]
- 30.Tsuboi A, Oka Y, Ogawa H, Elisseeva OA, Tamaki H, Oji Y, et al. Constitutive expression of the Wilms’ tumor gene WT1 inhibits the differentiation of myeloid progenitor cells but promotes their proliferation in response to granulocyte-colony stimulating factor (G-CSF). Leuk Res. 1999;23:499–505. doi: 10.1016/s0145-2126(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 31.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by CD8+ cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 32.Gao L, Ballantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, et al. Selective elimination of leukemia CD34+ progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 33.Vonderheide RH, Schultze JL, Anderson KS, maecker B, Butler MO, Xia Z, et al. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res. 2001;61:8366–8370. [PubMed] [Google Scholar]
- 34.Andersen MH, Pedersen LO, Capeller B, Bröker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 35.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 36.Raptis A, Clave E, Mavroudis D, Molldrem J, Van Rhee F, Barrett AJ. Polymorphism in CD33 and CD34 genes: a source of minor histocompatibility antigens on haematopoietic progenitor cells? Br J Haematol. 1998;102:1354–1358. doi: 10.1046/j.1365-2141.1998.00906.x. [DOI] [PubMed] [Google Scholar]
- 37.Amrolia PJ, Reid SD, Gao L, Schultheis B, Dotti G, Brenner MK, et al. Allorestricted cytotoxic T cells specific for human CD45 show potent antileukemic activity. Blood. 2003;101:1007–1014. doi: 10.1182/blood-2002-02-0525. [DOI] [PubMed] [Google Scholar]
- 38.Traversari C, van der Bruggen P, Luescher IF, Lurquin C, Chomez P, Van Pel A, et al. A nanopeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigens MZ2-E. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P, et al. BAGE: a new gene encoding an antigen recognized on human melanoma by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 40.Greiner J, Ronghoffer M, Simikopinko O, Szmaraqowska A, Huebsch S, Maurer U, et al. Simultaneous expression of different immunogenic antigens in acute myeloid leukemia. Exp Hematol. 2000;28:1413–1422. doi: 10.1016/s0301-472x(00)00550-6. [DOI] [PubMed] [Google Scholar]
- 41.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Steveley-O'Carroll K, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphocyte progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 42.Sotomayor EM, Borrello I, Levitsky HI. Tolerance and cancer: a critical issue in tumor immunology. Crit Rev Oncol. 1996;7:433–456. doi: 10.1615/critrevoncog.v7.i5-6.30. [DOI] [PubMed] [Google Scholar]
- 43.Bocchia M, Korontsvit T, Xu Q, Mackinnon S, Yang SY, Sette A, et al. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood. 1996;87:3587–3592. [PubMed] [Google Scholar]
- 44.Cathcart K, Pinilla-Ibarz J, Korontsvit T, Schwartz J, Zakhaleva V, Papadopoulos EB, et al. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103:1037–1042. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 45.Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, et al. Effect of p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet. 2005;365:657–662. doi: 10.1016/S0140-6736(05)17945-8. [DOI] [PubMed] [Google Scholar]
- 46.Mailaender V, Scheibenbogen C, Thiel E, Letsch A, Blau IW, Keilholz U. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004;18:165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 47.Letsch A, Scheibenbogen C, Busse A, Asemissen A, Schmittel WK, Hofmann L, et al. Phase II trial of vaccination with WT1 peptide, GM-CSF, and KHL in patients with acute myeloid leukemia and myelodysplasia: final immunological, molecular, and clinical results. Abstract J Clin Oncol. 2007;25:3800. (Abstract No. 3008) [Google Scholar]
- 48.Li Z, Qiao Y, Liu B, Laska EJ, Chakravarthi P, Kulko JM, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–4468. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 49.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 50.Huang AY, Bruce AT, Pardoll DM, Levitsky H. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J Exp Med. 1996;183:769–776. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to express granulocyte-macrophage colony stimulating factor stimulates potent, specific, and long-lasting antitumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitsky HI, Montgomery J, Ahmedzahed M, Staveley-O'Carroll K, Guarnieri F, Longo DM, et al. Immunization with granulocyte-macrophage colony stimulating factor transduced, but not B7-1-transduced, lymphoma cells primes idiotype-specific immune responses and generates potent systemic antitumor immunity. J Immunol. 1996;156:3858–3865. [PubMed] [Google Scholar]
- 53.Borrello I, Sotomayor EM, Cooke S, Levitsky H. A universal granulocyte-macrophage colony stimulating factor-producing bystander cell line for the use in the formulation of formulation of tumor cell based vaccines. Hum Gene Ther. 1999;10:1983–1991. doi: 10.1089/10430349950017347. [DOI] [PubMed] [Google Scholar]
- 54.Smith BD, Kasamon YL, Miller CB, Chia CY, Murphy K, Kowalski J, et al. K562/GM-CSF vaccination reduces tumor burden, including achieving molecular remissions, in chronic myeloid leukemia (CML) patients with residual disease on imatinib mesylate (IM). American society of hematology annual meeting. Blood. 2005;106:801a. (Abstract No. 6509) [Google Scholar]
- 55.Kennedy-Nasser AA, Brenner MK. T-cell therapy after hematopoietic stem cell transplantation. Curr Opin Hematol. 2007;14:616–624. doi: 10.1097/MOH.0b013e3282ef615a. [DOI] [PubMed] [Google Scholar]
- 56.Ruggeri M, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T, et al. Natural killer cell alloreactivity in allogeneic transplantation. Curr Opin Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]
- 57.Wodnar-Filipowicz A, Kalberer CP. Function of natural killer cells in immune defense against human leukemia. Swiss Med Wkly. 2006;136:359–364. doi: 10.4414/smw.2006.11360. [DOI] [PubMed] [Google Scholar]
- 58.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kihslinger JE, Godley LA. The use of hypomethylating agents in the treatment of hematological malignancies. Leuk Lymphoma. 2007;48:1676–1695. doi: 10.1080/10428190701493910. [DOI] [PubMed] [Google Scholar]
- 60.Kessler T, Fehrmann F, Bieker R, Berdel WE, Mesters RM. Vascular endothelial growth factor and its receptors as drug targets in hematological malignancies. Curr Drug Targets. 2007;8:257–268. doi: 10.2174/138945007779940089. [DOI] [PubMed] [Google Scholar]