Abstract
Movement, the fundamental component of behavior and the principal extrinsic action of the brain, is produced when skeletal muscles contract and relax in response to patterns of action potentials generated by motoneurons. The processes that determine the firing behavior of motoneurons are therefore important in understanding the transformation of neural activity to motor behavior. Here, we review recent studies on the control of motoneuronal excitability, focusing on synaptic and cellular properties. We first present a background description of motoneurons: their development, anatomical organization, and membrane properties, both passive and active. We then describe the general anatomical organization of synaptic input to motoneurons, followed by a description of the major transmitter systems that affect motoneuronal excitability, including ligands, receptor distribution, pre- and postsynaptic actions, signal transduction, and functional role. Glutamate is the main excitatory, and GABA and glycine are the main inhibitory transmitters acting through ionotropic receptors. These amino acids signal the principal motor commands from peripheral, spinal, and supraspinal structures. Amines, such as serotonin and norepinephrine, and neuropeptides, as well as the glutamate and GABA acting at metabotropic receptors, modulate motoneuronal excitability through pre- and postsynaptic actions. Acting principally via second messenger systems, their actions converge on common effectors, e.g., leak K+ current, cationic inward current, hyperpolarization-activated inward current, Ca2+ channels, or presynaptic release processes. Together, these numerous inputs mediate and modify incoming motor commands, ultimately generating the coordinated firing patterns that underlie muscle contractions during motor behavior.
I. INTRODUCTION
Motoneurons transform the internal actions of the brain into behavior, translating patterns of interneuronal activity into commands for skeletal muscle contraction and relaxation. Every movement, whether simple (kneejerk reflex, postural maintenance), rhythmic (locomotion, respiration), or complex (playing the piano, hitting a baseball, speaking), is the consequence of a highly detailed and precise pattern of activity of many populations of motoneurons, convolved with the biomechanical properties of the skeletomuscle system. Although the signal processing underlying the distribution of inputs between and within motoneuron pools determines the basic features of any movement, the final arbiters of nervous system output are motoneurons. How motoneurons respond to their inputs, and how their responses are regulated, is of interest and the subject of this review.
Sherrington (1142) introduced the concept of motoneurons as the final common path, representing the penultimate link between the central nervous system (CNS) and motor behavior. Since then, motoneurons have attracted the attention of investigators studying the cellular physiology of central neurons for several reasons. 1) The function of motoneurons is well defined, i.e., causing contraction of striated muscle. 2) With the advent of intracellular recording techniques, it was possible to study the electrical properties of these large accessible neurons, which can be readily identified in physiological experiments by antidromic invasion from specific muscle nerves (141). 3) Multisensorial inputs from muscles, joints, and skin produce synaptic potentials in motoneurons, and experimental access to these pathways paved the way for studies of synaptic transmission in the CNS and of simple reflex pathways in mammals (317, 436). Numerous major reviews have been published on motoneuron physiology; among the more notable are References 49, 107, 161, 162, 475.
From Sherrington to Eccles, motoneurons were the paradigmatic neurons of the brain. Many fundamental and general properties of neurons and synaptic transmission were first identified in motoneurons, e.g., quantal release, inhibitory transmission, and the consequent conclusion that chemical neurotransmission is the principal form of interneuronal communication. In the past decade, interest in motoneurons has waned as intense investigation of other regions of the brain has led to an encyclopedic identification of neuronal properties not typically associated with motor control, e.g., long-term potentiation observed in hippocampal neurons and proposed to be a component of learning. One difficulty in contextualizing all of this information is that many of these well-characterized properties have been identified in neurons in the absence of data concerning how these neurons process signals in actual behavior. Here, motoneurons have a unique advantage since we know precisely 1) the information coding of their output signals, i.e., contraction of the innervated muscle fibers, and; 2) in many cases, their activity during complex behaviors, either indirectly by observing movements or recording muscle activity, or directly by recording from their axons or better yet their cell bodies. This provides a context for understanding and interpreting data that should be more highly valued. One goal in this review is to emphasize this perspective. We review studies, with emphasis on recent work, describing the passive and active membrane properties of spinal and cranial motoneurons, anatomical organization of their synaptic input, and the actions of different transmitter systems on the control of motoneuronal excitability (see Fig. 1). Most of these studies were done without addressing issues that were and remain the focus of many studies of motoneurons, such as the physiological relevance and characterization of 1) different motor unit types, e.g., fast fatigue resistant, fast fatigable, slow fatigue resistant; 2) the mechanisms underlying their orderly recruitment, i.e., the size principle; and 3) the synaptic inputs from multiple classes of afferent fibers. We refer the reader to several excellent reviews on these important issues (49, 107, 475, 1103). We further limit our discussion to mammalian α-motoneurons, with a few notable exceptions involving motoneurons from other vertebrates, where some motoneuronal properties have been studied in more detail. There are important differences between the properties of motoneurons in newborn compared with those in adult mammals, both with respect to cellular properties and actions of transmitters; where such differences may reflect on the control of excitability, we have separated the description of the two groups.
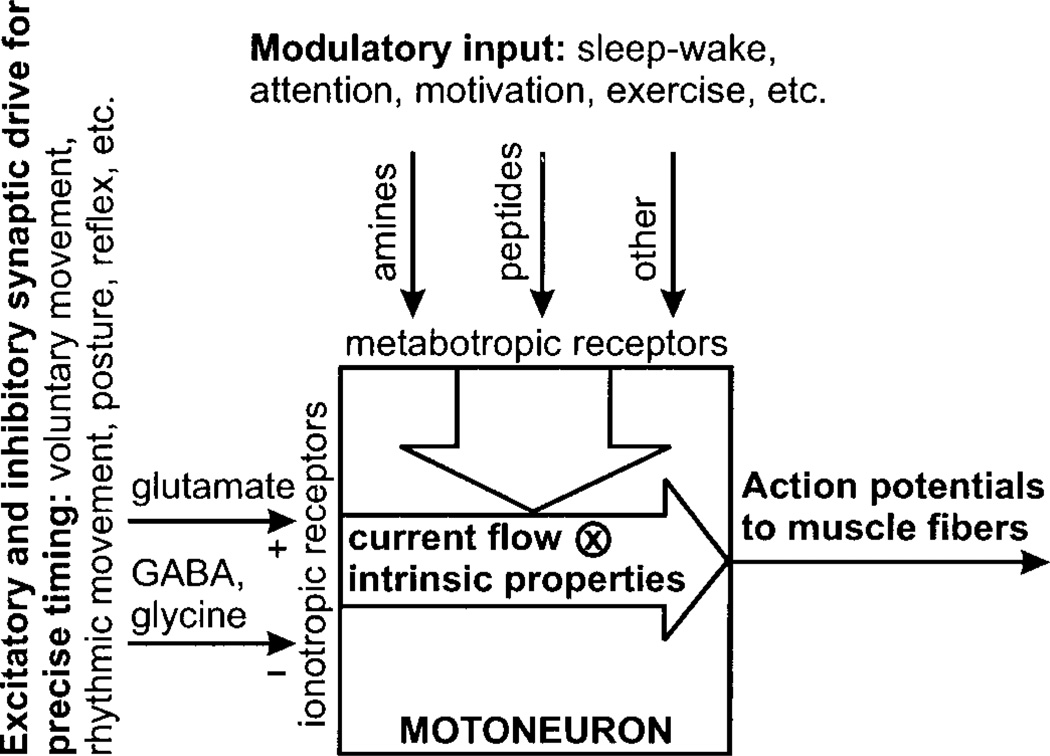
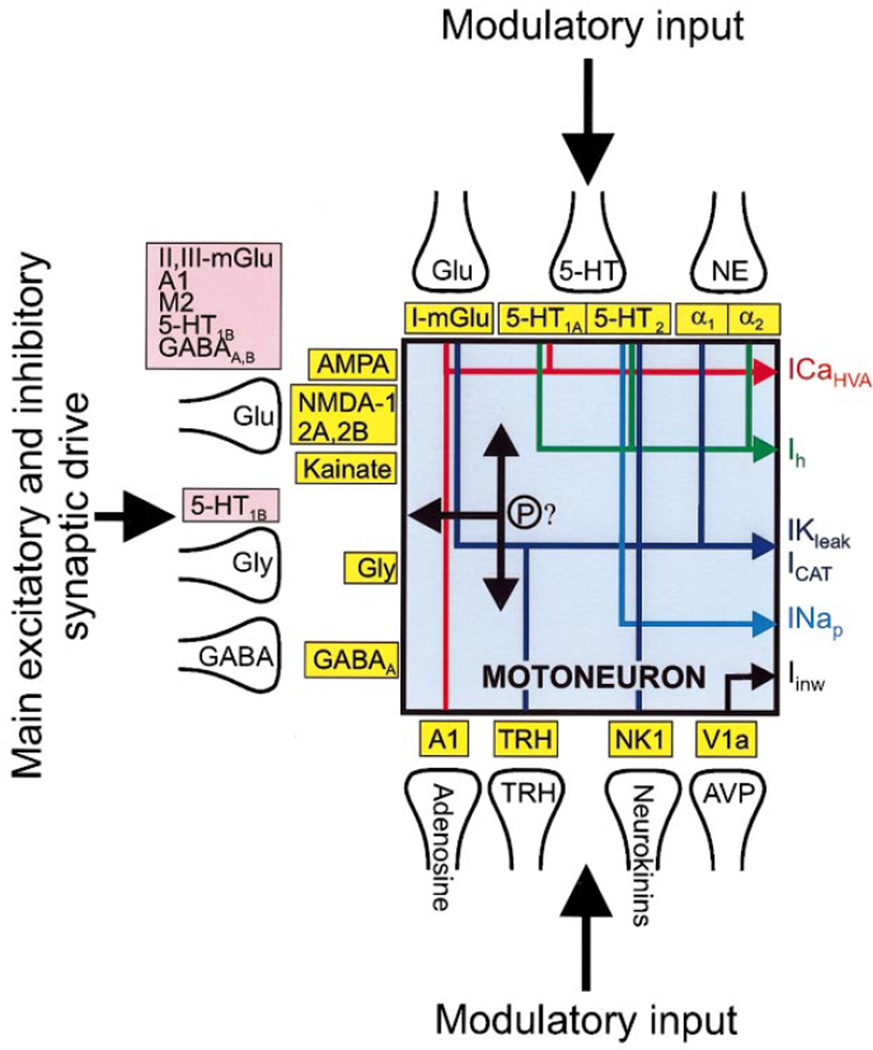
FIG. 1.
Overview of control of motoneuronal excitability. Precise timing of voluntary movements, rhythmic movements, afferent reflexes, and other motor acts is mediated primarily by excitatory and inhibitory synaptic drive to motoneurons using glutamate, GABA, and glycine. These transmitters activate ionotropic receptors generating synaptic current in motoneurons, which is convolved with intrinsic membrane properties to produce action potentials, which trigger muscle contraction. Modulatory systems, using amines, peptides, and other transmitters, act (mostly) through metabotropic receptors to modify excitability via changes in postsynaptic ion channel function and presynaptic release processes. These various modulatory systems produce changes in excitability related to the sleep-wake cycle, motivation, and exercise.
II. MOTONEURONS
A. Embryonic Development and Anatomical Organization of Motoneurons
Motoneurons are generated from progenitor cells in the ventral region of the neural tube early in embryonic development (145, 1230). The inductive signal in this process is the Sonic Hedgehog glycoprotein (SHH), which is secreted by axial mesodermal cells of the notochord (214, 319, 339, 340, 799, 1054, 1397). The extracellular matrix protein vitronectin may act as a downstream effector or a synergistic factor in SHH-induced motoneuron differentiation (803). The transcription factor MNR2 functions as a neural determination gene, and its expression initiates motoneuron differentiation (1233). Further motoneuronal differentiation requires expression of the LIM homeodomain transcription factor, Islet1 (Isl-1), since animals in which Isl-1 function has been eliminated do not generate motoneurons (980). Diversification of motoneuron subtypes in the spinal cord is controlled by the differential expression of four LIM homeodomain proteins (Isl-1, Isl-2, Lim-1, Lim-3) (32, 763, 1230, 1254, 1276). At the time of their birth, all classes of motoneurons express Isl-1 and Isl-2, but at the time of axon extension, the four LIM factors show a stereotyped expression pattern in functional subclasses of spinal motoneurons: 1) motoneurons innervating axial muscles express Isl-1, Isl-2, and Lim-3; 2) motoneurons innervating ventral limb muscles and body wall express Isl-1 and Isl-2; and 3) motoneurons innervating dorsal limb muscles express Lim-1 and Isl-2. Early-born motoneurons in the lateral motor column (LMC) at the brachial and lumbar levels synthesize a retinoid that induces late-born LMC motoneurons to migrate past the early-born LMC motoneurons to the ventrolateral spinal cord (1172); this suggests that neuronally released retinoids may coordinate subtype identity of spinal motoneurons innervating limb muscles. Genes of the LIM homeodomain class are also expressed differentially among cranial motor nuclei (1307). Their function is presently unknown; they may regulate receptors for guidance cues that direct axons selectively along distinct pathways to regions outside the spinal cord (763). Thus inductive signals from the notochord establish the identity of motoneurons, and local signals (possibly from the paraxial mesoderm) induce a differential expression pattern of LIM factors determining the motoneuronal subtype. Once the induction and functional differentiation of motoneurons has occurred, survival of developing motoneurons depends on muscle-derived factors and/or functional changes in the state of the motoneurons, i.e., from growing cells to transmitting cells (440, 936). The nature of the muscle-derived growth molecules is largely unknown, but one, hepatocyte growth factor/scatter factor, is necessary for survival of a subpopulation of limb-innervating motoneurons (1399). The molecular basis for motoneuronal innervation of specific muscles is being elucidated in model systems such as chick hindlimb muscles, zebrafish axial muscle, and Drosophila abdominal body wall muscle (cf. Ref. 325). However, the genetic determinants controlling subtype-specific development of motoneuronal morphology, intrinsic electrical properties, and CNS connectivity are largely unknown.
In the fully developed mammal, motoneuron groups are somatotopically organized (475, 502, 822, 916, 1055). Spinal cord motoneurons are in lamina IX of the ventral horn, divided into a medial and a lateral column. Motoneurons in the medial column innervate axial muscles, and those in the lateral column, present at the cervical, upper thoracic, and lumbosacral levels, innervate limb muscles. In the lateral column, motoneurons innervating distal muscles are more dorsal. In the rostrocaudal direction, motoneuron groups innervating single muscles span one to several spinal segments. Cranial, i.e., brain stem, motoneurons are not organized in a continuous column as in the spinal cord but form distinct nuclei, with an intrinsic somatotopic organization (297, 664).
The size and dendritic arborization of spinal and cranial motoneurons vary considerably. Consider, for example, cat hindlimb motoneurons. They have medium to large somata with a diameter of 30–70 µm (246, 1287, 1298, 1435) and 5–20 stem dendrites, which have a diameter of 0.5–19 µm and ramify extensively over a mean path length of ~1,200 µm, giving rise to ~150 dendritic terminations. The dendrites tend to project in the longitudinal direction (247), a phenomenon seen in many types of spinal motoneurons (178, 283, 1189).
B. Passive Membrane Properties of Motoneurons
The dendritic membrane constitutes >97% of the total membrane surface area in cat spinal motoneurons (246), with 61% of the stem dendrites and 12–33% of more distal dendrites covered by synaptic boutons (937). Consequently, the vast majority of synaptic inputs to motoneurons are dendritic, and integration of synaptic potentials is heavily influenced by the passive membrane properties of the dendrites (548, 1024). Synaptic current generated in a dendrite attenuates as it spreads electrotonically toward the soma, escaping through open channels in the membrane and charging the dendritic membrane capacitance. Determination of the geometrical features of the dendritic tree together with electrical parameters of the membrane [membrane resistance (Rm), membrane capacitance (Cm), and cytoplasmic resistivity (Ri)], provides an estimate of the attenuation of synaptic potentials. One useful parameter is the length constant, or space constant (λ), which is the distance over which a steady-state voltage is attenuated to 1/e (0.37) of its initial value.
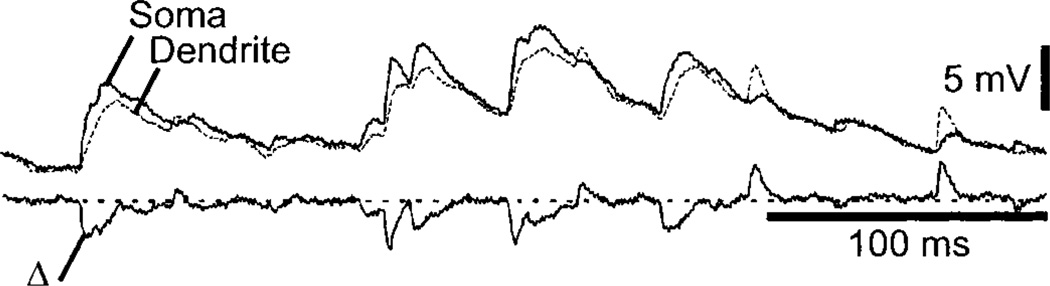
Estimates of the electrotonic length of cat spinal motoneurons, based on recordings with sharp intracellular electrodes (which introduces an artifactual somatic shunt) and morphological reconstructions of the dendritic tree, are between 1.1 and 1.6 λ (63–65, 336, 369, 1288). Whole cell patch-clamp recordings (reducing somatic shunt artifacts) with two electrodes on the same soma from spinal cord motoneurons in juvenile rats estimates electrotonic length of uncut dendrites at 0.85 λ (1247). Thus a substantial fraction of a direct current in the dendrite reaches the motoneuronal soma, making the neuron relatively compact electrotonically for slowly changing currents. However, synaptic potentials have fast rise times, and the low-pass filtering properties (due to membrane charging) of dendrites distort and attenuate a synaptic signal more strongly than an applied steady-state voltage. This strong filtering property in cat motoneurons is mainly the result of a large membrane capacitance, and peak attenuations measured for fast synaptic currents in the distal dendrites range over 20- to 30-fold. A more direct approach to the measurement of dendritic attenuation is to study spinal motoneurons in culture (Fig. 2) (706, 1290). The electrotonic length of these neurons is ~0.7 λ; dual recordings (one electrode on the soma and one on a dendrite) give a 1/e attenuation corresponding to ~260 µm for excitatory postsynaptic potentials (EPSP) travelling from the dendrite to the soma (706). Interestingly, EPSP travelling from the soma to the dendrite are attenuated much less (1/e attenuation of ~710 µm), a result predicted by cable theory (188). The overall picture of the passive properties of motoneurons in culture is that the soma and dendrites are roughly isopotential for slowly varying potentials with fast voltage transients arising from synaptically generated currents more strongly filtered. Thus dendritic EPSP should produce smaller and more slowly changing somatic depolarization that could bring the membrane potential above threshold for firing but with a smeared temporal relation to underlying synaptic potentials. In contrast, EPSP in the somatic region should rise sufficiently fast that if they were above threshold, an impulse would be generated in close synchrony with the incoming synaptic potential.
FIG. 2.
Attenuation of synaptic potentials along a dendrite of a motoneuron in culture. Top traces: simultaneous recordings from 2 electrodes of spontaneous excitatory postsynaptic potentials (EPSP) from the soma and a dendrite. Bottom trace: difference of the 2 recordings. Note that early and late parts of the trace are dominated by somatic and dendritic EPSP, respectively. [Adapted from Larkum et al. (706).]
Spatial differences in the membrane resistivity will also affect integration of synaptic input. For example, in cat spinal motoneurons, the somatic membrane has a lower resistivity than the dendritic membrane (179, 369, 1288), perhaps due to the presence of somatic voltage-dependent K+ channels (179). However, this difference may instead be due to a shunt induced by the recording pipette (1247). Ongoing synaptic input will also change the resistance of the dendritic membrane and the driving force for the synaptic current, and consequently affect synaptic integration. An estimated fivefold decrease in the membrane resistivity will result if all of the excitatory boutons impinging on a spinal motoneuron release transmitter at a rate of 30 quanta/s (107). The synaptically generated shunt of the motoneuronal membrane in an intact behaving animal has yet to be measured.
C. Active Membrane Properties of Motoneurons
The dynamic regulation of motoneuronal excitability is largely determined by voltage-gated channels, which also are the targets for several neuromodulators affecting excitability (Table 1).
TABLE 1.
Membrane currents in motoneurons, their proposed function, and modulation by transmitters
| Current (Ions) | Proposed Function | Transmitter Modulation | Transmitter Reference No. |
|---|---|---|---|
| INa i (Na+) | Action potential | ||
| INa p (Na+) | Acceleration of membrane potential to spike threshold, amplify EPSP, linearize firing with increased current input | 5-HT | 527 |
| IK leak (K+) | Resting Vm | TRH, SP, NE, 5-HT, Glu (metabotropic) | 77, 287, 302, 367, 526, 703, 958, 1039 |
| IKir (K+) | Resting Vm, stabilize Vm around rest | ||
| IKdr (K+) | Action potential repolarization, fAHP | ||
| Ih (K+, Na+) | Resting Vm, stabilize Vm around rest, rebound potentials | 5-HT, NE | 526, 702, 959 |
| IA (K+) | Resting Vm, control onset of firing | NE | 958 |
| ICl leak (Cl−) | Resting Vm | ||
| IK Ca(BK) (K+) | Action potential repolarization | ||
| IK Ca(SK) (K+) | AHP | ||
| ICa HVA (Ca2+) | ADP, AHP, plateau potentials | 5-HT Adenosine, Glu (metabotropic) | 73, 285, 287, 521, 796, 882 |
| ICa LVA (Ca2+) | ADP, action potential repolarization | 5-HT | 93 |
| INa Ca (Na+) | Plateau potentials, afterdepolarization in specialized motoneurons | ||
| IK Na (K+) | Postdischarge hyperpolarization |
EPSP, excitatory postsynaptic potential; Vm, membrane potential; fAHP, fast afterhyperpolarization; AHP, afterhyperpolarization; ADP, afterdepolarization; 5-HT, 5-hydroxytryptamine; TRH, thyrotropin-releasing hormone; SP, substance P; NE, norepinephrine; Glu, glutamate.
1. Na+ currents
All motoneurons have a fast inactivating Na+ current (INa i), which underlies Na+-dependent action potentials. The exact membrane distribution of inactivating Na+ channels (Nai channels) is presently unknown. Because action potentials initiate in the unmyelinated initial axon segment, this region may have a higher concentration of Nai channels (34, 66, 141, 200, 240, 861, 1114). Normally, initial segment action potentials invade the somatodendritic membrane and give rise to action potentials, with inflections in typical intracellular recordings referred to as IS and SD spikes (66, 240, 454, 870, 919). Na+ channels in the dendritic membrane have not been demonstrated directly in motoneurons in vivo or in acute brain slices, but there is evidence for backpropagating Na+-dependent spikes in dendrites of cultured spinal motoneurons (707, 766). The voltage dependence and kinetics of activation and inactivation of Na+ channels in motoneurons are difficult to study, because motoneurons are not ideal for voltage-clamp techniques requiring a high time resolution. Because of their complex electrotonic structure, motoneuron membrane charging is slow; reliably controlling membrane voltage, i.e., obtaining a good “space clamp,” is not possible. In two-electrode voltage-clamp recordings from spinal motoneurons, the somatic INa i in spinal motoneurons activates and inactivates rapidly (τ ~1 ms; range, 0.1–1.3 ms depending on voltage) and exhibits some steady-state inactivation at resting membrane potential (66). The somatic membrane of neonatal rat spinal motoneurons has tetrodotoxin (TTX)-sensitive 14-pS conductance Nai channels (1068). Activation occurs between −60 and −20 mV, and inactivation kinetics are fitted by a single exponential function (τ ~1–4 ms) with a half-maximal potential of −82 mV. Interestingly, recovery from inactivation is rather slow (τ ~154 ms), suggesting that control of firing frequency in motoneurons is affected by recovery time from Nai channel inactivation. However, whether these values from the neonate are comparable to values in the adult CNS is unknown.
A persistent, i.e., noninactivating, Na+ current (INa p) is present in facial, hypoglossal, and trigeminal motoneurons (205, 870, 919). INa p activates below spike threshold, which would accelerate subthreshold membrane depolarization to spike threshold. INa p may represent Nai channels in a noninactivating gating mode, rather than a distinct type of Na+ channels (148, 243).
2. K+ currents
K+ channels are principal determinants of the subthreshold membrane behavior, action potential shape, and firing properties of motoneurons and are also important targets for neuromodulators affecting motoneuronal excitability. Several distinct types of K+ conductances are found in motoneurons (825).
The resting membrane potential of cat spinal motoneurons in vivo is typically between −65 and −75 mV (458, 459, 993, 1421), positive to the equilibrium potential for K+ (EK). This suggests that resting membrane potential is the result of balance between outward K+-, inward Na+-, and Cl−-leak conductances. The relative contribution of these leak currents in motoneurons under in vitro conditions is estimated as gNa/gK = 0.13 and gCl/gK = 0.25 (372). Other ionic currents, such as the inward rectifier K+ current (IKir), hyperpolarization-activated inward current (Ih), transient outward K+ current (IA), and Ca2+-activated K+ currents also contribute to the resting membrane potential (74, 525, 667, 701, 1261).
Inward rectifier currents, i.e., currents that reduce upon depolarization and increase with hyperpolarization, are mediated by Kir channels (481, 912, 1065). In some neurons, inward rectifiers are active at resting membrane potential, giving rise to a steady outward current, which is in balance with leak inward currents. When the membrane is relatively unperturbed, this equilibrium ensures that the membrane potential is stable near EK. However, if the membrane is depolarized, Kir channels close (due to a voltage-dependent block by polyamines and Mg2+), releasing the membrane to depolarize further (anomalous rectification). Transmitters acting on the Kir channels (through second messenger systems) can have a powerful influence on neuronal excitability by reducing the stabilizing action of the Kir current. Recently, several novel inwardly rectifying K+ channels (Kir2.1, Kir2.2, Kir2.4, GIRK1–3) have been identified in brain stem motoneurons (607, 1261). Remarkably, expression of Kir2.4 transcripts appears restricted to brain stem motor nuclei. Transcripts appear to be absent in higher brain structures. In hypoglossal motoneurons, block of IKir2.2 and IKir2.4 by extracellular Ba2+ leads to depolarization and firing, suggesting that these and related conductances indeed contribute to the motoneuronal resting membrane potential.
Delayed rectifier (IKdr), transient outward (IA), Ca2+-activated K+ [IK Ca(BK), IK Ca(SK)], and leak currents shape the membrane trajectory of action potentials and associated afterhyperpolarizations in motoneurons. The delayed rectifier is a sustained outward K+ current (1065) activated by depolarization, with slower activation kinetics than INa i (90% complete within 5 ms, Ref. 62). It contributes to the falling phase of the action potential and the fast afterhyperpolarization (fAHP). External tetraethyl-ammonium (TEA) blocks the delayed rectifier, lengthening action potential duration and blocking fAHP in motoneurons (62, 205, 523, 870, 919, 1113, 1213, 1311). Unitary K+ currents of the delayed rectifier type, observed in patches from the soma of neonatal spinal motoneurons (1068), have a ~10-pS channel conductance (in normal Ringer solution), activate between −70 and 0 mV, and deactivate slowly (60 ms at −60 mV).
IA is a transient, i.e., rapidly inactivating, outward K+ current activated by depolarization, that is deinactivated by hyperpolarization (481, 1065), affecting the onset and steady-state firing of motoneurons (525, 919, 1068, 1213, 1311). In trigeminal motoneurons (525), IA activates around −55 to −60 mV (preceded by a “priming” hyperpolarization), peaks within 5 ms, inactivates with a time constant of 6–8 ms, and is partially activated at resting membrane potential (525). In spinal motoneurons of neonatal rat, IA activates between −60 and −20 mV, quickly inactivates (τ ~15–60 ms), and has a conductance of ~19 pS in normal Ringer solution (1068). In motoneurons, 4-aminopyridine (4-AP) blocks IA, prolonging spike repolarization, and reducing fAHP (525, 919, 1213, 1311, 1425). However, it is unclear whether IA affects the interspike interval, since interspike hyperpolarizations in motoneurons may be too small to deinactivate IA (525). If IA indeed is active at resting membrane potential, it could affect the onset of firing by delaying the occurrence of the first spike in response to a strong depolarizing input.
Ca2+-activated K+ currents are ubiquitous, found in all neurons. They are the result of large (BK) and small conductance (SK) K+ channels gated by a rise in intracellular Ca2+, and in the case of the BK channels, also by voltage (1065). Both IK Ca(BK) and IK Ca(SK) are present in motoneurons (205, 463, 523, 647, 666, 667, 826, 870, 918, 919, 1070, 1213, 1291, 1311). IK Ca(BK), which also is called Ic, is selective for K+ and activates after an influx of Ca2+ during an action potential. In the absence of IK Ca(BK), the falling phase of the action potential is prolonged (1213, 1291, 1311). In patches from cultured mouse motoneurons and with symmetrical K+ concentrations (140 mM), BK channels have a large conductance (240 pS), a sigmoidal dependence on potential, an increased open probability with increased Ca2+ concentration, an inactivation time constant of 40 ms at low Ca2+ concentration (0.5 µM), and are blocked by external TEA (825, 826). Unitary Ca2+-activated K+ currents of the BK type in soma membrane patches of rat spinal motoneurons (1070) have a conductance of 82 pS in normal Ringer solution, are activated by intracellular Ca2+ and depolarization, activate rapidly (within 2–3 ms with 10−4 M Ca2+ internally and depolarization to −50 mV), do not inactivate in 100 µM internal Ca2+, and are blocked by external TEA and charybdotoxin.
IK Ca(SK) is a Ca2+-activated, voltage-independent K+ current blocked by the bee venom apamin and is the dominant conductance underlying afterhyperpolarizations. Spike afterhyperpolarization in motoneurons is blocked by inorganic Ca2+ blockers, intracellular injection of Ca2+ chelators, and notably by apamin (7, 205, 523, 647, 1311, 1426), suggesting that motoneurons express SK channels and that they play a critical role in the repetitive firing behavior of motoneurons. In mouse motoneurons in culture, unitary currents from SK channels have a ~18-pS conductance, a 3.5-ms mean open time, and show no voltage dependency (825).
Na+-activated K+ channels (IK Na) are found in membrane patches from spinal motoneurons (1069). IK Na does not appear to contribute to single action potentials but gives rise to the postdischarge hyperpolarization that follows trains of action potentials, due to accumulation of internal Na+.
A hyperpolarization-activated current (Ih, or IQ in some studies), which is a mixed cationic current carried by Na+ and K+, is found in motoneurons (7, 62, 74, 205, 523, 577, 870, 919, 957, 1214, 1313). Ih has relatively slow kinetics (τ ~100–400 ms, Ref. 74) and has a reversal potential positive to resting membrane potential (approximately −40 mV, Refs. 74, 1214), i.e., the net current is inward both at rest and at hyperpolarized potentials. Consequently, Ih opposes membrane hyperpolarizations, such as would be produced by inhibitory synaptic input. Activation-deactivation of Ih following hyperpolarizations gives rise to a postinhibitory rebound (PIR) and can lead to rebound bursts of action potentials. Ih may be partially active at rest, thereby contributing to the resting membrane potential (74, 701). A steady activation at rest also means that Ih affects motoneuron responses to depolarizing inputs. During depolarization, Ih deactivation will increase neuronal input resistance. When the depolarizing current or synaptic input is relieved, steady outward currents repolarize the membrane, generating an afterhyperpolarization because of the increased input resistance. The afterhyperpolarization then activates Ih, and the membrane returns to rest. (Fig. 3; Refs. 870, 957). Thus Ih seems to stabilize the membrane potential around rest and also underlies rebound depolarizations and hyperpolarizations. Spinal motoneurons in the newborn rat receive phasic excitatory and inhibitory synaptic input during neurochemically induced fictive locomotion (486), and Ih may play a role in generating rebound burst firing following phasic inhibitory synaptic input (98).
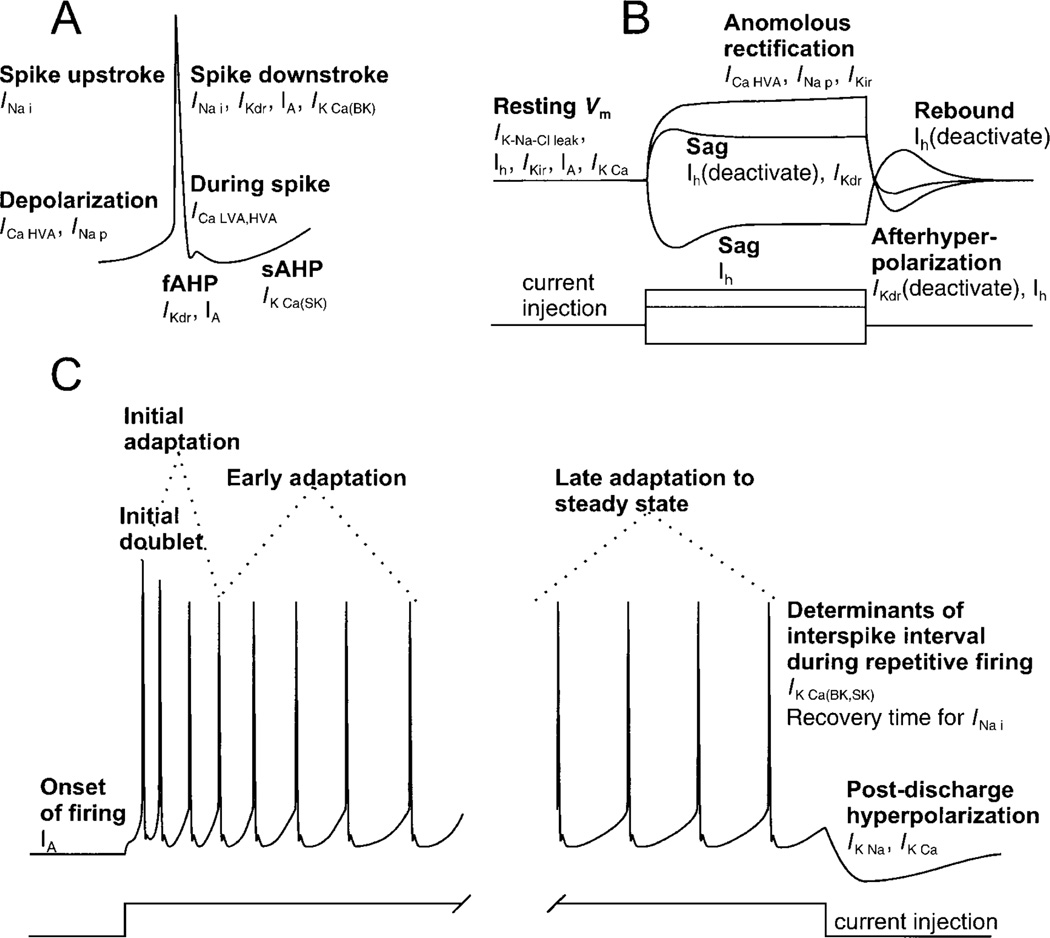
FIG. 3.
Supra- and subthreshold membrane behavior of motoneurons. A: ionic currents underlying the action potential waveform. B: ionic currents underlying subthreshold membrane behavior, in this case, elicited by a short-lasting depolarizing/hyperpolarizing square current pulse. C: different phases of adaptation during repetitive firing and postdischarge hyperpolarization after a long-lasting current pulse. Unless noted, currents are activated at times indicated. For definitions, see Table 1 and section iiC.
3. Ca2+ currents
At least six types of voltage-gated Ca2+ channels (L, N, P, Q, R, and T types) are expressed in CNS neurons (1030, 1275). The pharmacological and single-channel properties of motoneuronal Ca2+ channel subtypes have been worked out in greatest detail in neonatal hypoglossal motoneurons (1291, 1293, 1310). Three types of high-voltage-activated (HVA; L, N, and P type) and one type of low-voltage-activated (LVA; T type) Ca2+ channel types are present (1291), with single-channel conductances (with 110 mM Ba2+ as charge carrier) of 28 pS (L type), 14 pS (N type), 20 pS (P type), and 7 pS (T type). L- and P-type channels do not inactivate, whereas T- and N-type channels do (τ ~20 and 58 ms, respectively). Whether adult hypoglossal motoneurons express all of these types of Ca2+ channels is not known. Neonatal facial motoneurons also have HVA and LVA Ca2+ channel types in their somatic membrane (995). However, the HVA P-type channel is absent in the somatodendritic membrane, and a novel type (Rslow) carries a major component of the total HVA Ca2+ current (995). In this study, single-cell RT-PCR was used to detect mRNA for the α1A-subunit of HVA Ca2+ channel subtypes, along with other α1-subunit types. Because α1A-subunits are thought to combine to form P/Q-type Ca2+ channels, the absence of P-type channels in the soma suggests that P-type channels may be present elsewhere in the membrane of facial motoneurons, most likely in axon terminals (995). LVA and HVA Ca2+ channel types are also present in spinal motoneurons (524, 881). Ca2+ conductances affect the falling phase of the action potential, spike afterdepolarization (ADP), and afterhyperpolarization (AHP), in the latter case via IK Ca (463, 523, 524, 647, 870, 1291, 1310, 1311). The Ca2+ channel subtypes underlying ADP, which is most prominent in neonatal animals, and AHP can be distinguished pharmacologically and result from both LVA and HVA as well as HVA Ca2+ channels, respectively (647, 1291, 1311). The L-type Ca2+ channels play a central role in some motoneurons that are capable of generating plateau potentials (521, 527). In the absence of modulatory transmitters, inward current flowing through L-type Ca2+ channels is curtailed by outward currents (524), effectively generating a small inward rectification just subthreshold to spike initiation (870, 1112, 1111). Perturbations of the balance of inward and outward currents, e.g., in form of transmitter-induced reduction in outward K+ currents, can lead to plateau potentials and bistable membrane behavior by uncovering inward currents through L channels (121, 285, 521, 1200). In some specialized motoneurons, a separate mechanism operates to produce plateau potentials. Motoneurons in the rostral compact formation of the nucleus ambiguus, which innervate esophageal muscles, have a Ca2+-activated Na+ current (INa Ca) that produces prolonged plateaulike firing in response to brief afferent input or current injection. INa Ca resembles the Ca2+-activated nonspecific cationic current (ICAN) found in other CNS neurons (962). Spinal motoneurons in the turtle also have an ICAN, but in these motoneurons the current is not involved in generation of plateau potentials (970).
The spatial distribution of active conductances over the somatodendritic membrane is obviously important in determining the motoneuronal response to inputs. In turtle spinal motoneurons, Ca2+ conductances are present in dendrites (522), which may have important consequences for the transfer of synaptic input (1162), and in the generation and modulation of plateau potentials (521, 522, 524, 1200). In rat spinal motoneurons, L-type Ca2+ channels are present in the somatic and proximal dendritic membrane and N-type Ca2+ channels in both the dendritic and somatic membrane (1351).
D. Integration of Synaptic Input and Firing Modes of Motoneurons
The relationship between synaptic input to a motoneuron pool and the resulting muscle force, i.e., the motor pool input-output function, is generally described by a sigmoid curve (107). The initial upslope of the curve results from the orderly recruitment of motor units with increasing size, axonal conduction velocity, and fatigability, i.e., the size principle (107, 475). Rate modulation of the firing of motoneurons underlies further increases, with some contribution of continued recruitment. One of the main goals in the study of motoneuronal excitability is to understand how integration of synaptic input produces this input-output relationship, and how it is affected by neuromodulators. In broad terms, transformation of synaptic input to generation of impulses depends on the following: 1) motor unit type (types S, FR, FI, FF), 2) location of synaptic terminals, 3) character and distribution of active and passive membrane properties, and 4) effects of neuromodulators on synaptic transmission and on repetitive firing behavior.
In section iv, we discuss the mechanisms by which synaptic inputs affect the input-output relationship of motoneurons. In this section, we describe the basic firing properties of motoneurons.
Motoneurons fire repetitively in response to sustained suprathreshold excitatory input. This behavior has been studied mainly by injection of current pulses through an intracellular electrode. There are distinct phases of spike-frequency adaptation to such pulses, and a nonlinear rise in steady-state firing frequency with increasing current (Fig. 3; Refs. 437, 622, 625). At the onset of a long current pulse, an initial doublet [two spikes at short interval (<20 ms)] is often seen, which is part of the initial adaptation phase (0.5–1 s) during which the firing frequency drops sharply. A second phase of adaptation follows, which in some motoneurons (1087) is divided into an early (~2-s duration) and late phase (approaching steady state for long pulses). At the end of the current pulse, there is a postdischarge hyperpolarization followed by a return to resting potential.
Initial doublets, with instantaneous frequencies of 50–300 Hz, are seen in spinal motoneurons during endogenous motor behavior (488, 639, 673, 1417), suggesting that they are not an experimental artifact. Initial doublets may permit motoneurons to generate extra force at the onset of a contraction (476, 838), perhaps to overcome inertia, but the phenomenon is not necessarily correlated with a physiological need for strong contractions (639, 673). An increase in the magnitude and duration of spike AHP contributes to the initial adaptation (48, 62, 1312), dependent on Ca2+ entry (activating IK Ca) during the first few spikes (1088, 1301). However, blockade of Ca2+ influx does not entirely abolish initial adaptation. Other processes may contribute, such as deactivation of Ih at the onset of the depolarization, and changes in the threshold for action potential generation in the initial segment (1114). Late adaptation is also not abolished by Ca2+ channel blockers; in fact, it increases (1088, 1311), indicating that a number of Ca2+-independent mechanisms contribute to late adaptation. During repetitive firing, spike duration lengthens, and spike amplitude and rate of rise decrease (1312); this suggests that conductances that shape impulses change during maintained firing, leading to late adaptation. Late adaptation may be produced by a progressive increase/decrease in an unidentified outward/inward current (1088). Postdischarge hyperpolarization is blocked by ouabain, suggesting that it is due to an outward current generated by a Na+-K+ pump driven by local accumulation of Na+ (1088).
The relationship between steady-state firing rate and injected current (F-I relationship) increases in a linear fashion at low firing rates (primary range), then enters a steeper linear phase (secondary range) at higher firing rates (47, 623, 1109). In cat lumbosacral motoneurons, the secondary range starts when steady-state firing reaches ~50 spikes/s, with a slope 2–6 times that of the primary range (623). Many motoneurons lack the secondary range at steady state, with a linear F-I relationship over the entire firing range (573, 859, 870, 919). A persistent inward current (Ii, Ca2+ mediated) may underlie the secondary range (1003, 1114). The F-I relationship is highly dependent on the magnitude and duration of the spike AHP, since repetitive firing rate is directly related to the AHP duration (624). An effective way of modulating motoneuronal excitability is to modulate the magnitude of the AHP. For example, 5-hydroxytryptamine (5-HT) reduces the spike AHP (see sect. ivE) in cranial motoneurons, which leads to a dramatic increase in the slope of the F-I relationship (91, 526).
A particularly intriguing firing property of some motoneurons is the generation of repetitive firing that outlasts the period of excitatory input (239, 244, 326, 518–520, 527, 634, 1110). Plateau potentials underlie this behavior, which can be elicited in motoneurons by either short trains of excitatory synaptic input or current injection. Short-lasting inhibitory input can turn off the plateau potential, leading to bistable firing or membrane bistability under some circumstances. In most motoneurons, plateau potentials are not an endogenous membrane behavior but a latent property uncovered by activation of monoaminergic receptors (632, 634), and under modulatory control by other neurotransmitters (285, 1200). Thus motoneurons in spinalized cats (which are deprived of input from brain stem monoaminergic neurons) will exhibit plateau potentials when 5-HT and norepinephrine (NE) precursors are given intravenously (239, 244, 519), but not otherwise. A persistent Ca2+ current, possibly located in the dendrites, is the proposed ionic mechanism for generation of these plateau potentials in motoneurons (521, 522, 527, 720; see sect. ivE). During fictive locomotion (486, 1104) and in tonic muscle contractions associated with postural control (327, 633, 635), plateaulike firing is present in some motoneurons (486, 1104). Long-lasting plateau potentials are preferentially found in motoneurons with low thresholds for spike initiation and slow axonal conduction velocity, a hallmark of motoneurons of the S and FR type, which underlie most postural tasks (721, 722). The threshold for somatic activation (via current injection) of plateau potentials in cat spinal motoneurons is lowered by tonic excitatory afferent input (88). Because the plateau threshold can be lowered to the recruitment level of these motoneurons, plateau potentials under normal circumstances could play a role in amplifying the recruitment step rather than generating bistable behavior (88). An alternative view holds that plateau potentials serve to reduce the need for steady ongoing synaptic drive during maintained postural muscle contraction, through generation of self-sustained firing after transient synaptic input (634). Plateau generation in cat spinal motoneurons exhibits the phenomenon of “warm up,” i.e., a progressive lowering of threshold for plateau activation with repeated activation (3- to 6-s intervals) (89). Plateau warm up may represent a form of short-term plasticity in motoneurons that ensures an increased motoneuronal output during sustained motor acts such as repetitive movements, e.g., locomotion. Specialized motoneurons in the compact formation of the nucleus ambiguus (innervating the esophagus) show plateau potentials in response to short-lasting synaptic input or current injection (1041). This plateau potential is carried by an ICAN-like current and may generate prolonged spike activity in the ambiguus motoneurons during swallowing.
III. ORGANIZATION OF SYNAPTIC INPUT TO MOTONEURONS
Adult cat spinal motoneurons receive ~50,000–140,000 synaptic boutons (937), with >93% of the receptive membrane area in the dendrites. In L7 cat motoneurons, 61% of this space is covered by synaptic boutons. GABA/glycine-immunoreactive boutons dominate the stem dendrites, covering 69% of the membrane; glutamate-like immunoreactive terminals comprise 18% (ratio ~4). In more distal dendrites, the GABA/glycine-to-glutamate ratio falls to 1.5. About 6% of the dendritic boutons are not immunoreactive for GABA, glycine, or glutamate. Presumably these boutons contain other transmitters (937).
The origins of these synaptic inputs are of considerable interest, since they encode the functional significance of the incoming signals. In the following section we briefly summarize the anatomical organization of synaptic inputs to spinal and cranial motor nuclei. The majority of the cited studies are based on tracing techniques combined with immunohistochemical detection of putative transmitters. We do not attempt to give a complete description of all the known pathways; rather, we emphasize the major anatomical pathways and the organizational principles (Table 2, Fig. 4).
TABLE 2.
Major afferent inputs to brain stem and spinal motor nuclei
| Motor Nuclei | Reticular Formation/ Spinal Gray |
NTS | Spinal V Complex |
Vestibular Nuclei |
Peri-ambiguual Region |
Raphe Nuclei | Locus Coeruleus/ A7, A5 |
Pontine Nuclei |
PH/ RIMLF/ NIC |
PAG | Location of Premotor Neuron Unknown* |
Reference No. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oculomotor, abducens, trochlear (III, VI, IV) |
+ | + GABA | + | Angiotensin IV, bradykinin, endothelin, 5-HT, NE |
225, 281, 653, 684, 685, 699, 816, 849, 877, 891, 1176, 1344, 1346, 1383, 1420 |
|||||||
| Trigeminal (V) |
+ Met-Enk, ACh, GABA, Gly, Glu |
+ | + Glu | + 5-HT, SP, Met- Enk |
+ NE | + | ACh, angiotensin II, IV, endothelin, PTHRP, TRH |
83, 375, 451, 653, 737, 738, 740, 849, 982, 1210, 1263, 1277, 1278, 1315, 1342, 1416 |
||||
| Facial (VII) | + GABA, Gly |
+ | + GABA, Gly |
+ | + 5-HT, SP, Met- Enk |
+ NE | + | + | ACh, ADH, angiotensin IV, bombesin, prostaglandin, PTHRP, somatostatin, TRH |
192, 273, 376, 451, 482, 739, 740, 849, 850, 964, 1019, 1208, 1315, 1342, 1416, 1418 |
||
| Ambiguus | + ACh | + | + | + | + | + | ACh, ADH, CRF, GABA, Leu- Enk, NE, 5-HT, oxytocin, somatostatin, SP, TRH |
38, 159, 177, 281, 467, 574, 591, 698, 840, 892, 924, 998, 1096, 1144, 1315, 1393, 1427 |
||||
| Hypoglossal (XII) |
+ GABA, Gly |
+ | + GABA, Gly |
+ | + 5-HT, SP, Enk |
+ NE | + | ACh; adenosine; ADH; angiotensins II, III IV; ATP; DA; CRF; endothelin; NT; prostaglandin; PTHRP; somatostatin- 28; TRH |
12, 14, 85, 86, 122, 126, 235, 289, 399, 477, 653, 725, 739, 788, 789, 794, 836, 849, 935, 956, 1208, 1315, 1342, 1403 |
|||
| Spinal | + Glu, Gly | + | + | + GABA, Gly |
+ 5-HT, SP, Enk, CCK, NKA, Galanin, Glu |
+ NE Glu, Enk |
+ | Adenosine, Angiotensin IV, DA, vasopressin, CRF, NT, somatostatin |
41, 49, 84, 134, 209, 248, 300, 331, 354, 398, 409, 497, 501, 502, 504, 508, 659, 792, 848, 907, 908, 925, 939, 1012, 1128, 1161, 1183, 1267 |
+, Projection from premotoneurons to motoneurons; CRF, corticotropin-releasing factor; DA, dopamine; Gly, glycine; INC, interstitial nucleus of Cajal; Met-Enk, methionine-enkephalin; NKA, neurokinin A; NT, neurotensin; PTHRP, parathyroid hormone-related peptide; PAG, periaquaductal gray; PH, prepositus hypoglossi; riMLF, rostral interstitial nucleus of the medial longitudinal fasciculus; SP, substance P; TRH, thyrotropin-releasing hormone; ADH, vasopressin. Periambigual region is defined here as a region around and within the ambiguus nucleus in the ventrolateral medulla; locus coeruleus is defined as locus coeruleus and subcoeruleus nucleus; pontine nuclei include nucleus of the Kölliker-Fuse, parabrachial nucleus, pontine medial reticular formation.
Column indicates receptor expression, immunoreactivity, or physiological effect of putative transmitters within a motor nucleus, but with unknown location of the premotor neuron somata.
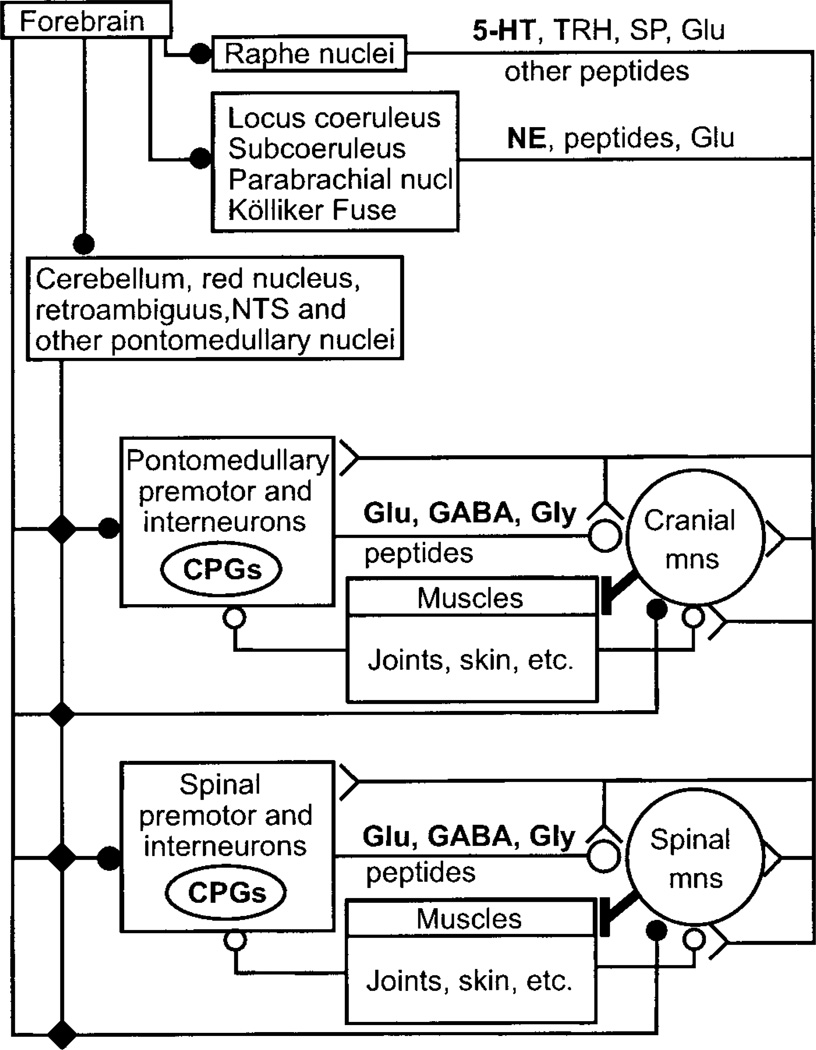
FIG. 4.
Anatomical organization of synaptic input to motoneurons. Main synaptic input to both cranial and spinal motoneurons comes from premotor and interneurons located close to the brain stem and spinal motoneuron pools; the few notable exceptions include direct corticospinal and rubrospinal inputs to motoneurons controlling the distal musculature, especially the digits, vestibulospinal projections to postural muscles, and bulbospinal projections transmitting inspiratory drive to phrenic motoneurons. Several cranial and spinal central pattern generators (CPG) are embedded in these premotor systems. The local premotor and interneurons also form the main gateway for relaying and integrating multisensorial afferent input from muscle, joints, skin, and descending synaptic information from forebrain, cerebellum, some brain stem nuclei, and the raphe, locus coeruleus, and other pontine/brain stem regions. Long projections from brain stem and pontine nuclei, both from diffusely projecting premotor groups, e.g., raphe and locus coeruleus, and from premotor groups involved in specialized motor tasks, e.g., respiration, equilibrium, posture, project directly to motoneurons, and to the local premotor and interneurons. Glutamate, GABA, and glycine are the principal transmitters of local premotor and interneurons but are also used in certain brain stem/pontine projections. 5-Hydroxytryptamine (5-HT), norepinephrine (NE), thyrotropin-releasing hormone (TRH), substance P (SP), and a host of other peptides are the main transmitters in the projections originating in brain stem/pontine nuclei, subserving modulatory functions in control of motoneuronal excitability. Symbols (solid circle, small open circle, large open circle, and fork shape) indicate different anatomical projection systems. Mns, motoneurons.
A. Afferent Projections to Spinal Motor Nuclei
Monosynaptic input to spinal motoneurons from sources outside the neuraxis originate exclusively from muscle spindle Ia and group II afferents (147, 502, 543, 830, 1184). The Ia projection likely uses glutamate as a transmitter (939). Propriospinal neurons provide the major synaptic input to spinal motoneurons (502). Labeling of spinal premotor neurons has been achieved by transneuronal transport of wheat germ agglutinin, virus, or retrograde labeling following discrete injection of tracers in motor columns. The pattern of premotor neuron labeling varies considerably depending on the motor group studied (21, 227, 513, 515, 566, 1012, 1264). Some spinal motoneurons receive synaptic input via recurrent axon collaterals from other motoneurons innervating the same or synergistic muscles (248, 249). Recurrent inhibition is mediated by Renshaw neurons that send inhibitory (GABAergic and glycinergic) projections to motoneurons innervating mainly proximal muscles (409, 1099, 1370). Interneurons and propriospinal neurons from most of the spinal cord laminae relay afferent signals from muscles, joints, and skin (49, 475, 563, 869, 1064), as well as segmental (22, 534, 1236) or supraspinal input to spinal motoneurons. They also generate coordinated, rhythmic patterns of activity such as locomotion and scratching that are ultimately transmitted to motoneurons (533, 1240, 1355); only a few studies have identified rhythmically active propriospinal interneurons with direct connections to spinal motoneurons (533, 1240).
Spinal motoneurons receive extensive projections from the brain stem. The raphe pallidus and obscurus contain premotor neurons that project directly to spinal motoneurons via the lateral and ventral funiculi (69, 508, 802). There are monosynaptic projections from the raphe pallidus to deltoid motoneurons (23) and medullary raphe nuclei to phrenic motoneurons (296). Raphe-spinal projections send off collaterals to the ventral horn of the spinal cord (529) and to the intermediolateral cell column (where the other class of nervous system efferents, preganglionic neurons, are located in the spinal cord), suggesting coordination of autonomic and somatic motor activity through common premotor neurons (20). Raphe premotor neurons are either serotonergic (265) or nonserotonergic (1161). Substance P (134, 209, 495), thyrotropin-releasing hormone (TRH) (134, 471), enkephalin-like peptides (134, 397, 497, 531, 723), cholecystokinin (792), neurokinin A (907), galanin (41), and glutamate or aspartate (908) colocalize with 5-HT (based on immunohistochemistry) in neuronal cell bodies in the caudal raphe and medial reticular formation, as well as in fibers and terminals in the ventral horn of the spinal cord (39, 41, 42, 133, 575, 576, 907, 908, 1034, 1237, 1297, 1348). Peptidergic immunoreactivity largely disappears from the inputs to motoneurons after destruction of serotonergic neurons with neurotoxins such as 5,6-dihydroxytryptamine (426, 576).
Noradrenergic premotor neurons projecting to spinal motoneurons are in the locus coeruleus, the subcoeruleus, the medial and lateral parabrachial nuclei, and the Kölliker-Fuse nuclei (222, 232, 503, 507, 585, 925, 1011, 1352, 1353). Met-enkephalin or glutamate is colocalized with NE in spinally projecting locus coeruleus neurons (395, 398, 749). Intraventricular injection of 6-hydroxydopamine reduces the ventral horn content of NE and the number of noradrenergic fibers by ~85%, suggesting that the brain stem premotor neurons are the main source of noradrenergic input to spinal motoneurons (715).
Spinal motoneurons also receive direct brain stem inputs from the retroambiguus nucleus (501, 1302), ventromedial medulla (GABAergic and glycinergic neurons; Refs. 504, 506), ventrolateral medulla (331, 354, 1047, 1248), and the nucleus tractus solitarius (NTS; Refs. 331, 874). On the basis of ultrastructural analysis of synapses in the ventral horn, a glutamatergic projection to spinal motoneurons originating in the ventromedial medulla may exist, but the location of the medullary glutamatergic premotor neurons is unknown (505). The vestibulospinal system, including neurons in the medial and lateral vestibular nuclei, projects to head and neck motoneurons and lumbosacral motoneurons (299, 448, 545, 1057, 1368).
Most corticospinal projections to motoneurons are indirect, typically via interneurons in the intermediate zone of the spinal cord. Direct corticospinal projections to some motoneuron groups innervating distal musculature and perhaps functionally involved in control of fine movement, are present in primates and to a limited extent in rats (125, 146, 252, 714, 741). Finally, the red nucleus projects monosynaptically to spinal motoneurons innervating distal muscles (391, 392, 500). Several other premotor neuron systems likely project to spinal motoneurons, since transmitter-like substances are found in fibers/boutons in spinal motor nuclei, and several transmitter receptors and physiological actions of transmitters have been demonstrated in motoneurons. The anatomical location of the premotor neurons using a particular transmitter remains unknown in most cases (Table 2).
B. Afferent Projections to Orofacial Motor Nuclei
Proprioceptive information from the muscles of mastication reaches the trigeminal and hypoglossal motor nuclei via the trigeminal mesencephalic nucleus (1015). Afferent information from other peripheral sensors enters the brain stem through vagal, glossopharyngeal, and accessory nerves and is conveyed to the trigeminal, facial, and hypoglossal nuclei via premotor neurons in the nucleus of the solitary tract (83, 122, 759, 1235, 1263). A third major sensory afferent input to orofacial nuclei is from the spinal trigeminal complex (122, 482, 544, 737, 1263). The largest concentration of premotor neurons to the orofacial motor nuclei is in the medullary and pontine reticular formation adjacent to the motor nuclei themselves. Thus hypoglossal premotor neurons are ventrolateral and dorsolateral in the medullary reticular formation (122, 297, 1263), and the majority of trigeminal and facial premotor neurons are in the pontomedullary and parvicellular reticular formations (482, 535, 850, 1263). Some of these premotor neurons are GABAergic, glycinergic, or glutamatergic (738, 739, 1210, 1277, 1278). In addition to these regions, a smaller number of premotor neurons to trigeminal, facial, and hypoglossal nuclei are located in 1) pontine nuclei (Kölliker-Fuse; parabrachial nucleus; and trigeminal, facial, and hypoglossal nuclei); 2) periaqueductal gray of the midbrain (facial and hypoglossal nuclei); 3) periambigual region (hypoglossal and facial nuclei); 4) vestibular nuclei (facial nucleus); 5) gigantocellular reticular nucleus (all 3 nuclei); and 6) paralemniscal zone in the lateral midbrain and external cuneate nucleus (facial nucleus) (175, 297, 474, 482, 544, 737–739, 935, 1209, 1263, 1404).
Noradrenergic input to the hypoglossal nucleus comes from neurons in three pontine regions, i.e., nucleus subcoeruleus, A7 and A5 cell groups (12, 14). The facial nucleus receives input from noradrenergic neurons in the A5 cell group (451) and trigeminal motor nucleus from the A7 cell group (451). This differential distribution of noradrenergic input to brain stem (and spinal cord) nuclei suggests that noradrenergic neurons can be divided into subgroups that differ in their connections and functional capacities (452).
The raphe pallidus, obscurus, and magnus are the main regions containing 5-HT-positive neurons projecting to the trigeminal, hypoglossal, and facial nucleus (376, 740, 789, 790). The raphe nuclei also contain premotor neurons positive for several neuropeptides. Substance P-like immunoreactive neurons in the caudal raphe project to the trigeminal, hypoglossal, and facial motor nucleus, and Met-enkephalin-like immunoreactive premotor neurons are in the caudal raphe and medial reticular formation (375, 376, 477).
Some premotor neuron groups projecting to the orofacial nuclei are involved in dedicated motor tasks and thus have more restricted projection patterns. The central subnucleus of the solitary tract contains the pattern generator for swallowing and conveys direct synaptic information to hypoglossal motoneurons and motoneurons forming the compact formation of the ambiguus nucleus (30, 67, 467). The Edinger-Westphal nucleus projects to the facial nucleus, forming part of the circuit mediating the corneal blink reflex (645).
The nucleus ambiguus contains esophageal, pharyngeal, and laryngeal motoneurons (106). Premotor neurons projecting to the ambiguus nucleus arise from the nucleus of the solitary tract (including the swallowing-related central subnucleus), zona intermedialis reticularis parvicellularis, pontine nuclei (Kölliker-Fuse, parabrachial nucleus), vestibular nuclei, periambigual regions, paraventricular hypothalamic nucleus, external cuneate nucleus, area postrema, and periaqueductal gray (67, 467, 591, 698, 924, 998, 1130, 1144, 1427, 1428).
Surprisingly, few projections have been demonstrated from the cortex to orofacial motor nuclei (1098), emphasizing the general scheme that voluntary motor commands to motoneurons likely pass through various groups of brain stem and/or spinal premotor neurons.
C. Afferent Projections to Oculomotor Nuclei
Eye muscles are innervated by motoneurons in the oculomotor, abducens, and trochlear motor nuclei. Several neuronal circuits in the brain stem and midbrain are dedicated to the coordination of these motor groups, and the organization of afferent input is consequently complex. Reticular formation premotor neurons projecting to the oculomotor nucleus are in the medial midbrain reticular formation (891), reticular formation of the mesodiencephalic junction (896, 1148), and the dorsal paragigantocellular reticular nucleus (191). Premotor neurons to the abducens and trochlear motor nuclei are also in the pontine reticular formation (699, 1115, 1344, 1420). All three motor nuclei receive input from the vestibular nuclei as part of the vestibulo-ocular reflex (225, 341, 434, 685, 699, 943, 1051, 1283). Some of these premotor neurons are GABAergic (superior vestibular nucleus) or cholinergic (the medial vestibular nucleus; Refs. 191, 685, 1346). Coordination between motoneurons in the oculomotor and abducens nuclei is partly mediated by GABAergic internuclear neurons projecting contralaterally between the nuclei (284, 817, 1346). The nucleus prepositus hypoglossi contains premotor neurons (possibly glycinergic) that project to all three oculomotor nuclei (341, 684, 699, 816, 1181, 1344). The oculomotor nucleus and the trochlear nucleus receive afferents from the rostral interstitial nucleus of the medial longitudinal fasciculus (GABAergic and glutamatergic; Refs. 1180, 1335) and interstitial nucleus of Cajal (654). A small number of neurons in the locus coeruleus, trigeminal sensory complex and olivary pretectal nucleus project to the oculomotor nucleus (191, 455, 646).
Several other premotor neuron systems likely project to cranial motor nuclei (see Table 2).
There are several common principles underlying the organization of synaptic input to spinal and cranial motoneurons (Fig. 4).
Multisensorial afferent input is conveyed via sensory nuclei in the brain stem and spinal gray.
Premotor neurons located close to the motoneuron groups in the reticular formation or spinal gray provide the major synaptic input to motoneurons. One notable exception is the phrenic motoneuron pool that receives inspiratory drive from premotor neurons in the medulla. These local premotor neurons form the main gateway for relaying and integrating multisensorial afferent input and descending synaptic information. Several spinal and brain stem central pattern generators (CPG) (locomotion, scratching, and others) are embedded in this premotor system.
Long projections from brain stem and pontine nuclei, both from diffusely projecting premotor groups (e.g., raphe, locus coeruleus) and from premotor groups involved in specialized motor tasks (e.g., respiration, eye movements, equilibrium), converge on brain stem and spinal premotor/interneurons as well as motoneurons.
Some motoneurons controlling distal limb muscles receive direct cortico- and rubrospinal synaptic input.
IV. TRANSMITTER MODULATION OF MOTONEURONAL EXCITABILITY
Motoneuronal inputs are affected by the presynaptic release of various transmitters (amino acids, amines, peptides) acting on postsynaptic receptors; several of these transmitters can also affect signaling by actions at presynaptic receptors. The integrative effect of these transmitters acting on their receptors, in concert with the intrinsic motoneuron properties, determine the generation of the efferent signals, action potentials propagated along peripheral motor nerve fibers and recurrent collaterals. The integration is complex. Actions at ionotropic receptors induce (or reduce) localized current flows that spread according to membrane properties and cellular morphology. Actions at metabotropic receptors initiate second messenger cascades that have myriad effects, including altering channel or receptor function. Many of these actions are convergent, that is, they ultimately act via the same signal transduction mechanisms, or affect the same target, such as a specific type of channel.
In this section, we review the principal neurotransmitter systems affecting motoneuronal excitability. In each section, we discuss the important ligands, the associated receptors, the effects on neuronal properties affecting excitability, the signal transduction pathways, and, where known, the function.
A. Glutamate: Ionotropic Actions
Glutamate is the principal fast excitatory neurotransmitter in the CNS (226, 854, 890). The potent excitatory action of glutamate on CNS neurons was first reported in 1960 for sensory and motoneurons of cat spinal cord (262). In this section we briefly review 1) glutamate receptor ligands; 2) the molecular biology of glutamate receptors; 3) the distribution of glutamate receptor subtypes/ subunits on different motoneuron pools; 4) functional implications of the structural diversity of glutamate receptors as it relates to motoneuron excitability in adult, during development, and in disease; 5) pre- and postsynaptic actions of glutamate; and 6) role of glutamate receptors in synaptic integration, production of oscillatory behavior and synaptic transmission.
1. Ligands
A major difficulty with establishing a role for glutamate and associated excitatory amino acids (EAA; l-aspartate, l-homocysteate; Ref. 342) in synaptic signaling is that they are also involved in metabolic processes. Thus determining whether their presence, synthesis, release, and transport underlies a metabolic or signaling function is difficult (469). The relative roles of the different EAA remain unclear. Limitations of the various techniques applied to the problem are reviewed elsewhere (469). Data are most consistent with l-glutamate as the main EAA at motoneuron synapses. Circumstantial evidence includes presence of high-affinity uptake mechanisms and high glutamine levels (456, 1151) in neurons and terminals that synapse on motoneurons (144, 808); detection of increased glutamate levels in perfusate from in vitro preparations following synaptic activation or glutamate uptake inhibition in rat and frog spinal cord (443, 614, 1223); potentiation of endogenous activity by EAA uptake inhibitors (142, 442, 818); and immunohistochemical detection of glutamate in boutons synapsing on motoneurons (878, 937, 1207). Furthermore, group Ia primary afferent boutons synapsing on retrogradely labeled motoneurons are enriched in glutamate-like immunoreactivity (939).
2. Receptors
Three ionotropic glutamate receptor subtypes, each assembled from an unknown combination of receptor subunits (most likely 5, but see Ref. 1059), are classified on the basis of pharmacological and functional properties as follows: 1) α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) [comprising glutamate receptor (GluR) 1–4 subunits]; 2) kainate (KA: high affinity; comprising GluR5–7 and KA1, KA2 subunits); and 3) N-methyl-d-aspartate (NMDA) receptors (comprising NMDAR1, NMDAR2A-D and NMDAR3A subunits). Two orphan subunits, δ1 and δ2, have also been identified (58, 100, 294, 499, 837, 853, 880, 1100, 1118, 1119).
The functional complexity of glutamate receptor-mediated synaptic signaling is conferred by variation in receptor subunit composition and further enhanced by posttranscriptional processing of gene transcripts through alternative splicing and RNA editing. All AMPA receptor subunits undergo alternative splicing of COOH-terminal sequences and flip and flop sequences near the M4 transmembrane domain. GluR2 subunit mRNA are edited at the Q/R site and GluR2, GluR3 and GluR4 subunit mRNA are edited at the R/G sites. Kainate subunit mRNA, GluR5 and GluR6, are edited at the Q/R site. GluR6 mRNA is also edited at I/V and Y/C sites. The NMDAR1 subunit exists as eight possible splice variants (NMDAR1a–4a and NMDAR1b–4b) generated through alternative splicing of one cassette in the NH2-terminal region and the individual or combined deletion of two cassettes in the COOH-terminal region (711, 887, 1194).
3. Distribution of receptors on motoneurons
AMPA, NMDA, and kainate receptors have been identified on motoneurons via receptor autoradiography (RAR), immunohistochemistry (ICC), and in situ hybridization (ISH). However, as receptor subunit composition and posttranscriptional modification contribute to the pharmacological and physiological properties of the GluR subtypes, we will focus on ICC and ISH studies. These data are summarized in Tables 3–5 and provide specific information on receptor subunit/splice variant expression within different motor nuclei and therefore may reveal a structural/molecular basis for motoneuron pool-specific differences in physiological properties and susceptibility to excitotoxicity/motoneuron disease. The functional implications are further explored in section ivA4.
TABLE 3.
Expression of AMPA receptor subunits in motoneurons
| GluR1 | GluR2 | GluR3 | GluR4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Motoneuron Nuclei | I | ISH | I | ISH | I | ISH | I | ISH | Reference No. |
| Edinger-Westphal | 1 | + | 2 | + | 1 | 1 | Rat 801* 1082 | ||
| Oculomotor (III) | 2.5 | nd | 3 | 3 | 3 | 3 | 2.5 | 2 | Rat 801* 978* 1082; human 1367 |
| Trochlear (IV) | nd | + | 3 | + | 3 | 2 | Rat 801* 1082 | ||
| Trigeminal (V) | 2 | nd | 3 | 3 | 3 | 4 | 3 | 3 | Rat 801 1279* 978* 1082 |
| Abducens (VI) | 2 | nd | 3 | 3 | 3 | 3 | 3 | 2 | Rat 801* 978* 1082 |
| Facial (VII) | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | Rat 801* 978* 1082 |
| Vagal (X) (DMV) | 1 | 1 | 2.5 | 2 | 2.5 | 1 | 2 | 1 | Rat 801* 978* 1082 human 1367 cat 28* |
| Ambigual | 1 | + | 3 | + | 2 | 2 | Rat 801* 1082 cat 28* | ||
| Hypoglossal (XII) | 1.5 | 2 | 3 | 3 | 3 | 2 | 2.5 | 3 | Rat 801* 978* 1082 human 1367 cat 28* |
| Spinal | 2 | 1 | 3.5 | 2 | 3.5 | 2.5 | 3.5 | 3 | Rat 801 966* 975 1082 1257† 407* 1206* 559 1206* 558* 1206, 1257, 1258* 1206* 1145 human 1367 |
Labeling intensity is relative, with 1 being low, 2 moderate, 3 strong, and 4 very strong. Values represent an approximate average of studies that have graded labeling intensity. Where (+) is indicated, study did not quantify expression levels. nd, not detected; I, immunohistochemistry; ISH, in situ hybridization.
Antibodies in above studies do not distinguish between GluR2 and -3.
Antibody to 2/3/4.
TABLE 5.
Expression of NMDA receptor subunits in motoneurons
| NMDAR1 | NMDAR2A | NMDAR2B | NMDAR2C | NMDAR2D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motoneuron Nuclei | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | Reference No. |
| Edinger-Westphal | 2 | 2 | 2 | Rat 977* 979 | |||||||
| Oculomotor (III) | 3 | 3 | 2 | 1 | 2 | nd | nd | nd | Rat 977* 979; mice 1340 | ||
| Trochlear (IV) | nd | nd | nd | Rat 977* 979 | |||||||
| Trigeminal (V) | 4 | 3 | 3 | 2 | 3 | nd | nd | nd | Rat 977* 979; mice 1279, 1340 | ||
| Abducens (VI) | 3 | 2 | 2 | Rat 977* 979 | |||||||
| Facial (VII) | 3 | 3 | 3 | 2 | 3 | nd | nd | nd | Rat 977* 979; mice 1340 | ||
| Vagal (X) | 2 | 2 | 2 | 1 | 2 | 1 | nd | nd | Rat 977* 979; mice 1340; cat 28 | ||
| Ambigual | 4 | 3 | 3 | 2 | 3 | nd | nd | nd | Rat 977* 979; mice 1340; cat 28 | ||
| Hypoglossal (XII) | 3 | 3 | 3 | 3 | 3 | 1 | nd | nd | Rat 977, 979; mice 1340; cat 28 | ||
| Spinal | 4 | 3 | 4 | 2 | 4 | 1 | nd | 0.5 | Rat 407, 765, 977, 1257* 979; mouse 1341; human 1078, 1145 | ||
I, immunohistochemistry; ISH, in situ hybridization; NMDA, N-methyl-d-aspartate; nd, not detected.
Antibody does not distinguish b/w NMDAR2A/B.
A) SPINAL MOTOR NUCLEI
Low levels of AMPA, kainate, and NMDA binding sites are seen in lamina IX of the spinal cord in rat (438, 558, 855), human (215, 565, 595, 1132–1134, 1136), and cat (845). Binding is typically <50% of the strongest labeling observed in the substantia gelatinosa and does not vary between different spinal cord levels. Although there are some inconsistencies between ICC and ISH studies, a general pattern emerges. For AMPA receptor subunits, ICC studies from rat (407, 558, 801, 1206) and human (1367) suggest that GluR4 ≥ GluR3/2 > GluR1. Similarly, ISH studies, which distinguish between GluR subunits 2 and 3, suggest that GluR4 = GluR3 > GluR2 > GluR1 (407, 559, 966, 1082, 1145, 1257, 1258), whereas recent studies in human spinal cord suggest low levels of GluR2 subunits (1137) and the complete absence of GluR2 mRNA expression (1366). Low-level GluR2 expression in spinal cord motoneurons is further supported by low GluR2 ICC relative to GluR3 and GluR4 in rat spinal cord (975). Analysis of GluR splice variants is limited to spinal motoneurons where expression of GluR2-flip, GluR3-flip and flop, and GluR4-flip and low levels of GluR1-flop in rat (559, 1238, 1257, 1258) differs from human, where all splice variants are present but flop isoforms predominate (1259).
The distribution of spinal cord kainate receptors has been least studied. KA1 and GluR5 are present in lumbar motoneurons (1257) and GluR5 is present in cervical motoneurons in rat (407). Detection of GluR6/7 and KA2 ICC in cervical motoneurons (976) contrasts with absence of ISH labeling of GluR6, GluR7, and KA2 in lumbar motoneurons (1257) and may reflect differential expression of subunits between cervical and lumbar motoneuron pools, but more likely reflects the greater sensitivity of ICC. There is much speculation on the cellular distribution and function of KA receptor subunits, since, with the exception of cultured hippocampal neurons (728, 729), dorsal root ganglia (528), and cerebellar granule cells (1170), presence of functional high affinity KA receptors in CNS neurons has not been confirmed. Recent developments of specific KA agonists and antagonists should facilitate functional characterization of KA receptors on motoneurons (217, 1201).
NMDAR1 and -2A subunits and transcripts are strongly expressed in cervical and lumbar motoneurons in rat (407, 765, 977, 979, 984, 1145) and mouse (1341), and NMDAR2A subunits are present in human (1078). The presence of NMDAR2B subunits remains uncertain as their localization with ICC in cervical spinal cord of rat is with an antibody that does not distinguish between NMDAR2A and -2B (977). In addition, ISH studies are inconsistent, showing low levels (765, 984) or absence of NMDAR2B transcript in rodent spinal cord (1257, 1341). Expression of NMDAR2D transcripts also varies from low levels (984, 1257) to none (765, 1145, 1341) in rodent spinal cord. NMDAR2C transcripts have not been detected (765, 1145, 1341). Thus the distribution of NMDA receptor subunits within the spinal cord appears to be NMDAR1 > NMDAR2A > NMDAR2B >> NMDAR2D > NMDAR2C with little variation along the rostrocaudal axis. NMDAR3A expression has yet to be examined. ISH analyses of the eight NMDAR1 splice variants (NMDAR1 1a,b to 4a,b) in the ventral horn of the cervical (765) and lumbar spinal cord (1255) are not entirely consistent. NMDAR1 type a and b splice variants are present within the ventral horn, as are NMDAR1–2 and NMDAR1–4 subunits. NMDAR1–3 expression is low in ventral horn cells. Furthermore, detection of multiple splice variants in single motoneurons supports heteromeric receptor assembly. Comparison of ISH images with functional analyses of heteromeric recombinant receptors and a more complete analysis of the properties of motoneurons will be required to determine whether splice variants of the NMDAR1 subunit contribute to heterogeneity of spinal motoneurons relevant to physiological and pathophysiological functions.
Assessment of the relative abundance of AMPA, KA, and NMDA receptors within spinal motoneuron pools is difficult, since most ICC and ISH studies do not simultaneously examine expression of all three receptor classes, nor do they assess labeling based on the summed expression of all receptor subunits. However, available data in rat indicate that AMPA ~ NMDA > KA (407, 1257), whereas data in rabbit suggest that AMPA ~ KA > NMDA (120).
B) CRANIAL MOTOR NUCLEI
As seen for spinal motoneurons, NMDA and non-NMDA receptor binding sites are low in brain stem motor nuclei relative to cortical regions (215, 795, 855, 856, 1135). NMDA receptor binding in motor nuclei subserving eye movements (III, IV, and VI nuclei) appears reduced relative to visceromotor nuclei [V, VII, and X (NA) nuclei] (1135) and the XII nucleus (855, 1135). In contrast, non-NMDA binding appears elevated in somatic motoneurons relative to visceromotor nuclei (215; see also Refs. 288, 865, 932, 1398).
With the exception of GluR1 subunits in the IV nucleus, GluR1–4 AMPA subunits and transcripts are present in all cranial motoneuron pools; GluR1 subunit expression is lowest, whereas levels of GluR2, -3, and -4 appear similar (28, 801, 978, 1082, 1367). Expression across functional groupings of cranial motoneurons is also similar, with the possible exception of a general reduction in expression of all subunits in general visceromotor nuclei (Edinger-Westfall, EW; dorsal motor nucleus of the vagus, DMV).
NMDA receptor subunit expression in spinal cord and brain stem motoneuron pools (EW, III, V, VII, X, NA and XII nuclei) are similar. In general, NMDAR1 > NMDAR2A > NMDAR2B (977, 979, 1340), with NMDAR2B identified in DMV and XII nucleus of mice only (1340). NMDAR2C and -D expression has only been examined in mice using ISH (1340), whereas NMDAR3A has not been examined. Again, differences in subunit expression across functional groups of cranial motoneurons are not obvious, aside from reduced signal in DMV and EW pools. Of note is that the IV nucleus fails to show immunoreactivity for NMDAR1 or NMDAR2A/B subunits.
Comparison of KA receptor subunit expression in spinal cord versus cranial motoneuron pools is premature. Expression of KA1 and GluR5 has not been examined, whereas moderate immunolabeling for GluR6/7 and KA2 has been observed in rat in all motoneuron pools examined (III, V, VI, VII, X, nucleus ambiguus, and XII nuclei). As seen for AMPA and NMDA subunit expression, ICC is reduced in EW relative to other motoneuron pools (976).
4. Physiological significance of glutamate receptor diversity
Molecular cloning and expression studies indicate that motoneuron responses to glutamatergic transmission, and to allosteric modulators, are determined by the type of subunits/splice variants that combine to form the glutamate receptor. Glutamate receptor expression is also dynamically regulated. Subunit expression can change during development (710, 711, 857), in response to afferent inputs and after ischemia (880). The physiological significance to motoneuron excitability of this potential diversity of glutamate receptors, however, is unclear. Continued development of subunit-/splice variant-specific agents such as Joro spider toxin (112), agriotoxin (478), Evans blue (616), and ifenprodil (1364, 1365; reviewed in Refs. 370, 427), combined with molecular physiological approaches similar to those used recently to introduce cDNA for the GluR1 subunit into motoneurons in vivo and in vitro (906), promise further insight into the role of specific glutamate subunits in controlling motoneuron excitability (906). Moreover, the implication that differential susceptibility of motoneurons to degeneration in motoneuron diseases may in part be attributable to molecular diversity of glutamate receptors (193, 559, 1082, 1134, 1135, 1258, 1366) should accelerate discovery in this area.
At present, the significance of specific subunits/splice variants to motoneuron excitability must be inferred by comparing the expression patterns in motoneurons with properties of 1) recombinant receptors studied in expression systems (e.g., Refs. 499, 752, 868) or 2) native receptors studied using a combination of whole cell recording and single-cell RT-PCR (e.g., Refs. 416, 584). This section examines key structural features of glutamate receptors most likely to be relevant to motoneuron excitability by focussing on patterns of glutamate subunit expression in motoneurons (see sect. ivA3) and the influence of these subunit/splice variants on channel kinetics (deactivation and desensitization), ionic permeability, and glutamate receptor modulation through phosphorylation. A complete discussion of the functional properties imparted to recombinant receptors by each of the 15 glutamate receptor subunits and their modified transcripts is available elsewhere (294).
A) AMPA RECEPTORS
I) Channel gating
Non-NMDA receptors activate and desensitize rapidly and are primarily responsible for fast excitatory synaptic transmission (294). Thus modulation of AMPA receptor properties can profoundly alter motoneuronal excitability. Subunit composition, alternative splicing of the flip/flop module, and RNA editing at the R/G site affect gating of AMPA receptors. The relative abundance of GluR2 (especially GluR2-flip) and GluR4 (especially the flop variant) subunits appears to be a major factor determining gating kinetics of native AMPA receptors (294, 416, 868). GluR2 flip subunits give rise to slowly gated channels, whereas GluR4 subunits determine rapidly gated AMPA channels. Thus high levels of GluR4 in spinal and cranial motoneurons in conjunction with reduced levels of GluR2 in spinal motoneurons may predict AMPA receptor complexes with rapid deactivation and desensitization kinetics (416, 868) as well as slow recovery rates from desensitization (752). For example, lumbar motoneurons in chicks have rapidly gated receptors with rapid desensitization kinetics (1166) and glutamatergic inspiratory drive to hypoglossal motoneurons is potentiated by cyclothiazide (which blocks AMPA receptor desensitization) (406). Not only are the rapid desensitization kinetics of AMPA receptors likely to play a role in shaping synaptic currents (50, 406, 1166), but rates of desensitization threefold faster than rates of resensitization can produce rapid frequency-dependent modulation of excitability, since synapses rich in this type of receptor should become much less excitable during high-frequency activation. Furthermore, if desensitization kinetics are subject to endogenous modulation similar to that produced by exogenous drugs (50, 406, 1126), allosteric modulation of desensitization kinetics can provide a powerful mechanism for modulating motoneuron excitability to glutamatergic inputs.
Posttranscriptional modification of motoneuron AMPA receptors may also be important in channel gating. For example, AMPA receptors assembled from flop variants generally have faster desensitization kinetics and slower recovery from desensitization than flip counterparts (416, 868, 1120). Thus developmental increases in expression of flop isoforms of GluR receptor subunits in rat (559) and preponderance of GluR flop variants in spinal motoneurons of human (1259) may increase the inability of motoneurons to reliably follow fast trains of stimuli, thereby decreasing excitability to glutamatergic inputs.
In contrast, RNA editing at the R/G site of GluR2, -3, and -4 subunits in brain increases developmentally and is associated with slower desensitization rates and faster recovery from desensitization (752). Thus, if R/G editing in motoneurons follows a similar pattern, it may enhance the excitability of motoneurons to glutamatergic inputs and increase their ability to follow high-frequency inputs. Thus interactions between receptor subunit, alternative splicing, and RNA editing may interact to control motoneuron responsiveness to glutamatergic inputs. Cells with rapidly desensitizing, slowly recovering AMPA receptors should respond only to the beginning of a sequence of fast inputs, whereas rapidly recovering, slowly desensitizing receptors should integrate incoming signals as they will transmit with greater reliability during trains of high-frequency stimuli.
II) Ionic permeability
Most AMPA receptors are permeable to Na+ and K+ but impermeable to Ca2+. Ca2+ impermeability is conferred by the presence of Q/R site-edited GluR2 subunits (167, 168, 498). Because virtually all (99%) GluR2 mRNA in the brain is edited at the Q/R site at all developmental stages, variation in Ca2+ permeability of AMPA receptors is determined by the presence or absence of the GluR2 subunit (167, 1120).
The relevance of Ca2+-permeable AMPA receptors to motoneuron physiology remains speculative. Potential consequences include modulation of repetitive firing behavior through effects on Ca2+-dependent K+ channels. Developmental increases in the amount of GluR2 relative to other subunits have important implications for activity-dependent development of motor circuits (559), whereas low-level expression of GluR2 subunits may contribute to the selective vulnerability of different motoneuron pools to neurodegeneration in conditions such as amytrophic lateral sclerosis (193, 559, 1082, 1137, 1258, 1318, 1366, but see Ref. 866).
III) Modulation of AMPA receptors by phosphorylation
Little is known of the importance of AMPA receptor phosphorylation in modulating motoneuron activity. However, given the prominent role of AMPA receptors in mediating synaptic drive to motoneurons, and that in many regions of the brain AMPA receptor function is regulated by phosphorylation of amino acid residues (serine, threonine, or tyrosine) of AMPA receptor subunits or associated proteins (68, 111, 363, 439, 655, 787, 821, 1052, 1229, 1331, 1396; for recent reviews, see Refs. 771, 871, 1053, 1173, 1174, 1256, 1395), phosphorylation may represent an important mechanism for modulating motor outflow from the CNS (e.g., Ref. 414).
Phosphorylation sites are not uniformly distributed on the different AMPA receptor subunits (583). Thus, in addition to physiological analyses of the effects of kinases and phosphatase inhibitors on AMPA-mediated synaptic inputs to motoneurons, determination of the receptor subunits that comprise AMPA receptors in motoneurons and identification of the phosphorylation sites in the AMPA receptor subunits are necessary steps to understanding the functional importance of phosphorylation, as well as subunit composition, in controlling motoneuron excitability. For example, although identification of multiple phosphorylation sites on the GluR1 subunit emphasize its importance in AMPA receptor regulation, its relevance to motoneuron control is questioned by the low-level expression of GluR1 in motoneurons. Potential phosphorylation sites on the remaining subunits (GluR2, -3, and -4, Ref. 895; GluR3, Ref. 617) may therefore be more relevant to control of motoneuron excitability.
B) NMDA RECEPTORS
NMDA receptors on motoneurons contribute to glutamatergic transmission, modulate repetitive firing behavior (through activation of IK Ca), contribute to nonlinear behavior (152, 314, 486), and reduce the voltage dependence of glutamatergic synaptic inputs (486, 745, but see Ref. 201). In addition, their long-duration excitatory postsynaptic currents (EPSC), Ca2+ permeability, and voltage dependence are important in the detection and reinforcement of weakly correlated synaptic inputs that is thought to contribute to use-dependent synaptic plasticity during development, as well in the adult (279, 837, 1094).
Given the involvement of NMDA receptors in control of motoneuron excitability, it is clear that modulation of NMDA channel properties through alterations in subunit composition, alternative splicing, and RNA editing will have significant impact on motoneuron behavior. The strength and sensitivity of the voltage-dependent Mg2+ block, channel kinetics, and glycine sensitivity vary, depending primarily on expression profiles of NMDAR2 subunits (2A–2D) (542, 683, 853, 857, 858, 1349).
For example, the voltage sensitivity of the Mg2+ block of heteromeric NMDA receptors (NMDAR1 to NMDAR2), as well as the magnitude of the block, is much stronger for receptors containing NMDAR2A or -2B relative to NMDAR2C or -2D subunits (857). Predominant expression of NMDAR2A and 2B subunits in cranial and spinal motoneurons (Table 5) should reduce the potential impact of NMDA-mediated Ca2+ influx on repetitive firing (through activation of IK Ca). The presence of significant levels of NMDAR2D subunits in lumbar motoneurons (1257), but not other motoneurons, may predict a greater susceptibility of spinal motoneurons to modulation by IK Ca.
Gating kinetics are also affected by subunit composition. The decay time constant of NMDA currents, which has significant implications for synaptic integration as well as use-dependent synaptic plasticity, varies 3- to 40-fold depending on subunit combination with the decay time constants of NMDAR1–2A recombinant receptors < NMDAR1–2B < NMDAR1–2C << NMDAR1–2D (857, 858). Cranial and spinal motoneurons, which predominantly express NMDAR2A and -2B subunits (Table 5), may experience shorter duration EPSC relative to NMDAR2D containing lumbar motoneurons (984, 1257). Longer duration postsynaptic potentials and weaker Mg2+ block of receptors composed of NMDAR1–2B, -2C, and -2D subunits would facilitate detection and reinforcement of weakly correlated inputs.
Phosphorylation of NMDA receptors by a variety of transduction pathways is also dependent on the presence of subunit-specific phosphorylation sites differentially distributed over NMDAR1 and -2 subunits (312, 542, 658, 871, 1076, 1249, 1250).
5. Pre- and postsynaptic actions of glutamate
A) PRESYNAPTIC ACTIONS
The initial observations of kainate-mediated depolarization of afferent terminals in the spinal cord (8), in conjunction with localization of presynaptic ionotropic glutamate receptors and their enhancement of transmitter release in hippocampus (218, 994), raised the intriguing possibility that synaptic transmission may be modulated by presynaptic ionotropic glutamate receptors. Evidence, however, is sparse (819) and currently does not support a role for such receptors at the level of motoneurons. In contrast, there is considerable evidence that metabotropic glutamate receptors modulate presynaptic transmission (see sect. ivB).
B) POSTSYNAPTIC ACTIONS
Generation of an inward current or depolarization of motoneurons by exogenously applied glutamate or its agonists is clearly established for all motoneurons examined and probably should be considered dogma. Currents induced by exogenous glutamate in neurons cultured from ventral spinal cord (810, 811, 1350) reverse near 0 mV, are relatively linear between −20 mV and more depolarized potentials, but show a little change in slope conductance at more hyperpolarized potentials. This rectification is due to the action of glutamate at AMPA and NMDA receptors. In general, AMPA-induced currents are nonrectifying, reverse near 0 mV, desensitize rapidly, and are mediated by Na+ and K+. AMPA receptors lacking GluR2 subunits, however, also pass Ca2+ and show doubly rectifying current-voltage (I–V) relationships. NMDA-induced currents, in contrast, exhibit a negative slope conductance in their I–V relationship between approximately −40 and −20 mV (due to a voltage-dependent Mg2+ block, Ref. 811), reverse near 0 mV, have slower kinetics of activation and longer decay than AMPA currents, and are mediated by Na+, K+, and Ca2+ (499, 1118, 1121).
Exogenous application of glutamate substantially increases motoneuronal conductance (up to 6-fold) (201, 745, 950, 1433). However, changes in conductance during synaptic activation of glutamate receptors are variable (201, 1139). During fictive locomotion in cat, motoneuron conductance can increase by ~20% relative to control. However, a conductance increase during the depolarized, relative to hyperpolarized, phase of the locomotor cycle occurs in only 8% of motoneurons (1139). Lack of phasic oscillation in input conductance in association with glutamatergic-mediated depolarization may reflect arrival of locomotor inputs on distal synapses where conductance changes are not detected with somatic intracellular recording (1162). Also, inhibitory inputs during the hyperpolarized phase of the cycle may produce increases in conductance similar to those produced during the depolarized phase by the excitatory inputs.
Despite the well-characterized nature of glutamate-induced currents, many potential sources of variability make description of a typical glutamatergic EPSP/C difficult. EPSP/C in motoneurons vary widely in shape, size, duration, onset and decay kinetics, voltage dependence, as well as antagonist sensitivity (86, 364, 412, 841, 926, 1158, 1433). The postsynaptic response to activation of a single glutamatergic synapse has been characterized in detail at few motoneuron synapses (412, 841, 1035). Stimulation of afferent pathways can lead to a distorted picture of individual synaptic inputs due to temporal dispersion in the arrival times of afferent impulses and difficulty in establishing an effective voltage clamp at distal synapses. Careful selection of somatic synapses for analysis is therefore required to obtain an accurate picture of evoked, spontaneous, or miniature EPSC. Differences between spinal and cranial motoneurons are not apparent. Activation of single group Ia afferent axons in vivo, primarily an AMPA receptor-mediated input (1327), produces an EPSC at somatic synapses with a peak amplitude of 330 pA (at resting membrane potential), a 10–90% rise time of 0.2 ms, and τ ~0.3–0.4 ms. The associated EPSP have a peak of ~100 V and, like the EPSC, reverse near 0 mV (364). AMPA-mediated EPSC evoked in spinal cord-spinal cord synapses in culture vary linearly in amplitude with membrane potential, have decay time constants between 0.6 ms (somal synapses) and 1–2 ms (proximal dendrites), and reverse near 0 mV (902). Combined AMPA/NMDA-mediated EPSC at similar spinal cord-spinal cord neuron synapses have decay time constants of 3.9 and 86 ms for the fast (AMPA) and slow (NMDA) components of the response, respectively (374). The slow component reverses near 0 mV, is blocked by NMDA antagonists, and is voltage dependent. Glutamate-mediated miniature EPSC (mEPSC) in lumbar motoneurons have a peak amplitude of 7.7 pA, a 1.2-ms rise time, and a 4-ms decay time constant (412). AMPA-mediated quantal EPSC in phrenic motoneurons have peak amplitudes of ~4 pA, whereas spontaneous EPSC have peak amplitudes of 5–50 pA, a mean rise time (10–90%) of 0.25–0.7 ms, and a decay time constant of 1.2–1.9 ms (746). In hypoglossal motoneurons, NMDA-mediated mEPSC average 16 pA in amplitude (range: ~5–60 pA), 10–90% rise time of 8 ms, and a decay time constant of >50 ms (at −50 mV). AMPA components of the mEPSC are markedly shorter, with decay time constants averaging ~10 ms (926).
Developmental changes in kinetic properties of mEPSC are also likely. Between embryonic day 17 and postnatal days 1–3, although mEPSC rise time does not change (412), mEPSC amplitude increases ~40% and the decay time constant almost doubles. Later changes in mEPSC properties have not been examined in detail. However, the time course of mEPSC in postnatal motoneurons (413) is within the range reported for afferent-evoked EPSP in spinal cord of adult cats (1036).
6. Synaptic integration
The spatiotemporal integration of glutamatergic synaptic inputs and production of action potentials as output by motoneurons, i.e., their transfer function, is poorly understood. Most models of motoneuron input-output have focused on steady-state conditions and are predicated on the (relatively) linear summation of synaptic (and injected) inputs (107, 108). Although linear summation at the soma is likely, experimental (163, 165, 1025) and simulation data (733, 1124) suggest that glutamatergic EPSP (1380) add in a nonlinear fashion. Active dendritic conductances and motoneuron bistability are present in many motoneurons (see sect. iiC) and, in conjunction with the voltage dependence of NMDA-mediated synaptic inputs (152, 374, 1433), will contribute to the nonlinearity (see below). Moreover, when glutamatergic inputs do add linearly, their combined effects on firing probability do not always sum in a linear fashion (359). In this light, the observation that unitary EPSP, or EPSP evoked from the same single fiber, vary up to eightfold in their rise times (reflecting dispersion of terminals over the dendritic tree, Refs. 33, 166, 1327) yet have similar amplitudes (33, 549) is also of interest. Motoneurons may therefore resemble hippocampal pyramidal neurons (197, 198) in having mechanisms for boosting the synaptic current at distal synapses to ensure equivalence in actions of synapses regardless of their location on the dendritic tree. Indeed, motoneurons may be geometrically arranged for optimal current transfer from dendrites to soma (859, 862).
The pattern of excitatory input may prove to be a very important factor in determining motoneuronal excitability (359, 1005). For example, transient glutamatergic synaptic inputs, such as the excitatory input from Ia afferents, appear to have a relatively greater effect on output than steady-state inputs (1004). Recent work where glutamatergic inspiratory drive currents to phrenic motoneurons were first recorded under voltage clamp and then reinjected under current clamp before and after removal of synchronous 20- to 40-Hz components, suggests that synchronized presynaptic activity that produces large, high-frequency components on rhythmic drive currents may play an important role in maximizing output for a given current input (960).
7. Role of glutamate receptors on motoneurons in production of rhythmic activity
NMDA receptor-mediated currents, by virtue of their voltage dependence, not only introduce nonlinearities into the synaptic integration process, but can contribute to oscillatory behavior. NMDA-induced currents underlie TTX-resistant bursting in abducens motoneurons (313, 314), mastication-related motoneurons (636), lamprey spinal locomotor motoneurons (449, 450), and neonatal rat spinal motoneurons (486). Depolarization is proposed to bring motoneurons into a voltage region where the Mg2+ block is removed, giving rise to a regenerative depolarization (328, 449, 450). As seen for 5-HT in motoneurons from embryonic amphibians (1155), modulation of NMDA channel function, as well as Ca2+-dependent K+ channels or voltage-dependent Ca2+ channels, can alter the balance of membrane properties to induce, or continuously regulate, oscillatory activity according to behavioral circumstances (326, 632). Thus, provided that NMDA receptors are endogenously activated during these behaviors (as seen for lamprey), motoneurons can actively shape motor output.
8. Role of glutamate in synaptic transmission
Unequivocal evidence that EAA act as transmitters at any motoneuron synapses is sparse (469). However, involvement of glutamate/glutamate receptors in synaptic transmission to motoneurons is strongly supported by electron microscopic analysis of synaptic inputs (878, 937), the inhibition by glutamate antagonists of excitatory synaptic activity in virtually all motoneurons examined, and the potentiation of endogenous activity by EAA uptake inhibitors (142, 442, 818).
Molecular physiological analysis of motoneuronal AMPA and NMDA receptors and the functional role of glutamate receptor diversity in mediating/modulating synaptic transmission is relatively unexplored. Ongoing development of agonists/antagonists specific for receptors containing certain receptor subunits/splice variants should foster new insight.
Current understanding of glutamatergic pharmacology at motoneuron synapses was advanced most significantly through development and application of specific NMDA and non-NMDA receptor antagonists (most recently the quinoxalinediones; Refs. 370, 514) to in vitro preparations of CNS tissue (ranging from thin slices of spinal cord/brain stem to rhythmically active brain stemspinal cord preparations in a variety of species; Refs. 94, 619–621, 813, 927, 944). Although it is important to remember that all receptors localized to a given synapse will act in concert to produce the postsynaptic response, considerable effort has been devoted to distinguish the relative roles of NMDA and non-NMDA receptors in mono- and polysynaptic transmission. This section considers some experimental difficulties of assessing the roles of these two receptor subtypes and then describes their contribution to the synaptic activation of motoneurons.
A) COMPLEXITIES OF ASSESSING NMDA AND NON-NMDA RECEPTOR CONTRIBUTIONS
I) Voltage-dependent Mg2+ block of the NMDA receptor
Experimental detection of the NMDA receptor contribution to monosynaptic inputs is complicated by the voltage-dependent Mg2+ block of the NMDA channel (811). Inclusion of high concentrations of the divalent cation Mg2+ in in vitro baths to isolate Ia monosynaptic inputs (557, 568) may obscure NMDA receptor contributions. Removal of Mg2+ in solutions bathing the neonatal rat spinal cord has inconsistent effects, revealing an NMDA component of the monosynaptic EPSP to motoneurons in one case (570) but not others (557, 568). More recent data, under conditions of normal Mg2+ levels, support a contribution of NMDA receptors to monosynaptic transmission (333, 335, 347, 368, 637, 991). The inability to observe a non-NMDA antagonist resistant component in monosynaptic reflexes (333, 347, 1190) does not rule out NMDA-receptor involvement. It may simply reflect the voltage-dependent block of NMDA receptors at resting membrane potentials (see below), i.e., the NMDA component does not show up in the absence of non-NMDA mediated, or experimental, depolarization (333, 347, 570, 637, 661, 991).
II) Developmental changes in NMDA receptors
Inconsistencies between studies may also result from a developmental decrease in the contribution of NMDA receptors to synaptic transmission. NMDA receptors are transiently expressed in high densities in the ventral horn during early postnatal development (432, 601). The magnitude of NMDA-induced depolarizations of spinal motoneurons decreases during development in neonatal rat between postnatal days 0–15 (516). In isolated spinal cord of embryonic rat, NMDA receptors contribute up to 50% of the short-latency dorsal root-activated EPSP, a contribution that decreases with age (1433). Complete loss of NMDA receptor involvement in monosynaptic transmission in mature mammals, however, is unlikely. Genes for NMDA receptor subunits are expressed in the ventral horn of adult rat cord, and a NMDA contribution has been observed in adult (335). Extension of experiments performed on adult rat cord in vitro (755–758) to include intracellular analysis of monosynaptic afferent inputs to motoneurons similar to that performed in neonates (661, 991) should help resolve this question.
III) Definition of mono- versus polysynaptic inputs
Establishing the relative roles of non-NMDA and NMDA receptors in mono- versus polysynaptic transmission is difficult, especially with stimulation of mixed afferent fibers. Stimulation of single, presynaptic axons can also be problematic because it produces postsynaptic effects with multiple (fast and slow) time courses. If the slow component is misinterpreted as polysynaptic, its depression by NMDA antagonists will not be seen to support involvement of NMDA receptors in monosynaptic transmission. Use of neonatal preparations in vitro advanced the pharmacological distinction of NMDA and non-NDMA receptors but perhaps clouded discrimination between mono- and polysynaptic inputs. Because of incomplete myelination, the conduction velocity of low-threshold fibers is ~10-fold slower in neonates than adults (1073). Differences in the threshold of activation for the different fiber types are also reduced. Although adult in vitro preparations address some of these issues, in vitro reductions in temperature to preserve spinal circuits may also affect response latency (109). Thus reference to mono- and polysynaptic transmission may be misleading and perhaps best reserved for paradigms where unequivocal distinction is possible; referring to early and late components of the afferent response (347) may be more appropriate.
B) ROLE OF GLUTAMATE IN TRANSMISSION OF PRIMARY AFFERENT FEEDBACK
I) Spinal reflex transmission
Primary afferent fibers have both mono- and polysynaptic projections to motoneurons, including those from Ia spindle afferents, Golgi tendon organs (Ib), and flexor reflex afferents (group II and III afferents from skin, joints, muscle, and group II spindle afferents), that mediate various reflexes. The general insensitivity of short-latency (presumptive monosynaptic) inputs and sensitivity of polysynaptic inputs to NMDA antagonists underlies the general consensus that non-NMDA receptors are primarily responsible for monosynaptic excitatory transmission while NMDA receptors underlie polysynaptic transmission (153, 277, 343, 346, 411, 470, 557, 568, 755–758, 996, 1160, 1246, 1381, 1382). Development of specific non-NMDA antagonists (514) confirmed the non-NMDA receptor involvement in monosynaptic (335, 347, 570, 637, 661, 758, 991, 1280) as well as polysynaptic afferent transmission (333, 347, 570, 637, 991).
The role of NMDA receptors in monosynaptic afferent transmission remains controversial. However, the existence of non-NMDA receptor antagonist-resistant components of monosynaptic inputs supports a NMDA receptor contribution (335, 570, 637, 991) (Fig. 5). For example, the Ia EPSP induced through muscle nerve stimulation in cats in vivo exhibits a GYKI 52466-insensitive component (335) that is weakly depressed by dl-2-amino-5-phosphonovaleric acid (APV) (368). In addition, the ventral root potential in adult rats in vivo (347), the monosynaptic EPSP in rat cord in vitro (637), and the fast EPSP associated with activation of ventrolateral spinal tracts (333) are depressed by NMDA antagonists. Similarly, a detailed intracellular analysis in isolated spinal cord of neonatal rat where polysynaptic inputs to motoneurons were reduced with mephenesin, reveals a significant NMDA component (991) (Fig. 5). Postsynaptic colocalization of NMDA and non-NMDA receptors at single afferent release sites mediating monosynaptic EPSP is further supported by similar sensitivity of NMDA and non-NMDA components of dorsal root or spinally evoked EPSC/EPSP to frequency-dependent changes in amplitude (661, 991, 1433).
FIG. 5.
dl-α-Amino-3-hydroxy-5-methylisoxazole-propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptor components of monosynaptic EPSP, elicited by stimulation of dorsal root filaments, in a L5 motoneuron in a hemisected spinal cord from neonatal rat. A: EPSP are shortened by bath application of 20 µM dl-2-amino-5-phosphonovaleric acid (APV; an NMDA antagonist). The remaining fast rising EPSP is blocked by addition of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; a non-NMDA antagonist) to the bath. B: addition of CNQX (10 µM) to the bathing medium eliminates a predominant non-NMDA receptor-mediated component of the monosynaptic EPSP, revealing a slow-rising EPSP, which is blocked by 20 mM APV. Bathing media contained 1 mM mephenesin (to reduce polysynaptic transmission), 5 µM strychnine, and 10 µM bicuculline. [Adapted from Pinco and Lev-Tov (991).]
While equivocal, taken as a whole the above data suggest that non-NMDA receptors underlie the majority of the short latency and perhaps even the longer latency reflex components, with a significant contribution from NMDA receptors to both components. The relative contributions of the two components may be dynamically regulated. NMDA receptor subunit composition and densities appear to decrease developmentally. In addition, rapid desensitization of AMPA receptors and their slow recovery relative to NMDA receptors (294) may dictate greater involvement of NMDA receptors in mediating high-frequency afferent inputs. Although NMDA receptors have traditionally been envisioned as important mediators of low-frequency activity (especially during development) because of the increased probability of summation associated with their prolonged EPSP (1094), further examination of frequency-dependent changes in the relative roles of NMDA and non-NMDA receptors is warranted.
II) Cranial reflex transmission
Data concerning the role of glutamate receptors in mediating primary afferent inputs to cranial motoneurons are sparse. In addition to the complexities discussed above, activation of primary afferent nerves is more difficult for cranial than spinal motoneurons because they are generally less accessible. Activation of specific functional classes of afferents through stimulation of nuclei or fiber tracts within supraspinal structures is a poor alternative, since stimulation of a common site of origin or defined fiber tract guarantees less functional identity than similar procedures in the spinal cord (164). Thus many of the glutamatergic afferent synapses on cranial motoneurons that have been studied cannot be functionally classified. Because afferent-evoked EPSP in cranial motoneurons are rarely induced from first-order primary afferent fibers, the relative roles of NMDA versus non-NMDA receptors in transmitting mono- versus polysynaptic primary afferent inputs has received limited attention. Recent development of in vitro preparations with intact bulbar reflex circuits should facilitate progress (55, 648, 829).
The relative contribution of non-NMDA and NMDA receptors varies among synapses to cranial motoneurons, but both are involved in transmitting afferent signals. Trigeminal-induced EPSP in rat abducens motoneurons are mediated by non-NMDA receptors (950). In contrast, monosynaptic EPSP to jaw-closer motoneurons elicited from spindle or periodontal receptor fibers in mesencephalic nucleus of V, when examined intracellularly (1273) rather than extracellularly (206, 656), show a small but significant NMDA component. The greater sensitivity to NMDA antagonists of extracellular potentials induced in trigeminal motoneurons through stimulation of tooth pulp versus oral mucosa further supports the differential involvement of NMDA and non-NMDA receptors (609). Similarly, the NMDA contribution to dual component EPSP elicited from the NTS fiber tract onto ambigual motoneurons in medullary slices, believed to be the efferent limb of the swallowing reflex, is larger than seen at most other cranial motoneuron synapses (1336).
C) ROLE OF GLUTAMATE IN TRANSMISSION OF DESCENDING AND PROPRIOSPINAL/PROPRIOBULBAR INPUTS
I) Spinal motoneurons
Many excitatory supraspinal and propriospinal motoneuron inputs are mediated by glutamate or a related EAA (31, 143, 156–158, 266, 267, 450, 928, see also Ref. 449). Presumptive monosynaptic EPSP/C evoked by stimulation of reticulospinal or propriospinal neurons/axons in the medial longitudinal fasciculus (MLF) (371), ventrolateral funiculus (VLF), and ventral horn (333, 992) are sensitive to NMDA and non-NMDA antagonists. Although pathway-specific variation in the relative contributions of non-NMDA and NMDA receptors is evident, non-NMDA antagonists primarily reduce EPSP amplitude while NMDA antagonists shorten EPSP duration. Parallel frequency-dependent changes in the amplitude of NMDA and non-NMDA components of EPSP/C evoked through VLF, MLF, and spinal cord (as well as dorsal root) stimulation, further supports the conclusion that NMDA and non-NMDA receptors are colocalized on the postsynaptic membrane (371, 661, 991, 992).
II) Cranial motoneurons
Postsynaptic colocalization of non-NMDA and NMDA receptors and their concurrent mediation of synaptic inputs are also apparent at synapses between propriobulbar neurons and cranial motoneurons. EPSP induced in ambigual motoneurons from presumptive NTS axons in vivo (1336) and mEPSC in hypoglossal motoneurons from unidentified sources (926) are sensitive to NMDA and non-NMDA antagonists (1336). That some mEPSP have kinetics and pharmacology consistent with exclusive involvement of NMDA receptors, however, again points to considerable diversity in the subtypes of glutamate receptors at different motoneuron synapses.
In summary, although the majority of data indicate involvement/colocalization of NMDA and non-NMDA receptors at motoneuron afferent synapses, the relative roles of the two major subtypes do not appear to be uniform. There is considerable diversity in the glutamate receptor complement of different motoneuron synapses. Although such diversity is undoubtedly important, its functional significance remains to be determined.
D) ROLE OF GLUTAMATE IN TRANSMISSION OF RHYTHMIC MOTOR DRIVE
Glutamate receptors play multiple essential roles in generation of rhythmic motor behaviors (356, 590). They mediate many of the afferent or descending signals that initiate these behaviors, which include locomotion (43, 590), chewing (608), and deglutition (105). Furthermore, exogenous glutamate can activate a variety of episodic rhythmic behaviors. Local application of glutamate into the NTS in vivo elicits deglutition (466, 627, 628), into the pontine micturition center elicits micturition (785, 963), and intrathecal glutamate activates locomotion (304). Bath application of glutamate agonists to various in vitro preparations is a common method of activating rhythmic motor networks underlying locomotion (202, 469, 486, 1168) as well as chewing (648).
Glutamate receptors are also essential for generation of rhythmic motor output from networks including those underlying locomotion (486, 1168), respiration (355, 357, 401, 405, 442, 1042, 1167), deglutition (105) and chewing (538, 648, 764). Distinguishing the role of glutamatergic receptors in rhythm generation from their role in transmission of the rhythmic drive to motoneurons, however, has been difficult due to the spatial proximity of many rhythm-generating networks to their output motoneurons. For example, application of EAA antagonists within the spinal cord to examine the pharmacology of rhythmic locomotor inputs to spinal motoneurons can interfere with rhythm generation itself.
I) Locomotion
Direct evidence for a role of glutamate receptors in signaling rhythmic locomotor drive potentials comes from experiments where the spatial proximity of locomotor rhythm-generating circuits and motoneurons has been overcome using muliticompartment perfusion chambers in vitro. Thus fictive locomotion is activated by local perfusion of discrete spinal cord segments with EAA agonists. Properties of rhythmic locomotor potentials in rat motoneurons outside the region of rhythm generation are consistent with NMDA and non-NMDA receptor involvement (201). Many motoneurons also receive tonic glutamatergic excitation during locomotion, which may serve to either increase motoneuron excitability, facilitate bistable behavior, or generate NMDA receptor-mediated pacemaker activity (see above).
II) Respiration
Spatial separation of cranial and especially spinal respiratory motoneurons from brain stem rhythm-generating networks has been exploited to establish the critical role of glutamate receptors in the transmission of excitatory inspiratory or expiratory drive (355, 401, 1026, 1042). The relative contribution of non-NMDA versus NMDA receptors is not firmly established. In vitro whole cell recording studies in rhythmic brain stem-spinal cord and transverse medullary slice preparations from neonatal rodents indicate that while phrenic (745) and hypoglossal motoneurons (405) possess NMDA receptors, inspiratory synaptic currents are unaffected by NMDA antagonists in the presence of normal Mg2+ concentrations (405, 745). In fact, inspiratory synaptic currents to hypoglossal motoneurons are similar in wild-type and transgenic neonatal mice lacking functional NMDA receptors (402). Furthermore, whole nerve (C4 or XII) inspiratory activity is not affected by NMDA antagonists (405, 442, 745) (in one case a minor decrease was observed, Ref. 818). Phrenic nerve output in adult rabbit and rat in vivo, however, is reduced by application of both non-NMDA and NMDA antagonists into the ventral horn (117, 216). Differences may reflect in vitro versus in vivo or neonate versus adult conditions. For example, in vitro, the concentration of glycine, an allosteric modulator of NMDA receptors, is reduced (96), and motoneurons may be relatively hyperpolarized due to deafferentation; consequently, NMDA channels may experience a greater Mg2+-dependent block. In addition, there are also developmental changes in expression of NMDA receptors. Although many brain regions show reductions in NMDA receptor expression, NMDA receptor binding in NTS and the ventrolateral medulla, regions containing premotor neurons which provide rhythmic respiratory drive to phrenic motoneurons, increases from postnatal day 0 and plateaus at approximately postnatal day 9 (1031).
III) Other
Glutamate receptor activation underlies myoclonic twitches in lumbar motoneurons that characterize REM sleep (1178), putative swallowing-related information transfer between NTS and ambigual motoneurons (1336), cortically evoked rhythmical masticatory inputs to jaw opener motoneurons (608), micturition (593, 805, 963, 1188, 1193, 1411–1413), and emetic (190, 278, 724) reflexes. The precise pharmacology of motoneuronal glutamate receptors involved in these rhythmic behaviors remains to be established.
9. Role of glutamate in activity-dependent development of motoneurons
Synaptic transmission during critical periods in early postnatal life plays a central role in acquisition of mature electrophysiological, morphological, and molecular properties of neurons (487). The propensity for activity-dependent (re)organization of circuits in the neonatal, but not the adult, nervous system underlies the hypotheses that 1) the molecular composition of neonatal circuits (including motor circuits) supports activity-dependent synaptic plasticity while maturational changes impede this process, and 2) features of glutamatergic transmission factor importantly in activity-dependent development of motor circuits. Activity-dependent restructuring of neuronal circuits has primarily been examined for thalamic and cortical sensory maps, but evidence for its role in motoneuron development is growing (361, 487, 599, 750, 899, 900). Developmental alterations in motoneuronal glutamate receptor subunit expression (558, 559), binding profiles (432, 595, 601), changing contributions of receptor subtypes to synaptic transmission (719, 1433), and reductions in NMDA-induced currents (516, 876) underscore not only the potential for developmental change in motoneuron excitability but the possible contribution of glutamate receptors to activity-dependent development of motoneurons.
Attention has focused on the role of NMDA receptors. Their long-duration EPSC, Ca2+ permeability, and voltage dependence factor prominently in theories of activity-dependent phenomena (279, 837, 1094). In non-motoneuron systems, NMDA receptor composition appears to be regulated to produce longer lasting EPSC in young tissue and facilitate activity-dependent synaptic plasticity (187, 479, 857, 1028, 1141, 1364, 1365, 1429).
NMDA receptors also appear to be involved in activity-dependent development at the motoneuron level. 1) Motoneuron somal growth and dendritic branching are inhibited by NMDA receptor antagonism in early postnatal life (594). 2) Afferent expression of Cat-301 proteoglycan, a proposed marker for activity-dependent development (596), is dependent on NMDA receptor activation (597), motoneuron activity (600), and activity in large-diameter afferent fibers (598). 3) Mouse dorsal root ganglion-ventral horn synapses undergo NMDA- and non-NMDA-mediated activity dependent modification in vitro (362, 901, 903). 4) Establishment of active sensorimotor synapses is correlated with increased motoneuron sensitivity to NMDA and kainate, and decreased sensitivity to glutamate, suggesting that dorsal root afferents affect the number, location, or binding affinity of glutamate receptors, perhaps through an activity-dependent process (1433). 5) NMDA antagonists extend the postnatal period where neonatal rats are able to recover from spinal cord injury (776). In fact, NMDA receptor properties and subunit composition may themselves be activity dependent. Early in development, when neurons receive few, weakly correlated inputs, long-duration NMDA-mediated events may be essential for synaptic organization. As development proceeds and synaptic inputs increase in strength and coherence, an activity-dependent change in NMDA receptor subunit expression/number favoring reduced NMDA currents may avoid excessive stimulation and excitotoxicity (1094). A developmental analysis of receptor subunit expression and properties is required to determine whether there is any transition in receptor properties to favor longer duration EPSP in motoneurons of younger animals.
Non-NMDA receptor properties may also be developmentally regulated to facilitate activity-dependent development of motoneurons. Higher levels of overall GluR subunit expression in neonates and a consistent bias of neonate motoneurons toward expression of flip GluR splice variants (559), which have higher gain than their flop counterparts, suggest greater excitability of neonatal motoneurons to glutamatergic inputs. The larger non-NMDA currents in neonates may lead to greater depolarization, greater activation of NMDA receptors, and greater Ca2+ influx. A greater Ca2+ permeability of neonatal AMPA receptors, suggested by the higher ratio of GluR1, -3, -4/GluR2 in neonatal motoneurons relative to adults (559), may also enhance plasticity in neonates.
In contrast to the postnatal period, disruption of glutamate/ NMDA-mediated synaptic activity during fetal life appears to be without major effect on motoneuron properties or synaptogenesis. Developmental changes in excitability of embryonic rat spinal motoneurons grown in culture, although affected by changes in electrical activity, are unaffected by glutamate antagonists (1392). Monosynaptic afferents connect appropriately with fetal chick or frog spinal cord motoneurons when synaptic activity is blocked (380). In the absence of functional NMDA receptors (NMDAR1 knockout mice), rhythmic respiratory motor output is present at birth and in vitro is undistinguishable from that of normal mice (402). A role for glutamatergic activity in the programmed decrease in motoneuron cell number that occurs during fetal development (apoptosis) (176, 386, 387, 695) remains possible (176).
The relative role of activity and glutamate receptors in the development of spinal versus cranial motoneurons, somatotopic organization of motoneuron pools (297, 997, 1343) and the cellular mechanisms underlying activity-dependent changes are all areas requiring further investigation.
B. Glutamate: Metabotropic Actions
Glutamate, in addition to its role as the principal fast excitatory neurotransmitter, also modulates neuronal excitability by activating a large family of metabotropic receptors.
1. Receptors
Metabotropic glutamate receptors (mGluR) are coupled through GTP-binding proteins to intracellular second messenger cascades (196, 1101, 1164, 1195, 1369). There are at least eight subtypes of mGluR (mGluR1 to -8) (3, 517, 804, 885, 931, 1231, 1232) that can be divided into three groups (groups I, II, and III) on the basis of sequence homology and pharmacological profiles (234, 989). Group I mGluR (mGluR-1, 5) are coupled to phospholipase C (PLC) and increase the synthesis of inositol 1,4,5-trisphosphate (IP3), and trigger intracellular Ca2+ release (3, 36, 804). Group II (mGluR2, -3) and group III mGluR (mGluR-4, 6–8) are negatively coupled to adenylyl cyclase and inhibit the formation of cAMP (315, 885, 931, 1085, 1231, 1232).
2. Cellular distribution of receptors
Metabotropic glutamate receptors are widely distributed in the CNS. There is a wide diversity and heterogeneous distribution of mGluR subtypes in different areas, with a unique differential cellular localization of receptor subtypes. Group I mGluR appear to be localized postsynaptically (71, 762, 800, 1146) where they act to increase neuronal excitability (905, 1105, 1108). Group II and III receptors are predominantly localized in presynaptic terminals (135, 638, 735, 1146, 1147) where they inhibit transmitter release (70, 373, 419, 773, 1075, 1107, 1272, 1314). This differential localization is by no means exclusive (419, 793, 1056). Nevertheless, the unique cellular localization of each mGluR subtype suggests that the precise placement of receptors is a crucial factor contributing to the control of neuronal excitability.
3. Modulation of synaptic transmission to motoneurons
Activation of mGluR causes a profound inhibition of synaptic transmission to spinal and cranial motoneurons (301, 561). Both group II and group III mGluR reduce spinal segmental transmission to lumbar motoneurons (181, 541, 562, 618, 1243) and bulbospinal inspiratory synaptic inputs to phrenic motoneurons (301, 302). Only synaptic events are depressed. These two groups of mGluR have no effect on postsynaptic depolarization induced by exogenous EAA (302, 541) or postsynaptic membrane intrinsic properties (181, 301). Moreover, the frequency of mEPSC is significantly reduced by both group II and group III agonists, whereas the mEPSC amplitude is unaffected (301, 302). This suggests that activation of presynaptic group II and group III mGluR inhibits glutamate release to motoneurons. Activation of mGluR can also enhance synaptic transmission, as seen in frog (433) and lamprey (224) spinal motoneurons.
The precise mechanisms underlying the presynaptic action of mGluR are not known. Inhibition of transmitter release from presynaptic terminals may result from the reduction of presynaptic Ca2+ influx (915). Inhibition of voltage-dependent Ca2+ currents after mGluR activation occurs in neuronal soma (219, 428, 730, 1089, 1185, 1204, 1272, 1410); perhaps a similar mechanism underlies the presynaptic inhibitory action of mGluR agonists. Another possible mechanism is the direct modulation of the exocytotic machinery by influencing the availability of vesicles or their probability of release (468). The activation of mGluR is effective in reducing the frequency of spontaneous mEPSC when presynaptic action potentials are blocked in motoneurons (301, 302) and in other neurons, such as in hippocampus (419, 779, 1091) and striatum (1282). These spontaneous mEPSC seem independent of presynaptic Ca2+ influx since they persist in the presence of Ca2+ channel blockers (419, 1090, 1091, 1102). Thus the inhibition of presynaptic Ca2+ currents does not seem obligatory for the reduction of transmitter release by mGluR. mGluR may inhibit transmitter release by interfering with the secretion cascade subsequent to presynaptic Ca2+ influx (419, 468, 1091, 1105, 1282).
Group II and group III mGluR both appear to be present in terminals presynaptic to motoneurons. Because their actions appear similar, what purpose is served by having both types present? One possibility is that each group of receptors is located in different sets of terminals. Another possibility is spatial segregation of each group within presynaptic elements (1146). In hippocampal neurons, group III receptors are predominantly located in presynaptic active zones, whereas group II receptors are found at the preterminal axon at a site away from the release sites (1146). Thus group III mGluR would be preferentially activated, whereas group II mGluR would be activated only under higher release conditions. However, because the identification of mGluR subtypes relies largely on the selectivity of agonists, and because the two groups show high homology, the possibility that various agonists act through the same receptor cannot be excluded.
In addition to presynaptic actions, modulation of postsynaptic ligand-gated receptors can alter synaptic transmission. In lumbar (1285) and cranial trigeminal (287) motoneurons, depression of synaptic transmission is mediated by postsynaptic mGluR that decrease currents through ionotropic glutamate receptors. Group I mGluR appear to be involved in this process (1285).
4. Modulation of intrinsic membrane properties
Activation of mGluR, most likely of group I, causes membrane depolarization (287, 301, 302, 316, 540, 560, 561, 1242, 1244). This effect persists after block of synaptic transmission, suggesting mediation by postsynaptic receptors. In rat phrenic (301, 302) and trigeminal (287) motoneurons, depolarization is due to an inward current produced by activation of postsynaptic group I mGluR (301). This inward current consists of at least two components: a dominant component resulting from the blockade of a Ba2+-sensitive resting K+ current (287, 302) and an unidentified Ba2+-resistant component (302). Inhibition of this tonic K+ current results in membrane depolarization and increase in membrane resistance, both effects increasing motoneuronal excitability. Thus motoneurons show enhanced firing in response to either synaptic current or injected current after activation of mGluR (301, 302).
mGluR modulation of postsynaptic Ca2+ currents can also increase motoneuronal excitability (287, 285). In turtle spinal motoneurons, mGluR sensitive to (R,S)-α-methyl-4-carboxyphenylglycine (MCPG), a nonselective mGluR antagonist, facilitate plateau potentials by increasing an L-type Ca2+ current (285). Furthermore, the facilitation induced by mGluR activation can be compartmentalized to affect only part of the motoneuron membrane, i.e., a plateau potential in a medial dendrite can be facilitated, with no simultaneous effect in a lateral dendrite (286).
5. Endogenous activation of mGluR in motoneurons
Motoneuron activity changes significantly after administration of mGluR antagonists. Synaptic currents in rat spinal lumbar and cervical motoneurons are enhanced by antagonists for group II and for group III mGluR (182, 183, 301). This is presumably caused by the blockade of presynaptic receptors, suggesting that endogenous activation of these receptors attenuates synaptic transmission. In phrenic motoneurons, the group I mGluR antagonist (R,S)-1-aminoindan-1,5,dicarboxylic acid (AIDCA) significantly reduces EPSC amplitude, suggesting that postsynaptic group I mGluR, along with various ionotropic receptors (441, 745), are activated by endogenously released glutamate during inspiration.
In summary, three groups of mGluR are functionally expressed in motoneurons to mediate differential effects on intrinsic and synaptic properties via distinct mechanisms operating at pre- or postsynaptic sites. The diversity of actions mediated by various receptor subtypes provides a wide dynamic range for modulation of motoneuron excitability.
C. GABA and Glycine: Ionotropic Actions
GABA (920, 1016) and glycine (675, 700, 1023) are the principal fast inhibitory transmitters in the mammalian CNS. They are involved in all aspects of nervous system function, including control of motoneuron excitability (87, 101, 675, 700, 782, 847, 1306).
In this section we briefly review 1) ligands responsible for endogenous activation of GABA and glycine receptors, 2) the molecular biology of ionotropic GABA and glycine receptors, 3) distribution of GABA and glycine receptors subunits amongst motoneuron pools, 4) the physiological significance of GABA/glycine receptor diversity to the control of motoneuron excitability, 5) post- and presynaptic actions of GABA and glycine, 6) functional role of GABA and glycine in modulating motoneuron excitability, and 7) development of ionotropic inhibitory systems.
1. Ligands
Compelling evidence indicates that GABA (920, 1016) and glycine (675, 700, 1023) are inhibitory transmitters in the mammalian CNS. β-Alanine is also a candidate ligand at GABA and glycine receptors (675, 1016); its effects are inhibited by GABA (SR-95531) and glycine antagonists (strychnine), and steroids potentiate the action of β-alanine at GABAA receptors (1384).
2. Receptors
A) GABA RECEPTORS
GABA produces its actions in the CNS by binding to two subclasses of ionotropic receptors, GABAA and GABAC, and one class of metabotropic receptor, GABAB (see sect. ivD). Pre- and postsynaptic GABAA receptors play prominent roles in modulating motoneuron excitability. GABAA receptors form Cl−-selective channels; have allosteric sites for benzodiazepines, barbiturates, neuroactive steroids; and are phosphorylated by protein kinase C (PKC) and protein kinase A (PKA) (59, 579, 592, 606, 893, 1152, 1363). They are hetero-oligomeric, pentameric, protein assemblies of unknown subunit combination or stoichiometry. At least 15 genetically distinct subunit subtypes (α 1–6; β 1–4; γ 1–4; δ) as well as alternatively spliced variants and additional posttranscriptional modifications underlie the molecular diversity of GABAA receptors (60, 242, 282, 309, 682, 867, 893, 1016).
GABAC receptors are ligand-gated Cl− channels that are insensitive to drugs that modulate GABAA and GABAB receptor function (123, 578, 580, 581). Compared with GABAA Cl− channels, they are more sensitive to GABA, less prone to desensitization, and have longer open times. Their molecular biology has not been fully characterized. It appears that ρ1–ρ2 subunits, which are typically referred to as GABAA receptor subunits, may form GABAC receptors (242, 338, 581). The role of GABAC receptors in regulation of motoneuron excitability is not known. GABAC type responses have been characterized predominantly in the retina. Furthermore, although ρ1 mRNA is exclusive to the retina, RT-PCR analysis of ρ2 indicates its presence throughout the brain, including the spinal cord. Its expression in, or presynaptic to, motoneurons remains to be established (338).
B) GLYCINE RECEPTORS
Glycine modulates motoneuron excitability through activation of a group of postsynaptic receptors belonging to a superfamily of ligand-gated ion channels that share homology with GABAA, nicotinic ACh, and 5-HT3 channels. Glycine receptors have been the subject of several recent reviews (81, 101–104, 674, 675, 700, 1023, 1306). They form Cl−-selective channels, possess allosteric sites for Zn2+, and are subject to positive (PKA, cAMP) and negative (PKC) modulation via receptor phosphorylation. The purified receptor contains two transmembrane subunits of 48 kDa (α) and 58 kDA (β) and a peripheral protein of 98 kDa (gephyrin). Glycine receptors form hetero-oligomeric, pentameric protein assemblies from α, which contain the glycine and strychnine binding sites, and β subunits. At least four genes code for separate α-subunits (α1, α2, α3, and α4), while diversity in β-subunits has not been found. Molecular diversity is further increased through alternate splicing. The α1-, α2-, and α3-subunits undergo alternative splicing to produce α1 ins, α2A and α2B, and α3K and α3L (917) splice variants, respectively, which modify pharmacological and electrophysiological properties of the receptor (82, 445, 676, 677). An additional subunit designated α2* differs from the α2A-subunit by only a single amino acid and likely represents an allelic variant of the α2-gene (81, 101–104, 674, 675, 700, 1023, 1306).
Gephyrin, a 93-kDa peripheral membrane polypeptide, is associated with the β-subunit (835). At least five splice variants exist (1008). Gephyrin is of primary significance because of its pivotal role in the formation of glycine receptor clusters, which most likely occurs through its anchoring of the receptor to the cytoskeleton (640, 641, 643, 1251, 1354). Although this protein may not be directly involved in signal transduction, its relevance to control of excitability is obvious due to the importance of synapse distribution over the somatodendritic tree in synaptic integration.
3. Distribution of inhibitory amino acid receptors on motoneurons
Understanding the types of receptors expressed by specific motoneuron pools and their spatial distribution on the surface of motoneuron in relation to functionally identified synapses (as well as the distribution of active dendritic conductances) is critical in understanding not only how motoneurons function as information processing units but how motoneurons target receptors to different synapse populations to effect various responses to input (831, 1162). This section 1) examines expression patterns of inhibitory amino acid receptor subtypes/subunits within the different motoneuron pools and 2) describes the spatial distribution of the amino acid receptors/ synapses over the somatodendritic tree.
A) GABAA RECEPTOR LOCALIZATION
I) Spinal motoneurons
Consistent with GABAA receptor binding studies (54, 349, 827, 1414, 1415), GABA or glutamate decarboxylase (GAD) immunostaining is widespread throughout the spinal cord, and lower in the ventral relative to the dorsal horn. GABAergic terminals are consistently found surrounding and contacting motoneuron cell bodies and proximal dendrites (186, 510, 775, 827, 875). GABA or its receptors are present in synaptic densities of cervical and lumbar motoneurons (290, 878, 937, 941, 942, 1027, 1197), at a low percentage of presynaptic (axoaxonic) terminals (290, 1197), and at extrasynaptic sites (1197).
α2- and α3-subunit mRNA are the most abundantly expressed of the α-subunits in the spinal cord. The α2-transcript (770, 971, 972, 1374) and protein (116) and the α5-subunit (116) are present in spinal motoneurons, whereas the α1-, α3-, and α6-transcripts (770, 971, 972) and α1-protein (115) are either absent or very sparse. Binding studies are generally consistent with these findings. Most spinal neurons have only type II benzodiazepine receptors (136) which, in transfected cells, are associated with α2- and α3-receptors (1009). Benzodiazepine type I pharmacology, which is not apparent in spinal neurons, is typically associated with α1-receptors.
β3-mRNA, but not β2 (971, 972, 1374), has been localized in spinal motoneurons. However, in contrast to dorsal horn cells and other regions of the spinal cord, the lack (116) or low level (25, 1045) of immunolabeling for β-subunit protein in spinal motoneurons questions a major role for β-subunits in native motoneuronal GABAA receptors.
Spinal motoneurons also express mRNA for the γ2-subunit (770, 971, 972, 1374) and γ2-immunolabeling (116, 1197). The γ1- and δ-transcripts have not been localized to spinal motoneurons (972). Differences in expression along the length of the cord are not apparent (116). Thus the combinations of GABAA subunits most likely to form receptors on spinal motoneurons are α2δ2 or α2b3δ2 in unknown stoichiometry. Continued development of subunit- specific antibodies (282) is required to confirm the presence of subunit protein.
II) Cranial motoneurons
GABAA receptors are also ubiquitous on cranial motoneurons (18, 875, 1177), but expression patterns of the various receptor subunits appear to differ between cranial and spinal motoneurons (Table 6). Facial and trigeminal motoneurons of adult rat express transcripts for α1-, α2-, β1–2-, and γ2-subunits but very low levels, or absence, of α3-, α5-, α6-, β1-, γ1-, and δ-transcripts (972). α1-mRNA is also strongly expressed in DMV, XII, facial, III, IV, VI, with only weak labeling in V (483). Immunohistochemical analyses with antibodies specific to α1–3-, α5-, β2/3-, γ2-, and δ-subunits indicate intense to moderate labeling for α1-, α2-, and γ2-subunits and weak labeling for α3 and α5 in III, V, VII, ambigual, DMV, and XII motoneurons. The only exceptions to this general pattern are that V and DMV show minimal labeling for α1 and that β2/3 has only been detected in the VII nucleus (388). Thus the major feature distinguishing cranial from spinal motor pools is the presence of α1- and α3-subunits in cranial motoneurons. Of considerable interest as well is the apparent lack of β2/3-subunits in all motoneuron pools (spinal and cranial), because β-subunits are widely expressed in many other neuron types. Differences between functional groups of cranial motoneurons are not yet apparent.
TABLE 6.
Expression of GABAA receptor subunits in motoneurons
| Motoneuron Nuclei | α1 | α2 | α3 | α5 | α6 | β1 | β2 | β3 | γ1 | γ2 | δ | Reference No. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | ||
| Cranial motoneurons | 388, 483, 972 | ||||||||||||||||||||||
| Oculomotor (III) | 2–3 | 3 | 2–3 | 1 | 1 | nd | nd | 2 | 2–3 | nd | |||||||||||||
| Trochlear (IV) | 3 | ||||||||||||||||||||||
| Trigeminal (V) | 1 | 1 | 2–3 | 1 | 1 | A/L | 1 | A/L | A/L | nd | 1 | 1 | 1 | 1 | A/L | 2 | 2–3 | nd | A/L | ||||
| Abducens (VI) | 3 | ||||||||||||||||||||||
| Facial (VII) | 2–3 | 2–3 | 2–3 | 2 | 1 | A/L | 1 | A/L | A/L | A/L | 1 | 1 | 1 | 1 | A/L | 2 | 2–3 | nd | A/L | ||||
| Vagal (X) (DMV) | 1 | 3 | 2–3 | 1 | 1 | nd | nd | 2 | 2–3 | nd | |||||||||||||
| Ambigual | 2–3 | 2–3 | 1 | 1 | nd | nd | 2 | 2–3 | nd | ||||||||||||||
| Hypoglossal (XII) | 2–3 | 3 | 2–3 | 1 | 1 | nd | nd | 2 | 2–3 | nd | |||||||||||||
| Spinal motoneurons | A/L | A/L | 2.5 | 3 | A/L | A/L | 1–2 | A/L | A/L | nd | A/L | 2 | nd | 2 | 2 | nd | 25, 115, 116, 770, 971, 972, 1045, 1197, 1374 | ||||||
Table 6 is based solely on data from immunohistochemical (I) and in situ hybridization (ISH) analyses. Subunits/transcripts for which data are not available have been excluded. Labeling intensity was reported as follows: nd, not detected; A/L, absent/low; 1, low; 2, moderate; 3, strong. Blank entry indicates data not available. Note: in general, antibodies to b2-subunits do not distinguish between β2 or β3.
B) GLYCINE RECEPTOR LOCALIZATION
Localization of glycinergic neurons and terminals has been examined by exploiting high-affinity uptake of [3H]glycine (494, 547) and glycine immunohistochemistry (180, 738, 739, 937, 941, 942, 1029, 1251, 1252, 1300, 1389). The postsynaptic localization of receptors on cranial and spinal motoneurons has been established through receptor autoradiography with [3H]glycine and [3H]strychnine (11, 140, 1010, 1061, 1419), immunohistochemistry (35, 115, 424, 1138, 1251–1253, 1270, 1271, 1300), and in situ hybridization (390, 642, 786, 1017, 1018, 1081, 1083, 1339). On cranial and spinal motoneurons, glycine receptors appear confined to the postsynaptic membrane, with few differences among the various motoneuron pools. One notable exception is the reduced labeling of visceromotor motoneurons (EW and DMV) relative to cranial motoneurons innervating striated muscle.
The differential expression of glycine receptor subunit proteins on motoneurons is poorly described. Specific antibodies are only available for α1-subunits and gephyrin. Few motoneuron studies have used the α1-antibody (80, 1270, 1271). Most have used a nonspecific antibody or the antibody to gephyrin (35, 115, 424, 1138, 1251, 1252, 1300; for description of antibody specificity, see Refs. 80, 981, 1271). Evidence that gephyrin may also be expressed at nonglycinergic synapses indicates the need for caution in using gephyrin immunoreactivity on its own as a marker for glycinergic synapses (1251).
Distribution of glycine receptor subunit transcripts assessed with in situ hybridization indicates marked developmental changes in expression patterns but similar patterns between the different motoneuron pools of the adult. In general, transcripts for α1- and β-subunits are highly expressed in adult cranial and spinal motoneurons (390, 786, 1081, 1083). α2-Transcripts are reduced relative to α1 (786, 1339) but show similar patterns of distribution (1017, 1018). Gephyrin transcripts are high in spinal motoneurons (642), whereas α3-mRNA is barely detectable in spinal ventral horn and hypoglossal motoneurons (786, 1159). Details of the α4-distribution are also unclear, but low levels are expressed in spinal cord of mouse. The only marked spatial difference in expression is that, relative to spinal and cranial motoneuron pools innervating striated muscle, α1-transcripts appear reduced in motoneurons innervating the eye muscles (1083). Consistent with immunohistochemical data, α1-, α2-, and β-transcripts are also reduced in visceromotor nuclei (EW and DMV) relative to motoneurons innervating striated muscle (390, 1081, 1083).
Subcellular distribution of glycine receptor transcripts may, however, differ between spinal and cranial motoneuron pools. In the majority of spinal cord motoneurons, α1- and α2-transcripts are localized in the soma and dendrites, whereas β-subunit and gephyrin mRNA are restricted to the soma (1017, 1018). In contrast, in facial, hypoglossal, and ambiguual motoneurons, α-transcripts are restricted to the soma (1018). Association of α-subunit mRNA in the dendrites with postsynaptic differentiations could provide dynamic modulation of synaptic efficacy (in spinal motoneurons but not cranial motoneurons) by changing composition and density of receptors at glycinergic synapses (1017).
C) SPATIAL DISTRIBUTION OF AMINO ACID RECEPTORS OVER THE SOMATODENDRITIC TREE
Ultrastructural characteristics of excitatory and inhibitory synapses on motoneurons are well established. Analysis of the distribution of presumptive excitatory (glutamatergic; S- or M-type terminals) and inhibitory (GABA- or glycinergic; F-type terminals) boutons (97, 114, 238) (139, 691, 1058), combined with postembedding immunohistochemical studies of amino acid transmitters in boutons synapsing with motoneurons, have provided important insight into the spatial distribution of synapses on motoneurons. Although many of the original studies focused on the soma and proximal dendrites (290, 291, 510, 941, 1027), extension of these studies to include distal dendrites has been essential, since dendrites comprise >90% of the motoneuron receptive domain.
A number of general organizational features are emerging. 1) Between 85 and 95% of boutons on spinal motoneurons (lumbar, phrenic, sacral) are immunoreactive for glutamate, GABA, and/or glycine (878, 937). 2) There is extensive colocalization of GABA and glycine with terminals that are exclusively glycinergic exceeding those that are exclusively GABAergic (582, 937, 941, 942, 1205). 3) Close to 60% of boutons apposing dendrites of spinal and brain stem motor nuclei of cat and rat contain glycine and/or GABA (291, 937, 941, 942, 1027, 1071, 1150, 1205). 4) Glutamate-enriched boutons appear to comprise just over one-third of boutons in lumbar and sacral motoneurons (937) and between ~48 and 58% of boutons in the phrenic nucleus (878, 1207). 5) The proximal compartment (soma and stem dendrites) appears to be under powerful glycine/GABA inhibitory influence. The proximal compartment of lumbar motoneurons has a glycine/GABA-to-glutamate synaptic ratio of 3.5– 4:1, and the distal compartment has a ratio of ~1.5:1 (937). The proportion of glutamate and inhibitory synapses does not appear to vary between proximal and distal dendritic compartments in phrenic motoneurons (878). However, exclusion of smaller distal dendrites from the sample raises the possibility that the proximal compartment of phrenic motoneurons is also under stronger inhibitory control. 6) Inhibitory synapses are not limited to the soma and proximal dendrites. Distal compartments of lumbar motoneurons show a uniform balance between inhibitory and excitatory synapses, with glutamate accounting for 40% of synapses (937). The importance of inhibitory synapses in dendrites has long been recognized. Not only can inhibition gate action potential production at the soma, but it can control the weight of excitatory inputs from different dendritic regions (1162).
The spatial distribution of functionally identified synapses and the molecular composition of glutamate/GABA/glycine receptors underlying these inputs are not yet known. Postembedding immunohistochemical studies with antibodies specific to the different receptor subunits in conjunction with electron microscopic analyses, similar to that used for NMDA receptor subunits in hippocampal and cerebellar neurons (977, 979), will greatly increase understanding of the ultrastructural localization of receptor subunit protein on the cell soma and dendrites of motoneurons (for reviews on factors controlling glutamate receptor distribution, see Refs. 324, 662, 1349). Description of the receptor subtypes/subunits mediating specific physiological inputs will require controlled activation of synapses at specific sites on specific dendritic branches, refinement of techniques for precise application of agonists/antagonists, and continued development of subtype/subunit-selective agonists/antagonists (370).
4. Physiological significance of GABA and glycine receptor diversity in modulation of motoneuron excitability
Inhibitory control is elaborated by GABA and glycine receptor diversity, heterogeneous spatial distribution of receptors, and colocalization of GABA and glycine. As previously discussed for glutamatergic transmission, the physiological significance to motoneuron excitability of the potential diversity of GABA and glycine receptors conferred by multiple subtypes, subunits, and posttranscriptional modification remains one of the major unanswered questions of amino acid transmission. Relatively little is known of the structure-function relationships of native GABA receptors in any neuron. In motoneurons, attempts to identify the native GABA receptors have been limited to immunoprecipitation studies (971) and immunochemical and in situ hybridization analysis of subunit expression. Attempts to match properties of native receptors to those of recombinant receptors have not yet identified a single native GABAA receptor in any neuron; however, analysis of the contribution of subunits to functional properties of recombinant receptors is far from complete. Physiological properties of recombinant receptors affected by subunit composition include GABA potency, conductance state, gating properties, modulation by steroids and phosphorylation, and intensity of benzodiazepine amplification of Cl− currents (150, 242, 772, 1016). The signal transduction mechanism, however, remains virtually unchanged in various receptor subtypes. The major functional implication of GABAA receptor diversity may be associated with changes in the channel-gating potency of GABA (242). The EC50 of 19 different subtypes of recombinant GABAA receptors varies from 0.3 to 15 µM (308). The importance of varied gating affinity of GABA receptors in control of motoneuron excitability is not clear, but in hippocampal and cortical pyramidal neurons, it is postulated to be critical for synchronizing the firing rate and coordinating neuronal interactions in columnary cortical activity (242).
The potential diversity of glycine receptors is less than that for GABAA receptors. However, variations in subunit composition affect gating properties and may account for the heterogeneity in the voltage dependence and desensitization properties of glycine responses (1023). The most obvious change in glycine receptor structure and function occurs during the first 2–3 postnatal weeks, when the fetal/neonatal receptor (most probably an homomeric α2-receptor) matures to the adult heteromeric form that lacks significant α2-subunit. The open time of recombinant α2-receptors is much greater than for homomeric α1- and native adult receptors (1159, 1219). These changes are consistent with developmental decreases in the decay time course of inhibitory postsynaptic currents (IPSC) in spinal neurons. In hypoglossal motoneurons, a postnatal switch from α2- to α1-glycine receptor subunit expression correlates with shorter channel open times and faster PSC/P decays, matching kinetic properties of glycinergic synaptic potentials to membrane properties of the motoneurons (1159). Thus changes in glycine receptor structure appear to significantly alter glycinergic transmission during development.
It is unlikely that any single approach will reveal the functional and molecular profiles of native GABA or glycine receptors in individual motoneurons (824, 1375); electrophysiology remains the best approach for assessing physiological, biophysical, and pharmacological properties of GABAA/glycine receptors (1407), but without subunit specific agonists/antagonists, it does not provide information on subunit composition. Difficulties of examining multiple subunits in a single neuron must be overcome to identify native receptors.
For a more thorough treatment of physiological properties that may be conferred by the presence or absence of specific receptors subunits on motoneuron membrane, we refer readers to our summary of receptor subtypes/subunits expressed on specific motoneuron pools and recent reviews on analysis of recombinant GABAA (242, 761, 824, 852, 1016, 1309) and glycine receptors (1023, 1306).
5. Post- and presynaptic actions of inhibitory amino acids
A) POSTSYNAPTIC ACTIONS: GABAA AND GLYCINE RECEPTORS
Inhibitory postsynaptic potentials were first recorded in cat motoneurons (241) and subsequently attributed to activation of glycine and GABAA receptors (257, 668, 1347). Activation of glycine (213, 257, 649, 668, 1211, 1215, 1221) or GABAA receptors (257, 275, 668, 782, 1221) in adult spinal and cranial motoneurons by exogenous or synaptically released agonists elicits similar responses comprising an opening of Cl−-selective ion channels, inward movement of Cl−, a decrease in membrane resistance, and membrane hyperpolarization. GABA and glycine receptors also have at least four similar conductance states, although the main conductance state differs (124, 462, 1169, 1218). The primary difference between the two receptors lies in their response kinetics; GABAA receptor-mediated responses decay more slowly and show greater desensitization.
Although predominantly hyperpolarizing, GABA and glycine responses can also be depolarizing if intracellular Cl− concentration is elevated. Thus, in neonatal (or fetal) motoneurons, where intracellular Cl− is elevated relative to adult, responses to GABAA (413, 1192, 1389) and glycine agonists, either exogenously applied (413, 1389) or synaptically released (412, 557, 582, 1159, 1211), are typically depolarizing ( flux does not contribute to the depolarizing response in motoneurons, Refs. 413, 967). Note that although these Cl−-dependent potentials are depolarizing, large decreases in input resistance are believed to underlie the fact that they remain inhibitory (412, 413, 1159, 1192, 1389).
The ontogeny of many aspects of GABA and glycinergic transmission has been examined in spinal motoneurons (412, 413, 1389); however, a clear description of the developmental stage where depolarizing GABA/glycine responses become hyperpolarizing is lacking. Whole cell recordings, while providing valuable information on kinetics of GABAergic and glycinergic IPSP/C of neonate and adult motoneurons (1215, 1218), are not well-suited for these measurements because of disruptions in internal Cl− concentration. Perforated-patch recordings using the Cl−-impermeant ionophore gramicidin indicate a change in reversal potential of glycinergic IPSC in hypoglossal motoneurons from −37 to −73 mV between postnatal days 0 and 18 (1159). A more complete developmental analysis using similar techniques in spinal as well as cranial motoneurons is required.
In summary, activation of GABAA and glycine receptors decreases motoneuron excitability, apparently regardless of whether the responses are depolarizing or hyperpolarizing. In adults, membrane hyperpolarization combines with a significant reduction in input resistance that shunts excitatory inputs to reduce excitability. In neonates, the reduction in input resistance and associated shunt has the dominant effect on excitability, reducing action potential output to injected and synaptic current despite the membrane depolarization (413, 668, 1192, 1389). Note, however, that a depolarizing versus hyperpolarizing shunt will have different effects on voltage-gated channels. The consequences of this differential activation of voltage-gated channels for motoneuron excitability remain to be studied.
B) COLOCALIZATION/CORELEASE OF GABA AND GLYCINE
A recurrent theme in studies of inhibitory transmission is the extensive degree of overlap between GABAergic and glycinergic systems. There is strong anatomical evidence for the colocalization of GABAA and glycine receptors at single postsynaptic densities (115, 1252, 1270) and for GABA and glycine colocalization in presynaptic terminals (937, 941, 942, 1150, 1205, 1252). Electrophysiological measurements are consistent with GABAergic and glycinergic contributions to IPSP/C in cranial motoneurons (656), as well as recurrent (1099), afferent, and descending inhibitory inputs to spinal motoneurons (412, 413, 991, 992, 1191, 1389). In fact, GABA and glycine can be coreleased from the same presynaptic vesicle (582) (see Fig. 6). The postsynaptic complement of receptors, however, is not constant between synapses. Analysis of evoked and mIPSC suggests three types of inhibitory synapses on spinal motoneurons: GABA only, glycine only, and mixed synapses comprising 15, 41, and 44% of the total input, respectively (582). These data raise a large number of important questions regarding how terminal type is determined, how transmitters are packaged, and how postsynaptic densities are constructed to match terminal type (914). The functional significance of this corelease to synaptic integration and motoneuron excitability is also uncertain. With the only major difference between the actions of GABA and glycine being the prolonged action of GABA, e.g., time constants of decay for the GABAA and glycine component in spinal motoneurons are ~59 and ~16 ms, respectively (582, cf. Ref. 413), the control of the relative amount of GABA versus glycine may regulate the time course of the inhibitory input, which would be of critical importance for motor coordination.
FIG. 6.
Corelease of glycine and GABA. Simultaneous whole cell patch-clamp recordings from a spinal interneuron and a (putative) motoneuron in a spinal cord slice. A: unitary inhibitory postsynaptic currents (IPSC) in this neuron pair before antagonist application. B: IPSC is partially blocked by 400 nM strychnine (glycine antagonist). C: remaining inhibitory synaptic current is blocked by adding 5 µM bicuculline (GABAA antagonist), demonstrating corelease of glycine and GABA in this motoneuronal synapse. Bottom traces in B and C are shown at an expanded amplitude scale. [From Jonas et al. (582). Copyright 1998 American Association for the Advancement of Science.]
C) PRESYNAPTIC ACTIONS OF GABAA RECEPTORS
Presynaptic inhibition by GABA receptors was first proposed to underlie the reduction in group Ia excitatory postsynaptic potentials in spinal motoneurons that could not be accounted for by postsynaptic mechanisms (381). The best anatomical evidence for GABAA receptor-mediated presynaptic inhibition of synaptic transmission to motoneurons is electron microscopic detection of GABA-like immunoreactivity in axoaxonic terminals presynaptic to afferent terminals in lamina VI and IX (280, 290, 809) and GABA receptor immunoreactivity on terminals presynaptic to motoneurons (1197; see also Refs. 824, 1187). Electrophysiological evidence for GABAA-mediated presynaptic inhibition includes indirect measurement of changes in the inhibition of ventral root reflexes or dorsal root potentials after activation of a conditioning stimulus (253, 318) and direct intracellular measurement of presynaptic inhibition of afferent-motoneuron EPSP (1191).
GABAA-mediated presynaptic inhibition appears to result from depolarization of primary afferent terminals in dorsal and ventral horns (256). Volleys of action potentials arriving in the spinal gray matter from segmental (afferent) or supraspinal (descending) sources initiate release of GABA from spinal interneurons. Subsequent activation of GABAA receptors on presynaptic terminals depolarizes the terminal and inhibits release of excitatory transmitter at the primary afferent-motoneuron synapses. GABAA receptor activation depolarizes primary afferent terminals because sensory neurons have reversal potentials for Cl− that are more positive than resting membrane potential (see review in Ref. 26). However, the precise mechanism by which primary afferent depolarization (PAD) inhibits transmitter release is not known. The prevailing hypothesis is that PAD inactivates Na+ channels sufficiently to block action potential invasion into the terminals (or reduce action potential amplitude), thereby suppressing transmitter release. Transmitter-induced membrane shunts (1326) and inactivation of Ca2+ currents may also contribute (254, 435, 697, 782, 819, 1123, 1328).
6. Functional role of GABA and glycine in modulating motoneuron excitability
A) POSTSYNAPTIC GABAA AND GLYCINE RECEPTORS
Activation of postsynaptic inhibitory receptors via local interneurons within or near cranial (199, 386, 387, 738, 739, 1029, 1277) and spinal (475, 1324, 1370) motoneuron pools, in conjunction with inhibitory projection neurons from the brain stem (504, 506) 1) control motoneuron responses to mono- and polysynaptic afferent and descending inputs, 2) help establish motoneuron recruitment order and gain, and 3) shape temporal and spatial patterns of activity in different motoneuron pools during reflexive and rhythmic behaviors and changes in state.
The contribution of postsynaptic GABAA and glycine receptors to afferent reflex and descending inhibitory control of motoneuron activity has been demonstrated in spinal motoneurons through stimulation of dorsal roots (570, 571, 991, 1389), ventral funiculi (333, 992), single afferent fibers, and premoto/interneurons (582, 1215). Cranial motoneurons are subject to similar inhibitory control (429, 631, 656, 886, 1221, 1222, 1406). However, considerable heterogeneity is apparent in these control systems. The relative contribution of GABA and glycine varies regionally between motoneuron pools and input pathways.
GABA- and glycine-mediated postsynaptic inhibition of motoneuron activity also contributes significantly to production of rhythmic behaviors, including respiration (353, 834), locomotion (201, 486, 644, 968), chewing (207, 337, 538), and swallowing (105), where motoneuron activity is characterized by sequential phases of excitation and inhibition. Inhibitory synaptic control of excitability during the active phase will affect the timing of burst onset and offset (affecting onset of muscle contraction and relaxation), firing pattern, and recruitment order (961, 969, 1049, 1377, 1379). Inhibitory inputs during the quiescent phase in a motoneuron, e.g., during the expiratory phase in an inspiratory neuron, will reduce the probability of spurious, and inappropriate, activation (1049). Inhibition seen at the end of each phase of activity is presumed to facilitate phase transitions but may not be obligatory to rhythm generation (355).
Inhibition may also provide phase-specific control of motoneuron gain, as suggested by the observation of a phasic inspiratory inhibition of phrenic motoneurons that matches the shape and time course of their inspiratory excitatory drive (961). Because phrenic motoneurons (444), like most motoneurons (292, 1084, 1143, 1221, 1394), participate in multiple behaviors, such a mechanism may contribute to the optimization of motoneuron excitability for different motor tasks.
Postsynaptic inhibition also modulates motoneuron excitability during behaviors such as micturition and over longer time scales associated with sleep state-dependent changes of muscle tone. In micturition, tonic activation of motoneurons innervating the external urethral sphincter during bladder filling (172, 351) is suppressed during voiding (350, 352), in part by a postsynaptic glycinergic mechanism (1138). Glycinergic inhibition of most spinal and some cranial motoneurons is also responsible for the atonia of rapid-eye-movement (REM) sleep (212, 485, 652, 1179). A perplexing aspect of this inhibition is its regional heterogeneity. Although most spinal motoneurons are inhibited, some inspiratory motoneurons are not, which is probably essential to avoid atonia of respiratory muscles. Among cranial motoneurons, the mechanism of inhibition varies during REM sleep. For example, in trigeminal motoneurons (649–651, 965, 1029), inhibition is glycinergic, whereas the inhibition of hypoglossal motoneuron activity results primarily from disfacilitation (669, 670, 672, 1378). The mechanisms underlying this differential control are incompletely understood but remain of considerable interest because of their potential involvement in conditions such as cataplexy, REM behavior disorder, and state-dependent respiratory disorders such as obstructive sleep apnea and perhaps sudden infant death syndrome.
B) PRESYNAPTIC GABAA RECEPTORS
Presynaptic inhibition mediated by GABAA (and GABAB; see sect. ivD3) receptors is not ubiquitous within the spinal cord. It appears to be a specialized feature of specific pathways that is mediated through complex interneuronal circuits (see Fig. 4 in Ref. 753). For example, GABAA receptor activation depolarizes afferent terminals from muscle (Ia, Ib, and type II) and skin but does not depolarize terminals of neurons projecting from the lateral vestibular (263) or red (260, 261) nuclei. Moreover, PAD of various classes of afferents is differentially affected by segmental and descending inputs (1062, 1063). Activation of group I afferents and vestibulospinal pathways induces PAD in Ia afferents, whereas cutaneous, reticulospinal, rubrospinal, and corticospinal pathways inhibit PAD. In contrast, stimulation of group Ib afferents, reticulospinal, rubrospinal, vestibulospinal, and corticospinal pathways induces PAD in Ib afferent terminals. Cutaneous afferents induce PAD in some Ib terminals and inhibit it in others. Part of this differential control is due to mediation of PAD in Ia and Ib afferents by different pools of last order GABAergic interneurons, with descending activity targeting specific sets of these interneurons (753). The observation that presynaptic inhibition induced through intraspinal stimulation differentially affects separate axonal branches of the same group I fiber (322, 323), and that ascending and segmental collaterals of individual spindle afferents can be separately controlled (753), suggests a complex spatial organization of presynaptic inhibitory circuits.
The functional significance of pathway-specific expression and modulation of PAD is not known, but it may provide a substrate for gating of different functional classes of afferents. The pattern of coactivation of axoaxonic synapses could presynaptically control the flow of information through the axonal arbor (696). Thus presynaptic inhibition may play an important role in the selection of sensory signals required for the execution of specific motor tasks (221, 753, 819, 904, 1062, 1063, 1154).
7. Development of GABA and glycine receptor systems
A) GABA DEVELOPMENT
The GABAergic system undergoes substantial developmental regulation (709, 769, 770, 823, 1001, 1002, 1033, 1106, 1391). Transcripts for α2, α3, α5, β2–3, and γ2–3 are all present in presumptive motoneurons of the mantle zone by embryonic day 13 in rat. Peak expression of subunits occurs between embryonic day 17 and 20 when mRNA for α2–5, β1–3, and γ1–3 subunits are all present. α3–5, β1–2, γ1, and γ3 then decrease and are almost absent by the end of the second postnatal week (770). Developmental changes in transcript expression appear to result in changes in the amount of receptor protein subunit (389, 712).
The precise role of GABAA receptors in development of motoneuron circuits and modulation of motoneuron excitability is not known. GABA itself can alter GABAA (and GABAB) receptor expression (748, 783, 784, 1106). In addition, the abundant expression of GABAA receptors in embryonic and neonatal spinal cord may serve a trophic role in regulation of neuronal differentiation and synaptogenesis (828). In early development, depolarization due to GABAA receptor activation could elevate intracellular Ca2+ levels, a potentially important signal in modulating early differentiation (1182). In addition, GABA- (and glycine-) mediated depolarization, in conjunction with the observation that in some systems glutamatergic synaptic transmission is strongly mediated by NMDA receptors early in development (1433), has led to the suggestion that in hippocampus GABA receptor activation may facilitate activation of NMDA receptors (87). In such a scheme, GABA- (and glycine- see below) mediated depolarization would play the role in activity-dependent organization of neuronal circuits conferred on AMPA receptors later in development. The relevance of such a mechanism at the motoneuron level is not known.
B) GLYCINE DEVELOPMENT
Glycine receptors show marked developmental heterogeneity. Relative to the adult form, the neonatal receptor has low affinity for strychnine (1376) and contains an α-subunit with different molecular mass and antigenic epitopes (700, 1023, 1306). Developmental changes in subunit expression from a neonatal receptor composed primarily of α2* are likely to underlie these differences, because α1, α3, and β subunits and transcripts are expressed at low levels in the fetus and neonate (786, 1339). Only α2* receptors have the low strychnine sensitivity characteristic of neonatal receptors, whereas the prolonged time course of fetal/neonatal postsynaptic potentials/currents is consistent with recombinant α2 receptors (1159, 1219). If the depolarizing actions of glycine (and GABA, see above) during development contribute to activity-dependent organization of motoneuronal circuits by removing the Mg2+ block of NMDA channels, the prolonged glycinergic postsynaptic potentials/currents in fetus and neonate (413, 1159) would facilitate this process.
D. GABA: Metabotropic Actions
GABA, in addition to its role as a fast inhibitory neurotransmitter, inhibits motoneuronal excitability by activating presynaptic metabotropic GABAB receptors.
1. GABAB receptors
GABAB receptors are coupled through G proteins to produce increases in K+ or decreases in Ca2+ currents and mediate slow synaptic inhibition. Two receptor genes have been identified by molecular cloning, GABABR1 and GABABR2. Interestingly, and unlike most G protein-coupled receptors which function as homomers, native GABAB receptors appear to be formed by heterodimerization of GABABR1 and GABABR2 (586, 613, 1356). Splice variants, GABABR1a and GABABR1b, for the GABABR1 clone have also been identified. These forms have similar pharmacological profiles but clearly a major goal of future studies is to define possible receptor subtypes (99, 128, 446, 612). Analysis of native receptors suggests at least five receptor subtypes, but it is not yet clear whether individual genes exist for each (251, 417). The cloning of the receptor should precipitate a vast increase in our understanding of GABAB receptor physiology including further delineation of its mechanisms of action (99) as well as its pre- and postsynaptic distribution within the CNS (118, 129, 626, 843, 873, 893).
2. GABAB receptor localization
GABAB receptor distribution has primarily been explored with receptor autoradiography (130, 132, 220, 415, 1006, 1007). Patterns of GABAA and GABAB receptor binding are not identical but do show considerable overlap. GABAB binding sites are distributed heterogeneously in the CNS. Within the spinal cord, the highest densities occur in the dorsal horn (11, 130, 783, 1006, 1007), but ventral horn binding is also present (11, 1006). Binding in the brain stem is highest in the spinal trigeminal nucleus.
A substantial presynaptic localization of GABAB receptors in the dorsal horn is suggested by 50% reductions in binding after capsaicin-induced degeneration of sensory afferents (1006, 1007). It is not known whether GABAB binding sites in the ventral horn are pre- or postsynaptic. Dorsal rhizotomy does not alter ventral horn binding, suggesting postsynaptic localization. GABAB agonists, however, have minimal effect on motoneuron properties (see below), leaving the possibility that ventral horn binding represents presynaptic sites on descending fibers (1006, 1007) or local interneurons.
3. Post- and presynaptic actions
A) POSTSYNAPTIC
In contrast to GABAA receptors, postsynaptic GABAB receptors appear to be of minimal importance in the regulation of motoneuron excitability. Application of the GABAB agonist baclofen at concentrations sufficient to depress excitatory and inhibitory synaptic activity is not associated with changes in motoneuron membrane potential, conductance, excitability, time constant, or EPSC decay (321, 572, 661, 731, 973, 985, 1317). Postsynaptic effects in mammalian motoneurons, including hyperpolarization, decreases in input resistance, and reductions in excitability and the afterhyperpolarization (276, 377, 1334; cf. Ref. 694), are only observed using high concentrations of baclofen.
Postsynaptic receptor involvement, however, cannot be completely excluded. GABAB agonists affect motoneurons of lower vertebrates (806). If the GABAB-mediated postsynaptic action occurs at distal dendrites, as appears to be the case in other neurons (843, 873), a GABAB receptor-mediated change in membrane potential/conductance may not be detected at the cell soma (686) but could still affect the neuronal response to other distal dendritic inputs.
B) PRESYNAPTIC
GABAB receptor activation with exogenous agonists such as baclofen is associated with presynaptic inhibition of excitatory synaptic transmission to cranial (461, 930) and spinal motoneurons. Glutamatergic Ia, Ib afferent, and dorsal root evoked potentials in spinal motoneurons are inhibited by baclofen (27, 259, 276, 321, 377, 661, 731, 734, 973, 985, 991, 1191, 1317, 1334, 1390). GABAB receptor activation is also associated with reductions in presynaptic Ca2+ influx (255) and in release of glutamate (604, 1239), substance P (780, 1239), and calcitonin gene-related peptide (CGRP) (781) from primary afferent terminals (127). A similar, although less potent, GABAB-mediated inhibition of transmission occurs at excitatory synapses descending through ventrolateral (321, 992) and ventromedial funiculi or from the reticular formation and vestibular nuclei (572). The apparent absence of presynaptic GABAB modulation of some descending inputs (261, 611) again highlights the potential for pathway-specific presynaptic modulation of motoneuron excitability.
The action of baclofen on inhibitory synaptic transmission to motoneurons is less consistent. Positive and negative results have been reported (276, 377, 611, 985, 1013). Suppression of spontaneous and evoked IPSP in rat spinal motoneurons by baclofen (1334) suggests that inhibitory inputs to motoneurons are under presynaptic control. However, the possibility that the inhibitory actions of baclofen on IPSP in these polysynaptic pathways is secondary to blockade of release of excitatory transmitters acting on inhibitory interneurons cannot be excluded.
GABAB agonists also suppress GABAA-mediated PAD (259, 1013). GABAB autoreceptors in terminals of last-order interneurons mediating PAD may function as a self-limiting mechanism controlling synaptic efficacy of these interneurons (843, 1013). Interestingly, the baclofen suppression of PAD is greater for Ib afferent-induced PAD than for PAD induced by descending reticular formation inputs. Thus last-order interneurons mediating PAD via segmental pathways may have a greater density of GABAB autoreceptors than those mediating PAD via descending pathways (1013). In spinal motoneurons of Xenopus, there is a GABAB receptor-mediated presynaptic inhibition of glycinergic inputs (1325); this has yet to be seen in mammalian motoneurons.
In addition to the effects on individual postsynaptic potential/currents, GABAB receptor activation is implicated in activity-dependent modulation of synaptic transmission. The efficacy of synaptic contacts can be modified by activity in the presynaptic neuron. For example, high-frequency activation of afferent inputs to spinal motoneurons can produce synaptic facilitation and depression and tetanic and posttetanic potentiation (973). For Ia afferent to motoneuron synapses, large-amplitude EPSP in motoneurons with large input resistance and small rheobase tend to show negative modulation (depression, a progressive decrease in EPSP amplitude during high-frequency stimulation). Small-amplitude EPSP in motoneurons with low input resistance and high rheobase undergo positive modulation (facilitation, Refs. 228, 229, 832, 1117). Both types of synapses are proposed to experience increased presynaptic Ca2+ and elevated probability of transmitter release. However, the greater susceptibility of synapses producing large EPSP to transmitter depletion is proposed to underlie their negative modulation. Reduced susceptibility of synapses producing small EPSP to transmitter depletion would show positive modulation. GABAB receptor activation with baclofen shifts negative modulation to positive (depression to facilitation) or increases the degree of positive modulation (facilitation) (731, 732, 973), presumably by decreasing Ca2+ entry into the terminal (255, 990, 1117), which decreases the probability of transmitter release and depletion.
The mechanism(s) by which baclofen reduces the release of transmitters from terminals presynaptic to motoneurons is not known. In dorsal root ganglion neurons, the presynaptic inhibitory action of GABAB receptors is proposed to result primarily from a G protein-mediated reduction of voltage-sensitive Ca2+ currents (255, 602, 731). An increase in K+ currents (410, 843, 873), and a third mechanism that is independent of effects on leak or voltage-gated currents identified in hippocampus, may also contribute (843).
4. Endogenous role for GABAB receptors in modulating motoneuron excitability?
Despite the clear demonstration that baclofen inhibits motoneuron activity, establishing an endogenous role for GABAB receptors in modulating motoneuron excitability has been slower. Relatively few studies have been performed at the motoneuron level. In addition, GABAB antagonists have limited efficacy in potentiating synaptic transmission, questioning the endogenous role of GABAB in modulating inputs to motoneurons (154, 1191, 1317, 1390; see also Ref. 843). Part of the limited efficacy, however, may reflect use of weak GABAB antagonists, such as 2-OH saclofen, or that GABAB antagonists applied at the cell soma do not reach distal synapses (1191). Intravenous administration of recently developed selective antagonists (154, 687, 688, 783) suggests a marked involvement of GABAB receptors in the prolonged inhibition of monosynaptic reflexes (258). Results on spinal and cranial motoneurons in vitro range from indicating substantial GABAB involvement (33, 183, 686) to no effect (154) and levels in between (1317). Discrepancies may reflect varying degrees of endogenous activation of the relevant pathways in different experimental models.
Another possibility is that GABAB receptors on afferent terminals are located extrasynaptically (1191). Under such conditions, activation of extrasynaptic receptors is only likely to occur during periods of massive GABA release (or reduced uptake) due to highly effective GABA uptake mechanisms. Paracrine-like activation of extrasynaptic GABAB receptors is present in hippocampal CA1 cells (539). Thus modulation of GABA uptake systems may play an important role in presynaptic inhibition by GABAB receptors.
In conclusion, GABAB receptors enable GABA to modulate excitability by inhibitory and disinhibitory effects. As a result of their coupling through second messenger systems and their differential effects on afferent and descending pathways, they have the potential to produce long-term changes in excitability in a pathway-specific manner, as suggested by the therapeutic value of baclofen and GABAB-related compounds. Baclofen in particular is used as a muscle relaxant in the treatment of spasticity of spinal origin (131, 781, 782, 784). Recent cloning of the GABAB receptor subunits (612, 613) will define the basis for introducing and improving the clinical profile of GABAB receptor ligands (128).
E. Serotonin
1. Ligands, receptors, and sources of 5-HT input to motoneurons
Soon after its initial discovery in the periphery (serum, intestinal mucosa), 5-HT was shown to be present in the mammalian CNS (29). Using the Falck-Hillarp histochemical fluorescence technique, Dahlstom and Fuxe (264, 408) demonstrated that 5-HT-positive cells are located in the midline of the brain stem and project to most of the brain. 5-HT-immunoreactive boutons are found in the ventral horn of the spinal cord, in cranial motor nuclei, and apposed to the somatodendritic membrane of cranial and spinal motoneurons, suggesting that 5-HT-containing neurons project directly to motoneurons (19, 24, 38, 39, 569, 657, 908, 988, 1072, 1186, 1224, 1286). On average, ~1,500 5-HT-immunoreactive boutons contact the dendritic area of individual spinal lumbar motoneurons in adult cats, with the vast majority of 5-HT input on the dendrites (24). Compared with the estimated total number of synaptic boutons on spinal motoneurons (50,000 –140,000; Ref. 937), this means that only 1–3% of synaptic boutons on spinal motoneurons are serotonergic. A large number of 5-HT-immunoreactive varicosities associated with spinal motoneurons also contain substance P, TRH, or other peptides (see sect. iii; Refs. 39, 133, 245, 908, 1237, 1286, 1348, 1388). Similar observations of 5-HT and substance P coexistence have been made in axonal varicosities in cranial motor pools (1225).
Some 15 5-HT receptor subtypes, which can be divided into seven subfamilies, have been characterized. All 5-HT receptors belong to the superfamily of G protein-coupled receptors, except for the 5-HT3 receptors, which are ligand-gated ion channels (420, 1296). The expression pattern of different 5-HT2A receptors or their transcripts has been mapped in some spinal and cranial motoneurons by immunocytochemistry, in situ hybridization, and PCR reactions detecting mRNA encoding for 5-HT receptors. In the spinal cord, immunodetection of 5-HT receptor subtypes using an anti-ideotypic antibody recognizing 5-HT1B, 5-HT2C, and 5-HT2A receptor subtypes reveals that one, two, or all of these receptors are present in the somatodendritic region of spinal motoneurons (1046).
Use of an antipeptide antibody (44, 629) reveals a striking distribution of 5-HT1A receptors in cervical spinal motoneurons (44, 629). The axon hillock is densely, and the soma is diffusely, labeled, whereas dendritic labeling is absent, suggesting that 5-HT signaling via 5-HT1A receptors is localized at the site of action potential generation (44, 629). Unassembled subunits of the 5-HT3 receptor, which is a ligand-gated ion channel, are found in the soma of both spinal and cranial motoneurons, but the location of the assembled 5-HT3 receptors is unclear (864). Cranial motor nuclei express transcripts for 5-HT1A and 5-HT2A receptors (1383); in the hypoglossal nucleus, transcripts encoding for 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3, 5-HT7, but not 5-HT1A receptors, are expressed (929). However, 5-HT1A receptors are expressed in motoneurons of neonatal animals (1228).
Pharmacological and electrophysiological studies have identified several 5-HT receptor subtypes in both spinal and cranial motoneurons, and in the following section we describe the pre- and postsynaptic actions of 5-HT on motoneurons and the receptors involved.
2. Pre- and postsynaptic actions of 5-HT on motoneurons
Application of 5-HT (by microiontophoresis) in the vicinity of spinal motoneurons in vivo or systemic injection of 5-HT precursors generally leads to the following: an increase in motoneuronal excitability (1362), tonic muscle electromyogram (EMG) activity increases (839, 1044), and some spinal motor reflexes are facilitated (223, 233, 1044, 1079, 1401). Locally applied 5-HT produces a small (2–6 mV) depolarization in spinal motoneurons in vivo, accompanied by an increase in membrane input resistance and a reduction in the spike AHP (1359).
The postsynaptic action of 5-HT in spinal motoneurons has been studied using in vitro preparations from neonatal or juvenile animals. Bath application of 5-HT depolarizes spinal motoneurons in spinal cord slices from the neonatal rat, an effect that persists after blockade of action potential-driven synaptic transmission (by adding TTX or replacing external Ca2+ with Mg2+) (236, 332, 1216, 1332, 1434). The 5-HT-induced current is carried by both Na+ and K+ through activation of an inwardly rectifying current (likely Ih); 5-HT1A receptors are involved in this action (1216). 5-HT also enhances LVA Ca2+ currents in neonatal rat spinal motoneurons (93). One study, however, reports depolarizations as well as hyperpolarizations induced by 5-HT in spinal motoneurons from neonatal rats, effects proposed to be mediated by decreasing and increasing a K+ current via 5-HT2 and 5-HT1A receptors, respectively (1332). Thus some spinal motoneurons in neonatal mammals may be inhibited rather than excited by 5-HT. Consistent with these observations, iontophoretic 5-HT applications to motoneurons in adult animals in vivo produce long-lasting hyperpolarizations following an initial short-lasting depolarization (1424). Neonatal rodent phrenic motoneurons are depolarized postsynaptically by 5-HT through activation of 5-HT2 receptors (Fig. 7) (744). In addition to this effect, exogenous 5-HT reduces the amplitude of inspiratory-modulated synaptic drive to these motoneurons, probably through activation of presynaptic 5-HT1B receptors, affecting synaptic release (295, 744).
FIG. 7.
5-HT depolarizes neonatal rat phrenic motoneurons (postsynaptic effect) and reduces inspiratory synaptic drive (presynaptic effect). Current and voltage-clamp recording from a phrenic motoneuron in a brain stem-spinal cord in vitro preparation, which generates spontaneous respiratory-like motor activity. One burst of inspiratory synaptic drive is captured in each trace. Traces show the following, left to right: control condition, after exposure to 20 µM DOI (5-HT2A,1C agonist), and after addition of 20 µM 5-HT (25 min after DOI). Note that DOI depolarizes the membrane and induces an inward current, and addition of 5-HT reduces the amplitude of the inspiratory synaptic drive. Inspiratory drive is transmitted by glutamate. This dual action of 5-HT may play a role in ensuring transmission of inspiratory drive regardless of variations in 5-HT release as part of the sleep-wake cycle (355). Vm, membrane potential; Im, membrane current. [Adapted from Lindsay and Feldman (744).]
5-HT induces plateau potentials in turtle spinal motoneurons under in vitro conditions (521, 1163). The plateau potential, which gives rise to long-lasting depolarizations and bistable firing behavior, is blocked by L-type Ca2+ channel antagonists. The underlying ionic mechanism may involve a reduction of outward K+ currents (primarily IK Ca), thereby uncovering a L-type Ca2+ current present both in the soma and dendrites of these motoneurons (522). However, a direct action on L-type Ca2+ channels is also possible (121, 522). Plateau potentials are found in spinal motoneurons in adult cats (see sect. iiD), when descending brain stem-spinal cord systems are intact or after spinal transection when 5-HT or NE precursors are given intravenously (239, 244, 519, 632). A reevaluation of the functional consequences of plateau potentials in hindlimb motoneurons in cats led to the suggestion that plateau potentials under normal circumstances play a role in ensuring effective recruitment rather than in generating bistable behavior (88). Finally, 5-HT reduces the amplitude of dorsal root evoked EPSP and IPSP via presynaptic 5-HT1 receptors (1387), a mode of action also found in several cranial motor pools.
The ionic mechanisms underlying 5-HT effects on cranial motoneurons have been worked out in greatest detail for facial, hypoglossal, and trigeminal motoneurons. In vivo or in vitro, facial motoneurons respond to exogenously applied 5-HT with a depolarization (5–8 mV) and an increase in membrane input resistance (705, 1303, 1304). The depolarization is unaffected by TTX, indicating a postsynaptic site of action (705). The receptor subtype mediating the depolarization is likely 5-HT2, but not 5-HT1A, receptors (1032). The underlying ionic mechanism is a 5-HT-induced enhancement of the hyperpolarization-activated Ih current (701, 702), concurrent with a decrease in a resting K+ current IK leak (701, 703).
Hypoglossal motoneurons are also excited by 5-HT (91, 305, 671), but mechanisms change developmentally. Exogenous application of 5-HT to hypoglossal motoneurons from neonatal rodents gives rise to a spike-initiating depolarization, with no clear change in membrane input resistance (91, 1228). Compared with adults, there is marked reduction in the spike AHP in these motoneurons (73, 91, 1228). However, hypoglossal motoneurons from juvenile animals respond to 5-HT with a depolarization associated with an increase in input resistance and show no effect of 5-HT on the spike AHP (1228). The discrepancy with regard to the spike AHP is explained by a reduction in the expression of 5-HT1A receptors in juvenile animals (79, 1228). In neonatal animals, activation of 5-HT1A receptors leads to a reduction in N- and P-type Ca2+ currents, which in turn leads to reduced spike AHP (73). Interestingly, 5-HT also acts presynaptically in the hypoglosssal nucleus of neonatal rats, reducing glutamatergic and glycinergic synaptic transmission through activation of presynaptic 5-HT1B receptors (1156, 1157, 1292).
Iontophoretic application of 5-HT to trigeminal motoneurons during cortically induced masticatory-like activity facilitates trigeminal discharge, suggesting an excitatory action of 5-HT (608). A detailed analysis of the underlying ionic mechanism in trigeminal motoneurons from juvenile guinea pigs under in vitro conditions shows that 5-HT reduces a resting K+ current (IK leak), enhances the hyperpolarization-activated cationic current (Ih, via activation of 5-HT2 receptors), and induces a Na+-dependent inward current (Iinw) (526). Furthermore, the medium-duration spike AHP is reduced by 5-HT (526), and, as found in spinal motoneurons, 5-HT can induce bistable membrane behavior (527). The bistable membrane behavior and plateau potentials are due to L-type Ca2+ channel activation with a contribution of the Na+-dependent inward current, but it is unclear whether 5-HT acts directly on L-type Ca2+ and Na+ channels, or indirectly by reducing opposing outward currents (527). In addition to these effects on the intrinsic properties of trigeminal motoneurons, 5-HT enhances the response to EAA (endogenous or exogenous) via 5-HT2 receptors (679, 1273). The facilitation could be a simple result of the increased input resistance, but may also involve direct actions of 5-HT on NMDA and AMPA channels (1273).
3. Signal transduction
Manipulations of intracellular signal transduction pathways suggest that 5-HT actions are mediated by receptors coupled to G proteins (except for the ionotropic 5-HT3 receptors), and a phosphorylation-independent action of cAMP (6, 702, 704). Intracellularly applied cAMP or forskolin mimics the action of 5-HT on Ih in neonatal spinal motoneurons, and a broad range of protein kinase inhibitors fail to block the action of 5-HT (702, 704), suggesting that activation of 5-HT receptors stimulate cAMP production, which in turn induces an inward current, perhaps by a phosphorylation-independent direct action on Ih (702, 704). Mechanisms that account for inhibition of leak K+ currents by 5-HT have not been determined but do not appear to involve PKC, at least in facial motoneurons (6).
4. Functional role of 5-HT in modulating motoneuronal excitability
Serotonergic neurons in the raphe nuclei are spontaneously active (1–5 spikes/s) during the normal waking state (552, 555). The discharge frequency changes during different motor behaviors, i.e., an increase in firing frequency is seen in raphe neurons in cats during treadmill locomotion compared with undisturbed waking and during oral activities such as chewing, biting, and licking (480, 1308). Conversely, a decrease in firing frequency is seen during REM sleep, when motor output is inhibited, and the release of 5-HT (measured by a microdialysis probe placed in the ventral horn) is reduced after exercise (421, 480, 556, 820). 5-HT exerts tonic facilitatory effects on spinal hindlimb motoneurons (635); after depletion of spinal monoamines in rats (using neurotoxins), there is a reduction in the total EMG activity of the soleus muscle and the duration and number of long-lasting gross-EMG episodes (635). Thus raphe neurons, projecting to spinal and cranial motoneurons, provide a tonic release of 5-HT during the normal waking state and increased cyclic release of 5-HT during gross repetitive types of motor behavior (552).
Local stimulation of the raphe nuclei with concurrent recordings from spinal motoneurons confirms the excitatory nature of the raphe-spinal 5-HT projection. Electrical stimulation of the nucleus raphe obscurus (for 1 min) induces a small depolarization in rat spinal lumbar motoneurons that is blocked by a systemic 5-HT1,2 receptor antagonist (1048). Brief stimulation (0.1– 0.7 ms) of the raphe pallidus induces subthreshold EPSP in cat spinal motoneurons (394). Stimulation of different raphe nuclei either facilitates or inhibits the activity of phrenic motoneurons (511, 512, 692, 693). The inhibitory effect is likely mediated by presynaptic 5-HT modulation of bulbospinal neurons transmitting inspiratory drive to these motoneurons, through activation of 5-HT1B receptors (295, 744). Laryngeal motoneurons are excited by stimulation of the raphe pallidus, an effect blocked by pretreatment with a 5-HT2 antagonist (37). Stimulation of the raphe pallidus-obscurus complex induces monosynaptic sub- or suprathreshold EPSP in trigeminal motoneurons (masseter and mylohyoid), and these potentials are reduced by a nonselective 5-HT antagonist (883).
In addition to these postsynaptic effects on spinal and cranial motoneurons, spinal and cranial reflex pathways are also affected by raphe stimulation. Stimulation of the raphe nuclei depress transmission from group II muscle afferents in the midlumbar spinal cord in cats (564), suggesting that release of 5-HT depresses transmission between spinal interneurons and motoneurons, or between the interneurons themselves (138). Crossed group II inhibitory reflex pathways (generating IPSP in contralateral extensor motoneurons) are under tonic control by descending serotonergic pathways, since the IPSP are abolished by lesions of descending pathways and restored by systemic administration of 5-HT1A agonists (4). In spinalized rats, monosynaptic transmission is inhibited or facilitated by activation of 5-HT1A and 5-HT2A/2C receptors, respectively (465). Under in vitro conditions, excitatory synaptic transmission induced by local electrical or dorsal root stimulation is facilitated via activation of 5-HT2A and/or 5-HT2C receptors (1400, 1434). Finally, 5-HT2A/2C agonists applied topically to the spinal cord of spinalized cats restore extensor reflex excitability, suggesting a facilitatory effect of 5-HT on the lumbar stretch reflex (839).
In conclusion, the dominant effect of the serotonergic raphe system is the enhancement of spinal and cranial motoneuron excitability. This action is mediated postsynaptically by activation of Ih, reduction of specific K+ conductances, uncovering of L-type Ca2+ currents, reduction of spike AHP amplitudes, and an increase in membrane input resistance. Because raphe serotonergic neurons have a slow and regular firing pattern in the normal waking state, a constant release of 5-HT may set the overall level of excitability of motoneurons, in particular in motoneuron pools innervating axial and postural muscles (552, 555). Upon changes in motor activity, such as initiation of locomotion, mastication, and other rhythmic motor behaviors, the activity of the serotonergic raphe system is increased, and consequently, there is an increase in the responsiveness and ease of recruitment in motoneurons. In addition, the serotonergic raphe system may regulate or gate afferent inputs to motoneurons pools by modulating synaptic transmission in specific reflex pathways and by direct actions on presynaptic terminals (355, 552). In this fashion, the raphe serotonergic system controls the excitability of motoneurons through direct actions on the motoneurons and through control of afferent input. These actions may be different in various motoneuron pools, especially those responsible for breathing movements (355).
F. Norepinephrine and Epinephrine
1. Ligands, receptors, and sources of NE input to motoneurons
Norepinephrine was initially established as a neurotransmitter in the periphery (490, 1320), and subsequently proposed to act centrally (1319). A large amount of work, neuroanatomical and neurophysiological, has since confirmed a prominent role for NE in controlling motoneuronal excitability.
All catecholamines, including dopamine, NE, and epinephrine, are synthesized in discrete populations of neurons by a series of enzymatic steps, beginning with the rate-limiting hydroxylation of the precursor amino acid tyrosine. The specific catecholaminergic neuronal phenotype is determined by the presence or absence of additional enzymes in the biosynthetic pathway: dopaminergic neurons express tyrosine hydroxylase (TH), but not dopamine β-hydroxylase (DBH); NE cells express TH and DBH, but not phenylethanolamine N-methyltransferase (PNMT); epinephrine-synthesizing neurons express all three (TH, DBH, and PNMT) (490). A brief discussion of dopamine and motoneuronal function will follow this section. Epinephrine and NE can both interact with adrenergic receptors at physiological concentrations. However, we limit our discussion to NE because neurons that make epinephrine have a more restricted brain distribution and provide little, if any, innervation of motoneurons (490).
The initial classification of adrenergic receptors into α- and β-subtypes (9) has expanded (173, 174, 909) to include three major classes of adrenoceptor (α1, α2, and β), each comprising at least three subtypes. These receptors are members of the rhodopsin family of G protein-coupled receptors. Members of each class of adrenoceptor share ~70–75% sequence homology, whereas sequence homology drops to ~40% between members of different classes (842). Pharmacological and molecular classifications of receptor subtypes do not, however, extend to clear functional differences among members of a given class, each of which utilizes similar transduction mechanisms (173, 174, 842).
Members of all three classes of adrenergic receptor are expressed in the CNS, with distinct and differential distributions for each receptor subtype. α1-Adrenoceptors are highly expressed on cranial and spinal motoneurons, as assayed by ligand-binding autoradiography (587); in situ hybridization experiments indicate that this binding is probably somatodendritic and could reflect α1A, α1B, and/or α1D expression, since transcripts for all three α1-receptor subtypes are present in motoneurons at high levels (298, 986). Radioligand binding to α1-receptors in motoneurons is detectable as early as postnatal days 1–5, and high levels of binding are maintained throughout development (588).
All three α2-adrenoceptor subtypes are present in rat brain, with the α2B-subtype expressed only in thalamic neurons (909, 910, 1095). Radioligand binding for α2-receptors, both α2A and α2C, are found on motoneurons, albeit at low levels, and transcripts representing these α2-subtypes are present in various motoneuronal populations (910, 1095, 1295). Immunohistochemical studies utilizing subtype-specific antibodies find somewhat different patterns of staining in motor nuclei; α2A-immunoreactivity is diffuse, perhaps reflecting staining on terminals (1227), whereas α2C-immunoreactivity is clearly somatic (1060). Furthermore, although α2A- and α2C-receptors are present at low levels in adult motoneurons, developmental studies suggest opposite developmental patterns of expression (1372, 1373). Thus α2A-receptors are transiently expressed in rat motoneurons at high levels during embryonic and early postnatal periods, reaching sustained low levels by approximately postnatal day 14 (1372); in contrast, α2C-expression increases from undetectable levels in the embryo and neonate to reach somewhat higher levels in the adult (1373).
The β1- and β2- but not the β3-adrenoceptors are expressed in brain (909, 911). However, there is little evidence for expression of β-adrenoceptors by motoneurons. Binding of β1- and β2-adrenoceptor ligands in motor nuclei is unremarkable (953, 1020), and neither β1- nor β2-adrenoceptor mRNA is found in motoneurons (909).
Motoneurons receive a relatively dense catecholaminergic input, the overwhelming majority of which represents NE (16, 863). NE fibers are present in spinal motor nuclei early in development (by embryonic day 16 in rat), but they increase in density over the early postnatal period (1021, 1234). The NE input to motoneurons derives primarily from the A5–A7 cell groups in the dorsolateral pontine tegmentum, particularly from the nucleus coeruleus (A6) and subcoeruleus (A6v) (14, 451, 768, 925; see sect. iii and Table 2). The relative inputs from these different NE-synthesizing cell groups to individual motor pools vary; trigeminal and spinal motoneurons receive most of their NE input from A5 and A7 cell groups, whereas the NE innervation of hypoglossal motoneurons emanates mostly from A6v cells (14, 451, 768). In addition, although NE fiber density is consistent in motor areas throughout most levels of the spinal cord and brain stem (863), a somatotopic distribution of innervation within individual motor nuclei is present. For example, fiber density is higher in the lateral part of the ventral horn at cervical and lumbar enlargements, where motoneurons that innervate primarily distal limb musculature are located (863); it is also higher in the ventral aspects of the hypoglossal nucleus, where tongue protrusor motoneurons are located (15, 16, 297). Thus NE projections may be preferentially directed from different pontine nuclei to specific motor pools, suggesting the potential for a signaling specificity affecting motor control. It is important to point out, however, that the majority of NE-containing profiles in motor nuclei are nonsynaptic (17, 185, 1021, 1022), and therefore, extrasynaptic (i.e., volume) transmission may abrogate some of the specificity coded in the differential somatotopic distribution of fibers (1022). Nevertheless, some synaptic profiles, predominantly axodendritic, are observed between catecholaminergic terminals and motoneurons (17, 185, 1021, 1022).
2. Pre- and postsynaptic actions of NE on motoneurons
Early work investigating the postsynaptic effects of NE on cat spinal motoneurons reported depressed excitability and/or membrane hyperpolarization, associated with a decrease in a mixed cationic current (334, 798, 983). In contrast, all recent work indicates that the predominant postsynaptic effects of NE are excitatory, suggesting that the initial findings resulted from a methodological artifact (1362). Thus, in cranial and spinal motoneurons, NE causes a slow membrane depolarization associated with a decrease in input conductance (332, 701, 958, 1303, 1360) and enhances firing responses to glutamatergic inputs, whether applied exogenously or released endogenously (395, 608, 609, 1360–1362). These effects of NE are postsynaptic, since they are preserved in the presence of TTX or a high-Mg2+, low-Ca2+ synaptic blockade medium.
The cellular mechanisms that mediate excitatory effects of NE have been investigated. An α1-adrenoceptor is involved since effects of NE are 1) blocked by α-adrenoceptor antagonists, prazosin and phentolamine, but not by the β-adrenoceptor antagonists propranolol or sotalol, and 2) mimicked by the α1-adrenoceptor agonist phenylephrine but not by either the α2-adrenoceptor agonist UK-14,304 or the β-adrenoceptor agonist isoproterenol (332, 608, 609, 958, 1323). On the basis of relative agonist/antagonist potencies and insensitivity of the response to chloroethylclonidine, the facilitation of spinal motoneuronal activity by NE may be mediated primarily by the α1A-adrenoceptor subtype (1323). If the excitatory effects of NE on motoneurons are indeed mediated by α1A-adrenoceptors, what is the role of the other α1-subtypes expressed by motoneurons? Perhaps individual subtypes are directed to different membrane locations, such as the motoneuronal terminals where α1-adrenoceptors mediate facilitation of acetylcholine release by NE (1171).
As suggested by its association with an increased input resistance (332, 958, 1303, 1360), the ionic mechanism that underlies motoneuronal depolarization by NE includes a decrease in a K+ current; the NE-modulated K+ current is relatively voltage insensitive and blocked by Ba2+ (701, 958), not unlike the leak K+ channel targeted by 5-HT, TRH, and substance P in motoneurons and by NE in other central neurons (e.g., Refs. 815, 1337). Furthermore, NE also activates a Ba2+-insensitive current component in motoneurons that is carried, at least in part, by Na+ (958) and that is also reminiscent of effects of 5-HT, TRH, and substance P in motoneurons (75, 91, 366, 367, 921). Indeed, the effects of TRH and NE occlude each other, suggesting a shared mechanism of action (958).
Two additional effects of NE influence firing responses (958): 1) NE induces a decrease in AHP amplitude in a subset of adult hypoglossal motoneurons, which is associated with an enhanced firing response to current injection (958); 2) NE causes an apparent enhancement of a transient K+ current in hypoglossal motoneurons that delays the firing response to intracellular current injection (958). Interestingly, NE acting via α1-adrenoceptors has the opposite effect on the transient K+ current (IA) in dorsal raphe neurons (5).
The possibility that NE mediates an endogenous excitatory effect on spinal motoneurons has been tested in rats and cats in vivo (204, 396). Electrical stimulation in the locus coeruleus increases excitability and generates a multicomponent postsynaptic potential in motoneurons (395). Similar to effects of exogenously applied NE on motoneurons, the increased excitability is associated with a lower current threshold to induce repetitive firing and a decreased spike AHP (393). Moreover, the motoneuronal excitation and the slow component of the electrically evoked EPSP are inhibited by α1-adrenoceptor antagonists (395). These results suggest that increased activity in locus coeruleus neurons will excite motoneurons via mechanisms similar to those induced in vitro by α1-adrenoceptor activation. The activity of locus coeruleus neurons in vivo is highly state dependent; these neurons also respond with increased activity to a variety of stress-inducing stimuli (553, 554). For example, firing of locus coeruleus neurons in cats increases dramatically in the presence of a dog (553, 554). This suggests that effects of noradrenergic systems may be important in facilitating motor activity during waking states, in general, and in providing additional support for motor activities that accompany physiological responses to stressors.
Although the predominant effect of NE on motoneurons is excitatory, resulting from activation of α1-adrenoceptors, there is some evidence for inhibitory effects mediated by α2-adrenoceptors. For example, muscle EMG activity and the flexor response to afferent nerve volleys in spinal rats are inhibited by the α2-adrenoceptor agonist clonidine (1074, 1265); in a subpopulation of trigeminal motoneurons, clonidine suppresses motoneuronal discharge induced by oral stimulation, an effect blocked by the α2-antagonist yohimbine (609). This effect of α2-adrenoceptor activation could be mediated by direct actions on premotor interneurons, or by pre- or postsynaptic actions at the level of the motoneurons. Certainly, presynaptic effects of α2-adrenoceptors are well described in other systems (e.g., see Ref. 1066), although α2-inhibition of synaptic inputs to motoneurons has not been directly demonstrated. On the other hand, clonidine causes a membrane hyperpolarization associated with a decreased conductance in rat spinal and hypoglossal motoneurons (237, 959). This direct inhibitory postsynaptic effect of clonidine in rat hypoglossal motoneurons is due to decreases in both the peak amplitude and activation kinetics of Ih attributed, in part, to α2-adrenoceptor activation (959).
3. Signal transduction
The α1-adrenoceptor signals primarily through pertussis toxin (PTX)-insensitive G proteins (Gαq/11 family) to activate PLC and cognate downstream signaling cascades, e.g., production of IP3, liberation of Ca2+ from intracellular stores, and activation of PKC (422, 423, 842, 1067). As with most other receptors, the signaling mechanisms are context dependent, and stimulation by α1-receptors of various additional phospholipases (e.g., PLA2, PLD) as well as adenylyl cyclase has also been reported (842). The relevance of any of these pathways to the direct α1-mediated effects on motoneurons remains to be determined, although a similar effect of α1-adrenoceptor activation on thalamic relay neurons (i.e., decreased leak K+ current) involves PTX-insensitive G proteins (814).
Activation of α2-adrenoceptors leads to PTX-sensitive inhibition of adenylyl cyclase and decreases in cAMP and PKA activity (1067), as well as direct activation of neuronal inwardly rectifying K+ current by α2-adrenoceptors (922). The latter mechanism is unlikely to account for the α2-mediated hyperpolarization in hypoglossal motoneurons because that effect is associated with a decrease, rather than an increase, in conductance (959). Because Ih is activated by increases in cAMP (537, 702), the inhibition of Ih reported to underlie the clonidine-induced hyperpolarization could have resulted from decreased cAMP. However, this does not seem to be the case, at least inasmuch as the effects on Ih are unaffected by the adenylyl cyclase inhibitor SQ-22536 (959).
4. Functional role in synaptic integration and rate control
There have been no direct tests of NE effects on synaptic inputs to motoneurons. An increase in synaptic activity often accompanies the depolarizing effects of NE in vitro, which could be due to enhanced synaptic activity resulting from effects on presynaptic terminals or on local premotor neurons. Some α2-adrenoceptors in motor nuclei, perhaps the diffusely staining α2A-subtype (1227), may be on presynaptic terminals, where they could modulate fast transmitter release. The postsynaptic effect of NE to decrease input conductance would, by analogy with effects of TRH, be expected to enhance postsynaptic potential amplitude summation during repetitive synaptic stimulation (1039).
The effects of NE on the generation of action potentials have been studied in adult rat hypoglossal motoneurons (958). Without exception, NE decreases the current necessary to induce repetitive firing, even after compensating for the NE-induced depolarization; this is due to the decreased input conductance that follows from inhibition of a leak K+ current. This effect is similar to that induced by TRH or 5-HT (75, 1228). In a subset of motoneurons, a decrease in the spike AHP is observed, and in these cells, there is also an increased slope of the firing frequency response to current inputs (958). These two mechanisms together predict more effective transduction of synaptic inputs into firing output. The superposition of an increased delay to firing suggests that spike patterning could be modified by NE (958); if this is due to enhanced IA, then this altered patterning would be more pronounced after a period of membrane hyperpolarization. Thus, although generally excitatory, NE may induce complex and state-dependent changes in motoneuronal input-output properties.
The effects of NE have been examined in the context of complex behaviors. For example, NE enhances inspiratory-related hypoglossal nerve burst discharge in a brain stem slice preparation that maintains a respiratory rhythm. These effects of NE are developmentally regulated, since the potentiation of hypoglossal activity increases dramatically during the early postnatal period (400, 404). These postnatal changes are largely due to effects mediated by α1-adrenoceptors, since phenylephrine mimics developmental changes observed with NE. Additional effects mediated by α2- and β-adrenoceptors, perhaps indirect, cause inhibition and excitation of hypoglossal nerve discharge, respectively; the α2-mediated effect decreases postnatally whereas the β-mediated effect increases (400, 1127). In cat spinal motoneurons, the α1-agonist methoxamine enhances motoneuronal excitability and facilitates a bistable behavior reminiscent of that seen in other vertebrate motoneurons in the presence of monoamines (520, 722). A bistablelike behavior can also be generated in mouse nucleus ambiguus motoneurons in the absence of NE or other monoamines (1041).
G. Dopamine
In contrast to the situation in lower vertebrates and invertebrates, where dopamine effects on motoneurons have been studied extensively (447, 464), effects of dopamine on mammalian motoneurons have received little attention. Traditionally, there has been little reason to suspect a major role for dopamine in the function of mammalian motoneurons, since they reportedly receive an extremely sparse dopaminergic innervation and show little, if any, dopamine receptor binding (110, 952). However, recent immunohistochemical work with new dopamine antibodies reveals a substantial dopaminergic innervation of spinal motoneurons (509, 1409). Motoneurons express D1 and D2 dopamine receptors (306, 1305, 1330, 1408), which are coupled, respectively, to activation and inhibition of adenylyl cyclase (844). Interestingly, the sexually dimorphic motor nuclei of the lumbar spinal cord (Onuf’s nucleus) receive a more dense dopaminergic input and express higher levels of D2 dopamine receptor than other motor pools (509, 1305); the physiological significance of this observation has not been determined.
Physiological investigations of dopamine effects on mammalian motoneurons have been limited. Dopamine enhances ventral horn field potentials during antidromic activation of motoneurons in rat lumbar spinal cord (53) but decreases the synaptic response to dorsal root stimulation in rat and cat motoneurons (189, 777). In addition, dopamine modulates Renshaw cell-mediated feedback inhibition of rat motoneurons via effects of both D1- and D2-type dopamine receptors (778, 1129). Intracellular studies on mammalian motoneurons are required to determine if mechanisms identified in lower vertebrates and invertebrates are retained in mammals. For example, dopamine inhibits Ca2+ currents and the spike AHP in lamprey motoneurons (447) and modulates IA and Ih in lobster motoneurons (464); in chick motoneurons, D1 receptor activation increases kainate-receptor currents via effects that involve increases in cAMP and PKA (1165). Clearly, much work will need to be done to clarify any role for dopamine in the control of motoneuronal excitability.
H. ACh
Motoneurons use ACh as their (primary) transmitter, but also receive cholinergic synaptic input, which in the spinal cord stems partly from axon collaterals from nearby homonymous motoneurons (248, 249), and in the brain stem from neurons in reticular formation and vestibular nuclei (191, 375, 1427). Cholinergic boutons on spinal and some cranial motoneurons are present (40, 532, 884, 1093); some are large varicosities forming en passant type contacts (884). Spinal and some cranial motoneurons have mRNA coding for the m2 subtype of muscarinic cholinergic receptors (1315), binding sites for muscarinic agonists (1092, 1316), and show labeling using m2-receptor antibodies (1345). Thus anatomical and molecular data suggest actions of ACh on both spinal and cranial motoneurons. However, studies demonstrating effects of cholinergic agonists on motoneurons are scarce. Spinal motoneurons in the adult cat and neonatal rat are depolarized by ACh or muscarinic agonists (680, 1431). Brain stem laryngeal motoneurons are depolarized by iontophoretically applied ACh (460), and motoneurons in the compact division of the nucleus ambiguus (swallowing motoneurons) are depolarized by cholinergic agonists acting at nicotinic receptors (1427). Finally, activation of presynaptic m2 receptors reduces the release of excitatory transmitters to hypoglossal motoneurons (86). Signal transduction pathways and effectors involved in these actions are presently unknown.
I. ATP
ATP has only recently been established as a transmitter within the CNS; its actions are mediated by two major types of P2 receptor families (1, 2, 169–171, 320, 345, 384, 923, 1077, 1432). P2x receptors, comprising seven receptor subtypes P2x1 to P2x7 and a variety of isoforms, are ligand-gated ion channels that mediate fast excitatory responses (344, 1198). P2y receptors, comprising at least 11 major subtypes P2y1 to P2y11, mediate slower responses via G proteins (61, 169, 170, 307). Purinergic synaptic signaling in the CNS is of growing interest because 1) P2 receptors are widely distributed (46, 113, 231, 605, 630, 1125, 1281, 1322), 2) P2x receptors mediate fast synaptic transmission and have a large Ca2+ conductance, and 3) extracellular hydrolysis of ATP produces adenosine, which modulates synaptic transmission through activation of adenosine receptors, e.g., in phrenic motoneurons (303).
An important role for ATP in motor control is implicated by the ubiquitous presence of ATP binding sites (1281) and mRNA for several P2x receptor subunits within cranial and spinal motor nuclei (231). Differential distribution of receptor subtypes between cranial and spinal motor nuclei is apparent from in situ hybridization data showing the following: P2x4 and P2x6 in EW, trochlear, motor trigeminal, and facial nuclei; P2x2, P2x4, and P2x6 in oculomotor, DMV, and hypoglossal nuclei; but P2x2,4–6 in spinal motoneuron pools. Immunohistochemical analysis of brain stem P2x receptor distribution using recently developed antibodies reveals even more extensive expression. Hypoglossal motoneurons, for example, express protein for P2x1–6 subunits (713). The functional significance of any differential distribution has not been established. P2 receptor-mediated excitation has only been demonstrated for inspiratory-modulated hypoglossal motoneurons in neonatal mouse (in vitro), and adult rat (in vivo) (403), motoneurons of the dorsal motor nucleus of vagus (1284) and phrenic motoneurons in vitro (M. A. Parkis and G. D. Funk, unpublished observations).
With the consideration that ATP acts as the principal fast excitatory transmitter at some central synapses (320, 345), ATP may directly mediate specific behavioral inputs. Alternatively, ATP may modulate glutamatergic synaptic transmission (736, 872) to motoneurons (403). P2y receptors are present in the CNS (61); however, their role in motor control remains to be established. Understanding the role that P2 receptors play in modulation of neuronal (and motoneuronal) excitability will be greatly facilitated by development of agonists and especially antagonists with greater specificity for the P2x and P2y receptor subtypes. Suramin, the most commonly used general P2 antagonist, and to a lesser degree pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), the most selective P2x receptor antagonist, also antagonize glutamatergic transmission and inhibit ecto-ATPase activity (45, 872, 894, 933; see also Ref. 530). Thus, although the actions of exogenous ATP on central neurons are under investigation, assessing the physiological significance of endogenously released ATP is more difficult. Pharmacological tools for selective manipulation of ecto-ATPases that rapidly degrade extracellular ATP will also be useful.
J. Adenosine
Adenosine, a constituent of brain extracellular fluid, is an important modulator of neuronal excitability, including motoneurons. Adenosine can be formed extracellularly following rapid hydrolysis of ATP released from axon terminals (250, 310) or formed intracellularly and released into extracellular space via specific transporters. Intracellular formation is believed to be the major source of adenosine (383). Four distinct subtypes of adenosine receptors, belonging to the superfamily of G protein-coupled receptors (384, 955), have been cloned and characterized: A1, A2A, A2B, and A3 (230, 269, 274, 358, 382, 384, 742, 743, 754, 934, 955, 1299, 1321). In brief, A1 receptors are coupled to PTX-sensitive G proteins (385, 882, 1274, 1423), inhibition of adenylyl cyclase and decreased production of cAMP. A1 receptors are the most abundant adenosine receptors and widely distributed in the brain. The effects of A1 receptors are primarily inhibitory, including decreasing neuronal excitability by altering postsynaptic membrane properties (311, 418, 1097, 1122, 1153, 1245, 1430) and inhibiting release of a wide variety of neurotransmitters (137, 360, 550, 589, 851, 879, 1050, 1090, 1289, 1294, 1385). The two adenosine A2 receptor subtypes (A2a and A2b) are both coupled to Gs proteins to stimulate adenylyl cyclase and consequently increase the formation of cAMP (268, 754). The physiological effects of A2 receptors are less clear than those of the A1 receptors. The function of A2b receptors in the brain has not been thoroughly investigated, and their physiological roles remain unclear. On the other hand, considerable evidence favors an excitatory role of A2a receptors in the CNS (149, 1116, 1422; cf. Ref. 708). Adenosine A3 receptors have very low affinity to adenosine. A3 receptors are coupled to Gαi-2, Gαi-3, and Gq-like proteins. Unlike other subtypes, A3 receptors are linked to two second messenger systems: decreasing cAMP through inhibition of adenylyl cyclase or increasing intracellular Ca2+ through stimulation of IP3 (see reviews in Refs. 155, 194, 1321). The physiological role of A3 receptors is unclear at present.
Adenosine effects on motoneurons are mainly mediated by A1 receptors. High levels of mRNA for A1 receptors are present in rat cranial and spinal motoneuron pools (1043) and in the areas containing hypoglossal premotoneurons (297), such as the reticular formation lateral to the hypoglossal nuclei, and in the ventrolateral medulla (1043). A1 receptors inhibit synaptic transmission to motoneurons via a presynaptic action. Activation of A1 receptors by exogenous agonists decreases evoked EPSP in hypoglossal motoneurons (85) and endogenous inspiratory-modulated EPSC in phrenic motoneurons (300); there is appreciable endogenous activity of A1 receptors, since A1 antagonists increase EPSP (85) or EPSC (300) amplitudes. The agonist significantly decreases mEPSC frequency (300), an event dependent on transmitter release probability (348, 1035), but has no effect on mEPSC amplitude, an event associated with postsynaptic responsiveness to the endogenously released transmitter (348, 1035; see also Ref. 678). Thus a presynaptic mechanism is likely for the inhibitory effect of A1 receptors on synaptic transmission to these motoneurons.
Activation of A1 receptors decreases both spontaneous and evoked firing of vagal motoneurons (796, 1262). Although the mechanism for the inhibition of spontaneous firing is unclear, the increase in AHP amplitude by postsynaptic A1 receptors, affecting IK Ca, is likely responsible for the decrease in evoked firing (796). Adenosine is reported to cause hyperpolarization in hypoglossal motoneurons (987), which is not observed with an A1 receptor agonist (85). However, the receptor subtype(s) mediating this hyperpolarization was not identified. In cultured motoneurons, activation of A1 receptors inhibits HVA Ca2+ current (mainly N type) (882); the functional role of this effect remains unclear.
At present, our understanding of the physiological role of the other adenosine receptor subtypes in modulating motoneurons is poor, primarily due to the lack of selective ligands and the diffuse distribution of these receptors.
K. TRH
1. Ligands, receptors, and sources of TRH input to motoneurons
The tripeptide TRH (pyroGlu-His-Pro-NH2) was the first hypophysiotropic hormone to be purified from hypothalamic extracts and characterized (1037). TRH is the product of posttranslational processing of a TRH precursor (preproTRH) mRNA that contains five copies of the TRH progenitor sequences (718). A number of other peptides in addition to TRH are derived from the same preproTRH (717, 718, 1386). These other peptides are synthesized and released from some neurons in a depolarization- and Ca2+-dependent manner and, in some systems, they affect target tissues with actions that are synergistic with TRH (160, 690). Effects of these additional preproTRH-derived peptides have not been reported on motoneurons.
Two mammalian TRH receptor genes, TRHR1 and TRHR2, have been identified (184, 423); they share ~50% sequence homology and are both members of the rhodopsin family of G protein-coupled receptors. The more recently identified TRHR2 has so far only been found in rat, where it is expressed in a pattern distinct from TRHR1; of particular relevance to this review, it appears that TRHR1, but not TRHR2, receptors are expressed in motoneurons (177, 184, 1416). TRHR1 receptors from rat, mouse, and human are nearly identical (~95% identity through residue 375) except for the COOH-terminal region where there is substantial variability (423). In the rat and mouse (but not human), two TRHR1 mRNA species have been identified. These arise by alternative mRNA splicing and differ in the COOH terminus, predicting a long and short isoform of the receptor. The significance of these TRHR1 receptor isoforms is unclear, since their expression in native tissue has not been demonstrated and the two isoforms appear functionally identical in heterologous expression systems (423).
Rat cranial and spinal motoneurons display dense TRH binding sites and express TRHR1 receptor mRNA at high levels (177, 1131, 1371, 1416). Expression of the TRH receptor is developmentally regulated, at least in some motoneuron pools. Thus TRH binding sites are at low levels in hypoglossal motoneurons of neonatal rat and increase in density over the early postnatal period to reach adult levels by approximately postnatal day 14 (78).
There is a relatively dense TRH input to brain stem and spinal cord motoneurons (489), and TRH fibers make axodendritic and, to a lesser degree, axosomatic contacts with motoneurons (1000). The TRH innervation appears to arise primarily from medullary raphe neurons rather than from hypothalamic cells (see sect. iii and Table 2). The medullary raphe nuclei and the hypothalamic paraventricular nuclei (PVN) are the two major areas in the brain in which TRH-containing somata are located (489, 576); these neurons send descending projections to regions of the brain stem and spinal cord that contain motoneurons (1202). However, combined histochemistry and retrograde tract tracing demonstrate that 1) TRH-expressing medullary raphe neurons project to spinal cord and the dorsal medulla (484, 767, 1080), 2) TRH-expressing PVN neurons are not retrogradely labeled from spinal cord or brain stem, and 3) spinally projecting PVN neurons do not express TRH (72). Furthermore, cutting the projection path to the dorsal medulla decreases the number of TRH-immunoreactive fibers in the hypoglossal nucleus (954), and electrical or chemical lesion of medullary raphe nuclei (471, 576) decreases TRH concentrations and TRH-IR fibers in spinal cord. On the other hand, disruption of descending pathways from the hypothalamus (151, 551, 954) or electrolytic lesion of the PVN (716) does not decrease TRH concentrations in the brain stem or spinal cord. Some differences in the density of TRH-immunoreactive fibers are noted among motoneuron pools and throughout postnatal development. For example, a greater number of TRH-immunoreactive fibers form close appositions to respiratory-related motoneurons of the nucleus ambiguus than to either nonrespiratory ambigual motoneurons or to hypoglossal or facial motoneurons (1196). In addition, there are developmental increases in the density of TRH-immunoreactive fibers in brain stem and spinal cord motor pools (78, 999); in the hypoglossal nucleus, the increased TRH innervation coincides temporally with the increased expression of TRH receptors by hypoglossal motoneurons (78).
2. Pre- and postsynaptic actions of TRH on motoneurons
TRH has direct excitatory effects on spinal and cranial motoneurons (75, 76, 913, 1039, 1212, 1333, 1357). In mammalian motoneurons, TRH causes a depolarization associated with a decrease in membrane conductance. At least two ionic mechanisms contribute to the depolarization: TRH inhibits a relatively voltage-insensitive resting leak K+ current and activates a mixed cationic current (75, 366, 921). This leak K+ current is insensitive to TEA, Cs+, 4-AP, or apamin and blocked by Ba2+ (75, 366, 921); the cationic current is partially inhibited by Cd2+, Mn2+, and Co2+, suggesting that it may be Ca2+ dependent (366). This combination of effects is common to a number of transmitters that act directly on motoneurons, i.e., qualitatively similar effects are seen with NE, substance P, and 5-HT. The effects of TRH occlude those of NE and substance P (367, 958), further suggesting a common mechanism.
Associated with its postsynaptic actions, exogenous TRH application to in vitro preparations often induces an increase in synaptic potentials recorded from motoneurons (75, 1039, 1333). The mechanism of this effect of TRH remains to be determined; it could reflect excitation and increased activity-dependent transmitter release from local premotor neurons, or a presynaptic effect on terminals that impinge on motoneurons (689, 1333).
TRH acts directly on motoneurons to cause a slow membrane depolarization when applied in vitro, suggesting that if it were released synaptically it might mediate a slow EPSP. In fact, electrical stimulation of the ventral funiculus in a neonatal spinal cord in vitro preparation to activate a descending TRH pathway evokes a slow EPSP in spinal motoneurons that is sensitive to TRH antibodies (1212). Identifying the role that such a TRH-mediated slow synaptic excitation plays in normal motor system function awaits the development of specific and selective TRH receptor antagonists (1358).
3. Signal transduction
The TRH receptor, like the α1-adrenoceptor, signals primarily through PTX-insensitive G proteins (Gαq/11 family) to activate PLC and cognate downstream signaling cascades, e.g., production of IP3, liberation of Ca2+ from intracellular stores, and activation of PKC (422, 423, 842, 1067). However, under some circumstances and in some cells, the TRH receptor may also couple to a variety of other G proteins, including Gαi, Gαo, and a Gαs-like protein to bring about additional effects (423). For example, antisense knockout studies indicate that Ca2+ channel activation by TRH in GH3 pituitary cells is mediated by the PTX-sensitive G proteins, Gαi-2 and Gαi-3 (430, 431). Interestingly, this effect is blocked by Gαq/11 antisense treatment and PKC inhibitors, suggesting that coordinate activation of both Gαq and Gαi signaling pathways may be necessary for Ca2+ channel activation by TRH (431). The intracellular mechanisms by which TRH modulates motoneuronal excitability have not been exhaustively studied but, insofar as they have been tested, there has been no evidence to support involvement of any of these pathways in mediating its direct postsynaptic effects. Thus, although TRH-induced depolarization of hypoglossal motoneurons involves G proteins, the TRH effect is independent of changes in intracellular Ca2+ or IP3 and unaffected by activation of PKC or PKA (77). Indeed, it is noteworthy that despite its widespread presence in many other neurons, the mechanism by which transmitters that act via Gαq-coupled receptors signal to cause inhibition of leak K+ channels and/or activation of cationic channels remains enigmatic.
4. Functional role of TRH in modulating motoneuronal excitability
TRH increases the duration and amplitude of trains of electrically evoked postsynaptic potentials in guinea pig hypoglossal motoneurons, presumably via postsynaptic effects on membrane input conductance and time constant (1039). Although not directly tested, the altered membrane properties induced by TRH would also be expected to enhance inhibitory synaptic inputs; interestingly, TRH also potentiates NMDA-induced depolarization and NMDA-mediated EPSP (1040). This is not due to a direct effect of TRH on the NMDA channels, since under voltage-clamp the NMDA current is unchanged; rather, the combined action of TRH and NMDA on the motoneuronal membrane leads to a region of negative slope conductance, potentiating NMDA-induced depolarizations (1040).
The input-output relationship of motoneurons is also enhanced by TRH; the curve relating depolarizing current input and firing frequency output in rat hypoglossal motoneurons is shifted to the left in the presence of TRH, i.e., previously subthreshold current inputs induce repetitive firing with the frequency of discharge increased at each suprathreshold current input (75). This is due to the combined effects of TRH-induced depolarization and decreased conductance; membrane depolarization brings the neuron closer to action potential threshold while the decreased input conductance allows more effective transformation of current inputs to voltage responses. The shift in the input-output relationship caused by TRH is not associated with a change in slope (i.e., gain) (75). This is consistent with the finding that TRH has no effect on the spike AHP, which largely determines interspike interval, and distinctly different from effects of the presumptive cotransmitter, 5-HT, which decreases the spike AHP and increases input-output gain in neonatal hypoglossal motoneurons (75, 91).
A number of changes take place during the early postnatal period that influence TRH effects on motoneurons. Expression of the TRH precursor mRNA increases markedly in raphe neurons within the first 2 wk after birth (78), as does the density of TRH-immunoreactive fibers in motor nuclei receiving input from the raphe nuclei (78, 999). Concomitant with this elevated innervation, the levels of TRH binding sites also increase to reach sustained high levels in rat hypoglossal motoneurons by postnatal day 14 (78). Thus both the presynaptic and postsynaptic elements of the raphe motoneuronal system mature over the first 2 wk via a process that is remarkably well matched in timing. Furthermore, the electrophysiological effects of TRH on motoneurons also change during this developmental period. An increasing fraction of TRHresponsive neurons are found through the early postnatal period, roughly paralleling the increase in receptor binding (78). However, a number of other changes also contribute to the functional maturation of hypoglossal motoneuron responses to TRH. Unlike adult hypoglossal motoneurons (75, 1039), in some responsive neonatal neurons TRH causes a depolarization that is not associated with decreased conductance, reminiscent of the TRH response after inhibition of the leak K+ current by Ba2+. The reason that TRH receptors do not appear to couple to K+ channels in this population of neurons remains to be determined. It is noteworthy that 5-HT, which modulates Ba2+-sensitive leak K+ channels in adult motoneurons (526, 1228), also fails to target those channels in neonatal hypoglossal motoneurons (91, 1228). Finally, there is a progressive increase in TRH current density that matches closely the increase in membrane conductance over the early postnatal period (78, 92, 1313). This latter effect ensures that the magnitude of the TRH-induced membrane depolarization will be comparable in adult and neonatal hypoglossal motoneurons, at least in the population of neonatal cells that are capable of responding (78) (see Fig. 8). Effects of TRH in the context of a more complex motor system are also enhanced with a similar postnatal time course; facilitatory effects of TRH on rhythmic inspiratory-related discharge measured on the hypoglossal nerve in vitro are greatly enhanced over the first 2 postnatal weeks (400).
FIG. 8.
Different neuromodulators can affect motoneuronal excitability via similar mechanisms. These records show the response of hypoglossal motoneurons to phenylephrine (PE), an α1-adrenoceptor agonist [top trace; from Parkis et al. (958)]; thyrotropin-releasing hormone [TRH; middle trace, from Bayliss et al. (75)]; and substance P (SP; bottom trace). All 3 transmitters induce a membrane depolarization, which can reach threshold for repetitive firing, e.g., see middle trace, spikes at the peak of the TRH response are truncated. The negative deflections in the sample traces, which represent responses to constant-amplitude current pulses, are enhanced by the transmitters, reflecting the transmitter-induced decrease in a resting K+ current. A second component, involving activation of a cationic current, also contributes to membrane depolarization by all 3 transmitters (data not shown). −d.c. indicates negative bias current used to bring the membrane potential back to control level.
L. Neurokinins
1. Ligands, receptors, and sources of neurokinin input to motoneurons
The neurokinins are the mammalian members of the tachykinin family of neuroactive peptides, which share a common structure that includes the COOH-terminal sequence Phe-Phe/Val-Gly Leu-Met-NH2 (453, 472, 889, 948). The undecapeptide substance P was the first member of this family to be purified and characterized (208); other mammalian members were identified only after some years and include neurokinin A (NKA; also known as substance K, neurokinin α, and neuromedin L) and neurokinin B (NKB; also known as neurokinin β, neuromedin K), as well as the recently discovered NH2-terminally extended versions of NKA, the so-called neuropeptide K (NPK) and neuropeptide γ (NPγ) (453, 472, 889, 948). In some tissues, fragments of NKA have been identified as well, e.g., NKA-(3—10) and NKA-(4—10), but it is unclear if these fragments are released or exist in these forms due to proteolytic degradation of NKA (1241).
All neurokinins identified to date derive from two closely related genes, preprotachykinin A (PPT-A) (615, 665, 897, 898) and preprotachykinin B (PPT-B) (119, 663), that may have arisen by duplication of a common ancestral gene (663, 888, 889). The PPT-A gene is differentially spliced, leading to three distinct mRNA isoforms: the α-form of PPT-A encodes only substance P, whereas the β- and γ-forms encode both substance P and NKA. The other neurokinin peptides, NPK and NPγ, are derived from β-PPT-A and γ-PPT-A mRNA, respectively (888). Although tissue-specific regulation of splicing has been suggested in other species, i.e., bovine (888, 898), in both rat and human CNS the preponderance of PPT-A mRNA exists in the γ- and/or the β-forms, with very little expressed as α-PPT-A mRNA in either species (195, 665). Thus preferential expression of the PPT-A mRNA isoforms that encode both substance P and NKA (as well as NPK or NPγ) suggests that individual neurons will usually coexpress multiple neurokinins. Indeed, with the use of specific antibodies, immunoreactivity for both substance P and NKA is seen in individual medullary raphe neurons that are known to project to motoneurons (907). In contrast to the complexity of PPT-A processing, with three splice variants and their multiple neurokinin products, the two (α and β) forms of the PPT-B mRNA give rise only to NKB among the currently known neurokinins (119, 663, 888).
As predicted from earlier autoradiographic studies (453), three neurokinin receptors that preferentially interact with each of the major tachykinins, the NK1 receptor with substance P, the NK2 receptor with NKA, and the NK3 receptor with NKB, have been identified by molecular cloning (889). All three cloned receptors are members of the rhodopsin family of G protein-coupled receptors. It is important to point out that the receptor-ligand associations described for the NK receptors are not absolute, since all known neurokinins can bind each of the NK receptor subtypes in nanomolar concentration ranges and activate each receptor with full potency (although EC50 values are ~1–2 orders higher) (536, 889). Moreover, a number of examples of mismatch between localization of NK receptors and preferred endogenous ligands have been noted (e.g., Refs. 293, 747, 892, 1149). Together, these observations suggest that there may be substantial cross-talk within the mammalian tachykininergic system.
The distribution of neurokinin receptors throughout the CNS, including motor nuclei, has been extensively investigated (90, 270–272, 293, 329, 774, 791, 892, 1014, 1149, 1405). The results of these studies are concordant with the conclusion that adult rat motoneurons predominantly express NK1 receptors and display substance P binding sites. The expression of NK1 receptors is not uniform among motoneuronal pools, however, implying that effects of substance P may vary depending on the particular motoneuronal pool. For example, some motoneuronal populations express very high levels, e.g., phrenic, pudendal motoneurons, whereas others express moderate, e.g., hypoglossal motoneurons, or only very low levels of NK1 receptor, e.g., facial, trigeminal motoneurons (210, 791, 892, 1405). The NK2 receptor is preferentially located in the periphery with only low levels of binding sites in the CNS, whereas the NK3 receptor has a widespread CNS distribution; neither NK2 or NK3 receptors, however, are prominent in motor nuclei (272, 293, 1149, 1405). The generalization regarding the predominant expression in motoneurons of the NK1 subtype among the NK receptors may not hold in all circumstances. For example, expression of each of the NK receptors is developmentally regulated (90, 211, 272, 1014) and, interestingly, high levels of NK3 binding sites are present in the ventral horn of the spinal cord up to at least postnatal day 10 (90). Whether these ventral horn NK3 binding sites represent transient somatodendritic NK3 expression by neonatal motoneurons or are associated with other neuronal elements remains to be determined.
The PPT-A and PPT-B genes are differentially expressed with overlapping but distinct patterns of expression (797, 1338). There is no significant NKB immunoreactivity in fibers innervating motoneurons (797, 833, 1260) and, consistent with this, no evidence for expression of its precursor PPT-B in cells that provide major projections to motoneurons, e.g., medullary raphe, sensory ganglia (797, 1338). Given that NK3 receptors have only been found on neonatal motoneurons (see above), it is unlikely that the endogenous PPT-B/NKB system has a major role in regulating motoneuronal function, at least in the adult.
In contrast, there is a dense innervation of all motoneuronal pools by substance P-immunoreactive fibers that is potentially derived from at least three sources: brain stem raphe neurons, primary afferent neurons, or substance P-expressing cells intrinsic to the spinal cord (751, see sect. iii and Table 2). Caudal raphe neurons, particularly in raphe obscurus and pallidus, express high levels of PPT-A mRNA, and individual spinally projecting raphe cells are immunoreactive for both substance P and NKA, the neurokinins encoded by PPT-A (576, 907, 1338). Descending inputs from medullary raphe neurons provide the most prominent neurokininergic innervation of motoneurons since the overwhelming majority of substance P and substance P-immunoreactive fibers in the ventral horn are lost after high spinal cord transection or chemical destruction of medullary raphe neurons (379, 473, 576, 603). Substance P is extensively colocalized with 5-HT and TRH in medullary raphe neurons and in fibers surrounding motoneurons (576, 951), and the density of substance P and 5-HT-immunoreactive fibers appears to increase during early postnatal development (951).
PPT-A is also expressed at high levels in many sensory ganglia neurons (1338), but dorsal rhizotomy causes only small decreases in substance P innervation of the ventral horn with much larger decreases in the density of dorsal horn substance P-immunoreactive fibers (492, 567, 1220). This is consistent with the preferential immunohistochemical localization of substance P to capsaicin-sensitive sensory neurons associated with small fibers in the C and Aδ range that carry nociceptive inputs and terminate primarily in the dorsal horn, and its absence in muscle afferents that project monosynaptically to spinal motoneurons (493, 812). A small amount of ventral horn substance P innervation may arise from spinal dorsal horn neurons that express PPT-A and substance P (751, 1338), and these may be important in conveying polysynaptic sensory inputs to the motoneurons (425).
2. Pre- and postsynaptic actions of neurokinins on motoneurons
Otsuka and colleagues (660, 945, 946, 1199, 1217) first demonstrated an excitatory effect of substance P on neonatal rat spinal cord motoneurons; they found that substance P causes a slow depolarization of the ventral root or the motoneuronal membrane potential. This is due to a direct postsynaptic effect on motoneurons because it persists, at least in part, after blockade of synaptic transmission in TTX or in a low-Ca2+/high-Mg2+ synaptic blockade medium (947, 1199). The depolarization of neonatal rat spinal motoneurons by substance P is associated with an apparent decrease in membrane conductance (365, 367), involving two ionic mechanisms: inhibition of a relatively voltage-independent resting K+ current and activation of a (presumably) mixed cationic current (367). The characteristics of these effects of substance P are highly reminiscent of those of TRH (see above), and accordingly, the effects of the two transmitters mutually occlude each other (367). Moreover, this mechanism of action involving a decrease in a K+ current combined with activation of a cationic current has also been described for substance P in locus coeruleus neurons, although in those cells the K+ current is inwardly rectifying (1140).
The identity of the neurokinin receptor that mediates the direct depolarizing effects of substance P on motoneurons is not yet entirely clear, although the bulk of the pharmacological evidence supports the aforementioned histochemical data that predicts involvement of NK1 receptors. Thus a number of selective NK1 agonists mimic effects of substance P on motoneurons, e.g., substance P methyl ester and [Sar9Met(O2)11]substance P, and those effects are blocked by some NK1 receptor antagonists, i.e., SR-140,333 (52, 365, 726). However, a number of additional NK1 antagonists are without effect on substance P responses, i.e., (±)-CP-96,345, RP-67580, and GR-82334, or display pharmacological profiles unlike that of the classical NK1 receptor, i.e., spantide (51, 726, 1402). Motoneurons may express a distinct isoform of the NK1 receptor that possesses atypical antagonist binding characteristics (1402). Alternatively, because most electrophysiological studies utilize preparations from neonatal rats in which more than one NK receptor may be expressed (especially both NK1 and NK3; see above), the atypical pharmacological results could simply reflect a mixed receptor population in those developing neurons.
The effects of the other neurokinins, NKA and NKB (and/or agonists selective for their cognate receptors), have also been studied on spinal motoneurons. In neonatal rat spinal motoneurons, NK2 and NK3 agonists cause a slow membrane depolarization that is reduced, although not completely, by TTX (365, 727, 807), suggesting these effects are mediated by premotor neurons. This is consistent with histochemical studies suggesting that ventral horn motoneurons do not express the NK2 or NK3 receptor subtypes, which are more prevalent in the dorsal horn. The small residual current occasionally seen in the presence of TTX may reflect effects mediated by NK2 or NK3 receptors expressed transiently on some neonatal motoneurons, or could be due to small crossover effects of the agonists onto NK1 receptors. The nature of the transmitter released onto motoneurons from spinal presynaptic neurons following NKA or NKB stimulation has not been determined, but it is notable that it causes a depolarization associated with an apparent decrease in input conductance, not unlike that produced directly by NK1 receptor activation (365). Of further interest, NK3-selective agonists (MePhe7)NKB and Senktide often induce a burst-firing behavior that is superimposed on the depolarization; the nature of this burst mechanism remains to be explored (365).
The effects of exogenously applied NK receptor agonists suggest that neurokinins, particularly substance P and other NK1 agonists, cause a slow membrane depolarization by acting directly on the motoneuron. Therefore, synaptically released substance P might be expected to mediate a slow EPSP in motoneurons. Indeed, electrical stimulation of upper cervical segments designed to activate descending substance P pathways to lumbar motoneurons in an isolated neonatal rat spinal cord induces a slow EPSP that is blocked by NK1 receptor antagonists and enhanced by a peptidase inhibitor (681). Moreover, a component of the slow EPSP is also sensitive to ketanserin, a 5-HT2 antagonist and the SP-dependent EPSP is virtually absent after treatment of the spinal cord with a chemical neurotoxin for 5-HT, suggesting that the substance P is released from fibers that also contained 5-HT (681). Thus this descending pathway from medullary serotonergic neurons that contain substance P probably represents the principal direct mechanism for substance P modulation of motoneuronal function. The release of substance P is apparently frequency dependent so that it may only be released at times of increased activity in raphe neurons (378), e.g., during increased locomotor or respiratory activity (1308). It is important to point out that reflex activation of motoneurons after stimulation of dorsal roots at intensities sufficient to activate small fibers can also induce a slow NK1-dependent EPSP in motoneurons (52, 457, 949). However, this effect is probably polysynaptic, since it is blocked by glutamate receptor antagonists and only the long-latency responses are sensitive to NK receptor antagonists (52).
3. Signal transduction
There is good evidence that all three NK receptors couple via PTX-insensitive G proteins to activation of PLC and production of IP3 and diacylglycerol as the major, although not only, signaling pathway (889, 948). In this respect, the NK1 receptor is similar to other receptors, e.g., 5-HT2, α1-adrenergic, TRH, that also mediate direct motoneuronal depolarization via inhibition of a resting K+ current and activation of a cationic current (75, 77, 526, 958). However, as in those other cases, the involvement of PLC and/or its downstream mediators in these effects remains to be demonstrated.
4. Functional role of neurokinins in modulating motoneuronal excitability
The effects of substance P on synaptic inputs to motoneurons have not been directly studied. There is currently no information as to whether substance P can modulate fast excitatory or inhibitory inputs onto motoneurons at a presynaptic site. Substance P may act presynaptically to enhance 5-HT release (846), perhaps by blocking a 5-HT autoreceptor on its own terminals (491, 496). The postsynaptic mechanisms of action of substance P, which are similar to those of TRH, suggest some additional possibilities. For example, the substance P-induced decrease in membrane conductance would be expected to increase the amplitude and duration of postsynaptic potentials by altering membrane characteristics, i.e., length and time constants, as shown for TRH (1039). This would be the case for both inhibitory and excitatory synaptic inputs. Furthermore, the firing response to current inputs is shifted to the left in the presence of substance P, presumably by virtue of the combined effects of substance P to depolarize and decrease the motoneuronal input conductance (726).
M. Arginine Vasopressin and Oxytocin
1. Ligands, receptors, and sources of arginine vasopressin and oxytocin input to motoneurons
Arginine vasopressin (AVP; also called antidiuretic hormone) and oxytocin are nine-amino acid posterior pituitary peptide hormones arranged in a six-amino acid disulfide ring with a three-amino acid side chain; they differ by only two amino acids (1038). AVP and oxytocin are cleaved from precursor hormones encoded by separate but closely linked genes that are likely derived from a common ancestral gene (546). The discovery of an extrahypothalamic distribution of AVP and oxytocin suggested CNS functions in addition to their well-known roles in regulating plasma osmolarity and increasing intramamallary pressure leading to milk ejection (1175).
Three receptors for AVP are suggested based on its distinct actions and pharmacology in different tissues: in vascular smooth muscle, the V1a (or V1) receptor causes vasoconstriction; in the anterior pituitary, the V1b (or V3) receptor induces ACTH release; and in the kidney, the V2 receptor enhances water reabsorption. To date, only a single oxytocin receptor has been identified. The existence of pharmacologically defined AVP receptor subtypes, as well as the oxytocin receptor, have now been verified by molecular cloning, and all are members of the rhodopsin family of G protein-coupled receptors (56, 57, 974).
The predominant AVP receptor in the CNS is the V1a subtype, and it is this isoform that appears to be expressed transiently on cranial motoneurons as identified by ligand binding autoradiography with either [3H]AVP or 125I-VPA (a selective V1a radioligand) (956, 1269). High levels of binding are detected in rat facial motoneurons from embryonic day 20 through the second postnatal week, whereupon AVP binding sites progressively diminish in density to reach low levels by postnatal day 19 and barely detectable levels in adulthood (1269). Likewise, V1a receptor binding is very low in most spinal cord motor nuclei of adult rats, although there are some exceptions; dorsolateral ventral horn motoneurons at the cervicothalamic spinal cord junction and medially located motoneurons throughout the lumbar cord show higher levels of V1a binding (1267). Most notable, however, are the sexually dimorphic pudendal motoneurons. In adult male rats, these motoneurons have much higher levels of V1a binding than any other spinal motoneurons. Moreover, the elevated levels of binding in pudendal motoneurons are apparently dependent on sex steroid hormones since binding is reduced by castration and is not elevated in the same motoneurons of female rats (1267). Interestingly, after axotomy of brain stem (facial, hypoglossal) and spinal motoneurons in the adult rat, receptor expression increases markedly, suggesting that those motoneurons revert to an immature neonatal-like phenotype after axotomy (1266).
Oxytocin receptors have not been studied as extensively as V1a receptors in the context of motoneurons. Nevertheless, oxytocin binding sites are not generally associated with adult motoneurons, although they are detected on hypoglossal motoneurons in the neonatal rat (956, 1268). Thus the expression of oxytocin receptors by motoneurons, at least by hypoglossal motoneurons, may be developmentally regulated in a manner somewhat analogous to V1a receptors.
AVP and oxytocin are synthesized in hypothalamic neurons, primarily but not exclusively in the paraventricular and supraoptic nuclei (1038). Descending projections from AVP- and oxytocin-containing hypothalamic neurons to the brain stem and spinal cord arise from the lateral parvicellular division of PVN (203, 1086). However, AVP- and oxytocin-immunoreactive fibers are localized to the dorsal horn of the spinal cord and to regions involved in sympathetic and parasympathetic autonomic regulation, e.g., intermediolateral cell column and dorsal motor nucleus of the vagus nerve, and are actually only sparsely represented in somatic motor nuclei (1175, 1203). Thus, if AVP and oxytocin elaborated from these fibers activate motoneurons, they may have to act at some distance from their release site. To our knowledge, a study of the motoneuronal innervation by these peptides throughout early postnatal development has not been reported. Therefore, it remains to be determined if the AVP and/or oxytocin inputs are more substantial in neonates when the cognate receptors are expressed at higher levels by motoneurons.
2. Pre- and postsynaptic actions of AVP and oxytocin on motoneurons
A direct, largely TTX-resistant postsynaptic depolarization of motoneurons by AVP was first demonstrated in a neonatal rat spinal cord preparation (1199); a depolarization in response to oxytocin was also noted, although that effect was somewhat more sensitive to TTX (1199). Subsequently, excitatory effects of both AVP and oxytocin have been documented in other neonatal motoneurons (956, 1019, 1269). Although the receptor and ionic mechanisms for effects of AVP have been studied most extensively, oxytocin and AVP each invokes inward currents that are similar in magnitude and time course in the same neonatal hypoglossal motoneurons, suggesting that they may share a common mechanism (956). Presynaptic effects of these peptides have not been demonstrated in motoneurons except insofar as showing that a component of their effects is inhibited by TTX or a low-Ca2+, high-Mg2+ synaptic blockade medium (956, 1199).
In neonatal rat brain stem slice preparations, AVP increases the firing rate of facial motoneurons and induces the development of an inward current in both facial and hypoglossal motoneurons (956, 1019, 1269). These effects are blocked by an AVP receptor antagonist and mimicked by a V1 but not a V2 receptor agonist (956, 1019, 1269). Together with the receptor binding data presented above (956, 1269), these results indicate that AVP acts via a V1a receptor to depolarize and increase the excitability of neonatal motoneurons. We are unaware of published reports of effects of either AVP or oxytocin on adult motoneurons, but effects on those older motoneurons are unlikely given the ephemeral nature of V1a and oxytocin receptor expression on those cells (1268, 1269).
The mechanism by which AVP modulates neonatal cranial motoneurons has been studied in vitro under voltage-clamp conditions. The AVP-induced current is voltage dependent, TTX insensitive, and carried, in part, by Na+ (10, 1019); the current in facial motoneurons is enhanced in low extracellular Ca2+, suggesting that it is partially inhibited at normal Ca2+ concentrations, i.e., 2 mM (10). This AVP-induced cationic current shares some features with the current induced by 5-HT in neonatal rat hypoglossal motoneurons (91) as well as with a Ba2+-resistant cationic current component induced by a number of transmitters in various adult motoneuronal preparations, e.g., 5-HT, TRH, and NE, although those other transmitter-induced currents are not noticeably voltage dependent, and their inhibition by extracellular Ca2+ has not been reported (526, 958). The voltage-dependent and kinetic characteristics of the AVP-sensitive current are distinctly different from those of Ih, a voltage-dependent cationic current that is modulated by 5-HT in some motoneurons (526, 701, 1216).
3. Signal transduction
The V1 and oxytocin receptors interact via Gαq/11 to activate PLC and downstream signaling cascades in vascular smooth muscle, corticotropes, and neurons (56, 57, 974); whether this signaling pathway contributes to effects of AVP and oxytocin on motoneurons remains to be determined. This same pathway is putatively activated by a host of other motoneuronal excitatory neurotransmitter receptors (e.g., see sect. iv, E, F, and K). Whereas these other transmitters inhibit resting K+ channels in addition to activating a cationic current, V1 receptors apparently target only the cationic channels (75, 526, 958). This may reflect the fact that effects of AVP and oxytocin were tested on neonatal motoneurons, since other transmitters that modulate both channels in adult motoneurons apparently activate only the cationic channel in neonatal motoneurons, e.g., 5-HT and TRH (78, 91, 1228). Thus it may be a general finding that ion channels modulated by Gαq/11-coupled receptors, and/or the transduction pathway from the receptor to those channels, are developmentally regulated in motoneurons (79).
4. Functional role of AVP and oxytocin in modulating motoneuronal excitability
The effects of both AVP and oxytocin on motoneurons are excitatory, although their precise role in control of motoneuronal function remains speculative. The high levels of V1a receptor binding in sexually dimorphic nuclei of adult male rats suggest a role for AVP in sexual reflexes (1267). The transient developmental pattern of AVP and oxytocin receptor expression in most other motoneuron pools suggests that effects of these neuropeptides may be important early in the motoneuronal maturation process (1269). Clearly, a number of important developmental changes occur in motoneurons and their targets during the early preweaning postnatal period when those receptors are strongly expressed (92, 95, 760, 1329). As mentioned, there does not appear to be a dense innervation of motor nuclei directly by either AVP or oxytocin (1175, 1203), and the source of endogenous peptides to interact with those transiently expressed motoneuronal receptors is unclear. In this regard, it would be interesting to know if a more dense plexus of AVP and oxytocin fibers surrounds motoneurons during this early period. Perhaps the early development of an excitatory AVP and/or oxytocin input to motoneurons serves to compensate for the delayed development of other excitatory neurotransmitter systems in the neonate (e.g., TRH, NE; Refs. 78, 400).
N. Other Neuropeptides
Numerous neuropeptides have been identified in fibers near motoneurons (330 and Table 2), but for the most part, their electrophysiological effects on motoneurons have not been systematically characterized. For example, an extensive enkephalin innervation of brain stem and spinal motoneurons suggests that they may be modulated by this peptide (13, 330, 477). Consistent with this, electrical stimulation in the region of enkephalin-synthesizing locus coeruleus neurons evokes a naloxone-sensitive IPSP (395). However, nothing is known of the mechanism by which enkephalin mediates this inhibition. Likewise, there has been little, if any, information regarding pre- or postsynaptic effects of other peptides. Almost 20 years ago, Suzue et al. (1199) showed that a number of peptides caused membrane depolarization in neonatal rat spinal motoneurons (e.g., bombesin, cholecystokinin, angiotensin II, neurotensin), but the receptor and ionic basis for effects of many of those transmitters still have not been elucidated.
V. CONCLUSIONS AND PERSPECTIVES
Motoneurons are specialized neurons, genetically programmed to form specific motor pools, which have a clearly defined and important role in brain function and behavior, i.e., control of skeletal muscle contraction and relaxation that underlies all movements. As such, the principles and mechanisms that determine their performance, especially the transformation of their inputs into action potentials to innervated motor units, are of interest. Moreover, insofar as motoneurons process signals like all other neurons, insights into their function may illuminate basic processes of brain function.
We have reviewed the basic properties of motoneurons and their synaptic inputs. Their common biophysical properties, e.g., voltage-dependent ion channels, and synaptic properties, e.g., amino acid, amine and peptide transmitters and associated receptors, are sufficiently rich that the combinatorial possibilities for different states of excitability are legion. The majority of these properties have been measured in highly reduced or constrained conditions that are amenable to experimental manipulation. Although these conditions may be distinctly different from those occurring during natural behaviors in unrestrained, undrugged mammals, a few transcendent principles and mechanisms have been revealed that are worth emphasizing.
The anatomical organization of inputs to cranial and spinal motoneurons follows several common features. Afferent input is conveyed via sensory nuclei in the brain stem and spinal gray. Premotor neurons are mostly located close to the motoneuron groups in the reticular formation or spinal gray. Long projections from brain stem and pontine nuclei converge on brain stem and spinal premotor/interneurons as well as motoneurons. The distributed innervation of motoneuron pools by supraspinal modulatory systems and local premotor/interneurons may be part of a general scheme where the excitability of brain stem and spinal functional units (composed of CPG, sensory interneurons, and motoneurons) are changed in a coordinated fashion during particular motor acts.
Inputs defining the precise timing of movement are mostly signaled by amino acids acting on ionotropic receptors. For example, signals related to voluntary movement, originating in the cortex, and relayed through a host of intermediate, and occasionally direct, pathways, appear to excite motoneurons via the release of glutamate and inhibit motoneurons via the release of GABA and glycine, all acting via ionotropic receptors. Similarly, rhythmic movements such as respiration, locomotion, and mastication, as well as postural and oligosynaptic reflexes, ultimately drive the appropriate movements by signals mediated by amino acid neurotransmitters acting via ionotropic receptors. With respect to the signaling of inputs related to the precise timing of movement, these neurotransmitters affect receptors that have fast onset and relatively fast offset of action. However, under certain conditions, e.g., plateau potentials or long-term facilitation, they may also trigger long-lasting changes in neuronal excitability. Insofar as functional signals transmitting information related to precise timing can be identified in other neurons, we propose that these too would be conveyed by amino acids acting at ionotropic receptors.
Inputs related to more generic aspects of movement, e.g., exercise, or state, such as related to or affected by the sleep-wake cycle, autonomic function, attention, or emotion (including fear), are conveyed to motoneurons by other neurotransmitters, such as the amines or peptides, mostly acting on metabotropic receptors. Modulatory inputs alter the excitability of motoneurons over a variety of time scales, affecting their responses to those inputs signaling precise timing signals for movement, but do not themselves code signals for movement. They play a basic role in determining the motoneuronal input-output relationship, which would be reflected functionally in the size and shape of bursts of activity that produce muscle contraction with timing and magnitude appropriate for performance of any coordinated movement.
Many modulators of motoneuronal excitability act through common effector mechanisms and may even share common second messenger pathways. For example, a) glutamate (metabotropic)-, NE-, 5-HT-, TRH-, and substance P-mediated signaling converge on a resting leak K+ current and/or a cationic inward current (Fig. 9). These effector mechanisms change motoneuronal excitability by altering two important electrophysiological parameters: membrane potential and input resistance. Activation of a steady inward current and reduction of leak outward K+ currents lead to depolarization, and the associated increased input resistance results in an electrotonically more compact neuron. b) NE and 5-HT systems converge on the hyperpolarization-activated inward current Ih, also increasing the level of motoneuronal excitability. c) Glutamate (metabotropic), 5-HT, and adenosine modulate Ca2+ channels. Influx of Ca2+ is a main determinant of the spike afterhyperpolarization (IK Ca), and the charge carried by Ca2+ can also lead to membrane depolarizations, e.g., plateau potentials, or changes in recruitment thresholds. Both of these effects can profoundly alter the input-output function of motoneurons. d) Glutamate- (metabotropic), adenosine-, ACh-, 5-HT-, and GABA-mediated synaptic transmission can modulate presynaptic transmitter release. At present, only glutamatergic and glycinergic signaling are known to be presynaptically modulated, but other transmitter system may be under similar control. The change in motoneuronal excitability elicited by these convergent transmitter systems has mostly been studied using exogenous application of different receptors ligands (iontophoresis, bath application). This represents a significant limitation, since very little data exist on endogenous release of any particular modulatory transmitter. Thus the duration and amplitude of the change in motoneuronal excitability due to activation of these modulatory systems during normal motor behavior is unknown.
FIG. 9.
Major transmitters (labeled in presynaptic terminals), presynaptic (red boxes) and postsynaptic (yellow boxes) receptors, and ion channel effectors involved in conveying main excitatory/inhibitory synaptic drive and modulatory input to motoneurons. Transmitter systems converge on 3 major effectors: ICa HVA, Ih, and IK leak-ICAT. Although graphically separated, several of the listed transmitters are colocalized in synaptic boutons. (P)?, possibility that phosphorylation of receptors or associated synaptic proteins play a role in regulating motoneuronal excitability; Glu, glutamate; Gly, glycine; AVP, arginine vasopressin.
There are many important and interesting questions for future study. These might include the following:
What is the particular role of the distributed inputs and conductances on the somatatodendritic membrane in ensuring proper signal processing in motoneurons for given movements? In principle, motoneurons have complex geometry and many highly nonlinear properties, but under some conditions, they appear to behave like spheres receiving inputs that are summed (nearly) linearly. Under what conditions do motoneurons exploit or minimize nonlinearities?
What are the actions of the multitude of peptide transmitter systems (Table 2) in ensuring the proper functioning of motoneurons? Under what conditions are peptides released, and what are their effects? Their actions may go beyond short-term regulation of excitability and may be related to longer term adaptation associated with learning, or to changes in motor unit or muscles properties with development, exercise, aging, or disease.
Are motoneuronal properties and/or synaptic processes modified on a short time scale during different motor behaviors? Motor networks in the brain stem and spinal cord are likely reorganizing, and not dedicated, networks, i.e., they probably undergo functional reorganization to generate different motor tasks. For example, respiratory motoneurons involved in normal quiet breathing also take part in producing numerous other motor acts such as phonation, coughing, emesis, and sneezing. Rapid changes in channel properties and synaptic functions may be involved in these transient reorganizations.
What are the cellular and molecular bases for intrinsic properties of motoneurons and their modulation by transmitters? Intrinsic membrane properties of motoneurons influence the manner by which synaptic inputs are transformed into the production of action potentials that ultimately dictates behavior. Numerous voltage-dependent and -independent currents contribute to motoneuronal membrane and transduction properties, and these currents are subject to modulation by transmitters. To understand these processes, it will be critical to 1) identify the molecular basis for intrinsic motoneuronal ionic currents, i.e., which of the myriad cloned channels underlie the measured currents, and 2) determine the cellular and molecular mechanisms that contribute to ion channel modulation by transmitters, i.e., which specific receptors and ion channels are involved, and what transduction pathways are interposed between receptor and channel. Finally, motoneuronal membrane properties and their modulation are not static during postnatal development. Thus it will also be important to characterize molecular and cellular mechanisms underlying motoneuronal membrane properties and their modulation at different developmental stages.
Do motoneurons exploit the molecular diversity of receptors to fine tune their phenotypic properties, especially because they may be useful in the control of particular muscles during particular (classes of) movements? Although many different receptors can be assembled, we do not know the significance of the combinatorial possibilities and if motoneurons exploit this potential. Differences in expression of glutamate receptor subtypes among different motoneuron groups (and within motor pools), together with the possibility of molecular modulation, e.g., phosphorylation of these subunits, would permit motoneuronal excitability to be regulated by differential expression patterns and posttranscriptional modification of receptor subunits or associated synaptic proteins. These mechanisms could contribute to the regulation of motoneuronal properties during development, aging, or disease.
Does phosphorylation of ionotropic amino acid receptors affect excitability to precisely control given movements or adapt motoneuronal function over longer time scales? The precise timing signals for movement are mediated by amino acid receptors. Specific regulation of their properties by phosphorylation of the receptors or associated synaptic proteins would provide a mechanism for controlling the underlying currents specifically, without generic alteration in motoneuronal properties.
In summary, control of motoneuronal excitability is an essential feature of all behavior. We are beginning to understand the grammar that underlies motoneuronal properties, but ultimately we need to understand their language as they act during behavior. This will require intense efforts and probably novel approaches.
TABLE 4.
Expression of kainate receptor subunits in motoneurons
| GluR5 | GluR6/7 | GluR7 | KA1 | KA2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motoneuron Nuclei | I | ISH | I | ISH | I | ISH | I | ISH | I | ISH | Reference No.* |
| Edinger-Westphal | 1 | 1 | 976 | ||||||||
| Oculomotor (III) | 2 | 2 | 976 | ||||||||
| Trochlear (IV) | |||||||||||
| Trigeminal (V) | 2 | 2 | 976 | ||||||||
| Abducens (VI) | 2 | 2 | 976 | ||||||||
| Facial (VII) | 2 | 2 | 976 | ||||||||
| Vagal (X) | 2 | 2 | 976 | ||||||||
| Ambigual | 2 | 2 | 976 | ||||||||
| Hypoglossal (XII) | 2 | 2 | 976 | ||||||||
| Spinal | 1 | 2 | nd | nd | 1 | 2 | nd | 407, 976, 1257 | |||
I, immunohistochemistry; ISH, in situ hybridization; nd, not detected; KA, kainate.
All data are from the rat.
Acknowledgments
We thank Dr. D. M. Robinson for assistance with compilation of references and Drs. H. Jahnsen, O. Kiehn, and J. Hounsgaard for valuable discussions.
The National Institutes of Health have generously supported our research, including RO1 Grants HL-37941, HL-40959, NS-24742, and NS-33583. J. C. Rekling is a Parker B. Francis Fellow in Pulmonary Research. G. D. Funk is supported by Health Research Council of New Zealand, the Marsden Fund, the Auckland Medical Research Foundation, the New Zealand Neurological Foundation, and New Zealand Lotteries Health Research. D. A. Bayliss is supported by National Institutes of Neurological Disorders and Stroke Grant NS-33583.
REFERENCES
- 1.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn. J. Physiol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 3.Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol. Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- 4.Aggelopoulos N, Burton M, Clarke R, Edgley S. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- 6.Aghajanian GK. Serotonin-induced inward current in rat facial motoneurons: evidence for mediation by G proteins but not protein kinase C. Brain Res. 1990;524:171–174. doi: 10.1016/0006-8993(90)90509-a. [DOI] [PubMed] [Google Scholar]
- 7.Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharmacol. 1986;87:354–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlquist RP. Historical perspective. Classification of adrenoreceptors. J Auton. Pharmacol. 1980;1:101–106. doi: 10.1111/j.1474-8673.1980.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 10.Alberi S, Dubois-Dauphin M, Dreifuss JJ, Raggenbass M. Modulation by divalent cations of the current generated by vasopressin in facial motoneurons. Brain Res. 1993;624:326–330. doi: 10.1016/0006-8993(93)90097-7. [DOI] [PubMed] [Google Scholar]
- 11.Albin RL, Hollingsworth Z, Sakurai SY, Gilman S. Inhibitory and excitatory amino acid neurotransmitter binding sites in cynomolgus monkey (Macaca fascicularis) cervical spinal cord. Brain Res. 1993;604:354–357. doi: 10.1016/0006-8993(93)90391-y. [DOI] [PubMed] [Google Scholar]
- 12.Aldes L. Topographically organized projections from the nucleus subceruleus to the hypoglossal nucleus in the rat: a light and electron microscopic study with complementary axonal transport techniques. J Comp. Neurol. 1990;302:643–656. doi: 10.1002/cne.903020318. [DOI] [PubMed] [Google Scholar]
- 13.Aldes L. The enkephalinergic innervation of the genioglossus musculature in the rat: implications for the respiratory control of the tongue. Brain Res. 1998;780:67–73. doi: 10.1016/s0006-8993(97)01126-8. [DOI] [PubMed] [Google Scholar]
- 14.Aldes L, Chapman M, Chronister R, Haycock J. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res. Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 15.Aldes L, Chronister R, Marco L, Haycock J, Thibault J. Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp. Brain Res. 1988;73:305–314. doi: 10.1007/BF00248222. [DOI] [PubMed] [Google Scholar]
- 16.Aldes L, Chronister R, Shelton CD, Haycock J, Marco L, Wong D. Catecholamine innervation of the rat hypoglossal nucleus. Brain Res. Bull. 1988;21:305–312. doi: 10.1016/0361-9230(88)90245-6. [DOI] [PubMed] [Google Scholar]
- 17.Aldes L, Shaw B, Chronister R, Haycock J. Catecholamine- containing axon terminals in the hypoglossal nucleus of the rat: an immuno-electronmicroscopic study. Exp. Brain Res. 1990;81:167–178. doi: 10.1007/BF00230113. [DOI] [PubMed] [Google Scholar]
- 18.Aldes LD, Chronister RB, Marco LA. Distribution of glutamic acid decarboxylase and gamma-aminobutyric acid in the hypoglossal nucleus in the rat. J Neurosci. Res. 1988;19:343–348. doi: 10.1002/jnr.490190309. [DOI] [PubMed] [Google Scholar]
- 19.Aldes LD, Marco LA, Chronister RB. Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immuno-electronmicroscopic study. Brain Res. Bull. 1989;23:249–256. doi: 10.1016/0361-9230(89)90154-8. [DOI] [PubMed] [Google Scholar]
- 20.Allen G, Cechetto D. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp. Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- 21.Alstermark B, Kummel H. Transneuronal transport of wheat germ agglutinin conjugated horseradish peroxidase into last order spinal interneurones projecting to acromio- and spinodeltoideus motoneurones in the cat. 1. Location of labelled interneurones and influence of synaptic activity on the transneuronal transport. Exp. Brain Res. 1990;80:83–95. doi: 10.1007/BF00228850. [DOI] [PubMed] [Google Scholar]
- 22.Alstermark B, Kummel H, Pinter M, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 17. Axonal projection and termination of C3–C4 propriospinal neurones in the C6-Th1 segments. Exp. Brain Res. 1990;81:447–461. doi: 10.1007/BF02423494. [DOI] [PubMed] [Google Scholar]
- 23.Alstermark B, Kummel H, Tantisira B. Monosynaptic raphespinal and reticulospinal projection to forelimb motoneurones in cats. Neurosci. Lett. 1987;74:286–290. doi: 10.1016/0304-3940(87)90311-9. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez F, Pearson J, Harrington D, Dewey D, Torbeck L, Fyffe R. Distribution of 5-hydroxytryptamineimmunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp. Neurol. 1998;393:69–83. [PubMed] [Google Scholar]
- 25.Alvarez FJ, Taylor-Blake B, Fyffe RE, de Blas AL, Light AR. Distribution of immunoreactivity for the beta 2 and beta 3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp. Neurol. 1996;365:392–412. doi: 10.1002/(SICI)1096-9861(19960212)365:3<392::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Leefmans F, Nani A, Marquez S. Chloride transport, osmotic balance and presynaptic inhibition. In: Rudomin P, editor. Presynaptic Inhibition and Neural Control. New York: Oxford Univ, Press; 1998. pp. 50–79. [Google Scholar]
- 27.Al-Zamil Z, Bagust J, Kerkut GA. The effect of bicuculline and baclofen on the dorsal root-ventral root reflex in the isolated spinal cord. Gen. Pharmacol. 1991;22:559–565. doi: 10.1016/0306-3623(91)90024-z. [DOI] [PubMed] [Google Scholar]
- 28.Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp. Neurol. 1998;402:75–92. [PubMed] [Google Scholar]
- 29.Amin A, Crawford B, Gaddum J. Distribution of 5-hydroxytryptamine and substance P in central nervous system. J Physiol. (Lond.) 1954;126:596–618. doi: 10.1113/jphysiol.1954.sp005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amri M, Car A. Projections from the medullary swallowing center to the hypoglossal motor nucleus: a neuroanatomical and electrophysiological study in sheep. Brain Res. 1988;441:119–126. doi: 10.1016/0006-8993(88)91389-3. [DOI] [PubMed] [Google Scholar]
- 31.Antonov SM, Kalinina NI, Kurchavyj GG, Magazanik LG, Shupliakov OV, Vesselkin NP. Identification of two types of excitatory monosynaptic inputs in frog spinal motoneurones. Neurosci. Lett. 1990;109:82–87. doi: 10.1016/0304-3940(90)90541-g. [DOI] [PubMed] [Google Scholar]
- 32.Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid I, Eisen J. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- 33.Appenteng K, Curtis JC, Grimwood PD, Min M-Y, Yang H-W. Excitatory synaptic transmission in the rat trigeminal motor nucleus. In: Morimoto T, Matsuya T, Takada K, editors. Brain and Oral Functions: Oral Motor Function and Dysfunction. Elsevier: Amsterdam; 1995. pp. 107–115. [Google Scholar]
- 34.Araki T, Terzuolo C. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- 35.Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- 36.Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- 37.Arita H, Ichikawa K, Sakamoto M. Serotonergic cells in nucleus raphe pallidus provide tonic drive to posterior cricoarytenoid motoneurons via 5-hydroxytryptamine2 receptors in cats. Neurosci. Lett. 1995;197:113–116. doi: 10.1016/0304-3940(95)11907-e. [DOI] [PubMed] [Google Scholar]
- 38.Arita H, Sakamoto M, Hirokawa Y, Okado N. Serotonin innervation patterns differ among the various medullary motoneuronal groups involved in upper airway control. Exp. Brain Res. 1993;95:100–110. doi: 10.1007/BF00229659. [DOI] [PubMed] [Google Scholar]
- 39.Arvidsson U, Cullheim S, Ulfhake B, Bennett G, Fone K, Cuello A, Verhofstad A, Visser T, Hökfelt T. 5-Hydroxytryptamine, substance P, and thyrotropin-releasing hormone in the adult cat spinal cord segment L7: immunohistochemical and chemical studies. Synapse. 1990;6:237–270. doi: 10.1002/syn.890060305. [DOI] [PubMed] [Google Scholar]
- 40.Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp. Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- 41.Arvidsson U, Ulfhake B, Cullheim S, Bergstrand A, Theodorson E, Hökfelt T. Distribution of 125I-galanin binding sites, immunoreactive galanin, and its coexistence with 5-hydroxytryptamine in the cat spinal cord: biochemical, histochemical, and experimental studies at the light and electron microscopic level. J Comp. Neurol. 1991;308:115–138. doi: 10.1002/cne.903080111. [DOI] [PubMed] [Google Scholar]
- 42.Arvidsson U, Ulfhake B, Cullheim S, Shupliakov O, Brodin E, Franck J, Bennett G, Fone K, Visser T, Hökfelt T. Thyrotropin-releasing hormone (TRH)-like immunoreactivity in the grey monkey (Macaca fascicularis) spinal cord and medulla oblongata with special emphasis on the bulbospinal tract. J Comp. Neurol. 1992;322:293–310. doi: 10.1002/cne.903220302. [DOI] [PubMed] [Google Scholar]
- 43.Atsuta Y, Abraham P, Iwahara T, Garcia-Rill E, Skinner RD. Control of locomotion in vitro. II. Chemical stimulation. Somatosensory Motor Res. 1991;8:55–63. doi: 10.3109/08990229109144729. [DOI] [PubMed] [Google Scholar]
- 44.Azmitia E, Gannon P, Kheck N, Whitaker-Azmitia P. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 45.Balcar VJ, Dias LS, Li Y, Bennett MR. Inhibition of [3H]CGP 39653 binding to NMDA receptors by a P2 antagonist, suramin. Neuroreport. 1995;7:69–72. [PubMed] [Google Scholar]
- 46.Balcar VJ, Li Y, Killinger S, Bennett MR. Autoradiography of P2x ATP receptors in the rat brain. Br. J. Pharmacol. 1995;115:302–306. doi: 10.1111/j.1476-5381.1995.tb15877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldissera F, Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course. First interval firing. Acta Physiol. Scand. 1974;91:528–544. doi: 10.1111/j.1748-1716.1974.tb05708.x. [DOI] [PubMed] [Google Scholar]
- 48.Baldissera F, Gustafsson B, Parmiggiani F. Saturating summation of the afterhyperpolarization conductance in spinal motoneurones: a mechanism for “secondary range” repetitive firing. Brain Res. 1978;146:69–82. doi: 10.1016/0006-8993(78)90218-4. [DOI] [PubMed] [Google Scholar]
- 49.Baldissera F, Hultborn H, Illert M. Handbook of Physiology. The Nervous System. Motor Systems. 12. II. Bethesda, MD: Am. Physiol. Soc; 1981. Integration in spinal neuronal systems; pp. 509–595. sect. I. [Google Scholar]
- 50.Ballerini L, Bracci E, Nistri A. Desensitization of AMPA receptors limits the amplitude of EPSPs and the excitability of motoneurons of the rat isolated spinal cord. Eur. J. Neurosci. 1995;7:1229–1234. doi: 10.1111/j.1460-9568.1995.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 51.Baranauskas G, Nistri A. Effects of RP 67580 on substance P-elicited responses and postsynaptic potentials of motoneurones of the rat isolated spinal cord. Peptides. 1995;16:357–359. doi: 10.1016/0196-9781(94)00194-4. [DOI] [PubMed] [Google Scholar]
- 52.Baranauskas G, Traversa U, Rosati A, Nistri A. An NK1 receptor-dependent component of the slow excitation recorded intracellularly from rat motoneurons following dorsal root stimulation. Eur. J. Neurosci. 1995;7:2409–2417. doi: 10.1111/j.1460-9568.1995.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 53.Barasi S, Roberts M. Responses of motoneurones to electrophoretically applied dopamine. Br. J. Pharmacol. 1977;60:29–34. doi: 10.1111/j.1476-5381.1977.tb16743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barber RP, Vaughn JE, Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Res. 1982;238:305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- 55.Barber WD, Yuan CS, Burks TF, Feldman JL, Greer JJ. In vitro brain stem-gastric preparation with intact vagi for study of primary visceral afferent input to dorsal vagal complex in caudal medulla. J Auton. Nerv. Syst. 1995;51:181–189. doi: 10.1016/0165-1838(94)00129-8. [DOI] [PubMed] [Google Scholar]
- 56.Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J Endocrinol. 1998;156:223–229. doi: 10.1677/joe.0.1560223. [DOI] [PubMed] [Google Scholar]
- 57.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit. Rev. Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 58.Barnard EA. Ionotropic glutamate receptors: new types and new concepts. Trends Pharmacol. Sci. 1997;18:141–148. doi: 10.1016/s0165-6147(97)01053-5. [DOI] [PubMed] [Google Scholar]
- 59.Barnard EA, Seeburg PH. Structural basis of the GABA-activated chloride channel: molecular biology and molecular electrophysiology. Adv. Biochem. Psychopharmacol. 1988;45:1–18. [PubMed] [Google Scholar]
- 60.Barnard EA, Sutherland M, Zaman S, Matsumoto M, Nayeem N, Green T, Darlison MG, Bateson AN. Multiplicity, structure, and function in GABAA receptors. Ann. NY Acad. Sci. 1993;707:116–125. doi: 10.1111/j.1749-6632.1993.tb38047.x. [DOI] [PubMed] [Google Scholar]
- 61.Barnard EA, Webb TE, Simon J, Kunapuli SP. The diverse series of recombinant P2Y purinoceptors. Ciba Found. Symp. 1996;198:166–188. doi: 10.1002/9780470514900.ch10. [DOI] [PubMed] [Google Scholar]
- 62.Barrett EF, Barrett JN, Crill WE. Voltage-sensitive outward currents in cat motoneurones. J Physiol. (Lond.) 1980;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrett JN, Crill WE. Specific membrane resistivity of dye-injected cat motoneurons. Brain Res. 1971;28:556–561. doi: 10.1016/0006-8993(71)90066-7. [DOI] [PubMed] [Google Scholar]
- 64.Barrett JN, Crill WE. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. (Lond.) 1974;239:325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett JN, Crill WE. Specific membrane properties of cat motoneurones. J Physiol. (Lond.) 1974;239:301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrett JN, Crill WE. Voltage clamp of cat motoneurone somata: properties of the fast inward current. J Physiol. (Lond.) 1980;304:231–249. doi: 10.1113/jphysiol.1980.sp013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrett R, Bao X, Miselis R, Altschuler S. Brain stem localization of rodent esophageal premotor neurons revealed by transneuronal passage of pseudorabies virus. Gastroenterology. 1994;107:728–737. doi: 10.1016/0016-5085(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 68.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 69.Basbaum A, Clanton C, Fields H. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp. Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- 70.Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. (Lond.) 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 72.Bayliss DA, Czyzyk-Krzeska M, Szymeczek-Seay C, Erickson J, Seroogy K, Milhorn DE. Detection of mRNA in neurons identified by projection or neurochemical content: in situ hydridization combined with retrograde tract-tracing and immunohistochemistry. In: Stumpf W, Solomon H, editors. Autoradiography and Correlative Imaging. San Diego, CA: Academic; 1995. pp. 343–355. [Google Scholar]
- 73.Bayliss DA, Umemiya M, Berger AJ. Inhibition of N-and P-type calcium currents and the after-hyperpolarization in rat motoneurones by serotonin. J Physiol. (Lond.) 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. J Neurophysiol. 1994;71:119–128. doi: 10.1152/jn.1994.71.1.119. [DOI] [PubMed] [Google Scholar]
- 75.Bayliss DA, Viana F, Berger AJ. Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992;68:1733–1745. doi: 10.1152/jn.1992.68.5.1733. [DOI] [PubMed] [Google Scholar]
- 76.Bayliss DA, Viana F, Berger AJ. Thyrotropin-releasing hormone causes excitation of rat hypoglossal motoneurons in vitro. Sleep. 1993;16:S49–S52. doi: 10.1093/sleep/16.suppl_8.s49. [DOI] [PubMed] [Google Scholar]
- 77.Bayliss DA, Viana F, Berger AJ. Effects of thyrotropin-releasing hormone on rat motoneurons are mediated by G proteins. Brain Res. 1994;668:220–229. doi: 10.1016/0006-8993(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 78.Bayliss DA, Viana F, Kanter R, Szymeczek-Seay C, Berger A, Millhorn DE. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994;14:821–833. doi: 10.1523/JNEUROSCI.14-02-00821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayliss DA, Viana F, Talley E, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir. Physiol. 1997;110:139–150. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 80.Bechade C, Colin I, Kirsch J, Betz H, Triller A. Expression of glycine receptor alpha subunits and gephyrin in cultured spinal neurons. Eur. J. Neurosci. 1996;8:429–435. doi: 10.1111/j.1460-9568.1996.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 81.Bechade C, Sur C, Triller A. The inhibitory neuronal glycine receptor. Bioessays. 1994;16:735–744. doi: 10.1002/bies.950161008. [DOI] [PubMed] [Google Scholar]
- 82.Becker C-M, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beckman M, Whitehead M. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 1991;557:265–279. doi: 10.1016/0006-8993(91)90143-j. [DOI] [PubMed] [Google Scholar]
- 84.Bell J, Desouza E. Functional corticotropin-releasing factor receptors in neonatal rat spinal cord. Peptides. 1988;9:1317–1322. doi: 10.1016/0196-9781(88)90198-2. [DOI] [PubMed] [Google Scholar]
- 85.Bellingham MC, Berger AJ. Adenosine suppresses excitatory glutamatergic inputs to rat hypoglossal motoneurons in vitro. Neurosci. Lett. 1994;177:143–146. doi: 10.1016/0304-3940(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 86.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- 87.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa J-L. GABAA, NMDA and AMPA receptors: a developmentally regulated “menage a trois”. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 88.Bennett D, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- 89.Bennett D, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- 90.Beresford IJ, Ireland SJ, Stables J, Hagan RM. Ontogeny and characterization of [125I]Bolton Hunter-eledoisin binding sites in rat spinal cord by quantitative autoradiography. Neuroscience. 1992;46:225–232. doi: 10.1016/0306-4522(92)90022-t. [DOI] [PubMed] [Google Scholar]
- 91.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci. Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 92.Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl. Physiol. 1996;81:1039–1048. doi: 10.1152/jappl.1996.81.3.1039. [DOI] [PubMed] [Google Scholar]
- 93.Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berger AJ. Recent advances in respiratory neurobiology using in vitro methods. Am. J. Physiol. Lung Cell. Mol. Physiol. 1990;259:L24–L29. doi: 10.1152/ajplung.1990.259.2.L24. [DOI] [PubMed] [Google Scholar]
- 95.Berger AJ, Bayliss DA, Bellingham MC, Umemiya M, Viana F. Postnatal development of hypoglossal motoneuron intrinsic properties. Adv. Exp. Med. Biol. 1995;381:63–71. doi: 10.1007/978-1-4615-1895-2_7. [DOI] [PubMed] [Google Scholar]
- 96.Berger AJ, Dieudonne S, Ascher P. Glycine uptake mechanisms govern glycine site occupancy at NDMA receptors of CNS excitatory synaspes. Soc. Neurosci. Abstr. 1998;24:341. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- 97.Bernstein JJ, Bernstein ME. Ventral horn synaptology in the rat. J Neurocytol. 1976;5:109–123. doi: 10.1007/BF01176185. [DOI] [PubMed] [Google Scholar]
- 98.Bertrand S, Cazalets JR. Postinhibitory rebound during locomotor-like activity in neonatal rat motoneurons in vitro. J Neurophysiol. 1998;79:342–351. doi: 10.1152/jn.1998.79.1.342. [DOI] [PubMed] [Google Scholar]
- 99.Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr. Opin. Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- 100.Bettler B, Mulle C. Review: neurotransmitter receptors. II. AMPA and kainate receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- 101.Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- 102.Betz H. Structure and function of inhibitory glycine receptors. Q Rev. Biophys. 1992;25:381–394. doi: 10.1017/s0033583500004340. [DOI] [PubMed] [Google Scholar]
- 103.Betz H, Langosch D, Hoch W, Prior P, Pribilla I, Kuhse J, Schmieden V, Malosio ML, Matzenbach B, Holzinger F. Structure and expression of inhibitory glycine receptors. Adv. Exp. Med. Biol. 1991;287:421–429. doi: 10.1007/978-1-4684-5907-4_37. [DOI] [PubMed] [Google Scholar]
- 104.Betz H, Langosch D, Rundstrom N, Bormann J, Kuryatov A, Kuhse J, Schmieden V, Matzenbach B, Kirsch J. Structure and biology of inhibitory glycine receptors. Ann. NY Acad. Sci. 1993;707:109–115. doi: 10.1111/j.1749-6632.1993.tb38046.x. [DOI] [PubMed] [Google Scholar]
- 105.Bieger D. Neuropharmacologic correlates of deglutition: lessons from fictive swallowing. Dysphagia. 1991;6:147–164. doi: 10.1007/BF02493518. [DOI] [PubMed] [Google Scholar]
- 106.Bieger D, Hopkins D. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp. Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- 107.Binder MD, Heckman CJ, Powers RK. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Vol. 1. Bethesda, MD: Am. Physiol. Soc; 1996. The physiological control of motoneuron activity; pp. 3–53. sect. 12. [Google Scholar]
- 108.Binder MD, Heckman CJ, Powers RK. How different afferent inputs control motoneuron discharge and the output of the motoneuron pool. Curr. Opin. Neurobiol. 1993;3:1028–1034. doi: 10.1016/0959-4388(93)90177-z. [DOI] [PubMed] [Google Scholar]
- 109.Biscoe TJ, Duchen MR. Synaptic physiology of spinal motoneurones of normal and spastic mice: an in vitro study. J Physiol. (Lond.) 1986;379:275–292. doi: 10.1113/jphysiol.1986.sp016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bjorklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Hokfelt T, Bjorklund A, editors. Classical Transmitters in the CNS. Elsevier: Amsterdam; 1984. pp. 55–122. [Google Scholar]
- 111.Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J Neurosci. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blaschke M, Keller BU, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc. Natl. Acad. Sci. USA. 1993;90:6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bo X, Burnstock G. Distribution of [3H]alpha,beta-methylene ATP binding sites in rat brain and spinal cord. Neuroreport. 1994;5:1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]
- 114.Bodian D. Origin of specific synaptic types in the motoneuron neuropil of the monkey. J Comp. Neurol. 1975;159:225–243. doi: 10.1002/cne.901590205. [DOI] [PubMed] [Google Scholar]
- 115.Bohlhalter S, Mohler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res. 1994;642:59–69. doi: 10.1016/0006-8993(94)90905-9. [DOI] [PubMed] [Google Scholar]
- 116.Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bohmer G, Schmid K, Schauer W. Evidence for an involvement of NMDA and non-NMDA receptors in synaptic excitation of phrenic motoneurons in the rabbit. Neurosci. Lett. 1991;130:271–274. doi: 10.1016/0304-3940(91)90413-n. [DOI] [PubMed] [Google Scholar]
- 118.Bonanno G, Raiteri M. Multiple GABAB receptors. Trends Pharmacol. Sci. 1993;14:259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- 119.Bonner TI, Affolter HU, Young AC, Young WSD. A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Brain Res. 1987;388:243–249. doi: 10.1016/0169-328x(87)90031-3. [DOI] [PubMed] [Google Scholar]
- 120.Bonnot A, Corio M, Tramu G, Viala D. Immunocytochemical distribution of ionotropic glutamate receptor subunits in the spinal cord of the rabbit. J Chem. Neuroanat. 1996;11:267–278. doi: 10.1016/s0891-0618(96)00173-1. [DOI] [PubMed] [Google Scholar]
- 121.Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J Neurophysiol. 1997;78:3371–3385. doi: 10.1152/jn.1997.78.6.3371. [DOI] [PubMed] [Google Scholar]
- 122.Borke R, Nau M, Ringler RJ. Brain stem afferents of hypoglossal neurons in the rat. Brain Res. 1983;269:47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- 123.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 124.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. (Lond.) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bortoff G, Strick P. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp. Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 127.Bowery NG. GABAB receptor pharmacology. Annu. Rev. Pharmacol. Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 128.Bowery NG. Metabotropic GABAB receptors cloned at last. Trends Pharmacol. Sci. 1997;18:103. [PubMed] [Google Scholar]
- 129.Bowery NG. Pharmacology of mammalian GABAB receptors. In: Enna SJ, Bowery NG, editors. The GABA Receptors. Totowa, NJ: Humana; 1997. pp. 209–236. [Google Scholar]
- 130.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 131.Bowery NG, Pratt GD. GABAB receptors as targets for drug action. Arzneimittel-Forschung. 1992;42:215–223. [PubMed] [Google Scholar]
- 132.Bowery NG, Price GW, Hudson AL, Hill DR, Wilkin GP, Turnbull MJ. GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS. Neuropharmacology. 1984;23:219–231. doi: 10.1016/0028-3908(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 133.Bowker R. Serotonergic and peptidergic inputs to the primate ventral spinal cord as visualized with multiple chromagens on the same tissue section. Brain Res. 1986;375:345–350. doi: 10.1016/0006-8993(86)90755-9. [DOI] [PubMed] [Google Scholar]
- 134.Bowker R, Westlund K, Sullivan M, Wilber J, Coulter J. Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: a multiple transmitter complex. Brain Res. 1983;288:33–48. doi: 10.1016/0006-8993(83)90079-3. [DOI] [PubMed] [Google Scholar]
- 135.Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braestrup C, Nielsen M. 3H-propyl-β-carboline-3-carboxylate as a selective radioligand for the BZI benzodiazepine receptor subclass. J Neurochem. 1981;37:333–341. doi: 10.1111/j.1471-4159.1981.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 137.Branisteanu D, Schubert P, Branisteanu DD. Modulatory effects of adenosine upon the transmitter release in the hippocampal slice preparation of rats. Physiologie. 1987;24:3–9. [PubMed] [Google Scholar]
- 138.Bras H, Cavallari P, Jankowska E, McCrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp. Brain Res. 1989;76:27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- 139.Bras H, Destombs J, Gogan P, Tyc-Dumont S. The dendrites of single brainstem motoneurons intracellularly labelled with horseradish peroxidase in the cat. An ultrastructural analysis of the synaptic covering and the microenvironment. Neuroscience. 1987;22:971–981. doi: 10.1016/0306-4522(87)92973-3. [DOI] [PubMed] [Google Scholar]
- 140.Bristow DR, Bowery NG, Woodruff GN. Light microscopic autoradiographic loaclization of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur. J. Pharmacol. 1986;126:303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- 141.Brock L, Coombs J, Eccles JC. Intracellular recording from antidromically activated motoneurones. J Physiol. (Lond.) 1953;122:429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brodin L, Grillner S. The role of putative excitatory amino acid neurotransmitters in the initiation of locomotion in the lamprey spinal cord. II. The effects of amino acid uptake inhibitors. Brain Res. 1985;360:149–158. doi: 10.1016/0006-8993(85)91230-2. [DOI] [PubMed] [Google Scholar]
- 143.Brodin L, Grillner S, Dubuc R, Ohta Y, Kasicki S, Hokfelt T. Reticulospinal neurons in lamprey: transmitters, synaptic interactions and their role during locomotion. Arch. Ital. Biol. 1988;126:317–345. [PubMed] [Google Scholar]
- 144.Brodin L, Ohta Y, Hokfelt T, Grillner S. Further evidence for excitatory amino acid transmission in lamprey reticulospinal neurons: selective retrograde labeling with (3H)d-aspartate. J Comp. Neurol. 1989;281:225–233. doi: 10.1002/cne.902810206. [DOI] [PubMed] [Google Scholar]
- 145.Bronner-Fraser M, Fraser S. Differentiation of the vertebrate neural tube. Curr. Opin. Cell Biol. 1997;9:885–891. doi: 10.1016/s0955-0674(97)80092-0. [DOI] [PubMed] [Google Scholar]
- 146.Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr. Clin. Neurophysiol. 1990;76:509–519. doi: 10.1016/0013-4694(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 147.Brown AG, Fyffe RE. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat’s lumbosacral spinal cord. J Physiol. (Lond.) 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brown AM, Schwindt PC, Crill WE. Different voltage dependence of transient and persistent Na+ currents is compatible with modal-gating hypothesis for sodium channels. J Neurophysiol. 1994;71:2562–2565. doi: 10.1152/jn.1994.71.6.2562. [DOI] [PubMed] [Google Scholar]
- 149.Brown SJ, James S, Reddington M, Richardson PJ. Both A1 and A2a purine receptors regulate striatal acetylcholine release. J Neurochem. 1990;55:31–38. doi: 10.1111/j.1471-4159.1990.tb08817.x. [DOI] [PubMed] [Google Scholar]
- 150.Browning MD, Endo S, Smith GB, Dudek EM, Olsen RW. Phosphorylation of the GABAA receptor by cAMP-dependent protein kinase and by protein kinase C: analysis of the substrate domain. Neurochem. Res. 1993;18:95–100. doi: 10.1007/BF00966927. [DOI] [PubMed] [Google Scholar]
- 151.Brownstein MJ, Utiger RD, Palkovits M, Kizer JS. Effect of hypothalamic deafferentation on thyrotropin-releasing hormone levels in rat brain. Proc. Natl. Acad. Sci. USA. 1975;72:4177–4179. doi: 10.1073/pnas.72.10.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp. Brain Res. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- 153.Brugger F, Evans RH, Hawkins NS. Effects of N-methyl-d-aspartate antagonists and spantide on spinal reflexes and responses to substance P and capsaicin in isolated spinal cord preparations from mouse and rat. Neuroscience. 1990;36:611–622. doi: 10.1016/0306-4522(90)90004-n. [DOI] [PubMed] [Google Scholar]
- 154.Brugger F, Wicki U, Olpe HR, Froestl W, Mickel S. The action of new potent GABAB receptor antagonists in the hemisected spinal cord preparation of the rat. Eur. J. Pharmacol. 1993;235:153–155. doi: 10.1016/0014-2999(93)90836-7. [DOI] [PubMed] [Google Scholar]
- 155.Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv. Pharmacol. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- 156.Buchanan JT, Brodin L, Dale N, Grillner S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987;408:321–325. doi: 10.1016/0006-8993(87)90397-0. [DOI] [PubMed] [Google Scholar]
- 157.Buchanan JT, Grillner S. Newly identified “glutamate interneurons” and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- 158.Buchanan JT, Grillner S, Cullheim S, Risling M. Identification of excitatory interneurons contributing to generation of locomotion in lamprey: structure, pharmacology, and function. J Neurophysiol. 1989;62:59–69. doi: 10.1152/jn.1989.62.1.59. [DOI] [PubMed] [Google Scholar]
- 159.Buijs R. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- 160.Bulant M, Roussel JP, Astier H, Nicolas P, Vaudry H. Processing of thyrotropin-releasing hormone prohormone (pro-TRH) generates a biologically active peptide, prepro-TRH-(160–169), which regulates TRH-induced thyrotropin secretion. Proc. Natl. Acad. Sci. USA. 1990;87:4439–4443. doi: 10.1073/pnas.87.12.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burke RE. Handbook of Physiology. The Nervous System. Motor Systems. 1. II. Bethesda, MD: Am. Physiol. Soc; 1981. Motor units: anatomy, physiology, and the functional organization; pp. 345–422. sect. I, chapt. 10. [Google Scholar]
- 162.Burke RE, Rudomin P. Handbook of Physiology. The Nervous System. Cellular Biology of Neurons. 2. I. Bethesda, MD: Am. Physiol. Soc; 1977. Spinal neurons and synapses; pp. 877–944. sect. I., chapt. 24. [Google Scholar]
- 163.Burke RE. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967;30:1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- 164.Burke RE. Synaptic efficacy and the control of neuronal input-output relations. Trends Neurosci. 1987;10:42–45. [Google Scholar]
- 165.Burke RE, Fedina L, Lundberg A. Spatial synaptic distribution of recurrent and group Ia inhibitory systems in cat spinal motoneurones. J Physiol. (Lond.) 1971;214:305–326. doi: 10.1113/jphysiol.1971.sp009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Burke RE, Rudomin P. Spinal neurons and synapses. In: Brookhart JM, Mountcastle VB, editors. The Nervous System. Cellular Biology of Neurons. 2. I. Baltimore, MD: Williams & Wilkins; 1977. pp. 877–944. [Google Scholar]
- 167.Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- 168.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 169.Burnstock G. Current state of purinoceptor research. Pharm. Acta Helv. 1995;69:231–242. doi: 10.1016/0031-6865(94)00043-u. [DOI] [PubMed] [Google Scholar]
- 170.Burnstock G. P2 purinoceptors: historical perspective and classification. Ciba Found. Symp. 1996;198:1–34. doi: 10.1002/9780470514900.ch1. [DOI] [PubMed] [Google Scholar]
- 171.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 172.Buss RR, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. J Physiol. (Lond.) 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bylund DB. Subtypes of alpha 1- and alpha 2-adrenergic receptors. FASEB J. 1992;6:832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- 174.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 175.Bystrzycka E, Nail B. The source of the respiratory drive to nasolabialis motoneurones in the rabbit; a HRP study. Brain Res. 1983;266:183–191. doi: 10.1016/0006-8993(83)90648-0. [DOI] [PubMed] [Google Scholar]
- 176.Caldero J, Ciutat D, Llado J, Castan E, Oppenheim RW, Esquerda JE. Effects of excitatory amino acids on neuromuscular development in the chick embryo. J Comp. Neurol. 1997;387:73–95. [PubMed] [Google Scholar]
- 177.Calza L, Giardino L, Ceccatelli S, Zanni M, Elde R, Hökfelt T. Distribution of thyrotropin-releasing hormone receptor messenger RNA in the rat brain: an in situ hybridization study. Neuroscience. 1992;51:891–909. doi: 10.1016/0306-4522(92)90528-a. [DOI] [PubMed] [Google Scholar]
- 178.Cameron W, Averill D, Berger A. Morphology of cat phrenic motoneurons as revealed by intracellular injection of horseradish peroxidase. J Comp. Neurol. 1983;219:70–80. doi: 10.1002/cne.902190107. [DOI] [PubMed] [Google Scholar]
- 179.Campbell D, Rose P. Contribution of voltage-dependent potassium channels to the somatic shunt in neck motoneurons of the cat. J Neurophysiol. 1997;77:1470–1486. doi: 10.1152/jn.1997.77.3.1470. [DOI] [PubMed] [Google Scholar]
- 180.Campistron G, Bujis RM, Geffard M. Glycine neurons in the brain and spinal cord. Antibody production and immunocytochemical localization. Brain Res. 1986;376:400–405. doi: 10.1016/0006-8993(86)90208-8. [DOI] [PubMed] [Google Scholar]
- 181.Cao C, Evans R, Headley P, Udvarhelyi P. A comparison of the effects of selective metabotropic glutamate receptor agonists on synaptically evoked whole cell currents of rat spinal ventral horn neurones in vitro. Br. J. Pharmacol. 1995;115:1469–1474. doi: 10.1111/j.1476-5381.1995.tb16639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao C, Tse H, Jane D, Evans R, Headley P. Antagonism of mGlu receptors and potentiation of EPSCs at rat spinal motoneurones in vitro. Neuropharmacology. 1997;36:313–318. doi: 10.1016/s0028-3908(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 183.Cao C, Tse H, Jane D, Evans R, Headley P. Metabotropic glutamate receptor antagonists, like GABA(B) antagonists, potentiate dorsal root-evoked excitatory synaptic transmission at neonatal rat spinal motoneurons in vitro. Neuroscience. 1997;78:243–250. doi: 10.1016/s0306-4522(96)00579-9. [DOI] [PubMed] [Google Scholar]
- 184.Cao J, O’Donnell D, Vu H, Payza K, Pou C, Godbout C, Jakob A, Pelletier M, Lembo P, Ahmad S, Walker P. Cloning and characterization of a cDNA encoding a novel subtype of rat thyrotropin-releasing hormone receptor. J Biol. Chem. 1998;273:32281–32287. doi: 10.1074/jbc.273.48.32281. [DOI] [PubMed] [Google Scholar]
- 185.Card J, Riley J, Moore R. The motor trigeminal nucleus of the rat: analysis of neuronal structure and the synaptic organization of noradrenergic afferents. J Comp. Neurol. 1986;250:469–484. doi: 10.1002/cne.902500406. [DOI] [PubMed] [Google Scholar]
- 186.Carlton SM, Hayes ES. Light microscopic and ultra-structural analysis of GABA-immunoreactive profiles in the monkey spinal cord. J Comp. Neurol. 1990;300:162–182. doi: 10.1002/cne.903000203. [DOI] [PubMed] [Google Scholar]
- 187.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 188.Carnevale NT, Johnston D. Electrophysiological characterization of remote chemical synapses. J Neurophysiol. 1982;47:606–621. doi: 10.1152/jn.1982.47.4.606. [DOI] [PubMed] [Google Scholar]
- 189.Carp JS, Anderson RJ. Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res. 1982;242:247–254. doi: 10.1016/0006-8993(82)90307-9. [DOI] [PubMed] [Google Scholar]
- 190.Carpenter DO, Briggs DB, Knox AP, Strominger N. Excitation of area postrema neurons by transmitters, peptides, and cyclic nucleotides. J Neurophysiol. 1988;59:358–369. doi: 10.1152/jn.1988.59.2.358. [DOI] [PubMed] [Google Scholar]
- 191.Carpenter M, Periera A, Guha N. Immunocytochemistry of oculomotor afferents in the squirrel monkey (Saimiri sciureus) J Hirnforsch. 1992;33:151–167. [PubMed] [Google Scholar]
- 192.Carpentier V, Vaudry H, Mallet E, Laquerriere A, Tayot J, Leroux P. Anatomical distribution of somatostatin receptors in the brainstem of the human fetus. Neuroscience. 1996;73:865–879. doi: 10.1016/0306-4522(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 193.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carruthers AM, Fozard JR. Adenosine A3 receptors: two into one won’t go. Trends Pharmacol. Sci. 1993;14:290–291. doi: 10.1016/0165-6147(93)90042-I. [DOI] [PubMed] [Google Scholar]
- 195.Carter MS, Krause JE. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci. 1990;10:2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cartmell J, Kemp JA, Alexander SP, Hill SJ, Kendall DA. Inhibition of forskolin-stimulated cyclic AMP formation by 1-aminocyclopentane-trans-1,3-dicarboxylate in guinea-pig cerebral cortical slices. J Neurochem. 1992;58:1964–1966. doi: 10.1111/j.1471-4159.1992.tb10077.x. [DOI] [PubMed] [Google Scholar]
- 197.Cash S, Yuste R. Input summation by cultured pyramidal neurons is linear and position-independent. J Neurosci. 1998;18:10–15. doi: 10.1523/JNEUROSCI.18-01-00010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999;22:383–394. doi: 10.1016/s0896-6273(00)81098-3. [DOI] [PubMed] [Google Scholar]
- 199.Castillo P, Pedroarena C, Chase MH, Morales FR. A medullary inhibitory region for trigeminal motoneurons in the cat. Brain Res. 1991;549:346–349. doi: 10.1016/0006-8993(91)90480-j. [DOI] [PubMed] [Google Scholar]
- 200.Catterall WA. Localization of sodium channels in cultured neural cells. J Neurosci. 1981;1:777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cazalets JR, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci. 1996;16:298–306. doi: 10.1523/JNEUROSCI.16-01-00298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. (Lond.) 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp. Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 204.Chan J, Fung S, Chan S, Barnes C. Facilitation of lumbar monosynaptic reflexes by locus coeruleus in the rat. Brain Res. 1986;369:103–109. doi: 10.1016/0006-8993(86)90517-2. [DOI] [PubMed] [Google Scholar]
- 205.Chandler SH, Hsaio C, Inoue T, Goldberg LJ. Electrophysiological properties of guinea pig trigeminal motoneurons recorded in vitro. J Neurophysiol. 1994;71:129–145. doi: 10.1152/jn.1994.71.1.129. [DOI] [PubMed] [Google Scholar]
- 206.Chandler SH. Evidence for excitatory amino acid transmission between mesencephalic nucleus of V afferents and jaw-closer motoneurons in the guinea pig. Brain Res. 1989;477:252–264. doi: 10.1016/0006-8993(89)91413-3. [DOI] [PubMed] [Google Scholar]
- 207.Chandler SH, Nielsen SA, Goldberg LJ. The effects of a glycine antagonist (strychnine) on cortically induced rhythmical jaw movements in the anesthetized guinea pig. Brain Res. 1985;325:181–186. doi: 10.1016/0006-8993(85)90314-2. [DOI] [PubMed] [Google Scholar]
- 208.Chang MM, Leeman SE, Niall HD. Amino-acid sequence of substance P. Nature New Biol. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 209.Chan-Palay V, Jonsson G, Palay S. Serotonin and substance P coexist in neurons of the rat’s central nervous system. Proc. Natl. Acad. Sci. USA. 1978;75:1582–1586. doi: 10.1073/pnas.75.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Charlton CG, Helke CJ. Autoradiographic localization and characterization of spinal cord substance P binding sites: high densities in sensory, autonomic, phrenic, and Onuf’s motor nuclei. J Neurosci. 1985;5:1653–1661. doi: 10.1523/JNEUROSCI.05-06-01653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Charlton CG, Helke CJ. Ontogeny of substance P receptors in rat spinal cord: quantitative changes in receptor number and differential expression in specific loci. Brain Res. 1986;394:81–91. doi: 10.1016/0165-3806(86)90084-2. [DOI] [PubMed] [Google Scholar]
- 212.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu. Rev. Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 213.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J. Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chiang C, Litingtung Y, Lee E, Young K, Corden J, Westphal H, Beachy P. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 215.Chinnery RM, Shaw PJ, Ince PG, Johnson M. Autoradiographic distribution of binding sites for the non-NMDA receptor antagonist [3H]CNQX in human motor cortex, brainstem and spinal cord. Brain Res. 1993;630:75–81. doi: 10.1016/0006-8993(93)90644-3. [DOI] [PubMed] [Google Scholar]
- 216.Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- 217.Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol. Sci. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- 218.Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- 219.Choi S, Lovinger DM. Metabotropic glutamate receptor modulation of voltage-gated Ca2+ channels involves multiple receptor subtypes in cortical neurons. J. Neurosci. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chu DC, Albin RL, Young AB, Penney JB. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34:341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- 221.Clarac F, El Manira A, Cattaert D. Presynaptic control as a mechanism of sensory-motor integration. Curr. Opin. Neurobiol. 1992;2:764–769. doi: 10.1016/0959-4388(92)90131-4. [DOI] [PubMed] [Google Scholar]
- 222.Clark F, Proudfit H. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;538:231–245. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- 223.Clarke R, Ogilvie J, Houghton A. Enhancement and depression of spinal reflexes by 8-hydroxy-2-(di-n-propylamino) tetralin in the decerebrated and spinalized rabbit: involvement of 5-HT1A- and non-5-HT1A-receptors. Br. J. Pharmacol. 1997;122:631–638. doi: 10.1038/sj.bjp.0701430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cochilla AJ, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 225.Coffey A, Kevetter G. Ipsilateral and contralateral projections to the trochlear nucleus arise from different subdivisions in the vestibular nuclei. Neurosci. Lett. 1989;99:274–280. doi: 10.1016/0304-3940(89)90459-x. [DOI] [PubMed] [Google Scholar]
- 226.Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol. Rev. 1989;41:143–210. [PubMed] [Google Scholar]
- 227.Collins WD, Erichsen J, Rose R. Pudendal motor and premotor neurons in the male rat: a WGA transneuronal study. J. Comp. Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- 228.Collins WD, Honig M, Mendell L. Heterogeneity of group Ia synapses on homonymous alpha-motoneurons as revealed by high-frequency stimulation of Ia afferent fibers. J. Neurophysiol. 1984;52:980–993. doi: 10.1152/jn.1984.52.5.980. [DOI] [PubMed] [Google Scholar]
- 229.Collins WFD, Davis BM, Mendell LM. Modulation of EPSP amplitude during high frequency stimulation depends on the correlation between potentiation, depression and facilitation. Brain Res. 1988;442:161–165. doi: 10.1016/0006-8993(88)91445-x. [DOI] [PubMed] [Google Scholar]
- 230.Collis MG, Hourani SM. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- 231.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Commissiong J, Hellstrom S, Neff N. A new projection from locus coeruleus to the spinal ventral columns: histochemical and biochemical evidence. Brain Res. 1978;148:207–213. doi: 10.1016/0006-8993(78)90391-8. [DOI] [PubMed] [Google Scholar]
- 233.Commissiong J, Sedgwick E. Modulation of the tonic stretch reflex by monoamines. Eur. J. Pharmacol. 1979;57:83–92. doi: 10.1016/0014-2999(79)90106-7. [DOI] [PubMed] [Google Scholar]
- 234.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 235.Connaughton M, Priestley J, Sofroniew M, Eckenstein F, Cuello A. Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocytochemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience. 1986;17:205–224. doi: 10.1016/0306-4522(86)90237-x. [DOI] [PubMed] [Google Scholar]
- 236.Connell L, Wallis D. Responses to 5-hydroxytryptamine evoked in the hemisected spinal cord of the neonate rat. Br. J. Pharmacol. 1988;94:1101–1114. doi: 10.1111/j.1476-5381.1988.tb11628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Connell LA, Majid A, Wallis DI. Involvement of alpha 1-adrenoceptors in the depolarizing but not the hyperpolarizing responses of motorneurones in the neonate rat to noradrenaline. Neuropharmacology. 1989;28:1399–1404. doi: 10.1016/0028-3908(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 238.Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol. Scand. Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- 239.Conway B, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of l-dopa and clonidine in the spinal cat. J. Physiol. (Lond.) 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J. Physiol. (Lond.) 1957;139:232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Coombs JS, Eccles JC, Fatt P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J. Physiol. (Lond.) 1955;130:326–373. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu. Rev. Pharmacol. Toxicol. 1998;38:321–350. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- 243.Crill WE. Persistent sodium current in mammalian central neurons. Annu. Rev. Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- 244.Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J. Physiol. (Lond.) 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cullheim S, Arvidsson U. The peptidergic innervation of spinal motoneurons via the bulbospinal 5-hydroxytryptamine pathway. Prog. Brain Res. 1995;104:21–40. doi: 10.1016/s0079-6123(08)61782-3. [DOI] [PubMed] [Google Scholar]
- 246.Cullheim S, Fleshman J, Glenn L, Burke R. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J. Comp. Neurol. 1987;255:68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- 247.Cullheim S, Fleshman J, Glenn L, Burke R. Three-dimensional architecture of dendritic trees in type-identified alpha-motoneurons. J. Comp. Neurol. 1987;255:82–96. doi: 10.1002/cne.902550107. [DOI] [PubMed] [Google Scholar]
- 248.Cullheim S, Kellerth J. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J. Physiol. (Lond.) 1978;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cullheim S, Kellerth J, Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977;132:1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- 250.Cunha RA, Sebasti AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J. Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cunningham MD, Enna SJ. Evidence for pharmacologically distinct GABAB receptors associated with cAMP production in rat brain. Brain Res. 1996;720:220–224. doi: 10.1016/0006-8993(96)00120-5. [DOI] [PubMed] [Google Scholar]
- 252.Curfs M, Gribnau A, Dederen P. Direct cortico-motoneuronal synaptic contacts are present in the adult rat cervical spinal cord and are first established at postnatal day 7. Neurosci. Lett. 1996;205:123–126. doi: 10.1016/0304-3940(96)12396-x. [DOI] [PubMed] [Google Scholar]
- 253.Curtis DR, Duggan AW, Felix D, Johnston GA. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971;32:69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- 254.Curtis DR, Gynther BD, Beattie DT, Lacey G. An in vivo electrophysiological investigation of group Ia afferent fibres and ventral horn terminations in the cat spinal cord. Exp. Brain Res. 1995;106:403–417. doi: 10.1007/BF00231063. [DOI] [PubMed] [Google Scholar]
- 255.Curtis DR, Gynter BD, Lacey G, Beattie DT. Baclofen-reduction of presynaptic calcium influx in the cat spinal cord in vivo. Exp. Brain Res. 1997;113:520–533. doi: 10.1007/pl00005604. [DOI] [PubMed] [Google Scholar]
- 256.Curtis DR, Gynther BD, Malik R. A pharmacological study of group I muscle afferent terminals and synaptic excitation in the intermediate nucleus and Clarke’s column of the cat spinal cord. Exp. Brain Res. 1986;64:105–113. doi: 10.1007/BF00238205. [DOI] [PubMed] [Google Scholar]
- 257.Curtis DR, Hosli L, Johnston GA, Johnston IH. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp. Brain Res. 1968;5:235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- 258.Curtis DR, Lacey G. GABA-B receptor-mediated spinal inhibition. Neuroreport. 1994;5:540–542. doi: 10.1097/00001756-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 259.Curtis DR, Lodge D, Bornstein JC, Peet MJ. Selective effects of (−)-baclofen on spinal synaptic transmission in the cat. Exp. Brain Res. 1981;42:158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- 260.Curtis DR, Malik R. The effect of GABA on lumbar terminations of rubrospinal neurons in the cat spinal cord. Proc. R. Soc. Lond. B Biol. Sci. 1984;223:25–33. doi: 10.1098/rspb.1984.0080. [DOI] [PubMed] [Google Scholar]
- 261.Curtis DR, Malik R. The differential effects of baclofen on segmental and descending excitation of spinal interneurones in the cat. Exp. Brain Res. 1985;58:333–337. doi: 10.1007/BF00235314. [DOI] [PubMed] [Google Scholar]
- 262.Curtis DR, Watkins JC. The excitation and depression of spinal neurons by structurally-related amino acids. J. Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- 263.Curtis DR, Wilson VJ, Malik R. The effect of GABA on the terminations of vestibulospinal neurons in the cat spinal cord. Brain Res. 1984;295:372–375. doi: 10.1016/0006-8993(84)90989-2. [DOI] [PubMed] [Google Scholar]
- 264.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiol. Scand. 1964;62:1–55. [PubMed] [Google Scholar]
- 265.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neuron systems. Acta Physiol. Scand. 1965;64:6–36. [PubMed] [Google Scholar]
- 266.Dale N, Grillner S. Dual-component synaptic potentials in the lamprey mediated by excitatory amino acid receptors. J. Neurosci. 1986;6:2653–2661. doi: 10.1523/JNEUROSCI.06-09-02653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dale N, Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J. Physiol. (Lond.) 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Daly JW, Butts-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell. Mol. Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PubMed] [Google Scholar]
- 269.Dalziel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacol. Rev. 1994;46:449–466. [PubMed] [Google Scholar]
- 270.Dam TV, Escher E, Quirion R. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Res. 1990;506:175–179. doi: 10.1016/0006-8993(90)91218-6. [DOI] [PubMed] [Google Scholar]
- 271.Dam TV, Martinelli B, Quirion R. Autoradiographic distribution of brain neurokinin-1/substance P receptors using a highly selective ligand [3H]-[Sar9, Met(O2)11]-substance P. Brain Res. 1990;531:333–337. doi: 10.1016/0006-8993(90)90796-e. [DOI] [PubMed] [Google Scholar]
- 272.Dam TV, Handelmann G, Quirion R. Neurokinin and substance P receptors in the developing rat central nervous system. In: Zagon I, McLaughlin P, editors. Receptors in the Developing Nervous System. New York: Chapman & Hall; 1993. pp. 55–96. [Google Scholar]
- 273.Daniel H, Billard J, Angaut P, Batini C. The inter-posito-rubrospinal system. Anatomical tracing of a motor control pathway in the rat. Neurosci. Res. 1987;5:87–112. doi: 10.1016/0168-0102(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 274.Daval JL, Nicolas F, Doriat JF. Adenosine physiology and pharmacology: how about A2 receptors? Pharmacol. Ther. 1996;71:325–335. doi: 10.1016/s0163-7258(96)00094-0. [DOI] [PubMed] [Google Scholar]
- 275.Davidoff RA, Hackman JC. Drugs, chemicals and toxins: their effects on the spinal cord. In: Davidoff RA, editor. Handbook of the Spinal Cord. New York: Dekker; 1983. pp. 409–476. [Google Scholar]
- 276.Davies J. Selective depression of synaptic excitation in cat spinal neurones by baclofen: an iontophoretic study. Br. J. Pharmacol. 1981;72:373–384. doi: 10.1111/j.1476-5381.1981.tb09137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Davies J, Evans RH, Jones AW, Smith DA, Watkins JC. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp. Biochem. Physiol. C Comp. Pharmacol. 1982;72:211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- 278.Davis CJ. Emesis research: a concise history of the critical concepts and experiments. J. R. Naval Med. Serv. 1997;83:31–41. [PubMed] [Google Scholar]
- 279.Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu. Rev. Neurosci. 1993;16:207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- 280.Dawe GS, Maxwell DJ. Contacts between group Ia muscle spindle afferent axons and GABA-immunoreactive perikarya in lamina VI of cat spinal cord. Exp. Physiol. 1991;76:461–464. doi: 10.1113/expphysiol.1991.sp003513. [DOI] [PubMed] [Google Scholar]
- 281.Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J. Chem. Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 282.de Blas AL. Brain GABAA receptors studied with subunit-specific antibodies. Mol. Neurobiol. 1996;12:55–71. doi: 10.1007/BF02740747. [DOI] [PubMed] [Google Scholar]
- 283.Dekker J, Lawrence D, Kuypers H. The location of longitudinally running dendrites in the ventral horn of the cat spinal cord. Brain Res. 1973;51:319–325. doi: 10.1016/0006-8993(73)90382-x. [DOI] [PubMed] [Google Scholar]
- 284.de la Cruz R, Pastor A, Martinez-Guijarro F, Lopez-Garcia C, Delgado-Garcia J. Role of GABA in the extraocular motor nuclei of the cat: a postembedding immunocytochemical study. Neuroscience. 1992;51:911–929. doi: 10.1016/0306-4522(92)90529-b. [DOI] [PubMed] [Google Scholar]
- 285.Delgado-Lezama R, Perrier J, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J. Physiol. (Lond.) 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Delgado-Lezama R, Perrier J-F, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J. Physiol. (Lond.) 1999;515:203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.del Negro CA, Chandler SH. Regulation of intrinsic and synaptic properties of neonatal rat trigeminal motoneurons by metabotropic glutamate receptors. J. Neurosci. 1998;18:9216–9226. doi: 10.1523/JNEUROSCI.18-22-09216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Dememes D, Raymond J. Radioautographic identification of [3H]glutamic acid labeled nerve endings in the cat oculomotor nucleus. Brain Res. 1982;231:433–437. doi: 10.1016/0006-8993(82)90379-1. [DOI] [PubMed] [Google Scholar]
- 289.de Souza E, Insel T, Perrin M, Rivier J, Vale W, Kuhar M. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J. Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Destombes J, Horcholle-Bossavit G, Simon M, Thiesson D. GABA-like immunoreactive terminals on lumbar motoneurons of the adult cat. A quantitative ultrastructural study. Neurosci. Res. 1996;24:123–130. doi: 10.1016/0168-0102(95)00980-9. [DOI] [PubMed] [Google Scholar]
- 291.Destombes J, Horcholle-Bossavit G, Thiesson D. Distribution of glycinergic terminals on lumbar motoneurons of the adult cat: an ultrstructural study. Brain Res. 1992;599:353–360. doi: 10.1016/0006-8993(92)90412-3. [DOI] [PubMed] [Google Scholar]
- 292.Dickinson PS. Interaction among neural networks for behavior. Curr. Opin. Neurobiol. 1995;5:792–798. doi: 10.1016/0959-4388(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 293.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J. Comp. Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 294.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 295.di Pasquale E, Lindsay AD, Feldman JL, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci. Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- 296.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 297.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J. Comp. Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- 298.Domyancic A, Morilak D. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J. Comp. Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 299.Donevan A, Neuber-Hess M, Rose P. Multiplicity of vestibulospinal projections to the upper cervical spinal cord of the cat: a study with the anterograde tracer Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1990;302:1–14. doi: 10.1002/cne.903020102. [DOI] [PubMed] [Google Scholar]
- 300.Dong X-W, Feldman JL. Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. J. Neurosci. 1995;15:3458–3467. doi: 10.1523/JNEUROSCI.15-05-03458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dong X-W, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J. Neurosci. 1999;19:5173–5184. doi: 10.1523/JNEUROSCI.19-13-05173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Dong X-W, Morin D, Feldman JL. Multiple actions of 1S,3R-ACPD in modulating endogenous synaptic transmission to spinal respiratory motoneurons. J. Neurosci. 1996;16:4971–4982. doi: 10.1523/JNEUROSCI.16-16-04971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dong X-W, Feldman JL. Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. J. Neurosci. 1995;15:3458–3467. doi: 10.1523/JNEUROSCI.15-05-03458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Douglas JR, Noga BR, Dai X, Jordan LM. The effects of intrathecal administration of excitatory amino acid agonists and antagonists on the initiation of locomotion in the adult cat. J. Neurosci. 1993;13:990–1000. doi: 10.1523/JNEUROSCI.13-03-00990.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Douse M, White D. Serotonergic effects on hypoglossal neural activity and reflex responses. Brain Res. 1996;726:213–222. [PubMed] [Google Scholar]
- 306.Dubois A, Savasta M, Curet O, Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986;19:125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 307.Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. Cell Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 308.Ducic I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. Gamma-aminobutyric acid gating of Cl− channels in recombinant GABAA receptors. J. Pharmacol. Exp. Ther. 1995;272:438–445. [PubMed] [Google Scholar]
- 309.Dunn SM, Bateson AN, Martin IL. Molecular neurobiology of the GABAA receptor. Int. Rev. Neurobiol. 1994;36:51–96. doi: 10.1016/s0074-7742(08)60303-7. [DOI] [PubMed] [Google Scholar]
- 310.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J. Pharmacol. Exp. Ther. 1989;249:31–37. [PubMed] [Google Scholar]
- 312.Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-d-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc. Natl. Acad. Sci. USA. 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Durand J. NMDA actions on rat abducens motoneurons. Eur. J. Neurosci. 1991;3:621–633. doi: 10.1111/j.1460-9568.1991.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 314.Durand J. Synaptic excitation triggers oscillations during NMDA receptor activation in rat abducens motoneurons. Eur. J. Neurosci. 1993;5:1389–1397. doi: 10.1111/j.1460-9568.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 315.Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J. Neurosci. 1995;15:3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Eaton S, Jane D, Jones P, Porter R, Pook P, Sunter D, Udvarhelyi P, Roberts P, Salt T, Watkins J. Competitive antagonism at metabotropic glutamate receptors by (S)-4-carboxyphenylglycine and (RS)-alpha-methyl-4-carboxyphenylglycine. Eur. J. Pharmacol. 1993;244:195–197. doi: 10.1016/0922-4106(93)90028-8. [DOI] [PubMed] [Google Scholar]
- 317.Eccles JC. The Physiology of Nerve Cells. Baltimore, MD: Johns Hopkins Press; 1957. [Google Scholar]
- 318.Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J. Physiol. (Lond.) 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Echelard Y, Epstein D, St-Jacques B, Shen L, Mohler J, McMahon J, McMahon A. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 320.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 321.Edwards FR, Harrison PJ, Jack JJ, Kullmann DM. Reduction by baclofen of monosynaptic EPSPs in lumbosacral motoneurones of the anaesthetized cat. J. Physiol. (Lond.) 1989;416:539–556. doi: 10.1113/jphysiol.1989.sp017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Eguibar JR, Quevedo J, Jiminez I, Rudomin P. Selective cortical control of information flow through different intraspinal collaterals of the same afferent fiber. Brain Res. 1994;643:328–333. doi: 10.1016/0006-8993(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 323.Eguibar JR, Quevedo J, Rudomin P. Selective cortical and segmental control of primary afferent depolarization of single muscle afferents in the cat spinal cord. Exp. Brain Res. 1997;113:411–430. doi: 10.1007/pl00005595. [DOI] [PubMed] [Google Scholar]
- 324.Ehlers MD, Mammen AL, Lau LF, Huganir RL. Synaptic targeting of glutamate receptors. Curr. Opin. Cell Biol. 1996;8:484–489. doi: 10.1016/s0955-0674(96)80024-x. [DOI] [PubMed] [Google Scholar]
- 325.Eisen J. Development of motoneuronal phenotype. Annu. Rev. Neurosci. 1994;17:1–30. doi: 10.1146/annurev.ne.17.030194.000245. [DOI] [PubMed] [Google Scholar]
- 326.Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog. Brain Res. 1989;80:257–267. doi: 10.1016/s0079-6123(08)62219-0. [DOI] [PubMed] [Google Scholar]
- 327.Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol. Scand. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- 328.El Manira A, Tegner J, Grillner S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J. Neurophysiol. 1994;72:1852–1861. doi: 10.1152/jn.1994.72.4.1852. [DOI] [PubMed] [Google Scholar]
- 329.Elde R, Schalling M, Ceccatelli S, Nakanishi S, Hökfelt T. Localization of neuropeptide receptor mRNA in rat brain: initial observations using probes for neurotensin and substance P receptors. Neurosci. Lett. 1990;120:134–138. doi: 10.1016/0304-3940(90)90187-e. [DOI] [PubMed] [Google Scholar]
- 330.Ellenberger HH, Vera P, Feldman JL, Holets V. Multiple putative neuromessenger inputs to the phrenic nucleus in rat. J. Chem. Neuroanat. 1992;5:375–382. doi: 10.1016/0891-0618(92)90053-s. [DOI] [PubMed] [Google Scholar]
- 331.Ellenberger HH, Vera P, Haselton J, Haselton C, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res. Bull. 1990;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- 332.Elliott P, Wallis D. Serotonin and l-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- 333.Elliott P, Wallis DI. Glutamatergic and non-glutamatergic responses evoked in neonatal rat lumbar motoneurons on stimulation of the lateroventral spinal cord surface. Neuroscience. 1993;56:189–197. doi: 10.1016/0306-4522(93)90573-x. [DOI] [PubMed] [Google Scholar]
- 334.Engberg I, Marshall K. Mechanism of noradrenaline hyperpolarization in spinal cord motoneurones of the cat. Acta Physiol. Scand. 1971;83:142–144. doi: 10.1111/j.1748-1716.1971.tb05061.x. [DOI] [PubMed] [Google Scholar]
- 335.Engberg I, Tarnawa I, Durand J, Ouardouz M. An analysis of synaptic transmission to motoneurones in the cat spinal cord using a new selective receptor blocker. Acta Physiol. Scand. 1993;148:97–100. doi: 10.1111/j.1748-1716.1993.tb09537.x. [DOI] [PubMed] [Google Scholar]
- 336.Engelhardt J, Morales F, Yamuy J, Chase M. Cable properties of spinal cord motoneurons in adult and aged cats. J. Neurophysiol. 1989;61:194–201. doi: 10.1152/jn.1989.61.1.194. [DOI] [PubMed] [Google Scholar]
- 337.Enomoto S, Katakura N, Sunada T, Katayama T, Hirose Y, Ishiwata Y, Nakamura Y. Cortically induced masticatory rhythm in masseter motoneurons after blocking inhibition by strychnine and tetanus toxin. Neurosci. Res. 1987;4:396–412. doi: 10.1016/0168-0102(87)90005-8. [DOI] [PubMed] [Google Scholar]
- 338.Enz R, Cutting GR. Molecular composition of GABAC receptors. Vision Res. 1998;38:1431–1441. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 339.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 340.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 341.Escudero M, de la Cruz R, Delgado-Garcia J. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J. Physiol. (Lond.) 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Evans RH. The pharmacology of segmental transmission in the spinal cord. Prog. Neurobiol. 1989;33:255–279. doi: 10.1016/0301-0082(89)90003-8. [DOI] [PubMed] [Google Scholar]
- 343.Evans RH, Francis AA, Jones AW, Smith DA, Watkins JC. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br. J. Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Evans RJ. The molecular biology of P2X receptors. J. Auton. Pharmacol. 1996;16:309–310. doi: 10.1111/j.1474-8673.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 345.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 346.Faber ES, Chambers JP, Brugger F, Evans RH. Depression of A and C fibre-evoked segmental reflexes by morphine and clonidine in the in vitro spinal cord of the neonatal rat. Br. J. Pharmacol. 1997;120:1390–1396. doi: 10.1038/sj.bjp.0701064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Farkas S, Ono H. Participation of NMDA and non-NMDA excitatory amino acid receptors in the mediation of spinal reflex potentials in rats: an in vivo study. Br. J. Pharmacol. 1995;114:1193–1205. doi: 10.1111/j.1476-5381.1995.tb13333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. (Lond.) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 349.Faull RL, Villiger JW. Benzodiazepine receptors in the human spinal cord: a detailed anatomical and pharmacological study. Neuroscience. 1986;17:791–802. doi: 10.1016/0306-4522(86)90045-x. [DOI] [PubMed] [Google Scholar]
- 350.Fedirchuk B, Downie JW, Shefchyk SJ. Reduction in perineal evoked excitatory postsynaptic potentials in cat lumbar and sacral motoneurons during micturition. J. Neurosci. 1994;14:6153–6159. doi: 10.1523/JNEUROSCI.14-10-06153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Fedirchuk B, Hochman S, Shefchyk SJ. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Exp. Brain Res. 1992;89:511–516. doi: 10.1007/BF00229875. [DOI] [PubMed] [Google Scholar]
- 352.Fedirchuk B, Shefchyk SJ. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. J. Neurosci. 1993;13:3090–3094. doi: 10.1523/JNEUROSCI.13-07-03090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Feldman JL. Handbook of Physiology. The Nervous System. Intrinsic Regulatory System in the Brain. 9. IV. Bethesda, MD: Am. Physiol. Soc; 1986. Neurophysiology of breathing in mammals; pp. 463–524. sect. 1. [Google Scholar]
- 354.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J. Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Feldman JL, Smith JC. Neural control of respiratory pattern in mammals: an overview. In: Dempsey J, Pack A, editors. Regulation of Breathing. New York: Dekker; 1995. pp. 39–69. [Google Scholar]
- 356.Feldman JL, Smith JC. Neural control of respiratory pattern in mammals: an overview. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Dekker; 1995. pp. 39–69. [Google Scholar]
- 357.Feldman JL, Smith JC, Liu G. Respiratory pattern generation in mammals: in vitro en bloc analyses. Curr. Opin. Neurobiol. 1991;1:590–594. doi: 10.1016/s0959-4388(05)80033-9. [DOI] [PubMed] [Google Scholar]
- 358.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol. Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 359.Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J. Physiol. (Lond.) 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Feuerstein TJ, Hertting G, Jackisch R. Modulation of hippocampal serotonin (5-HT) release by endogenous adenosine. Eur. J. Pharmacol. 1985;107:233–242. doi: 10.1016/0014-2999(85)90063-9. [DOI] [PubMed] [Google Scholar]
- 361.Fields RD, Nelson PG. Activity-dependent development of the vertebrate nervous system. Int. Rev. Neurobiol. 1992;34:133–214. doi: 10.1016/s0074-7742(08)60098-7. [DOI] [PubMed] [Google Scholar]
- 362.Fields RD, Yu C, Nelson PG. Calcium, network activity, and the role of NMDA channels in synaptic plasticity in vitro. J. Neurosci. 1991;11:134–146. doi: 10.1523/JNEUROSCI.11-01-00134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Figurov A, Boddeke H, Muller D. Enhancement of AMPA-mediated synaptic transmission by the protein phosphatase inhibitor calyculin A in rat hippocampal slices. Eur. J. Neurosci. 1993;5:1035–1041. doi: 10.1111/j.1460-9568.1993.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 364.Finkel AS, Redman SJ. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J. Physiol. (Lond.) 1983;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Fisher N, Baranauskas G, Nistri A. Multiple types of tachykinin receptor mediate a slow excitation of rat spinal motoneurones in vitro. Neurosci. Lett. 1994;165:84–88. doi: 10.1016/0304-3940(94)90715-3. [DOI] [PubMed] [Google Scholar]
- 366.Fisher N, Nistri A. A study of the barium-sensitive and -insensitive components of the action of thyrotropin-releasing hormone on lumbar motoneurons of the rat isolated spinal cord. Eur. J. Neurosci. 1993;5:1360–1369. doi: 10.1111/j.1460-9568.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 367.Fisher N, Nistri A. Substance P and TRH share a common effector pathway in rat spinal motoneurones: an in vitro electrophysiological investigation. Neurosci. Lett. 1993;153:115–119. doi: 10.1016/0304-3940(93)90090-8. [DOI] [PubMed] [Google Scholar]
- 368.Flatman JA, Durand J, Engberg I, Lambert JDC. Blocking the monosynaptic EPSP in spinal cord motoneurons with inhibitors of amino acid excitation. In: Hicks TP, Lodge D, McLennan H, editors. Excitatory Amino Acid Transmission. New York: Liss; 1987. pp. 285–292. [Google Scholar]
- 369.Fleshman J, Segev I, Burke R. Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J. Neurophysiol. 1988;60:60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- 370.Fletcher EJ, Lodge D. New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors. Pharmacol. Ther. 1996;70:65–89. doi: 10.1016/0163-7258(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 371.Floeter MK, Lev-Tov A. Excitation of lumbar motoneurons by the medial longitudinal fasciculus in the in vitro brain stem spinal cord preparation of the neonatal rat. J. Neurophysiol. 1993;70:2241–2250. doi: 10.1152/jn.1993.70.6.2241. [DOI] [PubMed] [Google Scholar]
- 372.Forsythe ID, Redman SJ. The dependence of motoneurone membrane potential on extracellular ion concentrations studied in isolated rat spinal cord. J. Physiol. (Lond.) 1988;404:83–99. doi: 10.1113/jphysiol.1988.sp017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Forsythe ID, Clements JD. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J. Physiol. (Lond.) 1990;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N-methyl-d-aspartate receptors on cultured mouse central neurones. J. Physiol. (Lond.) 1988;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Fort P, Luppi P, Sakai K, Salvert D, Jouvet M. Nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat trigeminal motor nucleus: a double-labeling study with cholera-toxin as a retrograde tracer. J. Comp. Neurol. 1990;301:262–275. doi: 10.1002/cne.903010209. [DOI] [PubMed] [Google Scholar]
- 376.Fort P, Sakai K, Luppi P, Salvert D, Jouvet M. Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. J. Comp. Neurol. 1989;283:285–302. doi: 10.1002/cne.902830209. [DOI] [PubMed] [Google Scholar]
- 377.Fox S, Krnjevic K, Morris ME, Puil E, Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3:495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- 378.Franck J, Brodin E, Fried G. Differential release of endogenous 5-hydroxytryptamine, substance P, and neurokinin A from rat ventral spinal cord in response to electrical stimulation. J. Neurochem. 1993;61:704–711. doi: 10.1111/j.1471-4159.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 379.Franck J, Brodin E, Fried K, Rosen A, Yamamoto Y, Fried G. The effect of selective serotonergic neurotoxin treatment on tachykinin levels in the rat ventral spinal cord. Neuroscience. 1991;45:339–345. doi: 10.1016/0306-4522(91)90231-c. [DOI] [PubMed] [Google Scholar]
- 380.Frank E, Mendelson B. Specification of synaptic connections between sensory and motor neurons in the developing spinal cord. J. Neurobiol. 1990;21:33–50. doi: 10.1002/neu.480210104. [DOI] [PubMed] [Google Scholar]
- 381.Frank K, Fuortes MGF. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. J. Physiol. (Lond.) 1957;16:39–40. [Google Scholar]
- 382.Fredholm BB. Adenosine receptors in the central nervous system. News Physiol. Sci. 1995;10:122–128. [Google Scholar]
- 383.Fredholm BB. ASTRA Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 384.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 385.Fredholm BB, Proctor W, van der Ploeg I, Dunwiddie TV. In vivo pertussis toxin treatment attenuates some, but not all, adenosine A1 effects in slices of the rat hippocampus. Eur. J. Pharmacol. 1989;172:249–262. doi: 10.1016/0922-4106(89)90055-2. [DOI] [PubMed] [Google Scholar]
- 386.Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, fast DiI and immunocytochemical study in the hypoglossal nucleus. J. Neurobiol. 1995;27:520–534. doi: 10.1002/neu.480270407. [DOI] [PubMed] [Google Scholar]
- 387.Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, fast DiI and immunocytochemical study of the nucleus ambiguus. J. Neurobiol. 1995;26:563–578. doi: 10.1002/neu.480260409. [DOI] [PubMed] [Google Scholar]
- 388.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 389.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J. Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Fujita M, Sato K, Sato M, Inoue T, Kozuka T, Tohyama M. Regional distribution of the cells expressing glycine receptor beta subunit mRNA in the rat brain. Brain Res. 1991;560:23–37. doi: 10.1016/0006-8993(91)91210-r. [DOI] [PubMed] [Google Scholar]
- 391.Fujito Y, Aoki M. Monosynaptic rubrospinal projections to distal forelimb motoneurons in the cat. Exp. Brain Res. 1995;105:181–190. doi: 10.1007/BF00240954. [DOI] [PubMed] [Google Scholar]
- 392.Fujito Y, Imai T, Aoki M. Monosynaptic excitation of motoneurons innervating forelimb muscles following stimulation of the red nucleus in cats. Neurosci. Lett. 1991;127:137–140. doi: 10.1016/0304-3940(91)90913-e. [DOI] [PubMed] [Google Scholar]
- 393.Fung SJ, Barnes CD. Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res. 1987;402:230–242. doi: 10.1016/0006-8993(87)90029-1. [DOI] [PubMed] [Google Scholar]
- 394.Fung SJ, Barnes CD. Raphe-produced excitation of spinal cord motoneurons in the cat. Neurosci. Lett. 1989;103:185–190. doi: 10.1016/0304-3940(89)90573-9. [DOI] [PubMed] [Google Scholar]
- 395.Fung SJ, Chan J, Manzoni D, White S, Lai Y, Strahlendorf H, Zhuo H, Liu R, Reddy V, Barnes CD. Cotransmitter-mediated locus coeruleus action on motoneurons. Brain Res. Bull. 1994;35:423–432. doi: 10.1016/0361-9230(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 396.Fung SJ, Manzoni D, Chan J, Pompeiano O, Barnes CD. Locus coeruleus control of spinal motor output. Prog. Brain Res. 1991;88:395–409. doi: 10.1016/s0079-6123(08)63825-x. [DOI] [PubMed] [Google Scholar]
- 397.Fung S, Reddy V, Zhuo H, Liu R, Barnes C. Bulbospinal neurons of the cat that co-contain serotonin and methionine enkephalin. Arch. Ital. Biol. 1994;132:61–72. [PubMed] [Google Scholar]
- 398.Fung S, Reddy V, Zhuo H, Liu R, Wang Z, Barnes C. Anatomical evidence for the presence of glutamate or enkephalin in noradrenergic projection neurons of the locus coeruleus. Microsc. Res. Tech. 1994;29:219–225. doi: 10.1002/jemt.1070290307. [DOI] [PubMed] [Google Scholar]
- 399.Funk GD, Kanjhan R, Walsh C, Lipski J, Comer A, Parkis M, Housley G. P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. J. Neurosci. 1997;17:6325–6337. doi: 10.1523/JNEUROSCI.17-16-06325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J. Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- 401.Funk GD, Feldman JL. Generation of respiratory rhythm and pattern in mammals: insights from developmental studies. Curr. Opin. Neurobiol. 1995;5:778–785. doi: 10.1016/0959-4388(95)80106-5. [DOI] [PubMed] [Google Scholar]
- 402.Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J. Neurophysiol. 1997;78:1414–1420. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- 403.Funk GD, Kanjhan R, Walsh C, Lipski J, Comer AM, Parkis MA, Housley GD. P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. J. Neurosci. 1997;17:6325–6337. doi: 10.1523/JNEUROSCI.17-16-06325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Funk GD, Parkis MA, Selvaratnam SR, Walsh C. Developmental modulation of glutamatergic inspiratory drive to hypoglossal motoneurons. Respir. Physiol. 1997;110:125–137. doi: 10.1016/s0034-5687(97)00078-9. [DOI] [PubMed] [Google Scholar]
- 405.Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J. Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- 406.Funk GD, Smith JC, Feldman JL. Modulation of neural network activity in vitro by cyclothiazide, a drug that blocks desensitization of AMPA receptors. J. Neurosci. 1995;15:4046–4056. doi: 10.1523/JNEUROSCI.15-05-04046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Furuyama T, Kiyama H, Sato K, Park HT, Maeno H, Takagi H, Tohyama M. Region-specific expression of subunits of ionotropic glutamate receptors (AMPA-type, KA-type and NMDA receptors) in the rat spinal cord with special reference to nociception. Brain Res. 1993;18:141–151. doi: 10.1016/0169-328x(93)90183-p. [DOI] [PubMed] [Google Scholar]
- 408.Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. The distribution of monoamine terminals in the central nervous system. Acta Physiol. Scand. 1965;64:41–85. [PubMed] [Google Scholar]
- 409.Fyffe RE. Glycine-like immunoreactivity in synaptic boutons of identified inhibitory interneurons in the mammalian spinal cord. Brain Res. 1991;547:175–179. doi: 10.1016/0006-8993(91)90590-r. [DOI] [PubMed] [Google Scholar]
- 410.Gage PW. Activation and modulation of neuronal K+ channels by GABA. Trends Neurosci. 1992;15:46–51. doi: 10.1016/0166-2236(92)90025-4. [DOI] [PubMed] [Google Scholar]
- 411.Ganong AH, Lanthorn TH, Cotman CW. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983;273:170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- 412.Gao BX, Cheng G, Ziskind-Conhaim L. Development of spontaneous synaptic transmission in the rat spinal cord. J. Neurophysiol. 1998;79:2277–2287. doi: 10.1152/jn.1998.79.5.2277. [DOI] [PubMed] [Google Scholar]
- 413.Gao BX, Ziskind-Conhaim L. Development of glycine- and GABA-gated currents in rat spinal motoneurons. J. Neurophysiol. 1995;74:113–121. doi: 10.1152/jn.1995.74.1.113. [DOI] [PubMed] [Google Scholar]
- 414.Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J. Physiol. (Lond.) 1998;509:255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gehlert DR, Yamamura HI, Wamsley JK. gamma-Aminobutyric acidB receptors in the rat brain: quantitative autoradiographic localization using [3H](−)-baclofen. Neurosci. Lett. 1985;56:183–188. doi: 10.1016/0304-3940(85)90126-0. [DOI] [PubMed] [Google Scholar]
- 416.Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 417.Gemignani A, Paudice P, Bonanno G, Raiteri M. Pharmacological discrimination between gamma-aminobutyric acid type B receptors regulating cholecystokinin and somatostatin release from rat neocortex synaptosomes. Mol. Pharmacol. 1994;46:558–562. [PubMed] [Google Scholar]
- 418.Gerber U, Greene RW, Haas HL, Stevens DR. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J. Physiol. (Lond.) 1989;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Gereau RWT, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J. Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Gerhardt CC, van Heerikhuizen H. Functional characteristics of heterologously expressed 5-HT receptors. Eur. J. Pharmacol. 1997;334:1–23. doi: 10.1016/s0014-2999(97)01115-1. [DOI] [PubMed] [Google Scholar]
- 421.Gerin C, Legrand A, Privat A. Study of 5-HT release with a chronically implanted microdialysis probe in the ventral horn of the spinal cord of unrestrained rats during exercise on a treadmill. J. Neurosci. Methods. 1994;52:129–141. doi: 10.1016/0165-0270(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 422.Gershengorn MC. Mechanism of signal transduction by TRH. Ann. NY Acad. Sci. 1989;553:191–196. doi: 10.1111/j.1749-6632.1989.tb46641.x. [DOI] [PubMed] [Google Scholar]
- 423.Gershengorn MC, Osman R. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol. Rev. 1996;76:175–191. doi: 10.1152/physrev.1996.76.1.175. [DOI] [PubMed] [Google Scholar]
- 424.Geyer SW, Gudden W, Betz H, Gnahn H, Weindl A. Co-localization of choline acetyltransferase and postsynaptic glycine receptors in motoneurons of rat spinal cord demonstrated by immunocytochemistry. Neurosci. Lett. 1987;82:11–15. doi: 10.1016/0304-3940(87)90163-7. [DOI] [PubMed] [Google Scholar]
- 425.Gibson S, Bloom S, Polak J. A novel substance P pathway linking the dorsal and ventral horn in the upper lumbar segments of the rat spinal cord. Brain Res. 1984;301:243–251. doi: 10.1016/0006-8993(84)91092-8. [DOI] [PubMed] [Google Scholar]
- 426.Gilbert R, Emson P, Hunt S, Bennett G, Marsden C, Sandberg B, Steinbusch H, Verhofstad A. The effects of monoamine neurotoxins on peptides in the rat spinal cord. Neuroscience. 1982;7:69–87. doi: 10.1016/0306-4522(82)90154-3. [DOI] [PubMed] [Google Scholar]
- 427.Gill R, Lodge D. Neuroprotective Agents and Cerebral Ischemia. Orlando, FL: Academic; 1997. Pharmacology of AMPA antagonists and their role in neuroprotection; pp. 197–232. [DOI] [PubMed] [Google Scholar]
- 428.Glaum SR, Miller RJ. Presynaptic metabotropic glutamate receptors modulate omega-conotoxin-GVIA-insensitive calcium channels in the rat medulla. Neuropharmacology. 1995;34:953–964. doi: 10.1016/0028-3908(95)00076-i. [DOI] [PubMed] [Google Scholar]
- 429.Goldberg LJ, Nakamura Y. Lingually induced inhibition of masseteric motoneurones. Experientia. 1968;24:371–373. doi: 10.1007/BF02140828. [DOI] [PubMed] [Google Scholar]
- 430.Gollasch M, Haller H, Schultz G, Hescheler J. Thyrotropin-releasing hormone induces opposite effects on Ca2+ channel currents in pituitary cells by two pathways. Proc. Natl. Acad. Sci. USA. 1991;88:10262–10266. doi: 10.1073/pnas.88.22.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Gollasch M, Kleuss C, Hescheler J, Wittig B, Schultz G. Gi2 and protein kinase C are required for thyrotropin-releasing hormone-induced stimulation of voltage-dependent Ca2+ channels in rat pituitary GH3 cells. Proc. Natl. Acad. Sci. USA. 1993;90:6265–6269. doi: 10.1073/pnas.90.13.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Gonzalez DL, Fuchs JL, Droge MH. Distribution of NMDA receptor binding in developing mouse spinal cord. Neurosci. Lett. 1993;151:134–137. doi: 10.1016/0304-3940(93)90004-5. [DOI] [PubMed] [Google Scholar]
- 433.Gotani H, Kuno M, Nakamura F, Matsuura S. Potentiation of excitatory postsynaptic potentials by a metabotropic glutamate receptor agonist (1S,3R-ACPD) in frog spinal motoneurons. Brain Res. 1995;689:281–288. doi: 10.1016/0006-8993(95)00580-j. [DOI] [PubMed] [Google Scholar]
- 434.Graf W, Ezure K. Morphology of vertical canal related second order vestibular neurons in the cat. Exp. Brain Res. 1986;63:35–48. doi: 10.1007/BF00235644. [DOI] [PubMed] [Google Scholar]
- 435.Graham B, Redman SJ. A simulation of action potentials in synaptic boutons during presynaptic inhibition. J. Neurophysiol. 1994;71:538–549. doi: 10.1152/jn.1994.71.2.538. [DOI] [PubMed] [Google Scholar]
- 436.Granit R. The Basis of Motor Control. New York: Academic; 1970. [Google Scholar]
- 437.Granit R, Kernell D, Shortness G. Quantitative aspect of repetitive firing of mammalian motoneurones, caused by injected currents. J. Physiol. (Lond.) 1963;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Greenamyre JT, Young AB, Penny JB. Quantitative autoradiographic distribution of l-[3H]glutamate-binding sites in the rat central nervous system. J. Neurosci. 1984;4:2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- 440.Greensmith L, Vrbova G. Motoneurone survival: a functional approach. Trends Neurosci. 1996;19:450–455. doi: 10.1016/s0166-2236(96)20034-7. [DOI] [PubMed] [Google Scholar]
- 441.Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J. Physiol. (Lond.) 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J. Physiol. (Lond.) 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Greer JJ, Smith JC, Feldman JL. Glutamate release and presynaptic action of AP4 during inspiratory drive to phrenic motoneurons. Brain Res. 1992;576:355–357. doi: 10.1016/0006-8993(92)90705-e. [DOI] [PubMed] [Google Scholar]
- 444.Grelot L, Milano S, Portillo F, Miller AD, Bianchi AL. Membrane potential changes of phrenic motoneurons during fictive vomiting, coughing, and swallowing in the decerebrate cat. J. Neurophysiol. 1992;68:2110–2119. doi: 10.1152/jn.1992.68.6.2110. [DOI] [PubMed] [Google Scholar]
- 445.Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Grifa A, Totaro A, Rommens JM, Carella M, Roetto A, Borgato L, Zelante L, Gasparini P. GABA (gamma-amino-butyric acid) neurotransmission: identification and fine mapping of the human GABAB receptor gene. Biochem. Biophys. Res. Commun. 1998;250:240–245. doi: 10.1006/bbrc.1998.9296. [DOI] [PubMed] [Google Scholar]
- 447.Grillner S, Deliagina T, Ekeberg O, El MA, Hill R, Lansner A, Orlovsky G, Wallen P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 448.Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from the vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Exp. Brain Res. 1971;12:457–479. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- 449.Grillner S, Wallen P, Brodin L, Lansner A. Neuronal network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties, and simulation. Annu. Rev. Neurosci. 1991;14:169–199. doi: 10.1146/annurev.ne.14.030191.001125. [DOI] [PubMed] [Google Scholar]
- 450.Grillner S, Wallen P, Dale N, Brodin L, Buchanan J, Hill R. Transmitters, membrane properties and network circuitry in the control of locomotion in lamprey. Trends Neurosci. 1987;10:34–49. [Google Scholar]
- 451.Grzanna R, Chee W, Akeyson E. Noradrenergic projections to brainstem nuclei: evidence for differential projections from noradrenergic subgroups. J. Comp. Neurol. 1987;263:76–91. doi: 10.1002/cne.902630107. [DOI] [PubMed] [Google Scholar]
- 452.Grzanna R, Fritschy J. Efferent projections of different subpopulations of central noradrenaline neurons. Prog. Brain Res. 1991;88:89–101. doi: 10.1016/s0079-6123(08)63801-7. [DOI] [PubMed] [Google Scholar]
- 453.Guard S, Watson S. Tachykinin receptor subtypes: classification and membrane signalling mechanisms. Neurochem. Int. 1991;18:149–165. doi: 10.1016/0197-0186(91)90180-l. [DOI] [PubMed] [Google Scholar]
- 454.Gueritaud J. Electrical activity of rat ocular motoneurons recorded in vitro. Neuroscience. 1988;24:837–852. doi: 10.1016/0306-4522(88)90072-3. [DOI] [PubMed] [Google Scholar]
- 455.Guerra-Seijas M, Labandeira GJ, Tobio J, Gonzalez F. Neurons located in the trigeminal sensory complex and the lateral pontine tegmentum project to the oculomotor nucleus in the rabbit. Brain Res. 1993;601:1–13. doi: 10.1016/0006-8993(93)91689-p. [DOI] [PubMed] [Google Scholar]
- 456.Gundersen V, Shupliakov O, Brodin L, Ottersen OP, Storm-Mathisen J. Quantification of excitatory amino acid uptake at intact glutamatergic synapses by immunocytochemistry of exogenous d-aspartate. J. Neurosci. 1995;15:4417–4428. doi: 10.1523/JNEUROSCI.15-06-04417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Guo JZ, Yoshioka K, Zhao FY, Hosoki R, Maehara T, Yanagisawa M, Hagan RM, Otsuka M. Pharmacological characterization of GR82334, a tachykinin NK1 receptor antagonist, in the isolated spinal cord of the neonatal rat. Eur. J. Pharmacol. 1995;281:49–54. doi: 10.1016/0014-2999(95)00228-d. [DOI] [PubMed] [Google Scholar]
- 458.Gustafsson B, Pinter M. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J. Physiol. (Lond.) 1984;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Gustafsson B, Pinter M. An investigation of threshold properties among cat spinal alpha-motoneurones. J. Physiol. (Lond.) 1984;357:453–483. doi: 10.1113/jphysiol.1984.sp015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Haji A, Furuichi S, Takeda R. Effects on iontophoretically applied acetylcholine on membrane potential and synaptic activity of bulbar respiratory neurones in decerebrate cats. Neuropharmacology. 1996;35:195–203. doi: 10.1016/0028-3908(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 461.Haji A, Takeda R. Microiontophoresis of baclofen on membrane potential and input resistance in bulbar respiratory neurons in the cat. Brain Res. 1993;622:294–298. doi: 10.1016/0006-8993(93)90832-8. [DOI] [PubMed] [Google Scholar]
- 462.Hamill OP, Bormann J, Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. Nature. 1983;305:805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- 463.Harada Y, Takahashi T. The calcium component of the action potential in spinal motoneurones of the rat. J. Physiol. (Lond.) 1983;335:89–100. doi: 10.1113/jphysiol.1983.sp014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J. Neurophysiol. 1995;74:1404–1420. doi: 10.1152/jn.1995.74.4.1404. [DOI] [PubMed] [Google Scholar]
- 465.Hasegawa Y, Ono H. Effects of 8-OH-DPAT, a 5-HT1A receptor agonist, and DOI, a 5-HT2A/2C agonist, on monosynaptic transmission in spinalized rats. Brain Res. 1996;738:158–161. doi: 10.1016/0006-8993(96)00991-2. [DOI] [PubMed] [Google Scholar]
- 466.Hashim MA, Bieger D. Excitatory amino acid receptor-mediated activation of solitarial deglutitive loci. Neuropharmacology. 1989;28:913–921. doi: 10.1016/0028-3908(89)90190-1. [DOI] [PubMed] [Google Scholar]
- 467.Hayakawa T, Zheng J, Yajima Y. Direct synaptic projections to esophageal motoneurons in the nucleus ambiguus from the nucleus of the solitary tract of the rat. J. Comp. Neurol. 1997;381:18–30. doi: 10.1002/(sici)1096-9861(19970428)381:1<18::aid-cne2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 468.Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- 469.Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol. Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 470.Headley PM, Parsons CG, West DC. The role of N-methylaspartate receptors in mediating responses of rat and cat spinal neurones to defined sensory stimuli. J. Physiol. (Lond.) 1987;385:169–188. doi: 10.1113/jphysiol.1987.sp016490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Helke C, Sayson S, Keeler J, Charlton C. Thyrotropin-releasing hormone-immunoreactive neurons project from the ventral medulla to the intermediolateral cell column: partial coexistence with serotonin. Brain Res. 1986;381:1–7. doi: 10.1016/0006-8993(86)90682-7. [DOI] [PubMed] [Google Scholar]
- 472.Helke CJ, Krause JE, Mantyh PW, Couture R, Bannon MJ. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. [PubMed] [Google Scholar]
- 473.Helke CJ, Neil JJ, Massari VJ, Loewy AD. Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res. 1982;243:147–152. doi: 10.1016/0006-8993(82)91128-3. [DOI] [PubMed] [Google Scholar]
- 474.Henkel C, Edwards S. The superior colliculus control of pinna movements in the cat: possible anatomical connections. J. Comp. Neurol. 1978;182:763–776. doi: 10.1002/cne.901820502. [DOI] [PubMed] [Google Scholar]
- 475.Henneman E, Mendell L. Handbook of Physiology. The Nervous System. Motor Systems. Bethesda, MD: Am. Physiol. Soc; 1981. Functional organization of motoneuron pool and its inputs; pp. 423–214. sect. I, vol. II, pt. 1, chapt. 11, [Google Scholar]
- 476.Hennig R, Lomo T. Gradation of force output in normal fast and slow muscles of the rat. Acta Physiol. Scand. 1987;130:133–142. doi: 10.1111/j.1748-1716.1987.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 477.Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypglossal nucleus in the rat. J. Comp. Neurol. 1998;391:491–505. [PubMed] [Google Scholar]
- 478.Herlitze S, Raditsch M, Ruppersberg JP, Jahn W, Monyer H, Schoepfer R, Witzemann V. Argiotoxin detects molecular differences in AMPA receptor channels. Neuron. 1993;10:1131–1140. doi: 10.1016/0896-6273(93)90061-u. [DOI] [PubMed] [Google Scholar]
- 479.Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 480.Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- 481.Hille B. Ionic Channels of Excitable Membranes. 2nd. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 482.Hinrichsen C, Watson C. Brain stem projections to the facial nucleus of the rat. Brain Behav. Evol. 1983;22:153–163. doi: 10.1159/000121514. [DOI] [PubMed] [Google Scholar]
- 483.Hironaka T, Morita Y, Hagihira S, Tateno E, Kita H, Tohyama M. Localization of GABAA-receptor alpha 1 subunit mRNA-containing neurons in the lower brainstem of the rat. Brain Res. 1990;7:335–345. doi: 10.1016/0169-328x(90)90083-p. [DOI] [PubMed] [Google Scholar]
- 484.Hirsch MD, Helke CJ. Bulbospinal thyrotropin-releasing hormone projections to the intermediolateral cell column: a double fluorescence immunohistochemical-retrograde tracing study in the rat. Neuroscience. 1988;25:625–637. doi: 10.1016/0306-4522(88)90264-3. [DOI] [PubMed] [Google Scholar]
- 485.Hishikawa Y, Shimizu T. Physiology of REM sleep, cataplexy, and sleep paralysis. Adv. Neurol. 1995;67:245–271. [PubMed] [Google Scholar]
- 486.Hochman S, Jordan LM, Schmidt BJ. TTX-resistant NMDA-receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J. Neurophysiol. 1994;72:2559–2562. doi: 10.1152/jn.1994.72.5.2559. [DOI] [PubMed] [Google Scholar]
- 487.Hockfield S, Kalb RG. Activity-dependent structural changes during neuronal development. Curr. Opin. Neurobiol. 1993;3:87–92. doi: 10.1016/0959-4388(93)90040-6. [DOI] [PubMed] [Google Scholar]
- 488.Hoffer J, Sugano N, Loeb G, Marks W, O’Donovan M, Pratt C. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J. Neurophysiol. 1987;57:530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- 489.Hökfelt T, Fuxe K, Johansson O, Jeffcoate S, White N. Distribution of thyrotropin-releasing hormone (TRH) in the central nervous system as revealed with immunohistochemistry. Eur. J. Pharmacol. 1975;34:389–392. doi: 10.1016/0014-2999(75)90269-1. [DOI] [PubMed] [Google Scholar]
- 490.Hökfelt T, Johansson O, Goldstein M. Central catecholamine neuron as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Hokfelt T, Bjorklund A, editors. Classical Transmitters in the CNS. Amsterdam: Elsevier; 1984. pp. 157–276. [Google Scholar]
- 491.Hökfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225:1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- 492.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975;100:235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- 493.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Substance P: localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 494.Hökfelt T, Ljungdahl A. Light and electron microscopic autoradiography on spinal cord slices after incubation with labeled glycine. Brain Res. 1971;32:189–194. doi: 10.1016/0006-8993(71)90163-6. [DOI] [PubMed] [Google Scholar]
- 495.Hökfelt T, Ljungdahl A, Steinbusch H, Verhofstad A, Nilsson G, Brodin E, Pernow B, Goldstein M. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3:517–538. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- 496.Hökfelt T, Millhorn D, Seroogy K, Tsuruo Y, Ceccatelli S, Lindh B, Meister B, Melander T, Schalling M, Bartfai T. Coexistence of peptides with classical neurotransmitters. Experientia. 1987;43:768–780. doi: 10.1007/BF01945354. [DOI] [PubMed] [Google Scholar]
- 497.Hökfelt T, Terenius L, Kuypers H, Dann O. Evidence for enkephalin immunoreactive neurons in the medulla oblongata projecting to the spinal cord. Neurosci. Lett. 1979;14:55–60. doi: 10.1016/0304-3940(79)95343-6. [DOI] [PubMed] [Google Scholar]
- 498.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 499.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 500.Holstege G. Anatomical evidence for an ipsilateral rubrospinal pathway and for direct rubrospinal projections to motoneurons in the cat. Neurosci. Lett. 1987;74:269–274. doi: 10.1016/0304-3940(87)90308-9. [DOI] [PubMed] [Google Scholar]
- 501.Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J. Comp. Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- 502.Holstege G. Descending motor pathways and the spinal motor system: limbic and non-limbic components. Prog. Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- 503.Holstege G, Kuypers HG, Boer RC. Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res. 1979;171:329–333. doi: 10.1016/0006-8993(79)90337-8. [DOI] [PubMed] [Google Scholar]
- 504.Holstege JC. Ultrastructural evidence for GABAergic brain stem projections to spinal motoneurons in the rat. J. Neurosci. 1991;11:159–167. doi: 10.1523/JNEUROSCI.11-01-00159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Holstege JC. The ventro-medial medullary projections to spinal motoneurons: ultrastructure, transmitters and functional aspects. Prog. Brain Res. 1996;107:159–181. doi: 10.1016/s0079-6123(08)61864-6. [DOI] [PubMed] [Google Scholar]
- 506.Holstege JC, Bongers C. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res. 1991;566:308–315. doi: 10.1016/0006-8993(91)91715-d. [DOI] [PubMed] [Google Scholar]
- 507.Holstege JC, Kuypers HG. Brainstem projections to lumbar motoneurons in rat. I. An ultrastructural study using autoradiography and the combination of autoradiography and horseradish peroxidase histochemistry. Neuroscience. 1987;21:345–367. doi: 10.1016/0306-4522(87)90126-6. [DOI] [PubMed] [Google Scholar]
- 508.Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 509.Holstege JC, van Dijken DH, Buijs R, Goedknegt H, Gosens T, Bongers C. Distribution of dopamine immunoreactivity in the rat, cat and monkey spinal cord. J. Comp. Neurol. 1996;376:631–652. doi: 10.1002/(SICI)1096-9861(19961223)376:4<631::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 510.Holstege JC, Calkoen F. The distribution of GABA in lumbar motoneuronal cell groups. A quantitative ultrastructural study in rat. Brain Res. 1990;530:130–137. doi: 10.1016/0006-8993(90)90669-3. [DOI] [PubMed] [Google Scholar]
- 511.Holtman JR, Jr, Dick TE, Berger AJ. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J. Neurosci. 1986;6:1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Holtman JR, Jr, Dick TE, Berger AJ. Serotonin-mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of the raphe obscurus. Brain Res. 1987;417:12–20. doi: 10.1016/0006-8993(87)90174-0. [DOI] [PubMed] [Google Scholar]
- 513.Hongo T, Kitazawa S, Ohki Y, Sasaki M, Xi M. A physiological and morphological study of premotor interneurones in the cutaneous reflex pathways in cats. Brain Res. 1989;505:163–166. doi: 10.1016/0006-8993(89)90131-5. [DOI] [PubMed] [Google Scholar]
- 514.Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competetive non-NMDA glutamate receptor antagonists. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 515.Hoover J, Durkovic R. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens. Motor Res. 1992;9:211–226. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- 516.Hori Y, Kanda K. Developmental alterations in NMDA receptor-mediated currents in neonatal rat spinal motoneurons. Neurosci. Lett. 1996;205:99–102. doi: 10.1016/0304-3940(96)12388-0. [DOI] [PubMed] [Google Scholar]
- 517.Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O’Hara PJ, Mulvihill ER, Almers W, Hagen FS. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 518.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp. Brain Res. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- 519.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J. Physiol. (Lond.) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Hounsgaard J, Hultborn H, Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Prog. Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- 521.Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J. Physiol. (Lond.) 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J. Physiol. (Lond.) 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J. Physiol. (Lond.) 1988;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J. Physiol. (Lond.) 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Hsiao CF, Chandler SH. Characteristics of a fast transient outward current in guinea pig trigeminal motoneurons. Brain Res. 1995;695:217–226. doi: 10.1016/0006-8993(95)00796-s. [DOI] [PubMed] [Google Scholar]
- 526.Hsiao CF, Trueblood P, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J. Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- 527.Hsiao CF, del Negro CA, Trueblood P, Chandler SH. The ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J. Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- 528.Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 529.Huisman A, Ververs B, Cavada C, Kuypers H. Collateralization of brainstem pathways in the spinal ventral horn in rat as demonstrated with the retrograde fluorescent double-labeling technique. Brain Res. 1984;300:362–367. doi: 10.1016/0006-8993(84)90847-3. [DOI] [PubMed] [Google Scholar]
- 530.Humphrey PP, Buell G, Kennedy I, Khakh BS, Michel AD, Surprenant A, Trezise DJ. New insights on P2x purinoceptors. Naunyn-Schmiedebergs Arch. Pharmacol. 1995;352:585–596. doi: 10.1007/BF00171316. [DOI] [PubMed] [Google Scholar]
- 531.Hunt SP, Lovick TA. The distribution of serotonin, met-enkephalin and beta-lipotropin-like immunoreactivity in neuronal perikarya of the cat brainstem. Neurosci. Lett. 1982;30:139–145. doi: 10.1016/0304-3940(82)90286-5. [DOI] [PubMed] [Google Scholar]
- 532.Ichikawa T, Shimizu T. Organization of choline acetyltransferase-containing structures in the cranial nerve motor nuclei and spinal cord of the monkey. Brain Res. 1998;779:96–103. doi: 10.1016/s0006-8993(97)01090-1. [DOI] [PubMed] [Google Scholar]
- 533.Ichikawa Y, Terakado Y, Yamaguchi T. Last-order interneurones controlling activity of elbow extensor motoneurones during forelimb fictive locomotion in the cat. Neurosci. Lett. 1991;121:37–39. doi: 10.1016/0304-3940(91)90643-8. [DOI] [PubMed] [Google Scholar]
- 534.Illert M, Lundberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3-C4 propriospinal neurones. Exp. Brain Res. 1978;33:101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- 535.Inagaki M, Takeshita K, Nakao S, Shiraishi Y, Oikawa T. An electrophysiologically defined trigemino-reticulo-facial pathway related to the blink reflex in the cat. Neurosci. Lett. 1989;96:64–69. doi: 10.1016/0304-3940(89)90244-9. [DOI] [PubMed] [Google Scholar]
- 536.Ingi T, Kitajima Y, Minamitake Y, Nakanishi S. Characterization of ligand-binding properties and selectivities of three rat tachykinin receptors by transfection and functional expression of their cloned cDNAs in mammalian cells. J. Pharmacol. Exp. Ther. 1991;259:968–975. [PubMed] [Google Scholar]
- 537.Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J. Physiol. (Lond.) 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Inoue T, Chandler SH, Goldberg LJ. Neuropharmacological mechanisms underlying rhythmical discharge in trigeminal interneurons during fictive mastication. J. Neurophysiol. 1994;71:2061–2073. doi: 10.1152/jn.1994.71.6.2061. [DOI] [PubMed] [Google Scholar]
- 539.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 540.Ishida M, Akagi H, Shimamoto K, Ohfune Y, Shinozaki H. A potent metabotropic glutamate receptor agonist: electrophysiological actions of a conformationally restricted glutamate analogue in the rat spinal cord and Xenopus oocytes. Brain Res. 1990;537:311–314. doi: 10.1016/0006-8993(90)90375-l. [DOI] [PubMed] [Google Scholar]
- 541.Ishida M, Saitoh T, Shimamoto K, Ohfune Y, Shinozaki H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the newborn rat isolated spinal cord. Br. J. Pharmacol. 1993;109:1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J. Biol. Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 543.Ishizuka N, Mannen H, Hongo T, Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J. Comp. Neurol. 1979;186:189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- 544.Isokawa-Akesson M, Komisaruk B. Difference in projections to the lateral and medial facial nucleus: anatomically separate pathways for rhythmical vibrissa movement in rats. Exp. Brain Res. 1987;65:385–398. doi: 10.1007/BF00236312. [DOI] [PubMed] [Google Scholar]
- 545.Isu N, Yokota J. Morphophysiological study on the divergent projection of axon collaterals of medial vestibular nucleus neurons in the cat. Exp. Brain Res. 1983;53:151–162. doi: 10.1007/BF00239407. [DOI] [PubMed] [Google Scholar]
- 546.Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc. Natl. Acad. Sci. USA. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Iversen LL, Bloom FE. Studies of the uptake of [3H]GABA and [3H]glycine in slices and homogenates of rat brain and spinal cord by electron microscopic autoradiography. Brain Res. 1972;41:131–143. doi: 10.1016/0006-8993(72)90621-x. [DOI] [PubMed] [Google Scholar]
- 548.Jack J, Noble D, Tsien R. Electric Current Flow in Excitable Cells. Oxford, UK: Clarendon; 1975. [Google Scholar]
- 549.Jack JJB, Redman SJ, Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J. Physiol. (Lond.) 1981;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Jackisch R, Strittmatter H, Kasakov L, Hertting G. Endogenous adenosine as a modulator of hippocampal acetylcholine release. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;327:319–325. doi: 10.1007/BF00506243. [DOI] [PubMed] [Google Scholar]
- 551.Jackson IM, Reichlin S. Brain thyrotrophin-releasing hormone is independent of the hypothalamus. Nature. 1977;267:853–854. doi: 10.1038/267853a0. [DOI] [PubMed] [Google Scholar]
- 552.Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- 553.Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog. Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 554.Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog. Brain Res. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- 555.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 556.Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol. Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- 557.Jahr CE, Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J. Physiol. (Lond.) 1986;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995;67:893–907. doi: 10.1016/0306-4522(95)00026-f. [DOI] [PubMed] [Google Scholar]
- 559.Jakowec MW, Yen L, Kalb RG. In situ hybridization analysis of AMPA receptor subunit gene expression in the developing rat spinal cord. Neuroscience. 1995;67:909–920. doi: 10.1016/0306-4522(95)00094-y. [DOI] [PubMed] [Google Scholar]
- 560.Jane D, Jones P, Pook P, Salt T, Sunter D, Watkins J. Stereospecific antagonism by (−)-alpha-methyl-4-carboxyphenylglycine (MCPG) of (1S,3R)-ACPD-induced effects in neonatal rat motoneurones and rat thalamic neurones. Neuropharmacology. 1993;32:725–727. doi: 10.1016/0028-3908(93)90088-k. [DOI] [PubMed] [Google Scholar]
- 561.Jane D, Jones P, Pook P, Tse H, Watkins J. Actions of two new antagonists showing selectivity for different subtypes of metabotropic glutamate receptor in the neonatal rat spinal cord. Br. J. Pharmacol. 1994;112:809–816. doi: 10.1111/j.1476-5381.1994.tb13151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Jane D, Pittaway K, Sunter D, Thomas N, Watkins J. New phenylglycine derivatives with potent and selective antagonist activity at presynaptic glutamate receptors in neonatal rat spinal cord. Neuropharmacology. 1995;34:851–856. doi: 10.1016/0028-3908(95)00063-c. [DOI] [PubMed] [Google Scholar]
- 563.Jankowska E, Noga B. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- 564.Jankowska E, Riddell J, Skoog B, Noga B. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J. Physiol. (Lond.) 1993;461:705–722. doi: 10.1113/jphysiol.1993.sp019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Jansen KL, Faull RL, Dragunow M, Waldvogel H. Autoradiographic localisation of NMDA, quisqualate and kainic acid receptors in human spinal cord. Neurosci. Lett. 1990;108:53–57. doi: 10.1016/0304-3940(90)90705-e. [DOI] [PubMed] [Google Scholar]
- 566.Jasmin L, Carstens E, Basbaum A. Interneurons presynaptic to rat tail-flick motoneurons as mapped by transneuronal transport of pseudorabies virus: few have long ascending collaterals. Neuroscience. 1997;76:859–876. doi: 10.1016/s0306-4522(96)00384-3. [DOI] [PubMed] [Google Scholar]
- 567.Jessell T, Tsunoo A, Kanazawa I, Otsuka M. Substance P: depletion in the dorsal horn of rat spinal cord after section of the peripheral processes of primary sensory neurons. Brain Res. 1979;168:247–259. doi: 10.1016/0006-8993(79)90167-7. [DOI] [PubMed] [Google Scholar]
- 568.Jessell TM, Yoshioka K, Jahr CE. Amino acid receptor-mediated transmission at primary afferent synapses in rat spinal cord. J. Exp. Biol. 1986;124:239–258. doi: 10.1242/jeb.124.1.239. [DOI] [PubMed] [Google Scholar]
- 569.Jiang C, Mitchell GS, Lipski J. Prolonged augmentation of respiratory discharge in hypoglossal motoneurons following superior laryngeal nerve stimulation. Brain Res. 1991;538:215–225. doi: 10.1016/0006-8993(91)90433-v. [DOI] [PubMed] [Google Scholar]
- 570.Jiang ZG, Shen E, Dun NJ. Excitatory and inhibitory transmission from dorsal root afferents to neonate rat motoneurons in vitro. Brain Res. 1990;535:110–118. doi: 10.1016/0006-8993(90)91829-6. [DOI] [PubMed] [Google Scholar]
- 571.Jiang ZG, Shen E, Wang MY, Dun NJ. Excitatory postsynaptic potentials evoked by ventral root stimulation in neonate rat motoneurons in vitro. J. Neurophysiol. 1991;65:57–66. doi: 10.1152/jn.1991.65.1.57. [DOI] [PubMed] [Google Scholar]
- 572.Jimenez I, Rudomin P, Enriquez M. Differential effects of (−)-baclofen on Ia and descending monosynaptic EPSPs. Exp. Brain Res. 1991;85:103–113. doi: 10.1007/BF00229991. [DOI] [PubMed] [Google Scholar]
- 573.Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J. Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- 574.Johansson O, Hökfelt T, Elde R. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience. 1984;13:265–339. doi: 10.1016/0306-4522(84)90233-1. [DOI] [PubMed] [Google Scholar]
- 575.Johansson O, Hökfelt T, Jeffcoate S, White N, Sternberger L. Ultrastructural localization of TRH-like immunoreactivity. Exp. Brain Res. 1980;38:1–10. doi: 10.1007/BF00237924. [DOI] [PubMed] [Google Scholar]
- 576.Johansson O, Hökfelt T, Pernow B, Jeffcoate S, White N, Steinbusch H, Verhofstad A, Emson P, Spindel E. Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P- and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience. 1981;6:1857–1881. doi: 10.1016/0306-4522(81)90028-2. [DOI] [PubMed] [Google Scholar]
- 577.Johnson SM, Getting PA. Electrophysiological properties of neurons within the nucleus ambiguus of adult guinea pigs. J. Neurophysiol. 1991;66:744–761. doi: 10.1152/jn.1991.66.3.744. [DOI] [PubMed] [Google Scholar]
- 578.Johnston GA. GABAC receptors. Prog. Brain Res. 1994;100:61–65. [PubMed] [Google Scholar]
- 579.Johnston GA. GABAA receptor pharmacology. Pharmacol. Ther. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 580.Johnston GA. GABAC receptors: relatively simple transmittergated ion channels? Trends Pharmacol. Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- 581.Johnston GAR. Molecular biology, pharmacology, and physiology of GABAC receptors. In: Enna SJ, Bowery NG, editors. The GABA Receptors. Totowa, NJ: Humana; 1997. pp. 297–323. [Google Scholar]
- 582.Jonas P, Bischofberger J, Sandkuhler J. Core-lease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 583.Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 584.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 585.Jones B, Yang T. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- 586.Jones KA. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 587.Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J. Comp. Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- 588.Jones LS, Gauger LL, Davis JN, Slotkin TA, Bartolome JV. Postnatal development of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]HEAT in normal rats and rats treated with alpha-difluoromethylornithine, a specific, irreversible inhibitor of ornithine decarboxylase. Neuroscience. 1985;15:1195–1202. doi: 10.1016/0306-4522(85)90262-3. [DOI] [PubMed] [Google Scholar]
- 589.Jonzon B, Fredholm BB. Adenosine receptor mediated inhibition of noradrenaline release from slices of the rat hippocampus. Life Sci. 1984;35:1971–1979. doi: 10.1016/0024-3205(84)90478-8. [DOI] [PubMed] [Google Scholar]
- 590.Jordan LM, Brownstone RM, Noga BR. Control of functional systems in the brainstem and spinal cord. Curr. Opin. Neurobiol. 1992;2:794–801. doi: 10.1016/0959-4388(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 591.Jurgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- 592.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 593.Kakizaki H, Yoshiyama M, Roppolo JR, Booth AM, De Groat WC. Role of spinal glutamatergic transmission in the ascending limb of the micturition reflex pathway in the rat. J. Pharmacol. Exp. Ther. 1998;285:22–27. [PubMed] [Google Scholar]
- 594.Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- 595.Kalb RG, Fox AJ. Synchronized overproduction of AMPA, kainate, and NMDA glutamate receptors during human spinal cord development. J. Comp. Neurol. 1997;384:200–210. doi: 10.1002/(sici)1096-9861(19970728)384:2<200::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 596.Kalb RG, Hockfield S. Molecular evidence for early activity-dependent development of hamster motor neurons. J. Neurosci. 1988;8:2350–2360. doi: 10.1523/JNEUROSCI.08-07-02350.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Kalb RG, Hockfield S. Induction of a neuronal proteoglycan by the NMDA receptor in the developing spinal cord. Science. 1990;250:294–296. doi: 10.1126/science.2145629. [DOI] [PubMed] [Google Scholar]
- 598.Kalb RG, Hockfield S. Large diameter primary afferent input is required for expression of the Cat-301 proteoglycan on the surface of motor neurons. Neuroscience. 1990;34:391–401. doi: 10.1016/0306-4522(90)90148-w. [DOI] [PubMed] [Google Scholar]
- 599.Kalb RG, Hockfield S. Activity-dependent development of spinal cord motor neurons. Brain Res. 1992;17:283–289. doi: 10.1016/0165-0173(92)90020-m. [DOI] [PubMed] [Google Scholar]
- 600.Kalb RG, Hockfield S. Electrical activity in the neuromuscular unit can influence the molecular development of motor neurons. Dev. Biol. 1994;162:539–548. doi: 10.1006/dbio.1994.1107. [DOI] [PubMed] [Google Scholar]
- 601.Kalb RG, Lidow MS, Halsted MJ, Hockfield S. N-methyl-d-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc. Natl. Acad. Sci. USA. 1992;89:8502–8506. doi: 10.1073/pnas.89.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Kamatchi GL, Ticku MK. Functional coupling of presynaptic GABAB receptors with voltage-gated Ca2+ channel: regulation by protein kinases A and C in cultured spinal cord neurons. Mol. Pharmacol. 1990;38:342–347. [PubMed] [Google Scholar]
- 603.Kanazawa I, Sutoo D, Oshima I, Saito S. Effect of transection on choline acetyltransferase, thyrotropin releasing hormone and substance P in the cat cervical cord. Neurosci. Lett. 1979;13:325–330. doi: 10.1016/0304-3940(79)91514-3. [DOI] [PubMed] [Google Scholar]
- 604.Kangrga I, Jiang MC, Randic M. Actions of (−)-baclofen on rat dorsal horn neurons. Brain Res. 1991;562:265–275. doi: 10.1016/0006-8993(91)90630-e. [DOI] [PubMed] [Google Scholar]
- 605.Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2x2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J. Comp. Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- 606.Kardos J. The GABAA receptor channel mediated chloride ion translocation through the plasma membrane: new insights from 36Cl− ion flux measurements. Synapse. 1993;13:74–93. doi: 10.1002/syn.890130110. [DOI] [PubMed] [Google Scholar]
- 607.Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J. Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Katakura N, Chandler SH. An iontophoretic analysis of the pharmacologic mechanisms responsible for trigeminal motoneuronal discharge during masticatory-like activity in the guinea pig. J. Neurophysiol. 1990;63:356–369. doi: 10.1152/jn.1990.63.2.356. [DOI] [PubMed] [Google Scholar]
- 609.Katakura N, Chandler SH. Iontophoretic analysis of the pharmacologic mechanisms responsible for initiation and modulation of trigeminal motoneuronal discharge evoked by intra-oral afferent stimulation. Brain Res. 1991;549:66–77. doi: 10.1016/0006-8993(91)90600-z. [DOI] [PubMed] [Google Scholar]
- 611.Kato M, Waldmann U, Murakami S. Effects of baclofen on spinal neurones of cats. Neuropharmacology. 1978;17:827–833. doi: 10.1016/0028-3908(78)90071-0. [DOI] [PubMed] [Google Scholar]
- 612.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 613.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 614.Kawagoe R, Onodera K, Takeuchi A. The release of endogenous glutamate from the newborn rat spinal cord induced by dorsal root stimulation and substance P. Biomed. Res. 1986;7:253–259. [Google Scholar]
- 615.Kawaguchi Y, Hoshimaru M, Nawa H, Nakanishi S. Sequence analysis of cloned cDNA for rat substance P precursor: existence of a third substance P precursor. Biochem. Biophys. Res. Commun. 1986;139:1040–1046. doi: 10.1016/s0006-291x(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 616.Keller BU, Blaschke M, Rivosecchi R, Hollmann M, Heinemann SF, Konnerth A. Identification of a subunit-specific antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate/kainate receptor channels. Proc. Natl. Acad. Sci. USA. 1993;90:605–609. doi: 10.1073/pnas.90.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Keller BU, Hollmann M, Heinemann S, Konnerth A. Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO J. 1992;11:891–896. doi: 10.1002/j.1460-2075.1992.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Kemp M, Roberts P, Pook P, Jane D, Jones A, Jones P, Sunter D, Udvarhelyi P, Watkins J. Antagonism of presynaptically mediated depressant responses and cyclic AMP-coupled metabotropic glutamate receptors. Eur. J. Pharmacol. 1994;266:187–192. doi: 10.1016/0922-4106(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 619.Kerkut GA. The development of isolated central nervous system preparations. Comp. Biochem. Physiol. C Pharmacol. 1982;72:161–169. doi: 10.1016/0306-4492(82)90080-6. [DOI] [PubMed] [Google Scholar]
- 620.Kerkut GA. Studying the isolated central nervous system; a report on 35 years: more inquisitive than acquisitive. Comp. Biochem. Physiol. A Physiol. 1989;93:9–24. doi: 10.1016/0300-9629(89)90187-4. [DOI] [PubMed] [Google Scholar]
- 621.Kerkut GA, Bagust J. The isolated mammalian spinal cord. Prog. Neurobiol. 1995;46:1–48. doi: 10.1016/0301-0082(94)00055-m. [DOI] [PubMed] [Google Scholar]
- 622.Kernell D. The adaptation and the relation between discharge frequency and current strenght of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol. Scand. 1965;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 623.Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol. Scand. 1965;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 624.Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol. Scand. 1965;65:87–100. [Google Scholar]
- 625.Kernell D, Monster A. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp. Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- 626.Kerr DI, Ong J. GABAB receptors. Pharmacol. Ther. 1995;67:187–246. doi: 10.1016/0163-7258(95)00016-a. [DOI] [PubMed] [Google Scholar]
- 627.Kessler JP, Cherkaoui N, Catalin D, Jean A. Swallowing responses induced by microinjection of glutamate and glutamate agonists into the nucleus tractus solitarius of ketamine-anesthetized rats. Exp. Brain Res. 1990;83:151–158. doi: 10.1007/BF00232203. [DOI] [PubMed] [Google Scholar]
- 628.Kessler JP, Jean A. Evidence that activation of N-methyl-d-aspartate (NMDA) and non-NMDA receptors within the nucleus tractus solitarii triggers swallowing. Eur. J. Pharmacol. 1991;201:59–67. doi: 10.1016/0014-2999(91)90323-i. [DOI] [PubMed] [Google Scholar]
- 629.Kheck N, Gannon P, Azmitia E. 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J. Comp. Neurol. 1995;355:211–220. doi: 10.1002/cne.903550205. [DOI] [PubMed] [Google Scholar]
- 630.Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. Localization of P2x purinoceptor transcripts in the rat nervous system. Mol. Pharmacol. 1995;48:569–573. [PubMed] [Google Scholar]
- 631.Kidokoro Y, Kubota K, Shuto S, Sumino R. Reflex organization of cat masticatory muscles. J. Neurophysiol. 1968;31:695–708. doi: 10.1152/jn.1968.31.5.695. [DOI] [PubMed] [Google Scholar]
- 632.Kiehn O. Plateau potentials and active integration in the “final common pathway” for motor behaviour. Trends Neurosci. 1991;14:68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- 633.Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J. Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- 634.Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr. Opin. Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- 635.Kiehn O, Erdal J, Eken T, Bruhn T. Selective depletion of spinal monoamines changes the rat soleus EMG from a tonic to a more phasic pattern. J. Physiol. (Lond.) 1996;492:173–184. doi: 10.1113/jphysiol.1996.sp021299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Kim YI, Chandler SH. NMDA-induced burst discharge in guinea pig trigeminal motoneurons in vitro. J. Neurophysiol. 1995;74:334–346. doi: 10.1152/jn.1995.74.1.334. [DOI] [PubMed] [Google Scholar]
- 637.King AE, Lopez-Garcia JA, Cumberbatch M. Antagonism of synaptic potentials in ventral horn neurones by 6-cyano-7-nitroquinoxaline-2,3-dione: a study in the rat spinal cord in vitro. Br. J. Pharmacol. 1992;107:375–381. doi: 10.1111/j.1476-5381.1992.tb12754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Kinoshita A, Ohishi H, Nomura S, Shigemoto R, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR4a, in the cerebellar cortex: a light and electron microscope study in the rat. Neurosci. Lett. 1996;207:199–202. doi: 10.1016/0304-3940(96)12519-2. [DOI] [PubMed] [Google Scholar]
- 639.Kirkwood P, Munson J. The incidence of initial doublets in the discharges of motoneurones of two different inspiratory muscles in the cat. J. Physiol. (Lond.) 1996;493:577–587. doi: 10.1113/jphysiol.1996.sp021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J. Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Kirsch J, Langosch D, Prior P, Littauer UZ, Schmitt B, Betz H. The 93-kDa glycine receptor-associated protein binds to tubulin. J. Biol. Chem. 1991;266:22242–22245. [PubMed] [Google Scholar]
- 642.Kirsch J, Malosio ML, Wolters I, Betz H. Distribution of gephyrin transcripts in the adult and developing rat brain. Eur. J. Neurosci. 1993;5:1109–1117. doi: 10.1111/j.1460-9568.1993.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 643.Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 644.Kjaerulff O, Kiehn O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J. Neurosci. 1997;17:9433–9447. doi: 10.1523/JNEUROSCI.17-24-09433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Klooster J, Beckers H, Vrensen G, van der Want J. The peripheral and central projections of the Edinger-Westphal nucleus in the rat. A light and electron microscopic tracing study. Brain Res. 1993;632:260–273. doi: 10.1016/0006-8993(93)91161-k. [DOI] [PubMed] [Google Scholar]
- 646.Klooster J, Vrensen G, Muller L, van der Want J. Efferent projections of the olivary pretectal nucleus in the albino rat subserving the pupillary light reflex and related reflexes. A light microscopic tracing study. Brain Res. 1995;688:34–46. doi: 10.1016/0006-8993(95)00497-e. [DOI] [PubMed] [Google Scholar]
- 647.Kobayashi M, Inoue T, Matsuo R, Masuda Y, Hidaka O, Kang Y, Morimoto T. Role of calcium conductances on spike afterpotentials in rat trigeminal motoneurons. J. Neurophysiol. 1997;77:3273–3283. doi: 10.1152/jn.1997.77.6.3273. [DOI] [PubMed] [Google Scholar]
- 648.Kogo M, Funk GD, Chandler SH. Rhythmical oralmotor activity recorded in an in vitro brainstem preparation. Somatosens. Motor Res. 1996;13:39–48. doi: 10.3109/08990229609028910. [DOI] [PubMed] [Google Scholar]
- 649.Kohlmeier KA, Lopez-Rodriguez F, Chase MH. Strychnine blocks inhibitory postsynaptic potentials elicited in masseter motoneurons by sensory stimuli during carbachol-induced motor atonia. Neuroscience. 1997;78:1195–1202. doi: 10.1016/s0306-4522(96)00627-6. [DOI] [PubMed] [Google Scholar]
- 650.Kohlmeier KA, Lopez-Rodriguez F, Liu RH, Morales FR, Chase MH. State-dependent phenomena in cat masseter motoneurons. Brain Res. 1996;722:30–38. doi: 10.1016/0006-8993(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 651.Kohlmeier KA, Lopez-Rodriguez F, Morales FR, Chase MH. Relationship between sensory stimuli-elicited IPSPs in motoneurons and PGO waves during cholinergically induced muscle atonia. J. Neurophysiol. 1997;78:2145–2155. doi: 10.1152/jn.1997.78.4.2145. [DOI] [PubMed] [Google Scholar]
- 652.Kohyama J, Shimohira M, Iwakawa Y. Brainstem control of phasic muscle activity during REM sleep: a review and hypothesis. Brain Dev. 1994;16:81–91. doi: 10.1016/0387-7604(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 653.Kohzuki M, Chai S, Paxinos G, Karavas A, Casley D, Johnston C, Mendelsohn F. Localization and characterization of endothelin receptor binding sites in the rat brain visualized by in vitro autoradiography. Neuroscience. 1991;42:245–260. doi: 10.1016/0306-4522(91)90162-h. [DOI] [PubMed] [Google Scholar]
- 654.Kokkoroyannis T, Scudder C, Balaban C, Highstein S, Moschovakis A. Anatomy and physiology of the primate interstitial nucleus of Cajal. I. Efferent projections. J. Neurophysiol. 1996;75:725–739. doi: 10.1152/jn.1996.75.2.725. [DOI] [PubMed] [Google Scholar]
- 655.Kolaj M, Cerne R, Cheng G, Brickey DA, Randic M. Alpha subunit of calcium/calmodulin-dependent protein kinase enhances excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. J. Neurophysiol. 1994;72:2525–2531. doi: 10.1152/jn.1994.72.5.2525. [DOI] [PubMed] [Google Scholar]
- 656.Kolta A. In vitro investigation of synaptic relations between interneurons surrounding the trigeminal motor nucleus and masseteric motoneurons. J. Neurophysiol. 1997;78:1720–1725. doi: 10.1152/jn.1997.78.3.1720. [DOI] [PubMed] [Google Scholar]
- 657.Kolta A, Dubuc R, Lund J. An immunocytochemical and autoradiographic investigation of the serotoninergic innervation of trigeminal mesencephalic and motor nuclei in the rabbit. Neuroscience. 1993;53:1113–1126. doi: 10.1016/0306-4522(93)90494-z. [DOI] [PubMed] [Google Scholar]
- 658.Koltchine VV, Anantharam V, Bayley H, Treistman SN. Alternative splicing of the NMDAR1 subunit affects modulation by calcium. Brain Res. 1996;39:99–108. doi: 10.1016/0169-328x(96)00012-5. [DOI] [PubMed] [Google Scholar]
- 659.Kondo M, Fujiwara H, Tanaka C. Autoradiographic evidence for dopaminergic innervation in guinea pig spinal cord. Jpn. J. Pharmacol. 1985;38:442–444. doi: 10.1254/jjp.38.442. [DOI] [PubMed] [Google Scholar]
- 660.Konishi S, Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974;252:734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- 661.Konnerth A, Keller BU, Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflügers Arch. 1990;417:285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- 662.Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr. Opin. Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 663.Kotani H, Hoshimaru M, Nawa H, Nakanishi S. Structure and gene organization of bovine neuromedin K precursor. Proc. Natl. Acad. Sci. USA. 1986;83:7074–7078. doi: 10.1073/pnas.83.18.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Krammer E, Rath T, Lischka M. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res. 1979;170:533–537. doi: 10.1016/0006-8993(79)90970-3. [DOI] [PubMed] [Google Scholar]
- 665.Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc. Natl. Acad. Sci. USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Krnjevic K, Lamour Y, MacDonald J, Nistri A. Effects of some divalent cations on motoneurones in cats. Can. J. Physiol. Pharmacol. 1979;57:944–956. doi: 10.1139/y79-144. [DOI] [PubMed] [Google Scholar]
- 667.Krnjevic K, Puil E, Werman R. Evidence for Ca2+-activated K+ conductance in cat spinal motoneurons from intracellular EGTA injections. Can. J. Physiol. Pharmacol. 1975;53:1214–1218. doi: 10.1139/y75-171. [DOI] [PubMed] [Google Scholar]
- 668.Krnjevic K, Puil E, Werman R. GABA and glycine actions on spinal motoneurons. Can. J. Physiol. Pharmacol. 1977;55:658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- 669.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 670.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 671.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci. Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 672.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- 673.Kudina L, Alexeeva N. Repetitive doublets of human motoneurones: analysis of interspike intervals and recruitment pattern. Electroencephalogr. Clin. Neurophysiol. 1992;85:243–247. doi: 10.1016/0168-5597(92)90112-o. [DOI] [PubMed] [Google Scholar]
- 674.Kuhse J, Becker CM, Schmieden V, Hoch W, Pribilla I, Langosch D, Malosio ML, Muntz M, Betz H. Heterogeneity of the inhibitory glycine receptor. Ann. NY Acad. Sci. 1991;625:129–135. doi: 10.1111/j.1749-6632.1991.tb33836.x. [DOI] [PubMed] [Google Scholar]
- 675.Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr. Opin. Neurobiol. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 676.Kuhse J, Schmieden V, Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 1990;265:22317–22320. [PubMed] [Google Scholar]
- 677.Kuhse J, Schmieden V, Betz H. A single amino acid exchange alters pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990;5:867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- 678.Kullmann DM, Siegelbaum SA. The site of expression of NMDA receptor-dependent LTP: new fuel for an old fire. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 679.Kurasawa I, Toda K, Nakamura Y. Non-reciprocal facilitation of trigeminal motoneurons innervating jaw-closing and jaw-opening muscles induced by iontophoretic application of serotonin in the guinea pig. Brain Res. 1990;515:126–134. doi: 10.1016/0006-8993(90)90586-z. [DOI] [PubMed] [Google Scholar]
- 680.Kurihara T, Suzuki H, Yanagisawa M, Yoshioka K. Muscarinic excitatory and inhibitory mechanisms involved in afferent fibre-evoked depolarization of motoneurones in the neonatal rat spinal cord. Br. J. Pharmacol. 1993;110:61–70. doi: 10.1111/j.1476-5381.1993.tb13772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Kurihara T, Yoshioka K, Otsuka M. Tachykininergic slow depolarization of motoneurones evoked by descending fibres in the neonatal rat spinal cord. J. Physiol. (Lond.) 1995;485:787–796. doi: 10.1113/jphysiol.1995.sp020769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Kuriyama K, Hirouchi M, Nakayasu H. Structure and function of cerebral GABAA and GABAB receptors. Neurosci. Res. 1993;17:91–99. doi: 10.1016/0168-0102(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 683.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 684.Labandeira-Garcia J, Guerra-Seijas M, Labandeira-Garcia J. Nucleus prepositus neurons projecting to the oculomotor and trochlear nuclei in rabbits. Brain Behav. Evol. 1990;35:16–22. doi: 10.1159/000115852. [DOI] [PubMed] [Google Scholar]
- 685.Labandeira-Garcia J, Guerra-Seijas M, Labandeira-Garcia J, Jorge-Barreiro F. Distribution of the vestibular neurons projecting to the oculomotor and trochlear nuclei in rabbits. Brain Behav. Evol. 1991;37:111–124. doi: 10.1159/000114352. [DOI] [PubMed] [Google Scholar]
- 686.Lacey G. Synaptic inhibition mediated by GABAB receptors in the neonatal rat spinal cord in vitro. Brain Res. 1996;717:76–80. doi: 10.1016/0006-8993(96)00534-3. [DOI] [PubMed] [Google Scholar]
- 687.Lacey G, Berthelot P, Vaccher C, Flouquet N, Vaccher MP, Debaert M, Curtis DR. Thienyl-GABA derivatives as specific baclofen agonists in the rat and cat spinal cord in vivo. Neurosci. Lett. 1993;159:64–66. doi: 10.1016/0304-3940(93)90799-q. [DOI] [PubMed] [Google Scholar]
- 688.Lacey G, Curtis DR. Phosphinic acid derivatives as baclofen agonists and antagonists in the mammalian spinal cord: an in vivo study. Exp. Brain Res. 1994;101:59–72. doi: 10.1007/BF00243217. [DOI] [PubMed] [Google Scholar]
- 689.Lacey G, Nistri A, Rhys-Maitland E. Large enhancement of excitatory postsynaptic potentials and currents by thyrotropin- releasing hormone (TRH) in frog spinal motoneurones. Brain Res. 1989;488:80–88. doi: 10.1016/0006-8993(89)90695-1. [DOI] [PubMed] [Google Scholar]
- 690.Ladram A, Bulant M, Delfour A, Montagne JJ, Vaudry H, Nicolas P. Modulation of the biological activity of thyrotropin-releasing hormone by alternate processing of pro-TRH. Biochimie. 1994;76:320–328. doi: 10.1016/0300-9084(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 691.Lagerback PA, Ulfhake B. Ultrastructural observations in beaded alpha-motoneuron dendrites. Acta Physiol. Scand. 1987;129:61–66. doi: 10.1111/j.1748-1716.1987.tb08040.x. [DOI] [PubMed] [Google Scholar]
- 692.Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J. Physiol. (Lond.) 1986;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J. Physiol. (Lond.) 1986;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Lalley PM. Biphasic effects of baclofen on phrenic motoneurons: possible involvment of two types of γ-aminobutyric acid (GABA) receptors. J. Pharmacol. Exp. Ther. 1983;226:616–624. [PubMed] [Google Scholar]
- 695.Lamb AH. Motoneuron death in the embryo. CRC Crit. Rev. Clin. Neurobiol. 1984;1:141–179. [PubMed] [Google Scholar]
- 696.Lamotte D’incamps B, Destombes J, Thiesson D, Hellio R, Lasserre X, Kouchtir-Devanne N, Jami L, Zytnicki D. Indications for GABA-immunoreactive axo-axonic contacts on the intraspinal arborization of a Ib fiber in cat: a confocal microscope study. J. Neurosci. 1998;18:10030–10036. doi: 10.1523/JNEUROSCI.18-23-10030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Lamotte D’incamps B, Meunier C, Monnet M-L, Jami L, Zytnicki D. Reduction of presynaptic action potentials by PAD: model and experimental study. J. Comp. Neurosci. 1998;5:141–156. doi: 10.1023/a:1008861815083. [DOI] [PubMed] [Google Scholar]
- 698.Lan C, Wen C, Tseng G, Tan C, Ling E, Shieh J. Efferent connections from the external cuneate nucleus to the medulla oblongata in the gerbil. Brain Res. 1994;668:107–116. doi: 10.1016/0006-8993(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 699.Langer T, Kaneko C, Scudder C, Fuchs A. Afferents to the abducens nucleus in the monkey and cat. J. Comp. Neurol. 1986;245:379–400. doi: 10.1002/cne.902450307. [DOI] [PubMed] [Google Scholar]
- 700.Langosch D, Becker CM, Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur. J. Biochem. 1990;194:1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- 701.Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J. Physiol. (Lond.) 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Larkman PM, Kelly JS. Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: mediation through a phosphorylation independent action of cAMP. Neuropharmacology. 1997;36:721–733. doi: 10.1016/s0028-3908(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 703.Larkman PM, Kelly JS. Characterization of 5-HT-sensitive potassium conductances in neonatal rat facial motoneurones in vitro. J. Physiol. (Lond.) 1998;508:67–81. doi: 10.1111/j.1469-7793.1998.067br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Larkman PM, Kelly JS, Takahashi T. Adenosine 3′:5′-cyclic monophosphate mediates a 5-hydroxytryptamine-induced response in neonatal rat motoneurones. Pflügers Arch. 1995;430:763–769. doi: 10.1007/BF00386174. [DOI] [PubMed] [Google Scholar]
- 705.Larkman PM, Penington NT, Kelly JS. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. J. Neurosci. Methods. 1989;28:133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- 706.Larkum M, Launey T, Dityatev A, Lüscher H-R. Integration of excitatory postsynaptic potentials in dendrites of motoneurons of rat spinal cord slice cultures. J. Neurophysiol. 1998;80:924–935. doi: 10.1152/jn.1998.80.2.924. [DOI] [PubMed] [Google Scholar]
- 707.Larkum ME, Rioult MG, Lüscher HR. Propagation of action potentials in the dendrites of neurons from rat spinal cord slice cultures. J. Neurophysiol. 1996;75:154–170. doi: 10.1152/jn.1996.75.1.154. [DOI] [PubMed] [Google Scholar]
- 708.Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. Gen. Pharmacol. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 709.Lauder JM, Han VKM, Henerson P, Verdoon T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brains: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- 710.Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 711.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Lawrence AJ, Bennett MR, Barden JA. Distribution of P2×-receptor immunoreactivity in adult rat brainstem (Abstract) Proc. Austr. Neurosci. Soc. 1999;10:167. [Google Scholar]
- 714.Lawrence D, Porter R, Redman S. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J. Comp. Neurol. 1985;232:499–510. doi: 10.1002/cne.902320407. [DOI] [PubMed] [Google Scholar]
- 715.Leanza G, MacCavino M, Stanzani S. Noradrenergic neurotransmission in the ventral spinal cord: basic characteristics and effects of denervating lesions, as studied in the awake rat by microdialysis. Brain Res. 1996;738:281–291. doi: 10.1016/s0006-8993(96)00796-2. [DOI] [PubMed] [Google Scholar]
- 716.Lechan RM, Snapper SB, Jackson IM. Evidence that spinal cord thyrotropin-releasing hormone is independent of the paraventricular nucleus. Neurosci. Lett. 1983;43:61–65. doi: 10.1016/0304-3940(83)90129-5. [DOI] [PubMed] [Google Scholar]
- 717.Lechan RM, Wu P, Jackson IM. Immunocytochemical distribution in rat brain of putative peptides derived from thyrotropin-releasing hormone prohormone. Endocrinology. 1987;121:1879–1891. doi: 10.1210/endo-121-5-1879. [DOI] [PubMed] [Google Scholar]
- 718.Lechan RM, Wu P, Jackson IM, Wolf H, Cooperman S, Mandel G, Goodman RH. Thyrotropin-releasing hormone precursor: characterization in rat brain. Science. 1986;231:159–161. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- 719.Lee MT, Koebbe MJ, O’Donovan MJ. The development of sensorimotor synaptic connections in the lumbosacral cord of the chick embryo. J. Neurosci. 1988;8:2530–2543. doi: 10.1523/JNEUROSCI.08-07-02530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J. Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- 721.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J. Neurophysiol. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- 722.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J. Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- 723.Leger L, Charnay Y, Dubois P, Jouvet M. Distribution of enkephalin-immunoreactive cell bodies in relation to serotonin-containing neurons in the raphe nuclei of the cat: immunohistochemical evidence for the coexistence of enkephalins and serotonin in certain cells. Brain Res. 1986;362:63–73. doi: 10.1016/0006-8993(86)91399-5. [DOI] [PubMed] [Google Scholar]
- 724.Lehmann A, Karrberg L. Effects of N-methyl-d-aspartate receptor antagonists on cisplatin-induced emesis in the ferret. Neuropharmacology. 1996;35:475–481. doi: 10.1016/0028-3908(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 725.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J. Comp. Neurol. 1996;373:322–339. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 726.Lepre M, Olpe H, Brugger F. The effects of neurokinin-1 receptor agonists on spinal motoneurones of the neonatal rat. Neuropharmacology. 1996;35:511–522. doi: 10.1016/0028-3908(96)00192-x. [DOI] [PubMed] [Google Scholar]
- 727.Lepre M, Olpe HR, Evans RH, Brugger F. Physiological and pharmacological characterization of the spinal tachykinin NK2 receptor. Eur. J. Pharmacol. 1994;258:23–31. doi: 10.1016/0014-2999(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 728.Lerma J, Morales M, Vicente MA, Herreras O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 1997;20:9–12. doi: 10.1016/S0166-2236(96)20055-4. [DOI] [PubMed] [Google Scholar]
- 729.Lerma J, Paternain AV, Naranjo JR, Mell-Strom B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Lester RA, Jahr CE. Quisqualate receptor-mediated depression of calcium currents in hippocampal neurons. Neuron. 1990;4:741–749. doi: 10.1016/0896-6273(90)90200-y. [DOI] [PubMed] [Google Scholar]
- 731.Lev-Tov A, Meyers DE, Burke RE. Activation of type B gamma-aminobutyric acid receptors in the intact mammalian spinal cord mimics the effects of reduced presynaptic Ca2+ influx. Proc. Natl. Acad. Sci. USA. 1988;85:5330–5334. doi: 10.1073/pnas.85.14.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Lev-Tov A, Meyers DE, Burke RE. GABAB receptors in the cat spinal cord. J. Basic Clin. Physiol. Pharmacol. 1990;1:87–94. doi: 10.1515/jbcpp.1990.1.1-4.87. [DOI] [PubMed] [Google Scholar]
- 733.Lev-Tov A, Miller JP, Burke RE, Rall W. Factors that control amplitude of EPSPs in dendritic neurons. J. Neurophysiol. 1983;50:399–412. doi: 10.1152/jn.1983.50.2.399. [DOI] [PubMed] [Google Scholar]
- 734.Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J. Physiol. (Lond.) 1992;447:149–169. doi: 10.1113/jphysiol.1992.sp018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Li H, Ohishi H, Kinoshita A, Shigemoto R, Nomura S, Mizuno N. Localization of a metabotropic glutamate receptor, mGluR7, in axon terminals of presumed nociceptive, primary afferent fibers in the superficial layers of the spinal dorsal horn: an electron microscope study in the rat. Neurosci. Lett. 1997;223:153–156. doi: 10.1016/s0304-3940(97)13429-2. [DOI] [PubMed] [Google Scholar]
- 736.Li J, Perl ER. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J. Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Li Y, Takada M, Kaneko T, Mizuno N. Premotor neurons for trigeminal motor nucleus neurons innervating the jaw-closing and jaw-opening muscles: differential distribution in the lower brainstem of the rat. J. Comp. Neurol. 1995;356:563–579. doi: 10.1002/cne.903560407. [DOI] [PubMed] [Google Scholar]
- 738.Li Y, Takada M, Kaneko T, Mizuno N. GABAergic and glycinergic neurons projecting to the trigeminal motor nucleus: a double labeling study in the rat. J. Comp. Neurol. 1996;373:498–510. doi: 10.1002/(SICI)1096-9861(19960930)373:4<498::AID-CNE3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 739.Li Y, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J. Comp. Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 740.Li Y, Takada M, Mizuno N. The sites of origin of serotoninergic afferent fibers in the trigeminal motor, facial, and hypoglossal nuclei in the rat. Neurosci. Res. 1993;17:307–313. doi: 10.1016/0168-0102(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 741.Liang F, Moret V, Wiesendanger M, Rouiller E. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J. Comp. Neurol. 1991;311:356–366. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- 742.Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- 743.Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol. Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 744.Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J. Physiol. (Lond.) 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J. Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- 746.Liu G, Feldman JL. Quantal synaptic transmission in phrenic motor nucleus. J. Neurophysiol. 1992;68:1468–1471. doi: 10.1152/jn.1992.68.4.1468. [DOI] [PubMed] [Google Scholar]
- 747.Liu H, Brown JL, Jasmin L, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc. Natl. Acad. Sci. USA. 1994;91:1009–1013. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Liu J, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABAA receptor antagonist bicuculline differentially alters expression of the GABAA receptor subunit mRNAs in fetal rat brainstem. Dev. Neurosci. 1998;20:83–92. doi: 10.1159/000017302. [DOI] [PubMed] [Google Scholar]
- 749.Liu R, Fung S, Reddy V, Barnes C. Localization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activity. Neuroscience. 1995;64:193–208. doi: 10.1016/0306-4522(94)00354-8. [DOI] [PubMed] [Google Scholar]
- 750.Liu Y, Fields RD, Fitzgerald S, Festoff BW, Nelson PG. Proteolytic activity, synapse elimination, and the Hebb synapse. J. Neurobiol. 1994;25:325–335. doi: 10.1002/neu.480250312. [DOI] [PubMed] [Google Scholar]
- 751.Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat. I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- 752.Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- 753.Lomeli J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature. 1998;395:600–604. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- 754.Londos C, Cooper DM, Wolff J. Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Long SK. Amino acid pharmacology in a mature rat spinal cord preparation in vitro. Comp. Biochem. Physiol. A Physiol. 1989;93:177–181. doi: 10.1016/0300-9629(89)90205-3. [DOI] [PubMed] [Google Scholar]
- 756.Long SK, Evans RH, Cull L, Krijzer F, Bevan P. An in vitro mature spinal cord preparation from the rat. Neuropharmacology. 1988;27:541–546. doi: 10.1016/0028-3908(88)90138-4. [DOI] [PubMed] [Google Scholar]
- 757.Long SK, Evans RH, Krijzer F. Effects of depressant amino acids and antagonists on an in vitro spinal cord preparation from the adult rat. Neuropharmacology. 1989;28:683–688. doi: 10.1016/0028-3908(89)90151-2. [DOI] [PubMed] [Google Scholar]
- 758.Long SK, Smith DA, Siarey RJ, Evans RH. Effect of 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline (CNQX) on dorsal root-, NMDA-, kainate- and quisqualate-mediated depolarization of rat motoneurones in vitro. Br. J. Pharmacol. 1990;100:850–854. doi: 10.1111/j.1476-5381.1990.tb14103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Lowe A. The neural regulation of tongue movements. Prog. Neurobiol. 1981;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- 760.Lowrie MB, Vrbová G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- 761.Luddens H, Korpi ER. Biological function of GABAA/benzodiazepine receptor heterogeneity. J. Psychiatr. Res. 1995;29:77–94. doi: 10.1016/0022-3956(94)00040-x. [DOI] [PubMed] [Google Scholar]
- 762.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 763.Lumsden A. Neural development. A “LIM code” for motor neurons? Curr. Biol. 1995;5:491–495. doi: 10.1016/s0960-9822(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 764.Lund JP. Mastication and its control by the brain stem. Crit. Rev. Oral Biol. Med. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- 765.Luque JM, Bleuel Z, Malherbe P, Richards JG. Alternatively spliced isoforms of the N-methyl-d-aspartate receptor subunit 1 are differentially distributed within the rat spinal cord. Neuroscience. 1994;63:629–635. doi: 10.1016/0306-4522(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 766.Lüscher H-R, Larkum ME. Modeling action potential initiation and back-propagation in dendrites of cultured rat motoneurons. J. Neurophysiol. 1998;80:715–729. doi: 10.1152/jn.1998.80.2.715. [DOI] [PubMed] [Google Scholar]
- 767.Lynn R, Kreider M, Miselis R. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J. Comp. Neurol. 1991;311:271–288. doi: 10.1002/cne.903110208. [DOI] [PubMed] [Google Scholar]
- 768.Lyons W, Grzanna R. Noradrenergic neurons with divergent projections to the motor trigeminal nucleus and the spinal cord: a double retrograde neuronal labeling study. Neuroscience. 1988;26:681–693. doi: 10.1016/0306-4522(88)90174-1. [DOI] [PubMed] [Google Scholar]
- 769.Ma W, Behar T, Barker JL. Transient expression of GABA immunoreactivity in the developing rat spinal cord. J. Comp. Neurol. 1992;235:271–290. doi: 10.1002/cne.903250210. [DOI] [PubMed] [Google Scholar]
- 770.Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J. Comp. Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- 771.MacDonald JF, Browning MD, Wang LY. Long-term enhancement of excitability and the regulation of glutamate receptors by protein kinases. Epilepsy Res. Suppl. 1996;12:275–282. [PubMed] [Google Scholar]
- 772.MacDonald RL, Olsen RW. GABAA receptor channels. Annu. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 773.Macek TA, Winder DG, Gereau RWT, Ladd CO, Conn PJ. Differential involvement of group II and group III mGluRs as autoreceptors at lateral and medial perforant path synapses. J. Neurophysiol. 1996;76:3798–3806. doi: 10.1152/jn.1996.76.6.3798. [DOI] [PubMed] [Google Scholar]
- 774.Maeno H, Kiyama H, Tohyama M. Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. Brain Res. 1993;18:43–58. doi: 10.1016/0169-328x(93)90172-l. [DOI] [PubMed] [Google Scholar]
- 775.Magoul R, Onteniente B, Geffard M, Calas A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience. 1987;20:1001–1009. doi: 10.1016/0306-4522(87)90258-2. [DOI] [PubMed] [Google Scholar]
- 776.Maier DL, Kalb RG, Stelzner DJ. NMDA antagonism during development extends sparing of hindlimb function to older spinally transected rats. Brain Res. 1995;87:135–144. doi: 10.1016/0165-3806(95)00065-l. [DOI] [PubMed] [Google Scholar]
- 777.Maitra K, Seth P, Ross H, Thewissen M, Ganguly D. Presynaptic dopaminergic inhibition of the spinal reflex in rats. Brain Res. Bull. 1992;28:817–819. doi: 10.1016/0361-9230(92)90266-z. [DOI] [PubMed] [Google Scholar]
- 778.Maitra KK, Seth P, Thewissen M, Ross HG, Ganguly DK. Dopaminergic influence on the excitability of antidromically activated Renshaw cells in the lumbar spinal cord of the rat. Acta Physiol. Scand. 1993;148:101–107. doi: 10.1111/j.1748-1716.1993.tb09538.x. [DOI] [PubMed] [Google Scholar]
- 779.Maki R, Robinson MB, Dichter MA. The glutamate uptake inhibitor l-trans-pyrrolidine-2,4-dicarboxylate depresses excitatory synaptic transmission via a presynaptic mechanism in cultured hippocampal neurons. J. Neurosci. 1994;14:6754–6762. doi: 10.1523/JNEUROSCI.14-11-06754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Malcangio M, Bowery NG. Gamma-aminobutyric acid B, but not gamma-aminobutyric acid A receptor activation, inhibits electrically evoked substance P-like immunoreactivity from the rat spinal cord in vitro. J. Pharamacol. Exp. Ther. 1993;299:1490–1496. [PubMed] [Google Scholar]
- 781.Malcangio M, Bowery NG. Possible therapeutic application of GABAB receptor agonists and antagonists. Clin. Neuropharmacol. 1995;18:285–305. doi: 10.1097/00002826-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 782.Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol. Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- 783.Malcangio M, Da Silva H, Bowery NG. Plasticity of GABAB receptor in rat spinal cord detected by autoradiography. Eur. J. Pharmacol. 1993;250:153–156. doi: 10.1016/0014-2999(93)90633-s. [DOI] [PubMed] [Google Scholar]
- 784.Malcangio M, Libri V, Teoh H, Constanti A, Bowery NG. Chronic (−)baclofen or CGP 36742 alters GABAB receptor sensitivity in rat brain and spinal cord. Neuroreport. 1995;6:399–403. doi: 10.1097/00001756-199501000-00042. [DOI] [PubMed] [Google Scholar]
- 785.Mallory BS, Roppolo JR, De Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- 786.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin- dependent kinase II. J. Biol. Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 788.Manaker S, Rizio G. Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J. Comp. Neurol. 1989;290:516–526. doi: 10.1002/cne.902900406. [DOI] [PubMed] [Google Scholar]
- 789.Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J. Comp. Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- 790.Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J. Comp. Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- 791.Manaker S, Zucchi PC. Autoradiographic localization of neurotransmitter binding sites in the hypoglossal and motor trigeminal nuclei of the rat. Synapse. 1998;28:44–59. doi: 10.1002/(SICI)1098-2396(199801)28:1<44::AID-SYN6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 792.Mantyh P, Hunt S. Evidence for cholecystokinin-like immunoreactive neurons in the rat medulla oblongata which project to the spinal cord. Brain Res. 1984;291:49–54. doi: 10.1016/0006-8993(84)90649-8. [DOI] [PubMed] [Google Scholar]
- 793.Manzoni O, Bockaert J. Metabotropic glutamate receptors inhibiting excitatory synapses in the CA1 area of rat hippocampus. Eur. J. Neurosci. 1995;7:2518–2523. doi: 10.1111/j.1460-9568.1995.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 794.Maqbool A, Batten T, Berry P, McWilliam P. Distribution of dopamine-containing neurons and fibres in the feline medulla oblongata: a comparative study using catecholamine-synthesizing enzyme and dopamine immunohistochemistry. Neuroscience. 1993;53:717–733. doi: 10.1016/0306-4522(93)90619-q. [DOI] [PubMed] [Google Scholar]
- 795.Maragos WF, Penney JB, Young AB. Anatomic correlation of NMDA and 3H-TCP-labeled receptors in rat brain. J. Neurosci. 1988;8:493–501. doi: 10.1523/JNEUROSCI.08-02-00493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Marks J, Donnelly D, Haddad G. Adenosine-induced inhibition of vagal motoneuron excitability: receptor subtype and mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 1993;264:L124–L132. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- 797.Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. J. Comp. Neurol. 1992;317:341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- 798.Marshall K, Engberg I. Reversal potential for noradrenaline- induced hyperpolarization of spinal motoneurons. Science. 1979;205:422–424. doi: 10.1126/science.451613. [DOI] [PubMed] [Google Scholar]
- 799.Marti E, Bumcrot D, Takada R, McMahon A. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- 800.Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- 801.Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- 802.Martin R, Jordan L, Willis W. Differential projections of cat medullary raphe neurons demonstrated by retrograde labelling following spinal cord lesions. J. Comp. Neurol. 1978;182:77–88. doi: 10.1002/cne.901820106. [DOI] [PubMed] [Google Scholar]
- 803.Martinez-Morales J, Barbas J, Marti E, Bovolenta P, Edgar D, Rodriguez-Tebar A. Vitronectin is expressed in the ventral region of the neural tube and promotes the differentiation of motor neurons. Development. 1997;124:5139–5147. doi: 10.1242/dev.124.24.5139. [DOI] [PubMed] [Google Scholar]
- 804.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 805.Matsumoto G, Hisamitsu T, De Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci. Lett. 1995;183:58–61. doi: 10.1016/0304-3940(94)11114-x. [DOI] [PubMed] [Google Scholar]
- 806.Matsushima T, Tegner J, Hill RH, Grillner S. GABAB receptor activation causes a depression of low- and high-voltage-activated Ca2+ currents, postinhibitory rebound, and postspike afterhyperpolarization in lamprey neurons. J. Neurophysiol. 1993;70:2606–2619. doi: 10.1152/jn.1993.70.6.2606. [DOI] [PubMed] [Google Scholar]
- 807.Matsuto T, Yanagisawa M, Otsuka M, Kanazawa I, Munekata E. The excitatory action of the newly-discovered mammalian tachykinins, neurokinin alpha and neurokinin beta, on neurons of the isolated spinal cord of the newborn rat. Neurosci. Res. 1984;2:105–110. doi: 10.1016/0168-0102(84)90008-7. [DOI] [PubMed] [Google Scholar]
- 808.Maxwell DJ, Christie WM, Ottersen OP, Storm-Mathisen J. Terminals of group Ia primary afferent fibres in Clarke’s column are enriched with L-glutamate-like immunoreactivity. Brain Res. 1990;510:346–350. doi: 10.1016/0006-8993(90)91389-x. [DOI] [PubMed] [Google Scholar]
- 809.Maxwell DJ, Christie WM, Short AD, Brown AG. Direct observations of synapses between GABA-immunoreactive boutons and muscle afferent terminals in lamina VI of the cat’s spinal cord. Brain Res. 1990;530:215–222. doi: 10.1016/0006-8993(90)91285-o. [DOI] [PubMed] [Google Scholar]
- 810.Mayer ML, Westbrook GL. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J. Physiol. (Lond.) 1984;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 812.McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience. 1989;28:745–753. doi: 10.1016/0306-4522(89)90019-5. [DOI] [PubMed] [Google Scholar]
- 813.McClellan AD. In vitro CNS preparations: unique approaches to the study of command and pattern generation systems in motor control. J. Neurosci. Methods. 1987;21:251–264. doi: 10.1016/0165-0270(87)90120-8. [DOI] [PubMed] [Google Scholar]
- 814.McCormick DA. Electrophysiological consequences of activation of adrenoceptors in the CNS. In: Szabadi E, Bradshaw C, editors. Adrenoceptors: Structure, Mechanisms, Function. Advances in Pharmacological Sciences. Basel: Birkhauser Verlag; 1991. pp. 159–169. [Google Scholar]
- 815.McCormick DA. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J. Neurosci. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.McCrea R, Baker R. Anatomical connections of the nucleus prepositus of the cat. J. Comp. Neurol. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- 817.McCrea R, Strassman A, Highstein S. Morphology and physiology of abducens motoneurons and internuclear neurons intracellularly injected with horseradish peroxidase in alert squirrel monkeys. J. Comp. Neurol. 1986;243:291–308. doi: 10.1002/cne.902430302. [DOI] [PubMed] [Google Scholar]
- 818.McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J. Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.McGehee DS, Role LW. Presynaptic ionotropic receptors. Curr. Opin. Neurobiol. 1996;6:342–349. doi: 10.1016/s0959-4388(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 820.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 821.McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- 822.McHanwell S, Biscoe T. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- 823.McKernan RM, Cox P, Whiting P. Differential expression of GABAA receptor α-subunits in rat brain during development. FEBS Lett. 1991;286:44–46. doi: 10.1016/0014-5793(91)80936-w. [DOI] [PubMed] [Google Scholar]
- 824.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 825.McLarnon JG. Potassium currents in motoneurones. Prog. Neurobiol. 1995;47:513–531. doi: 10.1016/0301-0082(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 826.McLarnon JG, Kim S, Michikawa M, Xu R. Properties of inward and outward potassium currents in cultured mouse motoneurons. Neuroscience. 1995;64:139–151. doi: 10.1016/0306-4522(95)90396-o. [DOI] [PubMed] [Google Scholar]
- 827.McLaughlin BJ, Barber R, Saito K, Roberts E, Wu JY. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J. Comp. Neurol. 1975;164:305–321. doi: 10.1002/cne.901640304. [DOI] [PubMed] [Google Scholar]
- 828.Meier E, Hertz L, Schousboe A. Neurotransmitters as neurochemical signals. Neurochem. Int. 1991;19:1–15. [Google Scholar]
- 829.Mellen NM, Feldman JL. Vagal stimulation induces expiratory lengthening in the in vitro neonate rat. J. Appl. Physiol. 1997;83:1607–1611. doi: 10.1152/jappl.1997.83.5.1607. [DOI] [PubMed] [Google Scholar]
- 830.Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J. Neurophysiol. 1971;34:171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- 831.Mendell LM, Collins WFI, Koerber HR. How are synapses distributed on spinal motoneurons to permit orderly recruitment? In: Binder MD, Mendell LM, editors. The Segmental Motor System. New York: Oxford Univ. Press; 1990. pp. 328–340. [Google Scholar]
- 832.Mendell LM, Taylor JS, Johnson RD, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia motoneuron synapse. J. Neurophysiol. 1995;73:662–673. doi: 10.1152/jn.1995.73.2.662. [DOI] [PubMed] [Google Scholar]
- 833.Merchenthaler I, Maderdrut JL, O’Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides. 1992;13:815–829. doi: 10.1016/0196-9781(92)90192-6. [DOI] [PubMed] [Google Scholar]
- 834.Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projections from Botzinger neurons. J. Neurosci. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 836.Meyerhof W, Wulfsen I, Schonrock C, Fehr S, Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc. Natl. Acad. Sci. USA. 1992;89:10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 838.Milano S, Grelot L, Bianchi A, Iscoe S. Discharge patterns of phrenic motoneurons during fictive coughing and vomiting in decerebrate cats. J. Appl. Physiol. 1992;73:1626–1636. doi: 10.1152/jappl.1992.73.4.1626. [DOI] [PubMed] [Google Scholar]
- 839.Miller J, Paul K, Lee R, Rymer W, Heckman C. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- 840.Milner TA, Okada J, Pickel VM. Monosynaptic input from Leu5-enkephalin-immunoreactive terminals to vagal motor neurons in the nucleus ambiguus: comparison with the dorsal motor nucleus of the vagus. J. Comp. Neurol. 1995;353:391–406. doi: 10.1002/cne.903530307. [DOI] [PubMed] [Google Scholar]
- 841.Min MY, Appenteng K. Multimodal distribution of amplitudes of miniature and spontaneous EPSPs recorded in rat trigeminal motoneurones. J Physiol. (Lond.) 1996;494:171–182. doi: 10.1113/jphysiol.1996.sp021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Minneman KP, Esbenshade TA. Alpha 1-adrenergic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1994;34:117–133. doi: 10.1146/annurev.pa.34.040194.001001. [DOI] [PubMed] [Google Scholar]
- 843.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog. Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 844.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 845.Mitchell JJ, Anderson KJ. Quantitative autoradio-graphic analysis of excitatory amino acid receptors in the cat spinal cord. Neurosci. Lett. 1991;124:269–272. doi: 10.1016/0304-3940(91)90110-f. [DOI] [PubMed] [Google Scholar]
- 846.Mitchell R, Fleetwood-Walker S. Substance P, but not TRH, modulates the 5-HT autoreceptor in ventral lumbar spinal cord. Eur. J. Pharmacol. 1981;76:119–120. doi: 10.1016/0014-2999(81)90020-0. [DOI] [PubMed] [Google Scholar]
- 847.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 848.Moeller I, Chai S, Oldfield B, McKinley M, Casley D, Mendelsohn F. Localization of angiotensin IV binding sites to motor and sensory neurons in the sheep spinal cord and hindbrain. Brain Res. 1995;701:301–306. doi: 10.1016/0006-8993(95)01128-0. [DOI] [PubMed] [Google Scholar]
- 849.Moeller I, Paxinos G, Mendelsohn F, Aldred G, Casley D, Chai S. Distribution of AT4 receptors in the Macaca fascicularis brain. Brain Res. 1996;712:307–324. doi: 10.1016/0006-8993(95)01482-9. [DOI] [PubMed] [Google Scholar]
- 850.Mogoseanu D, Smith A, Bolam J. Monosynaptic innervation of facial motoneurones by neurones of the parvicellular reticular formation. Exp. Brain Res. 1994;101:427–438. doi: 10.1007/BF00227336. [DOI] [PubMed] [Google Scholar]
- 851.Mogul DJ, Adams ME, Fox AP. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993;10:327–334. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- 852.Mohler H, Fritschy JM, Luscher B, Rudolph U, Benson J, Benke D. The GABAA receptors. From subunits to diverse functions. Ion Channels. 1996;4:89–113. [PubMed] [Google Scholar]
- 853.Molinoff PB, Williams K, Pritchett DB, Zhong J. Molecular pharmacology of NMDA receptors: modulatory role of NR2 subunits. Prog. Brain Res. 1994;100:39–45. doi: 10.1016/s0079-6123(08)60766-9. [DOI] [PubMed] [Google Scholar]
- 854.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 855.Monaghan DT, Cotman CW. Distribution of N-methyl-d-aspartate-sensitive l-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Monaghan DT, Yao D, Cotman CW. Distribution of [3H]AMPA binding sites in rat brain as determined by quantitative autoradiography. Brain Res. 1984;324:160–164. doi: 10.1016/0006-8993(84)90636-x. [DOI] [PubMed] [Google Scholar]
- 857.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 858.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 859.Moore JA, Appenteng K. The membrane properties and firing characteristics of rat jaw-elevator motoneurones. J Physiol. (Lond.) 1990;423:137–153. doi: 10.1113/jphysiol.1990.sp018015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Moore JA, Stockbridge N, Westerfield M. On the site of impulse initiation in a neurone. J Physiol. (Lond.) 1983;336:301–311. doi: 10.1113/jphysiol.1983.sp014582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Moore JA, Appenteng K. The morphology and electrical geometry of rat jaw-elevator motoneurones. J Physiol. (Lond.) 1991;440:325–343. doi: 10.1113/jphysiol.1991.sp018711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Moore R, Card J. Noradrenaline-containing neuron systems. In: Hokfelt T, Bjorklund A, editors. Classical Transmitters in the CNS. Amsterdam: Elsevier; 1984. pp. 123–156. [Google Scholar]
- 864.Morales M, Battenberg E, Bloom F. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp. Neurol. 1998:385–401. [PubMed] [Google Scholar]
- 865.Morin AM, Hattori H, Wasterlain CG, Thomson D. [3H]MK-801 binding sites in neonate rat brain. Brain Res. 1989;487:376–379. doi: 10.1016/0006-8993(89)90844-5. [DOI] [PubMed] [Google Scholar]
- 866.Morrison BM, Janssen WGM, Gordon JW, Morrison JH. Light and electron microscopic distribution of the AMPA receptor subunit, GluR2, in the spinal cord of control and G86R mutant superoxide dismutase transgenic mice. J Comp. Neurol. 1998;395:523–534. [PubMed] [Google Scholar]
- 867.Morrow AL. Regulation of GABAA receptor function and gene expression in the central nervous system. Int. Rev. Neurobiol. 1995;38:1–41. doi: 10.1016/s0074-7742(08)60523-1. [DOI] [PubMed] [Google Scholar]
- 868.Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeberg P, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1064. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- 869.Moschovakis A, Solodkin M, Burke R. Anatomical and physiological study of interneurons in an oligosynaptic cutaneous reflex pathway in the cat hindlimb. Brain Res. 1992;586:311–318. doi: 10.1016/0006-8993(92)91641-q. [DOI] [PubMed] [Google Scholar]
- 870.Mosfeldt Laursen A, Rekling JC. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience. 1989;30:619–637. doi: 10.1016/0306-4522(89)90156-5. [DOI] [PubMed] [Google Scholar]
- 871.Moss SJ, Smart TG. Modulation of amino acid-gated ion channels by protein phosphorylation. Int. Rev. Neurobiol. 1996;39:1–52. doi: 10.1016/s0074-7742(08)60662-5. [DOI] [PubMed] [Google Scholar]
- 872.Motin L, Bennett MR. Effect of P2-purinoceptor antagonists on glutamatergic transmission in the rat hippocampus. Br. J. Pharmacol. 1995;115:1276–1280. doi: 10.1111/j.1476-5381.1995.tb15036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. Int. Rev. Neurobiol. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 874.Mtui E, Anwar M, Gomez R, Reis D, Ruggiero D. Projections from the nucleus tractus solitarii to the spinal cord. J Comp. Neurol. 1993;337:231–252. doi: 10.1002/cne.903370205. [DOI] [PubMed] [Google Scholar]
- 875.Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. GABA and Neuropeptides in the CNS. Part I. New York: Elsevier; 1985. pp. 436–608. [Google Scholar]
- 876.Muramoto T, Mendelson B, Phelan KD, Garciarill E, Skinner RD, Puskarich-May C. Developmental changes in the effects of serotonin and N-methyl-d-aspartate on intrinsic membrane properties of embryonic chick motoneurons. Neuroscience. 1996;75:607–618. doi: 10.1016/0306-4522(96)00185-6. [DOI] [PubMed] [Google Scholar]
- 877.Murone C, Paxinos G, McKinley M, Oldfield B, Muller-Esterl W, Mendelsohn F, Chai S. Distribution of bradykinin B2 receptors in sheep brain and spinal cord visualized by in vitro autoradiography. J Comp. Neurol. 1997;381:203–218. doi: 10.1002/(sici)1096-9861(19970505)381:2<203::aid-cne7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 878.Murphy SM, Pilowsky PM, Llewellyn-Smith IJ. Vesicle shape and amino acids in synaptic inputs to phrenic motoneurons: do all inputs contain either glutamate or GABA? J Comp. Neurol. 1996;373:200–219. doi: 10.1002/(SICI)1096-9861(19960916)373:2<200::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 879.Myers S, Pugsley TA. Decrease in rat striatal dopamine synthesis and metabolism in vivo by metabolically stable adenosine receptor agonists. Brain Res. 1986;375:193–197. doi: 10.1016/0006-8993(86)90975-3. [DOI] [PubMed] [Google Scholar]
- 880.Myers SJ, Dingledine R, Borges K. Genetic regulation of glutamate receptor ion channels. Annu. Rev. Pharmacol. Toxicol. 1999;39:221–241. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- 881.Mynlieff M, Beam KG. Characterization of voltage-dependent calcium currents in mouse motoneurons. J Neurophysiol. 1992;68:85–92. doi: 10.1152/jn.1992.68.1.85. [DOI] [PubMed] [Google Scholar]
- 882.Mynlieff M, Beam KG. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Nagase Y, Moritani M, Nakagawa S, Yoshida A, Takemura M, Zhang L, Kida H, Shigenaga Y. Serotonergic axonal contacts on identified cat trigeminal motoneurons and their correlation with medullary raphe nucleus stimulation. J Comp. Neurol. 1997;384:443–455. doi: 10.1002/(sici)1096-9861(19970804)384:3<443::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 884.Nagy J, Yamamoto T, Jordan L. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- 885.Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for l-2-amino-4-phosphonobutyrate. J Biol. Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- 886.Nakamura Y, Goldberg LJ, Mizuno N, Clemente CD. Effects of hypoglossal afferent stimulation on masseteric motoneurons in cats. Exp. Neurol. 1978;61:1–14. doi: 10.1016/0014-4886(78)90177-2. [DOI] [PubMed] [Google Scholar]
- 887.Nakanishi N, Axel R, Shneider NA. Alternative splicing generates functionally distinct N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. USA. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Nakanishi S. Substance P precursor and kininogen: their structures, gene organizations, and regulation. Physiol. Rev. 1987;67:1117–1142. doi: 10.1152/physrev.1987.67.4.1117. [DOI] [PubMed] [Google Scholar]
- 889.Nakanishi S. Mammalian tachykinin receptors. Annu. Rev. Neurosci. 1991;14:123–136. doi: 10.1146/annurev.ne.14.030191.001011. [DOI] [PubMed] [Google Scholar]
- 890.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 891.Nakao S, Shiraishi Y, Miyara T. Direct projection of cat midbrain tegmentum neurons to the medial rectus subdivision of the oculomotor complex. Neurosci. Lett. 1986;64:123–128. doi: 10.1016/0304-3940(86)90086-8. [DOI] [PubMed] [Google Scholar]
- 892.Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp. Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- 893.Nakayasu H, Kimura H, Kuriyama K. Cerebral GABAA and GABAB receptors. Structure and function. Ann. NY Acad. Sci. 1995;757:516–527. doi: 10.1111/j.1749-6632.1995.tb17511.x. [DOI] [PubMed] [Google Scholar]
- 894.Nakazawa K, Inoue K, Ito K, Koizumi S, Inoue K. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn-Schmiedebergs Arch. Pharmacol. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- 895.Nakazawa K, Tadakuma T, Nokihara K, Ito M. Antibody specific for phosphorylated AMPA-type glutamate receptors at GluR2 Ser-696. Neurosci. Res. 1995;24:75–86. doi: 10.1016/0168-0102(95)00977-9. [DOI] [PubMed] [Google Scholar]
- 896.Nakoa S, Shiraishi Y. Direct projection of cat mesodi-encephalic neurons to the inferior rectus subdivision of the oculomotor nucleus. Neurosci. Lett. 1983;39:243–248. doi: 10.1016/0304-3940(83)90307-5. [DOI] [PubMed] [Google Scholar]
- 897.Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- 898.Nawa H, Kotani H, Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984;312:729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- 899.Nelson PG, Fields RD, Liu Y. Neural activity, neuronglia relationships, and synapse development. Perspect. Dev. Neurobiol. 1995;2:399–407. [PubMed] [Google Scholar]
- 900.Nelson PG, Fields RD, Yu C, Liu Y. Synapse elimination from the mouse neuromuscular junction in vitro: a non-Hebbian activity-dependent process. J Neurobiol. 1993;24:1517–1530. doi: 10.1002/neu.480241106. [DOI] [PubMed] [Google Scholar]
- 901.Nelson PG, Fields RD, Yu C, Neale EA. Mechanisms involved in activity-dependent synapse formation in mammalian central nervous system cell cultures. J Neurobiol. 1990;21:138–156. doi: 10.1002/neu.480210110. [DOI] [PubMed] [Google Scholar]
- 902.Nelson PG, Pun RY, Westbrook GL. Synaptic excitation in cultures of mouse spinal cord neurones: receptor pharmacology and behaviour of synaptic currents. J Physiol. (Lond.) 1986;372:169–190. doi: 10.1113/jphysiol.1986.sp016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Nelson PG, Yu C, Fields RD, Neale EA. Synaptic connections in vitro: modulation of number and efficacy by electrical activity. Science. 1989;244:585–587. doi: 10.1126/science.2717942. [DOI] [PubMed] [Google Scholar]
- 904.Nelson RJ. Interactions between motor commands and somatic perception in sensorimotor cortex. Curr. Opin. Neurobiol. 1996;6:810–810. doi: 10.1016/s0959-4388(96)80031-6. [DOI] [PubMed] [Google Scholar]
- 905.Netzeband JG, Parsons KL, Sweeney DD, Gruol DL. Metabotropic glutamate receptor agonists alter neuronal excitability and Ca2+ levels via the phospholipase C transduction pathway in cultured Purkinje neurons. J Neurophysiol. 1997;78:63–75. doi: 10.1152/jn.1997.78.1.63. [DOI] [PubMed] [Google Scholar]
- 906.Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 907.Nevin K, Zhuo H, Helke C. Neurokinin A coexists with substance P and serotonin in ventral medullary spinally projecting neurons of the rat. Peptides. 1994;15:1003–1011. doi: 10.1016/0196-9781(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 908.Nicholas AP, Pieribone VA, Arvidsson U, Hökfelt T. Serotonin-, substance P- and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neuroscience. 1992;48:545–559. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- 909.Nicholas AP, Hökfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol. Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- 910.Nicholas AP, Pieribone VA, Hökfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp. Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- 911.Nicholas AP, Pieribone VA, Hökfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience. 1993;56:1023–1039. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- 912.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 913.Nicoll RA. Excitatory action of TRH on spinal motoneurones. Nature. 1977;265:242–243. doi: 10.1038/265242a0. [DOI] [PubMed] [Google Scholar]
- 914.Nicoll RA, Malenka RC. A tale of two transmitters. Science. 1998;281:360–361. doi: 10.1126/science.281.5375.360. [DOI] [PubMed] [Google Scholar]
- 915.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol. Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 916.Nicolopoulos-Stournaras S, Iles J. Motor neuron columns in the lumbar spinal cord of the rat. J Comp. Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- 917.Nikolic Z, Laube B, Weber RG, Lichter P, Kioschis P, Poustka A, Mulhardt C, Becker CM. The human glycine receptor subunit alpha3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J Biol. Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- 918.Nishimura Y, Muramatsu M, Asahara T, Tanaka T, Yamamoto T. Electrophysiological properties and their modulation by norepinephrine in the ambiguus neurons of the guinea pig. Brain Res. 1995;702:213–222. doi: 10.1016/0006-8993(95)01058-4. [DOI] [PubMed] [Google Scholar]
- 919.Nishimura Y, Schwindt P, Crill W. Electrical properties of facial motoneurons in brainstem slices from guinea pig. Brain Res. 1989;502:127–142. doi: 10.1016/0006-8993(89)90468-x. [DOI] [PubMed] [Google Scholar]
- 920.Nistri A. Spinal cord pharmacology of GABA and chemically related amino acids. In: Davidoff RA, editor. Handbook of the Spinal Cord. Vol. 1. New York: Dekker; 1983. pp. 45–105. [Google Scholar]
- 921.Nistri A, Fisher N, Gurnell M. Block by the neuropeptide TRH of an apparently novel K+ conductance of rat motoneurones. Neurosci. Lett. 1990;120:25–30. doi: 10.1016/0304-3940(90)90159-7. [DOI] [PubMed] [Google Scholar]
- 922.North RA. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br. J. Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.North RA, Barnard EA. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 924.Nunez-Abades P, Portillo F, Pasaro R. Characterisation of afferent projections to the nucleus ambiguus of the rat by means of fluorescent double labelling. J Anat. 1990;172:1–15. [PMC free article] [PubMed] [Google Scholar]
- 925.Nygren L, Olson L. A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res. 1977;132:85–93. doi: 10.1016/0006-8993(77)90707-7. [DOI] [PubMed] [Google Scholar]
- 926.O’Brien JA, Isaacson JS, Berger AJ. NMDA and non-NMDA receptors are co-localized at excitatory synapses of rat hypoglossal motoneurons. Neurosci. Lett. 1997;227:5–8. doi: 10.1016/s0304-3940(97)00293-0. [DOI] [PubMed] [Google Scholar]
- 927.O’Donovan MJ. Developmental approaches to the analysis of vertebrate central pattern generators. J Neurosci. Methods. 1987;21:275–286. doi: 10.1016/0165-0270(87)90122-1. [DOI] [PubMed] [Google Scholar]
- 928.Ohta Y, Grillner S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J Neurophysiol. 1989;62:1079–1089. doi: 10.1152/jn.1989.62.5.1079. [DOI] [PubMed] [Google Scholar]
- 929.Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir. Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 930.Okabe S, Woch G, Kubin L. Role of GABAB receptors in the control of hypoglossal motoneurons in vivo. Neuroreport. 1994;5:2573–2576. doi: 10.1097/00001756-199412000-00042. [DOI] [PubMed] [Google Scholar]
- 931.Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol. Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- 932.Olsen RW, Szamraj O, Houser CR. [3H]AMPA binding to glutamate receptor subpopulations in rat brain. Brain Res. 1987;402:243–254. doi: 10.1016/0006-8993(87)90030-8. [DOI] [PubMed] [Google Scholar]
- 933.Ong WY, Motin LG, Hansen MA, Dias LS, Ayrout C, Bennett MR, Balcar VJ. P2 purinoceptor blocker suramin antagonises NMDA receptors and protects against excitatory behaviour caused by NMDA receptor agonist (RS)-(tetrazol-5-yl)-glycine in rats. J Neurosci. Res. 1997;49:627–638. doi: 10.1002/(SICI)1097-4547(19970901)49:5<627::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 934.Ongini E, Fredholm BB. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- 935.Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Hypoglossal premotor neurons with rhythmical inspiratory- related activity in the cat: localization and projection to the phrenic nucleus. Exp. Brain Res. 1994;98:1–12. doi: 10.1007/BF00229103. [DOI] [PubMed] [Google Scholar]
- 936.Oppenheim R. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 937.Örnung G, Ottersen O, Cullheim S, Ulfhake B. Distribution of glutamate-, glycine- and GABA-immunoreactive nerve terminals on dendrites in the cat spinal motor nucleus. Exp. Brain Res. 1998;118:517–532. doi: 10.1007/s002210050308. [DOI] [PubMed] [Google Scholar]
- 939.Örnung G, Ragnarson B, Grant G, Ottersen O, Storm-Mathisen J, Ulfhake B. Ia boutons to CCN neurones and motoneurones are enriched with glutamate-like immunoreactivity. Neuroreport. 1995;6:1975–1980. doi: 10.1097/00001756-199510010-00006. [DOI] [PubMed] [Google Scholar]
- 941.Örnung G, Shupliakov O, Linda H, Ottersen OP, Storm-Mathisen J, Ulfhake B, Cullheim S. Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: a postembedding electron microscopic study. J Comp. Neurol. 1996;365:413–426. doi: 10.1002/(SICI)1096-9861(19960212)365:3<413::AID-CNE6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 942.Örnung G, Shupliakov O, Otterson OP, Stormmathisen J, Cullheim S. Immunohistochemical evidence for coexistence of glycine and GABA in nerve terminals on cat spinal motoneurones: an ultrastructural study. Neuroreport. 1994;5:889–892. doi: 10.1097/00001756-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 943.Ostrowska A, Zimny R, Zguczynski L, Sikora E. The neurons of origin of non-cortical afferent connections to the oculomotor nucleus. A retrograde labeling study in the rabbit. Arch. Ital. Biol. 1991;129:239–258. [PubMed] [Google Scholar]
- 944.Otsuka M, Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974;252:733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- 945.Otsuka M, Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harbor Symp. Quant. Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- 946.Otsuka M, Konishi S, Takahashi T. Hypothalamic substance P as a candidate for transmitter of primary afferent neurons. Federation Proc. 1975;34:1922–1928. [PubMed] [Google Scholar]
- 947.Otsuka M, Yanagisawa M. The effects of substance P and baclofen on motoneurones of isolated spinal cord of the newborn rat. J Exp. Biol. 1980;89:201–214. doi: 10.1242/jeb.89.1.201. [DOI] [PubMed] [Google Scholar]
- 948.Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 949.Otsuka M, Yoshioka K, Yanagisawa M, Suzuki H, Zhao FY, Guo JZ, Hosoki R, Kurihara T. Use of NK1 receptor antagonists in the exploration of physiological functions of substance P and neurokinin A. Can. J. Physiol. Pharmacol. 1995;73:903–907. doi: 10.1139/y95-124. [DOI] [PubMed] [Google Scholar]
- 950.Ouardouz M, Durand J. Involvement of AMPA receptors in trigeminal post-synaptic potentials recorded in rat abducens motoneurons in vivo. Eur. J. Neurosci. 1994;6:1662–1668. doi: 10.1111/j.1460-9568.1994.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 951.Ozaki S, Kudo N, Okado N. Immunohistochemical study on development of serotonin-, substance P-, and enkephalin-positive fibers in the rat spinal motor nucleus. J Comp. Neurol. 1992;325:462–470. doi: 10.1002/cne.903250311. [DOI] [PubMed] [Google Scholar]
- 952.Palacios J, Wamsley J. Catecholamine receptors. In: Hokfelt T, Bjorklund A, editors. Classical Transmitters in the CNS. Amsterdam: Elsevier; 1984. pp. 325–351. [Google Scholar]
- 953.Palacios JM, Kuhar MJ. Beta-adrenergic-receptor localization by light microscopic autoradiography. Science. 1980;208:1378–1380. doi: 10.1126/science.6246585. [DOI] [PubMed] [Google Scholar]
- 954.Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- 955.Palmer TM, Stiles GL. Adenosine receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 956.Palouzier-Paulignan B, Dubois-Dauphin M, Tribollet E, Dreifuss J, Raggenbass M. Action of vasopressin on hypoglossal motoneurones of the rat: presynaptic and postsynaptic effects. Brain Res. 1994;650:117–126. doi: 10.1016/0006-8993(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 957.Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 958.Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- 959.Parkis MA, Berger AJ. Clonidine reduces hyperpolarization- activated inward current (Ih) in rat hypoglossal motoneurons. Brain Res. 1997;769:108–118. doi: 10.1016/s0006-8993(97)00677-x. [DOI] [PubMed] [Google Scholar]
- 960.Parkis MA, Dong X-W, Feldman JL, Funk GD. The 20–50 Hz bandwidth of inspiratory synaptic drive currents enhances action potential (AP) output from phrenic motoneurons (PMNs) Soc. Neurosci. Abstr. 1998;24:913. [Google Scholar]
- 961.Parkis MA, Dong X-W, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.Partridge LD, Müller TH, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Res. Rev. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 963.Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci. Lett. 1995;196:185–188. doi: 10.1016/0304-3940(95)11873-u. [DOI] [PubMed] [Google Scholar]
- 964.Pazos A, Cortes R, Palacios J. Thyrotropin-releasing hormone receptor binding sites: autoradiographic distribution in the rat and guinea pig brain. J Neurochem. 1985;45:1448–1463. doi: 10.1111/j.1471-4159.1985.tb07212.x. [DOI] [PubMed] [Google Scholar]
- 965.Pedroarena C, Castillo P, Chase MH, Morales FR. The control of jaw-opener motoneurons during active sleep. Brain Res. 1994;653:31–38. doi: 10.1016/0006-8993(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 966.Pellegrini-Giampietro DE, Fan S, Ault B, Miller BE, Zukin RS. Glutamate receptor gene expression in spinal cord of arthritic rats. J Neurosci. 1994;14:1576–1583. doi: 10.1523/JNEUROSCI.14-03-01576.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967.Perkins KL, Wong RK. The depolarizing GABA response. Can. J. Physiol. Pharmacol. 1997;75:516–519. [PubMed] [Google Scholar]
- 968.Perret C. Centrally generated pattern of motoneuron activity during locomotion in cat. Symp. Soc. Exp. Biol. 1983;27:405–422. [PubMed] [Google Scholar]
- 969.Perret C. Synaptic influences contributing to the pattern of limb motoneuron activity during fictive locomotion in the cat. In: Grillner S, editor. Neurobiology of Vertebrate Locomotion. New York: Macmillan; 1986. pp. 173–184. [Google Scholar]
- 970.Perrier J, Hounsgaard J. Ca2+-activated nonselective cationic current (ICAN) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- 971.Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- 972.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp. Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 973.Peshori KR, Collins WF, Mendell LM. EPSP amplitude modulation at the rat Ia-alpha motoneuron synapse: effects of GABAB receptor agonists and antagonists. J Neurophysiol. 1998;79:181–189. doi: 10.1152/jn.1998.79.1.181. [DOI] [PubMed] [Google Scholar]
- 974.Peter J, Burbach H, Adan RA, Lolait SJ, van Leeuwen FW, Mezey E, Palkovits M, Barberis C. Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cell. Mol. Neurobiol. 1995;15:573–595. doi: 10.1007/BF02071318. [DOI] [PubMed] [Google Scholar]
- 975.Petralia RS, Wang YX, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp. Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 976.Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp. Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 977.Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp. Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 979.Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 981.Pfeiffer F, Simler R, Grennigloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc. Natl. Acad. Sci. USA. 1984;81:7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Phillips M, Shen L, Richards E, Raizada M. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul. Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- 983.Phillis J, Tebecis A, York D. Depression of spinal motoneurones by noradrenaline, 5-hydroxytryptamine and histamine. Eur. J. Pharmacol. 1968;4:471–475. doi: 10.1016/0014-2999(68)90037-x. [DOI] [PubMed] [Google Scholar]
- 984.Piehl F, Tabar G, Cullheim S. Expression of NMDA receptor mRNAs in rat motoneurons is down-regulated after axotomy. Eur. J. Neurosci. 1995;7:2101–2110. doi: 10.1111/j.1460-9568.1995.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 985.Pierau F-K, Zimmerman P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cat’s spinal motoneurons. Brain Res. 1973;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- 986.Pieribone VA, Nicholas AP, Dagerlind A, Hökfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Pierrefiche O, Bischoff AM, Richter DW, Spyer KM. Hypoxic response of hypoglossal motoneurones in the in vivo cat. J Physiol. (Lond.) 1997;505:785–795. doi: 10.1111/j.1469-7793.1997.785ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Pilowsky PM, De Castro CD, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 990.Pinco M, Lev-Tov A. Modulation of monosynaptic excitation in the neonatal rat spinal cord. J Neurophysiol. 1993;70:1151–1158. doi: 10.1152/jn.1993.70.3.1151. [DOI] [PubMed] [Google Scholar]
- 991.Pinco M, Lev-Tov A. Synaptic excitation of alpha-motoneurons by dorsal root afferents in the neonatal rat spinal cord. J Neurophysiol. 1993;70:406–417. doi: 10.1152/jn.1993.70.1.406. [DOI] [PubMed] [Google Scholar]
- 992.Pinco M, Lev-Tov A. Synaptic transmission between ventrolateral funiculus axons and lumbar motoneurons in the isolated spinal cord of the neonatal rat. J Neurophysiol. 1994;72:2406–2419. doi: 10.1152/jn.1994.72.5.2406. [DOI] [PubMed] [Google Scholar]
- 993.Pinter M, Curtis R, Hosko M. Voltage threshold and excitability among variously sized cat hindlimb motoneurons. J Neurophysiol. 1983;50:644–657. doi: 10.1152/jn.1983.50.3.644. [DOI] [PubMed] [Google Scholar]
- 994.Pittaluga A, Thellung S, Maura G, Raiteri M. Characterization of two central AMPA-preferring receptors having distinct location, function and pharmacology. Naunyn-Schmiedebergs Arch. Pharmacol. 1994;349:555–558. doi: 10.1007/BF01258458. [DOI] [PubMed] [Google Scholar]
- 995.Plant T, Schirra C, Katz E, Uchitel O, Konnerth A. Single-cell RT-PCR and functional characterization of Ca2+ channels in motoneurons of the rat facial nucleus. J Neurosci. 1998;18:9573–9584. doi: 10.1523/JNEUROSCI.18-23-09573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Polc P. 2-Amino-7-phosphonoheptanoic acid depresses gamma-motoneurons and polysynaptic reflexes in the cat spinal cord. Eur. J. Pharmacol. 1985;117:387–389. doi: 10.1016/0014-2999(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 997.Populin LC, Yin TC. Topographical organization of the motoneuron pools that innervate the muscles of the pinna of the cat. J Comp. Neurol. 1995;363:600–614. doi: 10.1002/cne.903630407. [DOI] [PubMed] [Google Scholar]
- 998.Porter J, Balaban C. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib. Res. 1997;7:63–76. [PubMed] [Google Scholar]
- 999.Poulat P, Legrand A, Rajaofetra N, Marlier L, Privat A, Oliver C. Pre- and post-natal ontogeny of thyrotropin-releasing-hormone in the rat spinal cord: an immunocytochemical study. Dev. Brain Res. 1992;70:245–257. doi: 10.1016/0165-3806(92)90204-a. [DOI] [PubMed] [Google Scholar]
- 1000.Poulat P, Sandillon F, Marlier L, Rajaofetra N, Oliver C, Privat A. Distribution of thyrotropin-releasing hormone in the rat spinal cord with special reference to sympathetic nuclei: a light- and electron-microscopic immunocytochemical study. J Neurocytol. 1992;21:157–170. doi: 10.1007/BF01194975. [DOI] [PubMed] [Google Scholar]
- 1000.Poulter MO, Barker JL, O’Carrol AM, Lolait SJ, Mahan LC. Differential and transient expression of GABAA receptor a-subunit mRNAs in the developing rat CNS. J Neurosci. 1993;122:2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002.Poulter MO, Barker JL, O’Carroll AM, Lolait SJ, Mahan LC. Co-existent expression of GABAA receptor beta 2, beta 3 and gamma 2 subunit messenger RNAs during embryogenesis and early postnatal development of the rat central nervous system. Neuroscience. 1993;53:1019–1033. doi: 10.1016/0306-4522(93)90486-y. [DOI] [PubMed] [Google Scholar]
- 1003.Powers RK. A variable-threshold motoneuron model that incorporates time- and voltage-dependent potassium and calcium conductances. J Neurophysiol. 1993;70:246–262. doi: 10.1152/jn.1993.70.1.246. [DOI] [PubMed] [Google Scholar]
- 1004.Powers RK, Binder MD. Effective synaptic current and motoneuron firing rate modulation. J Neurophysiol. 1995;74:793–801. doi: 10.1152/jn.1995.74.2.793. [DOI] [PubMed] [Google Scholar]
- 1005.Powers RK, Binder MD. Experimental evaluation of input-output models of motoneuron discharge. J Neurophysiol. 1996;75:367–379. doi: 10.1152/jn.1996.75.1.367. [DOI] [PubMed] [Google Scholar]
- 1006.Price GW, Kelly JS, Bowery NG. The location of GABAB receptor binding sites in mammalian spinal cord. Synapse. 1987;1:530–538. doi: 10.1002/syn.890010605. [DOI] [PubMed] [Google Scholar]
- 1007.Price GW, Wilkin GP, Turnbull MJ, Bowery NG. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984;307:71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- 1008.Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 1009.Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA/benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- 1010.Probst A, Cortes R, Palacios JM. The distribution of glycine receptors in the human brain. A light microscopic autoradiographic study using [3H]strychnine. Neuroscience. 1986;17:11–35. doi: 10.1016/0306-4522(86)90222-8. [DOI] [PubMed] [Google Scholar]
- 1011.Proudfit H, Clark F. The projections of locus coeruleus neurons to the spinal cord. Prog. Brain Res. 1991;88:123–141. doi: 10.1016/s0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- 1012.Puskar Z, Antal M. Localization of last-order premotor interneurons in the lumbar spinal cord of rats. J Comp. Neurol. 1997;389:377–389. doi: 10.1002/(sici)1096-9861(19971222)389:3<377::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 1013.Quevedo J, Eguibar JR, Jimenez I, Rudomin P. Differential action of (−)-baclofen on the primary afferent depolarization produced by segmental and descending inputs. Exp. Brain Res. 1992;91:29–45. doi: 10.1007/BF00230011. [DOI] [PubMed] [Google Scholar]
- 1014.Quirion R, Dam TV. Ontogeny of substance P receptor binding sites in rat brain. J Neurosci. 1986;6:2187–2199. doi: 10.1523/JNEUROSCI.06-08-02187.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Raappana P, Arvidsson J. Location, morphology, and central projections of mesencephalic trigeminal neurons innervating rat masticatory muscles studied by axonal transport of choleragenoid-horseradish peroxidase. J Comp. Neurol. 1993;328:103–114. doi: 10.1002/cne.903280108. [DOI] [PubMed] [Google Scholar]
- 1016.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 1017.Racca C, Gardiol A, Triller A. Dendritic and postsynaptic localizations of glycine receptor alpha subunit mRNAs. J Neurosci. 1997;17:1691–1700. doi: 10.1523/JNEUROSCI.17-05-01691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Racca C, Gardiol A, Triller A. Cell-specific dendritic localization of glycine receptor alpha subunit messenger RNAs. Neuroscience. 1998;84:997–1012. doi: 10.1016/s0306-4522(97)00585-x. [DOI] [PubMed] [Google Scholar]
- 1019.Raggenbass M, Goumaz M, Sermasi E, Tribollet E, Dreifuss JJ. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991;11:1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc. Natl. Acad. Sci. USA. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.Rajaofetra N, Poulat P, Marlier L, Geffard M, Privat A. Pre- and postnatal development of noradrenergic projections to the rat spinal cord: an immunocytochemical study. Brain Res. 1992;67:237–246. doi: 10.1016/0165-3806(92)90224-k. [DOI] [PubMed] [Google Scholar]
- 1022.Rajaofetra N, Ridet J, Poulat P, Marlier L, Sandillon F, Geffard M, Privat A. Immunocytochemical mapping of noradrenergic projections to the rat spinal cord with an antiserum against noradrenaline. J Neurocytol. 1992;21:481–494. doi: 10.1007/BF01186952. [DOI] [PubMed] [Google Scholar]
- 1023.Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol. Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 1024.Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiol. Rev. 1992;72(Suppl):S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- 1025.Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- 1026.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr. Opin. Neurobiol. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- 1027.Ramirez-Leon V, Ulfhake B. GABA-like immunoreactive innervation and dendro-dendritic contacts in the ventrolateral dendritic bundle in the cat S1 spinal cord segment: an electron microscopic study. Exp. Brain Res. 1993;97:1–12. doi: 10.1007/BF00228812. [DOI] [PubMed] [Google Scholar]
- 1028.Ramoa AS, Prusky G. Retinal activity regulates developmental switches in functional properties and ifenprodil sensitivity of NMDA receptors in the lateral geniculate nucleus. Brain Res. 1997;101:165–175. doi: 10.1016/s0165-3806(97)00061-8. [DOI] [PubMed] [Google Scholar]
- 1029.Rampon C, Peyron C, Petit JM, Fort P, Gervasoni D, Luppi PH. Origin of the glycinergic innervation of the rat trigeminal motor nucleus. Neuroreport. 1996;7:3081–3085. doi: 10.1097/00001756-199611250-00058. [DOI] [PubMed] [Google Scholar]
- 1030.Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 1031.Rao H, Jean A, Kessler JP. Postnatal ontogeny of glutamate receptors in the rat nucleus tractus solitarii and ventrolateral medulla. J Auton. Nerv. Syst. 1997;65:25–32. doi: 10.1016/s0165-1838(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 1032.Rasmussen K, Aghajanian GK. Serotonin excitation of facial motoneurons: receptor subtype characterization. Synapse. 1990;5:324–332. doi: 10.1002/syn.890050409. [DOI] [PubMed] [Google Scholar]
- 1033.Redburn DA, Schousboe A. Neurotrophic Activity of GABA During Development. New York: Liss; 1987. [Google Scholar]
- 1034.Reddy V, Cassini P, Ho R, Martin G. Origins and terminations of bulbospinal axons that contain serotonin and either enkephalin or substance-P in the North American opossum. J Comp. Neurol. 1990;294:96–108. doi: 10.1002/cne.902940108. [DOI] [PubMed] [Google Scholar]
- 1035.Redman SJ. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol. Rev. 1990;70:165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- 1036.Redman SJ, Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. (Lond.) 1983;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1037.Reichlin S. TRH: historical aspects. Ann. NY Acad. Sci. 1989;553:1–6. doi: 10.1111/j.1749-6632.1989.tb46627.x. [DOI] [PubMed] [Google Scholar]
- 1038.Reichlin S. Neuroendocrinology. In: Wilson J, Foster D, editors. Williams Textbook of Endocrinology. Philadelphia, PA: Saunders; 1992. pp. 135–219. [Google Scholar]
- 1039.Rekling JC. Excitatory effects of thyrotropin-releasing hormone (TRH) in hypoglossal motoneurons. Brain Res. 1990;510:175–179. doi: 10.1016/0006-8993(90)90749-2. [DOI] [PubMed] [Google Scholar]
- 1040.Rekling JC. Interaction between thyrotropin-releasing hormone (TRH) and NMDA-receptor-mediated responses in hypoglossal motoneurones. Brain Res. 1992;578:289–296. doi: 10.1016/0006-8993(92)90260-g. [DOI] [PubMed] [Google Scholar]
- 1041.Rekling JC, Feldman JL. Calcium-dependent plateau potentials in rostral ambiguus neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1997;78:2483–2492. doi: 10.1152/jn.1997.78.5.2483. [DOI] [PubMed] [Google Scholar]
- 1042.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 1043.Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol. Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- 1044.Ribeiro-do-Valle L, Metzler C, Jacobs B. Facilitation of masseter EMG and masseteric (jaw-closure) reflex by serotonin in behaving cats. Brain Res. 1991;550:197–204. doi: 10.1016/0006-8993(91)91318-u. [DOI] [PubMed] [Google Scholar]
- 1045.Richards JG, Schoch P, Haring B, Mohler H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J Neurosci. 1987;7:1866–1886. doi: 10.1523/JNEUROSCI.07-06-01866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Ridet J, Tamir H, Privat A. Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-idiotypic antiserum. J Neurosci. Res. 1994;38:109–121. doi: 10.1002/jnr.490380114. [DOI] [PubMed] [Google Scholar]
- 1047.Rikard-Bell G, Bystrzycka E, Nail B. The identification of brainstem neurones projecting to thoracic respiratory motoneurones in the cat as demonstrated by retrograde transport of HRP. Brain Res. Bull. 1985;14:25–37. doi: 10.1016/0361-9230(85)90174-1. [DOI] [PubMed] [Google Scholar]
- 1048.Roberts M, Davies M, Girdlestone D, Foster G. Effects of 5-hydroxytryptamine agonists and antagonists on the responses of rat spinal motoneurones to raphe obscurus stimulation. Br. J. Pharmacol. 1988;95:437–448. doi: 10.1111/j.1476-5381.1988.tb11664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049.Robertson GA, Stein PS. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol. (Lond.) 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Robertson SJ, Edwards FA. ATP and glutamate are released from separate neurones in the rat medial habenula nucleus: frequency dependence and adenosine-mediated inhibition of release. J Physiol. (Lond.) 1998;508:691–701. doi: 10.1111/j.1469-7793.1998.691bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1051.Robinson F, Phillips J, Fuchs A. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J. Comp. Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- 1052.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 1053.Roche KW, Tingley WG, Huganir RL. Glutamate receptor phosphorylation and synaptic plasticity. Curr. Opin. Neurobiol. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 1054.Roelink H, Porter J, Chiang C, Tanabe Y, Chang D, Beachy P, Jessell T. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 1055.Romanes G. The motor cell columns of the lumbo-sacral spinal cord of the cat. J. Comp. Neurol. 1951;94:313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- 1056.Romano C, Sesma MA, McDonald CT, O’Malley K, van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 1057.Rose PK, Wainwright K, Neuber-Hess M. Connections from the lateral vestibular nucleus to the upper cervical spinal cord of the cat: a study with the anterograde tracer PHA-L. J. Comp. Neurol. 1992;321:312–324. doi: 10.1002/cne.903210210. [DOI] [PubMed] [Google Scholar]
- 1058.Rose PK, Neuber-Hess M. Morphology and frequency of axon terminals on the somata, proximal dendrites, and distal dendrites of dorsal neck motoneurons in the cat. J. Comp. Neurol. 1991;307:259–280. doi: 10.1002/cne.903070208. [DOI] [PubMed] [Google Scholar]
- 1059.Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- 1060.Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of alpha 2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 1061.Rotter A, Schultz CM, Frostholm A. Regulation of glycine receptor binding in the mouse hypoglossal nucleus in response to axotomy. Brain Res. Bull. 1984;13:487–492. doi: 10.1016/0361-9230(84)90029-7. [DOI] [PubMed] [Google Scholar]
- 1062.Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- 1063.Rudomin P, Quevedo J, Eguibar JR. Presynaptic modulation of spinal reflexes. Curr. Opin. Neurobiol. 1993;3:997–1004. doi: 10.1016/0959-4388(93)90173-v. [DOI] [PubMed] [Google Scholar]
- 1064.Rudomin P, Solodkin M, Jimenez I. Synaptic potentials of primary afferent fibers and motoneurons evoked by single intermediate nucleus interneurons in the cat spinal cord. J. Neurophysiol. 1987;57:1288–1313. doi: 10.1152/jn.1987.57.5.1288. [DOI] [PubMed] [Google Scholar]
- 1065.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 1066.Ruffolo RR., Jr Interactions of agonists with peripheral alpha-adrenergic receptors. Federation Proc. 1984;43:2910–2916. [PubMed] [Google Scholar]
- 1067.Ruffolo RR, Jr, Nichols AJ, Stadel JM, Hieble JP. Structure and function of alpha-adrenoceptors. Pharmacol. Rev. 1991;43:475–505. [PubMed] [Google Scholar]
- 1068.Safronov B, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. J. Physiol. (Lond.) 1995;487:91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069.Safronov BV, Vogel W. Properties and functions of Na+-activated K+ channels in the soma of rat motoneurones. J. Physiol. (Lond.) 1996;497:727–734. doi: 10.1113/jphysiol.1996.sp021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070.Safronov BV, Vogel W. Large conductance Ca2+-activated K+ channels in the soma of rat motoneurones. J. Membr. Biol. 1998;162:9–15. doi: 10.1007/s002329900337. [DOI] [PubMed] [Google Scholar]
- 1071.Saha S, Appenteng K, Balten TFC. Quantitative analysis and postsynaptic targets of GABA-immunoreactive boutons within the rat trigeminal motor nucleus. Brain Res. 1991;561:128–138. doi: 10.1016/0006-8993(91)90757-m. [DOI] [PubMed] [Google Scholar]
- 1072.Saha S, Appenteng K, Batten T. Light and electron microscopical localisation of 5-HT-immunoreactive boutons in the rat trigeminal motor nucleus. Brain Res. 1991;559:145–148. doi: 10.1016/0006-8993(91)90297-9. [DOI] [PubMed] [Google Scholar]
- 1073.Saito K. Development of spinal reflexes in the rat foetus studied in vitro. J. Physiol. (Lond.) 1979;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.Sakitama K. Intrathecal noradrenaline facilitates and inhibits the flexor reflex mediated by group II afferent fibers via alpha 1- and alpha 2-receptors, respectively. Jpn. J. Pharmacol. 1993;62:131–136. doi: 10.1254/jjp.62.131. [DOI] [PubMed] [Google Scholar]
- 1075.Salt TE, Eaton SA. Distinct presynaptic metabotropic receptors for l-AP4 and CCG1 on GABAergic terminals: pharmacological evidence using novel alpha-methyl derivative mGluR antagonists, MAP4 and MCCG, in the rat thalamus in vivo. Neuroscience. 1995;65:5–13. doi: 10.1016/0306-4522(94)00464-g. [DOI] [PubMed] [Google Scholar]
- 1076.Salter MW. SRC, N-methyl-d-aspartate (NMDA) receptors and synaptic plasticity. Biochem. Pharmacol. 1998;56:789–798. doi: 10.1016/s0006-2952(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 1077.Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog. Neurobiol. 1993;41:125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 1078.Samarasinghe S, Virgo L, De Belleroche J. Distribution of the N-methyl-d-aspartate glutamate receptor subunit NR2A in control and amyotrophic lateral sclerosis spinal cord. Brain Res. 1996;727:233–237. doi: 10.1016/0006-8993(96)00506-9. [DOI] [PubMed] [Google Scholar]
- 1079.Samathanam G, Duffy P, Kalivas PW, White SR. A comparison of 5-hydroxytryptophan effects on rat lumbar spinal cord serotonin release and monosynaptic response amplitude. Brain Res. 1989;501:179–182. doi: 10.1016/0006-8993(89)91040-8. [DOI] [PubMed] [Google Scholar]
- 1080.Sasek CA, Wessendorf MW, Helke CJ. Evidence for co-existence of thyrotropin-releasing hormone, substance P and serotonin in ventral medullary neurons that project to the intermediolateral cell column in the rat. Neuroscience. 1990;35:105–119. doi: 10.1016/0306-4522(90)90125-n. [DOI] [PubMed] [Google Scholar]
- 1081.Sato K, Kiyama H, Tohyama M. Regional distribution of cells expressing glycine receptor alpha 2 subunit mRNA in the rat brain. Brain Res. 1992;590:95–108. doi: 10.1016/0006-8993(92)91085-s. [DOI] [PubMed] [Google Scholar]
- 1082.Sato K, Kiyama H, Tohyama M. The differential expression patterns of messenger RNAs encoding non-N-methyl-d-aspartate glutamate receptor subunits (GluR1–4) in the rat brain. Neuroscience. 1993;52:515–539. doi: 10.1016/0306-4522(93)90403-3. [DOI] [PubMed] [Google Scholar]
- 1083.Sato K, Zhang JH, Saika T, Sato M, Tada K, Tohyama M. Localization of glycine receptor alpha 1 subunit mRNA-containing neurons in the rat brain: an analysis using in situ hybridization histochemistry. Neuroscience. 1991;43:381–395. doi: 10.1016/0306-4522(91)90302-5. [DOI] [PubMed] [Google Scholar]
- 1084.Satoh I, Kobayashi N, Nakajima Y, Konno A. Upper airway motor outputs during sneezing and coughing in decerebrate cats. Neurosci. Res. 1998;32:131–135. doi: 10.1016/s0168-0102(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 1085.Saugstad JA, Kinzie JM, Mulvihill ER, Segerson TP, Westbrook GL. Cloning and expression of a new member of the l-2-amino-4-phosphonobutyric acid-sensitive class of metabotropic glutamate receptors. Mol. Pharmacol. 1994;45:367–372. [PubMed] [Google Scholar]
- 1086.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 1087.Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J. Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- 1088.Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J. Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- 1089.Sayer RJ, Schwindt PC, Crill WE. Metabotropic glutamate receptor-mediated suppression of L-type calcium current in acutely isolated neocortical neurons. J. Neurophysiol. 1992;68:833–842. doi: 10.1152/jn.1992.68.3.833. [DOI] [PubMed] [Google Scholar]
- 1090.Scanziani M, Capogna M, Gähwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 1091.Scanziani M, Gahwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+ channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- 1092.Scatton B, Dubois A, Javoy-Agid F, Camus A. Autoradiographic localization of muscarinic cholinergic receptors at various segmental levels of the human spinal cord. Neurosci. Lett. 1984;49:239–245. doi: 10.1016/0304-3940(84)90296-9. [DOI] [PubMed] [Google Scholar]
- 1093.Schafer M, Eiden L, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system. Neuroscience. 1998;84:331–359. doi: 10.1016/s0306-4522(97)00516-2. [DOI] [PubMed] [Google Scholar]
- 1094.Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- 1095.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 1096.Schipper J, Steinbusch H, Vermes I, Tilders F. Mapping of CRF-immunoreactive nerve fibers in the medulla oblongata and spinal cord of the rat. Brain Res. 1983;267:145–150. doi: 10.1016/0006-8993(83)91048-x. [DOI] [PubMed] [Google Scholar]
- 1097.Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J. Physiol. (Lond.) 1995;483:769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Schmitt J, Gacek R. Anatomical investigation of the corticonuclear projections to the facial nerve nucleus in the cat. Laryngoscope. 1986;96:129–134. doi: 10.1288/00005537-198602000-00001. [DOI] [PubMed] [Google Scholar]
- 1099.Schneider S, Fyffe R. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J. Neurophysiol. 1992;68:397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- 1100.Schoepfer R, Monyer H, Sommer B, Wisden W, Sprengel R, Kuner T, Lomeli H, Herb A, Kohler M, Burnashev N. Molecular biology of glutamate receptors. Prog. Neurobiol. 1994;42:353–357. doi: 10.1016/0301-0082(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 1101.Schoepp DD, Johnson BG, Monn JA. Inhibition of cyclic AMP formation by a selective metabotropic glutamate receptor agonist. J. Neurochem. 1992;58:1184–1186. doi: 10.1111/j.1471-4159.1992.tb09381.x. [DOI] [PubMed] [Google Scholar]
- 1102.Scholz KP, Miller RJ. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- 1103.Schomburg E. Spinal sensorimotor systems and their supraspinal control. Neurosci. Res. 1990;7:265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- 1104.Schomburg E, Steffens H. Bistable characteristics of motoneurone activity during DOPA induced fictive locomotion in spinal cats. Neurosci. Res. 1996;26:47–56. doi: 10.1016/0168-0102(96)01073-5. [DOI] [PubMed] [Google Scholar]
- 1105.Schoppa NE, Westbrook GL. Modulation of mEPSCs in olfactory bulb mitral cells by metabotropic glutamate receptors. J. Neurophysiol. 1997;78:1468–1475. doi: 10.1152/jn.1997.78.3.1468. [DOI] [PubMed] [Google Scholar]
- 1106.Schousboe A, Redburn DA. Modulatory actions of gamma aminobutyric acid (GABA) on GABA type A receptor subunit expression and function. J. Neurosci. Res. 1995;41:1–7. doi: 10.1002/jnr.490410102. [DOI] [PubMed] [Google Scholar]
- 1107.Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J. Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- 1108.Schrader LA, Tasker JG. Modulation of multiple potassium currents by metabotropic glutamate receptors in neurons of the hypothalamic supraoptic nucleus. J. Neurophysiol. 1997;78:3428–3437. doi: 10.1152/jn.1997.78.6.3428. [DOI] [PubMed] [Google Scholar]
- 1109.Schwindt PC. Membrane-potential trajectories underlying motoneuron rhythmic firing at high rates. J. Neurophysiol. 1973;36:434–439. doi: 10.1152/jn.1973.36.3.434. [DOI] [PubMed] [Google Scholar]
- 1110.Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. J. Neurophysiol. 1980;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- 1111.Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J. Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- 1112.Schwindt PC, Crill WE. Effects of barium on cat spinal motoneurons studied by voltage clamp. J. Neurophysiol. 1980;44:827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- 1113.Schwindt PC, Crill WE. Differential effects of TEA and cations on outward ionic currents of cat motoneurons. J. Neurophysiol. 1981;46:1–16. doi: 10.1152/jn.1981.46.1.1. [DOI] [PubMed] [Google Scholar]
- 1114.Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J. Neurophysiol. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- 1115.Scudder C, Fuchs A, Langer T. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J. Neurophysiol. 1988;59:1430–1454. doi: 10.1152/jn.1988.59.5.1430. [DOI] [PubMed] [Google Scholar]
- 1116.Sebasti OAM, Ribeiro JA. Evidence for the presence of excitatory A2 adenosine receptors in the rat hippocampus. Neurosci. Lett. 1992;138:41–44. doi: 10.1016/0304-3940(92)90467-l. [DOI] [PubMed] [Google Scholar]
- 1117.Seebach BS, Mendell LM. Maturation in properties of motoneurons and their segmental input in the neonatal rat. J. Neurophysiol. 1996;76:3875–3885. doi: 10.1152/jn.1996.76.6.3875. [DOI] [PubMed] [Google Scholar]
- 1118.Seeburg PH. The TINS/TIPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 1119.Seeburg PH. The TIPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol. Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- 1120.Seeburg PH. The role of RNA editing in controlling glutamate receptor channel properties. J. Neurochem. 1996;66:1–5. doi: 10.1046/j.1471-4159.1996.66010001.x. [DOI] [PubMed] [Google Scholar]
- 1121.Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain receptor channels: mechanism and physiology. Brain Res. Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 1122.Segal M. Intracellular analysis of a postsynaptic action of adenosine in the rat hippocampus. Eur. J. Pharmacol. 1982;79:193–199. doi: 10.1016/0014-2999(82)90625-2. [DOI] [PubMed] [Google Scholar]
- 1123.Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axon terminals. J. Neurophysiol. 1990;63:987–998. doi: 10.1152/jn.1990.63.5.987. [DOI] [PubMed] [Google Scholar]
- 1124.Segev I, Fleshman JW, Jr, Burke RE. Computer simulation of group Ia EPSPs using morphologically realistic models of cat alpha-motoneurons. J. Neurophysiol. 1990;64:648–660. doi: 10.1152/jn.1990.64.2.648. [DOI] [PubMed] [Google Scholar]
- 1125.Seguela P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. J. Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. A novel allosteric potentiator of AMPA receptors: 4–2-(phenylsulfonylamino)ethylthio-2,6-difluoro-phenoxyaceta mide. J. Neurosci. 1997;17:5760–5771. doi: 10.1523/JNEUROSCI.17-15-05760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Selvaratnam S, Parkis MA, Funk GD. Developmental modulation of mouse hypoglossal nerve inspiratory output in vitro by noradrenergic receptor agonists. Brain Res. 1998;805:104–115. doi: 10.1016/s0006-8993(98)00673-8. [DOI] [PubMed] [Google Scholar]
- 1128.Senaris R, Schindler M, Humphrey P, Emson P. Expression of somatostatin receptor 3 mRNA in the motorneurones of the rat spinal cord, and the sensory neurones of the spinal ganglia. Brain Res. 1995;29:185–190. doi: 10.1016/0169-328x(94)00275-j. [DOI] [PubMed] [Google Scholar]
- 1129.Seth P, Gajendiran M, Maitra K, Ross H, Ganguly D. Evidence for D1 dopamine receptor-mediated modulation of the synaptic transmission from motor axon collaterals to Renshaw cells in the rat spinal cord. Neurosci. Lett. 1993;158:217–220. doi: 10.1016/0304-3940(93)90268-p. [DOI] [PubMed] [Google Scholar]
- 1130.Shapiro R, Miselis R. The central neural connections of the area postrema of the rat. J. Comp. Neurol. 1985;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 1131.Sharif NA. Quantitative autoradiography of TRH receptors in discrete brain regions of different mammalian species. Ann. NY Acad. Sci. 1989;553:147–175. doi: 10.1111/j.1749-6632.1989.tb46638.x. [DOI] [PubMed] [Google Scholar]
- 1132.Shaw PJ, Chinnery RM, Ince PG. Non-NMDA receptors in motor neuron disease (MND): a quantitative autoradiographic study in spinal cord and motor cortex using [3H]CNQX and [3H]kainate. Brain Res. 1994;655:186–194. doi: 10.1016/0006-8993(94)91613-6. [DOI] [PubMed] [Google Scholar]
- 1133.Shaw PJ, Ince PG. A quantitative autoradiographic study of [3H]kainate binding sites in the normal human spinal cord, brainstem and motor cortex. Brain Res. 1994;641:39–45. doi: 10.1016/0006-8993(94)91812-0. [DOI] [PubMed] [Google Scholar]
- 1134.Shaw PJ, Ince PG, Johnson M, Perry EK, Candy J. The quantitative autoradiographic distribution of [3H]MK-801 binding sites in the normal human spinal cord. Brain Res. 1991;539:164–168. doi: 10.1016/0006-8993(91)90701-v. [DOI] [PubMed] [Google Scholar]
- 1135.Shaw PJ, Ince PG, Johnson M, Perry EK, Candy JM. The quantitative autoradiographic distribution of [3H]MK-801 binding sites in the normal human brainstem in relation to motor neuron disease. Brain Res. 1992;572:276–280. doi: 10.1016/0006-8993(92)90484-q. [DOI] [PubMed] [Google Scholar]
- 1136.Shaw PJ, Ince PG, Matthews JN, Johnson M, Candy JM. N-methyl-d-aspartate (NMDA) receptors in the spinal cord and motor cortex in motor neuron disease: a quantitative autoradiographic study using [3H]MK-801. Brain Res. 1994;637:297–302. doi: 10.1016/0006-8993(94)91248-3. [DOI] [PubMed] [Google Scholar]
- 1137.Shaw PJ, Williams TL, Slade JY, Eggett CJ, Ince PG. Low expression of GluR2 AMPA receptor subunit protein by human motor neurons. Neuroreport. 1999;10:261–265. doi: 10.1097/00001756-199902050-00011. [DOI] [PubMed] [Google Scholar]
- 1138.Shefchyk SJ, Epsey MJ, Carr P, Nance D, Sawchuk M, Buss R. Evidence for a strychnine-sensitive mechanism and glycine receptors involved in the control of urethral sphincter activity during micturition in the cat. Exp. Brain Res. 1998;119:297–306. doi: 10.1007/s002210050345. [DOI] [PubMed] [Google Scholar]
- 1139.Shefchyk SJ, Jordan LM. Motoneuron input-resistance changes during fictive locomotion produced by stimulation of the mesencephalic locomotor region. J. Neurophysiol. 1985;54:1101–1108. doi: 10.1152/jn.1985.54.5.1101. [DOI] [PubMed] [Google Scholar]
- 1140.Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- 1141.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 1142.Sherrington C. The Integrative Action of the Nervous System. 2nd. New Haven, CT: Yale Univ. Press; 1947. [Google Scholar]
- 1143.Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J. Neurosci. 1999;19:2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1144.Shiba K, Umezaki T, Zheng Y, Miller A. The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp. Brain Res. 1997;115:513–519. doi: 10.1007/pl00005721. [DOI] [PubMed] [Google Scholar]
- 1145.Shibata T, Watanabe M, Ichikawa R, Inoue Y, Koyanagi T. Different expressions of alpha-amino-3-hydroxy-5-mehtyl-4-isoxazole propionic acid and N-methyl-d-aspartate receptor subunit mRNAs between visceromotor and somatomotor neurons of the rat lumbosacral spinal cord. J. Comp. Neurol. 1999;404:172–182. doi: 10.1002/(sici)1096-9861(19990208)404:2<172::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 1146.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 1148.Shiraishi Y, Nakao S. Differential locations in the midbrain of distinct groups of vertical eye movement-related neurones in cat: their projections and direct connections with oculomotor neurones. Acta Physiol. Scand. 1995;154:151–163. doi: 10.1111/j.1748-1716.1995.tb09897.x. [DOI] [PubMed] [Google Scholar]
- 1149.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J. Comp. Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 1150.Shupliakov O, Ornung G, Brodin L, Ulfhake B, Ottersen OP, Storm-Mathisen J, Cullheim S. Immunocytochemical localization of amino acid neurotransmitter candidates in the ventral horn of the cat spinal cord: a light microscopic study. Exp. Brain Res. 1993;96:404–418. doi: 10.1007/BF00234109. [DOI] [PubMed] [Google Scholar]
- 1151.Shupliakov O, Ottersen OP, Storm-Mathisen J, Brodin L. Glial and neuronal glutamine pools at glutamatergic synapses with distinct properties. Neuroscience. 1997;77:1201–1212. doi: 10.1016/s0306-4522(96)00537-4. [DOI] [PubMed] [Google Scholar]
- 1152.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 1153.Siggins GR, Schubert P. Adenosine depression of hippocampal neurons in vitro: an intracellular study of dose-dependent actions on synaptic and membrane potentials. Neurosci. Lett. 1981;23:55–60. doi: 10.1016/0304-3940(81)90186-5. [DOI] [PubMed] [Google Scholar]
- 1154.Sillar KT. Spinal pattern generation and sensory gating mechanisms. Curr. Opin. Neurobiol. 1991;1:583–589. doi: 10.1016/s0959-4388(05)80032-7. [DOI] [PubMed] [Google Scholar]
- 1155.Sillar KT, Simmers AJ. 5-HT induced NDMA receptor-mediated intrinsic osfcillations in embryonic amphibian spinal neurons. Proc. R. Soc. Lond. B Biol. Sci. 1994;255:139–145. doi: 10.1098/rspb.1994.0020. [DOI] [PubMed] [Google Scholar]
- 1156.Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J. Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- 1157.Singer JH, Berger AJ. Presynaptic inhibition by serotonin: a possible mechanism for switching motor output of the hypoglossal nucleus. Sleep. 1996;19(Suppl):S146–S149. doi: 10.1093/sleep/19.suppl_10.146. [DOI] [PubMed] [Google Scholar]
- 1158.Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J. Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- 1159.Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J. Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- 1160.Sivilotti LG, Gerber G, Rawat B, Woolf CJ. Morphine selectively depresses the slowest, NMDA-independent component of C-fibre-evoked synaptic activity in the rat spinal cord in vitro. Eur. J. Neurosci. 1995;7:12–18. doi: 10.1111/j.1460-9568.1995.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 1161.Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- 1162.Skydsgaard M, Hounsgaard J. Spatial integration of local transmitter responses in motoneurones of the turtle spinal cord in vitro. J. Physiol. (Lond.) 1994;479:233–246. doi: 10.1113/jphysiol.1994.sp020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Skydsgaard M, Hounsgaard J. Multiple actions of iontophoretically applied serotonin on motorneurones in the turtle spinal cord in vitro. Acta Physiol. Scand. 1996;158:301–310. doi: 10.1046/j.1365-201X.1996.558326000.x. [DOI] [PubMed] [Google Scholar]
- 1164.Sladeczek F, Pin JP, Récasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- 1165.Smith D, Lowe D, Temkin R, Jensen P, Hatt H. Dopamine enhances glutamate-activated currents in spinal motoneurons. J. Neurosci. 1995;15:3905–3912. doi: 10.1523/JNEUROSCI.15-05-03905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Smith DO, Franke C, Rosenheimer JL, Zufall F, Hatt H. Desensitization and resensitization rates of glutamate-activated channels may regulate motoneuron excitability. J. Neurophysiol. 1991;66:1166–1175. doi: 10.1152/jn.1991.66.4.1166. [DOI] [PubMed] [Google Scholar]
- 1167.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1168.Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 1988;2:2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- 1169.Smith SM, Zorec R, McBurney RN. Conductance states activated by glycine and GABA in cultured spinal neurones. J. Membr. Biol. 1989;108:45–52. doi: 10.1007/BF01870424. [DOI] [PubMed] [Google Scholar]
- 1170.Smith TC, Wang LY, Howe JR. Distinct kainate receptor phenotypes in immature and mature mouse cerebellar granule cells. J. Physiol. (Lond.) 1999;517:51–58. doi: 10.1111/j.1469-7793.1999.0051z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Snider RM, Gerald MC. Noradrenergic-mediated potentiation of acetylcholine release from the phrenic nerve: evidence for presynaptic alpha 1-adrenoceptor involvement. Life Sci. 1982;31:853–857. doi: 10.1016/0024-3205(82)90540-9. [DOI] [PubMed] [Google Scholar]
- 1172.Sockanathan S, Jessel T. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 1173.Soderling TR. Modulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Neurochem. Int. 1996;28:359–361. doi: 10.1016/0197-0186(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 1174.Soderling TR, Tan SE, McGlade-McCulloh E, Yamamoto H, Fukunaga K. Excitatory interactions between glutamate receptors and protein kinases. J. Neurobiol. 1994;25:304–311. doi: 10.1002/neu.480250310. [DOI] [PubMed] [Google Scholar]
- 1175.Sofroniew M. Vasopressin, oxytocin, and their related neurophysins. In: Bjorklund A, Hokfelt T, editors. GABA and Neuropeptides in the CNS. Amsterdam: Elsevier; 1985. pp. 93–165. [Google Scholar]
- 1176.Soghomonian J, Descarries L, Lanoir J. Monoamine innervation of the oculomotor nucleus in the rat. A radioautographic study. Neuroscience. 1986;17:1147–1157. doi: 10.1016/0306-4522(86)90084-9. [DOI] [PubMed] [Google Scholar]
- 1177.Soghomonian JJ, Pinard R, Lanoir J. GABA innervation in adult rat oculomotor nucleus: a radioautographic and immunocytochemical study. J. Neurocytol. 1989;18:319–331. doi: 10.1007/BF01190835. [DOI] [PubMed] [Google Scholar]
- 1178.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. Effects of excitatory amino acid antagonists on the phasic depolarizing events that occur in lumbar motoneurons during REM periods of active sleep. J. Neurosci. 1995;15:4068–4076. doi: 10.1523/JNEUROSCI.15-05-04068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1179.Soja PJ, Morales FR, Chase MH. Postsynaptic control of lumbar motoneurons during the atonia of active sleep. Prog. Clin. Biol. Res. 1990;345:9–22. [PubMed] [Google Scholar]
- 1180.Spencer R, Wang S. Immunohistochemical localization of neurotransmitters utilized by neurons in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) that project to the oculomotor and trochlear nuclei in the cat. J. Comp. Neurol. 1996;366:134–148. doi: 10.1002/(SICI)1096-9861(19960226)366:1<134::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 1181.Spencer R, Wenthold R, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular, reticular, and prepositus hypoglossi neurons that project to the cat abducens nucleus. J. Neurosci. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Spitzer NC. Spontaneous Ca2+ spikes and waves in embryonic neurons: signalling systems for differentiation. Trends Neurosci. 1994;17:115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 1183.Stanzione P, Zieglgansberger W. Action of neurotensin on spinal cord neurons in the rat. Brain Res. 1983;268:111–118. doi: 10.1016/0006-8993(83)90395-5. [DOI] [PubMed] [Google Scholar]
- 1184.Stauffer E, Watt D, Taylor A, Reinking R, Stuart D. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J. Neurophysiol. 1976;39:1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- 1185.Stefani A, Pisani A, Mercuri NB, Bernardi G, Calabresi P. Activation of metabotropic glutamate receptors inhibits calcium currents and GABA-mediated synaptic potentials in striatal neurons. J. Neurosci. 1994;14:6734–6743. doi: 10.1523/JNEUROSCI.14-11-06734.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 1187.Stephenson FA. The GABAA receptors. Biochem. J. 1995;310:1–9. doi: 10.1042/bj3100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1188.Stephenson JD. Pharmacology of the central control of micturition. Funct. Neurol. 1991;6:211–217. [PubMed] [Google Scholar]
- 1189.Sterling P, Kuypers H. Anatomical organization of the brachial spinal cord of the cat. II. The motoneuron plexus. Brain Res. 1967;4:16–32. doi: 10.1016/0006-8993(67)90145-x. [DOI] [PubMed] [Google Scholar]
- 1190.Streit J, Luscher HR. Miniature excitatory postsynaptic potentials in embryonic motoneurons grown in slice cultures of spinal cord, dorsal root ganglia and skeletal muscle. Exp. Brain Res. 1992;89:453–458. doi: 10.1007/BF00228262. [DOI] [PubMed] [Google Scholar]
- 1191.Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J. Physiol. (Lond.) 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192.Su C-K, C-Y C. GABAergic inhibition of neonatal rat phrenic motoneurons. Neurosci. Lett. 1998;248:191–194. doi: 10.1016/s0304-3940(98)00361-9. [DOI] [PubMed] [Google Scholar]
- 1193.Sugaya K, De Groat WC. Effects of MK-801 and CNQX, glutamate receptor antagonists, on bladder activity in neonatal rats. Brain Res. 1994;640:1–10. doi: 10.1016/0006-8993(94)91850-3. [DOI] [PubMed] [Google Scholar]
- 1194.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem. Biophys. Res. Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 1195.Sugiyama H, Ito I, Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987;325:531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- 1196.Sun Q, Pilowsky P, Llewellyn-Smith I. Thyrotropin-releasing hormone inputs are preferentially directed towards respiratory motoneurons in rat nucleus ambiguus. J. Comp. Neurol. 1995;362:320–330. doi: 10.1002/cne.903620303. [DOI] [PubMed] [Google Scholar]
- 1197.Sur C, McKernan R, Triller A. Subcellular localization of the GABAA receptor gamma 2 subunit in the rat spinal cord. Eur. J. Neurosci. 1995;7:1323–1332. doi: 10.1111/j.1460-9568.1995.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 1198.Surprenant A. Functional properties of native and cloned P2× receptors. Ciba Found. Symp. 1996;198:208–222. doi: 10.1002/9780470514900.ch12. [DOI] [PubMed] [Google Scholar]
- 1199.Suzue T, Yanaihara N, Otsuka M. Actions of vasopressin, gastrin releasing peptide and other peptides on neurons on newborn rat spinal cord in vitro. Neurosci. Lett. 1981;26:137–142. doi: 10.1016/0304-3940(81)90339-6. [DOI] [PubMed] [Google Scholar]
- 1200.Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J. Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- 1201.Swanson GT. Developing roles for kainate receptors in the cerebellum. J. Physiol. (Lond.) 1999;517:1. doi: 10.1111/j.1469-7793.1999.0001z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1202.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 1203.Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J. Comp. Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- 1204.Swartz KJ, Merritt A, Bean BP, Lovinger DM. Protein kinase C modulates glutamate receptor inhibition of Ca2+ channels and synaptic transmission. Nature. 1993;361:165–168. doi: 10.1038/361165a0. [DOI] [PubMed] [Google Scholar]
- 1205.Taal W, Holstege JC. GABA and glycine frequently colocalize in terminals on cat spinal motoneurons. Neuroreport. 1994;5:2225–2228. doi: 10.1097/00001756-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 1206.Tachibana M, Wenthold RJ, Morioka H, Petralia RS. Light and electron microscopic immunocytochemical localization of AMPA-selective glutamate receptors in the rat spinal cord. J. Comp. Neurol. 1994;344:431–454. doi: 10.1002/cne.903440307. [DOI] [PubMed] [Google Scholar]
- 1207.Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J. Comp. Neurol. 1996;372:343–355. doi: 10.1002/(SICI)1096-9861(19960826)372:3<343::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 1208.Tai T, Maclusky N, Adamson S. Ontogenesis of prostaglandin E2 binding sites in the brainstem of the sheep. Brain Res. 1994;652:28–39. doi: 10.1016/0006-8993(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 1209.Takada M, Itoh K, Yasui Y, Mitani A, Nomura S, Mizuno N. Distribution of premotor neurons for the hypoglossal nucleus in the cat. Neurosci. Lett. 1984;52:141–146. doi: 10.1016/0304-3940(84)90364-1. [DOI] [PubMed] [Google Scholar]
- 1210.Takahashi O, Satoda T, Uchida T. Distribution of GABA-immunoreactive premotor neurons projecting to the trigeminal motor nucleus in the rat. J. Hirnforsch. 1995;36:203–208. [PubMed] [Google Scholar]
- 1211.Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc. R. Soc. Lond. B Biol. Sci. 1984;221:103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- 1212.Takahashi T. Thyrotropin-releasing hormone mimics descending slow synaptic potentials in rat spinal motoneurons. Proc. R. Soc. Lond. B Biol. Sci. 1985;225:391–398. doi: 10.1098/rspb.1985.0068. [DOI] [PubMed] [Google Scholar]
- 1213.Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J. Physiol. (Lond.) 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1214.Takahashi T. Inward rectification in neonatal rat spinal motoneurones. J. Physiol. (Lond.) 1990;423:47–62. doi: 10.1113/jphysiol.1990.sp018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1215.Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J. Physiol. (Lond.) 1992;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1216.Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J. Physiol. (Lond.) 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217.Takahashi T, Konishi S, Powell D, Leeman SE, Otsuka M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Res. 1974;73:59–69. doi: 10.1016/0006-8993(74)91007-5. [DOI] [PubMed] [Google Scholar]
- 1218.Takahashi T, Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991;7:965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- 1219.Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- 1220.Takahashi T, Otsuka M. Regional distribution of substance P in the spinal cord and nerve roots of the cat and the effect of dorsal root section. Brain Res. 1975;87:1–11. doi: 10.1016/0006-8993(75)90774-x. [DOI] [PubMed] [Google Scholar]
- 1221.Takata M. Two types of inhibitory postsynaptic potentials in the hypoglossal motoneurons. Prog. Neurobiol. 1993;40:385–411. doi: 10.1016/0301-0082(93)90016-l. [DOI] [PubMed] [Google Scholar]
- 1222.Takata M, Fujita S. The properties of lingually induced IPSPs in the masseteric motoneurons. Brain Res. 1979;168:648–651. doi: 10.1016/0006-8993(79)90322-6. [DOI] [PubMed] [Google Scholar]
- 1223.Takeuchi A, Onodera R, Kawagoe R. The effects of dorsal root stimulation on the release of endogenous glutamate from the frog spinal cord. Proc. Jpn. Acad. 1983;59:88–92. [Google Scholar]
- 1224.Takeuchi Y, Kojima M, Matsuura T, Sano Y. Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat. Embryol. 1983;167:321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- 1225.Tallaksen-Greene S, Elde R, Wessendorf M. Regional distribution of serotonin and substance P co-existing in nerve fibers and terminals in the brainstem of the rat. Neuroscience. 1993;53:1127–1142. doi: 10.1016/0306-4522(93)90495-2. [DOI] [PubMed] [Google Scholar]
- 1227.Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of alpha 2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 1228.Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J. Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1229.Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J. Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1230.Tanabe Y, Jessel TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- 1231.Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992;8:169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- 1232.Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1233.Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- 1234.Tanaka H, Takahashi S, Miyamoto A, Oki J, Cho K, Okuno A. Developmental changes in the noradrenergic innervations of spinal motoneurons in neonatal rats. Pediatr. Neurol. 1996;14:21–27. doi: 10.1016/0887-8994(95)00258-8. [DOI] [PubMed] [Google Scholar]
- 1235.Tanaka T, Asahara T, Nishimura Y, Higuchi K, Yamamoto T. Afferent projections in the spinal accessory nerve to the facial motoneurons of the cat. Brain Res. 1992;585:377–380. doi: 10.1016/0006-8993(92)91240-f. [DOI] [PubMed] [Google Scholar]
- 1236.Tantisira B, Alstermark B, Isa T, Kummel H, Pinter M. Motoneuronal projection pattern of single C3–C4 propriospinal neurones. Can. J. Physiol. Pharmacol. 1996;74:518–530. [PubMed] [Google Scholar]
- 1237.Tashiro T, Ruda M. Immunocytochemical identification of axons containing coexistent serotonin and substance P in the cat lumbar spinal cord. Peptides. 1988;9:383–391. doi: 10.1016/0196-9781(88)90274-4. [DOI] [PubMed] [Google Scholar]
- 1238.Temkin R, Lowe D, Jensen P, Hatt H, Smith DO. Expression of glutamate receptor subunits in alpha-motoneurons. Mol. Brain Res. 1997;52:38–45. doi: 10.1016/s0169-328x(97)00249-0. [DOI] [PubMed] [Google Scholar]
- 1239.Teoh H, Malcangio M, Bowery NG. GABA, glutamate and substance P-like immunoreactivity release: effects of novel GABAB antagonists. Br. J. Pharmacol. 1996;118:1153–1160. doi: 10.1111/j.1476-5381.1996.tb15518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Terakado Y, Yamaguchi T. Last-order interneurones controlling activity of elbow flexor motoneurones during forelimb fictive locomotion in the cat. Neurosci. Lett. 1990;111:292–296. doi: 10.1016/0304-3940(90)90277-g. [DOI] [PubMed] [Google Scholar]
- 1241.Theodorsson-Norheim E, Jörnvall H, Andersson M, Norheim I, Oberg K, Jacobsson G. Isolation and characterization of neurokinin A, neurokinin A(3—10) and neurokinin A(4—10) from a neutral water extract of a metastatic ileal carcinoid tumour. Eur. J. Biochem. 1987;166:693–697. doi: 10.1111/j.1432-1033.1987.tb13567.x. [DOI] [PubMed] [Google Scholar]
- 1242.Thomas N, Clayton P, Jane D. Dicarboxyphenylglycines antagonize AMPA- but not kainate-induced depolarizations in neonatal rat motoneurones. Eur. J. Pharmacol. 1997;338:111–116. doi: 10.1016/s0014-2999(97)81937-1. [DOI] [PubMed] [Google Scholar]
- 1243.Thomas NK, Jane DE, Tse HW, Watkins JC. alpha-Methyl derivatives of serine-O-phosphate as novel, selective competitive metabotropic glutamate receptor antagonists. Neuropharmacology. 1996;35:637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- 1244.Thompson G, Jones P, Kilpatrick I. The actions of a range of excitatory amino acids at (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid-depolarizing receptors on neonatal rat motoneurones. Neuropharmacology. 1995;34:857–863. doi: 10.1016/0028-3908(95)00048-b. [DOI] [PubMed] [Google Scholar]
- 1245.Thompson SM, Haas HL, Gähwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol. (Lond.) 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1246.Thompson SWN, King AE, Woolf CJ. Activity-dependent changes in rat ventral horn neurons in vitro; summation of prolonged afferent evoked postsynaptic depolarizations produce a d-2-amino-5-ohosphonovaleric acid sensitive windup. Eur. J. Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 1247.Thurbon D, Lüscher HR, Hofstetter T, Redman SJ. Passive electrical properties of ventral horn neurons in rat spinal cord slices. J. Neurophysiol. 1998;79:2485–2502. doi: 10.1152/jn.1998.79.5.2485. [DOI] [PubMed] [Google Scholar]
- 1248.Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp. Brain Res. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- 1249.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 1250.Tingley WG, Roche KW, Thompson AK, Huganir RL. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993;364:70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- 1251.Todd AJ, Spike RC, Chong D, Neilson M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur. J. Neurosci. 1995;7:1–11. doi: 10.1111/j.1460-9568.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 1252.Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1253.Tohyama M, Wanaka A, Araki T, Betz H, Malbon CC. Localization of glycine and beta-adrenergic receptors in the rat brain. Arch. Histol. Cytol. 1989;52:39–48. doi: 10.1679/aohc.52.suppl_39. [DOI] [PubMed] [Google Scholar]
- 1254.Tokumoto M, Gong Z, Tsubokawa T, Hew C, Uyemura K, Hotta Y, Okamoto H. Molecular heterogeneity among primary motoneurons and within myotomes revealed by the differential mRNA expression of novel islet-1 homologs in embryonic zebrafish. Dev. Biol. 1995;171:578–589. doi: 10.1006/dbio.1995.1306. [DOI] [PubMed] [Google Scholar]
- 1255.Tolle TR, Berthele A, Laurie DJ, Seeburg PH, Zieglgansberger W. Cellular and subcellular distribution of NMDAR1 splice variant mRNA in the rat lumbar spinal cord. Eur. J. Neurosci. 1995;7:1235–1244. doi: 10.1111/j.1460-9568.1995.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 1256.Tolle TR, Berthele A, Schadrack J, Zieglgansberger W. Involvement of glutamatergic neurotransmission and protein kinase C in spinal plasticity and the development of chronic pain. Prog. Brain Res. 1996;110:193–206. doi: 10.1016/s0079-6123(08)62575-3. [DOI] [PubMed] [Google Scholar]
- 1257.Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J. Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1258.Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. Flip and Flop variants of AMPA receptors in the rat lumbar spinal cord. Eur. J. Neurosci. 1995;7:1414–1419. doi: 10.1111/j.1460-9568.1995.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 1259.Tomiyama M, Rodriguez-Puertas R, Cortes R, Christnacher A, Sommer B, Pazos A, Palacios JM, Mengod G. Differential regional distribution of AMPA receptor subunit messenger RNAs in the human spinal cord as visualized by in situ hybridization. Neuroscience. 1996;75:901–915. doi: 10.1016/0306-4522(96)00321-1. [DOI] [PubMed] [Google Scholar]
- 1260.Too HP, Maggio JE. Immunocytochemical localization of neuromedin K (neurokinin B) in rat spinal ganglia and cord. Peptides. 1991;12:431–443. doi: 10.1016/0196-9781(91)90081-y. [DOI] [PubMed] [Google Scholar]
- 1261.Topert C, Doring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J. Neurosci. 1998;18:4096–4105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262.Travagli R, Gillis R, Kellar K. S-adenosyl-l-methionine modulates firing rate of dorsal motor nucleus of the vagus neurones in vitro. Eur. J. Pharmacol. 1994;264:385–390. doi: 10.1016/0014-2999(94)00504-4. [DOI] [PubMed] [Google Scholar]
- 1263.Travers J, Norgren R. Afferent projections to the oral motor nuclei in the rat. J. Comp. Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- 1264.Tredici G, Migliorini C, Barajon I, Cavaletti G, Cece R. Anatomical organization of the spinal paths to the soleus and gastrocnemius muscles of the rat hind limb. J. Hirnforsch. 1996;37:81–89. [PubMed] [Google Scholar]
- 1265.Tremblay L, Bedard P. Effect of clonidine on motoneuron excitability in spinalized rats. Neuropharmacology. 1986;25:41–46. doi: 10.1016/0028-3908(86)90056-0. [DOI] [PubMed] [Google Scholar]
- 1266.Tribollet E, Arsenijevic Y, Marguerat A, Barberis C, Dreifuss JJ. Axotomy induces the expression of vasopressin receptors in cranial and spinal motor nuclei in the adult rat. Proc. Natl. Acad. Sci. USA. 1994;91:9636–9640. doi: 10.1073/pnas.91.20.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1267.Tribollet E, Barberis C, Arsenijevic Y. Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neuroscience. 1997;78:499–509. doi: 10.1016/s0306-4522(96)00591-x. [DOI] [PubMed] [Google Scholar]
- 1268.Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J. Neurosci. 1989;9:1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1269.Tribollet E, Goumaz M, Raggenbass M, Dubois-Dauphin M, Dreifuss JJ. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Dev. Brain Res. 1991;58:13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]
- 1270.Triller A, Cluzeaud F, Korn H. gamma-Aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. J. Cell Biol. 1987;104:947–956. doi: 10.1083/jcb.104.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Triller A, Cluzeaud F, Pfeiffer F, Betz H, Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J. Cell Biol. 1985;101:683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1272.Trombley PQ, Westbrook GL. l-AP4 inhibits calcium currents and synaptic transmission via a G-protein-coupled glutamate receptor. J. Neurosci. 1992;12:2043–2050. doi: 10.1523/JNEUROSCI.12-06-02043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273.Trueblood P, Levine MS, Chandler SH. Dual-component excitatory amino acid-mediated responses in trigeminal motoneurons and their modulation by serotonin in vitro. J. Neurophysiol. 1996;76:2461–2473. doi: 10.1152/jn.1996.76.4.2461. [DOI] [PubMed] [Google Scholar]
- 1274.Trussell LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J. Neurosci. 1987;7:3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1275.Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol. Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- 1276.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 1277.Turman J, Jr, Chandler SH. Immunohistochemical evidence for GABA and glycine-containing trigeminal premotoneurons in the guinea pig. Synapse. 1994;18:7–20. doi: 10.1002/syn.890180103. [DOI] [PubMed] [Google Scholar]
- 1278.Turman J, Jr, Chandler SH. Immunohistochemical localization of glutamate and glutaminase in guinea pig trigeminal premotoneurons. Brain Res. 1994;634:49–61. doi: 10.1016/0006-8993(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 1279.Turman J, Jr, Chandler SH. Expression of glutamate receptors in trigeminal neurons during postnatal development. In: Nakamura Y, editor. Symposium on Neurobiology of Mastication: From Molecular to Systems Approach. Amsterdam: Elsevier; 1999. [Google Scholar]
- 1280.Turski L, Bressler K, Klockgether T, Stephens DN. Differential effects of the excitatory amino acid antagonists, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 3-((−)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), on spinal reflex activity in mice. Neurosci. Lett. 1990;113:66–71. doi: 10.1016/0304-3940(90)90496-v. [DOI] [PubMed] [Google Scholar]
- 1281.Tuyau M, Hansen MA, Coleman MJ, Dampney RA, Balcar VJ, Bennett MR. Autoradiography of [3H]alpha,beta-methylene-ATP binding sites in medulla oblongata and spinal cord of the rat. Neurochem. Int. 1997;30:159–169. doi: 10.1016/s0197-0186(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 1282.Tyler EC, Lovinger DM. Metabotropic glutamate receptor modulation of synaptic transmission in corticostriatal co-cultures: role of calcium influx. Neuropharmacology. 1995;34:939–952. doi: 10.1016/0028-3908(95)00066-f. [DOI] [PubMed] [Google Scholar]
- 1283.Uchino Y, Hirai N, Suzuki S. Branching pattern and properties of vertical- and horizontal-related excitatory vestibuloocular neurons in the cat. J. Neurophysiol. 1982;48:891–903. doi: 10.1152/jn.1982.48.4.891. [DOI] [PubMed] [Google Scholar]
- 1284.Ueno S, Nabekura J, Akaike N, Inoue K. Responses of ATP and ACh in the identified neurons of dorsal motor nucleus of the vagus (Abstract) Drug Dev. Res. 1996;37:156. [Google Scholar]
- 1285.Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- 1286.Ulfhake B, Arvidsson U, Cullheim S, Hökfelt T, Brodin E, Verhofstad A, Visser T. An ultrastructural study of 5-hydroxytryptamine-, thyrotropin-releasing hormone- and substance P-immunoreactive axonal boutons in the motor nucleus of spinal cord segments L7-S1 in the adult cat. Neuroscience. 1987;23:917–929. doi: 10.1016/0306-4522(87)90168-0. [DOI] [PubMed] [Google Scholar]
- 1287.Ulfhake B, Kellerth J. A quantitative light microscopic study of the dendrites of cat spinal alpha-motoneurons after intracellular staining with horseradish peroxidase. J. Comp. Neurol. 1981;202:571–583. doi: 10.1002/cne.902020409. [DOI] [PubMed] [Google Scholar]
- 1288.Ulfhake B, Kellerth J. Electrophysiological and morphological measurements in cat gastrocnemius and soleus alpha-motoneurones. Brain Res. 1984;307:167–179. doi: 10.1016/0006-8993(84)90471-2. [DOI] [PubMed] [Google Scholar]
- 1289.Ulrich D, Huguenard JR. Purinergic inhibition of GABA and glutamate release in the thalamus: implications for thalamic network activity. Neuron. 1995;15:909–918. doi: 10.1016/0896-6273(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 1290.Ulrich D, Quadroni R, Luscher H. Electronic structure of motoneurons in spinal cord slice cultures: a comparison of compartmental and equivalent cylinder models. J. Neurophysiol. 1994;72:861–871. doi: 10.1152/jn.1994.72.2.861. [DOI] [PubMed] [Google Scholar]
- 1291.Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J. Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292.Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J. Neurophysiol. 1995;73:1192–1201. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- 1293.Umemiya M, Berger AJ. Single-channel properties of four calcium channel types in rat motoneurons. J. Neurosci. 1995;15:2218–2224. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1294.Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 1295.Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- 1296.Uphouse L. Multiple serotonin receptors: too many, not enough, or just the right number? Neurosci. Biobehav. Rev. 1997;21:679–698. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 1297.Vacca L, Hobbs J, Abrahams S, Naftchi E. Ultrastructural localization of substance P immunoreactivity in the ventral horn of the rat spinal cord. Histochemistry. 1982;76:33–49. doi: 10.1007/BF00493283. [DOI] [PubMed] [Google Scholar]
- 1298.van Buren J, Frank K. Correlation between the morphology and potential field of a spinal motor nucleus in the cat. Electroencephalogr. Clin. Neurophysiol. 1965;19:112–126. doi: 10.1016/0013-4694(65)90222-1. [DOI] [PubMed] [Google Scholar]
- 1299.van Calker D, Müller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 1300.van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J. Neurosci. 1988;8:472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301.van der Heyden MJ, Hilgevoord AA, Bour LJ, Ongerboer de Visser BW. Modeling motoneuron firing properties: dependency on size and calcium dynamics. Biol. Cybern. 1994;72:133–139. doi: 10.1007/BF00205977. [DOI] [PubMed] [Google Scholar]
- 1302.Vanderhorst V, De WH, Holstege G. Evidence for monosynaptic projections from the nucleus retroambiguus to hindlimb motoneurons in the cat. Neurosci. Lett. 1997;224:33–36. doi: 10.1016/s0304-3940(97)13449-8. [DOI] [PubMed] [Google Scholar]
- 1303.Vandermaelen CP, Aghajanian GK. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980;287:346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- 1304.Vandermaelen CP, Aghajanian GK. Serotonin-induced depolarization of rat facial motoneurons in vivo: comparison with amino acid transmitters. Brain Res. 1982;239:139–152. doi: 10.1016/0006-8993(82)90838-1. [DOI] [PubMed] [Google Scholar]
- 1305.van Dijken H, Dijk J, Voom P, Holstege JC. Localization of dopamine D2 receptor in rat spinal cord identified with immunocytochemistry and in situ hybridization. Eur. J. Neurosci. 1996;8:621–628. doi: 10.1111/j.1460-9568.1996.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 1306.Vannier C, Triller A. Biology of the postsynaptic glycine receptor. Int. Rev. Cytol. 1997;176:201–244. doi: 10.1016/s0074-7696(08)61611-3. [DOI] [PubMed] [Google Scholar]
- 1307.Varela-Echavarria A, Pfaff SL, Guthrie S. Differential expression of LIM homeobox genes among motor neuron subpopulations in the developing chick brain stem. Mol. Cell. Neurosci. 1996;8:242–257. doi: 10.1006/mcne.1996.0061. [DOI] [PubMed] [Google Scholar]
- 1308.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1309.Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 1310.Viana F, Bayliss DA, Berger AJ. Calcium conductances and their role in the firing behavior of neonatal rat hypoglossal motoneurons. J. Neurophysiol. 1993;69:2137–2149. doi: 10.1152/jn.1993.69.6.2137. [DOI] [PubMed] [Google Scholar]
- 1311.Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J. Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- 1312.Viana F, Bayliss DA, Berger AJ. Repetitive firing properties of developing rat brainstem motoneurones. J. Physiol. (Lond.) 1995;486:745–761. doi: 10.1113/jphysiol.1995.sp020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1313.Viana F, Bayliss DA, Berger AJ. Postnatal changes in rat hypoglossal motoneuron membrane properties. Neuroscience. 1994;59:131–148. doi: 10.1016/0306-4522(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 1314.Vignes M, Clarke VR, Davies CH, Chambers A, Jane DE, Watkins JC, Collingridge GL. Pharmacological evidence for an involvement of group II and group III mGluRs in the presynaptic regulation of excitatory synaptic responses in the CA1 region of rat hippocampal slices. Neuropharmacology. 1995;34:973–982. doi: 10.1016/0028-3908(95)00093-l. [DOI] [PubMed] [Google Scholar]
- 1315.Vilaro M, Wiederhold K, Palacios J, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47:367–393. doi: 10.1016/0306-4522(92)90253-x. [DOI] [PubMed] [Google Scholar]
- 1316.Villiger J, Faull R. Muscarinic cholinergic receptors in the human spinal cord: differential localization of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding sites. Brain Res. 1985;345:196–199. doi: 10.1016/0006-8993(85)90854-6. [DOI] [PubMed] [Google Scholar]
- 1317.Vinay L, Clarac F. CGP 35348 and CGP 55845A block the baclofen-induced depression of dorsal root evoked potentials in lumbar motoneurons of the neonatal rat. Neurosci. Lett. 1996;214:103–106. doi: 10.1016/0304-3940(96)12904-9. [DOI] [PubMed] [Google Scholar]
- 1318.Virgo L, Samarashinghe S, De Belleroche J. Analysis of AMPA receptor subunit mRNA expression in control and ALS spinal cord. Neuroreport. 1996;7:2507–2511. doi: 10.1097/00001756-199611040-00021. [DOI] [PubMed] [Google Scholar]
- 1319.Vogt M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after administration of drugs. J. Physiol. (Lond.) 1954;123:451–481. doi: 10.1113/jphysiol.1954.sp005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1320.von Euler U. The nature of adrenergic nerve mediators. Pharmacol. Rev. 1951;3:247–277. [PubMed] [Google Scholar]
- 1321.von Lubitz DK. Adenosine A3 receptor and brain. A culprit, a hero, or merely yet another receptor? Ann. NY Acad. Sci. 1997;825:49–67. doi: 10.1111/j.1749-6632.1997.tb48413.x. [DOI] [PubMed] [Google Scholar]
- 1322.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1323.Wada T, Hasegawa Y, Ono H. Characterization of alpha1-adrenoceptor subtypes in facilitation of rat spinal motoneuron activity. Eur. J. Pharmacol. 1997;340:45–52. doi: 10.1016/s0014-2999(97)01406-4. [DOI] [PubMed] [Google Scholar]
- 1324.Waldvogel HJ, Faull RL, Jansen KL, Dragunow M, Richards JG, Mohler H, Streit P. GABA, GABA receptors and benzodiazepine receptors in the human spinal cord: an autoradiographic and immunohistochemical study at the light and electron microscopic levels. Neuroscience. 1990;39:361–385. doi: 10.1016/0306-4522(90)90274-8. [DOI] [PubMed] [Google Scholar]
- 1325.Wall MJ, Dale N. GABAB receptors modulate glycinergic inhibition and spike threshold in Xenopus embryo spinal neurones. J. Physiol. (Lond.) 1993;469:275–290. doi: 10.1113/jphysiol.1993.sp019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1326.Wall PD. Do nerve impulses penetrate terminal arborizations? A pre-presynaptic control mechanism. Trends Neurosci. 1995;18:99–103. doi: 10.1016/0166-2236(95)93883-y. [DOI] [PubMed] [Google Scholar]
- 1327.Walmsley B, Bolton PS. An in vivo pharmacological study of single group Ia fibre contacts with motoneurones in the cat spinal cord. J. Physiol. (Lond.) 1994;481:731–741. doi: 10.1113/jphysiol.1994.sp020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1328.Walmsley B, Graham B, Nicol MJ. Serial E-M and simulation study of presynaptic inhibition along a group Ia collateral in the spinal cord. J. Neurophysiol. 1995;74:616–623. doi: 10.1152/jn.1995.74.2.616. [DOI] [PubMed] [Google Scholar]
- 1329.Walton KD, Lieberman D, Llinás A, Begin M, Llinás RR. Identification of a critical period for motor development in neonatal rats. Neuroscience. 1992;51:763–767. doi: 10.1016/0306-4522(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 1330.Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J. Chem. Neuroanat. 1989;2:119–137. [PubMed] [Google Scholar]
- 1331.Wang LY, Dudek EM, Browning MD, MacDonald JF. Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C. J. Physiol. (Lond.) 1994;475:431–437. doi: 10.1113/jphysiol.1994.sp020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1332.Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J. Physiol. (Lond.) 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333.Wang MY, Dun NJ. Direct and indirect actions of thyrotropin-releasing hormone on neonatal rat motoneurons in vitro. Neurosci. Lett. 1990;113:349–354. doi: 10.1016/0304-3940(90)90610-l. [DOI] [PubMed] [Google Scholar]
- 1334.Wang MY, Dun NJ. Phaclofen-insensitive presynaptic inhibitory action of (−/−)-baclofen in neonatal rat motoneurones in vitro. Br. J. Pharmacol. 1990;99:413–421. doi: 10.1111/j.1476-5381.1990.tb14718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335.Wang S, Spencer R. Spatial organization of premotor neurons related to vertical upward and downward saccadic eye movements in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) in the cat. J. Comp. Neurol. 1996;366:163–180. doi: 10.1002/(SICI)1096-9861(19960226)366:1<163::AID-CNE11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 1336.Wang YT, Bieger D, Neuman RS. Activation of NMDA receptors is necessary for fast information transfer at brainstem vagal motoneurons. Brain Res. 1991;567:260–266. doi: 10.1016/0006-8993(91)90804-5. [DOI] [PubMed] [Google Scholar]
- 1337.Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J. Neurosci. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1338.Warden MK, Young WS. Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J. Comp. Neurol. 1988;272:90–113. doi: 10.1002/cne.902720107. [DOI] [PubMed] [Google Scholar]
- 1339.Watanabe E, Akagi H. Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci. Res. 1995;23:377–382. doi: 10.1016/0168-0102(95)00972-V. [DOI] [PubMed] [Google Scholar]
- 1340.Watanabe M, Mishina M, Inoue Y. Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. J. Comp. Neurol. 1994;343:520–531. doi: 10.1002/cne.903430403. [DOI] [PubMed] [Google Scholar]
- 1341.Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal distributions of the N-methyl-d-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J. Comp. Neurol. 1994;345:314–319. doi: 10.1002/cne.903450212. [DOI] [PubMed] [Google Scholar]
- 1342.Weaver D, Deeds J, Lee K, Segre G. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Brain Res. 1995;28:296–310. doi: 10.1016/0169-328x(94)00222-z. [DOI] [PubMed] [Google Scholar]
- 1343.Weijs WA. Functional somatotopic organisation of motoneurons supplying the rabbit masseter muscle. J. Comp. Neurol. 1996;364:279–289. doi: 10.1002/(SICI)1096-9861(19960108)364:2<279::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 1344.Weiss C, Disterhoft J. Connections of the rabbit abducens nucleus. Brain Res. 1985;326:172–178. doi: 10.1016/0006-8993(85)91399-x. [DOI] [PubMed] [Google Scholar]
- 1345.Welton J, Stewart W, Kerr R, Maxwell D. Differential expression of the muscarinic m2 acetylcholine receptor by small and large motoneurons of the rat spinal cord. Brain Res. 1999;817:215–219. doi: 10.1016/s0006-8993(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 1346.Wentzel P, De ZC, Holstege J, Gerrits N. Inhibitory synaptic inputs to the oculomotor nucleus from vestibulo-ocular-reflex-related nuclei in the rabbit. Neuroscience. 1995;65:161–174. doi: 10.1016/0306-4522(94)00471-g. [DOI] [PubMed] [Google Scholar]
- 1347.Werman R, Davidoff RA, Aprison MH. Inhibitory action of glycine on spinal neurons in the cat. J. Neurophysiol. 1968;31:81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- 1348.Wessendorf M, Elde R. The coexistence of serotonin- and substance P-like immunoreactivity in the spinal cord of the rat as shown by immunofluorescent double labeling. J. Neurosci. 1987;7:2352–2363. [PMC free article] [PubMed] [Google Scholar]
- 1349.Westbrook GL, Krupp JJ, Vissel B. Cytoskeletal interactions with glutamate receptors at central synapses. Soc. Gen. Physiol. Ser. 1997;52:163–175. [PubMed] [Google Scholar]
- 1350.Westbrook GL, Mayer ML. Glutamate currents in mammalian spinal neurons: resolution of a paradox. Brain Res. 1984;301:375–379. doi: 10.1016/0006-8993(84)91107-7. [DOI] [PubMed] [Google Scholar]
- 1351.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and neve terminals. J. Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1352.Westlund KN, Bowker R, Ziegler M, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- 1353.Westlund K, Coulter J. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]
- 1354.Wheal HV, Chen Y, Mitchell J, Schachner M, Maerz W, Wieland H, van Rossum D, Kirsch J. Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity. Prog. Neurobiol. 1998;55:611–640. doi: 10.1016/s0301-0082(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 1355.Whelan PJ. Control of locomotion in the decerebrate cat. Prog. Neurobiol. 1996;49:481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 1356.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 1357.White S. A comparison of the effects of serotonin, substance P and thyrotropin-releasing hormone on excitability of rat spinal motoneurons in vivo. Brain Res. 1985;335:63–70. doi: 10.1016/0006-8993(85)90276-8. [DOI] [PubMed] [Google Scholar]
- 1358.White SR, Crane GK, Jackson DA. Thyrotropin-releasing hormone (TRH) effects on spinal cord neuronal excitability. Ann. NY Acad. Sci. 1989;553:337–350. doi: 10.1111/j.1749-6632.1989.tb46655.x. [DOI] [PubMed] [Google Scholar]
- 1359.White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989;502:205–213. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- 1360.White SR, Fung SJ, Barnes CD. Norepinephrine effects on spinal motoneurons. Prog. Brain Res. 1991;88:343–350. doi: 10.1016/s0079-6123(08)63821-2. [DOI] [PubMed] [Google Scholar]
- 1361.White SR, Neuman RS. Facilitation of spinal motoneurone excitability by 5-hydroxytryptamine and noradrenaline. Brain Res. 1980;188:119–127. doi: 10.1016/0006-8993(80)90561-2. [DOI] [PubMed] [Google Scholar]
- 1362.White SR, Fung SJ, Jackson DA, Imel KM. Serotonin, norepinephrine and associated neuropeptides: effects on somatic motoneuron excitability. Prog. Brain Res. 1996;107:183–199. doi: 10.1016/s0079-6123(08)61865-8. [DOI] [PubMed] [Google Scholar]
- 1363.Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int. Rev. Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 1364.Williams K. Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 1365.Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- 1366.Williams TL, Day NC, Ince PG, Kamboj RK, Shaw PJ. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]
- 1367.Williams TL, Ince PG, Oakley AE, Shaw PJ. An immunocytochemical study of the distribution of AMPA selective glutamate receptor subunits in the normal human motor system. Neuroscience. 1996;74:185–198. doi: 10.1016/0306-4522(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 1368.Wilson V, Yoshida M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp. Brain Res. 1969;9:365–380. doi: 10.1007/BF00235245. [DOI] [PubMed] [Google Scholar]
- 1369.Winder DG, Conn PJ. Activation of metabotropic glutamate receptors in the hippocampus increases cyclic AMP accumulation. J. Neurochem. 1992;59:375–378. doi: 10.1111/j.1471-4159.1992.tb08914.x. [DOI] [PubMed] [Google Scholar]
- 1370.Windhorst U. On the role of recurrent inhibitory feedback in motor control. Prog. Neurobiol. 1996;49:517–587. doi: 10.1016/0301-0082(96)00023-8. [DOI] [PubMed] [Google Scholar]
- 1371.Winokur A, Manaker S, Kreider MS. TRH and TRH receptors in the spinal cord. Ann. NY Acad. Sci. 1989;553:314–324. doi: 10.1111/j.1749-6632.1989.tb46653.x. [DOI] [PubMed] [Google Scholar]
- 1372.Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development. I. Alpha 2A messenger RNA expression. Neuroscience. 1997;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- 1373.Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development. II. Alpha 2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1997;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- 1374.Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res. 1991;10:179–183. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- 1375.Wisden W, Moss SJ. gamma-Aminobutyric acid type A receptor subunit assembly and sorting: gene targeting and cell biology approaches. Biochem. Soc. Trans. 1997;25:820–824. doi: 10.1042/bst0250820. [DOI] [PubMed] [Google Scholar]
- 1376.Withers MD, John PAST. Embryonic rat spinal cord neurons change expression of glycine receptor subtypes during development in vitro. J. Neurobiol. 1997;32:579–592. doi: 10.1002/(sici)1097-4695(19970605)32:6<579::aid-neu4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 1377.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 1378.Woch G, Davies RO, Pack AI, Kubin L. Behaviour of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J. Physiol. (Lond.) 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1379.Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport. 1995;6:2085–2088. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- 1380.Wolf EZY-Y, Roberts A. Non-linear summation of excitatory synaptic inputs to small neurones: a case study in spinal motoneurons of the young Xenopus tadpole. J. Physiol. (Lond.) 1998;511:871–886. doi: 10.1111/j.1469-7793.1998.871bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1381.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- 1382.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 1383.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 1384.Wu FS, Gibbs TT, Farb DH. Dual activation of GABAA and glycine receptors by beta-alanine: inverse modulation by progesterone and 5 alpha-pregnan-3 alpha-ol-20-one. Eur. J. Pharmacol. 1993;246:239–246. doi: 10.1016/0922-4106(93)90037-a. [DOI] [PubMed] [Google Scholar]
- 1385.Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 1386.Wu P, Lechan RM, Jackson IM. Identification and characterization of thyrotropin-releasing hormone precursor peptides in rat brain. Endocrinology. 1987;121:108–115. doi: 10.1210/endo-121-1-108. [DOI] [PubMed] [Google Scholar]
- 1387.Wu SY, Wang MY, Dun NJ. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991;554:111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- 1388.Wu W, Elde R, Wessendorf M. Organization of the serotonergic innervation of spinal neurons in rats. III. Differential serotonergic innervation of somatic and parasympathetic preganglionic motoneurons as determined by patterns of co-existing peptides. Neuroscience. 1993;55:223–233. doi: 10.1016/0306-4522(93)90468-u. [DOI] [PubMed] [Google Scholar]
- 1389.Wu WL, Ziskind-Conhaim L, Sweet MA. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J. Neurosci. 1992;12:3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1390.Wullner U, Klockgether T, Sontag KH. Phaclofen antagonizes the depressant effect of baclofen on spinal reflex transmission in rats. Brain Res. 1989;496:341–344. doi: 10.1016/0006-8993(89)91085-8. [DOI] [PubMed] [Google Scholar]
- 1391.Xia Y, Haddad GG. Ontogeny and distribution of GABAA receptors in rat brainstem and rostral brain regions. Neuroscience. 1992;49:973–989. doi: 10.1016/0306-4522(92)90373-a. [DOI] [PubMed] [Google Scholar]
- 1392.Xie H, Ziskind-Conhaim L. Blocking Ca(2+)-dependent synaptic release delays motoneuron differentiation in the rat spinal cord. J. Neurosci. 1995;15:5900–5911. doi: 10.1523/JNEUROSCI.15-09-05900.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393.Yajima Y, Hayashi Y. GABA(A) receptor-mediated inhibition in the nucleus ambiguus motoneuron. Neuroscience. 1997;79:1079–1088. doi: 10.1016/s0306-4522(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 1394.Yajima Y, Larson CR. Multifunctional properties of ambiguous neurons identified electrophysiologically during vocalization in the awake monkey. J. Neurophysiol. 1993;70:529–540. doi: 10.1152/jn.1993.70.2.529. [DOI] [PubMed] [Google Scholar]
- 1395.Yakel JL. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol. Sci. 1997;18:124–134. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]
- 1396.Yakel JL, Vissavajjhala P, Derkach VA, Brickey DA, Soderling TR. Identification of a Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-d-aspartate glutamate receptors. Proc. Natl. Acad. Sci. USA. 1995;92:1376–1380. doi: 10.1073/pnas.92.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- 1398.Yamaguchi T, Hayashi K, Murakami H, Maruyama S, Yamaguchi M. Distribution and characterization of the glutamate receptors in the CNS of ataxic mutant mouse. Neurochem. Res. 1984;9:497–505. doi: 10.1007/BF00964376. [DOI] [PubMed] [Google Scholar]
- 1399.Yamamoto Y, Livet J, Pollock R, Garces A, Arce V, Delapeyriere O, Henderson C. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development. 1997;124:2903–2913. doi: 10.1242/dev.124.15.2903. [DOI] [PubMed] [Google Scholar]
- 1400.Yamazaki J, Fukuda H, Nagao T, Ono H. 5-HT2/5-HT1C receptor-mediated facilitatory action on unit activity of ventral horn cells in rat spinal cord slices. Eur. J. Pharmacol. 1992;220:237–242. doi: 10.1016/0014-2999(92)90753-q. [DOI] [PubMed] [Google Scholar]
- 1401.Yamazaki J, Ono H, Nagao T. Stimulatory and inhibitory effects of serotonergic hallucinogens on spinal mono- and polysynaptic reflex pathways in the rat. Neuropharmacology. 1992;31:635–642. doi: 10.1016/0028-3908(92)90141-b. [DOI] [PubMed] [Google Scholar]
- 1402.Yanagisawa M, Otsuka M. Pharmacological profile of a tachykinin antagonist, spantide, as examined on rat spinal motoneurones. Br. J. Pharmacol. 1990;100:711–716. doi: 10.1111/j.1476-5381.1990.tb14080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1403.Yang CC, Chan JY, Chan SH. Central effect of angiotensin III on caudal hypoglossal neurons in rats. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 1995;268:R1242–R1248. doi: 10.1152/ajpregu.1995.268.5.R1242. [DOI] [PubMed] [Google Scholar]
- 1404.Yang CC, Chan JY, Chan SH. Excitatory innervation of caudal hypoglossal nucleus from nucleus reticularis gigantocellularis in the rat. Neuroscience. 1995;65:365–374. doi: 10.1016/0306-4522(94)00473-i. [DOI] [PubMed] [Google Scholar]
- 1405.Yashpal K, Dam TV, Quirion R. Quantitative autoradiographic distribution of multiple neurokinin binding sites in rat spinal cord. Brain Res. 1990;506:259–266. doi: 10.1016/0006-8993(90)91260-n. [DOI] [PubMed] [Google Scholar]
- 1406.Yasuda K. Electrophysiological and pharmacological properties of inhibitory postsynaptic potentials evoked in laryngeal motoneurons in decerebrate cats. J. Oto-Rhino-Laryngol. Soc. Jpn. 1997;100:227–235. doi: 10.3950/jibiinkoka.100.227. [DOI] [PubMed] [Google Scholar]
- 1407.Yeh HH, Grigorenko EV. Deciphering the native GABAA receptor: is there hope? J. Neurosci. Res. 1995;41:567–571. doi: 10.1002/jnr.490410502. [DOI] [PubMed] [Google Scholar]
- 1408.Yokoyama C, Okamura H, Nakajima T, Taguchi J, Ibata Y. Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J. Comp. Neurol. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]
- 1409.Yoshida M, Tanaka M. Existence of new dopaminergic terminal plexus in the rat spinal cord: assessment by immunohistochemistry using anti-dopamine serum. Neurosci. Lett. 1988;94:5–9. doi: 10.1016/0304-3940(88)90261-3. [DOI] [PubMed] [Google Scholar]
- 1410.Yoshino M, Kamiya H. Suppression of presynaptic calcium influx by metabotropic glutamate receptor agonists in neonatal rat hippocampus. Brain Res. 1995;695:179–185. doi: 10.1016/0006-8993(95)00743-a. [DOI] [PubMed] [Google Scholar]
- 1411.Yoshiyama M, Roppolo JR, De Groat WC. Effects of GYKI 52466 and CNQX, AMPA/kainate receptor antagonists, on the micturition reflex in the rat. Brain Res. 1995;691:185–194. doi: 10.1016/0006-8993(95)00671-c. [DOI] [PubMed] [Google Scholar]
- 1412.Yoshiyama M, Roppolo JR, De Groat WC. Interactions between NMDA and AMPA/kainate receptors in the control of micturition in the rat. Eur. J. Pharmacol. 1995;287:73–78. doi: 10.1016/0014-2999(95)00615-7. [DOI] [PubMed] [Google Scholar]
- 1413.Yoshiyama M, Roppolo JR, De Groat WC. Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole- 4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J. Pharmacol. Exp. Ther. 1997;280:894–904. [PubMed] [Google Scholar]
- 1414.Young WS, Kuhar MJ. Autoradiographic localisation of benzodiazepine recepotrs in the brains of humans and animals. Nature. 1979;280:393–395. doi: 10.1038/280393a0. [DOI] [PubMed] [Google Scholar]
- 1415.Young WS, Kuhar MJ. Radiohistochemical localization of benzodiazepine receptors in rat brain. J. Pharmacol. Exp. Ther. 1980;212:337–346. [PubMed] [Google Scholar]
- 1416.Zabavnik J, Arbuthnott G, Eidne K. Distribution of thyrotrophin-releasing hormone receptor messenger RNA in rat pituitary and brain. Neuroscience. 1993;53:877–887. doi: 10.1016/0306-4522(93)90632-p. [DOI] [PubMed] [Google Scholar]
- 1417.Zajac F, Young J. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J. Neurophysiol. 1980;43:1221–1235. doi: 10.1152/jn.1980.43.5.1221. [DOI] [PubMed] [Google Scholar]
- 1418.Zarbin M, Kuhar M, O’Donohue T, Wolf S, Moody T. Autoradiographic localization of (125I-Tyr4)bombesin-binding sites in rat brain. J. Neurosci. 1985;5:429–437. doi: 10.1523/JNEUROSCI.05-02-00429.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1419.Zarbin MA, Wamsley JK, Kuhar MJ. Glycine receptor: light microscopic and autoradiographic localizaion with [3H]strychnine. J. Neurosci. 1981;1:532–547. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1420.Zemlan F, Behbehani M, Beckstead R. Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res. 1984;292:207–220. doi: 10.1016/0006-8993(84)90757-1. [DOI] [PubMed] [Google Scholar]
- 1421.Zengel J, Reid S, Sypert G, Munson J. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J. Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- 1422.Zetterström T, Fillenz M. Adenosine agonists can both inhibit and enhance in vivo striatal dopamine release. Eur. J. Pharmacol. 1990;180:137–143. doi: 10.1016/0014-2999(90)90601-2. [DOI] [PubMed] [Google Scholar]
- 1423.Zgombick JM, Beck SG, Mahle CD, Craddockroyal B, Maayani S. Pertussis toxin-sensitive guanine nucleotide- binding protein(s) couple adenosine A1 and 5-hydroxytryptamine1A receptors to the same effector systems in rat hippocampus: biochemical and electrophysiological studies. Mol. Pharmacol. 1989;35:484–494. [PubMed] [Google Scholar]
- 1424.Zhang L. Effects of 5-hydroxytryptamine on cat spinal motoneurons. Can. J. Physiol. Pharmacol. 1991;69:154–163. doi: 10.1139/y91-022. [DOI] [PubMed] [Google Scholar]
- 1425.Zhang L, Krnjevic K. Effects of 4-aminopyridine on the action potential and the after-hyperpolarization of cat spinal motoneurons. Can. J. Physiol. Pharmacol. 1986;64:1402–1406. doi: 10.1139/y86-237. [DOI] [PubMed] [Google Scholar]
- 1426.Zhang L, Krnjevic K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neurosci. Lett. 1987;74:58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]
- 1427.Zhang M, Wang Y, Vyas D, Neuman R, Bieger D. Nicotinic cholinoceptor-mediated excitatory postsynaptic potentials in rat nucleus ambiguus. Exp. Brain Res. 1993;96:83–88. doi: 10.1007/BF00230441. [DOI] [PubMed] [Google Scholar]
- 1428.Zheng J, Seki M, Hayakawa T, Ito H, Zyo K. Descending projections from the paraventricular hypothalamic nucleus to the spinal cord: anterograde tracing study in the rat. Okajimas Folia Anat. Jpn. 1995;72:119–135. doi: 10.2535/ofaj1936.72.2-3_119. [DOI] [PubMed] [Google Scholar]
- 1429.Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J. Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- 1430.Zhu PJ, Krnjevic K. Adenosine release is a major cause of failure of synaptic transmission during hypoglycaemia in rat hippocampal slices. Neurosci. Lett. 1993;155:128–131. doi: 10.1016/0304-3940(93)90689-i. [DOI] [PubMed] [Google Scholar]
- 1431.Zieglgänsberger W, Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology. 1974;13:519–527. doi: 10.1016/0028-3908(74)90141-5. [DOI] [PubMed] [Google Scholar]
- 1432.Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 1433.Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J. Neurosci. 1990;10:125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434.Ziskind-Conhaim L, Seebach B, Gao B. Changes in serotonin-induced potentials during spinal cord development. J. Neurophysiol. 1993;69:1338–1349. doi: 10.1152/jn.1993.69.4.1338. [DOI] [PubMed] [Google Scholar]
- 1435.Zwaagstra B, Kernell D. Sizes of soma and stem dendrites in intracellularly labelled alpha-motoneurones of the cat. Brain Res. 1980;204:295–309. doi: 10.1016/0006-8993(81)90590-4. [DOI] [PubMed] [Google Scholar]