Abstract
The molecular circuitries controlling the process of skin wound healing have gained new significant insights in recent years. This knowledge is built on landmark studies on skin embryogenesis, maturation, and differentiation. Furthermore, the identification, characterization, and elucidation of the biological roles of adult skin epithelial stem cells and their influence in tissue homeostasis have provided the foundation for the overall understanding of the process of skin wound healing and tissue repair. Among numerous signaling pathways associated with epithelial functions, the PI3K/Akt/mTOR signaling route has gained substantial attention with the generation of animal models capable of dissecting individual components of the pathway, thereby providing a novel insight into the molecular framework underlying skin homeostasis and tissue regeneration. In this review we focus on recent findings regarding the mechanisms involved in wound healing associated with the upregulation of the activity of the PI3K/Akt/mTOR circuitry. This review highlights critical findings on the molecular mechanisms controlling the activation of mTOR, a downstream component of the PI3K-PTEN pathway, which is directly involved in epithelial migration and proliferation. We discuss how this emerging information can be exploited for the development of novel pharmacological intervention strategies to accelerate the healing of critical size wounds.
Keywords: PTEN, TSC1/TSC2, AKT, oral wound, wound therapy
Introduction
The skin is the largest organ in the body, with remarkable implications in physiology and homeostasis. Its main purpose is to maintain a barrier function, which prevents the loss water and protects the body against biological, physical, and chemical insults. Moreover, a healthy skin plays a crucial esthetical role, and often contributes to our social interactions and overall feeling of well-being (Elias, 1983; Elias and Choi, 2005; Proksch et al., 2008; Fuchs, 2009). These key functions require the integrity of the epidermal barrier. Upon injury, the fast response of the immune system, the deposition of a provisional matrix, and the coordinated engagement of all cellular components of the epidermis, including their tissue repopulating stem cells, are required for the rapid reestablishment of local homeostasis (Woodley et al., 1993; Singer and Clark, 1999; Elias and Choi, 2005; Fuchs and Nowak, 2008). Such capacity to heal injured tissues, however, may be compromised by large size wounds and can be aggravated by the presence of infections and medical conditions. This review presents an epitheliocentric view of the complex process of wound healing, with a special focus on the PI3K-mTOR signaling pathway, whose pharmacological perturbation can be exploited to promote accelerated epithelial healing.
The epidermis
The skin is formed during embryogenesis in a liquid interface with the amniotic fluid. Initial characteristics of skin are observed as early as 30 days after the estimated gestational age. By 6 weeks, the skin of a human embryo already shows a well-defined epidermis and dermis, tissue innervation and vascularization (Holbrook, 2011). The embryonic epidermis is composed by only two layers of epithelial cells separated from the dermis by a continuous basal lamina (Marinkovich et al., 1993). During the second trimester, the skin presents a much-improved organization with the development of appendances that progressively start to gain function.
During embryogenesis, studies using murine animal models have revealed two distinct phases of cell division in the epidermis (Lechler and Fuchs, 2005). At embryonic day 12.5 (E12.5), mice present most of the epidermis as a single cell layer, with the majority of the cellular divisions occurring laterally (92% of cells) thereby reflecting the direction of the rapidly growing embryo. Only a small percentage of the cells undergo a cellular division pattern with mitosis occurring perpendicular to the basal membrane; the resulting daughter cells therefore start to settle on top of the basal cells constituting the parabasal epithelial layer of the epidermis. Soon after, by day E15.5, there is a dramatic shift on the cellular division pattern with more than 70% of the cells presenting spindles perpendicular to the basal membrane. Such process results in asymmetrical cellular division, and the detachment of daughter cells from the basal membrane initiates the stratification process of the epidermis (Smart, 1970)(Poulson and Lechler, 2010).
The post-natal epidermis is organized in stratified layers presenting an outward progressive differentiation pattern (McGrath and Uitto, 2010). Epidermal stratification is essential in the development of a mechanical barrier against the external environment. The epidermis is composed by (i) the basal cellular layers responsible for conferring the regenerative capacity of the skin through the activation of symmetrical and asymmetrical cellular division during homeostasis or during the process of wound healing; (ii) the spinous cells located above the basal cells preceding the (iii) granular layer that is characterized by the intracellular accumulation of granules of keratohyalin. Finally, the (iv) stratum corneum is the outermost layer from the epidermis characterized by the absence of nuclei and cytoplasmatic organelles presenting flat keratin and filaggrin filaments. The last two stages of differentiation observed in the epidermis (accumulation of keratohyalin granules and accumulation of cross-linked flat keratin filaments) are important steps in the formation of the protective barrier of the skin mediated by an irreversible proteolytic cascade (List et al., 2002) (Miller and List, 2012).
The PI3K/AKT/mTOR signaling circuitry in skin development and homeostasis
The serine/threonine kinase AKT is an important component of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Overexpression and persistent activation of AKT are driven by oncogenes, growth factors, and cytokines. AKT activation converges in the phosphorylation of its Thr308 and Ser473 residues and, in turn, active AKT controls many cellular functions, ranging from cell growth and survival, to cell metabolism and growth (Alessi et al., 1996)(Andjelkovic et al., 1997). AKT functions are negatively regulated by molecules capable of antagonizing the PI3K signaling. Perhaps one of the most studied regulatory mechanism impinging on PI3K is the phosphatase PTEN (for phosphatase and tensin homolog deleted from Chromosome 10) (Li et al., 1997)(Steck et al., 1997). PTEN negatively regulates the PI3K pathway by converting phosphatidylinositol 3,4,5-triphosphate (PIP3) into phosphatidylinositol 4,5-biphosphate (PIP2), thus further restraining the activation status of AKT (Cantley and Neel, 1999).
Loss of function approaches have unveiled important functions of AKT during skin development and maintenance. Double knockout for Akt1 and Akt2 genes present translucent skin due to a proliferation impairment of the basal epithelial layer. However, no signs of changes in the differentiation pattern were observed. Double knockout mice are viable presenting a Mendelian distribution of traits, which indicates that Akt signaling is not essentially required during embryogenesis, but important for the maintenance of the normal architecture and homeostasis of the skin (Peng et al., 2003). Double knockout mice for Akt1 and Akt2 also result in a deficient activation of the mammalian target of Rapamycin (mTOR) (Gingras et al., 2001)(Simpson and Parsons, 2001).
The function of AKT signaling during skin homeostasis has been also indirectly demonstrated by Santos and colleagues in 2002 with the development of an animal model expressing cytokeratin 10 targeted to the basal layer of the epidermis driven by the bovine keratin K5 promoter (Santos et al., 2002). Cytokeratin 10 is an intermediated filament protein directly associated to the differentiation of the skin and found within the spinous layer of the epidermis. When expression of cytokeratin 10 was targeted to the proliferative layer of the epidermis (basal layers), it resulted in downregulation of the Akt activity along with reduced cellular proliferation, epidermal hypoplasia, and hyperkeratosis. These results support the physiological role of AKT in the maintenance of a viable and proliferative epidermis with preservation of the skin architecture. Indeed, the role of AKT during skin homeostasis have been partially addressed by the work of Paramio et al. where endogenous Akt was shown to interact with cytokeratin 10 (Paramio et al., 2001). Such interaction suggests that cytokeratin 10 regulates the translocation of AKT to the cell membrane, thereby preventing its activation by phosphorylation of the activation-loop threonine (Thr308), thus promoting the differentiation process of the spinous layer (CK10 positive cells) (Chou et al., 1998)(Kulik et al., 1997).
Gain of function assays have also helped the understanding of the role of the PI3K signaling in skin biology. Increased phosphorylation of AKT mediated by up regulation of the PI3K signaling subunits p110α and p110β results in the formation of a disorganized, hyperplastic, and poorly differentiated epithelium in organotypic skin cultures as a result of cytoskeleton reorganization, leading to an increased cellular migratory phenotype (Pankow et al., 2006). Interestingly, expression of an active AKT instead of the PI3K subunits results in growth arrest and differentiation of epithelial cells, while pharmacological interference of PI3K results in cell death and disruption of the machinery responsible for epithelial differentiation (Calautti et al., 2005). Thus, the modulation of the PI3K signaling pathway at the AKT level or upstream by the p110α and p110β PI3K subunits directly influences the epithelial response indicating a much broader function of the PI3K pathway in the control of skin homeostasis and probably wound healing.
Wound Healing
As the largest organ in the human body, the skin homeostasis is constantly disturbed by injuries affecting its most important function, the physical barrier. Such a role is maintained through a continuous self-renewal process that involves the maintenance of proliferative layer by epidermal stem cells (Oshima et al., 2001)(Tumbar et al., 2004) and the orchestration of a terminal differentiation process that involves the asymmetrical cellular division of basal keratinocytes (Lechler and Fuchs, 2005).
Disruption of tissue integrity with complete loss of continuity of epithelial cells results in the activation of an intricate process aiming at two distinct objectives; (i) quick covering and protection of wounded area; (ii) regaining the skin protective functions by tissue proliferation, migration, and ultimately remodeling. This process is mediated by an intricate mechanism that involves the formation of the blood clot, deposition of extracellular matrix (ECM) by stromal cells, and the induction of a well-regulated process of epithelial migration. Overall, the process of wound healing involves multiple interposed phases, including inflammation, re-epithelialization, formation of granulation tissue, vascularization, contraction, and finally, tissue remodeling (Woodley et al., 1993).
The inflammation phase is directly associated to the extravasation of blood to the wounded site and the formation of a fibrin clot, responsible for reestablishing tissue homeostasis. Shortly, increased concentration of growth factors initially secreted by platelets induces the differentiation of monocytes into macrophages. These cells are responsible for cleaning the wounded bed by the phagocytosis of cellular debris, foreign bodies and pathogens, and the differentiation of fibroblasts into myofibroblasts, which are essential for the process of tissue contraction (Singer and Clark, 1999). Short after the inflammation phase, the process of re-epithelialization is initially observed by the increase proliferation of the interfollicular epidermis (Singer and Clark, 1999) and the mobilization of hair follicle stem cells that migrate from their niche towards the wounded area (Ito et al., 2007)(Levy et al., 2007). The collaborative effort of the interfollicular and the follicular components of the skin during the re-epithelialization phase are evident by the recent characterization of the hair follicle stem cells as responsible for approximately 25% of cells found in the healed skin (Ito et al., 2005). These cells are essential for the accelerated healing during the initial phase of re-epithelialization (Langton et al., 2008). Underneath the re-epithelialization process, a new wound bed is formed by the migration of newly recruited fibroblasts and endothelial cells along with macrophages that together form the granulation tissue. This process is essential for the continuous migration of the epithelial cells and the progressive accumulation of extracellular matrix and collagen deposition. The neovascularization process then nurtures this newly formed granulous tissue, with endothelial cells originally guided by the deposition of extracellular matrix and angiogenic factors released by the fibroblasts. Finally, the differentiation of fibroblasts into myofibroblasts marks the phase of wound contraction and tissue remodeling that alone can take several months to complete. Overall, the acute phase of wound healing is fast and provides a protective barrier early on in the process, however full recovery of a wounded area including tissue remodeling is a longer process, which is often marked by a sub-optimal recovery of strength and appearance of the affected tissues.
The negative role of PI3K signaling in the control and maintenance of skin homeostasis is evident during the forced activation of oncogenes. Such process, known as oncogene-induced senescence (OIS) has been previously shown by our team (Castilho et al., 2009) to protect from the process of malignization of the epidermis through upregulation of mTOR function and the initiation of cell senescence programs. In this case, inducible upregulation of Wnt1 targeted to the basal layer of the skin using a cytokeratin 5 promoter results in activation of cellular senescence and differentiation of hair follicles, known to represent an important reservoir of stem cells in the skin. The process of cellular senescence in response to the conditional Wnt1 expression was initially documented by the overexpression of cytokeratin 10 in the hair follicles, which is not observed in control littermates reflecting increased cellular differentiation. Interestingly, mTOR function was also found upregulated in the hair follicle of transgenic mice expressing Wnt1 by the accumulation of the phosphorylated form of the ribosomal protein S6, an indirect downstream target of mTOR. Pharmacological interference of this pathway using the mTOR-specific allosteric inhibitor rapamycin resulted in complete abrogation of this phenotype, which demonstrated a new role of mTOR in the maintenance of skin homeostasis and its ability to trigger epithelial differentiation programs.
The PI3K/AKT/mTOR in wound healing
AKT
Numerous pathways and signaling networks have been studied and implicated in the control of skin response after injury. During normal wound healing in mice, we have observed a progressive upregulation of the Akt phosphorylated at serine 473 (ser473) towards the wound edge compared to the intact adjacent skin presenting a patched expression of active Akt in the differentiated layer of the epidermis (Squarize et al., 2010). Expression of Akt 473 subsequently extends to the spinous layer of the epidermis (cytokeratin 10 positive) in the transitional skin next to the epithelial tongue and is further expressed in all layers of the epidermis in the migratory epithelial tongue. Similar upregulation of Akt function during wound healing was also observed in a tail model of wound healing. In this model, overexpression of phosphorylated Akt was found confined to the proliferative and migratory areas of the wound, where Akt phosphorylation remains restricted to the outermost layer of the epidermis characterized by late stage cellular differentiation (Pankow et al., 2006). Activation of Akt resulting in augmented epithelial migration and proliferation is also observed upon disrupted contact of epithelial cells with the underlining connective tissue as observed in autoimmune blistering diseases (Pretel et al., 2009). Deletion of the extracellular matrix multidomain glycoprotein Emilin1, responsible for promoting α4β1 integrin–dependent cell adhesion, results in the proliferation of epithelial cells and accelerated wound healing (Danussi et al., 2011). Similarly, overexpression of the zinc finger transcription factor Klf5 in epithelial cells result in enhanced cellular migration through a direct interaction with integrin-liked kinases (ILK), which activates Akt (Yang et al., 2008). These results indicate a central importance of AKT as a molecule acting downstream from multiple pathways involved in wound healing.
PTEN
The Phosphatase and TENsin homolog (PTEN) gene was identified in 1997 as a tumor suppressor gene capable of antagonizing proto-oncogenes as the PI3K (Maehama and Dixon, 1998)(Maehama, 2007)(Stambolic et al., 1998). This phosphatase has as a substrate PIP3 that is dephosphorylated into PIP2, which results in the controlling of the AKT signaling activation. As a major regulator of the PI3K signaling, the role of PTEN in the control of physiological and pathological processes is becoming increasingly clear. This is the case for autoimmunity and lymphomagenesis (Newton and Turka, 2012), innate immune responses in mouse liver ischemia (Kamo et al., 2012), normal lung morphogenesis, and the prevention of lung carcinogenesis (Yanagi et al., 2007). Of interest for this review, in skin homeostasis loss of Pten results in progressive changes in the hair follicles with increased thickness of the outer root sheet and the development of multiple hyperproliferative lesions throughout the skin, especially around the nose, mouth, eyes, paws, limbs, and mucosa (Squarize et al., 2008). Such loss of function over the control of cellular proliferation caused by deletion of Pten denotes the importance of this tumor suppressor gene for the maintenance of tissue homeostasis, yet also indicates that transient downregulation of this tumor suppressive pathway may be of interest for regenerative therapies. Indeed, we have previously shown that excision of Pten from the epidermis using a CRE recombinase driven by the cytokeratin 14 promoter can dramatically influence the recovery of a wounded skin by accelerating the healing process (Fig. 1) (Squarize et al., 2010). As expected, deletion of Pten results in over activity of the PI3K signaling pathway and activation of the phosphorylated Akt and pS6 (Fig. 2) in the wounded area. Expression of active Akt and pS6, typically observed in the superficial layers of the epidermis (stratum corneum), was promptly upregulated in the spinous and partially in the basal layer at the transition skin, which is localized between normal skin and the wound edge. Akt and pS6 (Fig. 2) was further upregulated at the epithelial tongue throughout the entire thickness of the epidermis. Skin wounds from control mice also demonstrate an upregulation of Akt and pS6 in the transition and epithelial tongue, however much better compartmentalized within the differentiated layers of the epidermis than mice presenting deletion of Pten (Fig. 2) (Squarize et al., 2010). These findings suggest that physiological downregulation of Pten may be required for proper skin healing, and that further downregulation of other members of the PI3K signaling pathway, aiming at the activating mTOR, may also be a viable strategy to influence skin regeneration while preventing unwanted side effects.
Figure 1. Absence of Pten from epithelial cells of the skin resulted in accelerated wound healing.
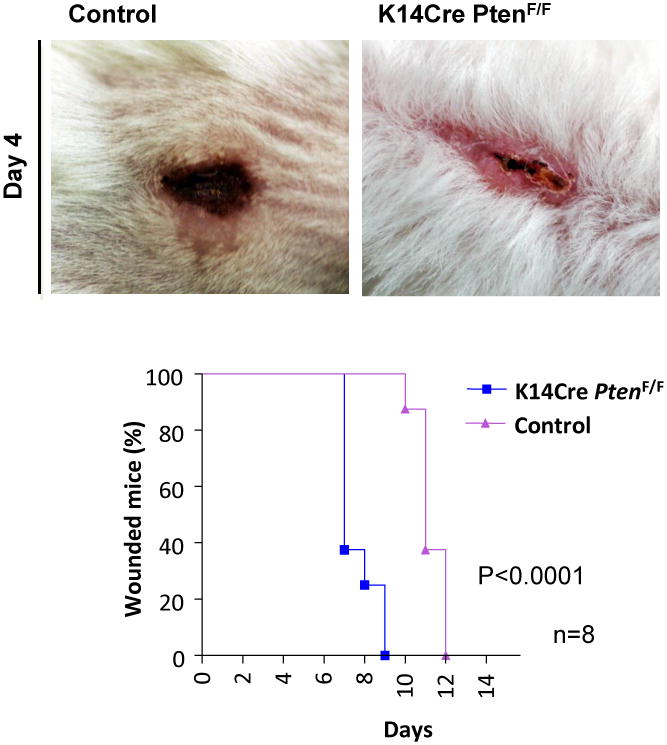
Pten is the main regulator of the PI3K/mTOR pathway. With the excision of Pten, epithelial cells proliferate and migrate faster than the epithelial cells from control mice. Accordingly, skin healing from K14Cre PtenF/F is dramatically accelerated after incisional surgical wounding. As seen here, wound closure of mice group with Pten epithelial-specific conditional deletion was completed by day 9, while control group exhibited wound closure by day 12 (p<0.0001, n=8) (data are from Squarize et al., 2010).
Figure 2. mTOR pathway is activated during the wound healing process.
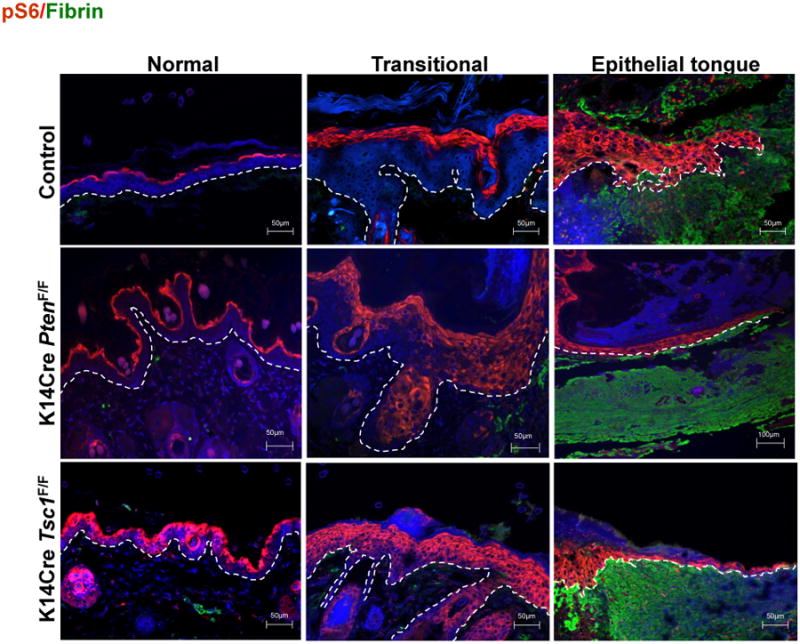
Upregulation of mTOR (pS6) is found in the transitional and epithelial tongue correspondent to the proliferative and migratory anatomical areas of normal wounds. In addition, histological analyses of the wounds demonstrate the upregulation of mTOR signaling on epithelial cells derived from Pten and Tsc1 deletion (K14Cre PtenF/F and K14Cre Tsc1F/F mice, respectively), which resulted in increased re-epithelization. (pS6, red (TRICT); fibrin clot, green (FITC), cells/nucleus, blue (DAPI)(data are from Squarize et al., 2010).
The tuberous sclerosis proteins
The tuberous sclerosis genes TSC1 and TSC2 code for two proteins named hamartin and tuberin, respectively. Their main function is to control cell growth and cell division. Both proteins also act as tumor growth suppressors by antagonizing the mTOR signaling pathway through the interaction with the Rheb1 GTPase. Loss of TSC1 or TSC2 gene function is caused by sporadic genetic mutations resulting in the genetic disorder known as tuberous sclerosis complex leading the accumulation of active Rheb1 and thereby constitutive activation of mTOR (Inoki and Guan, 2009). Therefore, transient suppression of the tuberous sclerosis complex constitutes a safe and attractive approach to induce mTOR signaling.
Indeed, we have shown that targeted excision of Tsc1 using a genetically defined approach exclusively to the epidermis results in the in vivo activation of mTOR signaling demonstrated by the phosphorylation of S6 protein, a hallmark of mTOR activity (Fig. 2) (Squarize et al., 2010). Moreover, by inhibiting the Tsc1 gene function and subsequently production of hamartin protein, we could observe an accelerated wound healing of conditional knockout mice with an overall reduction of the healing time of full thickness wounds similar to the phenotype observed in Pten conditional knockout mice (Fig. 3). Patients harboring mutations in the TSC gene develop hamartomas and non-neoplastic proliferative disorders; however they fail to develop tumors such as those observed in patients with Cowden disease which present loss of function mutations of the PTEN gene (Squarize et al., 2008). Similarly, long-term follow-up of mice presenting targeted mutation for Tsc1 show normal skin development and lack of tumor formation indicating that transient modulation of the PI3K signaling at the level of the tuberous sclerosis protein complex constitutes a viable and safe strategy to enhance tissue repair.
Figure 3. Deletion of Tsc1 from the epithelial cells of the skin resulted in acceleration of healing.
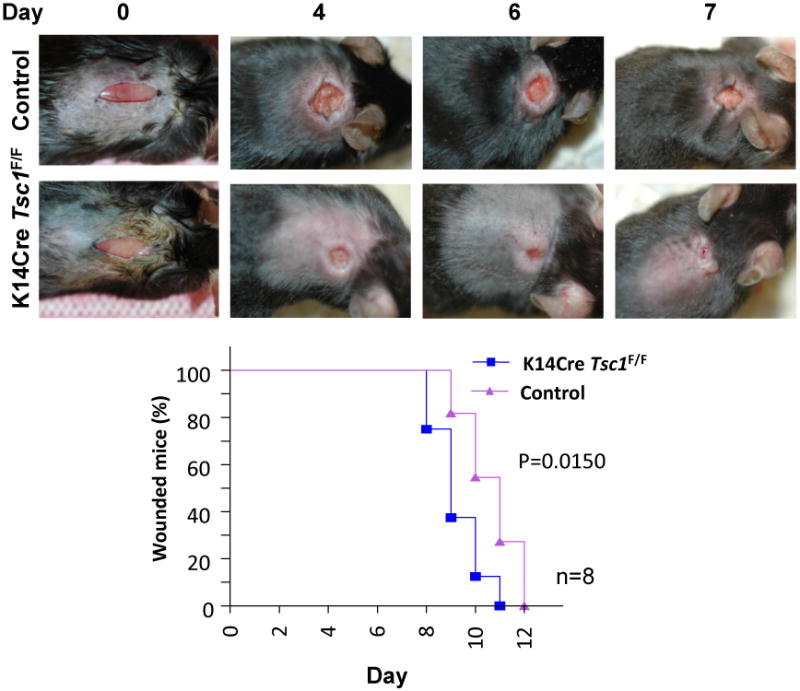
Tuberous sclerosis complex protein 1 (TSC1) forms a complex with TSC2. The inactivation of TSC2/TSC1 complex leads to the accumulation of the GTP-bound (active) form of Rheb1, which in turn promotes the direct activation of mTOR. With a genetically defined approach, when Tsc1 was eliminated from epithelial cell of the skin (using K14Cre Tsc1F/F mice) resulted in the acceleration of wound healing. The median wound closure time in K14Cre Tsc1F/F mice was 9 days after wound, as compared to day 11 in littermate control mice (p = 0.0150, n=8)(data are from Squarize et al., 2010).
Whereas interfering with the ability of normal regulatory mechanisms involved in the control of epithelial growth and cellular division may increase cancer risk, and hence it should be carefully taken in consideration, we have also observed that epithelial stem cells are endowed with protective mechanisms capable of inducing stem cell differentiation or senescence upon aberrant activation of mTOR (Castilho et al., 2009). Thus, strategies aiming at the indirect activation of mTOR though pharmacological or genetic downregulation of TSC proteins may represent a suitable strategy to enhance epithelial migration into the wound bed, thereby reducing the overall healing time and the development of secondary complications, without increasing cancer risk.
Periostin
The process of epithelial migration during wound healing is controlled not only by intrinsic factors, but also by extrinsic stimuli available in the wounded site produced during the process of blood clotting, or released by the stromal cells involved in the process of re-epithelialization. Periostin is one of the matricellular proteins normally expressed in adult skin, which is highly upregulated during wound healing (Hamilton, 2008)(Zhou et al., 2010)(Nishiyama et al., 2011)(Elliott et al., 2012). In fact, fibroblasts exposed to environmental changes found during wound healing differentiate into myofibroblasts, which present an increased secretion of periostin (Zhou et al., 2010). This matricellular protein is endowed of unique properties capable of responding to mechanical stress, as initially observed in the periodontal ligament and periosteum, where mechanical stresses play a central role in the dynamic of bone remodeling (Horiuchi et al., 1999). As a component of the extracellular matrix (ECM), periostin is known to interact with integrin molecules thereby activating the PI3K signaling pathway in tumor cells (Gillan et al., 2002)(Bao et al., 2004). Recently, Ontsuka et al. revealed that human dermal fibroblasts stimulated with recombinant periostin lead to the activation of several kinases including AKT, establishing the role of periostin in fibroblast-driven wound healing (Ontsuka et al., 2012). Although many functions of periostin have been identified, the signaling pathways involved in accelerated wound healing are still unknown. We have recently characterized the effects of periostin over epithelial cells at the biological and molecular levels (LK, RMC et al., manuscript in preparation). In this study, we found that periostin is capable of dramatically modulating the behavior of epithelial cells in vitro by increasing their migratory and proliferative capacity in a process that involves the upregulation of the AKT/mTOR signaling pathway. Therefore, the mitogenic and migratory capacity of epithelial cells observed during wound healing may not be solely driven by cell intrinsic signaling events, but it may rather result from the cooperative signaling from both the epithelial and mesenchymal components of the skin.
The emerging picture of the field is that a diversity of molecular signaling circuitries involved in the process of wound healing ultimately converges on the activation of the PI3K pathway resulting in activation of mTOR (Fig. 4). Although the process of healing is multifactorial and requires the cooperation between different cell types along with the deposition and maturation of the extracellular matrix, the sole enhancement of epithelial migration is feasible with obvious benefits for patients suffering of extensive wounds. Therefore, modulation of the PI3K pathway may represent a new therapeutic target for accelerating skin regeneration and the closure of critical life-threatening wounds.
Figure 4. The PI3K signaling pathway is highly activated during the wound healing process.
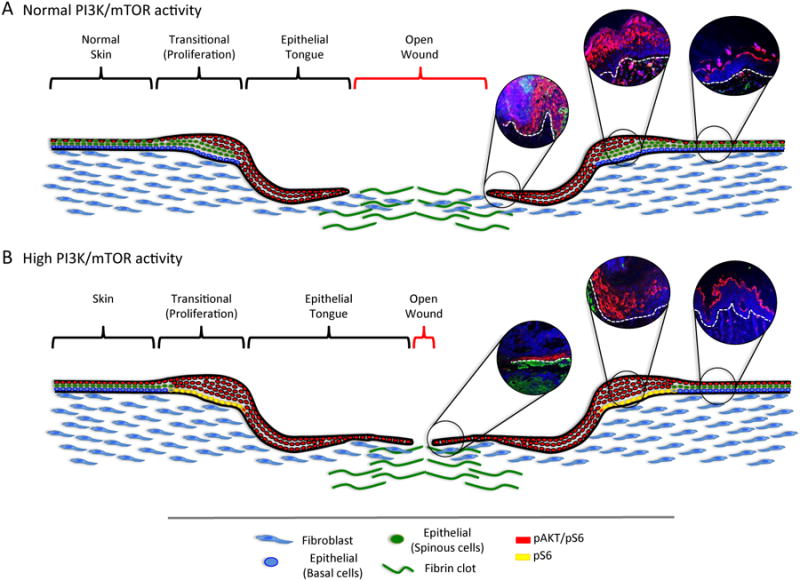
A. During the healing process, proliferation of epithelial cells occurs at the transitional area adjacent to the injury site, which displays increased pAKT (phosphorylated AKT) (immunofluorescence inserts) and mTOR activity mainly restricted to the differentiated layers of the epidermis. Normal skin is characterized by a patched expression of pAKT and pS6 (Normal skin-red cells). Accumulated epithelial cells migrate towards the center of the wound forming the epithelial tongue characterized by the co-overexpression of pAKT and pS6 (red cells). The epithelial tongue aims at covering the wounded area in the shortest time possible. Next, this thin layer of keratinocytes initiates a differentiation program that results in the stratification and formation of all skin layers comprised of basal, spinous, granular and cornified layers. B. Upon upregulation of the PI3K/mTOR signaling pathway, epithelial cells proliferate and migrate faster thereby accelerating the wound healing process. Upregulation of the PI3K pathway is characterized by overall increased expression of pAKT and pS6 in the skin. The proliferative anatomical area (transitional) of the wounded skin is now characterized by double expression of pS6 and pAKT in the spinous and parabasal layers of the epidermis while persistent expression of pS6 is now observed in the basal layer of the epidermis (yellow cells). Overexpression of the PI3K pathway results in the elongation of the epithelial tongue characterized by double expression of pS6 and pAKT (red cells). While the transient activation of PI3K/AKT/mTOR accelerates wound healing, the epithelial stem cells initiate cell senescence program if this pathway is persistently activated, thus protecting from hyperproliferation and tumor initiation.
Acknowledgments
The authors are supported by the National Institutes of Health (NIH/NCI) P50-CA97248 (University of Michigan Head and Neck SPORE) and by the Intramural Research Program of the US National Institutes of Health (NIH) and National Institute of Dental and Craniofacial Research (NIDCR).
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJC, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of Translocation in the Activation and Function of Protein Kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–89. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- Danussi C, Petrucco A, Wassermann B, Pivetta E, Modica TME, Belluz LDB, Colombatti A, Spessotto P. EMILIN1–α4/α9 integrin interaction inhibits dermal fibroblast and keratinocyte proliferation. J Cell Biol. 2011;195:131–145. doi: 10.1083/jcb.201008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80(Suppl):44s–49s. [PubMed] [Google Scholar]
- Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Elliott CG, Wang J, Guo X, Xu S, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Nowak JA. Building epithelial tissues from skin stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:333–350. doi: 10.1101/sqb.2008.73.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook KA. Embryogenesis of the Skin In Harper's Textbook of Pediatric Dermatology. Wiley-Blackwell; Oxford: 2011. pp. 2.1–2.41. [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Inoki K, Guan KL. Tuberous sclerosis complex, implication from a rare genetic disease to common cancer treatment. Hum Mol Genet. 2009;18:R94–100. doi: 10.1093/hmg/ddp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. Pten-mediated Akt/β-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2012 Jul 13; doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- Maehama T. PTEN: its deregulation and tumorigenesis. Biol Pharm Bull. 2007;30:1624–1627. doi: 10.1248/bpb.30.1624. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Keene DR, Rimberg CS, Burgeson RE. Cellular origin of the dermal-epidermal basement membrane. Dev Dyn. 1993;197:255–267. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Uitto J. Rook's Textbook of Dermatology. Wiley-Blackwell; Oxford: 2010. Anatomy and Organization of Human Skin; pp. 1–53. [Google Scholar]
- Miller G, List K. The matriptase-prostasin proteolytic cascade in epithelial development and pathology. Cell and Tissue Research. 2012:1–9. doi: 10.1007/s00441-012-1348-1. [DOI] [PubMed] [Google Scholar]
- Newton RH, Turka LA. Regulation of T cell homeostasis and responses by pten. Front Immunol. 2012;3:151. doi: 10.3389/fimmu.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Pankow S, Bamberger C, Klippel A, Werner S. Regulation of epidermal homeostasis and repair by phosphoinositide 3-kinase. J Cell Sci. 2006;119:4033–4046. doi: 10.1242/jcs.03175. [DOI] [PubMed] [Google Scholar]
- Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of Protein Kinase B (PKB) and PKC Mediates Keratin K10-Induced Cell Cycle Arrest. Molecular and Cellular Biology. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretel M, España A, Marquina M, Pelacho B, López-Picazo JM, López-Zabalza MJ. An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp Dermatol. 2009;18:771–780. doi: 10.1111/j.1600-0625.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Santos M, Paramio JM, Bravo A, Ramirez A, Jorcano JL. The Expression of Keratin K10 in the Basal Layer of the Epidermis Inhibits Cell Proliferation and Prevents Skin Tumorigenesis. J Biol Chem. 2002;277:19122–19130. doi: 10.1074/jbc.M201001200. [DOI] [PubMed] [Google Scholar]
- Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Smart IHM. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. British Journal of Dermatology. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Gutkind JS. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res. 2008;68:7066–7072. doi: 10.1158/0008-5472.CAN-08-0922. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Bugge TH, Gutkind JS. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS One. 2010;5:e10643. doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, De la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley DT, Chen JD, Kim JP, Sarret Y, Iwasaki T, Kim YH, O'Keefe EJ. Re-epithelialization. Human keratinocyte locomotion. Dermatol Clin. 1993;11:641–646. [PubMed] [Google Scholar]
- Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tetreault MP, Yermolina YA, Goldstein BG, Katz JP. Krüppel-like factor 5 controls keratinocyte migration via the integrin-linked kinase. J Biol Chem. 2008;283:18812–18820. doi: 10.1074/jbc.M801384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 2010;4:99–107. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


