Abstract
Objective
As smoking impacts physiological pathways in the central nervous system, it is important to consider the association between smoking and fibromyalgia, a pain condition caused predominantly by central nervous system dysfunction. The objectives were to assess the prevalence of current smoking among treatment-seeking chronic pain patients with (FM+) and without (FM−) a fibromyalgia-like phenotype; test the individual and combined influence of smoking and fibromyalgia on pain severity and interference; and examine depression as a mediator of these processes.
Methods
Questionnaire data from 1566 patients evaluated for a range of conditions at an outpatient pain clinic were used. The 2011 Survey Criteria for Fibromyalgia were used to assess the presence of symptoms associated with fibromyalgia.
Results
Current smoking was reported by 38.7% of FM+ patients compared to 24.7% of FM− patients. FM+ smokers reported higher pain and greater interference compared to FM+ nonsmokers, FM− smokers, and FM− nonsmokers. There was no interaction between smoking and fibromyalgia. Significant indirect effects of fibromyalgia and smoking via greater depression were observed for pain severity and interference.
Conclusions
Current smoking and positive fibromyalgia status were associated with greater pain and impairment among chronic pain patients, possibly as a function of depression. Although FM+ smokers report the most negative clinical symptomatology (i.e., high pain, greater interference) smoking does not appear to have a unique association with pain or functioning in FM+ patients, rather the effect is additive. The 38.7% smoking rate in FM+ patients is high, suggesting FM+ smokers present a significant clinical challenge.
Keywords: Tobacco Smoking, Chronic Pain, Fibromyalgia, Depression
Background
Chronic pain and tobacco dependence are both highly prevalent and comorbid disorders, and interactions between these conditions have been of increasing empirical interest [1]. There is accumulating evidence that smoking rates are elevated among persons with chronic pain [2], smoking has been identified a unique risk factor for chronic pain [3,4], pain has been shown to motivate smoking [5], and smokers (relative to non-smokers) tend to report more severe pain and greater functional impairment [6,7]. Potential mechanisms underlying increased pain among smokers include the direct effects of smoking on pain pathophysiology, and indirect effects via overlap with symptoms of depression [8–11]. The role of depression is of particular interest because chronic pain patients who smoke tobacco have consistently been shown to endorse greater levels of depression when compared with their nonsmoking counterparts [6,7]. Research has also shown that depression moderates the relationship between smoking and pain, suggesting that increased pain among smokers is largely driven by depression [11,12]. Consistent with these observations, a recently proposed reciprocal model suggests that pain, smoking, and depression may interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of tobacco dependence [1].
Despite growing scientific interest in pain-smoking relations, surprisingly little is known regarding the prevalence and influence of smoking across different types of chronic pain. Given evidence that nicotine exerts profound effects on physiological pathways in the central nervous system [13], researchers have begun to consider the role of smoking in patients with centralized pain (i.e., pain presumed to be caused predominantly by central nervous system dysfunction). The most well-studied centralized pain condition is fibromyalgia, a disorder characterized by chronic widespread pain, fatigue, sleep disruptions, depressive symptoms, and cognitive disturbances [14]. Nicotine has been shown to influence numerous central nervous system processes associated with fibromyalgia, including the endogenous opioid system [15,16], and serotonergic, noradrenergic and dopaminergic neurotransmitter systems [17,18]. One possibility is that smoking interacts with these central mechanisms, leading to worse pain outcomes in patients with fibromyalgia compared to patients who do not have fibromyalgia. A second, alternate possibility is that smoking has a similar association with pain regardless of whether a patient has fibromyalgia. That is, the negative pain outcomes associated with smoking in fibromyalgia patients may be a function of the combination of fibromyalgia and smoking. Distinguishing these possibilities is important both for understanding the potential mechanisms by which smoking influences pain and for informing effective treatment protocols when dealing with pain patients who also smoke.
The role of smoking in fibromyalgia is understudied relative to other types of chronic pain (e.g., low back pain and rheumatoid arthritis [3,4]). We are aware of only three studies that examined associations between smoking and fibromyalgia [19–21], and despite yielding initial evidence that smoking may be related to greater fibromyalgia-related pain and impairment, these studies were limited to within-group analyses (i.e., failed to include nonfibromyalgia comparisons) among relatively small samples of smokers (Ns = 33, 145, and 51, respectively), and failed to account for the role of depression as a potential mechanistic factor. The current lack of clarity regarding associations between pain, current smoking, and fibromyalgia status among treatment-seeking chronic pain patients represents a critical barrier to progress in this domain.
The objectives of the current study were to: 1) generate base rate data regarding the prevalence of current smoking among a large sample of treatment-seeking chronic pain patients with and without a fibromyalgia-like phenotype; 2) test the individual and combined influence of current tobacco use and fibromyalgia status on self-reported pain severity and pain interference; and 3) examine depression as a mediator of these outcomes. Specifically, we hypothesized that current smoking and positive fibromyalgia status would each be independently associated with greater self-reported pain severity and functional interference among persons with chronic pain, even after accounting for relevant socio-demographic factors. We further hypothesized that symptoms of depression would mediate associations between smoking/fibromyalgia status and pain severity/interference.
Materials and Methods
Participants
Participants included 1,566 noncancer chronic pain patients who sought treatment at a university-based outpatient pain medicine clinic between November 2010 and June 2012. As part of an ongoing clinical care and research initiative, all new patients were mailed a packet of questionnaires to complete prior to their initial visit. Consistent with IRB approval obtained prior to the start of this initiative, informed consent was waived, and questionnaire data was entered into the Assessment of Pain Outcomes Longitudinal Electronic Data Capture (APOLO EDC) system [22]. Although a total of 1,903 patients completed the questionnaires, 337 were excluded due to missing data on smoking status and/or the fibromyalgia scale. There were no other exclusion criteria.
Participants in this study were being evaluated for a range of primary diagnostic conditions, including chronic back pain (47%), musculoskeletal pain (15%), facial pain (8%), and disorders of the central nervous system (6%), among others. Given that a significant proportion of treatment-seeking pain patients can be expected to fall along a continuum of centralized pain processing dys-function [23,24], the 2011 Survey Criteria for Fibromyalgia allows for the assessment of fibromyalgia in clinical and epidemiological studies without requiring a tender point examination [25]. The measure is not intended to replace a clinical evaluation for the diagnosis of fibromyalgia, but can be used to place patients on the continuum of fibromyalgia-like symptomatology.
Measures
Smoking Status
Smoking status was assessed via a single item that asked participants to indicate whether they currently smoke cigarettes (Yes = current smoker, No = nonsmoker). Current smokers were further asked to indicate how many packs of cigarettes they smoke per day (<1 pack per day, ≥ 1 but < 2 packs per day, or ≥ 2 packs per day), and for how many years they have been smoking.
Fibromyalgia Status
The 2011 Survey Criteria for Fibromyalgia were used to assess the presence of symptoms associated with fibromyalgia. Although a definitive diagnosis of fibromyalgia cannot be made with survey criteria alone, the 2011 criteria are considered a reliable measure for classifying patients in survey research without the need for a physical exam [26]. The fibromyalgia survey criteria include the Widespread Pain Index (WPI) and Symptom Severity (SS) scale. The WPI was calculated using the Michigan Body Map [27], a one-sided body image with check boxes for 35 body areas, including the 19 body areas relevant to the WPI (scored 0–19). The SS scale was calculated by summing responses to six items (scored 0–12), that assessed: past-week fatigue, trouble thinking or remembering, and waking up tired (rated from 0 = no problem to 3 = severe); and the presence of pain or cramps in the lower abdomen, depression, and headache over the past 6 months (rated 1 = yes, 0 = no). The fibromyalgia survey allows for a clinical cut point for being termed “fibromyalgia-positive” (FM+). Patients were considered FM+ if they scored either ≥7 on the WPI and ≥5 on the SS scale, or between 3 and 6 on WPI and ≥9 on the SS [28]. Patients who did not meet these criteria were considered negative for fibromyalgia (FM−) [28]. Using a cutoff score to classify patients as FM+ or FM− aids interpretation of data and is also more likely to be clinically meaningful.
Pain Assessment
The pain assessment consisted of the Brief Pain Inventory (BPI). The BPI assesses both the severity of pain and its interference on common activities [29]. Pain severity on average, over the last week, and right now was rated on a scale of 0 (No Pain) to 10 (Pain as bad as you can imagine), and averaged to generate a single composite score (α = 0.88). The extent to which pain interfered with activity, mood, walking, work, relations with others, sleep, and enjoyment over the past week was rated on a scale of 0 (Does not interfere) to 10 (Completely interferes), and averaged to generate a single composite score (α = 0.89).
Symptoms of Depression
Symptoms of depression were assessed using the 7-item depression subscale from the Hospital Anxiety and Depression Scale (HADS) [30]. The HADS is a brief and widely used instrument to measure psychological distress in both general and medical populations [31]. It has been used in musculoskeletal populations [32], as well as in patients with chronic fatigue [33] and fibromyalgia [34]. A score of 0–7 is considered within the normal range; a score of 8–10 is suggestive of the presence of depression; and a score of 11 or higher indicates a high probability that depression is present [35].
Statistical Analyses
Data analyses for the entire sample included computing descriptive statistics for demographic variables (age, sex, race, education) and criterion variables (pain severity, pain interference, and depression) according to FM group and smoking status. Criterion variables were also tested for normality using Q-Q plots. Group differences were tested by performing analysis of variance (ANOVA) for the continuous demographic (significance = P < 0.01 to correct for multiple comparisons), and chi-square tests for the categorical demographic variables.
To evaluate the effects of smoking and FM status on pain severity and pain interference two analysis of covariance (ANCOVA) models were conducted. The main effects of smoking and FM status were included in the models along with the interaction of smoking with FM status to predict pain severity and pain interference. Demographic variables (sex, age, race, education) were included in each model as covariates. Additionally, planned comparisons assessing differences in pain interference and pain severity between smoking FM+ patients and all other patients were conducted. Bonferroni adjusted P -values were utilized and reported for each planned comparison.
Next, to examine the potential indirect effect of smoking/FM status on pain via depression, separate path analysis models were conducted (see results). Demographic variables (sex, age, race, education) were included as covariates in each model. Full information maximum likelihood was used to account for missing data [30], and bootstrapped standard errors were utilized. Mediation was assessed using the product of the coefficients method [36]. All statistical analyses were conducted using Stata 13.1 (StataCorp, College Station, TX, USA).
Results
Prevalence of Fibromyalgia-Like Phenotype and Current Smoking
Across the entire sample (N = 1,566), 35.2% of chronic pain patients were determined to be FM+ and 29.6% endorsed current tobacco smoking. Frequencies or means and standard deviations for demographic variables based on fibromyalgia and smoking status are presented in Table 1. FM+ patients were more likely to be younger, female, non-White, and to not have graduated college compared to FM− patients. Similarly, compared to non-smokers, current smokers were more likely to be younger and to not have graduated college.
Table 1.
Descriptive statistics for demographic, pain, and mood variables by fibromyalgia and smoking status
| Fibromyalgia Status |
Smoking Status |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Full Sample (N = 1,566) | FM + (n = 551) | FM – (n = 1,015) | P | Current Smoker (n = 464) | Nonsmoker (n = 1,102) | P |
| Age | 49.3 ± 15.4 | 46.6 ± 13.3 | 50.8 ± 16.2 | <0.001 | 43.9 ± 11.9 | 51.6 ± 16.1 | <0.001 |
| Sex (% female) | 59.3 | 66.4 | 55.5 | <0.001 | 58.6 | 59.6 | 0.71 |
| Ethnicity (% white) | 89.4 | 87.1 | 90.6 | 0.03 | 89.3 | 89.4 | 0.95 |
| Education (% college graduate) | 34.6 | 29.9 | 38.1 | 0.001 | 22.1 | 40.8 | < 0.001 |
| Marital status (% married) | 55.9 | 50.9 | 58.7 | 0.003 | 43.1 | 61.4 | <0.001 |
| Employment status (% employed) | 36.4 | 27.3 | 41.3 | <0.001 | 29.2 | 39.4 | <0.001 |
| Past smoking (% former smokers) | 43.9 | 43.6 | 44.1 | 0.890 | – | 43.9 | – |
| Duration of smoking (# of years) | 21.8±11.4 | 21.3±11.2 | 22.3±11.6 | 0.394 | 21.8±11.4 | – | – |
| Pain duration (% longer than 1 yr) | 70.7 | 79.1 | 66.4 | <0.001 | 75.1 | 68.9 | 0.048 |
| Pain severity (range = 0–10) | 6.36 ± 1.87 | 7.05 ± 1.54 | 5.99 ± 1.92 | <0.001 | 7.02 ± 1.62 | 6.08 ± 1.89 | <0.001 |
| Pain Interference (range = 0–10) | 6.85 ± 2.24 | 7.91 ± 1.71 | 6.27 ± 2.28 | <0.001 | 7.57 ± 1.88 | 6.54 ± 2.30 | <0.001 |
| Depression (range = 0–21) | 8.91 ± 4.60 | 11.13 ± 4.32 | 7.70 ± 4.29 | <0.001 | 10.60 ± 4.66 | 8.20 ± 4.39 | <0.001 |
Note: Values are the mean, SD unless otherwise indicated. FM+ = meets ACR survey criteria for fibromyalgia; FM– = does not meet ACR survey criteria for fibromyalgia. Ns may vary due to missing data on the demographic and criterion variables.
Prevalence of Current Smoking by Fibromyalgia Status
The rate of current smoking was significantly higher among FM+ patients (39%), relative to FM− patients (25%; χ2 = 33.23, P < 0.001). There was no difference between FM+ smokers (M = 0.90, SD = 0.48) and FM− smokers (M = 0.90, SD = 0.47) with regard to average number of daily cigarette packs smoked [t(424) = 0.04, P = 0.97]. Further, there was no difference in number of years smoking between FM+ (M = 21.32, SD = 0.83) and FM− smokers (M = 22.29, SD = 0.78) [t(398) = 0.85, P = 0.39].
Associations Between Fibromyalgia/Current Smoking Status and Pain Severity/Interference
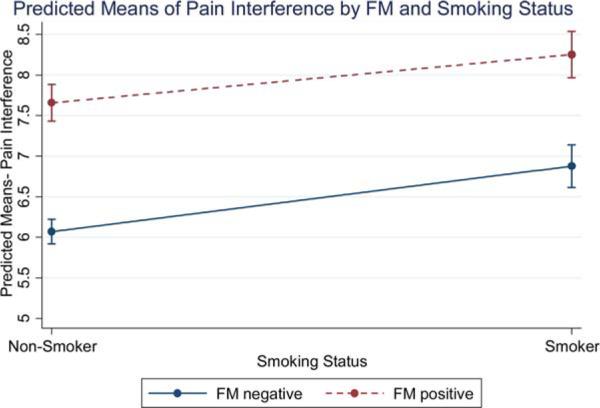
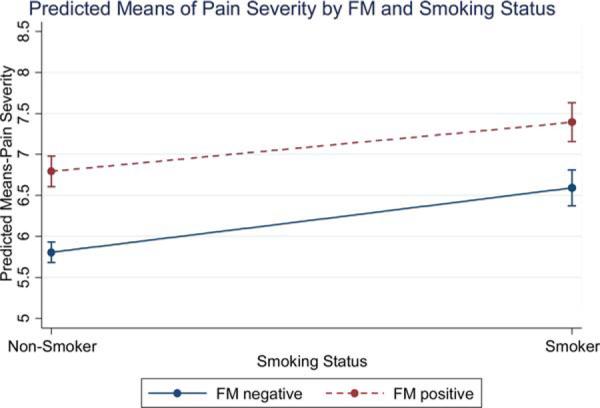
Unadjusted mean scores for the criterion variables within each FM group broken down by smoking status are reported in Table 2. The estimated marginal means of the ANCOVA models predicting pain severity and interference are presented in Figures 1 and 2. There were main effects of both FM status [F(1, 1420) = 60.51, P < 0.001] and smoking status [F(1, 1420) = 37.80, P < 0.001] on pain severity, such that current smokers and FM+ patients reported more severe pain than did current nonsmokers and FM-patients. Further, there were main effects of both FM status [F(1, 1406) = 131.56, P < 0.001] and smoking status [F(1, 1406) = 28.87, P < 0.001] on pain interference, such that current smokers and FM+ patients reported more pain interference. As seen in Figures 1 and 2, we observed no interaction between FM or smoking status for either pain interference [F(1, 1406) = 0.46, P = 0.50] or pain severity [F(1, 1420) = 0.77, P = 0.38]. Planned comparisons further revealed that pain ratings were highest for FM+ smokers, relative to FM+ nonsmokers (Mean difference = 0.56, SE = 0.16, Bonferroni adjusted P = 0.003), FM− smokers (Mean difference = 0.72, SE = 0.17, Bonferroni adjusted P < 0.001), and FM− nonsmokers (Mean difference = 1.46, SE = 0.15, Bonferroni adjusted P < 0.001). Similarly, interference ratings were highest for FM+ smokers, relative to FM+ nonsmokers (Mean difference = 0.60, SE = 0.19, Bonferroni adjusted P = 0.01), FM− smokers (Mean difference = 1.3, SE = 0.20, Bonferroni adjusted P < 0.001), and FM− nonsmokers (Mean difference = 2.09, SE = 0.17, Bonferroni adjusted P < 0.001).
Table 2.
Pain severity, pain interference, and symptoms of depression as a function of fibromyalgia and smoking status
| FM+ Current Smoker (n = 213) | FM+ Nonsmoker (n = 338) | FM– Current Smoker (n = 251) | FM– Nonsmoker (n = 764) | |
|---|---|---|---|---|
| Pain severity (range = 0–10) | 7.44 ± 1.32 | 6.80 ± 1.62* | 6.65 ± 1.76* | 5.77 ± 1.92* |
| Pain interference (range = 0–10) | 8.32 ± 1.38 | 7.66 ± 1.84* | 6.95 ± 2.01* | 6.04 ± 2.32* |
| Depression (% scoring 11 or above) | 69.19% | 52.10%* | 35.10%* | 22.19%* |
Note: Values are the unadjusted means, SD unless otherwise indicated. FM+ = meets ACR survey criteria for fibromyalgia; FM– = does not meet ACR survey criteria for fibromyalgia. Pain severity and pain interference were measured using the BPI. Symptoms of depression were assessed using the HADS. A score of 11 or higher on the HADS indicates a high probability that depression is present. Ns may vary due to missing data on the criterion variables.
indicates that the means or percentages were statistically significantly different from the FM+ smoker reference group at Bonferroni-adjusted P < 0.05 or less.
Figure 1.
Predicted means of pain interference by FM status and smoking status.
Note: Marginal means displayed at means of other covariates.[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
Predicted means of pain severity by FM status and smoking status.
Note: Marginal means displayed at means of other covariates. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Depression as a Mediator of Associations Between Fibromyalgia/Current Smoking Status and Pain Severity/Interference
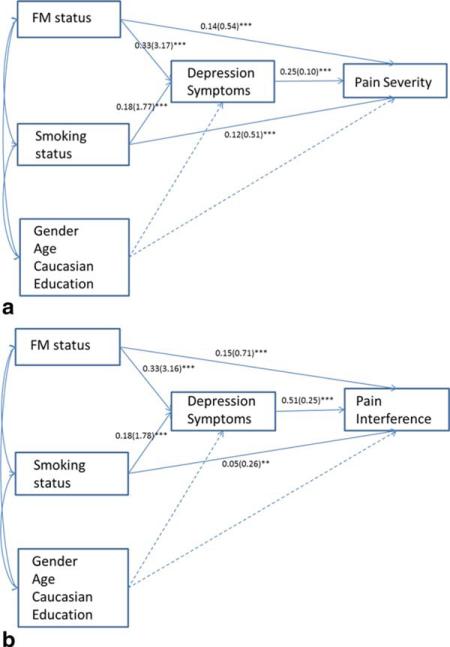
To determine the explanatory relevance of depression with regard to observed relations between fibromyalgia/smoking status and greater pain severity/interference, two separate path models were examined. Bootstrapping of the standard errors with 1,000 replications was employed in each model. Both standardized and unstandardized coefficients are presented in Figure 3a and 3b. As seen in Figures 3a and 3b, significant indirect effects of fibromyalgia and smoking status via greater depression were observed for both pain severity and interference outcomes, even after accounting for relevant sociodemo-graphic factors (i.e., gender, age, education, and race). Indirect effects reported here are the multiplicative product of the unstandardized coefficients from the predictor variable (e.g., smoking) to the mediator depression, and from the mediator to the outcome variable (e.g. pain severity). For pain severity (Figure 3a), the indirect effect of depression was 0.327 (P < 0.001; 95% CI: 0.243–0.410) for fibromyalgia status, and 0.183 (P < 0.001; 95% CI: 0.119–0.247) for current smoking status. Overall, this model accounted for 20% of the variance in pain severity and 18% of the variance in depression symptoms. For pain interference (Figure 3b), the indirect effect of depression was 0.778 (P < 0.001; 95% CI: 0.649–0.907) for fibromyalgia status, and 0.474 (P < 0.001; 95% CI: 0.347–0.601) for smoking status. Overall, this model accounted for 38% of the variance in pain interference, and 17% of the variance in depression symptoms.
Figure 3.
Path models testing mediation of FM status and smoking status through depression symptoms.
Note: Standardized coefficients are presented first and unstandardized path coefficients are presented in parentheses. *** is P < 0.001, ** is P < 0.01, * is P < 0.05. Dashed lines denote that paths from covariates gender, age, race, and education were estimated for both the mediator variable (depression) and outcome variables. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
To our knowledge, this is the first study to demonstrate that both current smoking and positive fibromyalgia status may be associated with greater pain and physical impairment among treatment-seeking chronic pain patients, possibly as a function of depression. Our path analysis models revealed significant indirect effects for current smoking and positive fibromyalgia status on both pain severity and pain interference, via greater scores on a measure of depression. As hypothesized, FM+ smokers reported more severe pain and functional impairment than did FM+ nonsmokers. Although these results are generally consistent with initial findings that smokers with fibromyalgia have reported greater pain and impairment than nonsmokers with fibromyalgia [19–21], these earlier studies failed to include nonfibromyalgia comparisons, thus prohibiting inferences regarding the relative importance of centralized pain in these outcomes.
To address gaps in the literature we tested a model that included main effects and interactions between smoking and fibromyalgia status. Both current smoking and having met survey criteria for fibromyalgia were independently associated with more severe pain and functional impairment among our sample of treatment-seeking chronic pain patients. As seen in Figures 1 and 2, the higher scores on pain severity and pain interference seen in FM+ smokers compared to FM+ nonsmokers were equivalent to the differences seen between smokers and nonsmokers who are FM−. Although we observed no interaction between smoking and fibromyalgia status for either outcome, planned comparisons revealed that FM+ smokers reported more severe pain and physical interference than did FM+ nonsmokers, FM− smokers, or FM− nonsmokers. Taken together, these findings support an additive effect of positive FM and smoking status on self-reported pain severity and pain interference. That is, we did not find that smoking has a unique association with pain outcomes among patients who were FM+, as has been hypothesized based on previous studies that did not include an FM− smoking group [20].
Depression has been considered as a key mechanistic factor in complex pain-smoking relations [1,13,37], and these findings are consistent with previous evidence that depression may help to explain why smokers with chronic pain tend to endorse more severe pain [11,12]. Indeed, it is possible that an underlying vulnerability (e.g., neurobiological, behavioral, affective, cognitive) is explanatory. For example, symptoms of depression are more prevalent among both smokers [38,39] and persons with chronic pain [40,41], often doubling rates observed in the general population. There is also some evidence that symptoms of depression and smoking behavior may be reciprocal in nature [42]. The role of depression is of particular interest because chronic pain patients who smoke tobacco have been shown to endorse greater levels of depression when compared with their nonsmoking counterparts [12], and depression has consistently been associated with more severe pain and functional impairment [9]. Consistent with these observations, abnormal levels of serotonin and norepinephrine have been found in patients who smoke, have depression, or experience chronic pain [34–36] suggesting that overlapping neurobiological factors likely influence the interaction. Medications that modulate central serotonin (in addition to nonpharmacological interventions such as cognitive-behavioral therapy) are evidence-based in the treatment of smoking cessation [43,44], depression [45,46], and fibromyalgia [47,48]. Future research should address the treatment implications of the affect-smoking–pain interaction, with particular focus on the role of depression.
Although recent data show that smoking prevalence in people with chronic pain may be twice that of the rate observed in the rate observed in the general population [2], little is known about how smoking rates may vary as a function of chronic pain type/source. These results indicated that the prevalence of current tobacco smoking was significantly higher among treatment-seeking chronic pain patients who also met diagnostic criteria for fibromyalgia (39% for FM+ vs 25% for FM−). Given that some of the most appropriate base rate information may be derived from clinical samples [49], these data further our understanding of how endorsement of centralized pain may confer increased risk for current tobacco smoking. Although previous studies reported smoking rates between 15% [21] and 26% [50] among fibromyalgia patients, it is important to note that the current data reflect rates of smoking among patients undergoing treatment for other primary pain complaints (e.g., chronic low back pain) who met the survey criteria for fibromyalgia [14,51]. Thus, although these findings may be applicable to persons who are formally diagnosed with fibromyalgia, the current data are most generalizable to a broader chronic pain population.
Given what is known about the health effects of smoking and the potential for smoking to influence pain, all persons with chronic pain should be encouraged to quit smoking. Although there has been little research regarding the efficacy of smoking cessation among persons with chronic pain [52] and the impact of smoking cessation on pain outcomes has yet to be established, there is evidence that smokers with pain are motivated to quit [53]. However, there is also some evidence that smokers in pain may experience greater difficulty when attempting to quit [54], and recurring pain has been prospectively linked to poor cessation outcomes [55]. Pain has been shown to be a potent motivator of smoking, [5] smoking may be used to cope with pain [44,45] and there is evidence that persons with chronic pain may smoke tobacco, in part, to distract themselves from pain and to better manage negative affect [56]. From a clinical perspective, the current study captured a subgroup of smokers who may struggle to quit, and better understanding why the smoking rate is so high in FM+ patients is important to inform smoking cessation. While this study did not assess patient beliefs about smoking, we hypothesize that FM+ smokers may be more likely than FM− smokers to report that smoking helps with affect regulation given that 69% of FM+ smokers endorsed symptoms suggestive of depression compared to 35% of FM− smokers. In general, smokers report that smoking helps with negative affect, yet smokers also report greater affective distress compared to nonsmokers [38]. This paradox suggests that smoking is an ineffective long term coping strategy for negative affect, yet the belief that smoking helps regulate mood is a barrier to cessation. Understanding differential motives for smoking and barriers to cessation among FM+ vs FM− smokers would help inform the development of tailored cessation programs.
Strengths of the current study include analysis of a large sample of treatment-seeking chronic pain patients, inclusion of both smokers and nonsmokers, application of established criteria for fibromyalgia, and our ability to account for relevant sociodemographic factors. Several limitations also bear noting. First, the cross-sectional design precludes causal inferences regarding temporal precedence. Thus, conclusions regarding the relative importance of fibromyalgia status above and beyond other pain and smoking relevant factors would be premature. We were also unable to determine when patients may have started smoking in relation to the onset of centralized pain symptoms. Second, patients were seen at an outpatient pain clinic in the Midwest and were predominantly Caucasian, thus limiting generalizability of these findings. Third, given that only pain over the last week was assessed, these data cannot speak to the potential covariation of pain and smoking behavior over time. Future studies using prospective designs to explore the temporal effects smoking, pain, and depression would be informative, as would studies on tailored interventions that address pain, smoking, and depression.
In conclusion, these findings shed light onto a potential concern with regards to the high smoking rate in treatment seeking patients who in addition to their primary pain diagnosis present with symptoms consistent with fibromyalgia. The smoking rate in FM+ patients was double the national smoking rate of 19.0% and significantly higher than the smoking rate of 20.5% in the state of Michigan [57], which suggests a need for further investigation into the smoking behavior in these patients. Additionally, the results of this study contribute new information about the additive association between smoking and fibromyalgia and provide the first evidence indicating that the association between smoking and clinical symptoms is similar in patients with and without fibromyalgia. Finally, depression emerged as an important factor in the association between smoking/fibromyalgia status and pain outcomes. Interrelations between tobacco smoking, depression, and chronic pain are likely complex and highly relevant to the treatment of both pain and smoking [12]. Future research should evaluate the utility of incorporating either pain- or smoking-relevant treatment components into existing interventions for tobacco dependence or chronic pain.
Acknowledgments
This study was supported by the Depart ment of Anesthesiology, University of Michigan. Dr. Hassett is a consultant for Bristol-Myers Squibb and Pfizer. Dr. Brummett receives research support from Neuros Medical, Inc. (Willoughby Hills, OH).
Footnotes
Disclosures: There are otherwise no relevant disclosures.
References
- 1.Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, Nicotine, and smoking: Research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–93. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tobacco Res. 2009;11(12):1407–14. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. Am J Med. 2010;123(1):87, e7–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Annu Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 5.Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects, of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117(2):467–72. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt MT, Hanscom B, Lauerman WC, Kang JD. Influence of smoking on the health status of spinal patients: The National Spine Network database. Spine (Phila Pa 1976) 2002;27(3):313–9. doi: 10.1097/00007632-200202010-00022. 1. [DOI] [PubMed] [Google Scholar]
- 7.Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11(5):643–53. [PubMed] [Google Scholar]
- 8.Ditre JW, Zale EL, Kosiba JD, Zvolensky MJ. A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Exp Clin Psychopharm. 2013;21(6):443–9. doi: 10.1037/a0034174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Arch Intern Med. 2003;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 10.Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993;33(2):129–37. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- 11.Goesling J, Brummett CM, Hassett AL. Cigarette smoking and pain: Depressive symptoms mediate smoking-related pain symptoms. Pain. 2012;153(8):1749–54. doi: 10.1016/j.pain.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–9. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: Pathophysiology and clinical implications. Anesthesiology. 2010;113(4):977–92. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- 14.Clauw DJ. Fibromyalgia: A clinical review. JAMA. 2014;311(15):1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 15.Scott DJ, Domino EF, Heitzeg MM, et al. Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacol. 2007;32(2):450–7. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- 16.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25(12):3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 18.Salokangas RKR, Vilkman H, Ilonen T, et al. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiat. 2000;157(4):632–4. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- 19.Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scand J Rheumatol. 2002;31(5):301–5. doi: 10.1080/030097402760375214. [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Kim SH, Nah SS, et al. Smoking habits influence pain and functional and psychiatric features in fibromyalgia. Joint Bone Spine. 2011;78(3):259–65. doi: 10.1016/j.jbspin.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, Oh TH. Impact of tobacco use in patients presenting to a multidiscipli-nary outpatient treatment program for fibromyalgia. Clin J Pain. Jan. 2009;25(1):39–43. doi: 10.1097/AJP.0b013e31817d105e. [DOI] [PubMed] [Google Scholar]
- 22.Hassett AL, Wasserman R, Goesling J, Rakovitis K, Shi B, Brummett CM. Longitudinal assessment of pain outcomes in the clinical setting: Development of the “APOLO” electronic data capture system. Reg Anesth Pain Med. 2012;37(4):398–402. doi: 10.1097/AAP.0b013e3182524672. [DOI] [PubMed] [Google Scholar]
- 23.Clauw DJ, Witter J. Pain and rheumatology: Thinking outside the joint. Arthritis Rheumatism. 2009;60(2):321–4. doi: 10.1002/art.24326. [DOI] [PubMed] [Google Scholar]
- 24.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 26.Hauser W, Jung E, Erbsloh-Moller B, et al. Validation of the fibromyalgia survey questionnaire within a cross-sectional survey. Plos One. 2012;7(5):e37504. doi: 10.1371/journal.pone.0037504. doi: 10.1371/journal.pone.0037504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brummett CM, Hassett AL, Brummett KA, Clauw DJ, Williams DA. The Michigan body map and its use in assessing the American College of Rheumatology Survey criteria for fibromyalgia. Arthritis Rheumatism. 2011;63(10):S368–S. [Google Scholar]
- 28.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 32.Pallant JF, Bailey CM. Assessment of the structure of the Hospital Anxiety and Depression Scale in musculoskeletal patients. Health Qual Life Outcomes. 2005;3:82. doi: 10.1186/1477-7525-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morriss RK, Wearden AJ. Screening instruments for psychiatric morbidity in chronic fatigue syndrome. J R Soc Med. 1998;91(7):365–8. doi: 10.1177/014107689809100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tin D, Bain LJ, Thorne JC, Nam S, Ginsburg L. Clinical Utility of the Hospital anxiety and depression scale for an outpatient fibromyalgia education program. Arthritis Rheumatism. 2012;64(Suppl 10):2402. doi: 10.1007/s10067-013-2377-1. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP. Introduction to Statistical Mediation Analysis. Routlage; New York: 2008. [Google Scholar]
- 37.Parkerson HA, Zvolensky MJ, Asmundson GJG. Understanding the relationship between smoking and pain. Expert Rev Neurother. 2013;13(12):1407–14. doi: 10.1586/14737175.2013.859524. [DOI] [PubMed] [Google Scholar]
- 38.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25(2):95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 39.Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addict Behav. 2004;29(6):1067–83. doi: 10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–8. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 41.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom Med. 2002;64(5):773–86. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 42.Boden JM, Fergusson DM, Norwood LJ. Cigarette smoking and depression: Tests of causal linkages using a longitudinal birth cohort. Brit J Psychiat. 2010;196(6):440–6. doi: 10.1192/bjp.bp.109.065912. [DOI] [PubMed] [Google Scholar]
- 43.Perkins KA, Conklin CA, Levine MD. Cognitive behavioral therapy for smoking cessation: A practical guidebook to the most effective treatments. Routledge; New York: 2007. [Google Scholar]
- 44.Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1) doi: 10.1002/14651858.CD000031.pub4. doi: 10.1002/14651858.CD000031.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin Psychol Rev. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiat. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 47.Bernardy K, Klose P, Busch AJ, Choy EHS, Hauser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9) doi: 10.1002/14651858.CD009796.pub2. doi: 10.1002/ 14651858.CD009796.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: A meta-analysis. JAMA. 2009;301(2):198–209. doi: 10.1001/jama.2008.944. [DOI] [PubMed] [Google Scholar]
- 49.Elwood RW. Psychological-tests and clinical discriminations - Beginning to address the base-rate problem. Clin Psychol Rev. 1993;13(5):409–19. [Google Scholar]
- 50.Pamuk ON, Donmez S, Cakar N. The frequency of smoking in fibromyalgia patients and its association with symptoms. Rheumatol Int. 2009;29(11):1311–4. doi: 10.1007/s00296-009-0851-5. [DOI] [PubMed] [Google Scholar]
- 51.Wolfe F. How to use the new American College of Rheumatology fibromyalgia diagnostic criteria. Arthritis Care Res (Hoboken) 2011;63(7):1073–4. doi: 10.1002/acr.20468. [DOI] [PubMed] [Google Scholar]
- 52.Hooten WM, Townsend CO, Hays JT, et al. A cognitive behavioral smoking abstinence intervention for adults with chronic pain: A randomized controlled pilot trial. Addict Behav. 2014;39(3):593–9. doi: 10.1016/j.addbeh.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn EJ, Rayens MK, Kirsh KL, Passik SD. Brief report: Pain and readiness to quit smoking cigarettes. Nicotine Tob Res. 2006;8(3):473–80. doi: 10.1080/14622200600670355. [DOI] [PubMed] [Google Scholar]
- 54.Zale EL, Ditre JW, Dorfman ML, Heckman BW, Brandon TH. Smokers in pain report lower confidence and greater difficulty quitting. Nicotine Tobacco Res. 2014 doi: 10.1093/ntr/ntu077. Available at: http://dx.doi.org/10.1093/ntr/ntu077. [DOI] [PMC free article] [PubMed]
- 55.Waldie KE, Mcgee R, Reeder AI, Poulton R. Associations between frequent headaches, persistent smoking, and attempts to quit. Headache. 2008;48(4):545–52. doi: 10.1111/j.1526-4610.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 56.Hooten WM, Vickers KS, Shi Y, et al. Smoking cessation and chronic pain: Patient and pain medicine physician attitudes. Pain Practice. 2011 doi: 10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention [December 2011];Tobacco Control State Highlights: Michigan. 2010 Available at: http://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2010/states/michigan/index.htm.