Abstract
There are two muscle fiber types in extraocular muscles: those receiving a single motor endplate, termed singly innervated fibers (SIFs), and those receiving multiple small terminals along their length, termed multiply innervated fibers (MIFs). In monkeys, these two fiber types receive input from different motoneuron pools: SIF motoneurons found within the extraocular motor nuclei, and MIF motoneurons found along their periphery. For the monkey medial rectus muscle, MIF motoneurons are found in the C-group, while SIF motoneurons lie in the A- and B-groups. We analyzed the somatodendritic morphology and ultrastructure of these three subgroups of macaque medial rectus motoneurons to better understand the structural determinants controlling the two muscle fiber types. The dendrites of A- and B-group motoneurons lay within the oculomotor nucleus, but those of the C-group motoneurons were located outside the nucleus, and extended into the preganglionic Edinger–Westphal nucleus. A- and B-group motoneurons were very similar ultrastructurally. In contrast, C-group motoneurons displayed significantly fewer synaptic contacts on their somata and proximal dendrites, and those contacts were smaller in size and lacked dense-cored vesicles. However, the synaptic structure of C-group distal dendrites was quite similar to that observed for A-and B-group motoneurons. Our anatomical findings suggest that C-group MIF motoneurons have different physiological properties than A- and B-group SIF motoneurons, paralleling their different muscle fiber targets. Moreover, primate C-group motoneurons have evolved a special relationship with the preganglionic Edinger–Westphal nucleus, suggesting these motoneurons play an important role in near triad convergence to support increased near work requirements.
INDEXING TERMS: oculomotor, vergence, extraocular, motor neurons, gaze, eye muscles, eye movements
The oculomotor system has provided a fertile model for neuroscientists seeking to better understand motor function due to the fact that the muscles involved are few in number and the behavioral repertoire of the eyes is relatively limited. Moreover, eye movements can be accurately measured, and the sensory signals for eliciting these behaviors can be readily manipulated. The six muscles that move the eye are controlled via three cranial nerves, the oculomotor, trochlear, and abducens, with the oculomotor driving four muscles (medial rectus, inferior rectus, superior rectus, and inferior oblique), and the latter two nerves driving the superior oblique and lateral rectus muscles, respectively. Investigations using retrograde tracers initially revealed that each of the muscles innervated by the oculomotor nerve is controlled by a pool of motoneurons within the oculomotor nucleus (Büttner-Ennever and Akert, 1981; Porter et al., 1983). However, one of the muscles in monkeys, the medial rectus, was observed to be distinct from the others, in that its motoneurons are distributed into three separate pools within the oculomotor nucleus, termed the A-, B-, and C-groups.
As with many supposedly “simple” systems, further investigation has uncovered considerable underlying complexity. Examination of the extraocular muscles themselves has revealed critical structural details. All six muscles can be subdivided into two compartments: an inner, global layer and an outer, orbital layer, with the outer layer inserting on the connective tissue pulleys in the orbit, and the inner layer inserting directly into the sclera of the eyeball (Demer et al., 2000). Furthermore, the muscle fibers within the extraocular muscles can be subdivided based on their mode of innervation (Mayr et al., 1975; Spencer and Porter, 1981; see Spencer and Porter, 2006, for review). The majority of fibers receive a single, plate-shaped (en plaque) ending from an axon near the middle of the fiber, and these are consequently termed singly innervated fibers (SIFs). However, a distinct minority of fibers are supplied by axons that extend along their entire length, displaying multiple, beaded (en passant and en grappe) terminals. These are consequently termed multiply innervated fibers (MIFs). Unlike the SIFs, which produce a unitary twitch in response to an action potential invading the motor endplate, MIFs are classified as non-twitch and are believed to be capable of graded, tonic contractions (Bondi and Chiarandini, 1983). The MIFs in the global layer are often characterized by the presence of a palisade ending where the muscle fibers insert into the tendon (Ruskell, 1978; Alvarado-Mallart and Pinçon-Raymond, 1979). There is no consensus as to whether the palisade endings are sensory or motor structures, but the cells that are the source of these axons have recently been demonstrated to reside with the MIF motoneurons (Lienbacher et al., 2011; Zimmermann et al., 2011, 2013), a complication that will be considered more fully in the Discussion. The MIFs of the orbital layer differ from those in the global layer in that they can also be supplied by an en plaque ending (Pachter, 1984).
The connection between medial rectus subgroups and extraocular muscle fiber structure was revealed through the groundbreaking work of Büttner-Ennever and colleagues (Büttner-Ennever et al., 2001; Eberhorn et al., 2005). By making injections into the monkey medial rectus muscle at the insertion, they were able to label just the MIF motoneurons supplying the global layer of the muscle, and so demonstrated that these lay within the medial rectus C-group located at the dorsomedial edge of the oculomotor nucleus. Furthermore, by making tendon injections in the other extraocular muscles, they demonstrated that each is supplied by a similar set of smaller motoneurons located along the periphery of the main nucleus. The inferior rectus, like the medial rectus, is supplied by C-group motoneurons, whereas the superior rectus and inferior oblique are supplied by small motoneurons located between the two oculomotor nuclei in the S-group. The relative size difference between MIF motoneurons and SIF motoneurons is also present in rodents, although the distribution difference seen in primates is not observed, since all the motoneurons are found within the borders of the extraocular motor nuclei (Eberhorn et al., 2006). These morphological differences strongly suggest that MIF motoneurons display as yet undescribed differences in their physiological characteristics with respect to SIF motoneurons. Indeed, there is evidence that MIF and SIF motoneurons differ significantly in terms of their inputs (Wasicky et al., 2004; Ugolini et al., 2006).
In light of the above evidence that MIF motoneurons in the C-group and the SIF motoneurons in the A- and B-groups differ in terms of their cell size, axonal targets, and likely inputs, we determined to investigate these motoneurons more thoroughly. By use of retrograde tracers in macaque monkeys, we characterized the dendritic morphology of the cells supplying the medial rectus muscle. We then extended this comparison to the ultrastructural features of the cells in each group. The findings of this study lend further credence to the belief that the MIF and SIF motoneuron populations are specialized to support distinct extraocular muscle fiber functions. Brief reports of this analysis have appeared earlier (Erichsen et al., 1998; May et al., 2000).
MATERIALS AND METHODS
These experiments were performed on adult and young adult, male Macaca fascicularis monkeys (n = 4). The surgical procedures were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee. They were fully compliant with National Institutes of Health (NIH) policies as described in Principles for Laboratory Care. Animals were initially sedated with ketamine HCl (10 mg/kg, IM), and then anesthetized with isoflurane (1–3%). Dexamethasone (1.0 mg/kg, IV) was administered to inhibit swelling, and atropine sulfate (0.05 mg/kg, IM) was given to decrease respiratory tract secretions. The temperature, heart rate, and blood gases were kept within normal ranges during the surgical operation. At the end of the surgery, Sensorcaine was infused around the wound edges, and Buprenex (0.01 mg/kg, IM) was administered as a postsurgical analgesic.
Using sterile surgical techniques, the medial rectus was approached by making an incision above the brow. The orbicularis oculi muscle was dis-inserted from the supraorbital ridge and an incision made in the underlying connective tissue. An ophthalmic muscle hook was used to draw the medial rectus muscle insertion forward. The sharpened needle of a 10 μl Hamilton syringe was inserted into the muscle belly, and 7 μl of a tracer solution containing 2% wheat germ agglutinin conjugated horseradish peroxidase (Sigma, St. Louis, MO) and 10% horseradish peroxidase (Sigma) (WGA-HRP) was pressure-injected. The orbicularis oculi muscle was reattached to its insertion using 4-0 suture and the incision was closed. Following a 48-hour survival period, the animals were again sedated with ketamine HCl, and then deeply anesthetized with sodium pentobarbital (50 mg/kg, IP). When areflexic, they were perfused through the heart with a buffered saline rinse followed by a fixative containing 1.0% paraformaldehyde (Fisher Chemical, Pittsburgh, PA) and 1.25% glutaraldehyde (Fisher Chemical) in pH 7.2, 0.1 M phosphate buffer (PB). The brainstem was blocked in the frontal plane and postfixed in the same solution for 1 hour.
Sections were cut on a vibratome (VT100S, Leica, Deerfield, IL) at 100 μm. Two sets, each containing an ordered series of sections 300 μm apart, were reacted to reveal the presence of the tracer using previously described methods (Chen and May, 2002; Wang et al., 2010). Briefly, the sections were incubated in a solution containing 0.25% ammonium molybdate (Fisher Chemical), 0.005% tetramethylbenzidene (TMB) (Fisher Chemical), and 2.5% ethanol in pH 6.0, 0.1 M PB. The reaction was initiated by the addition of 0.0125% hydrogen peroxide, and allowed to continue for 18 hours at 4°C. The blue TMB reaction product was stabilized by incubation in 5.0% ammonium molybdate in pH 6.0, 0.1 M PB.
One set of sections was then mounted out of pH 6.0, 0.1 M PB onto glass slides, counterstained with cresyl violet, dehydrated, cleared, and coverslipped to allow light microscopic analysis of the labeling pattern. Labeled cells were photographed using a Nikon Eclipse E600 microscope equipped with a Nikon DXM1200F digital camera. The images were acquired using Metamorph software (Universal Imaging, West Chester, PA), and the brightness, contrast, and color of the images were adjusted to match the viewed image using Photo-shop (Adobe Systems, San Jose, CA). Labeled cells were also drawn using an Olympus BH2 microscope equipped with a drawing tube.
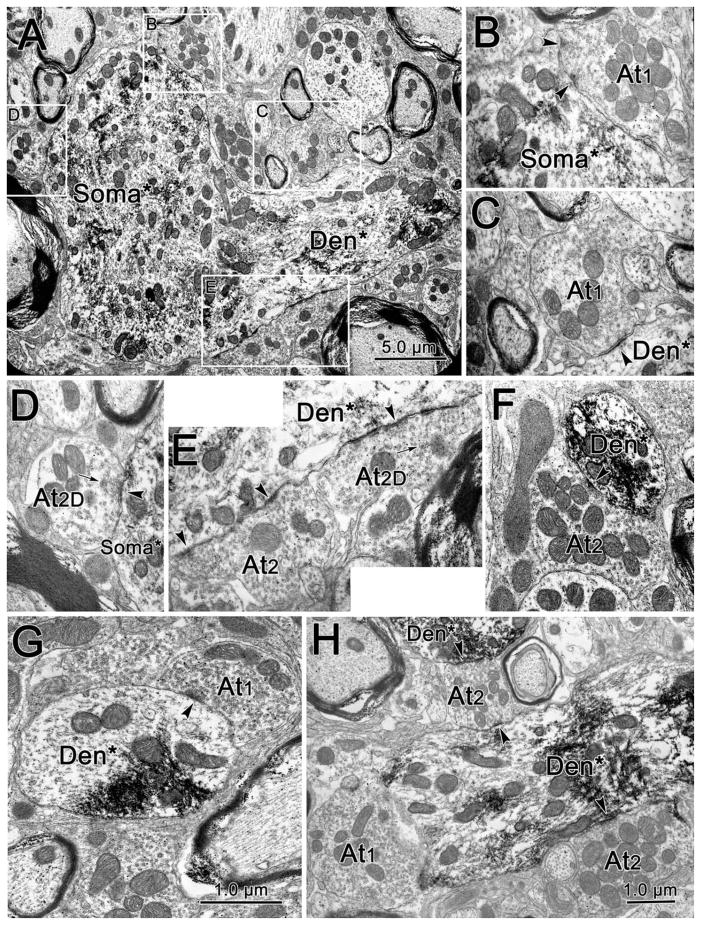
The other set of sections was processed further to stabilize the TMB reaction product for electron microscopy. After incubation in a solution of 1.0% diaminobenzidene (DAB) (Sigma) in pH 7.2, 0.1 M PB, the sections were reacted by the addition of 0.0125% hydrogen peroxide. Under a Wild M-8 stereoscope (Leica), samples containing label were cut from free-floating sections for electron microscopic processing. Initially, the samples were cut so that they just contained either the A-group (Fig. 1C), B-group (Fig. 1G), or C-group (Fig. 1D). In later experiments, additional samples (Fig 1E) were cut that contained the preganglionic Edinger–Westphal nucleus (EWpg). The sections were then mounted, counterstained with cresyl violet, and coverslipped to provide a record of the sampled regions. The blocks underwent routine electron microscopic processing. They were fixed and stained with 1.0% osmium tetroxide (EM Sciences, Fort Washington, PA) in pH 7.2, 0.1 M PB and 2.0% uranyl acetate (EM Sciences). They were dehydrated in ethanols and embedded in Epon Araldite (EM Sciences) blocks. Semithin sections were cut and stained with toluidine to allow inspection and trimming of the blocks to include just the target area. Ultrathin sections were cut with a diamond knife (Diatome, Fort Washington, PA) on an ultramicrotome (Leica Ultracut UCT), placed on copper mesh grids, and stained with lead citrate. The labeled cells were observed and photographed with a Zeiss 906 transmission electron microscope. Photoshop (Adobe) was used to match the tone of the plates assembled into each figure. Images of the labeled cells found in the A-, B-, and C-group samples were stored as TIF files and were quantitatively analyzed using NIH Image for Macintosh. Quantification was done blind to the sample area. The images were first scaled for the correct magnification, and then different image features were identified with different colors. The borders of the somata, dendrites, and axon terminals were defined, and the following aspects were quantified: their cross-sectional area, perimeter, major and minor axes, their length of presynaptic to postsynaptic profile apposition, and the length of synaptic contact. Due to the fact that cross-sectional area, perimeter, major and minor axes were strongly correlated, only area will be presented in the Results. The membrane coverage was computed by adding the apposition lengths on a postsynaptic profile and dividing by its perimeter. Student’s t-tests were used to compare measurements from the three motoneuron groups.
Figure 1.
Distribution of retrogradely labeled motoneurons (dots) observed following an injection of WGA-HRP into the belly of the medial rectus muscle. Motoneurons are found within three groups: A, B, and C. Trapezoids indicate typical locations for extracting samples in preparation for electron microscopy. CC, caudal central subdivision; EWpg, Edinger–Westphal preganglionic nucleus; III, oculomotor nucleus; MLF, medial longitudinal fasciculus; nD, nucleus of Darkschewitsch; PAG, periaqueductal gray; SOA, supraoculomotor area.
RESULTS
Light Microscopic Observations
The distribution of retrogradely labeled neurons is charted with respect to the borders of the oculomotor nucleus in Figure 1. The C-group neurons hug the dorsomedial edge of the oculomotor nucleus (III), lying in the supraoculomotor area (SOA) between the nucleus proper and the EWpg. At the rostral pole of III (Fig. 1A), C-group cells maintain this relation with EWpg, even as III shrinks. At the caudal end of the nucleus (Fig. 1G), they are not present once the caudal central subdivision (CC) appears. The A-group fills the rostral pole of III (Fig. 1A), but takes a progressively more ventral position as one moves caudally in III, even extending into the medial longitudinal fasciculus (MLF). This division has all but disappeared when the B-group appears in the dorsal aspect of III (Fig. 1F). The B-group takes up much of the caudal pole of the III (Fig. 1G). The distribution charted here closely resembles that previously described (Büttner-Ennever and Akert, 1981).
The morphology of the motoneurons is demonstrated in Figures 2, 3. The cells in all three groups were for the most part multipolar, with 3 to 5 primary dendrites emerging from the soma. There was no obvious common orientation to the multipolar somata. However, even visual examination reveals a clear difference in the soma size among the groups. Larger cells are numerous within the A-group (Fig. 2A) and B-group (Fig. 2B). However, the cells in the C-group (Fig. 2C) are more homogeneous in size, and the largest cells within this group appear to be about the same size as the smallest cells in the A- and B-groups. This qualitative view is supported by previous quantitative studies (Eberhorn et al., 2005).
Figure 2.
Photomicrographs of labeled motoneurons in the A-group (A), B-group (B), and C-group (C). Note that the C-group motoneurons are generally smaller than A- and B-group cells. III, oculomotor nucleus; MLF, medial longitudinal fasciculus; SOA, supraoculomotor area.
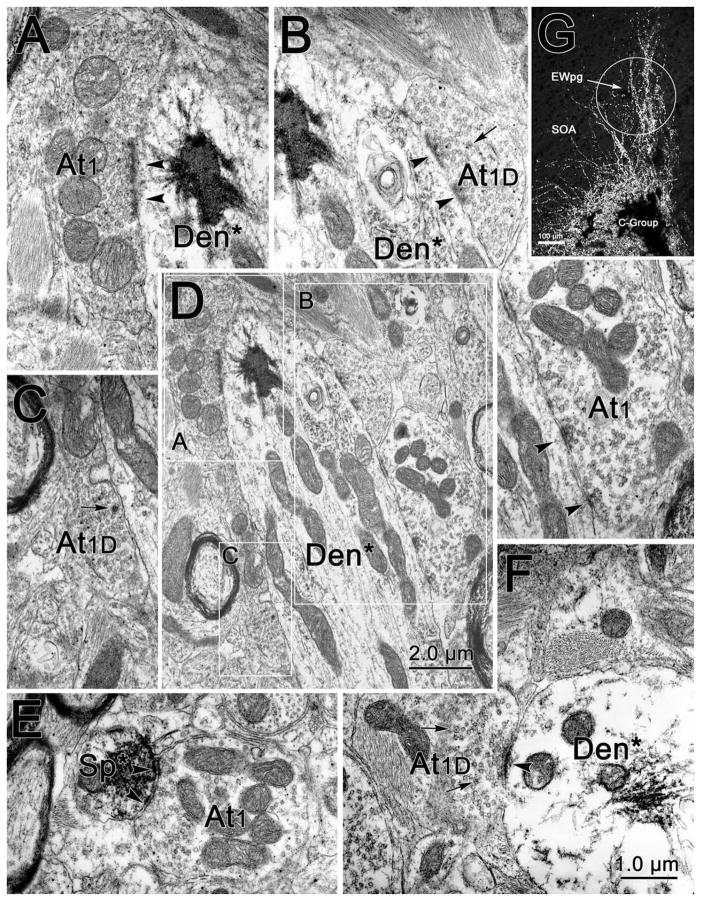
Figure 3.
Illustration of the dendritic relationships of C-group motoneurons. Note that the dendrites often extend into the preganglionic Edinger–Westphal nucleus (EWpg) or the supraoculomotor area (SOA), but rarely extend ventrolaterally towards the oculomotor nucleus (III).
We further examined the morphology of the dendrites of these cells in a case with good dendritic filling. The dendrites of the A-group cells extended within the oculomotor nucleus and between the fasicles of the MLF. Only at the rostral pole did A-group dendrites extend into SOA. The dendritic fields were densest in the immediate area of the retrogradely labeled cells. The dendrites of the B-group cells were also mainly found within the borders of the oculomotor nucleus, despite the proximity of the labeled cells to the SOA. However, the C-group dendrites displayed a very different distribution, as illustrated in Figure 3. Well-filled dendrites were observed to extend dorsally into the EWpg (Fig. 3A–C) and into the adjacent SOA (Fig. 3B,D). Some extended ventrally into the S-group region between the nuclei. However, dendrites extending into III proper were rarely, if ever, observed. At the rostral end of the nucleus (Fig. 3A), the dendritic fields of these motoneurons tended to have a more dorsoventral alignment.
Ultrastructural observations
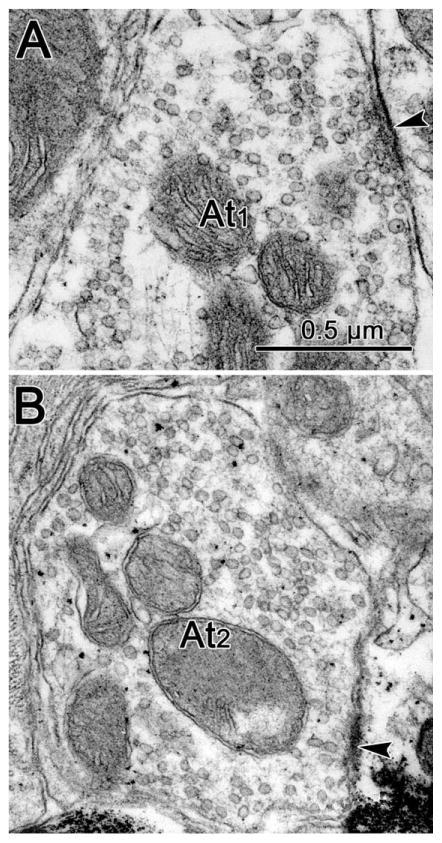
In observing the ultrastructure of the three groups of motoneurons, we attempted to divide the profiles synapsing upon them into categories based on their vesicular contents (Fig. 4). Profiles dominated by circular vesicular profiles were designated as At1 profiles (Fig. 4A). Those in which irregular or pleomorphic vesicle profiles were quite common were designated as At2 profiles (Fig. 4B). As high osmolar solutions were not used during osmication, the differences between these two profile types were subtle, and could represent a subjective division of a continuous distribution. Those profiles with numerous dense-cored vesicles were designated as At3 profiles, but these were so extremely rare that they will not be considered further here. In some cases, one or two scattered dense-cored vesicles were seen in profiles that otherwise fit into the first two categories. These profiles were designated At1D and At2D, respectively. It is, of course, possible that the At1 and At2 terminals just represent slices through the terminal in regions that did not contain the sparse dense-cored vesicles. Generally, terminals with round vesicles are associated with asymmetric synaptic densities, and those with pleomorphic vesicles are associated with symmetric synaptic densities. Although we often saw such associations, we did not require them for our categorization. These categories have considerable precedent (Peters et al., 1991). Terminals characterized by spherical clear vesicles and asymmetric synaptic junctions are usually excitatory in character, while those characterized by pleomorphic clear vesicles and symmetric junctions are usually inhibitory in nature. Dense-cored vesicles are associated with the presence of peptide neuromodulators.
Figure 4.
Ultrastructure of axon terminals seen contacting (arrowhead) retrogradely labeled medial rectus motoneurons. A: Some terminals had predominantly spherical vesicles (At1). B: Other terminals displayed more pleomorphic vesicles (At2).
The ultrastructure of examples of labeled (*) A-group cells is demonstrated in Figure 5. The WGA-HRP reaction product presents as flocculent, electron-dense crystals in the cytoplasm of the labeled cells. In the example shown in Figure 5D, it is particularly prevalent around the nucleus of the soma. Reaction product could also be observed in dendrites like the medium-sized example shown in Figure 5F and was even present in small processes likely to be spines (Fig. 5E). The somata of A-group cells generally presented with numerous axosomatic contacts (Fig. 5A,B,D), as did the proximal dendrites (Fig. 5C). The amount of membrane covered by synaptic terminals was even greater on more distal portions of the dendritic tree, as shown in the labeled dendrite (Den*) in Figure 5F. Although not numerous, spines were a definite feature of these cells (Fig. 5E,F). The profiles shown here contacting (arrowhead) the retrogradely labeled profiles feature terminals with clear round vesicles (At1). One of these terminals also contains a dense-cored vesicle (arrow) (At1D, Fig. 5F). The diameters of terminals varied considerably, even within the same category (compare Fig. 5C and 5A,B). In some cases, there was evidence that an individual axon made multiple contacts on the same cell (Fig. 5B).
Figure 5.
Examples of axon terminals (At) contacting (arrowhead) retrogradely labeled A-group medial rectus motoneurons. The WGA-HRP reaction product is flocculent electron-dense material located in the somata (Soma) and dendrites (Den) of retrogradely labeled (*) profiles. Examples of terminals contacting the somata (B,C) and a proximal dendrite (A) of the cell shown in D are shown at higher magnification. All were At1 type terminals, and in some cases they appeared to belong to the same axon (B). Additional examples of At1and At1D terminals contacting labeled dendrites and dendritic spines (Sp) are shown in E,F.
Samples taken from the B-group had ultrastructural features very similar to those of the A-group, as shown in Figure 6. Labeled somata (Soma*, Fig. 6A) and dendrites (Den*, Fig. 6A,F–H) were characterized by flocculent, electron-dense reaction product. Both the somata and proximal dendrites had numerous terminal contacts arrayed along their plasmalemmas (Fig. 6A). Examples of contacts containing both clear, spherical (At1, Fig. 6B,C,G,H) and clear, pleomorphic (At2, Fig. 6E,H) vesicles were observed contacting (arrowheads) the labeled B-group motoneurons, as were terminals that contained scattered dense-cored vesicles (arrows) (At2D), in addition to the clear vesicles (Fig. 6D,E). Terminals appeared to contact small (Fig. 6F), medium (Fig. 6G,H), and large (Fig. 6A) labeled dendrites with about the same frequency.
Figure 6.
Examples of axon terminals (At) contacting (arrowhead) retrogradely labeled B-group medial rectus motoneurons. The WGA-HRP reaction product is flocculent electron-dense material located in the somata (Soma) and dendrites (Den) of retrogradely labeled (*) profiles. Examples of terminals contacting the somata (B,D) and proximal dendrite (C,E) of the cell shown in A are shown at higher magnification. At1 (B,C), At2 (E), and At2D (E,D) type terminals were observed contacting the cell in A. Examples of At1 and At2 terminals contacting labeled dendrites of medium (H) and small (F,G) caliber are also shown.
The ultrastructural features of labeled cells found in samples taken from the C-group were somewhat different, as shown in Figure 7. The electron-dense reaction product could be seen in both the dendrites (Den*, Fig. 7A–C,E,F) and somata (Soma*, Fig. 7A,D) of labeled C-group motoneurons. However, very few terminal profiles were observed in synaptic contact (arrowheads) with either the somata (Fig. 7A,D) or proximal dendrites (Fig. 7A,B) of these retrogradely labeled cells. Instead, terminals were mainly seen in synaptic contact with small (Fig. 7F) and medium diameter (Fig. 7C,E) dendrites. Terminals with both clear round vesicles (At1, Fig. 7E) and clear pleomorphic vesicles (At2, Fig. 7C,E,F) were observed synapsing on C-group motoneurons.
Figure 7.
Examples of axon terminals (At) contacting (arrowhead) retrogradely labeled C-group medial rectus motoneurons. The WGA-HRP reaction product is flocculent electron-dense material located in the somata (Soma) and dendrites (Den) of retrogradely labeled (*) profiles. Very few synaptic contacts were observed on the somata and proximal dendrites, as shown in the low-magnification view of two motoneuron somata (A,D) and a longitudinal cut through a dendrite (B). More synaptic contacts were seen on medium-sized (C,E) and small (F) dendrites. Both At1 (E) and At2 (C,F) terminals were present.
Quantitative measures
We further investigated the similarities and differences among the three medial rectus subgroups by quantifying various aspects of their ultrastructure. As shown in Figure 8A, there was an evident difference between motoneurons in the C-group samples and those in the A- and B-group samples with respect to the extent of synaptic coverage on the plasma membranes of their somata and dendrites. Most C-group cells had less that 15% of their membrane covered, while values in the 20–30% range were the most common for the A- and B-groups. The means ± SDs for the three groups were: A-group (23.0 ± 12.4, n = 12), B-group (23.6 ± 13.3, n = 18), and C-group (11.2 ± 13.8, n = 84). There was no significant difference between the A- and B-groups (P = 0.5), but the C-group was significantly different from the combined A- and B-groups (P < 0.002) with respect to the coverage of the dendritic membrane by synaptic profiles. This difference was large enough to be evident from visual inspection (Compare Figs. 5, 6 with 7).
Figure 8.
Histograms showing the results of a quantitative analysis of medial rectus subgroup ultrastructure. A: While the A-group (light gray) and B-group (dark gray) samples have similar levels of somatodendritic coverage, C-group (white) samples display much less coverage of labeled motoneurons. B: The frequency with which the different terminals types contact labeled motoneurons in each of the different subgroup samples. Types 1 and 2 terminals were observed with about the same frequency, and they made up similar percentages of the contacts on each of the subgroups. Terminals with dense-cored vesicles contacted labeled motoneurons far less often than terminals without dense-cored vesicles in A-group and B-group samples, and terminals containing dense-cored vesicles were not observed to contact labeled motoneurons in C-group samples. C: The percent of terminals for each of the subgroups that lay in different terminal area ranges. The terminals observed contacting labeled motoneurons in C-group samples tended to be smaller than those contacting labeled motoneurons in A-group and B-group samples.
We also noted differences in the types and sizes of the synaptic terminals found in the different motoneuron subgroup samples. As shown in Figure 8B, terminals that contained dense-cored vesicles were rarely observed in synaptic contact with labeled medial rectus motoneurons. However, these terminals were essentially absent in the C-group samples. As shown in Figure 8C, the terminals contacting different subgroup motoneurons had the same overall range of sizes: 0.25 μm2 to over 5 μm2 (Fig. 7C). However, those terminals contacting C-group motoneurons were smaller on average (1.3 μm2 ± 0.07, n = 121) than those contacting A-group (2.7 μm2 ± 0.18, n = 35) and B-group (2.4 μm2 ± 0.20, n = 64) motoneurons. In fact, there was no significant difference between the A- and B-groups (P > 0.328), but the C-group population was significantly different from the combined A- and B-group populations (P < 0.0001). Part of this difference is due to a substantial number of quite large terminals (>3.0 μm2) present only on the A- and B-group motoneurons, as noted in Figure 5.
C-group dendritic ultrastructure within the EWpg
We were struck by two observations: 1) In the C-group samples, the percentage of postsynaptic membrane contacted by terminals was greater on small- and medium-sized dendrites than on somata and proximal dendrites (Fig. 7). 2) The dendrites of C-group motoneurons often extended up into the EWpg. This point is emphasized by the light micrograph shown in Figure 9G. Labeled dendrites extend through EWpg into the surrounding SOA. Thus, a significant portion of the synaptic space on these cells is found within EWpg. In monkeys, the EWpg contains cholinergic, preganglionic motoneurons projecting to the ciliary ganglion for the control of lens and pupil function, as opposed to peptidergic neurons that project centrally. The latter neurons lie in a separate, centrally projecting Edinger–Westphal nucleus (EWcp). This segregation has been demonstrated by immunohistochemical and tracer studies (Horn et al., 2008; May et al., 2008a,b; Kozicz et al., 2011).
Figure 9.
Examples of axon terminals (At) contacting (arrowhead) retrogradely labeled C-group medial rectus motoneuron dendrites within the EWpg. The WGA-HRP reaction product is flocculent electron-dense material located in the dendrites (Den) and spines (Sp) of retrogradely labeled (*) profiles. The extent of coverage can be appreciated in the low-magnification view of a longitudinally sectioned dendrite (D). This dendrite is contacted by terminals of the At1 (A,B) and At1D (B,C) type. Examples of terminals contacting a smaller dendrite (F) and a spine (E) are also shown. G: An LM photomicrograph in which crossed polarizers were used to show the extent of the dendritic field extending into EWpg in C-group cells that were extensively labeled with tracer.
In view of this dendritic organization, we elected to take additional samples from the region of the EWpg (Fig. 1E) following medial rectus muscle injections and to prepare them for ultrastructural analysis. Examples of the labeled profiles found in these samples are shown in Figure 9. Small-caliber dendrites (Den*, Fig. 9D,F) and even spines (Sp*, Fig. 9E) that contained patches of flocculent, electron-dense label were observed. The extent to which the dendrites were covered by synaptic terminals (Fig. 9D) was more similar to that seen in the A- and B-group samples than in the C-group samples. Furthermore, in addition to synaptic profiles containing just clear vesicles (At1, Fig. 9A,B,E), terminals that also contained scattered dense-cored vesicles (arrows) (At1D, Fig. 9B,C,F) were observed making synaptic contacts (arrowheads) with the labeled dendrites and spines. Again, this organization was more similar to the organization seen in the A- and B-group samples.
DISCUSSION
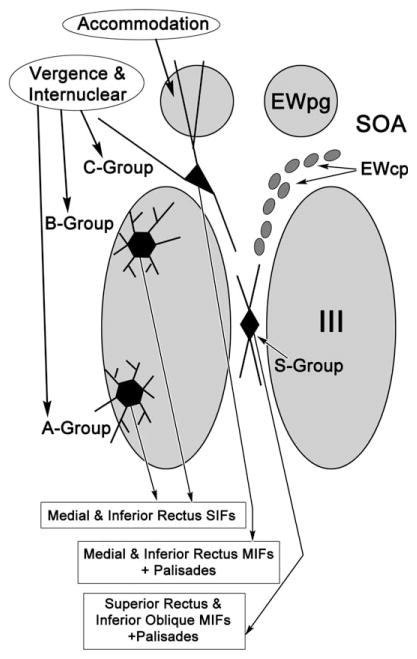
The results presented here confirm and extend previous light microscopic studies that indicated differences between C-group motoneurons and A- and B-group motoneurons. Specifically, we have shown that the smaller, C-group motoneurons found at the dorsomedial edge of the oculomotor nucleus (III) have dendrites that largely avoid the nucleus proper, and instead extend into the SOA and into the EWpg. In contrast, the dendrites of the larger, A- and B-group motoneurons are primarily distributed within III. Ultrastructural differences are also present. The C-group motoneurons have relatively fewer, smaller terminals on their somata and proximal dendrites than A- and B-group motoneurons. They also lack contacts from synaptic profiles containing dense-cored vesicles. However, those distal dendrites located in EWpg have ultrastructural characteristics similar to those of A- and B-group motoneurons. These findings are summarized in Figure 10.
Figure 10.
Schematic diagram that places the findings of the current study about medial rectus motoneurons in the context of their axonal targets and possible inputs. All three groups of medial rectus motoneurons found in and above the oculomotor nucleus (III) receive vergence signals, as well as conjugate horizontal gaze signals via the abducens internuclear projection. However, the C-group neurons are likely to also receive an accommodation signal due to the fact they extend their dendrites into the Edinger–Westphal preganglionic nucleus (EWpg). The C-group motoneurons lie very near the peptidergic cells of the centrally projecting Edinger–Westphal population (EWcp).
Cell location and dendritic morphology
The presence of subgroups within the medial rectus motoneuron population was first observed in monkeys by Büttner-Ennever and Akert (1981), and later confirmed by Porter et al. (1983). Büttner-Ennever et al. (2001) provided the first significant breakthrough in our understanding of these subgroups by injecting the muscle insertion in order to retrogradely label just the axons that make multiple endings on the fibers of the global layer of the extraocular muscles. These experiments in monkeys showed that C-group motoneurons are responsible for supplying these MIFs, while the larger motoneurons found within the oculomotor nucleus proper supply SIFs (Fig. 10). They further demonstrated that the global layer MIFs of each extraocular muscle are supplied by populations of small motoneurons located at the periphery of their respective extraocular motor nuclei. Further examination of inputs to the extraocular motoneurons has revealed that SIF and MIF motoneurons may receive different patterns of input. Anterograde tracer experiments indicate that the pretectal projection appears to be concentrated over the MIF motoneurons of III (Wasicky et al., 2004). Experiments using trans-neuronal transport of rabies virus in monkeys suggest that the abducens MIF motoneurons of the lateral rectus muscle are supplied by the nucleus prepositus hypo-glossi and the medial, parvocellular portion of the medial vestibular nucleus, while the abducens SIF motoneurons are supplied by the more lateral, magnocellular portion of the medial vestibular nucleus and the premotor burst neurons in the pontine reticular formation (Ugolini et al., 2006). However, anterograde tracer experiments indicate that the abducens internuclear neurons supply all three groups of medial rectus motoneurons (Büttner-Ennever and Akert, 1981; Wasicky et al., 2004) and that vestibular inputs supply both the MIF and SIF populations of III (Wasicky et al., 2004).
In the present study, the organization of the dendrites supports the idea that medial rectus MIF motoneurons have different inputs than medial rectus SIF motoneurons. Specifically, most of the C-group dendrites extended up into the EWpg and the SOA. The former contains preganglionic motoneurons that control the lens and pupil (Warwick, 1954; Akert et al., 1980; Burde, 1988; May et al., 2008b), while the latter is believed to be the site of vergence-related premotor neurons (Judge and Cumming, 1986; Mays et al., 1986; Zhang et al., 1991). Moreover, the ultrastructural analysis indicates that the majority of the synaptic input to the MIF motoneurons occurs on these same distal dendrites. Thus, the dendritic and synaptic organization of these motoneurons strongly suggests that they receive their major synaptic drives from the same sources as the preganglionic motoneurons in EWpg. In this manner, the dendritic distribution of C-group motoneurons may represent a structural correlate of accommodation-driven vergence. This also suggests that tonic activity within MIFs is crucial for the finely controlled tonic adjustments of vergence angle with respect to the accommodative drive that characterizes the near triad of primates (Fig. 10). Indeed, one might argue that fine control of the near triad is a prerequisite to complex tool production and use. In this light, the displacement of MIF motoneurons into a separate C-group may be an outcome of neural evolution in support of the increased requirements for near work in primates.
It is not known why the other occupant of the C-group, inferior rectus MIF motoneurons, colocalizes with the medial rectus population (Fig. 10). Both populations adduct the eye, but so do the superior rectus MIF motoneurons, which lie in the S-group. Some of the dendrites of the medial rectus motoneurons do extend into the S-group region. Consequently, it is likely that some inputs are shared among the entire MIF motoneuron population. For example, the pretectal projection is shared by both the C- and S-groups (Büttner-Ennever and Akert, 1996; Wasicky et al., 2004). Not all signals are shared, as abducens internuclear terminals are distributed to C-group, but not S-group, motoneurons (Büttner-Ennever and Akert, 1981). Clearly, until the signals carried by MIF motoneurons have been physiologically defined, it is difficult to produce a cogent argument as to which inputs are distinct and which might be shared among MIF and SIF motoneurons. Nevertheless, the fact that the medial rectus MIF motoneurons do receive abducens internuclear input onto their somata and proximal dendrites, like medial rectus SIF motoneurons, but extend their dendrites into EWpg and SOA, suggests that the medial rectus MIF and SIF motoneurons may have similar activity during conjugate eye movements, but differ in their activity during vergence movements. The fact that stereoscopic vision may be most critical when working with the hands in the lower medial visual field may explain why the medial and inferior rectus MIF motoneurons should lie together in the C-group and presumably share inputs.
Cell ultrastructure and histochemistry
Eberhorn et al. (2005) showed that SIF motoneurons are on average 6–8 μm larger in mean diameter than MIF motoneurons in monkeys and that they also differ in their histochemistry. Specifically, they demonstrated that, whereas MIF motoneurons lack nonphosphorylated neurofilament and perineuronal nets and do not stain for parvalbumin, SIF motoneurons have all these features. We have previously observed that in monkeys the levels of cytochrome oxidase expression, as revealed histochemically, are much lower in MIF motoneurons than in SIF motoneurons (May and Fratkin, 2002). It is interesting that, while a number of differences have been noted between SIF and MIF motoneurons, none of these studies have indicated differences other than location between A-group and B-group medial rectus motoneurons. Similarly, no evidence for ultrastructural differences was found in the present study. More recent studies indicate that a distinguishable MIF motoneuron population is also present in other species. In the rat, topographically separate A-, B-, and C-group medial rectus motoneuron populations are not present (Eberhorn et al., 2006). However, distal insertion injections in rats do label a subset of motoneurons that are, on average, smaller than the cells labeled by belly injections, although both groups are mixed together within III. Rat MIF motoneurons also share histochemical characteristics with monkey MIF motoneurons (Eberhorn et al., 2006). In cats, we have observed a similar, smaller motoneuron population labeled from insertion injections that lie within III (Bohlen et al., 2012). In birds, which also have foveas, a set of smaller medial rectus motoneurons is located in a separate subnucleus found, like the C-group, dorsomedial to the main oculomotor nucleus (Erichsen and Evinger, 2006).
The present study adds a number of other features to those listed above. Specifically, the ultrastructure of the C-group motoneurons is notable for the small number of contacts seen on the somata and proximal dendrites of these cells. If this is a general feature of MIF motoneurons, it may suggest that the neuronal nets observed around SIF motoneuron somata are related to synaptic contact (Eberhorn et al., 2005, 2006). Our preliminary data do indeed suggest that S-group motoneurons supplying the superior rectus muscle display relatively fewer synaptic contacts on their somata and proximal dendrites than SIF motoneurons within the nucleus proper (Warren et al., 2011). It is also noteworthy that the MIF motoneurons lie adjacent to a population of NADP-diaphorase-positive cells in EWcp (Fig. 10) (Erichsen and May, 2012). This presents the intriguing possibility that, while the MIF motoneurons may not have many synapses on their somata, their activity may be modulated by nonsynaptic means through the release of nitric oxide by this adjacent cell population. It has, however, been argued that oculomotor neurons lack receptors for nitric oxide (Moreno-López et al., 1996), although it remains unclear whether this characteristic extends specifically to monkey MIF motoneurons. The nitridergic population can also be characterized by immunohistochemical stains for peptides, in particular urocortin 1 (Horn et al., 2008; May et al., 2008a; Kozicz et al., 2011). This colocalization may indicate that these peptidergic cells share inputs with MIF motoneurons or, alternatively, the lack of inputs on C-group somata and proximal dendrites may indicate that they avoid sharing inputs with the adjacent peptidergic neurons.
In general, the ultrastructural features we observed in macaque SIF motoneurons are in agreement with the features previously described for the cat oculomotor nucleus (Tredici et al., 1976) and, specifically, for cat medial rectus motoneurons (de la Cruz et al., 1992), although we identified a higher percentage of profiles containing pleomorphic vesicles than Tredici et al. Furthermore, the synaptology of the medial rectus SIF motoneurons we observed in Macaca fascicularis was generally the same as that observed for the Macaca mulatta oculomotor nucleus (Waxman and Pappas, 1979). These authors did not characterize neurons like the MIF motoneurons described here. However, these would not have been in the samples taken from the core of the oculomotor nucleus in the rhesus monkey. Tredici et al. (1976) noted a class of smaller neurons in III, but it is unclear whether these represent MIF motoneurons found within the cat nucleus (Bohlen et al., 2012) or oculomotor internuclear neurons. The difference in synaptic coverage we observed here for the SIF and MIF motoneurons (23% and 11%, respectively) are similar to those observed for alpha and gamma motoneurons (34% and 16%, respectively) in the cat spinal cord (Johnson, 1986). This correlation is consistent with the suggestion that palisade endings, found at the ends of MIF, act as an inverted muscle spindle subserving eye muscle proprioception (Lienbacher and Horn, 2012). On the other hand, the role of palisade endings in proprioception has recently been called into question because palisade endings have vesicle-containing profiles, are cholinergic, and have immunohistochemical profiles suggesting synaptic release (Eberhorn et al., 2005; Blumer et al., 2009; Rungaldier et al., 2009). Furthermore, injections of anterograde tracer into the extraocular motor nuclei result in labeling of the palisade axons, some of which are in continuity with axons supplying the MIFs (Lienbacher et al., 2011a; Zimmermann et al., 2011, 2013). Taken together, these studies indicate the palisade ending may not be a component of a spindle-like apparatus responsible for eye muscle proprioception, thus reducing the likelihood that MIF motoneurons function as a type of gamma motoneuron. However, it has recently been suggested that a subpopulation of C-group motoneurons may specifically innervate the palisades (Lienbacher et al., 2011b; Lienbacher and Horn, 2012). This report noted that cells labeled from insertion injections were located at the rostral end of III, and had a fusiform or pseudounipolar appearance. We reviewed our collection of medial rectus injection cases, including cases with insertion injections, and did not note any obvious morphological differences related to the rostrocaudal location in the C-group population, beyond the fact that cells and dendrites tend to be more dorsoventrally oriented at the rostral pole of III (Fig. 3). However, we did not just inject the tendon and did not separately analyze rostral samples for electron microscopy, so further work is necessary to settle this issue.
In summary, these findings support the contention that MIF motoneurons likely differ from SIF motoneurons in terms of their inputs and physiological characteristics. They further suggest that C-group motoneurons are likely to carry more signals related to the vergence component of the near triad than A- and B-group motoneurons.
Acknowledgments
Grant sponsor: National Eye Institute (NEI); Grant number: EY014263 (to P.J.M.); Grant sponsor: National Lotteries Charity Board; Grant number: RB217495 (to J.T.E.).
The authors thank Glen Hoskins for excellent assistance in preparing the tissue for ultrastructural analysis, and Dr. Susan Warren for critically reading earlier drafts of the article. We also thank the anonymous reviewers for insightful suggestions.
Footnotes
CONFLICT OF INTEREST
The authors have no real or potential conflicts of interest that could influence or be perceived to influence the work.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: PJM, JTE. Acquisition of data: PJM, NFW. Analysis and interpretation of data: PJM, NFW, JTE. Drafting of the article: PJM. Critical revision of the article for important intellectual content: PJM, JTE. Statistical analysis: NFW. Obtained funding: PJM, JTE. Administrative, technical, and material support: PJM, JTE. Study supervision: PJM, JTE.
LITERATURE CITED
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980;184:491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Mallart RM, Pinçon-Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–584. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- Blumer R, Konakci KZ, Pomikal C, Wieczorek G, Lukas JR, Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009;50:1176–1186. doi: 10.1167/iovs.08-2748. [DOI] [PubMed] [Google Scholar]
- Bohlen MO, Warren S, Mustari M, May PJ. Are there subgroups of rectus motoneurons related to fiber type and layer in the cat? Soc Neurosci Abst. 2012;38:371.02. [Google Scholar]
- Bondi AY, Chiarandini DJ. Morphologic and electrophysiologic identification of multiply innervated fibers in rat extraocular muscles. Invest Ophthalmol Vis Sci. 1983;24:516–519. [PubMed] [Google Scholar]
- Burde RM. Disparate visceral neuronal pools subserve spinal cord and ciliary ganglion in the monkey: a double labeling approach. Brain Res. 1988;440:177–180. doi: 10.1016/0006-8993(88)91173-0. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Akert K. Medial rectus subgroups of the oculomotor nucleus and their abducens internuclear input in the monkey. J Comp Neurol. 1981;197:17–27. doi: 10.1002/cne.901970103. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AKE, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AK, Scherberger H, D’Ascanio P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438:318–335. doi: 10.1002/cne.1318. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor control of eyelid movements in conjunction with vertical saccades in the cat: the rostral interstitial nucleus of the medial longitudinal fasciculus. J Comp Neurol. 2002;450:183–202. doi: 10.1002/cne.10313. [DOI] [PubMed] [Google Scholar]
- de la Cruz RR, Pastor AM, Martínez-Guijarro FJ, López-García C, Delgado-García JM. Role of GABA in the extra-ocular motor nuclei of the cat: a postembedding immunocytochemical study. Neuroscience. 1992;51:911–929. doi: 10.1016/0306-4522(92)90529-b. [DOI] [PubMed] [Google Scholar]
- Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- Eberhorn AC, Ardeleanu P, Büttner-Ennever JA, Horn AK. Histochemical differences between motoneurons supplying multiply and singly innervated extraocular muscle fibers. J Comp Neurol. 2005;491:352–366. doi: 10.1002/cne.20715. [DOI] [PubMed] [Google Scholar]
- Eberhorn AC, Büttner-Ennever JA, Horn AK. Identification of motoneurons supplying multiply- or singly-innervated extraocular muscle fibers in the rat. Neuroscience. 2006;137:891–903. doi: 10.1016/j.neuroscience.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, Evinger LC. Dual innervation of the medial rectus muscle in pigeon. Soc Neurosci Abst. 2006;32:345.23. [Google Scholar]
- Erichsen JT, May PJ. A perioculomotor nitridergic population in the macaque and cat. Invest Ophthalmol Vis Sci. 2012;53:5751–5761. doi: 10.1167/iovs.12-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen JT, Wright NF, May PJ. Light and electron microscopic observations on motoneurons subserving the near triad in the macaque. Invest Ophthal Vis Sci. 1998;39:S1049. [Google Scholar]
- Horn AK, Eberhorn A, Härtig W, Ardeleanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Johnson IP. A quantitative ultrastructural comparison of alpha and gamma motoneurons in the thoracic region of the spinal cord of the adult cat. J Anat. 1986;147:55–72. [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J Comp Neurol. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienbacher K, Horn AK. Palisade endings and proprioception in extraocular muscles: a comparison with skeletal muscles. Biol Cybern. 2012;106:643–655. doi: 10.1007/s00422-012-0519-1. [DOI] [PubMed] [Google Scholar]
- Lienbacher K, Mustari M, Hess B, Büttner-Ennever J, Horn AK. Is there any sense in the palisade endings of eye muscles? Ann N Y Acad Sci. 2011a;1233:1–7. doi: 10.1111/j.1749-6632.2011.06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienbacher K, Mustari M, Ying HS, Büttner-Ennever JA, Horn AK. Do palisade endings in extraocular muscles arise from neurons in the motor nuclei? Invest Ophthalmol Vis Sci. 2011b;2:2510–2519. doi: 10.1167/iovs.10-6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Fratkin JD. Chemoidentification of components of the oculomotor complex in macaque and man. Soc Neurosci Abst. 2002;28:463.16. [Google Scholar]
- May PJ, Wright NF, Lin RC-S, RC-S, Erichsen JT. Light and electron microscopic features of medial rectus C-subgroup motoneurons in macaques suggest near triad specialization. Invest Ophthalmol Vis Sci. 2000;41:S820. [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008a;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Sun W, Erichsen JT. Defining the pupillary component of the perioculomotor preganglionic population within a unitary primate Edinger-Westphal nucleus. Prog Brain Res. 2008b;171:97–106. doi: 10.1016/S0079-6123(08)00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr R, Gottschall J, Gruber H, Neuhuber W. Internal structure of cat extraocular muscle. Anat Embryol (Berl) 1975;148:25–34. doi: 10.1007/BF00315560. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- Moreno-López B, Escudero M, Delgado-Garcia JM, Estrada C. Nitric oxide production by brain stem neurons is required for normal performance of eye movements in alert animals. Neuron. 1996;17:739–745. doi: 10.1016/s0896-6273(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Pachter BR. Rat extraocular muscle. 3. Histochemical variability along the length of multiply-innervated fibers of the orbital surface layer. Histochemistry. 1984;80:535–538. [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and the supporting cells. 3. New York: Oxford University Press; 1991. [Google Scholar]
- Porter JD, Guthrie BL, Sparks DL. Innervation of monkey extraocular muscles: localization of sensory and motor neurons by retrograde transport of horseradish peroxidase. J Comp Neurol. 1983;218:208–219. doi: 10.1002/cne.902180208. [DOI] [PubMed] [Google Scholar]
- Rungaldier S, Heiligenbrunner S, Mayer R, Hanefl-Krivanek C, Lipowec M, Streicher J, Blumer R. Ultrastructural and molecular biologic comparison of classic proprioceptors and palisade endings in sheep extraocular muscles. Invest Ophthalmol Vis Sci. 2009;50:5697–5706. doi: 10.1167/iovs.09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Innervation and structure of extraocular muscles in the monkey in comparison to those of the cat. J Comp Neurol. 1981;198:649–665. doi: 10.1002/cne.901980407. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Tredici G, Pizzini G, Milanesi S. The ultrastructure of the nucleus of the oculomotor nerve (somatic efferent portion) of the cat. Anat Embryol (Berl) 1976;149:323–346. doi: 10.1007/BF00315448. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Doldan Dans M, Dubayle D, Brandi AM, Büttner-Ennever J, Graf W. Horizontal eye movement networks in primates as revealed by retrograde trans-neuronal transfer of rabies virus: differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol. 2006;498:762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- Wang N, Warren S, May PJ. The macaque midbrain reticular formation sends side-specific feedback to the superior colliculus. Exp Brain Res. 2010;201:701–717. doi: 10.1007/s00221-009-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Horn AKE, Bohlen MO, May PJ. Does the central mesencephalic reticular formation project to vertical gaze motoneurons? Soc Neurosci Abst. 2011;37:699.05. [Google Scholar]
- Warwick R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat. 1954;88:71–93. [PMC free article] [PubMed] [Google Scholar]
- Wasicky R, Horn AK, Büttner-Ennever JA. Twitch and non-twitch motoneuron subgroups in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Pappas GD. Ultrastructure of synapses and cellular relationships in the oculomotor nucleus of the rhesus monkey. Cell Tissue Res. 1979;204:161–169. doi: 10.1007/BF00234630. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gamlin PD, Mays LE. Antidromic identification of midbrain near response cells projecting to the oculomotor nucleus. Exp Brain Res. 1991;84:525–528. doi: 10.1007/BF00230964. [DOI] [PubMed] [Google Scholar]
- Zimmermann L, May PJ, Pastor AM, Streicher J, Blumer R. Evidence that the extraocular motor nuclei innervate monkey palisade endings. Neurosci Lett. 2011;489:89–93. doi: 10.1016/j.neulet.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L, Morado-Díaz CJ, Davis-Löpez de Carrizosa MA, de la Cruz RR, May PJ, Streicher J, Pastor AM, Blumer R. Axons giving rise to the palisade endings of feline extraocular muscles display motor features. J Neurosci. 2013;33:2784–2793. doi: 10.1523/JNEUROSCI.4116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]