Abstract
A hallmark of chronic metabolic diseases, such as diabetes and metabolic syndrome, and oxidative stress, as occurs in chronic inflammatory and degenerative conditions, is the presence of extensive protein post-translational modifications, including glycation, glycoxidation, carbonylation and nitrosylation. These modifications have been detected on structural cartilage proteins in joints and intervertebral discs, where they are known to affect protein folding, induce protein aggregation and, ultimately, generate microanatomical changes in the proteoglycan–collagen network that surrounds chondrocytes. Many of these modifications have also been shown to promote oxidative cleavage as well as enzymatically-mediated matrix degradation. Overall, a general picture starts to emerge indicating that biochemical changes in proteins constitute an early event that compromises the anatomical organization and viscoelasticity of cartilage, thereby affecting its ability to sustain pressure and, ultimately, impeding its overall bio-performance.
Introduction
The health of the joints of the peripheral and axial skeleton, including the intervertebral discs, depends on the integrity of cartilaginous tissues,1,2 which, in turn, is influenced by the ability of chondrocytes to maintain the extracellular matrix (ECM) of cartilage. Numerous investigations have aimed to identify mechanisms by which ageing and mechanically-driven stresses lead to fissuring, fibrillation and wear of hyaline cartilage and fibrocartilage (Box 1); however, a newer line of enquiry focuses on the roles of metabolically-driven processes in cartilage degeneration. Conditions of prolonged oxidative stress (which occur, for example, during ageing and chronic inflammation) and metabolic stress (through diabetes and metabolic syndrome, for example) can induce biochemical changes, including glycation, carbonylation, lipoxidation and nitrosylation, in cartilage structural proteins. These post-translational modifications induce aggregation and/or unfolding of cartilage matrix proteins, which increases their susceptibility to enzymatic cleavage and degradation.3,4 Loss of these matrix proteins impairs the ability of cartilage to withstand mechanical stresses and therefore renders it even more susceptible to breakdown.5–8 The resulting loss of cartilage, observed as joint-space narrowing by radiography, in association with corresponding remodelling of subchondral bone, osteophyte formation and variable levels of joint pain, compromises articular performance and represents the common clinical picture of osteoarthritis (OA), the most common joint disease worldwide.
Box 1. Cartilage.
There are three types of cartilage: hyaline cartilage, fibrocartilage and elastic cartilage. Hyaline cartilage is characterized by an abundant glassy matrix and is found covering bone surfaces in synovial joints, within tracheal rings, and as part of the larynx and nose; fibrocartilage, which is found in menisci, the labra of the shoulder and hip, the annulus fibrosus of intervertebral discs and in the pubic symphysis, is typified by abundant collagen bundles; and elastic cartilage, which is found in the external ear, the eustachian tube and the larynx, appears as dense networks of elastin fibres. Hyaline cartilage and fibrocartilage are exquisitely designed for the purpose of distributing mechanical forces across articulating surfaces, absorbing shock and minimizing friction during joint motion.10,25,98 In this respect, articular cartilage is able to remodel itself to achieve a best-fit articulation that optimally distributes mechanical stresses to subchondral bone.99
In this Review, we summarize the current knowledge of the biochemical changes in the articular cartilage matrix—focusing on cartilage matrix proteins—that are associated with diabetes, metabolic syndrome and chronic oxidative stress. Although we acknowledge that both oxidative stress and metabolic diseases can directly affect chondrocytes and, in turn, matrix biosynthesis, an in-depth discussion of the effects of these stresses on chondrocytes is beyond the scope of this article.
Articular cartilage
Articular cartilage provides two essential functions for the joint. In the healthy state, this tissue ensures an extremely low coefficient of friction during joint motion, as well as controlling joint alignment and regulating the distribution of mechanical forces across the joint. The mechanical properties required for this function are critically dependent on the molecular architecture of this tissue.
The architecture of cartilage
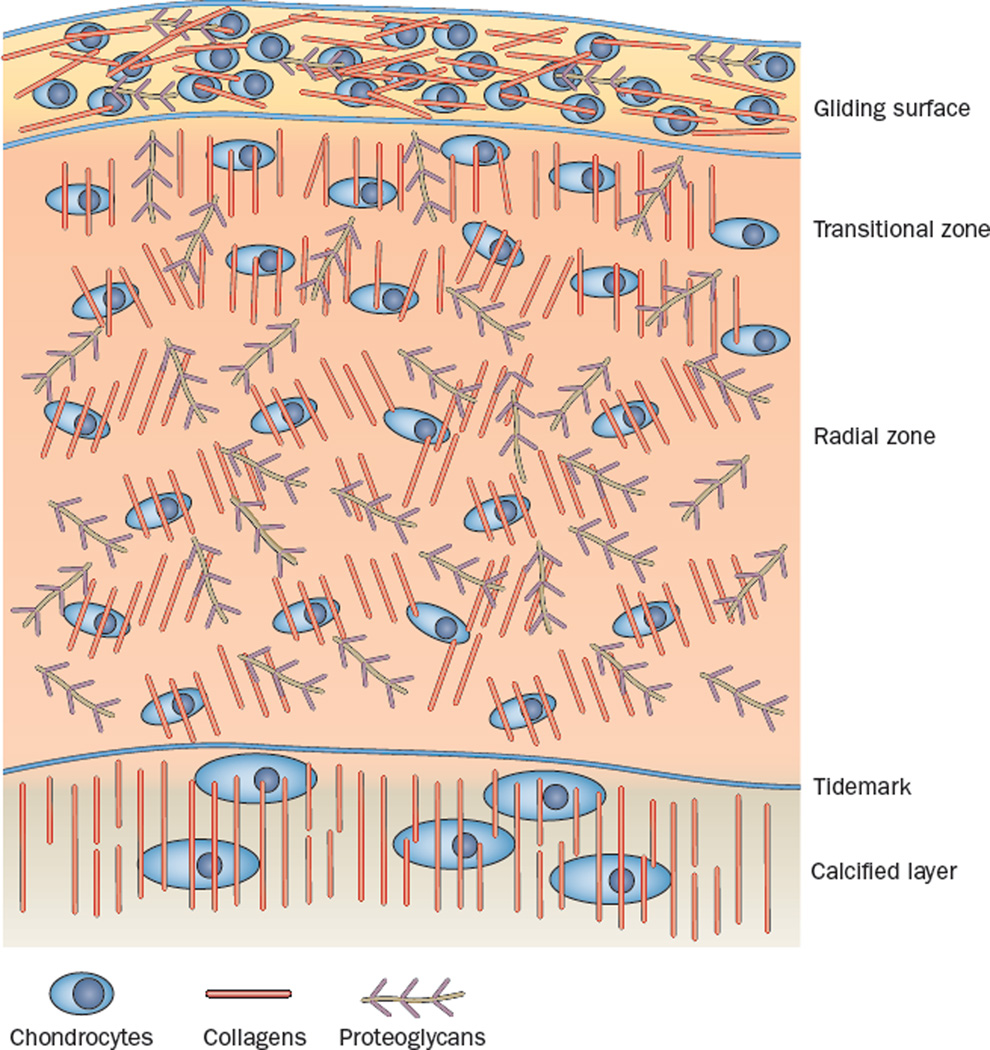
Although seemingly structurally simple when viewed by microscopy at low levels of magnification, articular cartilage is composed of a complex ECM that is highly organized to serve its biological purposes.9 On the basis of studies of cartilage samples taken from different species and different joints, four distinct zones can be distinguished, each with its own characteristic matrix appearance (Figure 1).5,10 The top layer, the superficial zone, known as the gliding surface, has been shown by transmission electron microscopy and scanning electron microscopy to consist of a layer of amorphous material containing lipids, proteins and proteoglycans, which covers a layer of woven type I and type II collagen fibres oriented in parallel to the surface. The gliding surface confers lubrication and resistance to sheer stress. The second layer, the transitional zone, contains flattened chondrocytes and bundles of type II collagen fibres that are oriented tangentially to the cartilage surface. This zone provides an enclosure for proteoglycan aggregates (formed mainly of aggrecan), which bind water molecules and provide resistance to compression. The radial (deep) zone, forming the third layer, comprises type II collagen bundles arranged radially, as well as chondrocytes and aggrecan. Here, the fibres tend to arborize as they extend outward from the bone surface. This zone anchors the cartilage into the fourth zone, the calcified layer that is immediately beneath the tidemark and above the subchondral bone.
Figure 1.
A schematic representation of articular cartilage. Articular cartilage is organized into four different zones. The surface layer (zone 1), also known as the gliding surface, contains collagen fibres that are organized in parallel to the joint surface, which favours the distribution of joint compressive loads. Zone 1 also contains the highest amount of chondrocytes, which promote matrix synthesis and tissue repair. Zone 2 (transitional) and zone 3 (radial) have a low density of chondrocytes, and collagen fibres are organized obliquely and radially to the joint surface to increase resistance to compressive forces. The radial zone is the most enriched in proteoglycans. The tidemark distinguishes the radial zone from the calcified cartilage, which anchors the collagen fibrils to the subchondral bone. In the calcified layer, chondrocytes are rare and mostly hypertrophic.
The molecular make-up of articular cartilage
In general, about 70% of the wet weight of cartilage comprises water and about 30% results from the presence of collagens and proteoglycans, upon which the mechanical properties of the tissue are dependent; a large number of other molecules also have important regulatory and organizational roles.
Collagens
Collagens provide the tissue with tensile strength and are arranged in specific configurations to maximize this property. The principal collagen constituent of articular cartilage is type II, although smaller amounts of types III, IX and XI are also found.11–13 Several other types of collagen are also present at trace amounts at specific times and in selective locations within articular cartilage.
Proteoglycans
Aggrecan, an enormous molecule of 1–3 × 106 kDa, is the principal proteoglycan component of the ECM. The aggrecan core protein is a 225-kDa structure with three globular domains (G1, G2 and G3) enclosing two extended regions that provide recognition sequences for modification by approximately 30 keratan sulfate chains and 100 chondroitin sulfate chains.14–16 G1 at the amino (N) terminus mediates binding of the aggrecan molecule to hyaluronic acid, an interaction that is stabilized by link protein.14 G2, which is highly conserved, does not interact with hyaluronic acid, and might have some role in aggrecan secretion. G3 has been proposed to interact with tenascins and fibulins to organize proteoglycans within the ECM.17 Aggrecan is important for its fixed negative charge density, which establishes repulsive forces among the chondroitin sulfate and keratin sulfate side chains and results in a high osmotic pressure within the matrix, consequently drawing water into the tissue. The resultant swelling is restrained by the collagen network.18
Other molecules
Additional molecules, such as link protein, decorin, biglycan, matrilins, cartilage oligomeric matrix protein (COMP), tenascins, fibulins and many more, are found throughout the cartilage matrix and are known to carry out critical roles in the formation and organization of, as well as intermolecular interactions within, this tissue. It is, therefore, easy to appreciate how impeding the function of these molecules can adversely affect the mechanical properties of healthy cartilage.
The cellular component of cartilage
Cartilage homeostasis is dependent on the biological activities of the embedded chondrocytes, the only cellular component of cartilage. These cells are dependent upon membrane transporters for nutrients and a series of molecules that enable survival in the face of low oxygen tension.19 The metabolic activities of chondrocytes are responsive to mechanical load,20 growth factors,21 and inflammatory cytokines.22
Chondrocytes are dispersed widely throughout the avascular matrix; the properties of this matrix vary according to the distance from the embedded chondrocytes. The matrix that immediately surrounds individual chondrocytes—the pericellular domain—contains specialized molecules such as collagen VI, perlecan and little, if any, type II collagen. It is now understood that the pericellular domain functions to connect the metabolic activity of chondrocytes with the mechanical environment of the joint.11 The matrix further away from the chondrocytes, referred to as the interterritorial domain, contains collagen type II fibrils that are interacting with collagens IX and XI. It is this part of the matrix that confers tensile strength on the articular cartilage.5
Protein oxidative modification
Post-translational modifications of collagen and aggrecan in articular cartilage are especially important because these molecules have low rates of turnover, so the functional effects of protein modifications on the molecular conformation, stability and function of cartilage persist for a long time. Studies of cartilage in its steady state indicate that the half-life of type II collagen is in the range of 100 years.23 Turnover of the aggrecan monomer seems to be more rapid, with a half-life of about 3.5 years.24 However, the aggrecan G1 domain, which binds to hyaluronic acid, seems to turn over much more slowly, with a half-life of about 25 years. It follows, then, that aggrecan turnover generates proportionately more G1 domain fragments and, that, because these fragments occupy binding sites on hyaluronic acid, aggrecan structures of lower quality are formed with ageing. In conditions of stress, turnover rates seem to accelerate as much as 10-fold.25 Turnover rates also vary in different regions of articular cartilage and are responsive to mechanical load,26 growth factors,27 and inflammatory and degenerative conditions, and seem to be more rapid in the chondrocyte territorial domain.28 Glycation, glycoxidation and lipoxidation reactions are especially important in metabolic disorders, such as diabetes and metabolic syndrome,29 whereas carbonylation and nitrosylation are often observed in chronic inflammatory conditions.3
Stress-associated protein modifications
Oxidation and reduction, or the redox reaction, involves the transfer of an electron from one molecule (the reducing agent) to another (the oxidizing agent). Protein oxidation involves the covalent modification of the primary sequence of a protein directly by reactive oxygen species (ROS) or reactive nitrogen species (RNS), or indirectly by secondary by-products of oxidative stress (Figure 2).30 Post-translational oxidative modifications can be classified as reversible or irreversible, and biologically can be beneficial, promoting cell survival and tissue regeneration, or detrimental, causing tissue degeneration and cell death.31,32 Beneficial oxidative modifications are most often associated with reversible alterations in protein structure, and occur during relatively brief periods of oxidative stress, whereas detrimental alterations typically arise in response to sustained oxidative conditions.
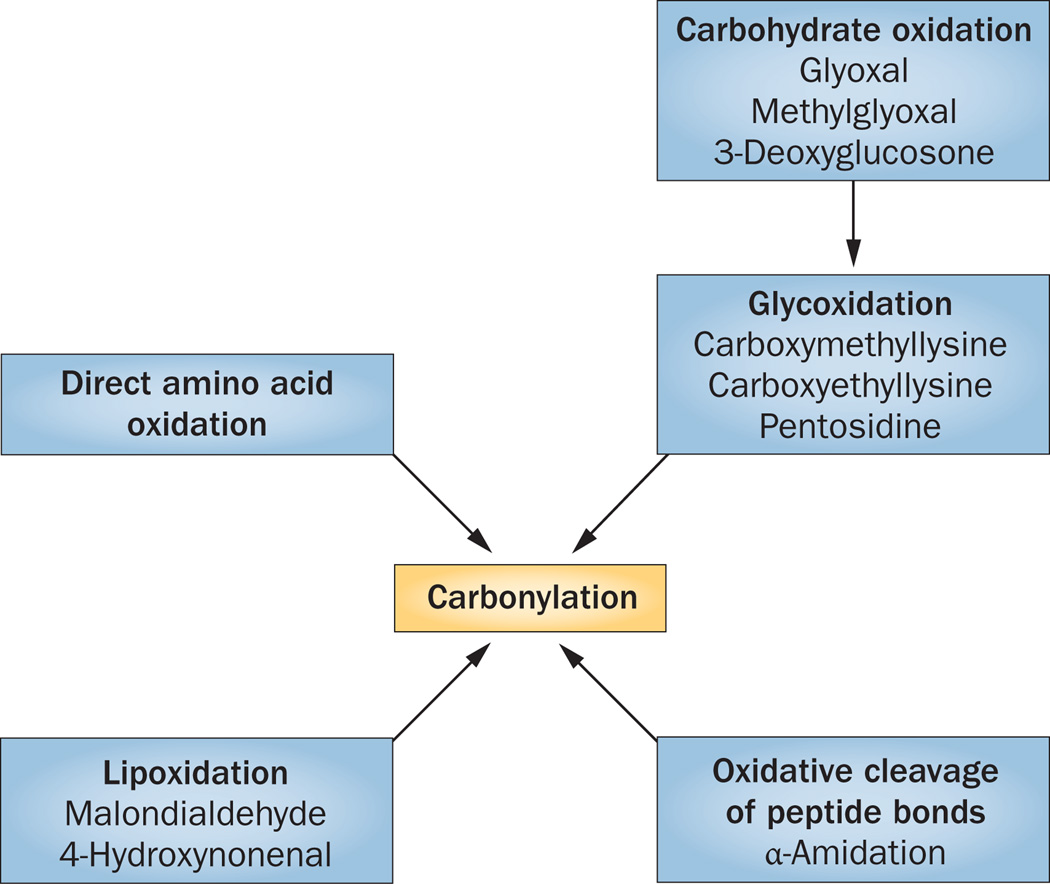
Figure 2.
Overview of the different mechanisms by which proteins can become carbonylated. Carbonyl groups (C=O) can directly modify proteins through direct oxidation of the amino acid side chain or by inducing oxidative cleavage of the peptide bond. Indirect oxidation can occur when carbonyl groups of a previously oxidized lipid (for example, 4-oxo-2-nonenal [ONE], malondialdehyde, acrolein, 2-proponal) or oxidized carbohydrate (glyoxal, methylglyoxal, 3-deoxyglucosone) react with cysteine, histidine and lysine residues, inducing lipoxidation and glycoxidation, respectively.
Carbonylation
Protein carbonylation is the most common oxidative reaction. It involves the conversion of N-terminal amino acids or amino-acid side chains into aldehyde or ketone groups by the direct action of either ROS or RNS.33 Protein carbonyl derivatives can also be formed indirectly by reactive carbonyl compounds created by glycoxidation of carbohydrates, lipoxidation of lipids, and by advanced glycation/lipoxidation end-products. These modifications are mostly irreversible and are widely used biomarkers of oxidative stress in ageing and have been assessed in several chronic and degenerative conditions as contributors to tissue degeneration.34–36 However, the results of studies carried out over the past 10 years indicate that, in certain conditions, carbonylation can have a beneficial role in signal transduction or can offer protection against reperfusion-induced injuries.32,37
Although all amino acids are susceptible to oxidative alterations, arginine, histidine, lysine, phenylalanine, tyrosine, proline, threonine, tryptophan, methionine and cysteine are more prone to undergo oxidation of their side chains than are other amino acids.36 Cysteine and methionine side chains contain a reactive sulfur group that is a primary target for reversible or irreversible ROS and RNS modifications. Reversible redox modifications of the thiol group of cysteine include S-sulfenation, S-nitrosylation, and S-glutathionylation, as well as the formation of disulfides.38,39 These reversible cysteine modifications not only have a beneficial role in protecting target proteins from ROS and RNS oxidative damage, they also influence enzyme activity as well as being important in cellular biological functions, such as cell growth and proliferation. By contrast, the formation of sulfonic and sulfenic acids during chronic oxidative stress constitute irreversible modifications that are associated with tissue damage and loss of protein function.40
The oxidation of methionine to methionine sulfoxide does not appear to have biological consequences for protein functionality and can be reversed by the action of a cytosolic reductase. Consequently, methionine residues on protein surfaces have been considered to function as ROS scavengers that protect proteins from irreversible oxidative injury.41 However, the formation of methionine sulfone, albeit a reversible modification through the activity of methionine reductase, has been shown to decrease the activity of plasma protease inhibitors.
Histidine residues are converted to 2-oxohistidine during prolonged oxidative stress, and this modification has been observed for several catalytic enzymes. The indole ring of tryptophan can be readily oxidized by ROS to form kynurenine and formylkynurenine, and by RNS to form nitrotryptophan. Formylkynurenine and nitrotryptophan have been associated with a decrease in protein function. Although phenylalanine is not as reactive as tyrosine, it can also be oxidized to tyrosine or nitrophenylalanine by ROS and RNS, respectively. In the presence of ROS or RNS, tyrosine forms tyrosinyl radicals, which can dimerize to form dityrosine or couple with hydroxyl radicals to form L-3,4-dihydroxyphenylalanine (L-DOPA) through a non-enzymatic reaction. The interaction of tyrosine with nitrogen dioxide will largely form nitrotyrosine. These tyrosine modifications are often observed during acute and chronic inflammation, when levels of ROS and RNS are elevated. Additionally, these tyrosine modifications are all irreversible and very damaging to protein biological activity. In addition to causing protein unfolding and aggregation, they impair tyrosine phosphorylation, a key mechanism in signal transduction (Figure 2).
The most commonly studied oxidative changes in aliphatic amino acids are lysine to allysine aldehyde (which occurs naturally in collagen and elastine through the action of lysyl oxidase); proline to 2-pyrrolidone; and both proline and arginine to their glutamic semialdehydes. The presence of these amino acids in the oxidized form is indicative of prolonged oxidative stress.
Glycation
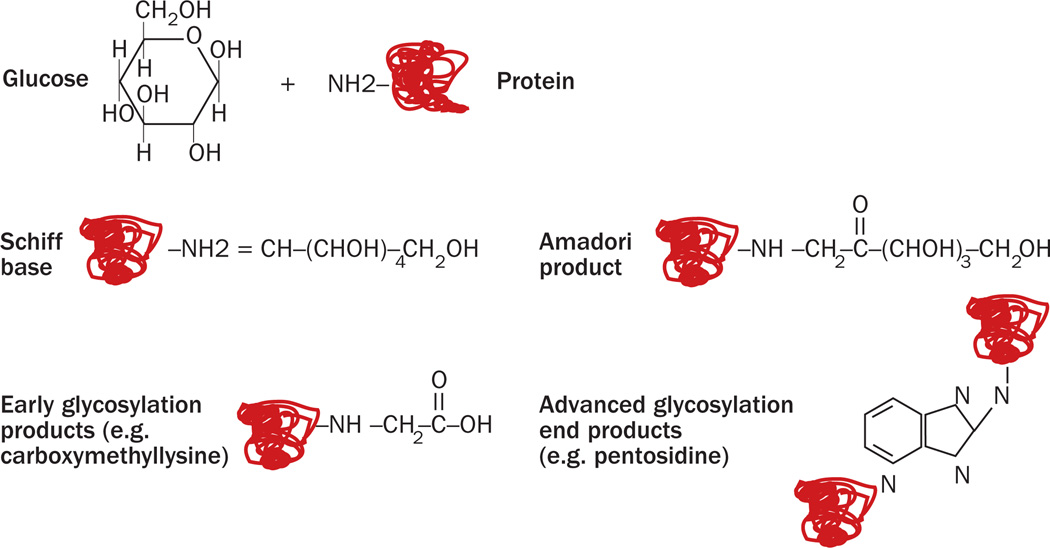
Glycation, a non-enzymatic reaction that covalently attaches a reducing sugar to an amino acid, occurs mainly when an aldose group of a monosaccharide or a polysaccharide binds to an amino group within a protein. The process mostly involves lysine and arginine side chains, leading to the formation of a Schiff base (Figure 3).42 This initial reaction is still reversible, and it is strictly dependent on the environmental sugar concentration. Over a period of days, the reactants will undergo chemical rearrangement and form early glycation products, which are known as Amadori products (more than 100 of which have been described). These early glycation steps are still reversible on decreasing the environmental sugar concentration. However, subsequent chemical modifications including oxidation, reduction and hydration can induce protein aggregation and crosslinking. This process, known as the Maillard reaction, takes place over a period of months or years, is irreversible, and leads to the formation of advanced glycation end products (AGEs) including hexitol-lysine, carboxymethyl-lysine, pentosidine, and pyrraline (Figure 3; Box 2).43,44
Figure 3.
Step-by-step mechanisms by which advanced glycation end products (AGEs) are formed. In the presence of high levels of circulating monosaccharides and polysaccharides, as occurs in diabetes and metabolic syndrome, the aldehyde group of an aldose (shown here as glucose) can bind to a protein amino group. This non-enzymatic reaction generates a covalent bond (Schiff base). Over time, several chemical rearrangements occur, where the OH group, close to carbon-nitrogen bond binds the nitrogen forming a ketone. Over one hundred rearrangements, known as Amadori products, have been mapped. Subsequent chemical rearrangements, which include reduction, hydration and further oxidation, will generate early and advanced glycation end products.
Box 2. Post-translational cartilage protein modifications.
-
▪
Allysine
-
▪
2-pyrrolidone
-
▪
Carboxymethyllysine
-
▪
Pentosidine
-
▪
Deoxyglucosone
-
▪
Glucosepane
-
▪
Furosine
-
▪
Cysteic acid
-
▪
Arginine oxidation to glutamic semialdehyde
Glycoxidation
Glycoxidation is the reaction that occurs between an amino acid and an oxidized sugar.45 Glycoxidation leads to the formation of AGEs when glucose, fructose or lipids are oxidized into dicarbonyl derivatives known as α-oxaldehydes (for example, glyoxal, methylglyoxal and 3-deoxyglucosone). These intermediates directly interact with lysine and arginine side chains to form imine linkages that will then be converted to α-amino-ketones through Amadori rearrangements.46
AGEs, which are very stable, are particularly damaging in two ways: they directly interact with proteins to alter their structure and function; and they interact with the AGE receptor (RAGE), which leads to proinflammatory effects through the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signalling pathway and nuclear factor κB (NFκB) activity.47
Additional effects on proteins
Oxidative stress and metabolic stress, in addition to causing protein oxidative modifications, can induce amino-acid side-chain cleavage, oxidative cleavage of the protein backbone, and protein crosslinking as new disulfide bonds form between two oxidized cysteines or Schiff-base formation occurs between carbonyl groups on different amino acids (Figure 4).40 Cleavage and crosslinking both result in the irreversible loss of the biological activity of the protein.48–50
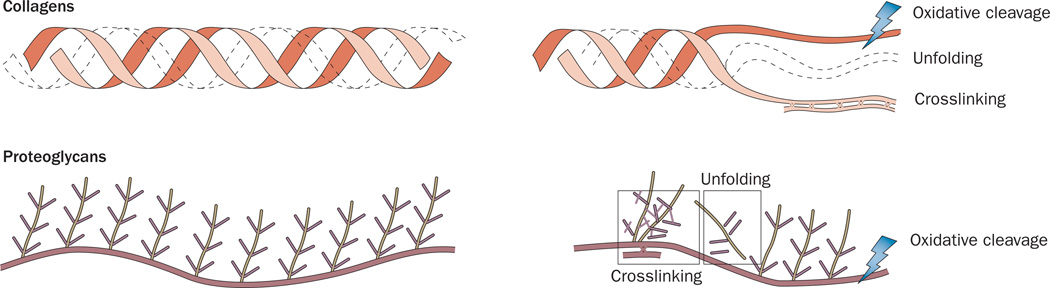
Figure 4.
The effects of oxidative post-translational modifications on the structure of collagens and proteoglycans. Reactive oxygen species and reactive nitrogen species can induce oxidative cleavage by breaking the protein amino-acid bonds or amino-acid side chains. Oxidative post-translational modifications can also induce protein unfolding by steric hindrance or by changing the hydrogen bonds and electrostatic interactions, which keep the proteins correctly folded. Finally, crosslinking between amino acids on the same protein or neighbouring proteins can occur between a free amino group and a carbonyl group.
Oxidative stress and cartilage structure
At the clinical level
Several clinical conditions vividly illustrate the impact of oxidative stress on connective tissue such as the articular cartilage matrix. An important example is type 2 diabetes mellitus, which affects more than 200 million people worldwide. Although much attention has been focused on glycation-mediated injury to blood vessels, eyes and kidneys, it is now clear that the metabolic consequences of type 2 diabetes, including hyperglycaemia and hyperlipidaemia, affect every organ in the human body, including articular cartilage. This effect on articular cartilage is highlighted by the increased prevalence of OA among people with diabetes independent of their age and BMI, and by a doubling of the need for total joint arthroplasty among this patient population.51 Indeed, OA is now viewed as part of the metabolic syndrome, which leads to OA in both weight-bearing and non-weight-bearing joints such as those of the hands and shoulders.2,51–59 Similarly, a high-fat diet has been shown to accelerate post-traumatic OA progression in both weight-bearing and non-weight-bearing joints.60 Moreover, in a mouse model of obesity-associated OA, exercise was shown to protect against OA progression even in the absence of weight reduction.61 Improvements in glucose tolerance and expression of proinflammatory cytokines that result from exercise seem to act independently of mechanical stress reduction in ameliorating OA.62
Several experimental models provide additional evidence that metabolic abnormalities can damage cartilage.63–67 Moreover, incubating cartilage tissues with threose, ribose or methylglyoxal has been shown to induce the formation and accumulation of AGEs in collagen and proteoglycan molecules.68–71 Likewise, chondrocytes cultured in high-glucose conditions generate more ROS, which increases oxidative stress in the local environment.72 Such in vitro data have been confirmed in animal studies, as the same array of post-translational modifications occurs on proteins following intra-articular injection of ribose and threose in models of surgically induced OA.73 Likewise, conditions associated with prolonged oxidative stress, such as chronic infections and chronic inflammatory processes, are associated with early signs of cartilage degeneration, even when the joints are not the primary targets of the autoimmune or inflammatory process.74
At the anatomical level
Several anatomical changes have been associated with oxidative injury to cartilage. The superficial layer of cartilage seems most susceptible to change, probably as a result of direct contact with the synovial fluid and the higher proinflammatory activity of the chondrocytes present in this layer.75 The macroanatomy of cartilage tissue during prolonged metabolic stress and oxidative stress exhibits early changes in the gliding zone, with a thinning of the proteoglycan and collagen layers as well as disorganization of collagen fibre orientation.64,76 These changes are followed by more extensive degenerative changes in the deeper transitional and radial zones. Microanatomically, a loss of matrix proteins and chondrocytes is observed in all these zones.64 For example, in streptozotocin-induced diabetes, a statistically significant decrease in the levels of type II collagen and proteoglycans and a concomitant increase in collagen XI levels were observed in articular cartilage.64 Similarly, the nucleus pulposus from diabetic rats has been reported to show thinning and increased expression of matrix metalloproteinases (MMPs) and RAGE.77 Additionally, oxidative stress has been reported to depolymerize glycosaminoglycans and to be associated with the degradation of hyaluronan.78 The sustained release of RNS during chronic inflammatory processes has also been shown to decrease the synthesis of proteoglycans and collagen in cartilage cultures and of glycosaminoglycan in an in vivo arthritis model.79,80 The decrease in the levels of these cartilage ECM components can be attributed to a decrease in chondrocyte synthetic activity as well as ROS-induced and RNS-induced protein oxidative cleavage and the increased activity of MMPs. Additionally, glycation or carbonylation of collagens and proteoglycans induces protein crosslinking, which changes protein secondary and tertiary structure, alters the spatial orientation of fibres and bundles and changes the surface charge and hydrophilic tension of proteins. Overall, the combined experimental data support the notion that, in conditions of increased oxidative and metabolic stress, cartilage undergoes important changes in its composition and molecular organization, including changes in the proportion of the different collagens and alterations in the ratio of collagen to proteoglycan, disruption of spatial orientation and increased protein crosslinking, which all alter its functional capabilities. These microanatomical alterations have profound effects that disrupt the carefully designed molecular arrangement of articular cartilage.64
Imaging techniques have been used to assess early changes in cartilage tissue. New MRI techniques, such as T1-rho (T1ρ) and T2 MRI, assess the interaction of water molecules with glycosaminoglycans in articular cartilage and indirectly provide information regarding the integrity of the collagen and aggrecan structure of cartilage. Both T1ρ and T2 MRI have been shown to be predictors for progressive cartilage loss in OA.81,82 The results from a 2013 study indicate that diabetes and increased abdominal girth are associated with higher T2 values (which correspond to an increased loss of collagen integrity).83 These results show progress towards the development of imaging techniques that could detect very early biochemical damage to cartilage tissues at a time when many of the protein post-translational modifications are still reversible. Thus, early detection could aid therapeutic interventions that are aimed at reversing early glycation, glycoxidation and carbonylation in otherwise healthy cartilage.
At the biochemical level
On a biochemical level, lysine to allysine aldehyde, proline to 2-pyrrolidone, and proline and arginine to their glutamic semialdehyde are the most commonly reported modifications. Pentosidine and 5,6-dioxoglucosone are also often observed on lysine, arginine and histidine side chains in collagen. Glucosepane, a derivative of 6-glucose that is carbon-crosslinked to lysine and arginine, is one of the main AGEs observed in cartilage proteins from patients with diabetes or metabolic syndrome. Over time, such modifications induce collagen and proteoglycan crosslinking as well as oxidative cleavage, both of which change the biomechanical properties of the tissue, causing stiffening of the collagen network.
Oxidative stress and cartilage function
Several studies have assessed the impact of metabolic stress on the mechanical properties of articular cartilage (Box 3). For example, cartilage from the ankle joints of individuals with diabetes was reported to be softer and more permeable than cartilage from those without diabetes.84 This observation contrasts with an increased stiffness in cartilage resulting from increased collagen crosslinking observed when AGEs are induced in vitro.85 However, the AGEs are likely to act over a very short period of time in vitro to mainly cross-link proteins, whereas the broader effects observed in vivo are probably mediated through a wider range of matrix protein modifications as well as altered to mechanosignals.86 Indeed, prolonged oxidative stress reduces the synthesis of proteoglycan and collagen through effects that involve modulation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN). PTEN functions as a negative regulator of phosphoinositol-3-kinase (PI3K)–AKT and ERK/MAPK signalling pathways, which instruct chondrocytes to upregulate matrix protein synthesis.87,88 PTEN has been shown to be upregulated in chondrocytes prepared from cartilage affected by OA.88 Additionally, the interaction of AGEs with RAGE in joint tissues is expected to enhance the local expression of inflammatory molecules, such as IL-1α/β and TNF, which are known to inhibit chondrocyte function. It is not surprising, therefore, that metabolic stresses mediated through oxidative pathways would alter the ECM of articular cartilage in ways that compromise the ability of this tissue to effectively withstand mechanical forces involved with locomotion and, together, predispose to cartilage degeneration. In this regard, it is noteworthy that joints with such compromised cartilage are more prone to the development of OA when coupled with other causes of this disease. For example, inducing AGEs in the dog knee by directly injecting ribose was found to increase the severity of subsequent OA following transection of the anterior cruciate ligament.89 Similarly, tears of the anterior cruciate ligament lead to more severe OA in older humans than in younger individuals.90
Box 3. Cartilage and mechanical stress.
In response to the application of a mechanical stress, cartilage does not respond immediately as would a metal spring. Rather, its response is determined by the spring-like properties of its molecular components and the rate at which fluid components egress. Thus, when cartilage is placed under a constant load (creep mode), compression occurs over a period of time. Similarly when cartilage is immediately compressed to a specified degree (relaxation mode), the force required to maintain that compression can be determined as a function of time. These mechanical features of articular cartilage form the triphasic theory for the swelling and deformation behaviours of articular cartilage.100 The large array of negative charges distributed over proteoglycans attracts positively charged ions and water molecules, and the density of the matrix restricts egress of water molecules during compression. In addition, the fixed negative charges on the chondroitin sulfate and keratin sulfate side chains of aggrecan repel each other electrostatically. These electrostatic factors work together to create the stiffness of healthy cartilage.101 Their importance is illustrated by changes in compressibility of cartilage during bovine development and human ageing.18 During bovine growth from the fetal calf to adult cow, the proteoglycan content of articular cartilage was found to remain constant while the collagen content increased 2–3-fold, resulting in a doubling of the compressive modulus (stiffness).102–104 By contrast, in the ageing human joint, the overall proteoglycan concentration decreases, aggrecan sidechains become shortened, and the collagen framework becomes disrupted, facilitating increased permeability and increased water content, with a corresponding decrease in the compressive modulus.5
Conclusions
In the past few years, improved proteomic approaches have enabled the analysis of cartilage protein post-translational modifications that are associated with conditions of chronic metabolic and oxidative stress. A picture emerges of a multitude of protein post-translational modifications that remodel cartilage. Although at present the evidence linking glycation, glycoxidation and carbonylation to impaired cartilage performance is largely correlative, it should be noted that the treatment of animal models of diabetes using agents that inhibit the formation or action of AGEs (aminoguanidine, pyridoxamine, LR-90, metformin, ARB inhibitors and hydralazine) has been shown to decrease diabetic complications,91 including degenerative changes in cartilage.92 A future goal would be the comprehensive mapping of each of these modifications to generate a cartilage ‘molecular signature’ for each pathological condition and to understand the effects of each signature on the primary, secondary and tertiary structures of cartilage matrix proteins, with the ultimate aim of gaining a better understanding of the relationship between biophysical alterations in proteins and the corresponding changes in tissue microanatomy and functionality.
In terms of the relationship between OA and the accumulation of oxidative modifications in cartilage, several studies indicate that this relationship is more complex than previously thought. Indeed, although all of these oxidative modifications promote cartilage degeneration through their effects on cartilage ECM proteins, a second set of signals is required to induce inflammation and bona fide degenerative OA. These signals could be provided by any of several means. First, AGEs could bind to RAGE present on tissue-resident macrophages, chondrocytes and osteoclasts to activate an inflammatory programme through NFκB and ERK/MAPK signalling.93,94 Second, ROS could activate NFκB signalling, leading to the production of proinflammatory cytokines, such as IL-1α/β and TNF. Third, ROS and AGEs might upregulate the expression of MMPs, collagenases and aggrecanases, which enhance the activity of catabolic pathways leading to matrix degradation. And, finally, the synthetic activity of chondrocytes could be decreased by oxidative or metabolic stress.95,96 Thus, the metabolic and oxidative imbalances observed in several chronic diseases initially cause the post-translational modification of cartilage proteins, which is followed by a second event that generates low-grade inflammation; together, both events promote degenerative changes, leading to the OA phenotype.97
Key points.
-
▪
Diabetes, metabolic syndrome and chronic infections increase glycaemia, lipidaemia and cellular levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS)
-
▪
Increased glycaemia, lipidaemia, ROS and RNS induce glycation, glycoxidation, carbonylation and nitrosylation of cartilage proteins
-
▪
Biochemical changes in proteins compromise the anatomical organization of cartilage
-
▪
Changes in the anatomical organization of cartilage compromise tissue visco-elasticity and, ultimately, the ability of cartilage to sustain pressure and its overall performance
-
▪
Protein biochemical changes are an early event in cartilage degeneration
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J.H. and L.S. researched the data for the article and wrote the manuscript. All authors (J.H., N.C. and L.S.) contributed substantially to discussions of the article content, and review or editing of manuscript before submission.
Contributor Information
John A. Hardin, Department of Orthopedic Surgery, Montefiore Medical Centre, 1250 Waters Place, New York, NY 10467, USA
Neil Cobelli, Department of Orthopedic Surgery, Montefiore Medical Centre, 1250 Waters Place, New York, NY 10467, USA.
Laura Santambrogio, Departments of Pathology, Microbiology and Immunology and Orthopedic Surgery, Albert Einstein College of Medicine, 1300 Morris Park Avenue, New York, NY 10461, USA.
References
- 1.Loeser RF. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 2013;25:108–113. doi: 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012;8:729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 3.Scharf B, et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem. Biol. 2013;20:922–934. doi: 10.1016/j.chembiol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8:7–9. doi: 10.1007/s11420-011-9250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 6.Wen CY, et al. Collagen fibril stiffening in osteoarthritic cartilage of human beings revealed by atomic force microscopy. Osteoarthritis Cartilage. 2012;20:916–922. doi: 10.1016/j.joca.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Martel-Pelletier J, Lajeunesse D, Fahmi H, Tardif G, Pelletier JP. New thoughts on the pathophysiology of osteoarthritis: one more step toward new therapeutic targets. Curr. Rheumatol. Rep. 2006;8:30–36. doi: 10.1007/s11926-006-0022-6. [DOI] [PubMed] [Google Scholar]
- 8.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem. J. 1998;330:345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujioka R, Aoyama T, Takakuwa T. The layered structure of the articular surface. Osteoarthritis Cartilage. 2013;21:1092–1098. doi: 10.1016/j.joca.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre DR, Weis MA, Wu JJ. Articular cartilage collagen: an irreplaceable framework? Eur. Cell. Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 15.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- 16.Hardingham TE, Fosang AJ, Dudhia J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur. J. Clin. Chem. Clin. Biochem. 1994;32:249–257. [PubMed] [Google Scholar]
- 17.Aspberg A. The different roles of aggrecan interaction domains. J. Histochem. Cytochem. 2012;60:987–996. doi: 10.1369/0022155412464376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han EH, Chen SS, Klisch SM, Sah RL. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys. J. 2011;101:916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins RJ, Browning JA, Ellory JC. Surviving in a matrix: membrane transport in articular chondrocytes. J. Membr. Biol. 2000;177:95–108. doi: 10.1007/s002320001103. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins RJ, Browning JA, Urban JP. Chondrocyte regulation by mechanical load. Biorheology. 2000;37:67–74. [PubMed] [Google Scholar]
- 21.Chubinskaya S, et al. Response of human chondrocytes prepared for autologous implantation to growth factors. J. Knee Surg. 2008;21:192–199. doi: 10.1055/s-0030-1247818. [DOI] [PubMed] [Google Scholar]
- 22.Mobasheri A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr. Rheumatol. Rep. 2013;15:385. doi: 10.1007/s11926-013-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 24.Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch. Biochem. Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 25.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand. J. Med. Sci. Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 27.Kopesky PW, et al. Sustained delivery of bioactive TGF-β1 from self-assembling peptide hydrogels induces chondrogenesis of encapsulated bone marrow stromal cells. J. Biomed. Mater. Res. A. 2014;102:1275–1285. doi: 10.1002/jbm.a.34789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013;15:375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucala R. Diabetes, aging, and their tissue complications. J. Clin. Invest. 2014;124:1887–1888. doi: 10.1172/JCI75224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadtman ER, Levine RL. Protein oxidation. Ann. N. Y. Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 31.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008;16(Suppl. 2):S15–S20. doi: 10.1016/S1063-4584(08)60008-4. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z, Yan LJ. Protein oxidative modifications: beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 2013;1:15–26. [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis JM, et al. A. Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 2012;23:399–406. doi: 10.1016/j.tem.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor-1 and osteogenic protein-1. Arthritis Rheumatol. 2014;66:2201–2209. doi: 10.1002/art.38641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 37.Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hohn A, Konig J, Grune T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteomics. 2013;92:132–159. doi: 10.1016/j.jprot.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc. Natl Acad. Sci. USA. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arena S, Salzano AM, Renzone G, D’Ambrosio C, Scaloni A. Non-enzymatic glycation and glycoxidation protein products in foods and diseases: an interconnected, complex scenario fully open to innovative proteomic studies. Mass Spectrom. Rev. 2014;33:49–77. doi: 10.1002/mas.21378. [DOI] [PubMed] [Google Scholar]
- 43.Tessier FJ. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. (Paris) 2010;58:214–219. doi: 10.1016/j.patbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Basle E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem. Biol. 2010;17:213–227. doi: 10.1016/j.chembiol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Vistoli G, et al. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 2013;47(Suppl. 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 46.Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulsen MW, et al. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 48.Baraibar MA, Liu L, Ahmed EK, Friguet B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid. Med. Cell Longev. 2012;2012:919832. doi: 10.1155/2012/919832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terman A, Brunk UT. Aging as a catabolic malfunction. Int. J. Biochem. Cell Biol. 2004;36:2365–2375. doi: 10.1016/j.biocel.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Grune T, Merker K, Jung T, Sitte N, Davies KJ. Protein oxidation and degradation during postmitotic senescence. Free Radic. Biol. Med. 2005;39:1208–1215. doi: 10.1016/j.freeradbiomed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Schett G, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36:403–409. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann. Rheum. Dis. 2011;70:1354–1356. doi: 10.1136/ard.2010.146399. [DOI] [PubMed] [Google Scholar]
- 53.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 54.Pottie P, et al. Obesity and osteoarthritis: more complex than predicted! Ann. Rheum. Dis. 2006;65:1403–1405. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad. Med. 2009;121:9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 56.Cimmino MA, Cutolo M. Plasma glucose concentration in symptomatic osteoarthritis: a clinical and epidemiological survey. Clin. Exp. Rheumatol. 1990;8:251–257. [PubMed] [Google Scholar]
- 57.Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vila LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J. Clin. Rheumatol. 2013;19:1–6. doi: 10.1097/RHU.0b013e31827cd578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17:168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Velasquez MT, Katz JD. Osteoarthritis: another component of metabolic syndrome? Metab. Syndr. Relat. Disord. 2010;8:295–305. doi: 10.1089/met.2009.0110. [DOI] [PubMed] [Google Scholar]
- 60.Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res. Ther. 2011;13:R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol. Aging Age Relat. Dis. 2012;2:17470. doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kayal RA, et al. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44:357–363. doi: 10.1016/j.bone.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atayde SA, et al. Experimental diabetes modulates collagen remodelling of joints in rats. Histol. Histopathol. 2012;27:1471–1479. doi: 10.14670/HH-27.1471. [DOI] [PubMed] [Google Scholar]
- 65.DeGroot J, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthritis Cartilage. 2001;9:720–726. doi: 10.1053/joca.2001.0469. [DOI] [PubMed] [Google Scholar]
- 66.Verzijl N, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 2000;350:381–387. [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss RE, Gorn AH, Nimni ME. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes. 1981;30:670–677. doi: 10.2337/diab.30.8.670. [DOI] [PubMed] [Google Scholar]
- 68.DeGroot J, et al. Accumulation of advanced glycation end products decreases collagen turnover by bovine chondrocytes. Exp. Cell Res. 2001;266:303–310. doi: 10.1006/excr.2001.5224. [DOI] [PubMed] [Google Scholar]
- 69.DeGroot J, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 70.Verzijl N, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 71.Saudek DM, Kay J. Advanced glycation endproducts and osteoarthritis. Curr. Rheumatol. Rep. 2003;5:33–40. doi: 10.1007/s11926-003-0081-x. [DOI] [PubMed] [Google Scholar]
- 72.Heywood HK, Nalesso G, Lee DA, Dell’accio F. Culture expansion in low-glucose conditions preserves chondrocyte differentiation and enhances their subsequent capacity to form cartilage tissue in three-dimensional culture. Biores. Open Access. 2014;3:9–18. doi: 10.1089/biores.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vos PA, et al. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J. Orthop. Res. 2012;30:1398–1404. doi: 10.1002/jor.22092. [DOI] [PubMed] [Google Scholar]
- 74.Soltes L, et al. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 75.Hauselmann HJ, Stefanovic-Racic M, Michel BA, Evans CH. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J. Immunol. 1998;160:1444–1448. [PubMed] [Google Scholar]
- 76.Onur T, Wu R, Metz L, Dang A. Characterisation of osteoarthritis in a small animal model of type 2 diabetes mellitus. Bone Joint Res. 2014;3:203–211. doi: 10.1302/2046-3758.36.2000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai TT, et al. Advanced glycation end products in degenerative nucleus pulposus with diabetes. J. Orthop. Res. 2014;32:238–244. doi: 10.1002/jor.22508. [DOI] [PubMed] [Google Scholar]
- 78.Mendoza G, et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr. Res. 2007;342:96–102. doi: 10.1016/j.carres.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 79.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem. Biophys. Res. Commun. 1994;200:142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 80.Bezerra MM, et al. Reactive nitrogen species scavenging, rather than nitric oxide inhibition, protects from articular cartilage damage in rat zymosan-induced arthritis. Br. J. Pharmacol. 2004;141:172–182. doi: 10.1038/sj.bjp.0705600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin. Musculoskelet. Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 82.Borthakur A, Reddy R. Imaging cartilage physiology. Top. Magn. Reson. Imaging. 2010;21:291–296. doi: 10.1097/RMR.0b013e31823dfe2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jungmann PM, et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res. (Hoboken) 2013;65:1942–1950. doi: 10.1002/acr.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Athanasiou KA, et al. Effects of diabetes mellitus on the biomechanical properties of human ankle cartilage. Clin. Orthop. Relat. Res. 1999;368:182–189. [PubMed] [Google Scholar]
- 85.Verzijl N, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 86.Fick JM, Huttu MR, Lammi MJ, Korhonen RK. In vitro glycation of articular cartilage alters the biomechanical response of chondrocytes in a depth-dependent manner. Osteoarthritis Cartilage. 2014;22:1410–1418. doi: 10.1016/j.joca.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 87.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 2009;284:31972–31981. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwasa K, et al. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J. Orthop. Res. 2014;32:231–237. doi: 10.1002/jor.22506. [DOI] [PubMed] [Google Scholar]
- 89.DeGroot J, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 90.Legnani C, Terzaghi C, Borgo E, Ventura A. Management of anterior cruciate ligament rupture in patients aged 40 years and older. J. Orthop. Traumatol. 2011;12:177–184. doi: 10.1007/s10195-011-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monnier VM. Intervention against the Maillard reaction in vivo. Arch. Biochem. Biophys. 2003;419:1–15. doi: 10.1016/j.abb.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 92.Figarola JL, et al. LR-90 a new advanced glycation endproduct inhibitor prevents progression of diabetic nephropathy in streptozotocin-diabetic rats. Diabetologia. 2003;46:1140–1152. doi: 10.1007/s00125-003-1162-0. [DOI] [PubMed] [Google Scholar]
- 93.Steenvoorden MM, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 94.Loeser RF, et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 96.Rosa SC, et al. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res. Ther. 2009;11:R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abramson SB, Berenbaum F, Hochberg MC, Moskowitz RW. Introduction to OARSI FDA initiative OAC special edition. Osteoarthritis Cartilage. 2011;19:475–477. doi: 10.1016/j.joca.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 98.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 2014;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 100.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 101.Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim. Biophys. Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 102.Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J. Orthop. Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 103.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surg. Am. 1982;64:88–94. [PubMed] [Google Scholar]
- 104.Temple MM, Xue Y, Chen MQ, Sah RL. Interleukin-1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 2006;54:3267–3276. doi: 10.1002/art.22145. [DOI] [PubMed] [Google Scholar]