Abstract
Objective
To evaluate the risk of and risk factors for retinal neovascularization (NV) in cases of uveitis.
Design
Retrospective cohort study.
Participants
Patients with uveitis at four US academic ocular inflammation subspecialty practices.
Methods
Data were ascertained by standardized chart review. Prevalence data analysis used logistic regression. Incidence data analysis used survival analysis with time-updated covariates where appropriate.
Main Outcome Measures
Prevalence and incidence of NV.
Results
Among uveitic eyes of 8931 patients presenting for initial evaluation, 106/13,810 eyes had NV (prevalence=0.77%, 95% confidence interval (CI): 0.60%–0.90%). Eighty-eight more eyes developed NV over 26,465 eye-years (incidence=0.33%/eye-year, 95% CI: 0.27–0.41%). Factors associated with incident NV include age <35 as compared to >35 years (adjusted hazard ratio (aHR) = 2.4, 95% CI: 1.5–3.9), current cigarette smoking (aHR=1.9, 95% CI: 1.1–3.4), and systemic lupus erythematosus (aHR=3.5, 95% CI: 1.1–11). Recent diagnosis of uveitis was associated with an increased incidence of NV (compared to patients diagnosed >5 years ago, aHR=2.4 (95% CI 1.1–5.0) and aHR=2.6 (95%CI 1.2–6.0) for diagnosis within <1 year vs. 1–5 years respectively). Compared to anterior uveitis, intermediate uveitis (aHR=3.1, 95% CI: 1.5–6.6), posterior uveitis (aHR=5.2, 95% CI: 2.5–11), and panuveitis (aHR=4.3, 95% CI: 2.0–9.3) were associated with a similar degree of increased NV incidence. Active (aHR=2.1, 95%CI: 1.2–3.7) and slightly active (aHR=2.4, 95%CI: 1.3–4.4) inflammation were associated with an increased incidence of NV as compared to inactive inflammation. NV incidence also was increased with retinal vascular occlusions (aHR=10, 95% CI: 3.0–33), retinal vascular sheathing (aHR=2.6, 95% CI: 1.4–4.9), and exudative retinal detachment (aHR=4.1, 95% CI: 1.3–13). Diabetes mellitus was associated with a somewhat increased incidence of retinal NV (aHR=2.3, 95% CI: 1.1–4.9); and systemic hypertension (aHR 1.5; 95% CI:0.89–2.4) was associated with non-significantly increased NV incidence. Results were similar in sensitivity analyses excluding the small minority of patients with diabetes mellitus.
Conclusions
Retinal neovascularization is a rare complication of uveitis, which occurs more frequently in younger patients; smokers; and those with intermediate/posterior/panuveitis, systemic vasculopathy and/or retinal vascular disease; and active inflammation. Inflammation and retinal neovascularization likely are linked; additional studies are needed to further elucidate this connection.
Introduction
Patients with uveitis can develop retinal neovascularization (NV), putatively through ischemic and inflammatory mechanisms, and in association with a number of systemic conditions1,2. The literature contains case series of retinal NV in association with Behçet Disease3, juvenile idiopathic arthritis2, sarcoidosis4, pars planitis5, Eales’ disease6, Crohn’s disease7, systemic lupus erythematosus8 (SLE) and idiopathic retinal vasculitis9, 10. Some of these cases had retinal ischemia demonstrated on fluorescein angiography, but others had no demonstrable retinal ischemia.
The current paradigm for the development of retinal NV posits that ischemic retina releases pro-angiogenic molecules such as vascular endothelial growth factor (VEGF); these molecules stimulate the growth of abnormal vessels.11, 12 In addition, there is now substantial evidence implicating inflammation in pathologic angiogenesis. Tumors that incite inflammation stimulate angiogenesis more than tumors without inflammation.13 In the retina, capillary nonperfusion from diabetes is associated with leukostasis, increased vascular permeability, and increases in pro-inflammatory transcription factors and cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β), interleukin 6 (IL-6), and interleukin 8 (IL-8).12, 14 In animal models of proliferative diabetic retinopathy, monocytes were found in neovascular fronds, and inhibition of monocytes led to a reduction in neovascularization.15 These observations suggest that inflammation may contribute to many neovascular diseases. However, despite significant intraocular inflammation, clinical impression suggests that only a small proportion of patients with uveitis develop retinal NV.
In an effort to better characterize the risk of retinal NV in the context of uveitis, here we report the prevalence, incidence and systemic and ocular risk factors associated with retinal NV in a large cohort of patients with uveitis.
Methods
STUDY POPULATION
The design of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study has been detailed elsewhere.16 In brief, the SITE Cohort Study is a retrospective cohort study of patients with inflammatory eye diseases seen at five tertiary ocular inflammation centers in the United States from the inception of these centers. One of these centers often used a co-management approach, which resulted in a different pattern of ascertainment of some clinical outcomes than the other four centers. Patients reported here were seen between 1978 and 2007 at the other four centers. Only data from patients with non infectious uveitis were included in this report; patients with known HIV infection had been excluded from the parent study.
DATA COLLECTION
Information on patients with inflammatory eye disease was entered into a database from clinic medical records using a computer-based standardized data entry form set specifically prepared for the SITE Cohort Study, with quality control checks requiring real-time error correction, so as to optimize data quality. In some previous reports from the parent study, enrollment at the largest site was limited to an approximate 40% random sample of patients due to logistical and funding constraints; however, data entry was subsequently completed, and the entire population was used in this analysis.
Data collected and used in this analysis include: demographic characteristics, ocular inflammatory diagnoses, diagnosis of systemic inflammatory disease(s), ophthalmologic examination findings, and ocular surgeries. HLA-B27 and HLA-A29 status, which had been tested when clinically indicated based on symptoms and clinical findings, also were considered. The possibility of systemic inflammatory disease diagnoses coexisting with ocular inflammation had been aggressively pursued by routine questioning; laboratory testing and consultations were obtained when indicated. Systemic inflammatory diagnoses specifically evaluated in the study included Behçet Disease, Cogan’s Syndrome, Crohn’s Disease, dermatomyositis, erythema nodosa, familial systemic granulomatosis, juvenile idiopathic arthritis, mucous membrane pemphigoid, polyarteritis nodosa, polymyositis, rheumatoid arthritis, relapsing polychondritis, sarcoidosis, systemic lupus erythematosus (SLE), scleröderma, Sjogren’s Syndrome, spondyloarthropathies, temporal arteritis, Takayasu’s Disease, ulcerative colitis, and granulomatosis with polyangiitis. For several of the rarer of these conditions, few cases had been identified even in this large cohort, limiting the ability to assess association with retinal NV risk. Ophthalmologic examinations at each visit documented inflammatory disease activity and the presence of inflammatory disease sequelae including macular edema, epiretinal membrane, exudative retinal detachment, presence of active inflammatory chorioretinal lesions, choroidal neovascularization, presence of vitreous snowballs, retinal vascular sheathing and retinal vascular occlusion.
MAIN OUTCOME MEASURES
For the purposes of the study, diagnosis of retinal NV required documentation of visualization of the NV clinically or via ancillary studies; patients with vitreous hemorrhage alone were not classified as having NV, neither was vitreous hemorrhage required for diagnosis of NV. While diagnosis of retinal NV could be made or confirmed by fluorescein angiography, ancillary testing was not required for the diagnosis to be accepted, consistent with reporting criteria for retrospective data adopted in the field of uveitis.17
STATISTICAL ANALYSIS
The overall prevalence of retinal NV was determined at cohort entry as the proportion of eyes with retinal NV out of all eyes with uveitis. Using logistic regression, crude and adjusted odds ratios were calculated for each demographic, systemic or ocular factor. Relationships to the demographic, systemic and ocular factors listed above are reported as crude and adjusted odds ratios (cOR and aOR, respectively).
The incidence of retinal neovascularization was calculated for all eyes free of retinal NV at cohort entry and with at least one follow-up evaluation as a rate per eye-year, censoring follow up at either the point where retinal NV was diagnosed or as of the last visit free of retinal NV. Cox proportional hazard models were used to calculate crude hazard ratios (cHR) and adjusted hazard ratios (aHR) for incident NV in relation to the demographic, systemic and ocular factors listed above. Because characteristics such as the degree of activity could change over the course of the study, the same eye could exist in more than one category; each interval of follow-up time was assigned to the appropriate category in a time-varying manner.
All adjusted models omitted variables that neither were associated with the outcome nor were judged scientifically relevant, in the interest of parsimony. Final models were adjusted for primary ocular location of inflammation, SLE, bilateral inflammation, smoking status, diabetes mellitus, systemic hypertension, age category, time from diagnosis and gender. Proportions and rates are presented along with 95% confidence intervals (CIs), accounting for non independence of eyes of the same person using standard methods. Because patients with diabetes mellitus can develop retinal neovascularization from proliferative diabetic retinopathy, a sensitivity analysis (not shown) was conducted excluding all patients with diabetes; the results were similar.
In cases where there were zero events in a group being examined, we were unable to estimate a 95% CI using Wald confidence intervals following our standard method of analysis. To get a population without the clustering of the two eyes of bilaterally uveitic patients we randomly selected 1 eye from each patient and found the 95% CI using the profile likelihood method. To get a more accurate and stable upper limit to the confidence interval for these variables with zero events, we took 100 random samples of one eye per patient. What is reported is the mean upper limit for the confidence interval of those 100 randomly selected populations. We tested this method in some of the variables where we were able to compute a Wald CI, and the results were more conservative than the Wald CI.
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina, USA).
The Institutional Review Boards of each institution granted approval for this p roject, including a waiver of consent allowing all patients living and deceased to be included. The project was conducted in accordance with the principles of the Declaration of Helsinki.
Results
CHARACTERISTICS OF THE STUDY POPULATION
13,704 eyes from 8931 patients with uveitis were included in the analysis of prevalence. The median age was 41 years with a 25th–75th percentile range (IQR) of 29 to 53 years. The population was 63% female. Eyes with anterior uveitis accounted for 57% of the population, while 16% had intermediate, 15% had posterior, and 11% had panuveitis. At the time of entry into the study, 45% of these eyes were classified as having active and 14% slightly active uveitis. Eleven percent of patients were known to be HLA-B27 positive.
PREVALENCE OF RETINAL NEOVASCULARIZATION
Among the 13,704 eyes, 106 presented with retinal NV, yielding a prevalence of 0.77% (95% CI: 0.60%–0.90%). Eyes presenting with retinal NV were more likely to belong to subjects younger versus older than 35 years of age (aOR = 2.0, 95% CI 1.2–3.4; see Table 1), and to be associated with bilateral inflammation (2.6; 1.3–5.3). Eyes diagnosed with uveitis between 1 and 5 years prior to presentation were more likely to have retinal NV than those diagnosed more than 5 years prior (aOR = 2.2, 95%CI 1.0 – 4.6). However, those eyes with less than 1 year since diagnosis did not carry a statistically significantly increased risk (aOR = 1.7, 95%CI 0.81 – 3.6). Patients with slightly active inflammation were more likely to have NV (aOR = 2.3; 95%CI 1.2–4.6) as compared to patients with inactive inflammation (reference group). Patients with active inflammation had a higher prevalence in the unadjusted analysis (crude OR = 2.1; 95% CI 1.2–3.8) but this association was not significant after adjustment for other variables (aOR = 1.5; 95%CI 0.84–2.6). Diabetes mellitus (aOR=1.4, 95%CI: 0.61–3.2) and hypertension (aOR= 1.3, 95%CI: 0.71 – 2.4) were not significantly associated with retinal NV in the prevalence analysis. Data regarding prevalence and risk for additional conditions of interest which were not associated with retinal NV are given in Table 2 (available online at http://aaojournal.org).
Table 1.
Prevalence of retinal NV and associated risk factors†
| Characteristics | No Retinal NV | Retinal NV | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Number of Eyes | 13,704 | 106 (0.77%) | ||
| Age<35 (compared to age>35) | 5,357 (39%) | 57 (54%) | 1.8 (1.2 – 2.9)* | 2.0 (1.2 – 3.4)* |
| Female (compared to male) | 8,598 (63%) | 64 (60%) | 0.90 (0.57 – 1.4) | 0.90 (0.56 – 1.5) |
| Behçet Disease | 322 (2.4%) | 4 (3.8%) | 1.6 (0.59 – 4.5) | 0.63 (0.22 – 1.8) |
| Systemic lupus erythematosus | 114 (0.83%) | 4 (3.8%) | 4.7 (1.1 – 20)* | 2.9 (0.69 – 13) |
| Sarcoidosis | 911 (6.7%) | 9 (8.5%) | 1.3 (0.57 – 3.0) | 1.3 (0.55 – 2.9) |
| HLA-B27+ | 1550 (11%) | 0 | 0 (0 – 2.8) †† | 0 (0 – 0.9) †† |
| HLA-A29+ | 275 (2.0%) | 1 (0.94%) | 0.47 (0.060 – 3.4) | 0.15 (0.020 – 1.1) |
| Hypertension | 2,587 (19%) | 23 (21%) | 1.2 (0.68 – 2.1) | 1.3 (0.71 – 2.4) |
| Diabetes | 783 (5.7%) | 7 (6.4%) | 1.2 (0.53 – 2.5) | 1.4 (0.61 – 3.2) |
| Bilateral uveitis | 9,661 (71%) | 97 (92%) | 4.5 (2.3 – 9.0)** | 2.6 (1.3 – 5.3)** |
| Current Smoker (compared to never smoker) | 2,364 (17%) | 24 (23%) | 1.8 (1.0 – 3.9)* | 1.5 (0.81 – 2.6) |
| Former Smoker (compared to never smoker) | 1,386 (10%) | 8 (7.6%) | 1.0 (0.39 – 2.7) | 0.99 (0.36 – 2.7) |
| Uveitis diagnosis ≤ 1 year (vs >5 years) | 6,505 (47%) | 53 (50%) | 2.1 (0.99–4.3) | 1.7 (0.81 – 3.6) |
| Uveitis diagnosis between 1 and 5 years (vs >5 years) | 3,900 (28%) | 40 (38%) | 2.6 (1.2 – 5.5)* | 2.2 (1.0 – 4.6)* |
| Site of inflammation | ||||
| Anterior uveitis | 7,820 (57%) | 12 (11%) | 1.0 (reference group) | 1.0 (reference group) |
| Intermediate uveitis | 2,197 (16%) | 21 (20%) | 6.2 (2.7 – 14)** | 4.5 (1.9 – 10)** |
| Posterior uveitis | 2,116 (15%) | 52 (49%) | 16 (7.7 – 33)** | 12 (5.9 – 26)** |
| Panuveitis | 1,571 (11%) | 21 (20%) | 8.7 (3.8 – 20)** | 6.6 (2.8 – 15)** |
| Activity | ||||
| Inactive inflammation | 4,781 (42%) | 24 (23%) | 1.0 (reference group) | 1.0 (reference group) |
| Slightly active inflammation | 1,569 (14%) | 25 (24%) | 3.2 (1.6 – 6.2)** | 2.3 (1.2 – 4.6)* |
| Active inflammation | 5,128 (45%) | 55 (53%) | 2.1 (1.2 – 3.8)* | 1.5 (0.84 – 2.6) |
| Macular edema | 1,603 (11%) | 30 (28%) | 3.0 (1.8 – 4.9)** | 2.1 (1.2 – 3.5)** |
| Epiretinal membrane | 586 (4.3%) | 23 (22%) | 6.2 (3.7 – 11)** | 4.5 (2.6 – 7.8)** |
| Exudative retinal detachment | 162 (1.2%) | 5 (4.7%) | 4.1 (1.7 – 10.2)** | 2.1 (0.83 – 5.5) |
| Choroidal NV | 77 (0.56%) | 4 (3.8%) | 6.9 (2.5 – 19)** | 2.7 (0.90 – 8.1) |
| Snowballs present | 788 (5.8%) | 3 (2.8%) | 0.48 (0.15 – 1.5) | 0.30 (0.090 – 0.98)* |
| Retinal vascular sheathing | 859 (6.3%) | 16 (15%) | 2.7 (1.4 – 5.0)** | 1.1 (0.6 – 2.0) |
| Retinal vascular occlusion | 73 (0.53%) | 8 (7.6%) | 15 (6.6 – 35)** | 4.7 (1.9 – 11)** |
| Active inflammatory chorioretinal lesions | 904 (6.6%) | 9 (8.5%) | 1.3 (0.6 – 3.0) | 0.40 (0.17 – 0.92)* |
Adjusted analysis adjusts for age category, gender, bilateral inflammation, smoking status, hypertension, diabetes, time from diagnosis, and systemic lupus erythematosus.
For characteristics which were not present in any patients with retinal NV, an odds ratio (OR) of zero is given but an estimated confidence interval is calculated using a profile likelihood method (see text for details).
These conditions with low numbers of retinal neovascularization are available in supplemental tables online and omitted from the print versions for brevity. Data reporting risk based on gradings of anterior chamber cell, vitreous cell, and vitreous haze are available in the supplemental table.
Takayasu arteritis and dermatomyositis had fewer than 5 observations in both the prevalence and incidence analyses and are omitted from the tables.
=p<0.05
=p<0.01
More posteriorly located inflammation was associated with a higher prevalence of retinal NV. The prevalence of retinal NV was 0.1% in eyes with anterior uveitis, 1% in eyes with intermediate uveitis, 2.4% in eyes with posterior uveitis, and 1.3% in eyes with panuveitis. As compared with anterior uveitis, intermediate uveitis carried an aOR of 4.5 (95%CI 1.9–10), posterior uveitis 12 (95%CI 5.9–26), and panuveitis 6.6 (95%CI 2.8–15). Amongst eyes with posterior or panuveitis, those with retinal vasculitis had the highest prevalence of retinal NV (5.7%, 95%CI: 4.1%–7.7%). Prevalence for additional posterior or panuveitis subtypes is given in Table 3 (available online at http://aaojournal.org).
Other retinal findings positively associated with prevalent retinal NV included macular edema (aOR = 2.1, 95%CI 1.2 – 3.5) and epiretinal membrane (aOR = 4.5; 95%CI 2.6–7.8). Retinal vascular occlusions (aOR = 4.7, 95%CI 1.9 – 11) were present significantly more often in eyes presenting with retinal NV but retinal vascular sheathing was not associated in the prevalence analysis (aOR = 1.1; 95%CI 0.55–2). Presence of snowballs in the vitreous was associated with a slightly decreased prevalence of NV (aOR = 0.30; 95%CI 0.10–0.98) as was the presence of active inflammatory chorioretinal lesions (aOR = 0.40; 95%CI 0.18–0.92). Choroidal NV tended to be associated with increased odds of NV, but the association was not statistically significant in the adjusted model (crude OR = 6.9, 95%CI: 2.5–19; aOR=2.7, 95%CI: 0.90 – 8.1). Exudative retinal detachment also was associated with increased crude odds but not significantly increased adjusted odds of prevalent NV (crude OR = 4.1, 95%CI: 1.7–10; aOR=2.1, 95%CI: 0.83–5.5).
INCIDENCE OF RETINAL NEOVASCULARIZATION
Among the 13,704 eyes included in the prevalence analysis, the 8,487 which did not have retinal NV at entry and had at least one follow-up visit were evaluated over time for incidence of retinal NV. Demographic and clinical characteristics of the prevalence and incidence cohorts were similar (data not shown).
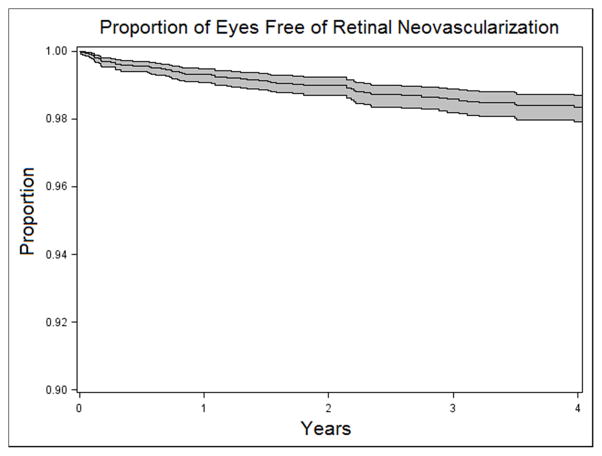
Over 26,465 eye-years (median follow-up 1.4 eye-years), 88 eyes developed retinal NV (Incidence rate=0.33% per eye-year, 95% CI: 0.27–0.41%; see Figure 1). As in the prevalence analysis, patients younger than 35 years were more likely to develop retinal NV than older patients (adjusted hazard ratio = 2.4; 95% CI 1.5–3.9; see Table 4). Diagnosis of uveitis within the last five years was associated with an increased prevalence of NV (compared to patients diagnosed >5 years ago) with diagnosis less than 1 year carrying an adjusted odds ratio of 2.1 (95% CI 0.99–4.3) and diagnosis between 1 and 5 years carrying an adjusted odds ratio of 2.6 (95%CI 1.2 – 5.5). Bilateral inflammation also was significantly associated with higher risk of retinal NV in the unadjusted model (cHR = 3.3; 95% CI 1.6–6.8), but unlike the result in the prevalence analysis, the association fell just below the level of statistical significance in the adjusted model (aHR = 2.0; 95% CI 0.95–4.2).
Figure 1.
Kaplan-Meier curve showing the estimated proportion and 95%CI free of retinal neovascularization over time among 8,487 uveitic eyes initially free of retinal neovascularization.
Table 4.
Incidence of retinal NV and associated risk factors†
| Characteristic | Number at Risk | Incident NV Cases | Incidence Rate | Crude hazard ratio (HR) (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Number of eyes | 8487 | 88 | 0.0033 | ||
| Age<35 | 3342 | 48 | 0.0044 | 1.77 (1.1– 2.8)* | 2.4 (1.5 – 3.9)** |
| Female (compared to male) | 5399 | 52 | 0.0031 | 0.81 (0.51 – 1.3) | 0.75 (0.47 – 1.2) |
| Behçet disease | 226 | 5 | 0.0042 | 1.5 (0.6 – 3.8) | 0.63 (0.26 – 1.5) |
| Systemic lupus erythematosus | 78 | 5 | 0.012 | 4.6 (1.6 – 14)** | 3.5 (1.1 – 11)* |
| Sarcoidosis | 647 | 8 | 0.0030 | 0.99 (0.41 – 2.4) | 0.94 (0.39 – 2.3) |
| HLA-B27+ | 1100 | 6 | 0.0017 | 0.47 (0.21 – 1.1) | 1.3 (0.55 – 3.1) |
| HLA-A29+ | 179 | 2 | 0.0026 | 0.84 (0.21 – 3.4) | 0.44 (0.10 – 1.9) |
| Hypertension | 1791 | 30 | 0.0040 | 1.5 (0.93 – 2.5) | 1.5 (0.89 – 2.4) |
| Diabetes | 550 | 12 | 0.0061 | 2.1 (0.98 – 4.3) | 2.3 (1.1 – 4.9)* |
| Bilateral | 5590 | 80 | 0.0039 | 3.3 (1.6 – 6.8)** | 2.0 (0.95 – 4.2) |
| Current Smoker (vs. never smoker) | 1545 | 24 | 0.0052 | 2.3 (1.3 – 4.1)** | 1.9 (1.1 – 3.4)* |
| Former Smoker (vs. never smoker) | 889 | 12 | 0.0039 | 1.8 (0.84 – 3.8) | 1.7 (0.79 – 3.7) |
| Uveitis diagnosis ≤ 1 years (vs >5 years) | 4387 | 49 | 0.0039 | 2.6 (1.3 – 5.4)** | 2.4 (1.1 – 5.0)* |
| Uveitis diagnosis between 1 and 5 years (vs >5 years) | 2238 | 30 | 0.0040 | 2.8 (1.3 – 6.0)** | 2.6 (1.2 – 6.0)* |
| Site of uveitis | |||||
| Anterior | 4857 | 15 | 0.0011 | 1.0 (reference group) | 1.0 (reference group) |
| Intermediate | 1358 | 20 | 0.0044 | 4.0 (2.0 – 8.4)** | 3.1 (1.5 – 6.6)** |
| Posterior | 1242 | 30 | 0.0068 | 6.7 (3.3 – 14)** | 5.2 (2.5 – 11)** |
| Panuveitis | 1030 | 23 | 0.0060 | 5.8 (2.8 – 12)** | 4.3 (2.0 – 9.3)** |
| Activity | |||||
| Inactive | -- | 40 | 0.0022 | 1.00 (reference group) | 1.0 (reference group) |
| Slightly active | -- | 18 | 0.0080 | 2.8 (1.5 – 5.0)** | 2.4 (1.3 – 4.4)** |
| Active | -- | 29 | 0.0086 | 2.7 (1.6 – 4.5)** | 2.1 (1.2 – 3.7)** |
| Macular edema | -- | 14 | 0.0072 | 2.0 (1.1 – 3.8)* | 1.5 (0.75 – 3.1) |
| Epiretinal membrane | -- | 6 | 0.0032 | 1.2 (0.51 – 2.7) | 0.83 (0.37 – 1.9) |
| Exudative retinal detachment | -- | 3 | 0.025 | 6.3 (2.1 – 19)** | 4.1 (1.3 – 13)* |
| Choroidal neovascularization | -- | 2 | 0.030 | 6.7 (0.90 – 49) | 4.1 (0.46 – 36) |
| Snowballs present | -- | 7 | 0.012 | 2.9 (1.4 – 6.3)** | 2.3 (1.0 – 5.4) |
| Retinal vascular sheathing | -- | 14 | 0.018 | 5.2 (2.7 – 10)** | 2.6 (1.4 – 4.9)** |
| Retinal vascular occlusion | -- | 4 | 0.090 | 20 (7.3 – 57)** | 10 (3.0 – 33)** |
| Active inflammatory chorioretinal lesions | -- | 1 | 0.0017 | 0.35 (0.05 – 2.6) | 0.16 (0.020 – 1.2) |
Adjusted analysis adjusts for age category, gender, bilateral inflammation, smoking status, hypertension, diabetes, primary ocular location of inflammation, systemic lupus erythematosus, and time from diagnosis. For associated characteristics which could vary from visit to visit (such as activity), the analysis was done using a time varying approach. For these characteristics, no value for number of eyes is given for variables analyzed as time-dependent covariates.
For characteristics which were not present in any patients with retinal NV, a hazard ratio (HR) of zero is given but an estimated confidence interval is calculated using a profile likelihood method (see text for details).
These conditions with low numbers of retinal neovascularization are available in supplemental tables online and omitted from the print versions for brevity. Data reporting risk based on gradings of anterior chamber cell, vitreous cell, and vitreous haze are available in the supplemental table.
Takayasu arteritis and dermatomyositis had fewer than 5 observations in both the prevalence and incidence analyses and are omitted from the tables. Although there were 8 eyes with familial systemic granulomatosis in the prevalence analysis, none were eligible for the incidence analysis.
=p<0.05
=p<0.01
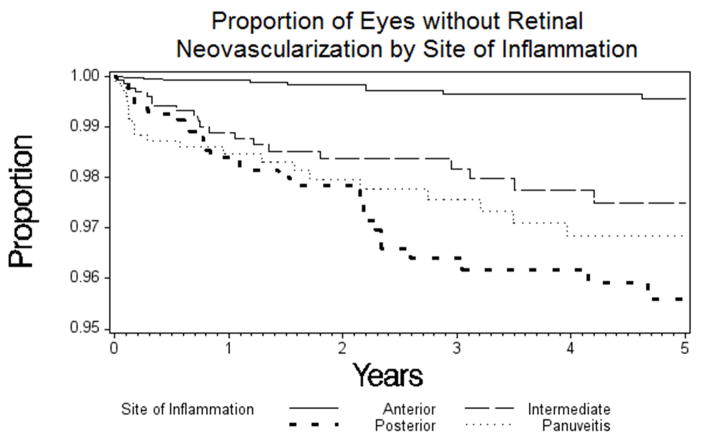
The incidence analysis also confirmed that diagnosis with a form of inflammation involving the posterior segment of the eye was associated with higher risk of retinal NV. The incidence rate per eye-year of developing retinal NV was 0.11 for anterior uveitis, 0.44% for intermediate uveitis, 0.68% for posterior uveitis, and 0.60% for panuveitis. In comparison with anterior uveitis, intermediate uveitis carried an aHR of 3.1 (95% CI: 1.5–6.6), posterior uveitis 5.2 (95% CI: 2.5–11), and panuveitis 4.3 (95% CI: 2.0–9.3) (see Figure 2). Several posterior segment complications of posterior inflammation also were associated with increased risk of retinal NV, including exudative retinal detachment (aHR=4.1; 95% CI 1.3–13), retinal vascular occlusions (aHR=10; 95% CI 3.0–33) and vascular sheathing (aHR = 2.6; 95% CI 1.4–4.9; the last in contrast to the prevalence analysis). There was no statistically significant association between choroidal NV and retinal NV in the incidence analysis (aHR=4.1; 95% CI 0.46–36), both of which were uncommon events. Unlike in the prevalence analysis, presence of snowballs was associated with an increased risk of retinal NV, although this fell just at the level of significance (aHR = 2.3; 95%CI 1.0–5.4). Active inflammatory chorioretinal lesions, which were associated with decreased risk in the prevalence analysis, also tended to be associated with lower risk in this incidence analysis, but not to a statistically significant degree (aHR = 0.16; 95%CI 0.020–1.2).
Figure 2.
Kaplan-Meier curve estimating the proportion of uveitic eyes free of retinal neovascularization over time by International Uveitis Study Group/Standardization of Uveitis Nomenclature17 site of uveitic inflammation classification. The risk of developing retinal neovascularization with anterior uveitis was much less than the risk of developing it with inflammation involving the posterior segment. However, the differences between intermediate, posterior, and panuveitis cases were not statistically significant (see Table 4).
Using a time-updated analysis approach, active inflammation—whether graded as overall activity or according to grading of anterior chamber cells, vitreous cells, or vitreous haze—tended to be associated with greater incidence of retinal NV. In comparison with an overall activity grading of inactive, a grading of active was associated with an increased risk (aHR = 2.1; 95%CI 1.2–3.7) as was a grading of slightly active (aHR = 2.4; 95%CI 1.3–4.4). Gradings of anterior chamber cells showed a dose-response relationship with stronger association at higher gradings. While 0.5+ and 1+ anterior chamber cell grades were not associated with increased risk, 2+ cell was associated with higher risk (aHR = 2.7; 95%CI 1.2–6.0) as was 3+ cells (aHR = 3.9; 95%CI 1.3 – 11). Vitreous cell was only associated at the 1+ level with a crude hazard radio of 2.3 (95%CI 1.3 – 4.2) however this relationship dropped below the level of significance in the adjusted analysis. 1+ vitreous haze was associated with a higher risk (aHR = 2.3; 95%CI 1.1–5.0) however this association was not seen at higher gradings. The numbers of eyes in the higher vitreous cell and vitreous haze groups was small which may limit the ability to detect associations. (Complete data omitted from print Table 4, but available in online Table 5, available at http://aaojournal.org).
The power of the study to detect associations with systemic inflammatory diseases was limited by the relatively small numbers of patients with several of the systemic conditions. Nevertheless, diagnosis with systemic lupus erythematosus (aHR = 3.5; 95% CI 1.1–11) or polyarteritis nodosa (aHR = 7.2; 95% CI: 1.2–44) was associated with a significantly increased risk of retinal NV.
Amongst eyes with posterior or panuveitis, those with retinal vasculitis had the highest incidence of retinal NV (2.0% per eye-year, 95%CI: 1.3–3.0%). As compared to the incidence rate for anterior uveitis of 0.11% per eye-year, Vogt-Koyanagi-Harada Syndrome was also associated with a higher than average incidence of retinal NV (1.0% per eye-year, 95%CI 0.37–2.2%) (Table 6, available online at http://aaojournal.org). Several systemic disease conditions of interest such as granulomatosis with polyangiitis (previously known as Wegener’s granulomatosis) and Takayasu arteritis were too infrequent to assess association with incident retinal NV with reasonable power. No association was detected for the other conditions assessed (see Table 5).
Regarding risk factors for systemic vascular disease, current cigarette smoking was associated with an almost two-fold increased incidence of retinal NV (aHR = 1.9; 95% CI: 1.1–3.4) and there was a non-significant tendency towards higher retinal NV risk with prior cigarette smoking (aHR = 1.7; 95% CI: 0.79–3.7). Hypertension tended to be associated with greater risk of retinal NV, but not to a statistically significant degree (aHR 1.5; 95% CI:0.89–2.4). Diabetes mellitus also carried an increased risk (aHR = 2.3; 95%CI 1.1–4.9) unlike in the prevalence analysis. When eyes of patients with diabetes mellitus were excluded in a sensitivity analysis, all results were similar (data not shown).
Discussion
Retinal NV was an infrequent (though clinically important) complication in our cohort of tertiary uveitis cases, which was associated with several risk factors that may be relevant to understanding the pathogenesis of the condition and predicting risk in cases of uveitis. In other disease conditions, retinal neovascularization occurs most commonly in response to retinal ischemia. In this study, factors representing retinal ischemia (including retinal vascular occlusions, systemic lupus erythematosus, and cigarette smoking) were associated with increased risk of retinal NV. Previous reports from members of this group have shown that current smoking is associated with an increased risk of disease recurrence, as well as increased severity and greater likelihood for bilateral disease18 suggesting that smoking may affect ocular inflammation as well as having adverse vascular effects. Smoking is a risk factor for choroidal neovascularization in age-related macular degeneration,19 suggesting a potentially proangiogenic effect of some component of tobacco smoke, which our data tend to support. However, even after adjustment for these ischemic risk factors, inflammatory activity continued to be significantly associated with risk of neovascularization. This observation suggests that ischemia does not entirely explain the incidence of retinal NV in the setting of uveitis.
At a molecular level, inflammation works in concert with ischemia in promoting neovascularization. In diabetes, inflammatory molecules such as interleukin 1-beta (IL-1β) have a role in promoting apoptosis of endothelial cells.20 Some of the same cytokines which cause capillary loss, including IL-1β and TNF-α, can promote endothelial proliferation and pathologic angiogenesis 12. Additional molecules such as IL-6 and IL-8 also are involved in the neovascular phase12. Many of these same cytokines are upregulated in ocular inflammation, including IL-6, IL-8, and TNF-α.21 While it is possible that some of these molecules could be coincidentally upregulated rather than necessary for the development of retinal NV, corneal models have shown that IL-8 and TNF-α can cause neovascularization directly.22,23 This molecular link between inflammation and ischemia may provide a mechanism to explain our observations between the presence of inflammatory signs and retinal NV.
The presence of currently active inflammation was associated with retinal NV in our study. The degree of increased risk was similar between active and slightly active eyes, there appeared to be a dose-response relationship with anterior chamber inflammation, and there was no clear relationship with measures of vitreous inflammation. Several interpretations are possible for the lack of a consistent linear relationship with increased activity. In the timevarying analysis, eyes could switch from active to inactive and between cell or haze grades from visit to visit, so activity would only be correlated to NV if activity had a rapid effect on the development of NV. It is possible that retinal NV is driven by more long-term alterations in inflammatory pathways or by structural changes which take time to develop, tending to obscure associations. The incidence analysis may also have been less powerful than the prevalence analysis for this question because inflammation at presentation may have been more severe and persistent than during follow-up (tertiary management might have resulted in better control of inflammation, on average). Treatment data were not analyzed in this study, because of potential indications-for-treatment bias, but the impact of effective treatment can be inferred by evaluating the relationship between [in]activity and retinal NV, as effective treatment would result in control of uveitis activity.
Another explanation for the pattern of association we observed between inflammatory attributes and retinal NV is that proangiogenic molecules could persist even after active inflammation is controlled. For example, Paroli et al. found elevated vascular endothelial growth factor (VEGF) levels in the aqueous humor of patients with quiescent uveitis24. If so, the presence of retinal NV would not always be a sign of disease activity and would not necessarily require increased immunosuppressive therapy. Rather, therapy should be directed against the neovascular process. However, it seems likely that consistent inflammatory control over time would reduce the risk of retinal NV, in addition to the other benefits conveyed by consistent control.
If accumulation of proangiogenic molecules was a significant contributor to development of retinal NV, one would expect the incidence to increase with time since diagnosis. Our data show the reverse, with younger patients and those with more recent diagnosis were more likely to develop retinal NV. Previous clinical observations seem to support our results; in pars planitis, for example, vitreous hemorrhage presumably due to retinal NV was much more likely in patients diagnosed prior to age 17 than those diagnosed after 17, perhaps because the vitreous of younger patients may exert stronger tractional forces on the retinal vessels.25,26
In ocular inflammatory disease, peripheral retinal photocoagulation as a treatment for retinal NV has been studied in small, observational series.3,10,27,28 Synthesizing these and our observations, it is possible that photocoagulation would help those patients where ischemic retina is driving neovascularization. In those patients without ischemia scatter photocoagulation appears less likely to help, and anti-inflammatory treatment may be more useful. Because the patients in our study were observed mostly in the era prior to widespread use of anti-VEGF agents, we did not evaluate their outcomes. Several reports have shown promising results of anti-VEGF therapy for retinal NV in the setting of uveitis.30–32 In one study, bevacizumab was supplemented with panretinal photocoagulation, but when and how often this treatment was applied was not described.32 Future studies could incorporate ultra-wide field fluorescein angiography, which can assess a broader area of retina for disease activity and ischemia. 29 Additional studies will be necessary to determine the best combination of treatments for patients with uveitis and retinal neovascularization.
As in all retrospective studies, our results have important limitations. Our results regarding the incidence of retinal NV are anticipated to be more reliable than the results regarding the prevalence of retinal NV, because patients may have been referred for tertiary evaluation in part because of the occurrence of complications of inflammation such as retinal NV (prevalence-incidence bias). However, the fact that risk factor associations followed a similar pattern in the two analyses suggest that such bias likely did not vary between subsets of patients/eyes, and provides reassurance of the validity of the associations observed. The fact that fewer associations were statistically significant in the incidence analysis likely reflects less statistical power than in the prevalence analysis because there were fewer cases, and/or mitigation of risk by clinical management. Because cases were retrospectively ascertained, we have missed some cases of retinal NV. Because such misclassification would be expected to blunt associations, it is unlikely that any such errors would have led to spuriously positive associations, although it is possible that it reduced our power to detect associations. We also did not assess the effect of anti-inflammatory treatment on retinal NV, due to indications-for434 treatment biases which would require very logistically challenging clinical trials to evaluate optimally. However, to the extent that treatment effects are mediated via control of inflammation, it should be possible to infer the benefits of treatment based on the degree of relationship between inflammatory activity and retinal NV. Future studies also would benefit from capturing more information on retinal ischemia using wide field imaging. Because of the rarity of retinal NV in patients with uveitis (an uncommon condition itself), prospective studies would be challenging to implement. By using a retrospective design with a strong data quality control system, we were able to capture a large amount of data, which allowed the risk of NV to be estimated with reasonable confidence and permitted identification of several risk factors.
In summary, retinal NV is a rare complication of uveitis for which posterior segment involvement (intermediate, posterior, and panuveitis), younger age, and relatively recent diagnosis are strong but unmodifiable risk factors. Systemic conditions such as smoking, diabetes and some forms of systemic vasculitis (most clearly systemic lupus erythematosus) are associated with increased risk of NV; mitigation of these conditions by smoking cessation or appropriate treatment might mitigate retinal NV risk, in addition to providing several other benefits. Disease activity is associated with retinal NV, although the temporal relationship and degree of activity required to induce NV is not certain. When treatment of retinal NV is indicated, based on the available information, it seems reasonable to treat cases with widespread ischemia with treatments directed against ischemia (anti-VEGF therapy and scatter retinal photocoagulation when ischemia is expected to be long-lasting), whereas cases lacking demonstrable ischemia may reasonably be approached first with anti-inflammatory therapy, although even in these cases anecdotal evidence suggests anti-VEGF therapy may be helpful. Anti-inflammatory therapy will be indicated for any case with active uveitis, and it may be acceptable in lower risk circumstances to try such therapy first rather than scatter photocoagulation or anti-VEGF therapy even in ischemic cases. If immediate clearing of retinal NV is required, use of both approaches together could be valuable in an appropriate clinical setting. Because effective treatment of the underlying uveitis may alter the clinical course, it is possible that treatment of NV in the context of uveitis may not need to be as aggressive as treatment for NV primarily of ischemic etiology. Further studies evaluating outcomes of these patients in relationship to anti-inflammatory and anti-angiogenic therapies would be valuable to refine the management approach.
Supplementary Material
Acknowledgments
Financial Support:
This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness, the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C was previously supported by and RBN and HNS continue to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
Footnotes
Conflict of Interest: None
Financial Disclosure(s):
The author(s) have made the following disclosure(s): C. Stephen Foster: (equity owner) Eyegate, (consultant, lecturer) Allergan; (consultant, lecturer) Bausch & Lomb; (consultant) Sirion; (lecturer) Alcon; (lecturer) Inspire; (lecturer) Ista; (lecturer) Centocor; Douglas A. Jabs: (Data and Safety Monitoring Committee (DSMC) member) Applied Genetic Technologies Corporation; (DSMC member) Novartis Pharmaceutical Corp ; (consultant) Santen; James Rosenbaum: (consultant) Abbvie; (consultant) Santen; (consultant) Sanofi; (consultant) EMD Serono; (consultant) Genentech; (consultant) Regeneron; (consultant) Allergan; (consultant) Novartis; consultant (UCB); Jennifer Thorne: (consultant) AbbVie; (consultant) Gilead; (consultant) Xoma; (grant recipient) Allergan; John H. Kempen: (consultant) Abbvie; (consultant) Allergan; (consultant) Alcon; (consultant) Clearside; (consultant) Can-Fite; (consultant) Lux Biosciences; (consultant) Roche; (consultant) Xoma; (consultant) Vitae; (grant recipient) Eyegate;
Contributions
Design and conduct of the study (AKP, JHK); collection, management, analysis, and interpretation of the data (all authors); preparation, review, or approval of the manuscript (All Authors)
Institutional Review Board Approval
The project was conducted in accordance with the principles of the Declaration of Helsinki, with the approval of the governing Institutional Review Boards of each institution, each of which has granted waiver of consent and HIPAA exemption, allowing all living and deceased patients to be included.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuo IC, Cunningham ET., Jr Ocular neovascularization in patients with uveitis. Int Ophthalmol Clin. 2000;40(2):111–26. doi: 10.1097/00004397-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Gray T, Kanski J, Lightman S. Steroid responsive disc neovascularisation in uveitis associated with juvenile chronic arthritis. Br J Ophthalmol. 1998;82(3):327–8. doi: 10.1136/bjo.82.3.326b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, et al. Neovascularization of the optic disc in behcet’s disease. Jpn J Ophthalmol. 2006;50(3):256–65. doi: 10.1007/s10384-005-0307-8. [DOI] [PubMed] [Google Scholar]
- 4.Miyao A, Ikeda T, Matsumoto Y, et al. Histopathological findings in proliferative membrane from a patient with sarcoid uveitis. Jpn J Ophthalmol. 1999;43(3):209–12. doi: 10.1016/s0021-5155(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 5.Kalina PH, Pach JM, Buettner H, et al. Neovascularization of the disc in pars planitis. Retina. 1990;10(4):269–73. doi: 10.1097/00006982-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Biswas J, Sharma T, Gopal L, et al. Eales disease--an update. Surv Ophthalmol. 2002;47(3):197–214. doi: 10.1016/s0039-6257(02)00288-6. [DOI] [PubMed] [Google Scholar]
- 7.Saatci OA, Kocak N, Durak I, et al. Unilateral retinal vasculitis, branch retinal artery occlusion and subsequent retinal neovascularization in crohn’s disease. Int Ophthalmol. 2001;24(2):89–92. doi: 10.1023/a:1016351800466. [DOI] [PubMed] [Google Scholar]
- 8.Vine AK, Barr CC. Proliferative lupus retinopathy. Arch Ophthalmol. 1984;102(6):852–4. doi: 10.1001/archopht.1984.01040030672015. [DOI] [PubMed] [Google Scholar]
- 9.Graham EM, Stanford MR, Shilling JS, et al. Neovascularisation associated with posterior uveitis. Br J Ophthalmol. 1987;71(11):826–33. doi: 10.1136/bjo.71.11.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanislo SR, Lowder CY, Kaiser PK, et al. Corticosteroid therapy for optic disc neovascularization secondary to chronic uveitis. Am J Ophthalmol. 2000;130(6):724–31. doi: 10.1016/s0002-9394(00)00598-5. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell M, Uusitalo-Jarvinen H, Aguilar E, et al. Ocular neovascularization: Basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52(Suppl 1):S3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Sapieha P, Hamel D, Shao Z, et al. Proliferative retinopathies: Angiogenesis that blinds. Int J Biochem Cell Biol. 2010;42(1):5–12. doi: 10.1016/j.biocel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007;13(10):2825–30. doi: 10.1158/1078-0432.CCR-06-2416. [DOI] [PubMed] [Google Scholar]
- 14.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007:200795103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30(2):65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 16.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: The systemic immunosuppressive therapy for eye diseases (SITE) cohort study. Ophthalmic Epidemiol. 2008;15(1):47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galor A, Feuer W, Kempen JH, et al. Adverse effects of smoking on patients with ocular inflammation. Br J Ophthalmol. 2010;94(7):848–53. doi: 10.1136/bjo.2009.174466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: The number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90(1):75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88(10):1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curnow SJ, Murray PI. Inflammatory mediators of uveitis: Cytokines and chemokines. Curr Opin Ophthalmol. 2006;17(6):532–7. doi: 10.1097/ICU.0b013e32801094b5. [DOI] [PubMed] [Google Scholar]
- 22.Strieter RM, Kunkel SL, Elner VM, et al. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141(6):1279–84. [PMC free article] [PubMed] [Google Scholar]
- 23.Lu P, Li L, Liu G, et al. Critical role of TNF-α induced macrophage VEGF and iNOS production in the experimental corneal neovascularization. IOVS. 2012;53(7):3516–26. doi: 10.1167/iovs.10-5548. [DOI] [PubMed] [Google Scholar]
- 24.Paroli MP, Teodori C, D’Alessandro M, et al. Increased vascular endothelial growth factor levels in aqueous humor and serum of patients with quiescent uveitis. Eur J Ophthalmol. 2007;17(6):938–42. doi: 10.1177/112067210701700611. [DOI] [PubMed] [Google Scholar]
- 25.Lauer AK, Smith JR, Robertson JE, et al. Vitreous hemorrhage is a common complication of pediatric pars planitis. Ophthalmology. 2002;109(1):95–8. doi: 10.1016/s0161-6420(01)00866-1. [DOI] [PubMed] [Google Scholar]
- 26.Hardwick C, Morris R, Witherspoon D, et al. Pathologic human vitreous promotes contraction by fibroblasts. Implications for proliferative vitreoretinopathy. Arch Ophthalmol. 1995;113:1545–53. doi: 10.1001/archopht.1995.01100120075013. [DOI] [PubMed] [Google Scholar]
- 27.Park SE, Mieler WF, Pulido JS. Peripheral scatter photocoagulation for neovascularization associated with pars planitis. Arch Ophthalmol. 1995;113(10):1277–80. doi: 10.1001/archopht.1995.01100100065030. [DOI] [PubMed] [Google Scholar]
- 28.Biswas J, Ravi RK, Narayansamy A, et al. Eales’ disease – current concepts in diagnosis and management. J Ophthalmic Inflamm Infect. 2013;3(1):11. doi: 10.1186/1869-5760-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell JP, Leder HA, Sepah YJ, et al. Wide-field retinal imaging in the management of non-infectious posterior uveitis. Am J Ophthalmol. 2012;154(5):908–911. doi: 10.1016/j.ajo.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Kurup S, Lew J, Byrnes G, et al. Therapeutic efficacy of intravitreal bevacizumab on posterior uveitis complicated by neovascularization. Acta Ophthalmol. 2009;87(3):349–52. doi: 10.1111/j.1755-3768.2008.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansour AM, Mackensen F, Arevalo JF, et al. Intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2008;146(3):410–6. doi: 10.1016/j.ajo.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Mansour AM, Arevalo JF, Fardeau C, et al. Three-year visual and anatomic results of administrating intravitreal bevacizumab in inflammatory ocular neovascularization. Can J Ophthalmol. 2012;47(3):269–74. doi: 10.1016/j.jcjo.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Tempest-Roe S, Joshi L, Dick AD, et al. Local therapies for inflammatory eye disease in translation: Past, present and future. BMC Ophthalmol. 2013;13(1):39. doi: 10.1186/1471-2415-13-39. 2415–13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.