Abstract
Neuronal senescence caused by diabetic neuropathy is considered a common complication of diabetes mellitus. Neuronal senescence leads to the secretion of pro-inflammatory cytokines, the production of reactive oxygen species, and the alteration of cellular homeostasis. Agmatine, which is biosynthesized by arginine decarboxylation, has been reported in previous in vitro to exert a protective effect against various stresses. In present study, agmatine attenuated the cell death and the expression of pro-inflammatory cytokines such as IL-6, TNF-alpha and CCL2 in high glucose in vitro conditions. Moreover, the senescence associated-β-galatosidase's activity in high glucose exposed neuronal cells was reduced by agmatine. Increased p21 and reduced p53 in high glucose conditioned cells were changed by agmatine. Ultimately, agmatine inhibits the neuronal cell senescence through the activation of p53 and the inhibition of p21. Here, we propose that agmatine may ameliorate neuronal cell senescence in hyperglycemia.
Keywords: Agmatine, High glucose, Hyperglycemia, Cell death, Senescence, Pro-inflammatory cytokines, p53, p21
INTRODUCTION
Diabetes is a growing public health concern with the increase in the worldwide population [1]. Hyperglycemia can lead to inflammation-induced metabolic disorders and various catabolism dysfunctions [2]. Hyperglycemia causes an acceleration of age-related damage of neurons [3]. Cellular senescence is defined as an irreversible proliferation arrest and contributes to age-associated decline in cellular homeostasis in diverse tissue [4]. Age-related pathologies are accompanied by an increase in proinflammatory cytokines, including tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 [5]. Specifically, the release of IL-6, IL-1, TNF-alpha, and chemokine (C-C motif) ligand 2 (CCL2) has been reported to be involved in p53-mediated cellular senescence [6]. p53 is as a transcription factor that mediates cell cycle arrest, cell senescence, and apoptosis [7]. In the central nervous system (CNS), p53 induction has been shown to correlate with neuronal death and DNA damage and senescence [8] in in vitro and in in vivo studies. In high glucose condition, the p53 cascade is strongly associated with the cell senescence [9] and cell metabolism. In addition, the activation of p21 has been reported that it leads to the aggravation of cellular senescence by reactive oxygen species (ROS) production [10] and cytokine's secretion [11]. Not only p21, there are inducers of cell senescence such as p35, Cdk 5 [12] which are related to cell cycle arrest and they are also increased in cellular senescence condition, strongly related with p21.
Agmatine is a cationic polyamine peptide that is synthesized by decarboxylation of L-arginine by arginine decarboxylase (ADC) [13]. Results of experimental studies have shown that agmatine has neuroprotective effects in CNS disorders including cerebral ischemia [14], Alzheimer's disease [15] and Parkinson's disease [16]. In diabetic rats with middle cerebral artery occlusion, posttreatment with agmatine reduces neurobehavioral dysfunction [17]. Moreover, DNA fragmentation and expression of proapoptotic proteins such as cleaved caspase-3 were significantly reduced in ADC-transfected neural stem cells against H2O2 stress [18]. Given that amine peptides are linked with the cell senescence through p53 and p21 cascade, we hypothesized that agmatine may alleviate the cell senescence by regulating p21 and p53 signaling. Here, we aimed to study the effects of agmatine on high glucose-induced neuronal cellular damage, with a focus on the p21 and p53 pathway, to test the hypothesis that agmatine could reverse high glucose-induced cellular senescence.
MATERIALS AND METHODS
Neuro2A cell culture and drug treatment
N2A cells possess some of the properties of neuronal stem cells and are capable of differentiating into neuron-like cells in the presence of retinoic acid (RA) (Sigma-Aldrich, St. Louis, MO, USA). Undifferentiated N2A cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 100 µg/mL penicillin-streptomycin (Gibco, Grand Island, NY, USA). N2A cells were passaged at least twice and then plated at 1×104 cells/mL in DMEM supplemented with 10% FBS for 24 hours. After that, the medium was changed to DMEM supplemented with 1% FBS and 5 µM RA for differentiation. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37℃. Cells were treated with D-glucose (Sigma-Aldrich, St. Louis, MO, USA) and agmatine (100 µM) (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours before sampling. As several in vitro studies [19], we experimented in the concentration of glucose (25~200 mM).
Cell viability (MTT assay)
N2A cells were seeded at 1×104 cells/mL in 96-well plates to examine the effects of all experimental treatments. Cells were then rinsed with phosphate-buffered saline (PBS), and culture medium was replaced with serum-free medium. Next, 100 µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA) solution (5 mg/ml in PBS) was added per well. After 1 hour 30 min of incubation, the medium was removed, and dimethyl sulfoxide was added to solubilize the purple formazan product of the MTT reaction. The supernatant from each well was measured at a wavelength of 570 nm with background subtraction at 650 nm. All experiments were repeated at least 5 times. Cell viability was expressed as a percentage relative to the normal control group value.
Determination of intracellular reactive oxygen species (ROS)
The level of ROS in N2A cells was measured using a fluorescent probe, 2' 7'-dichlorodihydrofluorescein diacetate (DCF-DA; Invitrogen, Carlsbad, CA, USA), as previously described [20]. N2A cells were passaged at least twice and then plated at 1 × 104 cells/mL in DMEM supplemented with 10% FBS for 24 hours. After that, the medium was changed to DMEM supplemented with 1% FBS and 5 µM RA for differentiation. Cells were treated with glucose and agmatine for 24 hours. Then, N2A cells were treated with 5 µM DCF-DA for 30 min at 37℃. After washing with PBS, fluorescence was measured with a microscope (Olympus, Korea) equipped with a CCD camera (Hamamatsu Photonics, Japan).
Western blot analysis
After agmatine treatment and exposure to high glucose stress, N2A cells were washed twice with ice-cold PBS, scraped, and collected. N2A cell pellets were lysed with ice-cold RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA). The lysates were centrifuged at 16,100g for 1 hour at 4℃ to produce whole-cell extracts. Protein content was quantified using the BCA protein assay kit (Pierce, IL, USA). Protein (35 µg) was separated on a 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% bovine serum albumin in TBS/Tween (20 nM Tris (pH 7.2), 150 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature, immunoblots were incubated overnight at 4℃ with primary antibodies that specifically detect cleaved caspase 3 (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), caspase 3 (1:2000, Abcam, Cambridge, MA, USA), phosphor-p53 (1:2000, Abcam, Cambridge, MA, USA), p53 (1:2000, Abcam, Cambridge, MA, USA), or β-actin (1:2000, Cell Signaling, Danvers, MA, USA). Blots were then incubated with horseradish peroxidase-linked anti-mouse or -rabbit IgG antibodies (Abcam, Cambridge, MA, USA) for 2 hours at room temperature. Protein bands were detected and analyzed using enhanced chemiluminescence (ECL; Pierce, IL, USA).
Reverse transcription-PCR
The expression of Bax, TNF-alpha, CCL2, p21, cyclin A, PARP-1, p35, Cdk 5 in N2A cells treated with high glucose and agmatine was examined using reverse transcription PCR with the corresponding primers. Samples were lysed with Trizol reagent (Invitrogen, Carlsbad, CA, USA). PCR was performed using the following thermal cycling conditions: 10 min at 95℃; 35 cycles of denaturing at 95℃ for 15 sec; annealing at 63℃for 30 sec and elongation at 72℃ for 30 sec; final extension at 72℃ for 10 min; and holding at 4℃. PCR was performed using the following primers (5' to 3'): TNF-alpha (F) CAA GGG ACA AGG CTG CCC CG, (R) GCA GGG GCT CTT GAC GGC AG, CCL2 (F) CTC GAG ATG CAG GTC CCT GTC AT, (R) AAG CTT CTA GTT CAC TGT CAC ACT G, p21 (F) AGT GTG CCG TTG TCT CTT CG, (R) ACA CCA GAG TGC AAG ACA GC, Cyclin A (F) AGT ACC TGC CTT CAC TCA TTG CTG, (R) TCT GGT GAA GGT CCA CAA GAC AAG, PARP-1 (F) AGG CCC TAA AGG CTC AGA AT, (R) CTA GGT TTC TGT GTC TTG AC, Cdk 5 (F) GGC TAA AAA CCG GGA AAC TC, (R) CCA TTG CAG CTG TCG AAA TA, p35 (F) AAG AAC GCC AAG GAC AAG AA, (R) TCA TTG TTG AGG TGC GTG AT, β-actin (F) TCT GGC ACC ACA CCT TCT A, (R) AGG CAT ACA GGG ACA GCA C. PCR products were separated by gel electrophoresis using 1.5% agarose gels and stained with ethidium bromide.
Quantitative real time-PCR
To examine the amount of IL-6 mRNA in conditioned N2A cells, quantitative real time-PCR was performed using IL-6 primers. A One Step SYBR® Prime Script TM RT-PCR Kit II (Takara, Japan) was used to conduct qRT-PCR. PCR was performed using the following primers (5' to 3'): IL-6 (F) AAC GAT GA TGC ACT TGC AGA, (R) CTC TGA AGG ACT CTG GCT TTG, β-actin (F) TCT GGC ACC ACA CCT TCT A, (R) AGG CAT ACA GGG ACA GCA C. Denaturing was carried out at 95℃ for 3 min; 40 cycles of 95℃ for 20 sec; annealing at 60℃ for 20 sec; and extension at 72℃ for 20 sec. At each extension step at 72℃, fluorescence was detected at 585 nm. The expression of IL-6 was assessed using an ABI prism 7500 Real-Time PCR System (Life Technologies Corporation, CA, USA) and analyzed with comparative Ct quantification. β-actin was amplified as an internal control. The ΔCt values of high glucose-exposed N2A cells were compared with the ΔCt values of normal N2A cells.
β-Galactosidase staining (Cell senescence assay)
N2A cells under the experimental condition were fixed in 0.2% glutaraldehyde solution for 15 min at room temperature. To detect β-galactosidase activation, we were performed β-galactosidase staining (Cell Signaling Technology, Beverly, MA, USA) at pH 6.0 [21]. For quantification, approximately five randomly chosen microscope fields in three independent cultures were examined at ×20 magnification so that at least 30 cells were considered as being either β-galactosidase positive or negative. β-galactosidase positive had cytoplasmic staining that appeared dark blue. All the experiments were repeated 3 times. N2A cells were then visualized under a confocal microscope (Zeiss LSM 700, Carl Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical analyses were conducted using SPSS 18.0 software (IBM Corp., Armonk, NY, USA). Data are expressed as the mean±standard error of the mean of three independent experiments. The statistical significance of group differences was determined by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc multiple comparison tests. Differences compared with normal control were considered statistically significant at *p<0.05, **p<0.001 and differences within groups were marked using lines and drew * and ** to show significancy.
RESULTS
Agmatine reduces neuronal cell death and ROS generation under high glucose condition
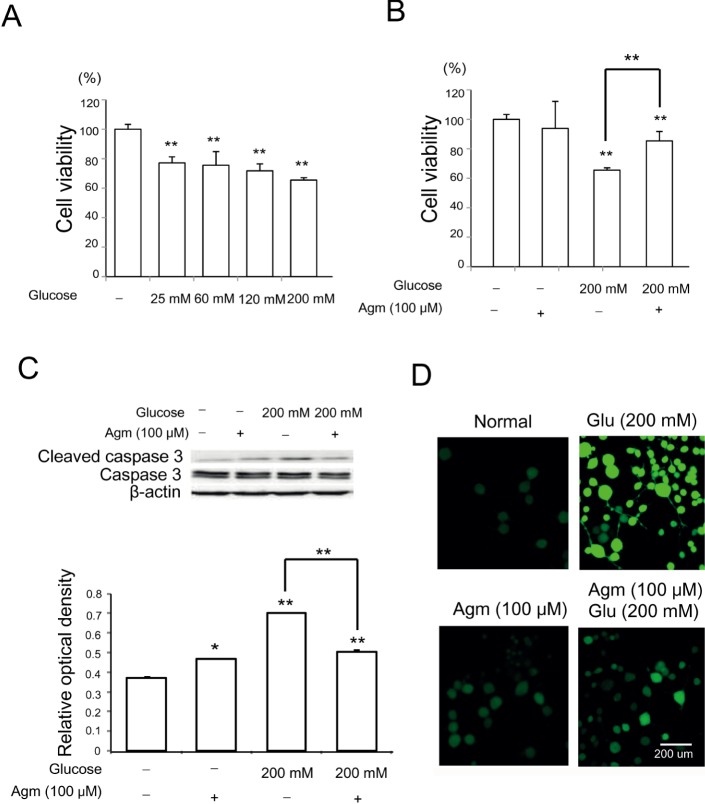
To examine the effect of high glucose stress on neuronal cell, we conducted a cell viability assay (Fig. 1A and 1B), western blot analysis (Fig. 1C), DCF-DA assay (Fig. 1D). In the cell viability assay, we observed markedly decreased cell viability depending on glucose concentration compared to control treatment (Fig. 1A). Based on MTT assay, we choose 200 mM of glucose as a high glucose stress condition because it induces high glucose stress as much as possible but not causes severe cell death. Fig. 1B showed the agmatine's effect on the cell viability under glucose 200 mM treatment (Fig. 1B). The increase in cleaved caspase-3 protein levels induced by high glucose condition was reduced by agmatine (Fig. 1C). ROS generation was measured using DCF-DA reagent in conditioned N2A cells (Fig. 1D). High glucose (200 mM) treatment induced significantly ROS generation in N2A cells (Fig. 1D). In comparison, agmatine attenuated the generation of ROS in N2A (Fig. 1D) cells in high glucose stress condition. Given our results, we hypothesize that agmatine may rescue neuronal cells from cell death under high glucose conditions.
Fig. 1. The measurement of the cell death, ROS generation in N2A cells under high glucose condition and agmatine treatment. (A) The graph showed that glucose concentration dependent decrease of cell viability of N2A cell compared to the normal control group. The more concentration of glucose was added, the more cells were dead. Each experiment included the 6 repeats per condition. (B) The cells treated with both glucose 200 mM and agmatine were exhibited increase in cell viability compared to the same glucose stress. Each experiment included the 6 repeats per condition. (C) Western blotting experiments showed that the relative protein level of cleaved caspase 3 /caspase 3 significantly was reduced in the glucose with agmatine 200 mM treatment group compared to the glucose treatment group. Each experiment included 4 repeats per condition. (D) ROS levels of conditioned N2A cells were measured using DCF-DA. ROS levels in agamatine treatment group were almost the same with the normal control group. ROS levels in N2A cells were increased in high glucose injury compared to the normal control group. Under high glucose condition, ROS levels in the agmatine teatment group tend to attenuate compared to high glucose exposed group. Agmatine reduced the high glucose-induced increase in DCF-DA-positive cells (green). Each experiment included 4 repeats per condition. 2', 7'-dichlorodihydrofluorescein diacetate (DCF-DA): green, Scale bar=200 µm.
Agmatine prohibits β-galactosidase activation under high glucose condition
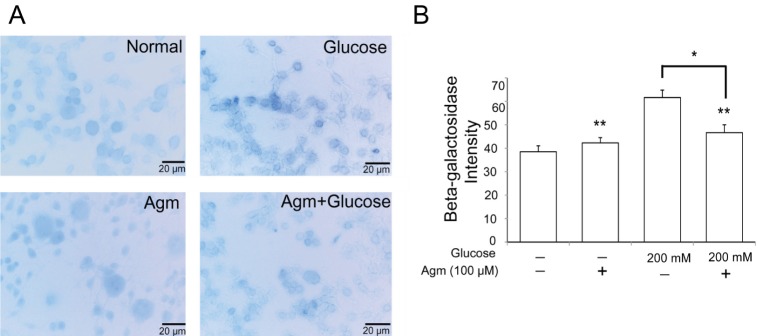
To determine the β-galactosidase as a hydrolase enzyme which accumulated in senescence cells under high glucose, we conducted β-Galactosidase staining (Fig. 2A and 2B). By comparison with the normal control group, β-galactosidase positive cells in high glucose condition were observed more positive cells (Fig. 2A and 2B). In contrast to the increased β-Galactosidase positive cells (blue) in high glucose group, β-Galactosidase positive cells were consciously reduced by agmatine treatment (Fig. 2B). Judging from β-galactosidase was expressed in senescence cells, our findings indicate that high glucose generates the cellular senescence and agmatine protects the cellular senescence against high glucose stress.
Fig. 2. The measurement of the β-galactosidase activity in N2A cells under high glucose condition and agmatine treatment. (A, B) The activity of β-galactosidase in experimental N2A cells was measured by β-galactosidase staining kit. The β-galactosidase positive cells (blue) were markedly increased in the glucose treatment group. Agmatine treatment were reduced the β-galactosidase positive cells in spite of the high glucose condition. Data were expressed as mean±S.E.M, and each experiment included 3 repeats per condition. Differences were considered significant at *p<0.05, **p<0.001. Glucose: glucose 200 mM treatment group, Agm: agmatine 100 µM treatment group, Agm+ Glucose: agmatine 100 µM and glucose 200 mM treatment group, senescence associated β-galactosidase (β-Gal): blue.
Agmatine prevents the expression of pro-inflammatory cytokines in high glucose exposed N2A cells
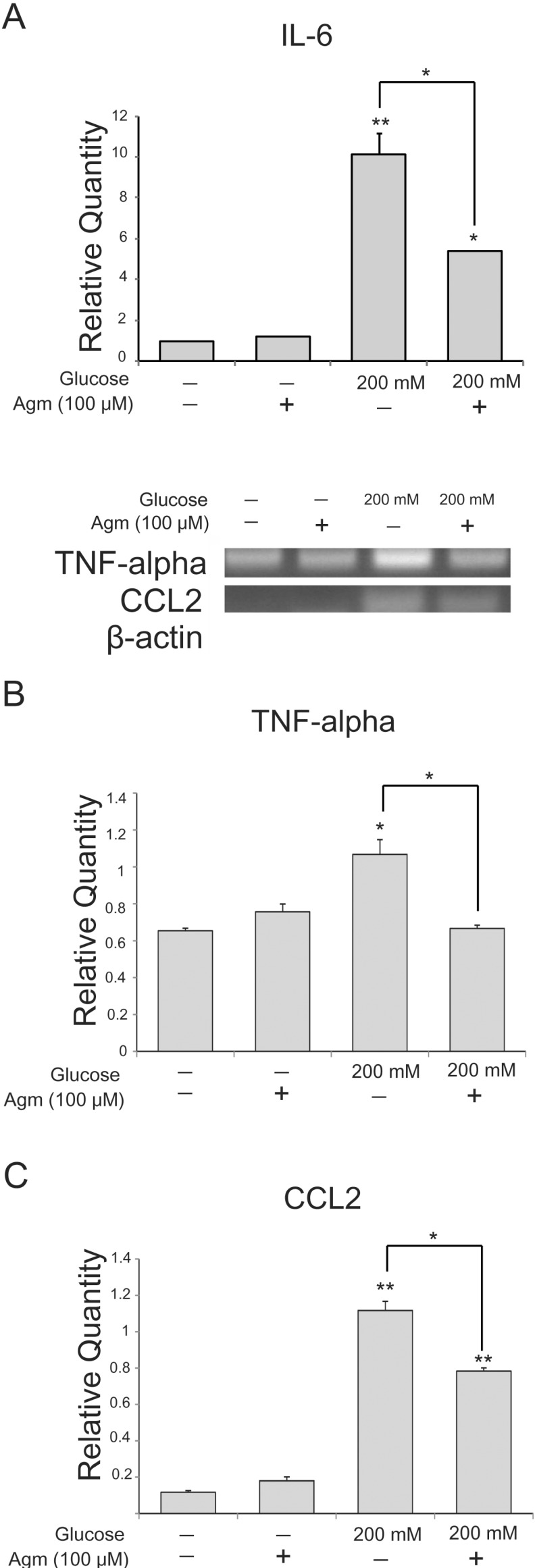
To estimate the alteration of inflammatory cytokine expression in neuronal cells exposed to high glucose conditions, we conducted real-time PCR (Fig. 3A) and reverse transcription PCR (Fig. 3B and 3C). Under high glucose condition, the mRNA expression of pro-inflammatory cytokines such as IL-6 (Fig. 3A), TNF-alpha (Fig. 3B), and CCL2 (Fig. 3C) was increased. However, agmatine treatment reduced the high glucose-induced increase in IL-6 (Fig. 3A), TNF-alpha (Fig. 3B), and CCL2 (Fig. 3C) mRNA levels compared to neuronal cells exposed to high glucose condition alone. Our results showed that increased expression of pro-inflammatory cytokines caused by high glucose was reduced by agmatine.
Fig. 3. The measurement of pro-inflammatory cytokines in N2A cells under high glucose condition and agmatine treatment. (A) Pro-inflammatory cytokine IL-6 mRNA level was measured by using quantitative real time-PCR. In glucose 200 µM group, mRNA level of IL-6 was increased compared to the normal control group whereas the agmatine treatment groups showed lesser mRNA levels of IL-6 in spite of the high glucose toxicity. (B) TNF-alpha mRNA level was measured by using reverse transcription-PCR. In high glucose group, mRNA level of TNF-alpha was increased compared to the normal control group. The agmatine treatment group showed lesser mRNA level of TNF-alpha in spite of the high glucose toxic condition. (C) CCL2 mRNA level was measured by using reverse transcription-PCR. In high glucose group, mRNA level of CCL2 was increased compared to the normal control group. The agmatine treatment group showed lesser mRNA level of CCL2 in spite of the high glucose toxic condition. Data were expressed as mean±S.E.M, and each experiment included 4 repeats per condition. beta-actin was used as an internal control. Differences were considered significant at *p<0.05, **p<0.001. IL-6: interleukin-6, CCL2: chemokine (C-C motif) ligand 2, TNF-alpha: tumor suppressor factor-alpha.
Agmatine alleviates the p21 mediated pathway in neuronal cells exposed to high glucose condition
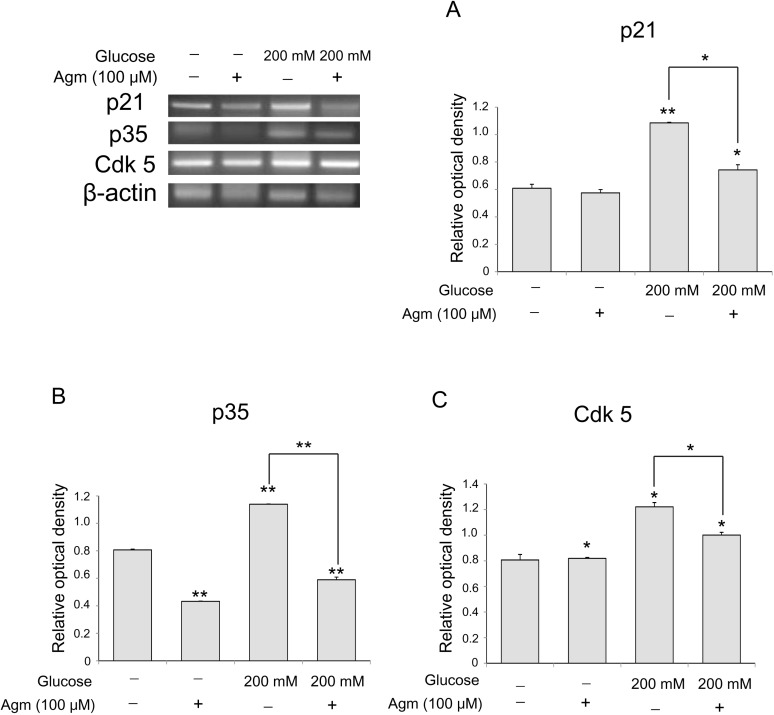
To investigate p21 signaling related to cellular senescence in neuronal cells under the high glucose condition, we performed reverse transcription PCR (Fig. 4). In the high glucose condition, mRNA levels of p21 (Fig. 4A) increased with regard to cellular senescence in comparison with the normal control group (Fig. 4A). Agmatine treatment reduced the increase in mRNA levels of p21 in the high glucose-exposed N2A cells (Fig. 4A). Moreover, the mRNA levels of p35 (Fig. 3B), Cdk 5 (Fig. 4C), were significantly increased in the high glucose condition, and were attenuated by agmatine treatment (Fig. 4B and 4C). In this regard, agmatine may inhibit neuronal cell senescence via p21-mediated cell senescence signaling in hyperglycemia.
Fig. 4. The measurement of p21, p35, Cdk 5 level in N2A cells under high glucose condition and agmatine treatment. (A) p21 mRNA level was measured by using reverse transcription-PCR. In high glucose group, mRNA level of p21 was increased compared to the normal control group. The agmatine treatment group showed lesser mRNA levels of p21 in spite of the high glucose toxic condition. (B) p35 mRNA level was measured by using reverse transcription-PCR. In high glucose group, mRNA level of p35 was increased compared to the normal control group. The agmatine treatment group showed lesser mRNA level of Cyc p35 in spite of the high glucose toxic condition. (C) Cdk 5 mRNA level was measured by using reverse transcription-PCR. In high glucose group, mRNA level of Cdk 5 was increased compared to the normal control group. The agmatine treatment group showed lesser mRNA level of Cdk 5 in spite of the high glucose toxic condition. Data were expressed as mean±S.E.M, and each experiment included 3 repeats per condition. β-actin was used as an internal control. Differences were considered significant at *p<0.05, *p<0.001.
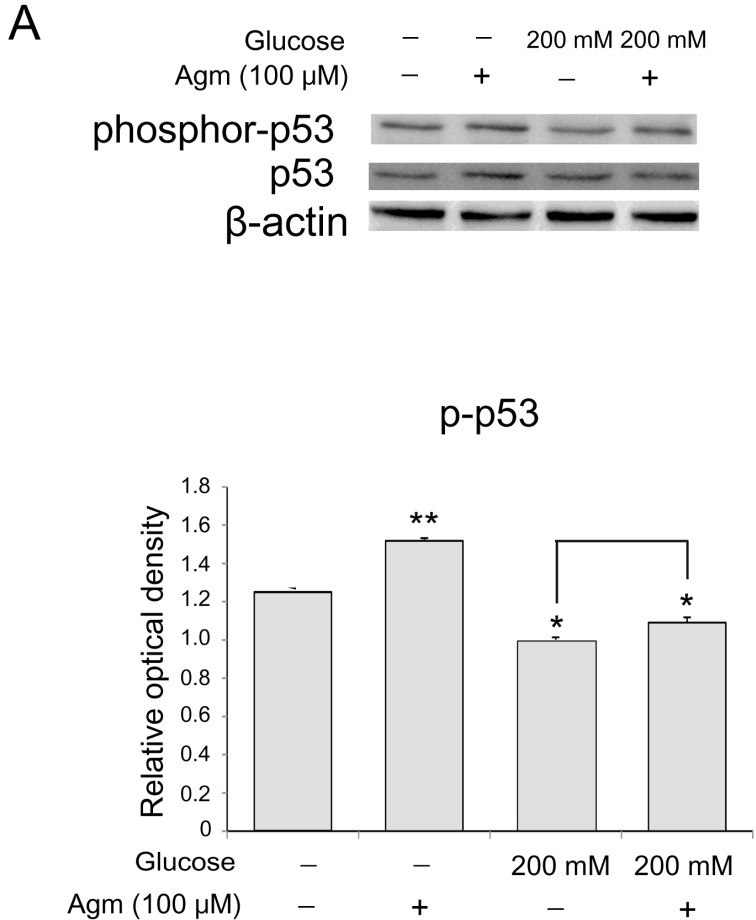
Agmatine inhibits activation of p53 in neuronal cells exposed to high glucose condition
To assess the phosphorylation of p53 in N2A cells, we checked the protein level of phosphorylated p53 using western blotting. Agmatine results in the increase of p53 phosphorylation (Fig. 5C) with regard to cellular senescence in comparison with the normal control group (Fig. 5C). In high glucose condition, agmatine increased phosphorylation of p53 in the N2A cells (Fig. 5C). Taken together, agmatine may inhibit neuronal cell senescence by activating p53 in hyperglycemia.
Fig. 5. The measurement of phosphorylation of p53 level in N2A cells under high glucose condition and agmatine treatment. (A, B) p53 phosphorylation levels were measured by using western blotting. In agmatine treatment group, the protein level of p-p53 was increased compared to the normal control group. The high glucose injured group showed lesser protein levels of p-p53 than normal control group. In high glucose condition, agmatine treatment increased the phosphorylation of p53 in the cells. Data were expressed as mean±S.E.M, and each experiment included 3 repeats per condition. beta-actin was used as an internal control. Differences were considered significant at *p<0.05, **p<0.001.
DISCUSSION
Hyperglycemia leads to cell death, oxidative stress and cellular senescence, which is defined as an irreversible cessation of mitosis via p21-dependent signaling that occurs in the early stages of diabetic nephropathy [11]. In the cell, caspase 3 activation showed the cell death in response to the stress. In the present study, decrease of cleaved caspase 3 expression in high glucose conditioned N2A cells by agmatine indicates that agmatine may rescue the cells against the high glucose-induced cell death (Fig. 1C). Several researches demonstrated that the cellular senescence is commonly characterized by high β-galactosidase activity [22]. In many researches, β-galactosidase activity has been used as the important marker of cell senescence in vitro and in vivo. In present study, we found that cells were attenuated the level of β-galactosidase under high glucose condition (Fig. 2). In counterpoint to the high glucose group, agmatine treatment in high glucose condition group evidently attenuated the increase β-galactosidase activity caused by high glucose stress. Because cellular senescence have been reported previously as the reduction of telomere length and increased expression of cyclin-dependent kinase (CDK) inhibitors such as p21. The CDK inhibitor p21 is essential to arrest cells in G1 after cellular damage [23]. Several studies showed that p21 results in the increased expression of genes associated with lysosome enzymes and mitochondrial proteins, and this is involved in the induction of senescence [24]. As byproducts of cellular oxidative processes, ROS have been shown to be markers of cell senescence. p21 has been reported that it is involved in the ROS generation, leading to the aggravation of cellular senescence [10]. Increased expression of p21 leads to increased production of ROS in cells and subsequently, senescence. Based on our results, decrease of p21 could be linked to the reduction of both cell senescence and ROS generation by agmatine. Moreover, decrease of p35, Cdk 5 known as related with p21-mediated cellular senescence [12] by agmatine in high glucose condition supports our hypothesis that agmatine could alleviate the p21 mediated cell senescence signaling [11].
Neuronal senescence results in cytokine dysregulation with an increase of pro-inflammatory cytokines and reduction of anti-inflammatory cytokines, leading to neurodegenerative diseases. Increased levels of TNF-alpha, IL-1, and IL-6 aggravate the process of neurodegenerative pathology [5]. Some studies demonstrated that cell senescence via the p21 pathway causes an increase in pro-inflammatory cytokine levels in patients [5]. Specifically, pro-inflammatory cytokines TNF-α [25], IL-6 [26], and CCL2 [27] have been shown to increase excitotoxic neuronal damage. In present study, our results assume that agmatine may attenuate the high glucose-induced increase of pro-inflammatory cytokines such as IL-6 (Fig. 3A), TNF-alpha (Fig. 3B), and CCL2 (Fig. 3C) through inhibition of p21-mediated signaling, suggesting that these pro-inflammatory cytokines were regulated by the p21 pathway.
The activation of p53 has been reported that it could promote cell cycle arrest and DNA repair [7]. In addition, p53 activates numerous anti-oxidant genes such as GPX1, ALDH4, Acad11, TIGAR, and TP53INP1 related to the inhibition of ROS accumulation and cell survival [28]. p53 also has known as the regulator of the autophagy pathway and the booster of cell survival and protects cells from metabolic stress [29]. Current study suggested that spermine among amines like agmatine could promote the activity of autophagy in cells through the activation of p53 [30].
Consequently, in present study, our results indicate that agmatine alleviates the neuronal cell senescence by high glucose by downregulaing p21 and by activating p53.
Thus, in accordance with our findings, we propose that further research is needed to elucidate that the effect of agmatine on the p21 and p53-medaiated signaling, which may serve as a target for the development of promising therapeutic strategies in diabetes-induced neuropathy.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea. (HI14C2173).
Footnotes
Author contributions: Juhyun Song conducted the experiments, and wrote the preliminary draft and wrote the manuscript. Somang Kang conducted the experiments and finished the manuscript. Byeori Lee and Yumi Oh conducted the experiments, and helped discussing the design of the study. Eosu Kim, Chul-Hoon Kim and Ho-Take Song revised the manuscript. Jong Eun Lee revised the manuscript, and provided overall supervision for the project.
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 3.Smith-West C, Garris DR. Diabetes-associated hypothalamic neuronal depopulation in the aging Chinese hamster. Brain Res. 1983;285:385–389. doi: 10.1016/0165-3806(83)90036-6. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 6.Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A, Chouaid C, Dubois-Rande JL, Damotte D, Adnot S. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011;109:543–553. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui X, Han W, Pan H. p53-induced autophagy and senescence. Oncotarget. 2015;6:11723–11724. doi: 10.18632/oncotarget.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein I, Yizhak K, Madar S, Goldfinger N, Ruppin E, Rotter V. p53 promotes the expression of gluconeogenesisrelated genes and enhances hepatic glucose production. Cancer Metab. 2013;1:9. doi: 10.1186/2049-3002-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, Tulchinsky E, Jones GD, Roninson IB, Macip S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem. 2012;287:9845–9854. doi: 10.1074/jbc.M111.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications. 2014;28:604–611. doi: 10.1016/j.jdiacomp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao D, Hinds PW. p35 is required for CDK5 activation in cellular senescence. J Biol Chem. 2010;285:14671–14680. doi: 10.1074/jbc.M109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 14.Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:PL 41–PL 46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 15.Song J, Hur BE, Bokara KK, Yang W, Cho HJ, Park KA, Lee WT, Lee KM, Lee JE. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocininduced Alzheimer rat model. Yonsei Med J. 2014;55:689–699. doi: 10.3349/ymj.2014.55.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilad GM, Gilad VH, Finberg JP, Rabey JM. Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity. Neurochem Res. 2005;30:713–719. doi: 10.1007/s11064-005-6865-9. [DOI] [PubMed] [Google Scholar]
- 17.Cui H, Lee JH, Kim JY, Koo BN, Lee JE. The neuroprotective effect of agmatine after focal cerebral ischemia in diabetic rats. J Neurosurg Anesthesiol. 2012;24:39–50. doi: 10.1097/ANA.0b013e318235af18. [DOI] [PubMed] [Google Scholar]
- 18.Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA, Lee JE. Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev. 2011;20:527–537. doi: 10.1089/scd.2010.0312. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Li B, Zheng Z, Kang T, Zeng M, Liu Y, Xia B. Protective effects of Notch1 signaling activation against high glucose-induced myocardial cell injury: analysis of its mechanisms of action. Int J Mol Med. 2015;36:897–903. doi: 10.3892/ijmm.2015.2294. [DOI] [PubMed] [Google Scholar]
- 20.Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 21.Burn SF. Detection of β-galactosidase activity: X-gal staining. Methods Mol Biol. 2012;886:241–250. doi: 10.1007/978-1-61779-851-1_21. [DOI] [PubMed] [Google Scholar]
- 22.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 23.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 24.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci U S A. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA. p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol. 2012;181:1306–1315. doi: 10.1016/j.ajpath.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volonte D, Zou H, Bartholomew JN, Liu Z, Morel PA, Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) J Biol Chem. 2015;290:4202–4214. doi: 10.1074/jbc.M114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang X, Asano M, O'Reilly A, Farquhar A, Yang Y, Amar S. p53 is an important regulator of CCL2 gene expression. Curr Mol Med. 2012;12:929–943. doi: 10.2174/156652412802480844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 29.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 30.Chae YB, Kim MM. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int J Biol Macromol. 2014;63:56–63. doi: 10.1016/j.ijbiomac.2013.10.041. [DOI] [PubMed] [Google Scholar]