Abstract
Background
Trypanosoma brucei is a eukaryotic pathogen which causes African trypanosomiasis. It is notable for its variant surface glycoprotein (VSG) coat, which undergoes antigenic variation enabled by a large suite of VSG pseudogenes, allowing for persistent evasion of host adaptive immunity. While Trypanosoma brucei rhodesiense (Tbr) and T. b gambiense (Tbg) are human infective, related T. b. brucei (Tbb) is cleared by human sera. A single gene, the Serum Resistance Associated (SRA) gene, confers Tbr its human infectivity phenotype. Potential genetic recombination of this gene between Tbr and non-human infective Tbb strains has significant epidemiological consequences for Human African Trypanosomiasis outbreaks.
Results
Using long and short read whole genome sequencing, we generated a hybrid de novo assembly of a Tbr strain, producing 4,210 scaffolds totaling approximately 38.8 megabases, which comprise a significant proportion of the Tbr genome, and thus represents a valuable tool for a comparative genomics analyses among human and non-human infective T. brucei and future complete genome assembly. We detected 5,970 putative genes, of which two, an alcohol oxidoreductase and a pentatricopeptide repeat-containing protein, were members of gene families common to all T. brucei subspecies, but variants specific to the Tbr strain sequenced in this study. Our findings confirmed the extremely high level of genomic similarity between the two parasite subspecies found in other studies.
Conclusions
We confirm at the whole genome level high similarity between the two Tbb and Tbr strains studied. The discovery of extremely minor genomic differentiation between Tbb and Tbr suggests that the transference of the SRA gene via genetic recombination could potentially result in novel human infective strains, thus all genetic backgrounds of T. brucei should be considered potentially human infective in regions where Tbr is prevalent.
Introduction
African trypanosomiasis is a disease of humans and livestock in sub-Saharan Africa caused by protozoan parasites of the Trypanosoma brucei complex, which are transmitted between mammalian hosts by their tsetse fly (Glossina sp.) vector [1]. Human-infective members of the Trypanosoma brucei complex are the causative agents of Human African Trypanosomiasis (HAT), or sleeping sickness [2]. T. b. rhodesiense (Tbr) causes an acute form of HAT in eastern Africa, characterized by punctuated outbreaks in discrete disease foci [3], while T. b. gambiense (Tbg) causes a chronic form of the disease in western and central Africa and accounts for over 95% of reported cases [4]. T. b. brucei (Tbb), is not infective to humans, but, together with other animal trypanosome species, causes the livestock wasting disease, Nagana, across a range that overlaps with that of the human-infective parasites [2]. According to recent estimates from the World Health Organization, 50 million people in Africa are at risk of acquiring sleeping sickness. Although the number of new HAT cases has recently fallen below 10,000 for the first time in decades, the disease has a long history of cyclical emergence patterns [5]. This, coupled with the lack of a vaccine against HAT and high toxicity of late stage drug treatments [6], poses a significant challenge to the proposed goal of eliminating HAT as a public health problem by 2020 [7].
Two complete genome assemblies exist for one strain each of two of the three subspecies within the T. brucei group, Tbb [8] and Tbg [9]. A comparison of these genomes has revealed that, despite the substantial difference in disease caused by them, they are very similar at a genomic level—with 99.2% of sequence identity in coding regions and only a single oxidoreductase gene present in Tbb but not in Tbg [9]. Population level genomic comparison of 39 isolates sampled across the three named subspecies within the T. brucei group (Tbb, Tbr and Tbg) confirms a high degree of similarity, with only 2.33% of nucleotides being variable across the group, and no fixed SNP differences between them [10]. This genome wide analysis also confirms previous microsatellite data [11–15] that suggested that, while Tbg strains are genetically distinct from Tbb/Tbr, these strains are indistinguishable from one another [10]. Additionally, shared heterozygosity between a Tbb and a Tbr strain at the genomic scale [16] strongly suggests that horizontal transfer between the two subspecies occurs in the field. Conversely, a study of 7 microsatellite loci did not find evidence of gene flow between Tbb and Tbr [17]. This finding is in contradiction with numerous other population level studies that show that sympatric strains of Tbb and Tbr are more closely related to each other than to allopatric strains from the same named taxon [11–15]. This apparent contradiction is potentially due to the use of a small number of makers characterized by low diversity leading to limited ability to detect gene flow, rather than genuine reproductive isolation.
The ability of T. brucei to evade mammalian host adaptive immune response is through remarkable antigenic variation of its VSG coat, enabled by a suite of non-expressed VSG genes largely located in subtelomeric cassettes and on a variable number of small to intermediate sized chromosomes [8]. It is thought that replacement of expressed VSG genes with novel copies through ectopic recombination allows for the expression of a novel protein coat approximately once every 100 cell doublings during clonal replication [18]. ESAGs are co-transcribed with VSG genes, and pseudogenic copies are prevalent in sub-telomeric VSG arrays [19]. While the function of ESAG3 is not explicitly known, ESAGs are involved in recombination driven antigenic variation [20].
Despite high relative variability in the VSG and ESAG regions of the T. brucei genome, the number of genomic differences between subspeciess is remarkably low [9,21]. However, there are critical functional differences between T. brucei subspecies—specifically the ability of the human infective forms to evade human innate resistance via the action of the trypanosome lytic factors (TLF) present in the serum. Both Tbg and Tbr have independently evolved distinct mechanisms to evade the human immune system. Tbg evades lysis by TLF through a modified haptoglobin-haemoglobin (HpHbr) receptor and through the presence of a specific, truncated VSG (TgsGP) [22,23] that allows for reduced uptake and efficacy of TLF [19,24]. In Tbr, another truncated VSG (SRA) prevents cell lysis by binding to the TLF protein apolipoprotein L-1 (ApoL-1), the trypanolytic component of TLF [3,25]. Heterogeneous expression of SRA in previously susceptible Tbb strains renders them resistant to lysis by human serum [25]. Interestingly, no other genetic differences between the two subspecies are known and in laboratory tests Tbr and Tbb can sexually recombine in the tsetse fly vector to produce viable, recombinant offspring [26–28]. The possibility that the SRA gene is the only differentiating feature between Tbb and Tbr subspecies indicates that, if recombination occurs in wild populations of T. brucei, Tbb strains which are currently un-infective in humans, could potentially acquire SRA via genetic recombination, thus becoming infective [4,28]. This has significant epidemiological implications for at least two reasons: (1) As Rhodesian HAT is characterized by temporally and geographically localized outbreak foci [3], they may arise from recombination with previously un-infective Tbb strains, and thus not necessarily require Tbr movement between disease foci; (2) all Tbb genetic backgrounds must be considered potentially infective, when trying to predict and control outbreaks of Rhodesian HAT.
Although assembled and annotated genomes of Tbb and Tbg exist [8,9], it is not currently known if the SRA gene is the only gene specific to Tbr, because a Tbr genome is not yet available. Moreover, previous genomic studies of isolates from all three subspecies were based on short-read Illumina data aligned against the published Tbb genome, thus impeding our ability to detect Tbr specific variants not found in the Tbb genome. In the current study we produced a Tbr hybrid de novo assembly taking advantage of long-read Pacific Biosciences and short-read, high throughput Illumina sequence data. We used these sequences to extract putative genes from the Tbr genome to compare with the existing Tbb and Tbg genomes [8,9] to determine if any are only found in the Tbr genome. If genes specific to Tbr other than SRA are present, it would suggest that functions other than that conferred by the SRA gene are involved in the life history and disease type differences associated with Tbr. If no unique genes other than SRA are discovered, SRA gene is likely to be solely responsible for the human infectivity of Tbr. This would further suggest that any Tbb strain from independent evolutionary backgrounds could become human infective upon acquiring the SRA gene through horizontal transfer events.
Results and Discussion
Sequencing and Assembly
Short read high throughput sequencing of the STIB900 Tbr strain produced 48,975,696 individual reads for an expected coverage based on the TREU 927/4 Tbb genome of approximately 122x. Long read high throughput sequencing produced a total of 570,319 sequences. Read length of the Pacific Biosciences long read sequencing ranged from 116–9,729 bp (S1 Fig). Hybrid de novo assembly of the data resulted in 4,210 individual scaffolds ranging in length from 1,256–243,494 bp (S1 Appendix). The total number of base pairs included in scaffolds was 38,771,836. The number of reads vs read length and number of scaffolds vs scaffold length is shown in S1 Fig.
Given that the shortest of the 11 megabase (mb) chromosomes of the Tbb nuclear genome is approximately 1.1mb in length [8], the hybrid de novo assembly method we implemented was unable to recover full-length sequences of these chromosomes. This is probably in part due to the fact that the T. brucei genome is known to be extremely repetitive [8,9], presenting significant challenges to current methodologies for genome assembly. However, a promising result from our data is that the total length of our scaffolds (38.8mb) is considerably longer than the annotated Tbb genome (30.2mb), suggesting that the scaffolds produced in this study comprise a significant proportion of the Tbr genome, and thus are a valuable tool for a future complete genome assembly. However, the highly repetitive nature of the VSG subtelomeric libraries, which can comprise up to 30% of the T. brucei genome [8], makes these regions inherently difficult to accurately assemble, meaning that additional curation and resequencing is likely to be necessary to accurately construct them.
Detection of Novel Genes
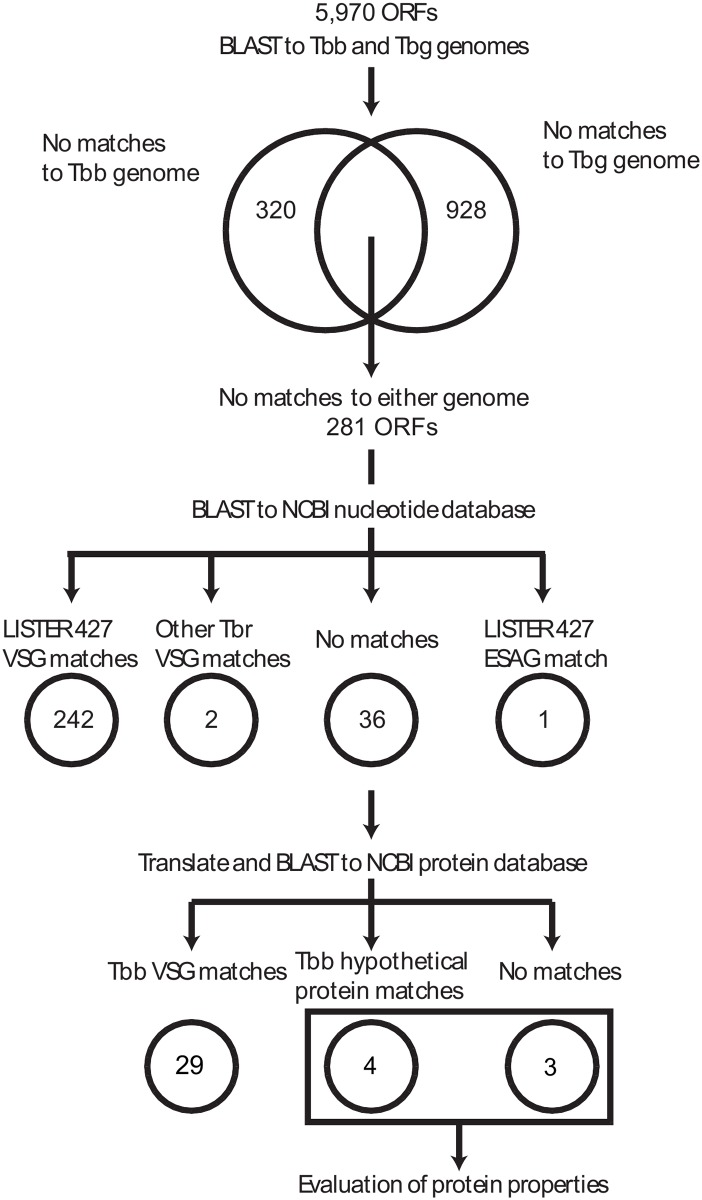
To look for Tbr genes not found in the published Tbb or Tbg genomes we focused our analyses on the longest scaffolds (1,817 scaffolds >5000bp in length), which comprised 30.8mb of the Tbr genome. We detected a total of 5,970 open reading frames (ORFs) >1,000bp in length (S2 Appendix), which are likely to represent a significant portion of the Tbr genes, given that the number of genes in the Tbb genome is 9,898 [8]. In support of this we found that 85.9% of reads mapped to the Tbb genome [8] and that they were evenly distributed across the 11 chromosomes of the genome (S3 Appendix). The BLAST [29] searches and progressive filtering steps from the initial 5,970 ORFs to the final Tbr specific putative genes are summarized in Fig 1. We found 320 and 928 ORFs that did not have a match in the Tbb TREU 927/4 and Tbg DAL 972 genomes, respectively, confirming the higher level of similarity of Tbr with Tbb than Tbg found in previous studies [10–12]. Interestingly, we also found 281 ORFs in the Tbr strain we sequenced with no match to either the published Tbb or the Tbg genomes. When these were compared to the Genbank nucleotide database, 242 ORFs matched variant surface glycoprotein (VSG) pseudogenes from the well-studied LISTER 427 Tbb strain [30] and 2 ORFs matched VSG genes from clones from other Tbr strains. This complements significant efforts that have been made the characterize VSG variation across T. brucei [20,31] which has demonstrated that the majority of subspecies specificity in T. brucei lies in these gene regions, such that these 242 ORFs did not match to either reference genome, but did match to the LISTER 427 Tbb strain. It is also possible that despite painstaking efforts to characterize VSG cassettes in the T. brucei genome [20,31] the assembly of VSG cassettes in the annotated genomes of Tbb and Tbg are incomplete due to the inherent difficulties in assembling these highly repetitive genomic regions, thus not allowing for an exhaustive comparison of the three subspecies. We also found one ORF that matched to a LISTER 427 expression site associated gene (ESAG) pseudogene—ESAG3. Of the remaining 36 ORFs, which did not have a nucleotide match in the Tbb or Tbg genomes, 29 still matched to VSG genes, when translated into amino acid sequences and searched against the NCBI protein database. The accumulation of synonymous substitutions in these genes was probably sufficient to prevent a nucleotide, but not a protein match. This result implies functional conservation across the Tbr VSG library and also suggests a potential role for purifying selection operating on VSG arrays. Our analyses also revealed that over 96% of ORFs, which did not match to either the Tbb or Tbr genomes, were matches to VSG pseudogenes.
Fig 1. Flow chart documenting the series of BLAST searches and filtering leading from the initial 5,970 ORFs detected from the de novo assembly of the STIB900 T. b. rhodesiense genome to the final two genes specific to this Tbr strain.
Of note is the high number of variant surface glycoprotein (VSG) genes, which show substantial specificity to the T. b. rhodesiense strain and were detected at each level of filtering.
Four of the seven remaining ORFs (ORF 4–7, Table 1) have confident (>99% identity, 100% coverage) matches to hypothetical proteins present in the Tbb genome in the NCBI protein database. This implies that these genes are likely to be orthologous to coding genes in the Tbb genome, but with enough synonymous variation to prevent a confident nucleotide level match in either Tbb or Tbg. The remaining three ORFs (ORFs 1–3, Table 1) did not have matches in the NCBI protein database, and appear to represent putatively novel genes specific to the STIB900 Tbr genome. The first two of these three ORFs (ORFs 1 and 2, Table 1) are identical at the amino acid level, indicating multiple copies of this putative gene in the Tbr genome.
Table 1. Summary of statistics calculated, putative function and expression levels for genes of unknown function with no nucleotide level matches outside of the STIB900 Tbr genome.
A) Statistics reported include length in nucleotides, Gravy Index (GI), Instability index (II), Isoelectric Point (IP), number of coiled coils (CC), longest disorder region in amino acids (DR), Percentage of coil structure (CS), number of trans-membrane helices (TH), number of signal peptides (SP), Insertion score (IS), number of homologs in the NCBI non-redundant protein database (HNr), number of homologs in the RCSB protein database (HPDB). B) Putative function is listed, as determined using a meta-prediction search and the method used to determine putative function.
| A | B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF | Length | GI | II | IE | CC | DR | CS | TH | SP | IS | HNr | HPDB | Putative function | Source |
| 1 | 1749 | -0.5 | 53 | 9 | 0 | 11 | 26 | No | No | 0 | 1012 | 40 | pentatricopeptide repeat-containing protein | Hhsearch |
| 2 | 1749 | -0.5 | 53 | 9 | 0 | 11 | 26 | No | No | 0 | 1012 | 40 | pentatricopeptide repeat-containing protein | HHsearch |
| 3 | 1038 | 0.3 | 54 | 6 | 0 | 25 | 30 | 1 | 39 | - | 2 | 0 | Alcohol Oxidoreductase | EzyPred |
| 4 | 1452 | -0.3 | 58 | 6 | 0 | 73 | 45 | No | No | - | 2 | 0 | Hydrolase acting on ester bonds | EzyPred |
| 5 | 2799 | -0.3 | 53 | 8 | 0 | 35 | 51 | No | No | 0 | 15 | 0 | Hydrolase acting on ester bonds | EzyPred |
| 6 | 1251 | 0.2 | 28 | 8 | 0 | 54 | 35 | 10 | No | 0 | 810 | 0 | Solute carrier family 35 | Homolog in SWISS-PROT Database |
| 7 | 1530 | -0.6 | 61 | 6 | 21 | 115 | 47 | No | No | - | 1 | 0 | None detected |
Structure and Function of Novel Genes
We used the Xtalpred RF server [32] to investigate the biochemical and biophysical properties of the seven genes with unknown functions (ORFs 1–7). Table 1 shows for each of these ORFs the gravy and instability indices, isoelectric points, numbers of coiled coils, longest disorder region in amino acids, percentages of coil structure, numbers of trans-membrane helices, numbers of signal peptides, insertion scores, and numbers of homologs in the NCBI non-redundant protein database and in the RCSB protein database. We used Meta-Server for protein sequence analysis (MESSA) [33] to predict their function. As four ORFs have close homologs in the Tbb genome (ORFs 4–7, Table 1), we focused on the putative function of the remaining three ORFs for which no match in the Tbr/Tbg genomes was found. The results of this analysis suggest that the ORFs 1 and 2 encode a pentatricopeptide repeat-containing (PPR) protein, a family of genes critical in facilitating mitochondrial translation in trypanosomes [34], while ORF 3 encodes a putative alcohol oxidoreductase, an enzyme involved in alcohol metabolism in many organisms and implicated in drug resistance in Trypanosoma cruzi [35]. Of note is the fact that the single gene present in the published Tbb genome and absent from the Tbg genome is also an oxidoreductase gene [9]. This would suggest that while the conservation of oxidoreductase genes is characteristic of African trypanosomes [36], some specificity of function of oxidoreductase in certain strains may exist. It should be noted that translocation may explain the appearance of these seemingly novel gene variants—of note is observed high rates of translocation in PPR genes in plant genomes [37]. Possible translocation of these genes is warrants further analysis pending a more complete Tbr genome assembly.
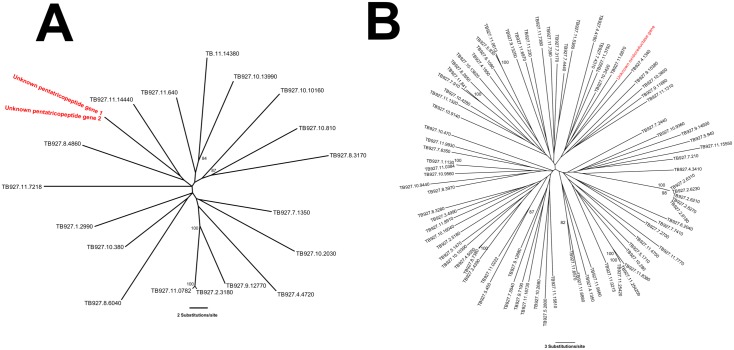
We carried out phylogenetic analyses using RaxMLv7.7.6 [38] to: (1) clarify the evolutionary relationships between the ORFs 1–3 (Table 1) and their respective homologs in the Tbr genome, and (2) demonstrate that these ORFs are related to, but not identical to other PTP repeat containing and oxidoreductase proteins in the Tbb and Tbg genomes, respectively. Fig 2 shows the results for the two gene families. Both trees have low bootstrap support values on several nodes, limiting interpretations on the specific relationships among the different genes. This is likely due to relatively high levels of sequence divergence amongst genes causing phylogenetic saturation in the alignments. Nevertheless, regardless of the weakness of most topological relationships, this analysis clearly shows that ORF 1–3 are related but not identical to genes with similar functions in the Tbb genome. This further supports the possibility that these three ORFs, while members of the above mentioned gene families, may be Tbr specific variants. This possibility is also strengthened by considering the alignment of the flanking regions of each ORF to the Tbb and Tbg genomes. As the first two ORFs are located on a single scaffold, we aligned the regions of the scaffold before, between, and after the two ORFs. These regions all aligned to the first chromosome of the Tbb and Tbg genomes between 321,685–328,598bp, demonstrating that the flanking regions overlap with no ORF between them (S2 and S3 Figs, S4 Appendix). However, it is important to also note that this alignment is characterized by low pairwise identity (46.4%), suggesting that it may be misaligned. This could be due to the repetitive nature of the ORFs and flanking regions, which may be syntenic with a poorly assembled repetitive region of the Tbb/Tbg genome, or potentially the entire region is specific to Tbr. The region before and after ORF 3 aligned to chromosome 9 of the Tbb and Tbg genomes between 1,929,544–1,935,562bp. The flanking regions do not overlap, however the gap between them is 313bp—considerably shorter than the ORF (1,038bp), suggesting that the ORF is not present in either the Tbb or Tbg genome.
Fig 2. Phylogenies of A) ORF 1–2 with annotated pentatricopeptide repeat-containing protein genes in the TREU927/4 Tbb genome; and B) ORF3 with oxidoreductase genes in the TREU927/4 Tbb genome.
These phylogenies confirm that these Tbr ORFs are related to members of these two gene families, but are phylogenetically distinct variants specific to the STIB900 Tbr strain. The unknown ORFs detected in our study are shown in red, known genes are shown in black, encoded using their TriTrypDB [36] database names. Bootstrap support values are shown for nodes with support >70.
Epidemiological and Evolutionary Implications
The de novo approach presented here provides the final proof of the genetic similarity between Tbb and Tbr, which was suggested by previous studies based on a microsatellite [11–15] and genomic comparisons [10,16]. The important implications of this result are at least twofold. First, considering Tbb and Tbr separate subspecies, although accepted in epidemiological practice, is misleading, because of the implicit assertion that taxonomic designation reflects independent evolutionary trajectories [28,39]. Second, this finding implies that all Tbb strains circulating in T. brucei non-human host have the potential to acquire the SRA gene and thus become human infective. Admittedly, given that recombination can only happen in the tsetse salivary glands, the likelihood of this happening frequently is relatively low, depending on how often tsetse flies are infected with both subspecies. However, since we have evidence of gene exchanges among sympatric Tbb and Tbr subspecies [11,15], this must have occurred over evolutionary times. Thus, this possibility and its epidemiological implications cannot be dismissed, as it suggests that epidemiological studies and control efforts would be significantly aided by a population scale analysis of the rate of gene flow between Tbb and Tbr subspecies in wild populations.
The investigation of almost 6,000 ORFs in the STIB900 Tbr strain reveals that only three 3 genes (ORF 1–3, Table 1) aside from the SRA gene are putatively specific to Tbr. This supports the previous suggestion that Tbb, Tbg, and Tbr are genetically highly similar [10–12,14] and that their observed differences in life history traits and disease outcomes are due to variation in genes present in all of them [9]. The fact that even the three genes (ORFS 1–3) found only in Tbr strain used in this study are members of gene families known to be abundant in trypanosomatids [9,34] further supports this point. Although these three ORFs seem to be Tbr specific, their role in directly facilitating human host infections is unclear, given that multiple copies of these genes are also found in Tbb. A similar pattern could also have been generated from convergent selection pressures due to similar selection regimes from exposure to the same host after independent strains of Tbb infected humans, following acquisition of the SRA gene. Moreover, as our analyses was based on the comparison of only one strain each for Tbb, Tbg, and Tbr, we cannot conclusively state that they are Tbr specific, as multiple strains for each subspecies from different geographic locations are necessary to test this. However, given the data we have so far, it remains plausible that acquisition of SRA is the only event required to allow a previously non-zoonotic Tbb strain to become human infective. Nevertheless, the finding of several VSG related ORFs and a few novel genes that appear to be Tbr specific suggest further research directions to better understand both their functional significance and evolutionary origin, as this may yield important insights for the development of novel treatments for Rhodesian HAT.
Methods
Sequencing and Assembly
We extracted DNA from a Tbr isolate (STIB900) from cryobanks at the Swiss Tropical and Public Health Institute, Basel. This strain was isolated from a patient in Ifakara, Tanzania in 1982 and had undergone minimal laboratory passaging. The presence of the SRA gene and thus as Tbr via PCR using the protocols outlined in [40]. Fragmentation and library preparation for both short and long read sequencing was carried out at the Yale Center for Genome Analysis (YCGA). Short read library preparation was conducted using an Illumina Paired-End DNA Sample Prep Kit (Illumina Inc., USA) and paired end (2x75bp) sequencing performed using the Illumina HiSeq 2000 platform. Quality control of reads was done using FastQC [41]. Long read library preparation was conducted using a Pacific Biosciences DNA template prep kit (Pacific Biosciences, USA), and 16 cells of Single Molecule, Real-Time (SMRT) sequence data were produced using a Pacific Biosciences RS II sequencer (YCGA).
We used the two-step PBcR (PacBio corrected reads) error correction and de novo assembly process described in Koren et al. [42]. This process, implemented in the Celera Assembler, trims and corrects individual long read sequences from Pacific Biosciences sequencing by mapping short read sequences from the Illumina platform to them to produce highly accurate, long read sequence for de novo assembly. De novo assembly was conducted using the Celera Assembler [43] using the default settings for long reads.
Detection of open reading frames and blast search strategy
Scaffolds >5000 base pairs (bp) in length were imported into Geneiousv6.05 [35] to detect ORFs of at least 1000 bp—representing putative genes in the Tbr genome. ORFs were exported from Geneious and BLASTv2.27 [29] was used to detect matches to both the TREU 927/4 Tbb genome [8] and the DAL972 Tbg genome [9]. For all BLAST searches, we used an e-value of 1−5, a minimum length of 800bp, reporting only the best match for each ORF. Additionally, a BLAST search of the NCBI nucleotide database was conducted for ORFs for which no matches were found in either the Tbb or Tbg genomes. This was followed by a BLAST search against the NCBI protein database of the ORFs with no nucleotide match after translating then into amino acid sequences to identify potential functional matches. In order to confirm representative coverage of the genome, we aligned the detected ORFs to the Tbb genome [8] using BWAv0.7.12 [44]. Coverage for each chromosome was evaluated using Geneiousv6.05 [45] and detailed results for each chromosome reported in S3 Appendix.
Determination of function and homology of novel genes
To understand more about three ORFs for which no confident match could be found (ORFs 1–3) and the four which matched genes of unknown function in the Tbb genome (ORF 4–7), we analyzed them with the program Xtalpred [32]. Xtlapred uses a logarithmic opinion pool method to determine the feasibility of a given protein to crystallize and estimates a number of parameters relevant to the secondary structure of the protein. We used the Meta Server for Sequence Analysis (MESSA) [33] to predict the putative function of each ORF. This method implements a variety of search strategies to predict the structure and function of a protein from its amino acid sequence.
To further examine genes with no close match in either the Tbb or Tbg genome, we extracted all genes annotated with the same predicted function (i.e. pentatricopeptide containing protein genes (ORF 1–2) (n = 20) and oxidoreductase genes (ORF 3) (n = 89) from the TREU 927/4 Tbb genome [8] and performed two separate alignments with the ORFs detected in our study using MUSCLEv3.8.31 [46]. Partitioning scheme and substitution model selection for each alignment was conducted using PartitionFinder v1.1.1 [47] which identified a model with 3rd codon positions as a separate partition as optimal for both alignments and at GTR + I model as optimal for both 1st and 2nd codon partitions, and GTR and GTR+I+G models as optimal for the 3rd positions of the ORF 1 and 2, and ORF 3 alignments respectively. A maximum likelihood phylogeny was constructed for each of the two alignments using RaxMLv7.7.6 [38] with 1000 bootstrap replicates. The consensus trees was visualized using Figtreev1.3.1 [48].
Additionally, we aligned the flanking regions of each of the genes with no close match in in either the Tbb or Tbg genome to those respective genomes to verify the absence of each ORF. Each alignment was conducted with MUSCLEv3.8.31 [46] and are shown in S4 Appendix.
Ethics Statement
The isolate used for this study (STIB900) was collected by Dr Mantel Tanner as part of a diagnostic procedure in adherence to the medical ethics and the procedures of the Helsinki declaration for routine medical procedures.
Supporting Information
(FASTA)
(CSV)
(XLSX)
A) The region before, between and after ORF 1 and 2 aligned with the first chromosome of the Tbb and Tbg genomes B) The region before and after ORF 3 aligned with the ninth chromosome of the Tbb and Tbg genomes.
(NEX)
A) The number Raw PacBio SMRT sequencing reads recovered plotted against read length in base pairs B) All scaffolds resulting from hybrid de novo assembly of combined PacBio SMRT read and Illumina short read data plotted against scaffold length in base pairs.
(PDF)
The first three rows show the Tbr sequence before (first row), between (second row), and after (third row), compared to the sequence for chromosome 1 for Tbb (fourth row) and Tbg (fifth row). Colors indicate nucleotides (A—red, C—blue, G—orange, T—green). Dashed black lines represent putative indels. Numbers indicate genome positions in the Tbb and Tbg genomes and respective alignment positions in the flanking sequences.
(PDF)
The first three rows show the Tbr sequence before (first row), between (second row), and after (third row), compared to the sequence for chromosome 9 for Tbb (fourth row) and Tbg (fifth row). Colors indicate nucleotides (A—red, C—blue, G—orange, T—green). Dashed black lines represent putative indels. Numbers indicate respective positions in the Tbb and Tbg genomes and respective alignment positions in the flanking sequences.
(PDF)
Acknowledgments
This work was supported by NIH R21 grant AI094615-01 awarded to A.C. and S.A. The Yale University Biomedical High Performance Computing Center was used for all analyses.
Data Availability
Data have been deposited to GenBank: PRJNA310248.
Funding Statement
This work was supported by NIH R21 grant AI094615-01 awarded to A.C. and S.A. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010;375: 148–159. 10.1016/S0140-6736(09)60829-1 [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Neglected Tropical Diseases. 2009. 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson W. The SRA gene: the key to understanding the nature of Trypanosoma brucei rhodesiense. Parasitology. 2005;131: 143 [DOI] [PubMed] [Google Scholar]
- 4.Gibson WC. Will the real Trypanosoma b. gambiense please stand up. Parasitol Today. 1986;2: 255–257. 10.1016/0169-4758(86)90011-6 [DOI] [PubMed] [Google Scholar]
- 5.Simarro PP, Franco JR, Diarra A, Ruiz Postigo JA Jannin J. Diversity of human African trypanosomiasis epidemiological settings requires fine-tuning control strategies to facilitate disease elimination. Res Rep Trop Med. 2013;4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jannin J, Cattand P. Treatment and control of human African trypanosomiasis. Curr Opin Infect Dis. 2004;17: 565–571. 10.1097/00001432-200412000-00009 [DOI] [PubMed] [Google Scholar]
- 7.Organization WH. Uniting to combat neglected tropical diseases [Internet]. Available: www.unitingtocombatntds.org
- 8.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309: 416–422. 10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- 9.Jackson AP, Sanders M, Berry A, McQuillan J, Aslett MA, Quail MA, et al. The genome sequence of Trypanosoma brucei gambiense, causative agent of chronic human African Trypanosomiasis. PLoS Negl Trop Dis. 2010;4 10.1371/journal.pntd.0000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sistrom M, Evans B, Bjronson R, Gibson W, Balmer O, Maser P, et al. Comparative genomics reveals multiple genetic backgrounds of human pathogenicity in the Trypanosoma brucei complex. Genome Biol Evol. 2014;6: 2811–2819. 10.1093/gbe/evu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balmer O, Beadell JS, Gibson W, Caccone A. Phylogeography and taxonomy of Trypanosoma brucei. PLoS Negl Trop Dis. 2011;5 10.1371/journal.pntd.0000961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson WC, de C Marshall TF, Godfrey DG. Numerical analysis of enzyme polymorphism: a new approach to the epidemiology and taxonomy of trypanosomes of the subgenus Trypanozoon. Adv Parasitol. 1980;18: 175–246. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey DG, Baker RD, Rickman LR, Mehlitz D. The Distribution, Relationships and Identification of Enzymic Variants within the Subgenus Trypanozoon In: Baker JR MR, editor. Advances in Parasitology. 29th ed Academic Press; 1990. pp. 1–74. [DOI] [PubMed] [Google Scholar]
- 14.MacLeod A, Tait A, Turner CM. The population genetics of Trypanosoma brucei and the origin of human infectivity. Philos Trans R Soc Lond B Biol Sci. 2001;356: 1035–1044. 10.1098/rstb.2001.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echodu R, Sistrom M, Bateta R, Murilla G, Okedi L, Aksoy S, et al. Genetic Diversity and Population Structure of Trypanosoma brucei in Uganda: Implications for the Epidemiology of Sleeping Sickness and Nagana. PLoS Negl Trop Dis. Public Library of Science; 2015;9: e0003353 Available: doi: 10.1371%2Fjournal.pntd.0003353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodhead I, Capewell P, Wendi Bailey J, Beament T, Chance M, Kay S, et al. Whole-genome sequencing of Trypanosoma brucei reveals introgression between subspecies that is associated with virulence. MBio. 2013;4 10.1128/mBio.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy CW, MacLean L, Sweeney L, Cooper A, Turner CMR, Tait A, et al. Population Genetics of Trypanosoma brucei rhodesiense: Clonality and Diversity within and between Foci. PLoS Negl Trop Dis. 2013;7 10.1371/journal.pntd.0002526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn D, Cross GAM. Analysis of Trypanosoma brucei vsg expression site switching in vitro. Mol Biochem Parasitol. 1997;84: 189–201. 10.1016/S0166-6851(96)02794-6 [DOI] [PubMed] [Google Scholar]
- 19.Jackson AP, Berry A, Aslett M, Allison HC, Burton P, Vavrova-Anderson J, et al. Antigenic diversity is generated by distinct evolutionary mechanisms in African trypanosome species. Proc Natl Acad Sci. 2012;109: 3416–3421. 10.1073/pnas.1117313109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcello L, Barry JD. Analysis of the VSG gene silent archive in Trypanosoma brucei reveals that mosaic gene expression is prominent in antigenic variation and is favored by archive substructure. Genome Res. 2007;17: 1344–1352. 10.1101/gr.6421207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berriman M, Hall N, Sheader K, Bringaud F, Tiwari B, Isobe T, et al. The architecture of variant surface glycoprotein gene expression sites in Trypanosoma brucei. Mol Biochem Parasitol. 2002;122: 131–140. 10.1016/S0166-6851(02)00092-0 [DOI] [PubMed] [Google Scholar]
- 22.Capewell P, Clucas C, DeJesus E, Kieft R, Hajduk S, Veitch N, et al. The TgsGP Gene Is Essential for Resistance to Human Serum in Trypanosoma brucei gambiense. PLoS Pathog. 2013;9 10.1371/journal.ppat.1003686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uzureau P, Uzureau S, Lecordier L, Fontaine F, Tebabi P, Homblé F, et al. Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature. 2013;501: 430–4. 10.1038/nature12516 [DOI] [PubMed] [Google Scholar]
- 24.Capewell P, Veitch NJ, Turner CMR, Raper J, Berriman M, Hajduk SL, et al. Differences between Trypanosoma brucei gambiense groups 1 and 2 in their resistance to killing by trypanolytic factor 1. PLoS Negl Trop Dis. 2011;5 10.1371/journal.pntd.0001287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Xong H, Vanhamme L, Chamekh M, Chimfwembe CE, Van Den Abbeele J, Pays A, et al. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell. 1998;95: 839–846. 10.1016/S0092-8674(00)81706-7 [DOI] [PubMed] [Google Scholar]
- 26.Gibson W, Garside L, Bailey M. Trisomy and chromosome size changes in hybrid trypanosomes from a genetic cross between Trypanosoma brucei rhodesiense and T. b. brucei. Mol Biochem Parasitol. 1992;51: 189–199. 10.1016/0166-6851(92)90069-V [DOI] [PubMed] [Google Scholar]
- 27.Gibson WC. Analysis of a genetic cross between Trypanosoma brucei rhodesiense and T. b. brucei. Parasitology. 1989;99 Pt 3: 391–402. 10.1017/S0031182000059114 [DOI] [PubMed] [Google Scholar]
- 28.Gibson W, Peacock L, Ferris V, Fischer K, Livingstone J, Thomas J, et al. Genetic Recombination between Human and Animal Parasites Creates Novel Strains of Human Pathogen. PLoS Negl Trop Dis. Public Library of Science; 2015;9: e0003665 Available: doi: 10.1371%2Fjournal.pntd.0003665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 30.Brems S, Guilbride DL, Gundlesdodjir-Planck D, Busold C, Luu VD, Schanne M, et al. The transcriptomes of Trypanosoma brucei Lister 427 and TREU927 bloodstream and procyclic trypomastigotes. Mol Biochem Parasitol. 2005;139: 163–172. 10.1016/j.molbiopara.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 31.Cross GA, Kim HS, Wickstead B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol Biochem Parasitol. 2014;195: 59–73. 10.1016/j.molbiopara.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 32.Slabinski L, Jaroszewski L, Rychlewski L, Wilson IA, Lesley SA, Godzik A. XtalPred: A web server for prediction of protein crystallizability. Bioinformatics. 2007;23: 3403–3405. 10.1093/bioinformatics/btm477 [DOI] [PubMed] [Google Scholar]
- 33.Cong Q, Grishin NV. MESSA: Meta-Server for protein Sequence Analysis. BMC Biology. 2012. p. 82 10.1186/1741-7007-10-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aphasizheva I, Maslov D, Wang X, Huang L, Aphasizhev R. Pentatricopeptide Repeat Proteins Stimulate mRNA Adenylation/Uridylation to Activate Mitochondrial Translation in Trypanosomes. Mol Cell. 2011;42: 106–117. 10.1016/j.molcel.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos FMF, Liarte DB, Mortara RA, Romanha AJ, Murta SMF. Characterization of a gene encoding alcohol dehydrogenase in benznidazole-susceptible and -resistant populations of Trypanosoma cruzi. Acta Trop. 2009;111: 56–63. 10.1016/j.actatropica.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Fujioka S, Fukumoto S, Inoue N, Sakamoto K, Hirata H, et al. Trypanosome alternative oxidase, a potential therapeutic target for sleeping sickness, is conserved among Trypanosoma brucei subspecies. Parasitol Int. 2010;59: 560–564. 10.1016/j.parint.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 37.Geddy R, Brown GG. Genes encoding pentatricopeptide repeat (PPR) proteins are not conserved in location in plant genomes and may be subject to diversifying selection. BMC Genomics. 2007;8: 130 10.1186/1471-2164-8-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 39.Gibson W. Species concepts for trypanosomes: from morphological to molecular definitions? Kinetoplastid Biol Dis. 2003;2: 10 10.1186/1475-9292-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson W, Backhouse T, Griffiths A. The human serum resistance associated gene is ubiquitous and conserved in Trypanosoma brucei rhodesiense throughout East Africa. Infect Genet Evol. 2002;1: 207–214. 10.1016/S1567-1348(02)00028-X [DOI] [PubMed] [Google Scholar]
- 41.Andrews S. FastQC: A quality control tool for high throughput sequence data. Available: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. 2010.
- 42.Koren S, Schatz MC, Walenz BP, Martin J, Howard JT, Ganapathy G, et al. Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nature Biotechnology. 2012. pp. 693–700. 10.1038/nbt.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers EW, Sutton GG, Delcher AL, Dew IM, Fasulo DP, Flanigan MJ, et al. A whole-genome assembly of Drosophila. Science. 2000;287: 2196–2204. 10.1126/science.287.5461.2196 [DOI] [PubMed] [Google Scholar]
- 44.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5: 113 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- 48.Rambaut A. FigTree, a graphical viewer of phylogenetic trees. Inst Evol Biol Univ Edinburgh. 2009; Available: http://tree.bio.ed.ac.uk/software/figtree/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(FASTA)
(CSV)
(XLSX)
A) The region before, between and after ORF 1 and 2 aligned with the first chromosome of the Tbb and Tbg genomes B) The region before and after ORF 3 aligned with the ninth chromosome of the Tbb and Tbg genomes.
(NEX)
A) The number Raw PacBio SMRT sequencing reads recovered plotted against read length in base pairs B) All scaffolds resulting from hybrid de novo assembly of combined PacBio SMRT read and Illumina short read data plotted against scaffold length in base pairs.
(PDF)
The first three rows show the Tbr sequence before (first row), between (second row), and after (third row), compared to the sequence for chromosome 1 for Tbb (fourth row) and Tbg (fifth row). Colors indicate nucleotides (A—red, C—blue, G—orange, T—green). Dashed black lines represent putative indels. Numbers indicate genome positions in the Tbb and Tbg genomes and respective alignment positions in the flanking sequences.
(PDF)
The first three rows show the Tbr sequence before (first row), between (second row), and after (third row), compared to the sequence for chromosome 9 for Tbb (fourth row) and Tbg (fifth row). Colors indicate nucleotides (A—red, C—blue, G—orange, T—green). Dashed black lines represent putative indels. Numbers indicate respective positions in the Tbb and Tbg genomes and respective alignment positions in the flanking sequences.
(PDF)
Data Availability Statement
Data have been deposited to GenBank: PRJNA310248.