Abstract
Maternal obesity is associated with increased risk of developing diabetes, obesity and premature death in adult offspring. Mid-life diabetes, hypertension and hypercholesterolaemia are risk factors for the development of sporadic Alzheimer’s disease (AD). A key pathogenic feature of AD is the accumulation of β-amyloid (Aβ) in the brain. The purpose of this study was to investigate the effect of high fat diet feeding during early life on Aβ pathology in the Tg2576 mouse model of AD. Female mice were fed a standard (C) or high fat (HF) diet before mating and during gestation and lactation. At weaning, male offspring were fed a C diet. Significantly higher levels of guanidine-soluble Aβ and plaque loads were observed in the hippocampi of 11-month old Tg2576 mice born to mothers fed a HF diet. Changes in the extracellular matrix led to increased retention of Aβ within the parenchyma. These data support a role for maternal and gestational health on the health of the aged brain and pathologies associated with AD and may provide a novel target for both the prevention and treatment of AD.
Over 2.1 billion adults are currently estimated to be overweight or obese, of which approximately 38% are women of childbearing age1. Obesity is associated with an intake of diets that are high in saturated fat, typical of a Western diet2,3. A large body of experimental and epidemiological evidence supports a relationship between maternal diet, gestational environment and health in adulthood4. In particular, maternal obesity is associated with increased risk of coronary heart disease, diabetes, obesity, attention deficit hyperactivity disorder and premature death in adult offspring5,6,7. However, there is sparse evidence on how maternal obesity affects the susceptibility to neurodegenerative diseases such as Alzheimer’s disease (AD).
In addition to advanced age, the presence of diabetes, hypertension and hypercholesterolemia in mid-life increases the risk of developing AD8. AD is characterized pathologically by the intracellular accumulation of tau and the extracellular deposition of β-amyloid (Aβ) peptides in the parenchyma as plaques and in the walls of arteries and capillaries as cerebral amyloid angiopathy (CAA)9. Aβ42 is predominantly found in parenchymal plaques, while CAA is composed principally of Aβ4010. Aβ peptides of 38–42 amino acids are produced following the proteolytic cleavage of the amyloid precursor protein (APP) by β- and γ-secretase. Removal of Aβ from the brain is mediated by several mechanisms, including enzymatic cleavage by neprilysin and insulin degrading enzyme (IDE), uptake by microglia and perivascular macrophages, transcytosis across the blood-brain barrier and perivascular drainage along basement membranes of capillaries and arteries11,12,13,14,15,16.
Cerebrovascular basement membranes (CVBM) are specialized sheets of extracellular matrix that are composed of laminins, collagen IV and heparin sulphate proteoglycans such as perlecan and fibronectin and are in direct communication with the extracellular spaces of the parenchyma17. The composition of CVBM and the extracellular matrix influence the kinetics of Aβ aggregation. In particular, laminin, nidogens and collagen IV inhibit the aggregation of Aβ and destabilize pre-formed fibrils, while fibronectin and perlecan accelerate the aggregation of Aβ via high-affinity interactions18,19,20. In the aged and AD brain, there is a shift in the relative expression of basement membrane proteins towards those that promote Aβ aggregation, which contributes to a failure of perivascular drainage of Aβ from the brain and the development of CAA and dementia13,21,22,23,24,25,26.
The correlation between maternal obesity and the development of metabolic syndrome in adulthood and the increased risk of AD in individuals with metabolic syndrome in mid-life, suggests that a link may exist between maternal obesity and increased risk of AD. In a previous related study we demonstrated that maternal high fat diet resulted in failure of perivascular clearance of Aβ in normal offspring27. The aim of the current study was to investigate the effect of prenatal high fat diet exposure on Aβ pathology in the Tg2576 mouse model of AD.
Results
Prenatal HF increases levels of guanidine-soluble Aβ40 and Aβ42
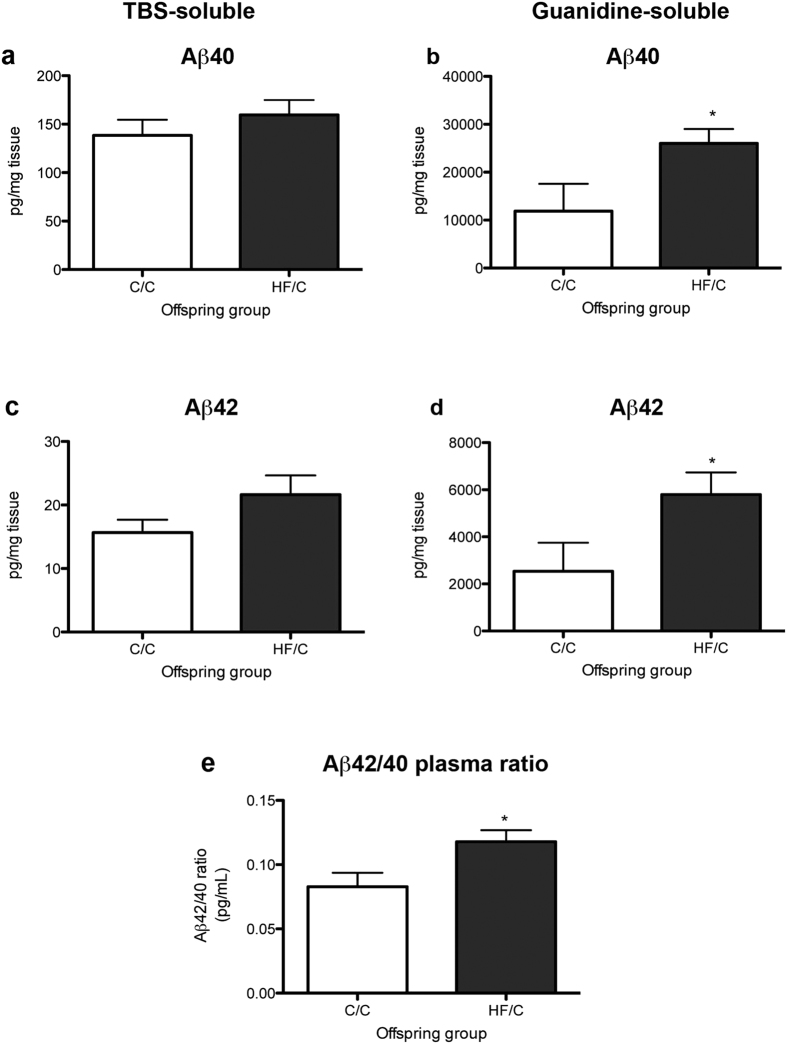
To determine the effect of early life high fat feeding on levels of Aβ, the hippocampi of 11-month old Tg2576 mice born to mothers fed a normal (C/C) and high fat diet (HF/C) were analysed for TBS- and guanidine-soluble human Aβ40 and Aβ42 by ELISA28. No differences were detected in the levels of TBS-soluble Aβ40 or Aβ42 between C/C and HF/C offspring (Fig. 1a,c). However, levels of guanidine-soluble Aβ40 and Aβ42 were both significantly higher in the brains of HF/C mice compared to C/C animals (Fig. 1b,d). Plasma Aβ42/40 ratios were also significantly elevated in HF/C vs. C/C mice (Fig. 1e).
Figure 1.
(a–d) Levels of hippocampal TBS-soluble human Aβ40 (a) and Aβ42 (c) did not differ between 11-month old C/C and HF/C Tg2576 mice. However, HF/C mice had significantly higher levels of guanidine-soluble Aβ40 (b) and Aβ42 (d) compared to C/C animals. (e) The plasma ratio of Aβ42:Aβ40 was also higher in HF/C vs. C/C mice. *p < 0.05.
Parenchymal Aβ40 plaque load and size are increased in HF/C mice
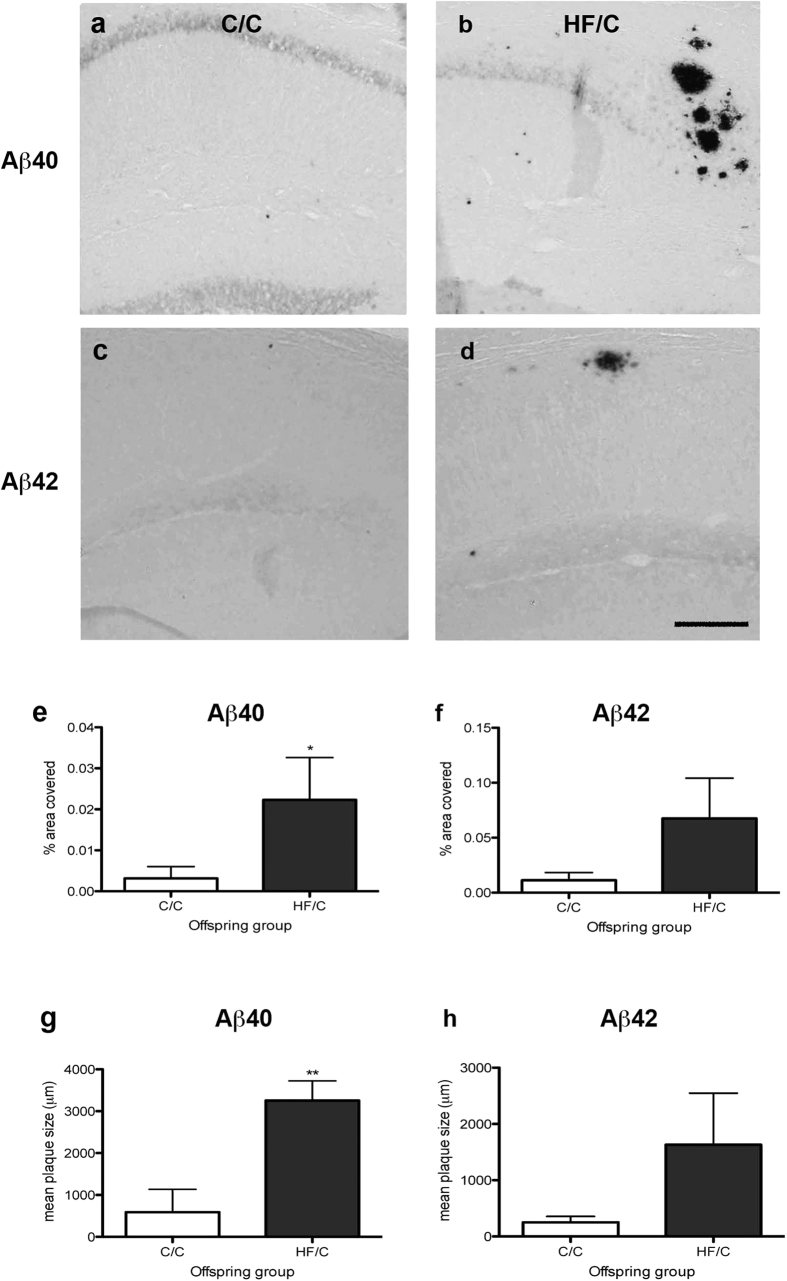
To confirm that the elevated levels of guanidine-soluble Aβ in HF/C mice were associated with increased Aβ pathology, brains tissue sections were processed for immunohistochemistry using antibodies against human Aβ40 and Aβ42. A few Aβ-positive dense-core parenchymal plaques were observed in the hippocampus of both C/C and HF/C mice, consistent with the amount of insoluble plaque deposition at this age29 (Fig. 2a–d). The percentage area covered by Aβ40-positive plaques within the hippocampus was significantly higher in HF/C mice compared to C/C offspring (Fig. 2e). A similar, but non-significant trend was also observed for Aβ42 (Fig. 2f). Analysis of average plaque size showed that Aβ40- and Aβ42-positive plaques were approximately 5.5 and 6.1 times larger, respectively, in HF/C mice than those observed in C/C animals (Fig. 2g,h).
Figure 2.
(a–d) Photomicrographs of staining of Aβ40- (a,b) and Aβ42-positive plaques (c,d) in the hippocampus of C/C (a,c) and HF/C (b,d) 11-month old Tg2576 mice. (e–h) Both percentage area and average size of Aβ40-positive plaques was significantly higher in HF/C mice compared to C/C offspring (e, g) with a similar non-significant trend observed for Aβ42 (f,h). Scale bar = 500 μm. *p < 0.05.
APP levels are decreased in HF/C mice
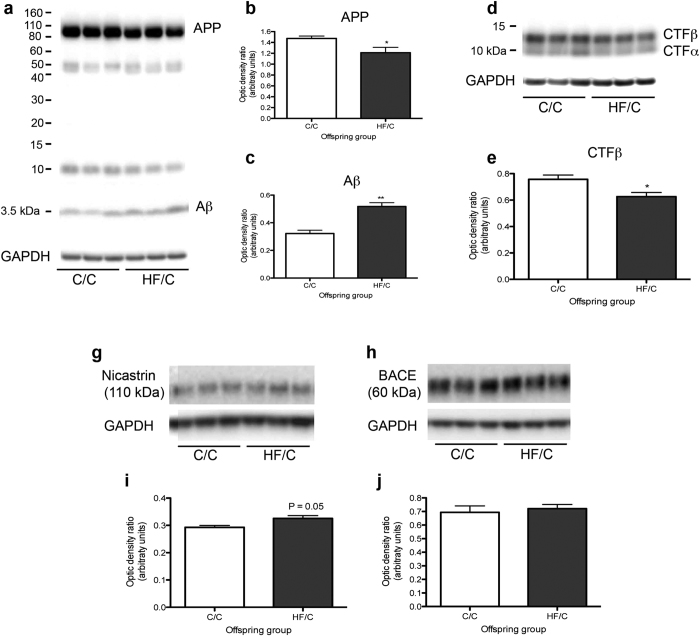
Elevated levels of Aβ may be due to increased APP processing and/or decreased Aβ clearance from the brain. To determine if levels of APP were altered between C/C and HF/C mice, hippocampi from Tg2576 mice were processed by Western blotting with antibodies against full-length and C-terminal fragments of APP (APP-CTF), Aβ1-16, BACE and the γ-secretase component nicastrin. Levels of full-length APP and APP-CTFβ were significantly decreased in the brains of HF/C mice, while levels of 4 kDa Aβ were increased (Fig. 3a–e). An increase in nicastrin expression, which bordered statistical significance (p = 0.05), was also noted in HF/C animals (Fig. 3g,i). Levels of BACE did not differ between offspring groups (Fig. 3h,j).
Figure 3.
(a–c) Levels of full-length APP were significantly decreased and levels of 4 kDa Aβ were increased in the hippocampus of 11-month old Tg2676 HF/C mice compared to C/C mice. (d,e) Levels of APP-CTFβ were also significantly decreased in the brains of HF/C mice (d, e) while APP-CTFα levels were unaltered between diet groups. (g–j) An increase in nicastrin expression, which bordered statistical significance (p = 0.05), was also noted in HF/C animals (g–i). No significant differences were noted in the levels of BACE (h,j). *p < 0.05, **p < 0.01.
Diffusion of Aβ within the extracellular matrix is reduced in HF/C mice
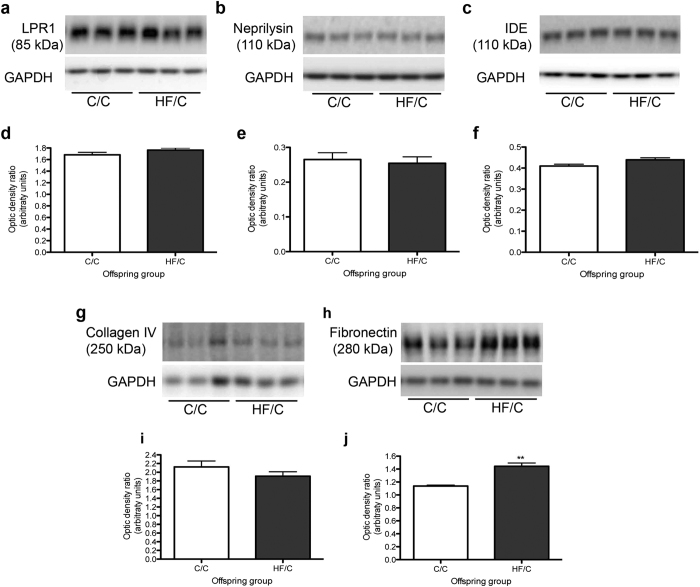
To determine whether the clearance of Aβ was affected by prenatal exposure to high fat, the levels of enzymes that degrade Aβ (neprilysin, insulin degrading enzyme (IDE)), the endothelial transporter low-density receptor-related protein-1 (LRP-1), as well as basement membrane proteins collagen IV and fibronectin were assessed by Western blotting. No significant differences were noted in the levels of LRP-1, neprilysin or IDE between C/C and HF/C mice (Fig. 4a–f). Collagen IV expression was also unchanged between diet groups (Fig. 4g,i). However, levels of fibronectin were significantly higher in the hippocampi of HF/C mice compared to the C/C group (Fig. 4j,j).
Figure 4.
(a–f) Levels of hippocampal expression of LRP-1 (a,d), neprilysin (b,e) and IDE (c,f) did not differ between C/C and HF/C mice. (g–j) Levels of collagen IV (g,i) were also unaltered between offspring groups. However fibronectin levels were significantly higher in the hippocampus of HF/C mice compared to C/C offspring (h,j). **p < 0.01.
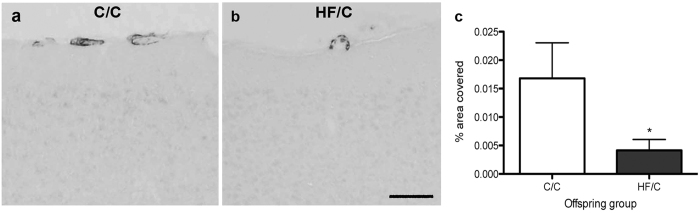
We have previously shown that cerebrovascular basement membranes are an extension of the parenchymal extracellular matrix17 and act as lymphatic drainage pathways along which Aβ is removed from the brain13. Failure of this clearance mechanism contributes to the accumulation of Aβ as CAA25. To evaluate if vascular Aβ was also affected by prenatal HF exposure, CAA severity was evaluated in cortical leptomeningeal arteries. As demonstrated in Fig. 5, vessel coverage by Aβ40-positive CAA was significantly lower in HF/C mice than in C/C mice (Fig. 5a–c). Quantification of Aβ42-positive vascular deposits was not possible as these deposits were rarely observed in both C/C and HF/C mice.
Figure 5.
(a,b) Photomicrographs of Aβ40-positive CAA staining in cortical leptomeningeal vessels of 11-month old C/C (a) and HF/C (b) Tg2576 mice. (c) Vessel coverage by Aβ40-positive staining was significantly lower in HF/C mice than in C/C mice. Scale bar = 500 μm. *p < 0.05.
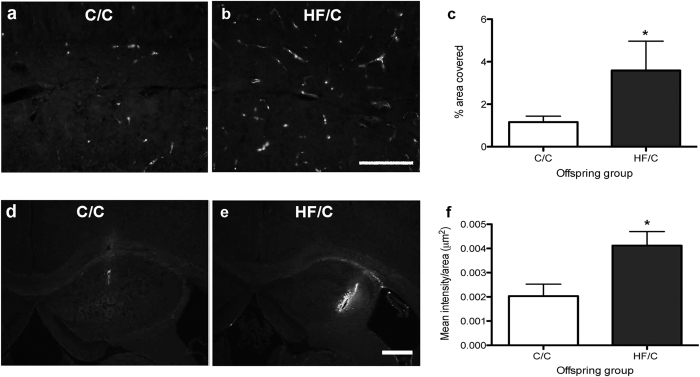
To determine if reduced CAA severity was due to diet-induced changes in the extracellular matrix that increase the retention of Aβ in the parenchyma, we first evaluated the distribution of fibronectin in the hippocampus of C/C and HF/C mice by immunohistochemistry. Consistent with the Western blotting data, fibronectin staining covered a significantly higher percentage of the hippocampus in HF/C mice compared to C/C animals (Fig. 6a–c). To evaluate if the diffusion of Aβ within the extracellular matrix was affected by HF diet independent of plaque pathology, 5-month old wildtype C/C and HF/C mice were injected with human Aβ40. Within 5 minutes post-injection, the majority of the Aβ had diffused away from the injection site of C/C mice (Fig. 6d). Over the same time period, significantly more Aβ was retained in the hippocampi of HF/C mice, as indicated by the increased amount of fluorescence measured at the site of injection (Fig. 6e,f).
Figure 6.
(a–c) Photomicrographs of fibronectin staining in the hippocampus of 11-month old C/C (a) and HF/C (b) Tg2576 mice. Fibronectin covered a significantly greater area in HF/C mice compared to C/C animals (c). (d,e) Photomicrographs of exogenous HilyteFluor-488 human Aβ40 injected into the hippocampus of 5-month old wildtype C/C (d) and HF/C mice (e). (f) Significantly more Aβ was retained at the injection site in the hippocampi of HF/C mice, as indicated by the increased mean fluorescence intensity measured at the site of injection. Scale bars: a and b = 500 μm, d and e = 400 μm. *p < 0.05.
Discussion
Maternal diet is key for the long-term health of the offspring30. In the present study, we found that 11-month old Tg2576 mice born to mothers fed a high fat diet during gestation and lactation developed higher levels of guanidine-soluble Aβ and hippocampal plaque load compared to mice born to mothers on a normal diet. Increased Aβ pathology was due in part to a failure of diffusion and increased retention of Aβ within the parenchyma. These data support a key role for early life environment on the health and function of the aged brain and pathologies associated with AD.
The risk of developing sporadic AD is influenced by modifiable factors such as smoking, obesity, and physical and cognitive activity31. Among these risk factors, obesity has emerged as a global issue, with approximately 40% of all adults currently estimated to have a body mass index over 25kg/m2 1. The majority of studies investigating the link between obesity and risk of AD have used models of postnatal high fat diet feeding. Pathological features related to AD, in association with decreased cognitive function have been reported in various transgenic mouse models of AD following consumption of diets high in saturated fats and/or cholesterol32,33,34,35.
Despite the established link between maternal obesity and the development in adulthood of conditions that themselves increase the risk of AD, such as obesity and diabetes8,36, very little work has been done to investigate the relationship between maternal obesity and risk of AD. We have previously reported that exposure to a high fat diet during brain development affects the expression of markers of the neurovascular unit and impairs the efficiency of Aβ clearance from the brain along cerebral basement membranes in adult offspring27. The present study expands upon these findings by demonstrating that Aβ pathology is higher in Tg2576 mice born to obese mothers, due in part to increased retention of Aβ in the parenchyma. Although Aβ production may also have been affected in HF/C mice, more detailed experiments are needed to definitively determine if the observed increase in levels of 4-kDa Aβ were due to increased APP processing or increased retention.
The findings of this study are in contrast to those of Martin et al.37, who reported decreased cognitive performance of 12-month old female 3xTg mice exposed to a high fat diet during gestation and lactation in the absence of changes in Aβ plaque load. Although the reasons for this discrepancy are unknown, they may relate to differences in diet composition, sex and/or transgene expression. Nevertheless, both studies support a role for maternal obesity in the etiology of AD-related pathology.
We have previously demonstrated that Aβ contained with the interstitial fluid of the brain is rapidly cleared from the parenchyma along cerebrovascular basement membranes which invaginate the extracellular space13,17,38. Impaired elimination of Aβ and other metabolites along perivascular pathways is hypothesized to trigger the rise in Aβ oligomers that drives the amyloid cascade with seeding of Aβ plaques, hyperphosphorylation of tau and propagation of neurofibrillary tangles, leading to synaptic dysfunction and death39. Changes in the glycoprotein and proteoglycan composition of the basement membrane and extracellular matrix influence the kinetics of Aβ aggregation and the efficiency by which Aβ is removed from the brain21,22,23,24,25. In particular, incubation of Aβ with fibronectin in vitro accelerates fibril formation21 and increased expression of vascular fibronectin has been shown to precede the development of CAA in vivo40. In the present study, fibronectin expression was increased in HF/C mice in association with decreased diffusion of Aβ within the extracellular matrix at 5 minutes after injection, suggesting that Aβ was retained within the parenchyma of HF/C mice. This is further supported by the findings that the parenchymal plaques in HF/C mice were significantly larger than those in C/C mice and that CAA severity in cortical leptomeningeal vessels was decreased in HF/C mice. This is in agreement with our previous findings that perivascular drainage of Aβ is disrupted in the presence of pre-existing plaques, which are bound by solutes contained within the ISF25. Collectively, these results suggest that early life exposure to a HF diet resulted in alterations in the extracellular matrix and lead to the retention of Aβ within the parenchyma. However, as the present study used only one time point to evaluate the diffusion of Aβ from the parenchyma, further work is needed to determine the time-course of Aβ clearance from the brains of HF/C mice.
Findings from the present study support the Latent Early-Life Associated Regulation hypothesis of AD proposed by Lahiri et al.41,42,43, which suggests that maternal diet influences early epigenetic changes that contribute to disease susceptibility in late life. As the incidence of obesity continues to rise in both girls and women of childbearing age1, it is imperative that the epigenetic and biochemical pathways that are influenced by maternal obesity are identified. Such information may provide a novel target for both the prevention and treatment of AD.
Methods
Mouse prenatal high fat feeding
Female C57Bl/6 and SJLxC57Bl/6 mice were fed a standard (C, 20% kcal fat, 17% kcal protein, 63% kcal carbohydrate, n = 6) or high fat diet (HF, 45% kcal fat, 20% kcal protein, 35% kcal carbohydrate; Special Diet Services, UK, n = 6), for four weeks before mating, as described previously27. Females were then mated with either wildtype or Tg2576 male mice. Tg2576 mice express the human Swedish APP mutation and show elevated levels of SDS-insoluble Aβ starting at 6 months of age29. Diffuse parenchymal plaques appear around 10 months of age in the cortex and hippocampus and as CAA in cortical leptomeningeal arteries29. Dams were kept on the diet during gestation and lactation, with litters standardized to 8 pups to avoid nutritional biases. Male offspring were fed a C diet from weaning, generating 2 offspring groups, C/C and HF/C, representing the pre- and post-weaning diet. Wildtype mice were aged to 5 months while the Tg2576 offspring were sacrificed at 11 months of age. All animal experiments were approved by the local Animal Welfare and Ethical Review Body at the University of Southampton and carried out in accordance with approved guidelines of the Home Office (30/3095).
Sandwich Aβ ELISA
Tg2576 mice (n = 3/group) were perfused intracardially with 0.01M phosphate buffered saline (PBS), brains rapidly removed and snap frozen. The hippocampus was dissected and sonicated in TBS extraction buffer (140 mM NaCl, 3 mM KCl, 25 mM Tris (pH 7.4), 1% Nonidet P-40, 5 mM EDTA) containing a protease inhibitor cocktail (Sigma Aldrich, Gillingham, UK). Homogenates were centrifuged (20 820 g, 15 mins, 4 °C) and supernatant collected as the soluble fraction. Pellets were sonicated in 5M guanidine HCl in 50 mM Tris (pH 8.0), incubated for 3 hours at room temperature, spun down (20 820 g, 20 mins, 4 °C) and supernatant collected as the guanidine-soluble fraction. Samples were diluted and analyzed using commercially available sandwich ELISA kits (Life Technologies, Paisley, UK) as per manufacturer’s instructions. Plasma samples obtained immediately after sacrifice were also assessed for Aβ levels. Samples were measured in duplicate, with mean values ± S.E.M. reported for treatment groups.
Immunohistochemistry
Mice (n = 5/group) were perfused intracardially with 0.01M PBS, followed by 4% paraformaldehyde, brains removed and post-fixed overnight. For enzyme-linked immunohistochemistry, brain tissue sections were washed in 0.01M PBS, treated with formic acid (70%, 30 sec) and incubated overnight at 4 °C with antibodies against human anti-Aβ40 (1:250, Merck Millipore, Watford, UK) and anti-Aβ42 (1:250, Merck Millipore). Sections were washed in 0.01M PBS, incubated with biotinylated anti-rabbit (1:400, Vector Labs, Peterborough, UK) and developed using glucose oxidase enhancement with DAB as chromagen. For fluorescent immunohistochemistry, tissue sections were washed in 0.01M PBS, treated with 1 mg/mL pepsin in 0.2N HCl (45 sec, 37 °C) and incubated overnight at 4 °C with anti-fibronectin (1:400, AbD Serotec, Kidlington, UK). Sections were washed in 0.01M PBS, incubated 2 hrs with AlexaFluor 488-conjugated anti-rabbit (1:200; Life Technologies) and coverslipped with mowiol. Images were captured on a Nikon microscope and exported to Photoshop CS. For quantification of percentage area covered by staining, micrographs were converted to binary images, thresholded to eliminate background staining and evaluated by densitometry using Image J software (NIH, Maryland, USA).
Western blotting
The hippocampi of Tg2576 mice (n = 3/group) were sonicated in Ripa lysis buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Igepal, 50 mM NaF, 1 mM NaVO3) containing a protease inhibitor cocktail. Proteins (15–45 μg) were separated by gel electrophoresis on 3–8% Tris-acetate or 10–20% Tris-Tricine gels (Life Technologies), transferred to nitrocellulose membranes and incubated with the following antibodies: anti-APP CTF (1:1000, BioLegend, London, UK), anti-Aβ1-16 (clone 6E10, 1:500, BioLegend), anti-nicastrin (1:500, New England Biolabs, Hitchin, UK), anti-β-secretase-1 (BACE, 1:750, New England Biolabs), anti-low-density receptor-related protein-1 (LRP-1, 1:2500, Insight Biotechnology, Wembley, UK), anti-IDE (1:750, Abcam, Cambridge, UK), anti-neprilysin (1:500, Abcam, Cambridge, UK), anti-fibronectin (1:750, AbD Serotec) and anti-collagen IV (1:500, Sigma Aldrich). Membranes were stripped and reprobed with anti-glyceraldehyde-3-PDH (GAPDH) antibody (1:50,000; Sigma Aldrich) to ensure equal protein loading. Immunoblots were repeated twice per antibody and quantified by densitometry using Image J software. Means ± S.E.M. were calculated as an optical density ratio of protein levels normalized to GAPDH levels.
Intracerebral injections
Wildtype mice (n = 5/group) were injected stereotaxically with HilyteFluor-488 human Aβ40 (100 μM, 0.5 μL, Anaspec, Fremont, USA) into the left hippocampus (coordinates from Bregma: AP = −1.9 mm; ML = 1.5 mm and DV = 1.7 mm). Injection pipettes were left in situ for 2 minutes to prevent reflux and mice were sacrificed 5 minutes post-injection. Mice were perfused intracardially with 0.01M PBS, followed by 4% paraformaldehyde, brains sectioned and coverslipped with Mowiol containing citiflor anti-fading reagent. Images were captured on a Nikon microscope and exported to Image J. The size of Aβ bolus was quantified by subtracting the mean grey value intensity of Aβ at the site of injection from the mean grey value intensity of the contralateral, non-injected hippocampus. Intensity values were then divided by the total hippocampal area of each section and expressed as mean intensity/μm2.
Statistical analysis
All mice were coded and measurements were carried out in a blinded fashion. Data obtained from ELISA and immunohistochemistry experiments were analyzed using a one-tailed Student’s t-test, where Aβ pathology was predicted to be greater in the HF/C group. Western blot and injection site data were analyzed using a two-tailed Student’s t-test. All data are presented as mean ± S.E.M. and in all cases significance was set at p < 0.05. Analyses were carried using GraphPad Prizm software.
Additional Information
How to cite this article: Nizari, S. et al. Increased Aβ pathology in aged Tg2576 mice born to mothers fed a high fat diet. Sci. Rep. 6, 21981; doi: 10.1038/srep21981 (2016).
Acknowledgments
This work was supported by Alzheimer’s Research UK and the Wessex Medical Trust.
Footnotes
Author Contributions C.H. and R.C. conceived the experiments, S.N. and C.H. conducted the experiments, S.N. and C.H. analysed the results and C.H. wrote the manuscript. All authors reviewed the manuscript.
References
- Ng M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781, 10.1016/S0140-6736(14)60460-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella D. et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr 141, 2219–2225, 10.3945/jn.111.143826 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. M. et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J Nutr 142, 824–831, 10.3945/jn.111.153460 (2012). [DOI] [PubMed] [Google Scholar]
- Barker D. J. & Martyn C. N. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health 46, 8–11 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlee S. D. & MacDougald O. A. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta 1842, 495–506, 10.1016/j.bbadis.2013.07.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. M. et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347, f4539, 10.1136/bmj.f4539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. L., Nousen E. K. & Chamlou K. A. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav 123, 236–242, 10.1016/j.physbeh.2012.07.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers K. et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry 30, 234–246, 10.1002/gps.4245 (2015). [DOI] [PubMed] [Google Scholar]
- Braak H. & Del Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer’s disease. Adv Anat Embryol Cell Biol 215, 1–162 (2015). [PubMed] [Google Scholar]
- Soontornniyomkij V., Choi C., Pomakian J. & Vinters H. V. High-definition characterization of cerebral beta-amyloid angiopathy in Alzheimer’s disease. Human pathology 41, 1601–1608, 10.1016/j.humpath.2010.04.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. D. & Zlokovic B. V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta neuropathol 118, 103–113, 10.1007/s00401-009-0522-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C. A., Deng L., Fenili D., Nitz M. & McLaurin J. In vivo uptake of beta-amyloid by non-plaque associated microglia. Current Alzheimer Res 9, 890–901, CAR-EPUB-20120123-021 (2012). [DOI] [PubMed] [Google Scholar]
- Hawkes C. A. et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-beta from the mouse brain. Aging cell 12, 224–236, 10.1111/acel.12045 (2013). [DOI] [PubMed] [Google Scholar]
- Hawkes C. A. & McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA 106, 1261–1266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners J. S. et al. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol 18, 240–252, BPA132 10.1111/j.1750-3639.2008.00132.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J. J. et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Science translational medicine 4, 147ra111, 10.1126/scitranslmed.3003748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. W., Carare R. O., Schreiber S. & Hawkes C. A. The Cerebrovascular Basement Membrane: Role in the Clearance of beta-amyloid and Cerebral Amyloid Angiopathy. Front Aging Neurosci 6, 251, 10.3389/fnagi.2014.00251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman F. C., Alvarez A., Morgan C. & Inestrosa N. C. Laminin blocks the assembly of wild-type A beta and the Dutch variant peptide into Alzheimer’s fibrils. Amyloid 5, 16–23 (1998). [DOI] [PubMed] [Google Scholar]
- Castillo G. M., Ngo C., Cummings J., Wight T. N. & Snow A. D. Perlecan binds to the beta-amyloid proteins (A beta) of Alzheimer’s disease, accelerates A beta fibril formation, and maintains A beta fibril stability. J Neurochem 69, 2452–2465 (1997). [DOI] [PubMed] [Google Scholar]
- Cotman S. L., Halfter W. & Cole G. J. Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer’s disease brain. Mol Cell Neurosci 15, 183–198, 10.1006/mcne.1999.0816 (2000). [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Ozawa D., Ookoshi T. & Naiki H. Surface-bound basement membrane components accelerate amyloid-beta peptide nucleation in air-free wells: an in vitro model of cerebral amyloid angiopathy. Biochim Biophys Acta 1834, 1624–1631, 10.1016/j.bbapap.2013.04.011 (2013). [DOI] [PubMed] [Google Scholar]
- Castillo G. M. et al. Laminin inhibition of beta-amyloid protein (Abeta) fibrillogenesis and identification of an Abeta binding site localized to the globular domain repeats on the laminin a chain. J Neurosci.Res. 62, 451–462 (2000). [DOI] [PubMed] [Google Scholar]
- Castillo G. M., Ngo C., Cummings J., Wight T. N. & Snow A. D. Perlecan binds to the beta-amyloid proteins (A beta) of Alzheimer’s disease, accelerates A beta fibril formation, and maintains A beta fibril stability. J Neurochem. 69, 2452–2465 (1997). [DOI] [PubMed] [Google Scholar]
- Snow A. D. et al. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron 12, 219–234 (1994). [DOI] [PubMed] [Google Scholar]
- Hawkes C. A. et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta neuropathol 121, 431–443, 10.1007/s00401-011-0801-7 (2011). [DOI] [PubMed] [Google Scholar]
- Mawuenyega K. G. et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774, 10.1126/science.1197623 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C. A., Gentleman S. M., Nicoll J. A. & Carare R. O. Prenatal high-fat diet alters the cerebrovasculature and clearance of beta-amyloid in adult offspring. J Pathol 235, 619–631, 10.1002/path.4468 (2015). [DOI] [PubMed] [Google Scholar]
- van Helmond Z., Miners J. S., Kehoe P. G. & Love S. Oligomeric Abeta in Alzheimer’s disease: relationship to plaque and tangle pathology, APOE genotype and cerebral amyloid angiopathy. Brain pathology 20, 468–480, 10.1111/j.1750-3639.2009.00321.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T. et al. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci 21, 372–381, 21/2/372 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hajj N., Schneider E., Lehnen H. & Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 148, R111–120, 10.1530/REP-14-0334 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W. Lifestyle neuropathology: how our behavior harms our brains and what we can do about it. Acta neuropathol 127, 1, 10.1007/s00401-013-1234-2 (2014). [DOI] [PubMed] [Google Scholar]
- Herculano B. et al. beta-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J Alzheimer Dis 33, 983–997, 10.3233/JAD-2012-121324 (2013). [DOI] [PubMed] [Google Scholar]
- Julien C. et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiology of aging 31, 1516–1531, 10.1016/j.neurobiolaging.2008.08.022 (2010). [DOI] [PubMed] [Google Scholar]
- Knight E. M., Martins I. V., Gumusgoz S., Allan S. M. & Lawrence C. B. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol aging 35, 1821–1832, 10.1016/j.neurobiolaging.2014.02.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesako M. et al. Continuation of exercise is necessary to inhibit high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. PloS one 8, e72796, 10.1371/journal.pone.0072796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake A. J. & Reynolds R. M. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 140, 387–398, 10.1530/REP-10-0077 (2010). [DOI] [PubMed] [Google Scholar]
- Martin S. A., Jameson C. H., Allan S. M. & Lawrence C. B. Maternal high-fat diet worsens memory deficits in the triple-transgenic (3xTgAD) mouse model of Alzheimer’s disease. PloS one 9, e99226, 10.1371/journal.pone.0099226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C. A. et al. Disruption of Arterial Perivascular Drainage of Amyloid-beta from the Brains of Mice Expressing the Human APOE epsilon4 Allele. PloS one 7, e41636, 10.1371/journal.pone.0041636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R. O., Hawkes C. A., Carare R. O. & Hardy J. Does the difference between PART and Alzheimer’s disease lie in the age-related changes in cerebral arteries that trigger the accumulation of Abeta and propagation of tau? Acta neuropathol 129, 763–766, 10.1007/s00401-015-1416-1 (2015). [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T., Lin C., Sanan D. A., Mucke L. & Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156, 139–150 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D. K., Zawia N. H., Greig N. H., Sambamurti K. & Maloney B. Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology 9, 375–379, 10.1007/s10522-008-9162-6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D. K., Maloney B. & Zawia N. H. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry 14, 992–1003, 10.1038/mp.2009.82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D. K., Maloney B., Basha M. R., Ge Y. W. & Zawia N. H. How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Current Alzheimer Res 4, 219–228 (2007). [DOI] [PubMed] [Google Scholar]