Abstract
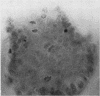
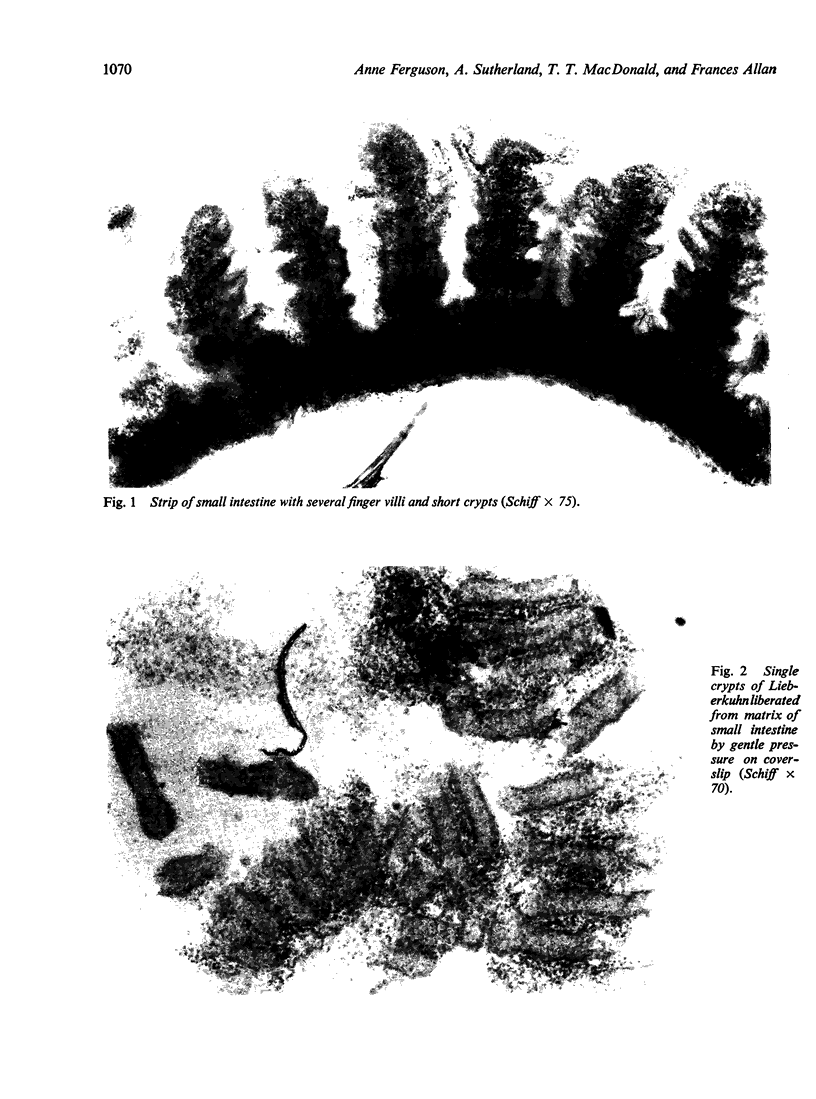
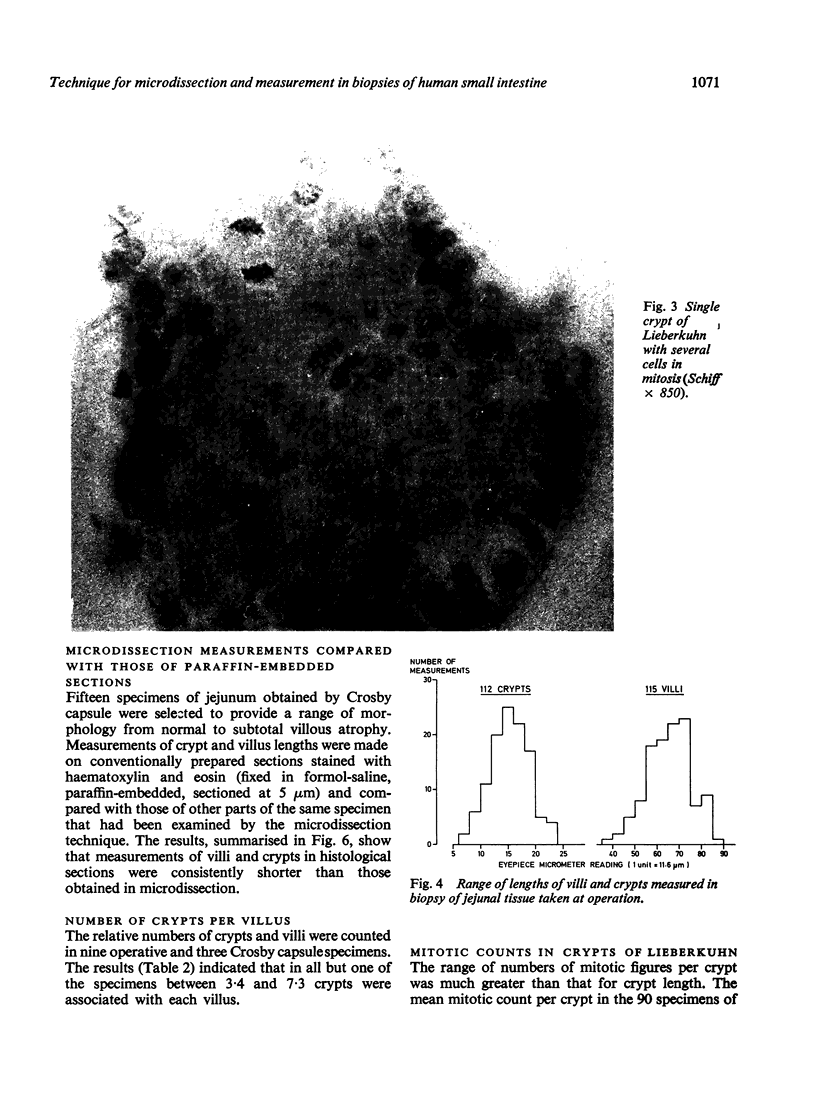
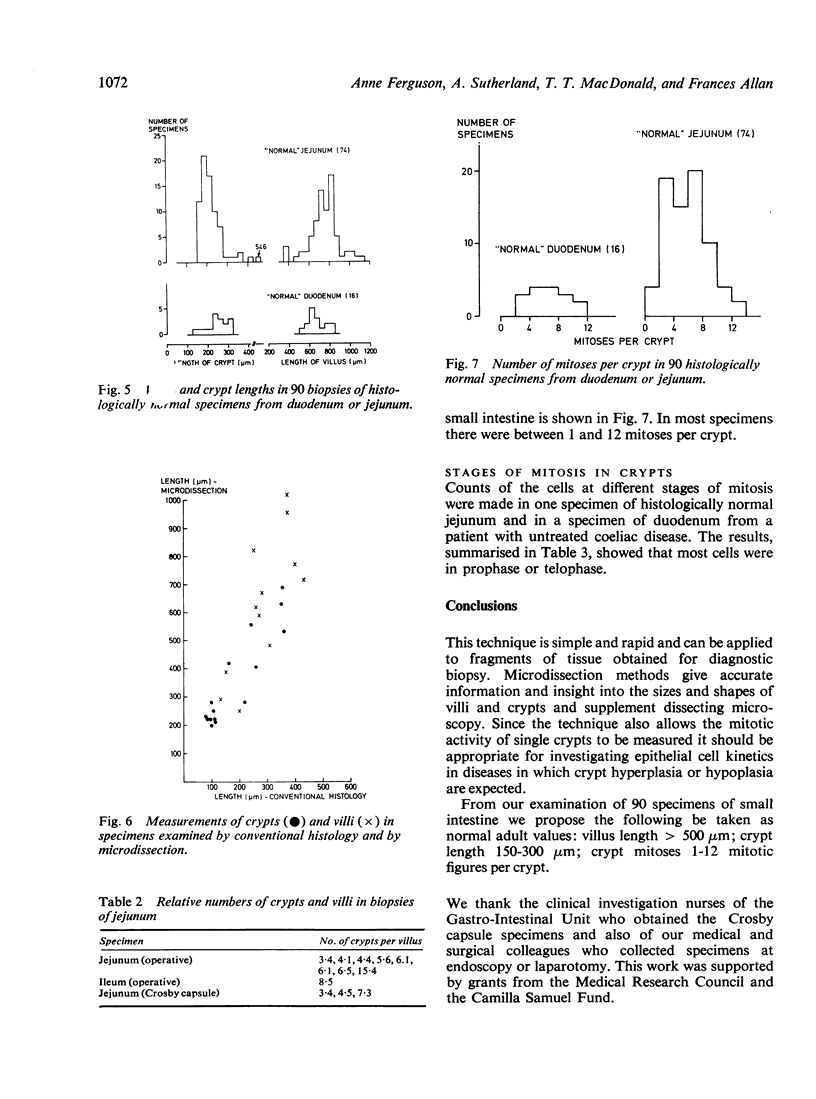
A microdissection and measurement technique has been adapted for biopsy of human small intestine. Specimens fixed in alcohol and acetic acid are Schiff stained in bulk. Villi and crypts are then dissected out under a dissecting microscope, placed under a coverslip, examined, measured, and the number of mitoses in individual crypts counted. With this method specimens of normal small intestine have been found to have villi 500 microns to 1100 microns long and crypts 150 microns to 300 microns. These values were double those obtained when measuring sections of the same specimens stained with haematoxylin and eosin. The mean number of mitoses per crypt in normal duodenum and jejunum ranged from 1 to 12 and most of the cells in mitosis were in prophase or telophase. This rapid, sensitive, and inexpensive technique complements the available methods of measuring small intestinal architecture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairnie A. B., Millen B. H. Fission of crypts in the small intestine of the irradiated mouse. Cell Tissue Kinet. 1975 Mar;8(2):189–196. doi: 10.1111/j.1365-2184.1975.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Clark R. M. Evidence for both luminal and systemic factors in the control of rat intestinal epithelial replacement. Clin Sci Mol Med. 1976 Feb;50(2):139–144. doi: 10.1042/cs0500139. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Control of intestinal epithelial replacement: lack of evidence for a tissue-specific blood-borne factor. Cell Tissue Kinet. 1974 May;7(3):241–250. doi: 10.1111/j.1365-2184.1974.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Diet, mucosal architecture and epithelial cell production in the small intestine of specified-pathogen-free and conventional rats. Lab Anim. 1975 Jul;9(3):201–209. doi: 10.1258/002367775780994600. [DOI] [PubMed] [Google Scholar]
- Clarke R. M., Ecknauer R., Feyerabend G. Analysis of the effects of food and of digestive secretions on the small intestine of the rat. 1. Mucosal morphology and epithelial replacement. Gut. 1976 Nov;17(11):895–899. doi: 10.1136/gut.17.11.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. Progress in measuring epithelial turnover in the villus of the small intestine. Digestion. 1973;8(2):161–175. doi: 10.1159/000197311. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. The effect of growth and of fasting on the number of villi and crypts in the small intestine of the albino rat. J Anat. 1972 May;112(Pt 1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. The time-course of changes in mucosal architecture and epithelial cell production and cell shedding in the small intestine of the rat fed after fasting. J Anat. 1975 Nov;120(Pt 2):321–327. [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Hypersensitivity reactions in the small intestine. III. The effects of allograft rejection and of graft-versus-host disease on epithelial cell kinetics. Cell Tissue Kinet. 1977 Jul;10(4):301–312. [PubMed] [Google Scholar]