Abstract
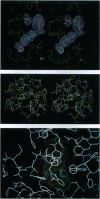
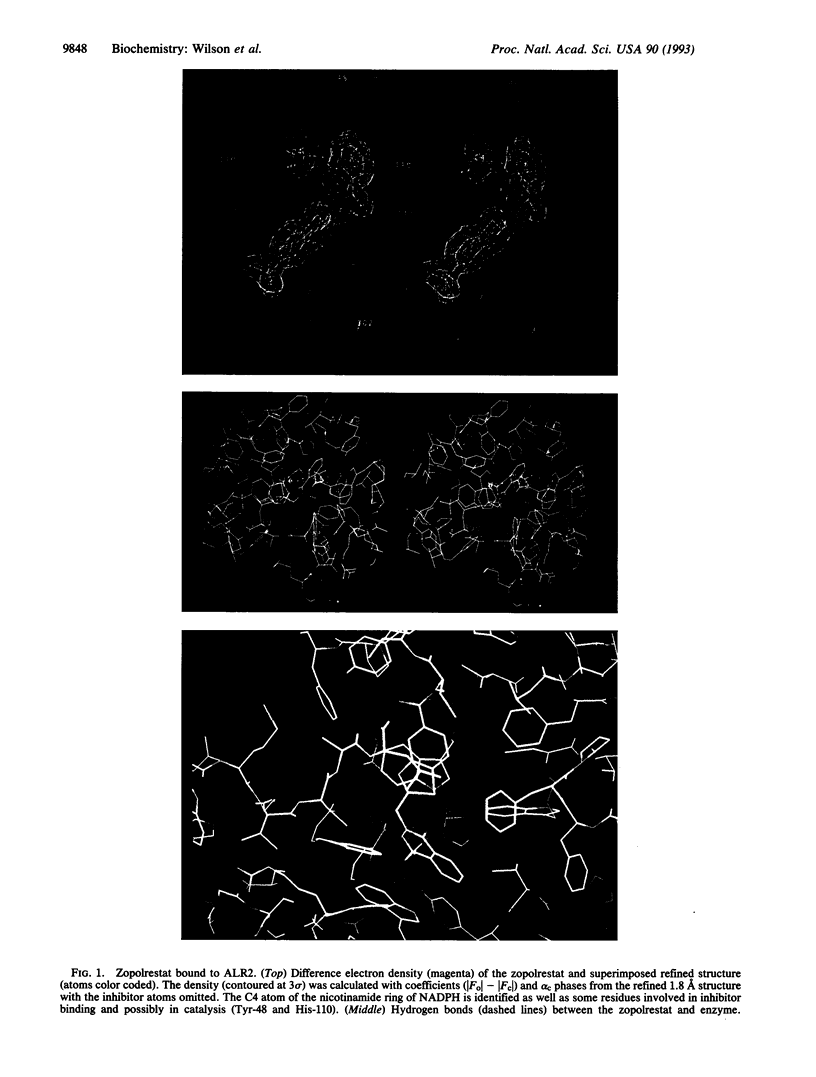
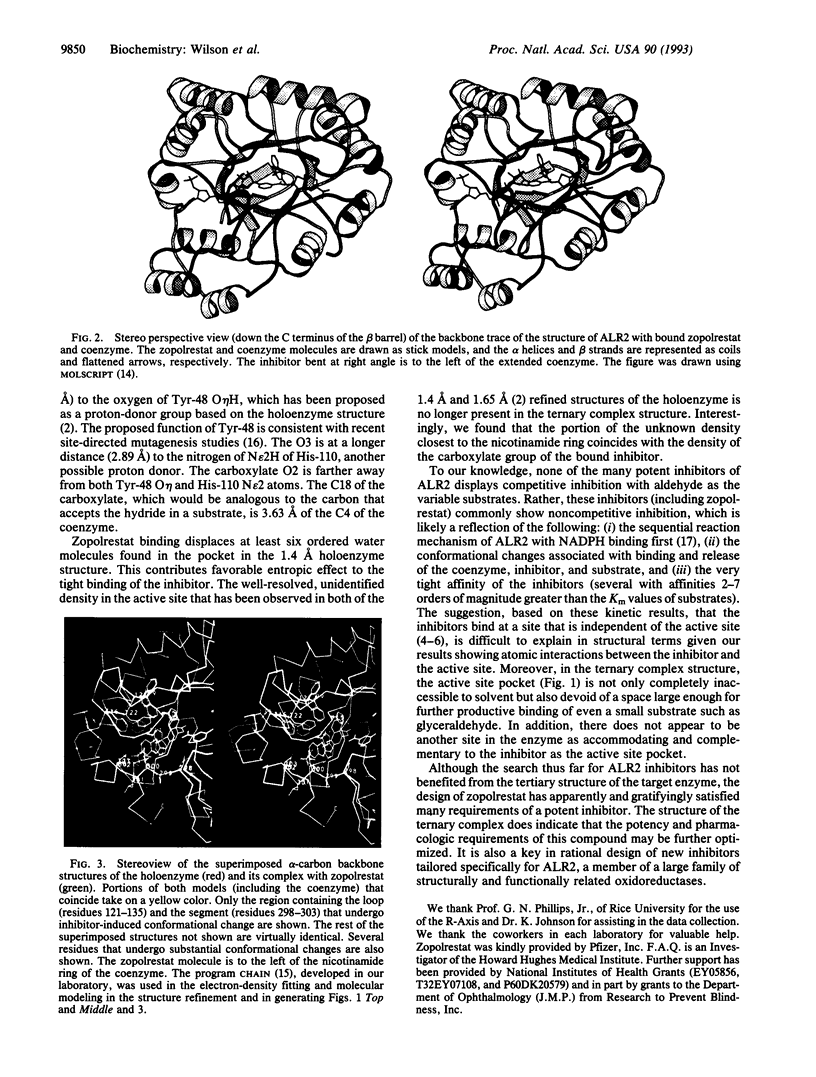
As the action of aldose reductase (EC 1.1.1.21) is believed to be linked to the pathogenesis of diabetic complications affecting the nervous, renal, and visual systems, the development of therapeutic agents has attracted intense effort. We report the refined 1.8 A x-ray structure of the human holoenzyme complexed with zopolrestat, one of the most potent noncompetitive inhibitors. The zopolrestat fits snugly in the hydrophobic active site pocket and induces a hinge-flap motion of two peptide segments that closes the pocket. Excellent complementarity and affinity are achieved on inhibitor binding by the formation of 110 contacts (< or = 4 A) with 15 residues (10 hydrophobic), 13 with the NADPH coenzyme and 9 with four water molecules. The structure is key to understanding the mode of action of this class of inhibitors and for rational design of better therapeutics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borhani D. W., Harter T. M., Petrash J. M. The crystal structure of the aldose reductase.NADPH binary complex. J Biol Chem. 1992 Dec 5;267(34):24841–24847. doi: 10.2210/pdb1abn/pdb. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Sharpless N. E. Pharmacophor requirements of the aldose reductase inhibitor site. Mol Pharmacol. 1983 Nov;24(3):521–531. [PubMed] [Google Scholar]
- Kador P. F. The role of aldose reductase in the development of diabetic complications. Med Res Rev. 1988 Jul-Sep;8(3):325–352. doi: 10.1002/med.2610080302. [DOI] [PubMed] [Google Scholar]
- Kubiseski T. J., Hyndman D. J., Morjana N. A., Flynn T. G. Studies on pig muscle aldose reductase. Kinetic mechanism and evidence for a slow conformational change upon coenzyme binding. J Biol Chem. 1992 Apr 5;267(10):6510–6517. [PubMed] [Google Scholar]
- Morjana N. A., Flynn T. G. Aldose reductase from human psoas muscle. Purification, substrate specificity, immunological characterization, and effect of drugs and inhibitors. J Biol Chem. 1989 Feb 15;264(5):2906–2911. [PubMed] [Google Scholar]
- Mylari B. L., Larson E. R., Beyer T. A., Zembrowski W. J., Aldinger C. E., Dee M. F., Siegel T. W., Singleton D. H. Novel, potent aldose reductase inhibitors: 3,4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl] methyl]-1-phthalazineacetic acid (zopolrestat) and congeners. J Med Chem. 1991 Jan;34(1):108–122. doi: 10.1021/jm00105a018. [DOI] [PubMed] [Google Scholar]
- Petrash J. M., Harter T. M., Devine C. S., Olins P. O., Bhatnagar A., Liu S., Srivastava S. K. Involvement of cysteine residues in catalysis and inhibition of human aldose reductase. Site-directed mutagenesis of Cys-80, -298, and -303. J Biol Chem. 1992 Dec 5;267(34):24833–24840. [PubMed] [Google Scholar]
- Rondeau J. M., Tête-Favier F., Podjarny A., Reymann J. M., Barth P., Biellmann J. F., Moras D. Novel NADPH-binding domain revealed by the crystal structure of aldose reductase. Nature. 1992 Jan 30;355(6359):469–472. doi: 10.1038/355469a0. [DOI] [PubMed] [Google Scholar]
- Sarges R., Oates P. J. Aldose reductase inhibitors: recent developments. Prog Drug Res. 1993;40:99–161. doi: 10.1007/978-3-0348-7147-1_5. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Murdock G. L., Ma Y., Goodman S. R., Zimmer W. E. Molecular cloning of testicular 20 alpha-hydroxysteroid dehydrogenase: identity with aldose reductase. Biochemistry. 1993 Feb 16;32(6):1401–1406. doi: 10.1021/bi00057a003. [DOI] [PubMed] [Google Scholar]
- Wilson D. K., Bohren K. M., Gabbay K. H., Quiocho F. A. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992 Jul 3;257(5066):81–84. doi: 10.1126/science.1621098. [DOI] [PubMed] [Google Scholar]